Abstract
Aims
In contrast to operations performed for other fractures, there is a high incidence rate of surgical site infection (SSI) post-open reduction and internal fixation (ORIF) done for tibial plateau fractures (TPFs). This study investigates the effect of induced membrane technique combined with internal fixation for managing SSI in TPF patients who underwent ORIF.
Methods
From April 2013 to May 2017, 46 consecutive patients with SSI post-ORIF for TPFs were managed in our centre with an induced membrane technique. Of these, 35 patients were included for this study, with data analyzed in a retrospective manner.
Results
All participants were monitored for a mean of 36 months (24 to 62). None were subjected to amputations. A total of 21 patients underwent two-stage surgeries (Group A), with 14 patients who did not receive second-stage surgery (Group B). Group A did not experience infection recurrence, and no implant or cement spacer loosening was noted in Group B for at least 24 months of follow-up. No significant difference was noted in the Lower Extremity Functional Scale (LEFS) and the Hospital for Special Surgery Knee Score (HSS) between the two groups. The clinical healing time was significantly shorter in Group B (p<0.001). Those with longer duration of infection had poorer functional status (p<0.001).
Conclusion
Management of SSI post-ORIF for TPF with induced membrane technique combined with internal fixation represents a feasible mode of treatment with satisfactory outcomes in terms of infection control and functional recovery.
Cite this article: Bone Joint Res 2021;10(7):380–387.
Keywords: Surgical site infection, Tibial plateau fractures, Induced membrane technique, Internal fixation
Article focus
Management of surgical site infection (SSI) post-open reduction and internal fixation (ORIF) for tibial plateau fractures (TPFs) with induced membrane technique in combination with internal fixation represents a feasible mode of treatment with satisfactory outcomes in terms of infection control and functional recovery.
Key messages
Radical debridement forms the basis of using internal fixation as a stabilizing method.
Usage of antibiotic cement to cover the internal fixation plate is a key step during the first-stage surgery.
Strengths and limitations
Management of SSI post-ORIF for TPF with internal fixation is beneficial for functional recovery without increasing the recurrence rate of infection.
The follow-up period of the patients with permanent spacers is relatively short, and complications may occur with increased duration of follow-up.
Introduction
Tibial plateau fractures (TPFs) are prone to postoperative surgical site infection (SSI).1 The incidence rate was previously reported to be between 2.6% and 45%,2 which is high in contrast to other fractures managed with open reduction and internal fixation (ORIF).3 Given its periarticular location, a SSI occurring after ORIF of TPF harbours disastrous consequences.2,4 Current treatment options include repeated radical debridement,5,6 irrigation6 and drainage combined with vacuum sealing drainage (VSD),7 removal or retention of internal fixation,6,8 flap or muscle flap coverage,9,10 and systemic6,8,11 and/or local12 antibiotics use. However, due to the relatively long treatment period and recurrent infection, the function of the knee joint is greatly affected.2
Orthopaedic implant-associated infection has been documented to be a result of biofilm formation by surface-adhering bacteria.13,14 Biofilm that has been firmly established on an implant is resistant to host immunity14 and antibiotics.15 Evidence shows that once biofilms have formed, the entire tissue bed may continue to contaminate further new implants, thereby propagating infection despite assumed clearance.16,17 Therefore, previously infected sites were once considered a contraindication for reuse of internal fixation.18 In 2007, Thonse and Conway19 reported an effective management of infected nonunion and segmental bone defects with antibiotic cement-coated interlocking nail. In 2017, our centre fashioned temporary internal fixators with an antibiotic cement-coated locking plate for the treatment of femoral osteomyelitis defects with initial induced membrane operation, achieving good clinical results.20 The current investigation analyzes a series of patients with SSI of TPF after ORIF who were subjected to first-stage internal fixation with antibiotic cement-coated locking plates, followed by second-stage induced membrane technique. We aimed to observe the effect of this method on infection control and the recovery of knee joint function.
Methods
Digital medical records of 46 consecutive patients with deep SSI after ORIF of TPFs, managed with induced membrane technique in our centre between April 2013 and May 2017, were selected for retrospective analysis. SSI was diagnosed based on a combination of clinical local swelling and bony pain, laboratory, histopathological, and microbiological investigations, as well as via imaging.21 We excluded 11 patients from the study, including those younger than 18 years or older than 70 years in three cases (source of autogenous bone was limited), Cierny-Mader Host C in two cases (not suitable for surgery),22 two cases who had less than 24 months of follow-up, treatment with external fixation in two cases, combined with knee joint infection in two cases (impact on knee function assessment). This study finally consisted of a total of 35 subjects. The Schatzker classification23 of all fractures and patient demographics are presented in Table I. A total of 21 of the 35 patients underwent two-stage surgery (Group A), while the other 14 patients did not receive reconstruction because of individual reasons (Group B). The basic details of each case are presented in Supplementary Table i.
