Abstract
Background
There is a significant prevalence of new onset neuropsychiatric symptoms (NPS), some severe and persistent, in patients with coronavirus disease 2019 (COVID-19).
Objective
This study reports on the use of electroconvulsive therapy (ECT) to treat NPS associated with COVID-19.
Methods
A review of the literature pertaining to the use of ECT in patients with COVID-19 and NPS was performed through PubMed, PsycINFO, and MEDLINE. Search terms included “Electroconvulsive Therapy” and “ECT,” combined with “COVID-19” and “Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV-2).” In addition, we present a case in which ECT was used to achieve complete remission in a patient who developed new onset, treatment-resistant depression, psychosis, and catatonia, associated with COVID-19.
Results
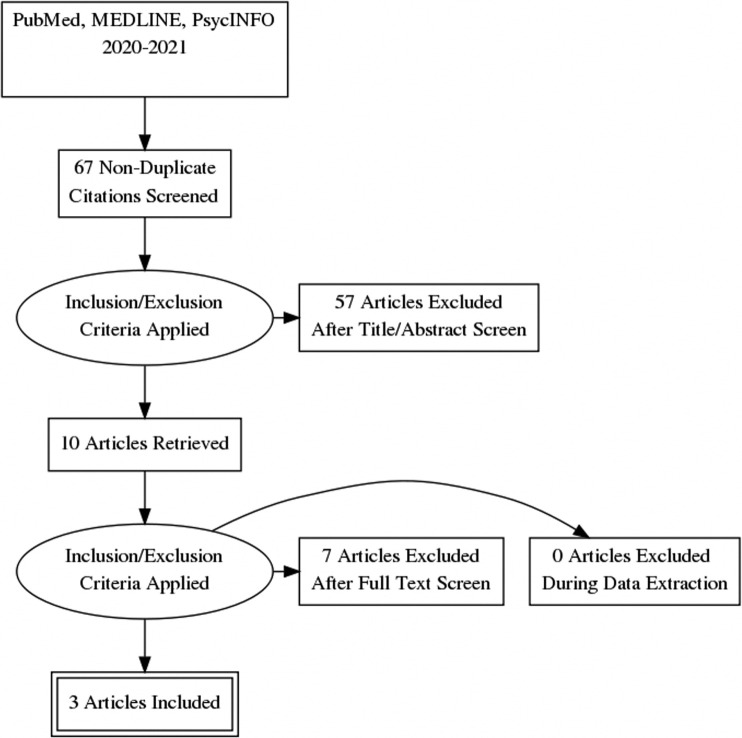
A total of 67 articles were reviewed with 3 selected for inclusion. These articles detailed 3 case reports of patients with new onset NPS (mania, psychosis and suicidality, and catatonia) that developed in the context of active COVID-19 and were treated successfully with ECT.
Conclusions
ECT, a broad-spectrum treatment that has been found to be effective in various NPS (independent of etiology), is shown in our case report and others, to be safe and effective for NPS associated with COVID-19. Although we identified only 3 other cases in the literature, we believe that the probable antiinflammatory mechanism of ECT, its safety and tolerability, and the faster time to symptom remission support the need for more research and increased clinician awareness about this life-saving procedure.
Key words: COVID-19, psychiatric, neuropsychiatric, psychosis, catatonia, electroconvulsive therapy
Introduction
Coronavirus disease 2019 (COVID-19), the illness caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, has been increasingly recognized to be associated with neuropsychiatric complications such as delirium, depression, psychosis, and catatonia.1 , 2 While the underlying pathophysiology of neuropsychiatric symptoms (NPS) in COVID-19 is not yet well understood, these complications are thought to be caused by direct CNS invasion or indirect neuroinflammation which develops during the acute infectious period.1 Reports on NPS in patients with COVID-19 demonstrate a significant prevalence, with some persisting long after acute illness resolution.3, 4, 5 In one study of 40,496 patients diagnosed with COVID-19, 22.5% were found to have NPS.3 In a surveillance study of 153 patients with COVID-19 in the United Kingdom, almost 60% of patients that presented with altered mental status were found to have NPS. Alarmingly, more than 90% of the neuropsychiatric presentations were classified as new diagnoses, which included psychosis, affective disorders, and “dementia-like” syndromes.4 The high prevalence of these complications, sometimes extending far beyond diagnosis and hospital stay,5 underscores the need for safe and effective treatment options.
