Abstract
Aims
Poly (ADP-ribose) polymerase (PARP) inhibitor has been reported to attenuate inflammatory response in rat models of inflammation. This study was designed to investigate the effect of PARP signalling in osteoarthritis (OA) cartilage inflammatory response in an OA rat model.
Methods
The OA model was established by anterior cruciate ligament transection with medial meniscectomy in Wistar rats. The poly (ADP-ribose) polymerase 1 (PARP-1) shRNA (short hairpin (sh)-PARP-1) and negative control shRNA (sh-NC) were delivered using a lentiviral vector and were intra-articularly injected into rats after surgery. The weight-bearing distribution of the hind limbs and the knee joint width were measured every two weeks. The expression levels of PARP-1, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) in cartilage were determined using real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) and Western blot. The serum concentrations of inflammatory cytokines were detected using enzyme-linked immunosorbent assay (ELISA).
Results
PARP-1 expression level significantly increased in the cartilage of the established OA rat model. sh-PARP-1 treatment suppressed PARP-1 levels, decreased the Δ Force (the difference between the weight on ipsilateral limb and contralateral limb) and the knee joint width, inhibited cartilage matrix catabolic enzymes, and ameliorated OA cartilage degradation and attenuated inflammatory response.
Conclusion
PARP-1 inhibition attenuates OA cartilage inflammatory response in the OA rat model.
Cite this article: Bone Joint Res 2021;10(7):401–410.
Keywords: PARP-1 inhibition, Osteoarthritis, Cartilage inflammatory response
Article focus
This study was designed to investigate the effect of poly (ADP-ribose) polymerase (PARP) signalling in osteoarthritis (OA) cartilage inflammatory response in OA rat model.
Key messages
Poly (ADP-ribose) polymerase 1 (PARP-1) expression level significantly increased in the cartilage of the established OA rat model.
PARP-1 shRNA (short hairpin (sh)-PARP-1) treatment suppressed PARP-1 levels.
PARP-1 inhibition attenuates OA cartilage inflammatory response.
Strengths and limitations
PARP-1 inhibition attenuates OA cartilage inflammatory response in the OA rat model.
Another OA animal model should be investigated to verify the findings.
Histological analysis could be provided to support the changes in the cartilage.
No immunofluorescence to identify whether sh-PARP-1 is just limited to the superficial layer of the cartilage or if it penetrates deeper.
The toxicity and tolerance of sh-PARP-1 on OA models would supply the evidence for the potential use of sh-PARP-1 for OA treatment in the clinic.
Gene expression within the synovium of rat knees was not examined.
Introduction
Osteoarthritis (OA) is a gradually degenerative disease and is a major source of disability and pain. OA is characterized by cartilage degradation, synovial inflammation, and bone remodelling, and represents the most commonly diagnosed joint disorder.1-3 Among people who are over 60 years old, around 10% of men and 18% of women suffer from OA.4,5 Generally, OA patients experience impaired mobility, joint pain, and inflammation. Joint pain is the most obvious symptom of OA, and almost all OA patients complain about pain even in some other parts of the body.6 Accordingly, OA-related pain results in disability and reduced life quality. Currently, the treatment for OA in the clinic includes steroids, acetaminophen, and non-steroidal anti-inflammatory drugs.7,8 However, these strategies for OA treatment are not satisfactory because although they are all designed to relieve OA-associated pain and OA-related inflammation, they cannot reverse the OA pathological process.9 Joint/knee arthroplasty surgery is another choice, but the risks of this procedure include side effects from the anaesthesia, blood clots, infection, pain, allergy, and complications.10
Cartilage degradation is widely believed to be crucial for OA development.11,12 The biochemical and structural alteration of articular cartilage such as insufficient synthesis of extracellular matrix (ECM) and progressive chondrocyte degradation was demonstrated by various studies.13-16 The ECM is a self-assembled macromolecule complex and supplies structural strength to tissues. ECM is a dynamic network among which cells secrete molecules, and these secreted molecules in turn mediate cell behaviour. Cartilage ECM produces inflammatory mediators, local factors, and matrix-degrading enzymes to regulate cartilage degradation.17 It is commonly believed that in arthritis and cartilage degradation, interleukin-1 (IL-1) is a crucial cytokine at both the early and late stages. Tumour necrosis factor alpha (TNF-α) is mainly involved in the early stages of arthritis and cartilage degradation. Some other cytokines may be regulatory (IL-6, IL-8) or inhibitory (IL-4, IL-10, IL-13, and interferon gamma (IFN-γ)) in arthritis and cartilage degradation.18,19 Due to the limited repair capability, the progress of cartilage degradation is incurable and irreversible. The mechanism of cartilage degradation has been explored by several groups,20-22 but the information that is needed for OA treatment is still limited. Therefore, it is urgent to further understand the precise mechanism of cartilage degradation and inflammation in OA development.