Table I.
Demographic analysis (total n = 35).
| Variable | Value |
|---|---|
| Mean age, yrs (range) | 46 (19 to 64) |
| Sex, n (%) | |
| Male | 30 (85.7) |
| Female | 5 (14.3) |
| Schatzker fracture types, n (%) | |
| Ⅳ | 2 (5.7) |
| Ⅴ | 24 (68.6) |
| Ⅵ | 9 (25.7) |
| Cierny-Mader type, n (%) | |
| A | 6 (17.1) |
| B | 29 (82.9) |
| Bacterial strains, n (%) | |
| Negative | 4 (11.4) |
| Positive | 31 (88.6) |
| Staphylococcus aureus (MRSA) | 18 (5) (51.4 (14.2)) |
| Pseudomonas aeruginosa | 2 (5.7) |
| Escherichia coli | 3 (8.6) |
| Acinetobacter baumannii | 1 (2.9) |
| > two kinds of bacteria | 7 (20) |
| Mean number of previous operations (range) | 2.1 (1 to 6) |
| Mean duration of infection, wks (range) | 55.6 (1 to 468) |
MRSA, methicillin-resistant Staphylococcus aureus.
Preoperative evaluation
Information regarding history of previous trauma and surgery, present laboratory diagnostic indicators, imaging procedures, and physical examination findings were extracted. In the case of the lack of knee joint infection, an estimate of the debridement scope, fixation mode, and coverage method based on radiography, radionuclide bone scan, MRI, and CT were recorded.
Surgical technique and postoperative treatment: the first stage
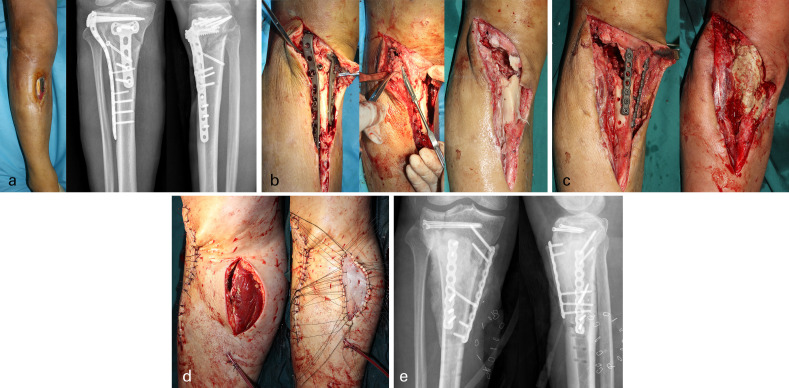
Tourniquet was used to ensure a relatively bloodless surgical field. Debridement range of soft-tissue was done with a 2 mm margin of healthy tissue and a 5 mm margin of healthy bone.24 Sinuses, scars, sequestrum, inflammatory granulation, and foreign bodies (including implants and allograft) were thoroughly cleared until healthy bone was achieved (Figure 1b).25 After radical debridement, antibiotic polymethyl methacrylate (PMMA) bone cement containing gentamicin (Heraeus, Germany) was used for filling bone defects (with the addition of 5 g vancomycin in 40 g gentamicin PMMA bone cement by our surgical team). Broken ends were stabilized with an antibiotic cement-coated locking plate (Figure 1c). If the skin defect was large, tension-free coverage was carried out through the use of a tissue flap (Figure 1d). Broad-spectrum antibiotics were empirically administered for anti-infective therapy. If bacterial culture was positive, the anti-infection plan was adjusted according to drug sensitivity. All patients received intravenous anti-infection therapy for two weeks, followed by four weeks of oral antibiotics. All patients were advised for range of motion knee exercises with strict prohibition of weight-bearing prior to the second operation.
Fig. 1.
First-stage surgical procedure. a) Photograph, and anteroposterior (AP) and lateral preoperative radiographs, of a 50-year-old male patient with a Schatzker VI fracture. b) The sinuses, scars, sequestrum, inflammatory granulation, and foreign bodies (including implants and allograft) were thoroughly cleared until healthy bone was achieved. c) An antibiotic bone cement spacer was used to fill the bone defect, followed by application of antibiotic cement coated locking plate. d) The skin defect was large, with tension-free coverage carried out using a flap. e) First-stage postoperative AP and lateral radiographs demonstrated that bone defect was filled with antibiotic bone cement spacer and the locking plate and hollow screw were used as internal fixator to limb stabilization.