Electroconvulsive therapy (ECT) has been used since the 1930s as a treatment for severe psychiatric conditions, particularly those resistant to pharmacotherapy.6 ECT has been found effective for depression, mania, psychosis, agitation in delirium, and catatonia.7 , 8 ECT has also been used to treat psychiatric symptoms that develop as a result of autoimmune-driven inflammatory processes.9 , 10 The nonspecific neuropsychiatric syndrome of catatonia can be caused by both medical and psychiatric conditions, including infection, and is responsive to ECT.11 , 12 COVID-19 may result in psychiatric sequelae, acute and chronic, which may be managed by ECT.
An overwhelmingly safe and well-tolerated procedure, ECT has no absolute contraindications.8 , 13 ECT is highly effective compared with medications and may provide the benefit of faster time to remission.6 Because of the increasing evidence of psychiatric manifestations of COVID-19, some of which can become severe and life-threatening, we believe that more clinicians need to be made aware of this important treatment option. Isolation requirements, limitation on procedures, and possibility of aerosolization specific to ECT have impaired access to this effective and necessary procedure during the pandemic.14 Despite these challenges, NPS of infectious diseases highlight the need for ECT to remain a non-elective procedure during global pandemics. We agree with the many articles already published that have voiced concern over this major problem in ECT access.14, 15, 16 The following case report and review of the literature emphasizes the important potential role of ECT in the treatment of COVID-19 NPS.
Case Report
A 52-year-old woman without prior psychiatric history was admitted to our hospital in March 2021 for depression and psychosis, which developed during mild COVID-19 illness. She had well-controlled type II diabetes, mild hypertension, and postviral reactive airway disease periodically requiring oral steroids. She was otherwise healthy and working full-time before symptom onset.
After testing positive for COVID-19 by polymerase chain reaction in early January 2021, her primary care provider prescribed azithromycin and prednisone 50 mg for mild upper respiratory symptoms. She developed insomnia and anxiety; symptoms progressed despite self-discontinuation of prednisone after 2 doses. In early February, she was hospitalized by her primary care provider for worsening depression and paranoia.
Magnetic resonance imaging with and without contrast of the brain, electroencephalography, serum rheumatologic panel (Smith Ab, DNA Ab, SS-A and SS-B, ANA, RF, Anti-TPO, C4, C3), cerebrospinal fluid (CSF) (cell counts, gram stain and cultures; PCR for West Nile, JCV, and COVID-19), and encephalitis panel (S AMPA-R CBA Ab, Ampiphysin Ab, Anti-glial nuclear Ab Type 1, Anti-neuronal nuclear Ab Type 1, 2, 3, CASPR-IgG CBA, CRMP-5-IgG, DPPX Ab IFA, GABA-B-R Ab CBA, GAD65 Ab Assay, GFAP IFA, IgLON5 IFA, LGI1-IgG CBA, mGluR1 Ab IFA, NIF IFA, NMDA-R Ab CFA, Purkinje Cell Cytoplasmic Ab Type Tr, 1, and 2, N-Type Calcium Channel Ab, P/Q-Type Calcium Channel Ab) were negative. Notably, CSF IgG was mildly elevated (4.15), as were ferritin (238 ng/mL), C-reactive protein (1.35 mg/dL), erythrocyte sedimentation rate (34 mm/hr), and interleukin-6 (17 pg/mL).
Given suspicion for a neuroinflammatory etiology of the symptoms, she was treated with IV methylprednisolone 1000 mg for 5 days, with minimal, temporary improvement. Psychopharmacological trials of short duration were attempted without significant change (escitalopram to 10 mg for 3 days, sertraline to 100 mg for 5 days, risperidone to 3 mg for 8 days, aripiprazole to 15 mg for 10 days, olanzapine to 15 mg for 10 days). She was discharged to a neurorehabilitation center and presented to our hospital within 48 hours for evaluation of suicidal thoughts.