Poly (ADP-ribose) polymerase (PARP) is one type of nuclear enzyme that is activated during the process of DNA strand breaks.23 PARP has been shown to be crucial in inflammation pathogenesis.24 In acute and chronic inflammation animal models, PARP inhibitor GPI 6150 was characterized to attenuate inflammatory response, organ injury, and paw swelling, partially via suppressing neutrophil recruitment to inflammatory sites.25 In this study, we investigated the effect of poly (ADP-ribose) polymerase 1 (PARP-1) inhibition via shRNA on the OA cartilage inflammatory response in anterior cruciate ligament transection (ACLT) with medial meniscectomy-induced OA model in rat. PARP-1 shRNA (short hairpin (sh)-PARP-1) suppressed cartilage matrix catabolic enzyme expression, attenuated cartilage degradation, and ameliorated the inflammatory response.
Methods
OA rat model and intra-articular injection of sh-PARP-1
The adult male Wistar rats with 330 g to 350 g of body weight (four-month-old) were purchased from GemPharmatech (China) and housed in an animal facility with standard conditions. Rats were randomly allocated to the experimental groups using. a random sequence table. A researcher who is not the authors was aware of the group allocation. OA was induced in the rats using ACLT with medial meniscectomy. Briefly, the rats were first anaesthetized by intraperitoneal injection of 65 mg/kg pentobarbital, followed by clipping the hair of the right knee. After making an incision in the medial aspect of the joint capsule, ACLT was performed on the rats, followed by removing the medial meniscus. Then, the joint was irrigated using normal saline, the capsule was sutured using 4 to 0 vicryl, and the skin was closed using 4 to 0 nylon mattress sutures. The Sham rats were given the same incision but without ACLT or medial meniscus removal (MMx). OA rats were randomly allocated into treatment and non-treatment groups. The allocation was blinded to all authors. The rats were supplied with supplemental heat and were monitored until recovery from anaesthesia. At day 84, the rats were euthanized using overdose of pentobarbital after all tests were completed. In Figure 1, five rats were needed at each timepoint in each group. In Figures 2 to Figure 5, 12 rats were needed in each group. For each experiment, the rats were age- and sex-matched. To show that the ARRIVE guidelines were adhered to in this study, we have provided an ARRIVE checklist in the Supplementary Material.
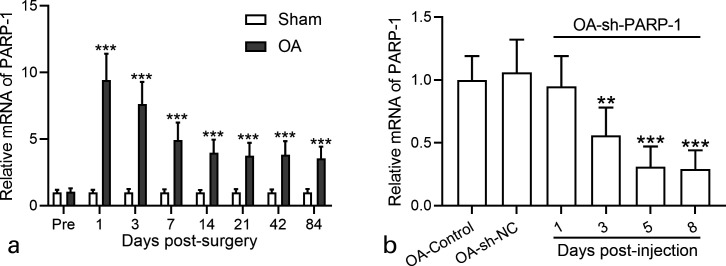
Fig. 1.
Poly (ADP-ribose) polymerase 1 (PARP-1) activity in the cartilage of osteoarthritis (OA) rats and effects of intra-articular injection of PARP-1 shRNA (short hairpin (sh)-PARP-1). a) Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) was used to analyze the messenger RNA (mRNA) expression levels of PARP-1 in the cartilage of OA rats at different timepoints after anterior cruciate ligament transection (ACLT) plus medial meniscus removal (MMx) surgery. N = 5 in each group at each timepoint. b) RT-qPCR was used to analyze the mRNA expression levels of PARP-1 in the cartilage of OA rats at different timepoints after intra-articular injection of sh-PARP-1. NC, negative control. N = 5 in each group at each timepoint. Data are presented as mean (standard deviation (SD)). **p < 0.01, ***p < 0.001 compared to sham or control. One-way analysis of variance (ANOVA) followed by Dunn's multiple comparisons test for b), and two-way ANOVA followed Tukey's multiple comparisons test for a).