Surgical technique and postoperative treatment: the second stage
Second-stage surgery was planned after six to eight weeks. Complete removal of bone cement and implants under the membrane was done, with the broken ends of the bone cleaned to form fresh wounds prior to fixation with a proper locking plate (Synthes, Switzerland), which was wrapped with antibiotic bone cement afterwards. An autograft was used to fill the bone defect (anterior or posterior iliac crest). All patients subsequently received two weeks of intravenous antibiotics. Non-weight-bearing functional exercises were allowed within three months after surgery. Three months later, radiographs were repeated to determine the feasibility of gradual weight-bearing.
Follow-up
A follow-up evaluation, encompassing radiological and clinical assessments, was done at one, two, three, four, six, nine, 12, 18, and 24 months. The Lower Extremity Functional Scale (LEFS)26 and Hospital for Special Surgery Knee Score (HSS)27 were used to quantify clinical results. During each follow-up clinic appointment, assessments of plain radiographs of the affected tibial plateau were carried out (medial-lateral and anterior-posterior planes). Radiological healing was determined by the formation of callus in at least three of four cortices.28 Patients who were able to fully weight-bear and were free of pain were defined as having achieved clinical healing.29
Statistical analysis
The SPSS v22.0 statistical software package (IBM, USA) was used to analyze all data. The chi-squared test and Fisher’s exact test were used to contrast categorical variables, while the independent-samples t-test was used to analyze continuous variables. Clinical healing times were assessed using Kaplan-Meier survival curves and log-rank test for comparison. A two-sided test that resulted in a p-value of less than 0.05 was taken to indicate statistical significance.
Results
All 35 patients achieved satisfactory outcomes during the mean follow-up time of 36 months (24 to 62), with none requiring amputation. A total of 21 patients underwent two-stage surgery (Group A), while the other 14 patients did not receive reconstruction (Group B). In Group A, the infection control was obtained through two repeated debridement sessions in two patients, with the remaining 19 undergoing a single debridement session only. After primary debridement, two patients required local flaps to achieve wound coverage. All 21 patients were treated with autografts in the second stage, and median radiological healing was achieved at 16 weeks (interquartile range 13.0 to 22.0) after the operation. There was no recurrence of infection after the reconstruction in Group A, and the mean LEFS and HSS at the last follow-up was 65.7 points (40 to 80) and 82.6 points (52 to 100), respectively (Figures 2a and 2b). In Group B, the infection control was obtained through two primary debridements in two patients, and a single primary debridement in the remaining 12 patients. There was no recurrence of infection, and no implant or cement spacer loosening for at least 24 months’ follow-up. The mean LEFS and HSS of the last follow-up was 68.9 points (51 to 80) and 86.0 points (67 to 100), respectively (Figures 3a and 3b). Demographic characteristics (p>0.05), previous operations (p = 0.343, independent-samples t-test) , duration of infection (p = 0.748, independent-samples t-test) , and bacterial strains (Staphylococcus aureus) (p = 0.305, Fisher’s exact test) were not significantly different between the two groups. LEFS (p = 0.376, independent-samples t-test) and HSS (p = 0.404, independent-samples t-test) did not differ markedly between the two groups. Interestingly, our analysis uncovered notable variability in clinical healing time. Through an analysis using the Kaplan-Meier survival curve (Figure 4), the clinical healing time in Group B was significantly less in contrast to that in Group A (p<0.001, log-rank test) (Table II). Factors such as age, duration of infection, and previous operations were analyzed in order to determine factors that were able to predict functional recovery (Table III), and patients with long duration of infection were more likely to suffer poor function (p<0.001, independent-samples t-test).
Fig. 2.
Two-stage treatment process of a 40-year-old male patient with a Schatzker V fracture. a) Anteroposterior (AP) and lateral radiographs before operation. Plate exposure seen prior to operation (photograph). First-stage postoperative AP and lateral radiograph showed that bone defect was filled with antibiotic bone cement spacer, and the locking plate and hollow screw were used as internal fixator to limb stabilization. Second-stage postoperative AP and lateral radiographs demonstrating placement of iliac crest autograft after removal of spacer and replacement of implant to limb stabilization. b) Radiographs of three, 12, 24, and 30 months after bone grafting, where the implants were removed at 30 months, demonstrating the duration of bony consolidation.