She presented with depressed mood, psychomotor slowing, paranoia, and nihilistic delusions. She was started on olanzapine 10 mg and sertraline 100 mg. Given symptom severity and minimal improvement with psychopharmacology, ECT consult was placed. Although initially hesitant, she demonstrated capacity for consent and received her first treatment of bitemporal ECT on hospital day (HD) 19. The first 3 treatments were not ideally spaced (QOD) owing to her delusions interfering with treatment.
ECT infrequency and disease progression likely contributed to worsening withdrawal and motor symptoms concerning for catatonia. Olanzapine was discontinued, but her oral intake continued to decline, and by HD 26 (after ECT #3), she developed full catatonia.
On HD 27, she was immobile, negativistic, and mute, with a Bush-Francis Catatonia Rating Scale (BFCRS) score of 18. She was transferred to a medical unit for fluids and IV lorazepam. By HD 30, she was able to consent to restart ECT, and she completed treatments 4–9 on a Monday, Wednesday, Friday schedule. Treatments were confounded by concurrent use of lorazepam, 8 mg total daily. Despite the use of flumazenil (up to 1mg), seizures were of short duration, and she was rapidly uptitrated to 100% energy (Somatics Thymatron System IV, pulse width 0.5 msec, current 0.91 amps, stimulus duration 8 sec, frequency 70 Hz, charge 508.6 mC.).
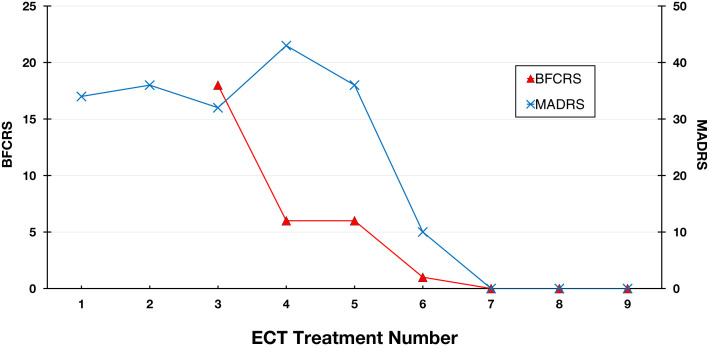
As the index course progressed, her mental status improved substantially. Her BFCRS and Montgomery-Asberg Depression Rating Scale (MADRS) scores dropped to 0 (Figure 1 ). After ECT, lorazepam was tapered completely, without recurrence of catatonia. She showed a marked improvement in affect, interpersonal engagement, and self-care. She was discharged to family on HD 47 and continued in her recovered state at a 1-month psychiatric follow-up.
Figure 1.
Symptom Rating Scales.
Methods
We conducted a review of available literature on the use of ECT in patients with psychiatric complications of COVID-19. PubMed, MEDLINE, and PsycINFO databases were searched independently by 2 of the authors (G.A. and R.L.). The following terms were used: “Electroconvulsive Therapy” and “ECT,” combined with “COVID-19” and “SARS-COV-2.” These searches were performed in May 2021 and included literature published from 2020 onward. A total of 67 results were returned, after removing duplicates. The full texts of English-language articles meeting inclusion criteria were reviewed. Details extracted from each case report included the patient's age, psychiatric diagnoses, onset of psychiatric illness in relation to COVID-19 infection, details of ECT treatment, and outcome of treatment.
Inclusion/Exclusion Criteria
Included texts described administration of ECT to patients with psychiatric illness that developed in the context of COVID-19 infection. Only articles available in English language were included. Texts excluded did not pertain to the use of ECT in patients with psychiatric complications of COVID-19 specifically. If the patient's psychiatric indication for ECT predated the COVID-19 infection, the article was excluded, even if there was a decompensation related to COVID-19 (Figure 2 ).