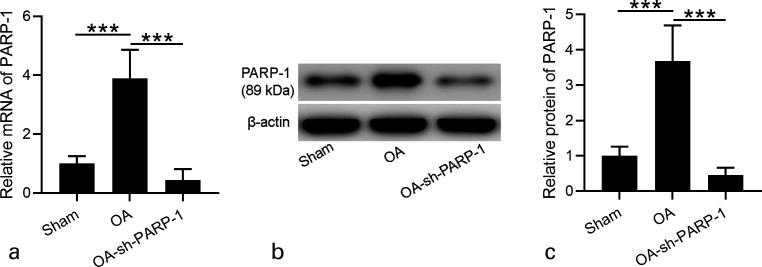
Fig. 2.
Effects of intra-articular injection of poly (ADP-ribose) polymerase 1 (PARP-1) shRNA (short hairpin (sh)-PARP-1) on the expression of PARP-1 in the cartilage of osteoarthritis (OA) rats 12 weeks after surgery. a) Real-time quantitative reverse transcription polymerase chain reaction was used to analyze the messenger RNA (mRNA) expression levels of PARP-1 in the cartilage of OA rats 12 weeks after surgery. b) Western blotting was used to assay the protein expression of PARP-1 in the cartilage of OA rats 12 weeks after surgery. c) The relative expressions were shown, which were normalized to sham; N = 12 in each group. Data are presented as mean (standard deviation (SD)). ***p < 0.001 between the indicated groups. One-way analysis of variance (ANOVA) followed by Dunn's multiple comparisons test.
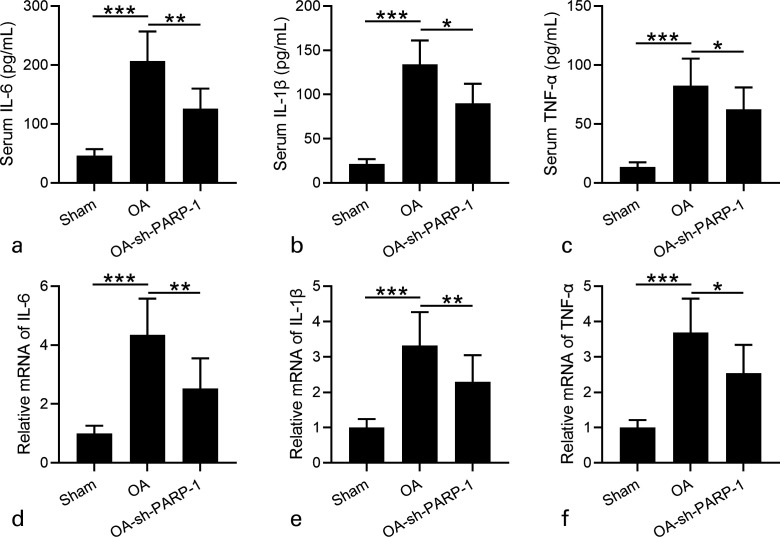
Fig. 5.
Effects of intra-articular injection of poly (ADP-ribose) polymerase 1 (PARP-1) shRNA (short hairpin (sh)-PARP-1) on the inflammatory responses in rats 12 weeks after anterior cruciate ligament transection (ACLT) plus medial meniscus removal (MMx). a) to c) Serum concentrations of interleukin (IL)-6, IL-1β, and tumour necrosis factor alpha (TNF-α) were measured by enzyme-linked immunosorbent assay (ELISA). d) to f) Real-time quantitative reverse transcription polymerase chain reaction was used to analyze the messenger RNA (mRNA) expression levels of IL-6, IL-1β, and TNF-α in cartilage. N = 12 in each group. Data are presented as mean (standard deviation (SD)). *p < 0.05, **p < 0.01, ***p < 0.001 between the indicated groups. One-way analysis of variance (ANOVA) followed by Dunn's multiple comparisons test.