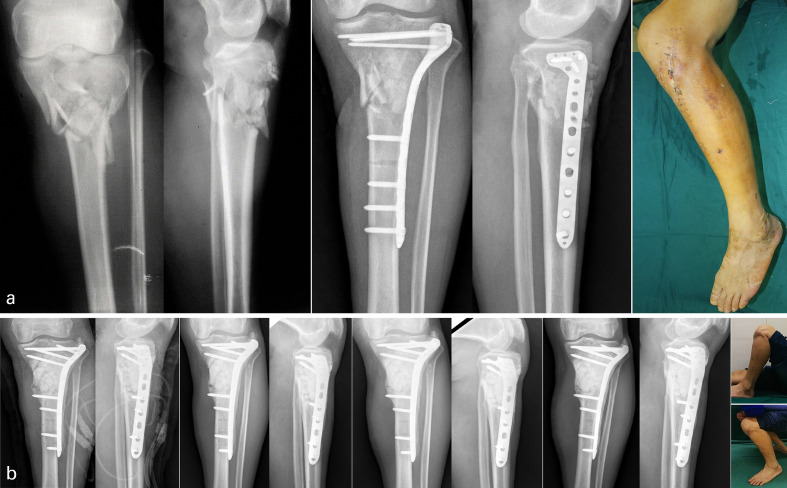
Fig. 3.
One-stage treatment process of a 61-year-old male patient with a Schatzker VI fracture. a) Anteroposterior (AP) and lateral radiographs of post-trauma. Photographs and AP and lateral radiographs after open reduction internal fixation demonstrated that knee function was limited and plate was exposed. b) First-stage postoperative AP and lateral radiographs demonstrated that bone defect was filled with antibiotic bone cement spacer, and the locking plate was used as internal fixator to limb stabilization. Radiographs of three, 12, and 24 months after first-stage surgery demonstrating no obvious changes in the proximal tibia. Photos demonstrated function recovery of knee joint at the last follow-up.
Fig. 4.
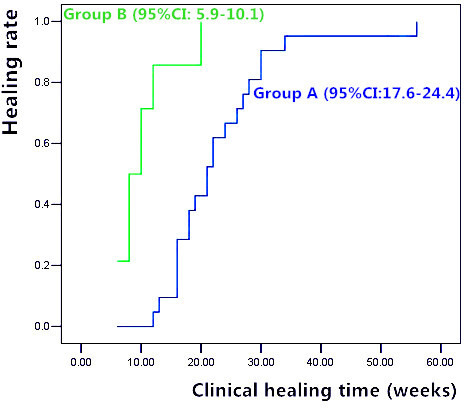
Kaplan-Meier survival curve for the comparison of clinical healing time between Group A and Group B. The clinical healing time in Group B was significantly less in comparison to that in Group A (p<0.001, log-rank test). CI, confidence interval.
Table II.
Comparison of two groups in demographic analysis and final results.
| Variables | Group A | Group B | p-value |
|---|---|---|---|
| Number of patients | 21 | 14 | |
| Mean age, yrs (SD) | 44.0 (11.8) | 48.6 (11.3) | 0.268* |
| Sex, n | 0.369† | ||
| Female | 2 | 3 | |
| Male | 19 | 11 | |
| Schatzker fracture types, n | 0.603‡ | ||
| IV | 1 | 1 | |
| V | 14 | 10 | |
| VI | 6 | 3 | |
| Cierny-Mader type, n | 0.664† | ||
| A | 3 | 3 | |
| B | 18 | 11 | |
| Mean previous operations, n (SD) | 2.2 (1.4) | 1.9 (0.7) | 0.343* |
| Mean duration of infection, wks (SD) | 61.2 (122.5) | 49.8 (59.5) | 0.748* |
| Bacterial strains (S. aureus), n | 9 | 9 | 0.305† |
| Mean functional recovery score (SD) | |||
| LEFS | 65.7 (10.8) | 68.9 (9.7) | 0.376* |
| HSS | 82.6 (12.4) | 86.0 (10.8) | 0.404* |
| Median bone healing time, wks (IQR) | |||
| Radiological healing | 16.0 (13.0 to 22.0) | N/A | N/A |
| Clinical healing | 21.0 (16.0 to 27.0) | 8.0 (8.0 to 12.0) | < 0.001§ |
Group B received no reconstruction and as such had no radiological healing time.
Independent-samples t-test.
Fisher’s exact test.
Chi-squared test (linear by linear).
Log-rank test.
HSS, Hospital for Special Surgery Knee Score; IQR, interquartile range; LEFS, Lower Extremity Functional Scale; N/A, not applicable; S. aureus, Staphylococcus aureus; SD, standard deviation.
Table III.