Figure 2.
Study Inclusion and Exclusion Methodology.
Results
After excluding 64 articles that did not meet our criteria, 3 articles were included in our review.17, 18, 19 Through this review, we identified 3 patients that developed psychiatric problems during COVID-19 infection and who were treated with ECT. Extracted data are summarized in Table 1 .
Table 1.
Literature Review of the Use of ECT in COVID-19 NPS
| Authors & year | Patient age and sex | Psychiatric comorbidities | Indication for ECT | Pharmacological treatment | Method of C-19 diagnosis | Notable laboratorty results | # Treatments | Treatment outcome |
|---|---|---|---|---|---|---|---|---|
| Kashaninasab et al., 202017 | 25-year-old male | None reported | Mania, severe, with psychotic features | Initially haloperidol 20 mg IM daily, biperiden 10 mg IM daily, and chlorpromazine 100 mg IM daily; gradually replaced by 1500 mg sodium valproate daily | Clinical; CT Chest | 6 | Patient was discharged in stable condition | |
| Chacko et al., 202018 | 52-year-old male | None | Psychosis, suicidality | Fluoxetine 20 mg daily, olanzapine 5 mg po BID, and lorazepam up to 2 mg po BID | Clinical; SARS-CoV-2 IgG (+), 3 weeks after discharge | Elevated LFTs and inflammatory markers (ESR = 40 mm/hr, CRP = 1.5 mg/dL, D-dimer = 1003 ng/mL) | 6 | Patient was discharged in improved condition with no thoughts of suicide |
| Deocleciano de Araujo et al., 202119 | 50-year-old male | Mild IDD, well-controlled epilepsy | Catatonia | Diazepam 10 mg IV QID, later substituted by lorazepam 2 mg po TID; sertraline 25 mg daily, and olanzapine 5 mg daily | PCR | CSF protein (55 mg/dL), CK 8819 U/l, WBC 20.8 x 109/L | 10 | Patient was discharged “fully recovered” and “back to his usual self” |
CRP = C-reactive protein; CSF = cerebrospinal fluid; ECT = electroconvulsive therapy; ESR = erythrocyte sedimentation rate; IDD = intellectual and developmental disabilities; PCR = polymerase chain reaction; SARS-CoV-2 = Severe Acute Respiratory Syndrome Coronavirus 2.
Case Summaries
Kashaninasab et al17 describe a 25-year-old man who experienced a “first episode” of severe mania with psychotic features in the context of COVID-19 illness. Acute symptoms were not responsive to antipsychotics, so ECT was started on HD 3. After 6 treatments, he was discharged on sodium valproate 1500 mg daily with controlled psychotic and mood symptoms.
Chacko et al18 report on a 52-year-old man with obstructive sleep apnea and no prior psychiatric history who developed psychosis and mutism after a COVID-19 exposure. Symptoms were initially relieved with lorazepam, and he was clinically diagnosed with COVID-19 and discharged on antibiotics. He was readmitted to the hospital for ongoing psychosis and suicidality. He remained suicidal despite pharmacotherapy; therefore, ECT was initiated. He was discharged on oral medications and in improved condition after 6 treatments.
Deocleciano de Araujo et al19 report on a 50-year-old man with intellectual and developmental disabilities and well-controlled epilepsy who presented with catatonia and COVID-19, diagnosed by polymerase chain reaction. Although initially in critical condition (requiring intensive care unit stay) and progression to malignant catatonia despite benzodiazepines, he returned to baseline after 10 treatments of ECT.