For intra-articular injection of sh-PARP-1, the knees after surgery were immediately intra-articularly injected with 50 μl of sh-PARP-1 (1 × 109 transducing units (TU)/ml) using a 26 G needle. Then, the intra-articular injection of sh-PARP-1 was performed once a week until the rats were euthanized for further examination.
All animal studies were approved by Hefei Affiliated Hospital of Anhui Medical University.
Construction of sh-PARP-1 lentiviral vector
The sh-PARP-1 sequences were designed using the Ambion shRNA design online tool (NM-013063) (Thermo Fisher Scientific, USA). The sequences are: sh-PARP-1: 5’-CCA AAG GAA TTC CGA GAA A-3’; and negative control shRNA (sh-NC): 5’-TTC TCC GAA CGT GTC ACG T-3’. The lentivirus vector construction and production were completed by GenScript (China). In brief, the lentivirus-sh-PARP-1 (5 μg) and viral packaging vectors (1 μl) were co-transfected into the 293 T cells. At 48 hours after transfection, the viral supernatant was obtained and filtered using a 0.45 μm pore size filter. Then, after centrifuging the supernatant (3,500 g, 25 minutes), the precipitate was resuspended in 500 μl phosphate-buffered saline. Finally, the obtained recombinant lentivirus-sh-PARP-1 was stored at -80°C. The control plasmid was treated using the same procedures.
Incapacitance test and knee joint width
The incapacitance apparatus (Linton Instrumentation, UK) was used to determine weight-bearing ability. Rats stood on a 65° angle from the horizontal inclined plane, which was on the incapacitance apparatus. The weight on each hind limb was independently detected using the apparatus. The mean weight from five detections was calculated. The difference between the weight from the ipsilateral limb and the weight from the injured limb contralateral (Δ Force) was presented. Δ Force in different groups was detected at 2, 4, 6, 8, 10, and 12 weeks post-surgery.
The knee joint width was detected using calipers at 2, 4, 6, 8, 10, and 12 weeks post-surgery. Then, the rats were euthanized using overdose of pentobarbital for further detection.
Cartilage isolation of the joint
Cartilage isolation of the joint was performed as previously described.26 Briefly, the rats were euthanized using overdose of pentobarbital at week 12. Then, cartilage was carefully harvested from the femoral condyle and tibial plateau of rats, and a quick microscopic examination was performed to confirm the absence of contaminating non-cartilaginous tissue. The freshly isolated cartilage was processed for real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) and western blot as described below.
RT-qPCR
Cartilage tissues were collected from pre-surgery and 1, 3, 7, 14, 21, 42, and 84 days post-surgery. RNA from cartilage was isolated using TRIzol reagent (Cat No. 15596018; Thermo Fisher Scientific), followed by RNA concentration detection. Then, cDNA Synthesis Kit (Qiagen, USA) was used for complementary DNA synthesis. RT-qPCR was performed using the SYBR Taq kit (Cat. No. 1708882; Bio-Rad, USA). The primers used in this study are listed in Table I. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was used as a control. The 2−△△Ct method was used to determine the relative expression levels of indicated genes.
Table I.
Oligonucleotide primer sequences for real-time quantitative reverse transcription polymerase chain reaction.
| Gene | Primer direction | Sequence (5′--3′) |
|---|---|---|
| PARP-1 | Forward | TCCCAGAACAAGGACGAAGC |
| Reverse | CCTCACACACGACTCGAACA | |
| Aggrecan | Forward | ATGATGGCGCTGTTCTGAAGG |
| Reverse | GAAGTGATGCATGGCATTGAGG | |
| Col2A1 | Forward | GGCAATAGCAGGTTCACGTACA |
| Reverse | GATAACAGTCTTGCCCCACTTACC | |
| MMP-1 | Forward | ACAGTTTCCCCGTGTTTCAG |
| Reverse | CCCACACCTAGGTTTCCTCA | |
| MMP-3 | Forward | TCTTTCACTCAGCCAATGCT |
| Reverse | GGGAGGTCCATAGAGGGATT | |
| IL-6 | Forward | GGATACCACCCACAACAGAC |
| Reverse | TTGCCGAGTAGACCTCATAG | |
| IL-1β | Forward | TCATTGTGGCTGTGGAGAAG |
| Reverse | CTATGTCCCGACCATTGCTG | |
| TNF-α | Forward | CCCCTTTATCGTCTACTCCTC |
| Reverse | GCTGGTAGTTTAGCTCCGTTT | |
| iNOS | Forward | GCATCCCAAGTACGAGTGGT |
| Reverse | GAAGGCGTAGCTGAACAAGG | |
| COX-2 | Forward | GTGGGATGACGAGCGACTGT |
| Reverse | TTTCAGGGAGAAGCGTTTGC | |
| GAPDH | Forward | GAAGGTGAAGGTCGGAGTC |
| Reverse | GAAGATGGTGATGGGATTTC |
Col2A1, collagen type II alpha 1 chain; COX-2, cyclooxygenase-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ICAM-1, intercellular cell adhesion molecule-1; IL, interleukin; iNOS, inducible nitric oxide synthase; MMP, matrix metalloproteinase; PARP-1, poly(ADP-ribose) polymerase-1; TNF-α, tumour necrosis factor alpha.