Factors that may be predictive of function recovery.
| Variables | Number of patients | LEFS (SD) | HSS (SD) |
|---|---|---|---|
| Duration of infection, wks | |||
| >10 | 19 | 59.9 (8.7) | 76.0 (9.7) |
| ≤ 10 | 16 | 75.4 (3.8) | 93.4 (4.8) |
| p-value | <0.001 | <0.001 | |
| Age, yrs | |||
| ≥ 50 | 18 | 65.1 (11.6) | 82.1 (13.2) |
| <50 | 17 | 69.1 (8.7) | 85.9 (9.9) |
| p-value | 0.259 | 0.334 | |
| Previous operations, n | |||
| ≥ 2 | 24 | 65.5 (10.2) | 82.4 (11.3) |
| <2 | 11 | 70.3 (10.4) | 87.3 (12.5) |
| p-value | 0.210 | 0.261 |
All p-values calculated using independent-samples t-test.
HSS, Hospital for Special Surgery Knee Score; LEFS, Lower Extremity Functional Scale.
Discussion
Surgical site infection of TPFs after ORIF carries substantial morbidity.4 In addition to meticulous infection control and bone defect repair, it also involves the prolonged rehabilitation to regain knee function. Therefore, the management of SSI of TPF after ORIF is very challenging. The current series of 35 patients with SSI of TPF after ORIF were managed with a two-stage surgery involving a first stage of antibiotic cement-coated locking plates as internal fixation and a second stage using the induced membrane technique. We achieved a high success rate of infection control (100%), bone union (100%), and satisfactory establishment of knee function across all subjects. This is the first study of its kind to demonstrate the successful use of an induced membrane technique in treating infected TPFs.
Bone infection is managed primarily with radical debridement.30 Incomplete debridement is the major reason of recurrent infection.20,30,31 Masquelet et al32 reported that inadequate debridement could be avoided through repeated debridement sessions and the usage of a spacer without antibiotics. Simpson et al24 suggested that all necrotic and infected bone should be excised with 5 mm or more healthy tissue margin in Cierny-Mader Host B, and less than 5 mm healthy tissue margin in Cierny-Mader Host A. The widely used 'paprika sign' is limited in its ability to determine satisfactory extent of bone resection.30 Therefore, the extent of bony infection in our study was demarcated using several modes of imaging including radionuclide bone scans, CT scans, and plain radiographs. Based on this system, the whole infected bone area was regarded as a cystic structure that contained sequestrum, pus, and implant, while the junction between infected tissue and normal tissue was regarded as the cyst wall. Radical debridement was done to achieve complete removal of this cystic structure with a 2 mm margin of normal soft-tissue around the cyst wall. Infected wounds were converted contaminated wounds,18 thereby optimizing conditions for employing internal fixation for stabilization. In this study, 88.6% (31/35) of the participants achieved satisfactory infection control with just a single surgery, indicating the benefits of this radical debridement technique.
In addition to radical debridement, local stabilization as well as microbiological specific local antibiotics application are very important.33 External fixators represented the first choice for gaining local stabilization in Masquelet’s clinical cases involving post-traumatic septic nonunions that occasionally required iterative excisions.32 The use of a transarticular external fixator often results in joint stiffness,34 which impacts patients’ ability to carry out activities of daily living35 and negatively affects their mental health.36 In 2007, Thonse and Conway19 first reported an effective use of an antibiotic cement-coated interlocking nail in managing infected nonunion and segmental bone defects. A study in 2017 demonstrated good clinical outcomes through first-stage induced membrane technique in combination of a temporary antibiotic cement-coated locking plate internal fixator in the management of femoral osteomyelitis defects.20 Wang et al30 also reported that antibiotic cement should completely envelop the surface of the implant to avoid biofilm formation. In this study, an internal fixator comprising an antibiotic cement-coated locking plate was implanted following radical debridement, which provides antibiotic delivery and bony stability.37 Compared with an external fixator, this fixation provides greater stability and comfort level without increasing the recurrence rate of infection.31 One limitation of this technique is the fact that the coating does not protect screws and fixation holes.38 Additionally, coating efficacy may be hindered by gentamicin/vancomycin resistance. In our series, infection control was obtained through re-debridement in four patients, who may have required the additional procedure either due to incomplete debridement or to the above reason.
In 1986, Masquelet et al32 serendipitously discovered the efficaciousness of the induced membrane technique in treating large diaphyseal defects. This concept was then further developed in 2010.39 Compared to the Ilizarov technique and vascularized fibular bone grafting, induced membrane technique mimics natural fracture healing in achieving bony reconstruction. In Group A of this series, all patients were treated with autogenous cancellous bone transplantation in the induced membrane at the second stage. The mean radiological healing time was 16 weeks, which was near to previously reported results.32 Refracture and infection recurrence are the major complications associated with induced membrane technique.32 Incomplete debridement20,30,32 and use of external fixation40 were important contributing factors, respectively. In our study, the bone healing rate reached 100%, and no refracture or infection recurrence, which may be a result of the use of combined radical debridement technique and internal fixation.