Discussion
Postinfectious NPS, including psychosis, catatonia, and affective disorders, have been documented as early as the 19th century.1 , 20 , 21 The association is especially strong for respiratory viruses such as influenza1; in 1926, Karl Menninger, writing about postinfluenza psychoses in a group of 200 patients, described influenza as having “almost unequal neurotoxicity.”22 The early 1900s encephalitis lethargic pandemic, temporally associated with 1918 influenza A virus (H1N1) but now thought to have been caused by another pathogen, caused catatonia and postencephalitic parkinsonism.23 Catatonia has been reported to be caused by numerous other viral infections.24 Exposure to certain viruses, including influenza and coronaviruses, has been found to be associated with mood disorders.21 , 25 Data from various studies have provided evidence of the potential of coronaviruses, a common cause of respiratory infections, to cause NPS.26 Reports of SARS-CoV-127 , 28, MERS-CoV,28 and now SARS-CoV-2 have identified specific NPS, including psychotic, mood, and cognitive symptoms that have been diagnosed during these illnesses.4 , 27 , 28
There are several potential mechanisms by which Sars-CoV-2 could cause NPS. The first mechanism is by direct invasion of the CNS. Coronaviruses are “neurotropic”: they are capable of infecting neurons.26 , 29 This can be accomplished through nasal mucosal invasion through the cribriform plate or through other peripheral nerves.30 Coronaviruses could also invade the CNS hematogenously.29
The second mechanism by which SARS-CoV-2 could cause NPS is by neuroinflammation secondary to a general immune response.1 , 27 Troyer et al further group this indirect neuroinflammation into cytokine network dysregulation, peripheral immune cell transmigration, and postinfectious autoimmunity. Case reports of catatonia in COVID-19 describe a rise in proinflammatory markers, hypothesized to alter basal ganglia function, that accompanies onset of catatonia.2 , 31 Similarly, Ferrando et al27 demonstrated the same pattern of rise in inflammatory markers in 3 patients that developed psychosis associated with asymptomatic COVID-19. Depression has also been found to be associated with increased inflammatory markers and may be a common psychiatric complication of infection.24
Our patient's history and examination suggest that she developed COVID-19–associated NPS because of neuroinflammation during acute infection. First, despite nasal swab SARS-CoV-2 polymerase chain reaction positivity persisting for months, there was no SARS-CoV-2 RNA found in her CSF. In addition, her laboratory results were mostly unremarkable, except for an increase in serum inflammatory markers: ferritin, erythrocyte sedimentation rate, C-reactive protein, interleukin-6: a pattern of findings described in other case reports, including those describing catatonia2 , 31 and psychosis27 secondary to COVID-19. Her CSF IgG was also above normal, suggesting a disruption in the blood-brain barrier. Notably, elevated inflammatory markers were also described in 1 of the 3 case reports identified by our literature review18 and elevated WBC and CSF protein in another.19
The mechanism of action of ECT is not well understood. Proposed mechanisms include changes in regional blood flow and metabolism, increased BDNF, angiogenesis and neurogenesis, normalization of cortisol levels, enhancement of dopamine transmission, and regulation of overactive glutamate transmission.32 Importantly, multiple sessions of ECT lead to a decline in levels of inflammatory mediators.32 ECT has been used effectively in treatment of psychiatric complications of autoimmune encephalitis9 and in other conditions, such as major depression and Parkinson's,33 in which there has been increasing interest in association with inflammation.24 If ECT partially or fully reverses the chemical, structural, and functional changes associated with infection-induced neuroinflammatory state, we argue that ECT should be considered as a treatment option to improve outcomes and prognosis in post-COVID-19 neuropsychiatric disorders.
Limitations
Limitations were found in our study. The methods of COVID-19 diagnosis used in the identified case reports are inconsistent. One did not clearly specify the method of diagnosis.17 In another, the patient was diagnosed clinically, and this was later confirmed with a positive IgG.18
A confounding factor in our case is the patient's use of prednisone 50 mg x 2 doses, which was temporally associated with onset of her symptoms. Although we initially considered steroid-induced mood and psychotic disorder, several important points in the history contradicted this: her prior use of prednisone without adverse effects, the low total dose of prednisone, and the persistence and worsening of symptoms despite steroid discontinuation. There is at least one large case-control study of patients with SARS-CoV-1 to support this: though nearly all cases of acute psychosis were attributed to steroid use, those patients were treated with much larger daily and cumulative doses of corticosteroid. In addition, the authors concluded that individual factors determining susceptibility to psychosis were also relevant.34 We speculate that at most, our patient's steroid use may have potentiated symptoms in an already susceptible individual.