Western blot
The proteins from the cartilage of rats from different groups were lysed by radioimmunoprecipitation assay (RIPA) buffer (Beyotime, China) containing protease inhibitors. Then, proteins were separated using 10% sodium dodecyl sulfate gel and transferred onto the polyvinylidene fluoride membrane. Subsequently, the membranes were blocked using 5% non-fat milk, followed by incubation with primary antibodies at 4°C overnight. The antibodies used in this study were: anti-PARP-1 (Cleaved PARP (Asp214) Antibody (Rat Specific) #9545, 1:1,000; Cell Signaling Technology (CST), USA); anti-iNOS (#32027, 1:1,000; CST); anti-COX-2 (#12282, 1:1,000; CST); anti-MMP-13 (#AB39012, 1:1,000; Abcam, UK); aggrecan (ab36861, 1:1,000; Abcam); type II collagen (ab188570, 1:1,000; Abcam); and anti-β-actin (#3700, 1:1,000; CST). Finally, the membranes were further incubated using horseradish peroxidase-secondary antibodies. The enhanced luminol-based chemiluminescence was used to detect protein bands. The expression level was quantified using ImageJ software (National Institutes of Health, USA). The β-actin expression level was used as a control.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of matrix metalloproteinase (MMP)-1 (Rat MMP-1 ELISA Kit, E-EL-R0617c), MMP-3 (Rat MMP-3 ELISA Kit, E-EL-R0619c), IL-6 (Rat IL-6 ELISA Kit, E-EL-R0015c), IL-1β (Rat IL-1β ELISA Kit, E-EL-R0012c), and TNF-α (Rat TNF-α ELISA Kit, E-EL-R2856c) in serum were detected using commercial ELISA kits from Elabscience Biotechnology Co. (China), following the manufacturer’s instructions.
Statistical analysis
GraphPad Prism v7.0 software (GraphPad Software, USA) was used to analyze raw data. Data are presented as mean (standard deviation (SD)). The differences in means among multiple groups were analyzed using one-way analysis of variance (ANOVA) followed by Dunn’s multiple comparisons test. If different experimental groups with different timepoints were analyzed, two-way ANOVA followed by Tukey’s multiple comparisons test was used. Statistical significance was set at p < 0.05.
Results
PARP-1 expression significantly increases in the cartilage of OA rats
The expression level of PARP-1 was first determined by RT-qPCR in the cartilage of ACLT plus surgery-induced OA rats and controlled Sham rats. As shown in Figure 1a, PARP-1 messenger RNA (mRNA) expression level greatly increased in the cartilage of OA rats at different time points after surgery (1, 3, 7, 14, 21, 42, and 84 days post-surgery) compared with Sham rats. PARP-1 expression sharply increased on day 1 post-surgery, and gradually decreased to the stable level 14 days post-surgery, suggesting the important role of PARP-1 in OA.
We next downregulated PARP-1 expression using shRNA (sh-PARP-1), which was delivered by lentiviral vector and detected PARP-1 mRNA expression level in the cartilage of different group rats after intra-articular injection of sh-PARP-1. As shown in Figure 1b, PARP-1 mRNA expression significantly decreased three days post-injection, as well as five days and eight days post-injection. These results demonstrated that PARP-1 expression in the cartilage significantly increased in OA rats and decreased after sh-PARP-1 injection. sh-PARP-1 suppressed PARP-1 expression levels 12 weeks after surgery.