In addition to controlling infection and reconstructing defects, restoring knee function is another important goal. Joint immobility for more than three to four weeks can lead to permanent knee stiffness in some patients.41,42 Prasarn et al43 noted that a long duration of infection resulted in compromised knee function. In our study, the longer the duration of infection, the worse the postoperative functional recovery (p<0.001, independent-samples t-test), which was similar to the reported results.43 However, all of the 35 patients in our study achieved good mid-term functional outcomes, and the mean LEFS and HSS of the last follow-up were 65.7 (40 to 80) and 82.6 points (52 to 100) in Group A, and 68.9 (51 to 80) and 86.0 points (67 to 100) points in Group B, respectively. One of the important factors is the choice of stabilization method. The antibiotic cement-coated locking plate as internal fixation functioned as a stabilizing internal structure without severely impacting knee joint function. Early non-weight-bearing functional exercises were feasible after the first-stage surgery.
Individualized treatment programmes are important for patients.30 Cierny et al44,45 reported a method of permanent spacers to treat osteomyelitis successfully. The permanent spacers were composites of various antibiotic-impregnated bone cements (poly-methyl-methacrylate) reinforced with a nail, pin, or plate.45 The rationale for these choices was not elaborated in the literature. In our study, 14 patients did not receive secondary surgery (Group B) because of individual reasons, such as economic reasons or patient refusal (fear of reoperation and satisfaction with pre-existing functional status). Antibiotic cement-coated locking plate was used to stabilize fractures as permanent spacers. Through at least two years' follow-up, there were no marked differences in LEFS and HSS between Group A and Group B. The clinical healing time in Group B was markedly reduced in contrast to that of Group A (p<0.001, log-rank test). The patients (Group B) had no bone healing time, and the antibiotic cement-coated locking plate as internal fixation functioned as a stabilizing internal structure, which could provide enough support to allow full weight-bearing earlier without refracture. The results were encouraging and similar to the literature.44,45 Nevertheless, permanent spacers, like prosthetic joints, often have a predetermined service life that requires further scrutiny through studies with longer follow-up durations.
This study is limited in its retrospective design and lack of a control group, with future prospective randomized controlled trials required. Our small case number and short follow-up period may also limit the ability of this study to fully capture the extent of long-term outcomes, especially for the patients of no secondary surgery.
Management of SSI post-ORIF for TPF with internal fixation is a reliable method, especially for infection control and functional recovery. The premise of using internal fixation as a stabilizing method is radical debridement, and usage of antibiotic cement to cover the internal fixation plate is a key procedure during the first-stage surgery.
Author contributions
J. Shen: Performed the operations and the statistical analysis, Wrote the manuscript.
D. Sun: Performed the operations and the statistical analysis, Reviewed and revised the manuscript.
J. Fu: Performed the operations, Reviewed the manuscript.
S. Wang: Collected the clinical data.
X. Wang: Performed the operations.
Z. Xie: Designed the study, Performed the operations, Reviewed the manuscript.
Funding statement
This work was supported by the National key R & D project (2016YFC1102005): Development of bioactive spinal and segmental bone defect repair devices; Research on repair technology and equipment of war injury (AWS17J004-02); Construction and application of bone infection case registration system based on case data and Internet + (SWH2017ZDCX4105). The authors report grants from Army Medical University (SWH2017ZDCX4105), Logistical Support Department of the People's Liberation Army (AWS17J004-02), and the Ministry of Science and Technology of the People's Republic of China (2016YFC1102005) for this study. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
All the authors declare that there is no conflict of interest.
Ethical review statement
This study was approved by the ethics committee of Southwest Hospital Chongqing, China (No. KY201878).
Supplementary material
Table displaying the basic details of each case.