Conclusion
This is the 4th case of COVID-19-associated severe psychiatric symptoms responsive to ECT. Our case is unique in that it describes acute and chronic psychiatric sequelae of COVID-19, all of which responded to ECT. The cases identified in our literature review, heterogeneous in patient characteristics and their psychiatric diagnoses (catatonia, psychotic, and mood disorders), support that ECT is effective in a “broad-spectrum” of psychiatric problems, and specifically, that it is safe, well-tolerated, and effective in NPS associated with COVID-19. As our case and the others have demonstrated, COVID-19–associated NPS can be severely disabling and even life-threatening: this suggests a role for early and aggressive use of ECT for psychiatric sequelae of COVID-19.
Footnotes
Disclosure: Dr Gordon is on the speaker's bureau for HEAL trafficking, AMWA PATH and SOAR. She does expert work on mental health and human trafficking. She has no investment, industry or pharmaceutical relationships to disclose. Dr. Livingston, Dr. Austgen, and Dr. Meyers report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheiner N.S., Smith A.K., Wohlleber M., Malone C., Schwartz A.C. COVID-19 and catatonia: a case series and systematic review of existing literature. J Acad Consult Psychiatry. 2021;21:S2667–S2960. doi: 10.1016/j.jaclp.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalleballe K., Reddy Onteddu S., Sharma R., et al. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav Immun. 2020;88:71–74. doi: 10.1016/j.bbi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varatharaj A., Thomas N., Ellul M.A., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo M.S., Malsy J., Pöttgen J., et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020;2:fcaa205. doi: 10.1093/braincomms/fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer J. In: Clinical Manual of Electroconvulsive Therapy. Mankad M., Beyer J., Weiner R., Krystal A., editors. American Psychiatric Publishing, Inc.; 2010. History of electroconvulsive therapy; pp. 3–8. [Google Scholar]
- 7.Beyer J. In: Clinical Manual of Electroconvulsive Therapy. Mankad M., Beyer J., Weiner R., Krystal A., editors. American Psychiatric Publishing, Inc.; 2010. Indications for use; pp. 9–25. [Google Scholar]
- 8.Meyer J.P., Swetter S.K., Kellner C.H. Electroconvulsive therapy in geriatric psychiatry: a selective review. Clin Geriatr Med. 2020;41:79–93. doi: 10.1016/j.cger.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Gough J.L., Coebergh J., Chandra B., Nilforooshan R. Electroconvulsive therapy and/or plasmapheresis in autoimmune encephalitis? World J Clin Cases. 2016;4:223–228. doi: 10.12998/wjcc.v4.i8.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones M., Gausche E., Reed E. A case of neuropsychiatric lupus with severe malignant catatonia that improved with daily electroconvulsive therapy. J Neuropsychiatry Clin Neurosci. 2016;28:e19–e20. doi: 10.1176/appi.neuropsych.15080211. [DOI] [PubMed] [Google Scholar]
- 11.Oldham M.A. The Probability that catatonia in the hospital has a medical cause and the relative proportions of its causes: a systematic review. Psychosomatics. 2018;59:333–340. doi: 10.1016/j.psym.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Denysenko L., Sica N., Penders T.M., et al. Catatonia in the medically ill: etiology, diagnosis, and treatment. The Academy of Consultation-Liaison Psychiatry Evidence-Based Medicine Subcommittee Monograph. Ann Clin Psychiatry. 2018;30:140–155. [PubMed] [Google Scholar]
- 13.Mankad M., Weiner R. In: Clinical Manual of Electroconvulsive Therapy. Mankad M., Beyer J., Weiner R., Krystal A., editors. American Psychiatric Publishing, Inc.; 2010. Adverse effects; pp. 139–148. [Google Scholar]