References
- 1. Bachoura A, Guitton TG, Smith RM, Vrahas MS, Zurakowski D, Ring D. Infirmity and injury complexity are risk factors for surgical-site infection after operative fracture care. Clin Orthop Relat Res. 2011;469(9):2621–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Henkelmann R, Frosch KH, Glaab R, et al. . Infection following fractures of the proximal tibia - a systematic review of incidence and outcome. BMC Musculoskelet Disord. 2017;18(1):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu S-G, Mao Z-G, Liu B-S, Zhu H-H, Pan H-L. Evaluating the use of antibiotic prophylaxis during open reduction and internal fixation surgery in patients at low risk of surgical site infection. Injury. 2015;46(2):184–188. [DOI] [PubMed] [Google Scholar]
- 4. Parkkinen M, Madanat R, Lindahl J, Mäkinen TJ. Risk factors for deep infection following plate fixation of proximal tibial fractures. J Bone Joint Surg Am. 2016;98-A(15):1292–1297. [DOI] [PubMed] [Google Scholar]
- 5. Morris BJ, Unger RZ, Archer KR, Mathis SL, Perdue AM, Obremskey WT. Risk factors of infection after ORIF of bicondylar tibial plateau fractures. J Orthop Trauma. 2013;27(9):e196–e200. [DOI] [PubMed] [Google Scholar]
- 6. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85–90. [DOI] [PubMed] [Google Scholar]
- 7. Leininger BE, Rasmussen TE, Smith DL, et al. . Experience with wound VAC and delayed primary closure of contaminated soft tissue injuries in Iraq. Injury. 2006;61(5):1207–1211. [DOI] [PubMed] [Google Scholar]
- 8. Papagelopoulos PJ, Partsinevelos AA, Themistocleous GS, Mavrogenis AF, Korres DS, Soucacos PN. Complications after tibia plateau fracture surgery. Injury. 2006;37(6):475–484. [DOI] [PubMed] [Google Scholar]
- 9. Pollak AN, McCarthy ML, Burgess AR. Short-Term wound complications after application of flaps for coverage of traumatic soft-tissue defects about the tibia. the lower extremity assessment project (leap) Study Group. J Bone Joint Surg Am. 2000;82-A(12):1681–1691. [PubMed] [Google Scholar]
- 10. Chou Y-C, Wu C-C, Chan Y-S, Chang C-H, Hsu Y-H, Huang Y-C. Medial gastrocnemius muscle flap for treating wound complications after double-plate fixation via two-incision approach for complex tibial plateau fractures. J Trauma. 2010;68(1):138–145. [DOI] [PubMed] [Google Scholar]
- 11. Fletcher N, Sofianos D'Mitri, Berkes MB, Obremskey WT. Prevention of perioperative infection. J Bone Joint Surg Am. 2007;89-A(7):1605–1618. [DOI] [PubMed] [Google Scholar]
- 12. McKee MD, Li-Bland EA, Wild LM, Schemitsch EH. A prospective, randomized clinical trial comparing an antibiotic-impregnated bioabsorbable bone substitute with standard antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis and infected nonunion. J Orthop Trauma. 2010;24(8):483–490. [DOI] [PubMed] [Google Scholar]
- 13. Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol. 2012;65(2):158–168. [DOI] [PubMed] [Google Scholar]
- 14. Valour F, Trouillet-Assant S, Rasigade J-P, et al. . Staphylococcus epidermidis in orthopedic device infections: the role of bacterial internalization in human osteoblasts and biofilm formation. PLoS One. 2013;8(6):e67240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2(2):114–122. [DOI] [PubMed] [Google Scholar]
- 16. Kinik H, Karaduman M. Cierny-Mader type III chronic osteomyelitis: the results of patients treated with debridement, irrigation, vancomycin beads and systemic antibiotics. Int Orthop. 2008;32(4):551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27(11):2331–2339. [DOI] [PubMed] [Google Scholar]
- 18. Luo F, Wang X, Wang S, Fu J, Xie Z. Induced membrane technique combined with two-stage internal fixation for the treatment of tibial osteomyelitis defects. Injury. 2017;48(7):1623–1627. [DOI] [PubMed] [Google Scholar]
- 19. Thonse R, Conway J. Antibiotic cement-coated interlocking nail for the treatment of infected nonunions and segmental bone defects. J Orthop Trauma. 2007;21(4):258–268. [DOI] [PubMed] [Google Scholar]
- 20. Yu X, Wu H, Li J, Xie Z. Antibiotic cement-coated locking plate as a temporary internal fixator for femoral osteomyelitis defects. Int Orthop. 2017;41(9):1851–1857. [DOI] [PubMed] [Google Scholar]
- 21. Mouzopoulos G, Kanakaris NK, Kontakis G, Obakponovwe O, Townsend R, Giannoudis PV. Management of bone infections in adults: the surgeon's and microbiologist's perspectives. Injury. 2011;42(42 Suppl 5):S18–S23. [DOI] [PubMed] [Google Scholar]
- 22. Cierny G, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;10(414):7–24. [DOI] [PubMed] [Google Scholar]
- 23. Schatzker J, Mcbroom R, Bruce D. The tibial plateau fracture. The Toronto experience 1968-1975. Clin Orthop Relat Res. 1979;138:94–104. [PubMed] [Google Scholar]
- 24. Simpson AH, Deakin M, Latham JM. Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J Bone Joint Surg Br. 2001;83-B(3):403–407. [DOI] [PubMed] [Google Scholar]
- 25. Han W, Shen J, Wu H, Yu S, Fu J, Xie Z. Induced membrane technique: advances in the management of bone defects. Int J Surg. 2017;42:110–116. [DOI] [PubMed] [Google Scholar]
- 26. Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther. 1999;79(4):371–383. [PubMed] [Google Scholar]
- 27. Insall JN, Ranawat CS, Aglietti P, Shine J. A comparison of four models of total knee-replacement prostheses. J Bone Joint Surg Am. 1976;58-A(6):754–765. [PubMed] [Google Scholar]
- 28. Blum ALL, BongioVanni JC, Morgan SJ, Flierl MA, dos Reis FB. Complications associated with distraction osteogenesis for infected nonunion of the femoral shaft in the presence of a bone defect: a retrospective series. J Bone Joint Surg Br. 2010;92-B(4):565–570. [DOI] [PubMed] [Google Scholar]
- 29. Stafford PR, Norris BL. Reamer-irrigator-aspirator bone graft and BI Masquelet technique for segmental bone defect nonunions: a review of 25 cases. Injury. 2010;41(Suppl 2):S72–S77. [DOI] [PubMed] [Google Scholar]
- 30. Wang X, Luo F, Huang K, Xie Z. Induced membrane technique for the treatment of bone defects due to post-traumatic osteomyelitis. Bone Joint Res. 2016;5(3):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jia C, Wang X, Yu S, et al. . An antibiotic cement-coated locking plate as a temporary fixation for treatment of infected bone defects: a new method of stabilization. J Orthop Surg Res. 2020;15(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet. 2000;45(3):346–353. [PubMed] [Google Scholar]
- 33. Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13(6):417–427. [DOI] [PubMed] [Google Scholar]
- 34. Masquelet AC, Kishi T, Benko PE. Very long-term results of post-traumatic bone defect reconstruction by the induced membrane technique. Orthop Traumatol Surg Res. 2019;105(1):159–166. [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Jiang H, Deng Z, et al. . Comparison of monolateral external fixation and internal fixation for skeletal stabilisation in the management of small tibial bone defects following successful treatment of chronic osteomyelitis. Biomed Res Int. 2017;2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abulaiti A, Yilihamu Y, Yasheng T, Alike Y, Yusufu A. The psychological impact of external fixation using the Ilizarov or Orthofix LRS method to treat tibial osteomyelitis with a bone defect. Injury. 2017;48(12):2842–2846. [DOI] [PubMed] [Google Scholar]
- 37. Conway JD, Hlad LM, Bark SE. Antibiotic cement-coated plates for management of infected fractures. Am J Orthop. 2015;44(2):E49–53. [PubMed] [Google Scholar]
- 38. Romanò CL, Tsuchiya H, Morelli I, Battaglia AG, Drago L. Antibacterial coating of implants: are we missing something? Bone Joint Res. 2019;8(5):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41(1):27–37. [DOI] [PubMed] [Google Scholar]
- 40. Taylor BC, French BG, Fowler TT, Russell J, Poka A. Induced membrane technique for reconstruction to manage bone loss. J Am Acad Orthop Surg. 2012;20(3):142–150. [DOI] [PubMed] [Google Scholar]
- 41. Thienpont E, Schmalzried T, Bellemans J. Ankylosis due to heterotopic ossification following primary total knee arthroplasty. Acta Orthop Belg. 2006;72(4):502–506. [PubMed] [Google Scholar]
- 42. Weigel DP, Marsh JL. High-Energy fractures of the tibial Plateau. knee function after longer follow-up. J Bone Joint Surg Am. 2002;84-A(9):1541–1551. [DOI] [PubMed] [Google Scholar]
- 43. Prasarn ML, Ahn J, Achor T, Matuszewski P, Lorich DG, Helfet DL. Management of infected femoral nonunions with a single-staged protocol utilizing internal fixation. Injury. 2009;40(11):1220–1225. [DOI] [PubMed] [Google Scholar]
- 44. Cierny G, DiPasquale D. Treatment of chronic infection. J Am Acad Orthop Surg. 2006;14(10):S105–S110. [DOI] [PubMed] [Google Scholar]
- 45. Cierny G. Surgical treatment of osteomyelitis. Plast Reconstr Surg. 2011;1(1):258–272. [DOI] [PubMed] [Google Scholar]