- 14.Sienaert P., Lambrichts S., Popleu L., Van Gerven E., Buggenhout S., Bouckaert F. Electroconvulsive therapy during COVID-19-times: our patients cannot Wait. Am J Geriatr Psychiatry. 2020;28:772–775. doi: 10.1016/j.jagp.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sagué-Vilavella M., Gil-Badenes J., Baldaquí Baeza N., et al. The other victims of COVID-19: the value of electroconvulsive therapy. J ECT. 2021;37:e1–e2. doi: 10.1097/YCT.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espinoza R.T., Kellner C.H., McCall W.V. Electroconvulsive therapy during COVID-19: an essential medical procedure-maintaining service viability and accessibility. J ECT. 2020;36:78–79. doi: 10.1097/YCT.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashaninasab F., Panahi Dashdebi R., Ghalehbandi M.F. Comorbidity of Coronavirus disease (COVID-19) and the first episode of bipolar disorder and its treatment challenges: a case report. Med J Islam Repub Iran. 2020;34:103. doi: 10.34171/mjiri.34.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chacko M., Job A., Caston F., George P., Yacoub A., Cáceda R. COVID-19-induced psychosis and suicidal behavior: case report. SN Compr Clin Med. 2020;26:1–5. doi: 10.1007/s42399-020-00530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deocleciano de Araujo C., Schlittler L.X.C., Sguario R.M., Tsukumo D.M., Dalgalarrondo P., Banzato C.E.M. Life-threatening catatonia associated with coronavirus disease 2019. Psychosomatics. 2020;62:256–257. doi: 10.1016/j.psym.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson C.J., Thomas R.H., Solomon T., Michael B.D., Nicholson T.R., Pollak T.A. COVID-19 and psychosis risk: real or delusional concern? Neurosci Lett. 2021;10:741. doi: 10.1016/j.neulet.2020.135491. [DOI] [PubMed] [Google Scholar]
- 21.Okusaga O., Yolken R.H., Langenberg P., et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130:220–225. doi: 10.1016/j.jad.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menninger K.A. Influenza and schizophrenia. Am J Psychiatry. 1926;82:469–529. doi: 10.1176/ajp.151.6.182. [DOI] [PubMed] [Google Scholar]
- 23.Shorter E. The first psychiatric pandemic: encephalitis lethargica, 1917–27. Med Hypotheses. 2021;146:110420. doi: 10.1016/j.mehy.2020.110420. [DOI] [PubMed] [Google Scholar]
- 24.Rogers J.P., Pollak T.A., Blackman G., David A.S. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6:620–630. doi: 10.1016/S2215-0366(19)30190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale S.D., Berrett A.N., Erickson L.D., Brown B.L., Hedges D.W. Association between virus exposure and depression in US adults. Psychiatry Res. 2018;261:73–79. doi: 10.1016/j.psychres.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Severance E.G., Dickerson F.B., Viscidi R.P., et al. Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr Bull. 2011;37:101–107. doi: 10.1093/schbul/sbp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrando S.J., Klepacz L., Lynch S., et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? Psychosomatics. 2020;61:551–555. doi: 10.1016/j.psym.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers J.P., Chesney E., Oliver D., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desforges M., Le Coupanec A., Dubeau P., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:1–28. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gouse B.M., Spears W.E., Nieves Archibald A., Montalvo C. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529–530. doi: 10.1016/j.bbi.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A., Kar S.K. How electroconvulsive therapy works?: Understanding the neurobiological mechanisms. Clin Psychopharmacol Neurosci. 2017;15:210–221. doi: 10.9758/cpn.2017.15.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacilio R.M., Livingston R.K., Gordon M.R. The use of electroconvulsive therapy in eating disorders: a systematic literature review and case report. J ECT. 2019;35:272–278. doi: 10.1097/YCT.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 34.Lee D.T.S., Wing Y.K., Leung H.C.M., et al. Factors associated with psychosis among patients with severe acute respiratory syndrome: a case-control study. Clin Infect Dis. 2004;39:1247–1249. doi: 10.1086/424016. [DOI] [PMC free article] [PubMed] [Google Scholar]