The results from Figure 1b indicate that sh-PARP-1 suppressed PARP-1 mRNA expression to a stable level seven days post-injection. So, we performed intra-articular injection of sh-PARP-1 once a week and determined PARP-1 expression 12 weeks after surgery. As expected, PARP-1 mRNA expression level in the cartilage of OA rats was much higher than in Sham rats, and sh-PARP-1 treatment significantly suppressed PARP-1 mRNA level in OA rats (Figure 2a).
The increased PARP-1 protein level in the cartilage of OA rats was dramatically suppressed after sh-PARP-1 treatment (Figures 2b and 2c). These results demonstrate that sh-PARP-1 suppressed PARP-1 mRNA expression 12 weeks after surgery. sh-PARP-1 attenuated weight-bearing distribution of the hind limbs and the knee joint width.
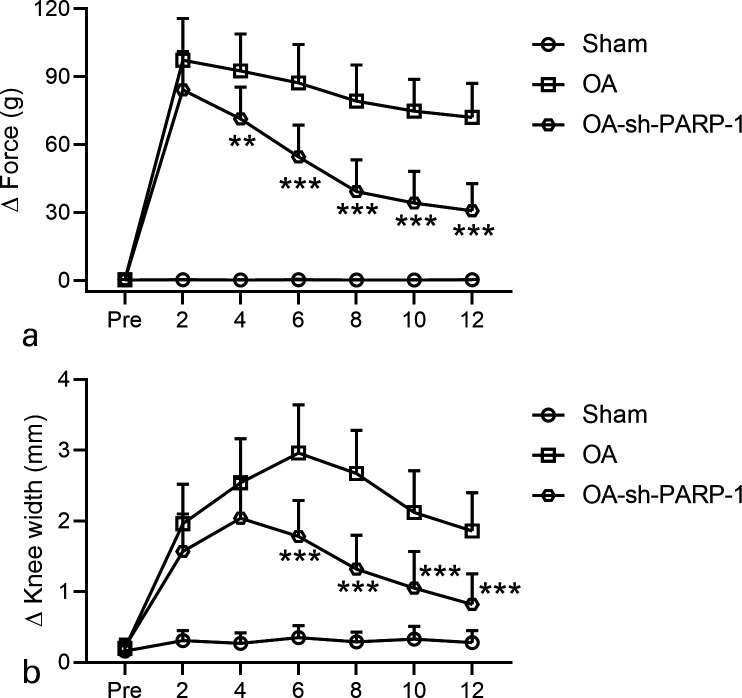
The effect of sh-PARP-1 treatment on the weight-bearing distribution of the hind limbs and the knee joint width was further explored. The Δ Force (g) was defined as the difference between the weight on ipsilateral limb and contralateral limb. As shown in Figure 3a, the Δ Force of OA rats is much higher than Sham rats at different time points after surgery (2, 4, 6, 8, 10, and 12 weeks), and sh-PARP-1 treatment significantly attenuated the Δ Force of OA rats at 12 weeks after surgery. The individual data of the Δ Force of OA rats are given in Supplementary Figure aa.
Fig. 3.
Effects of intra-articular injection of poly (ADP-ribose) polymerase 1 (PARP-1) shRNA (short hairpin (sh)-PARP-1) on the weight-bearing distribution of a) Δ force and b) knee joint width in rats that underwent anterior cruciate ligament transection (ACLT) plus medial meniscus removal (MMx). An incapacitance test was used to measure the weight-bearing of the hind limb preoperatively and every two weeks after surgery for 12 weeks. The widths of the bilateral joints were measured every two weeks after surgery for 12 weeks. The data are the difference between the weights and the widths of the contralateral and ipsilateral limbs. N = 12 in each group. Data are presented as mean (standard deviation (SD)).**p < 0.01, ***p < 0.001 compared to the osteoarthritis (OA) group. Two-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test.
Similarly, the knee joint width of OA rats at 12 weeks was much higher than that of Sham rats and was greatly attenuated by sh-PARP-1 treatment (Figure 3b). These data indicate that sh-PARP-1 treatment attenuated the weight-bearing distribution of the hind limbs and the knee joint width of OA rats. The individual data of knee joint width of OA rats are given in Supplementary Figure ab.
sh-PARP-1 ameliorates OA cartilage inflammation
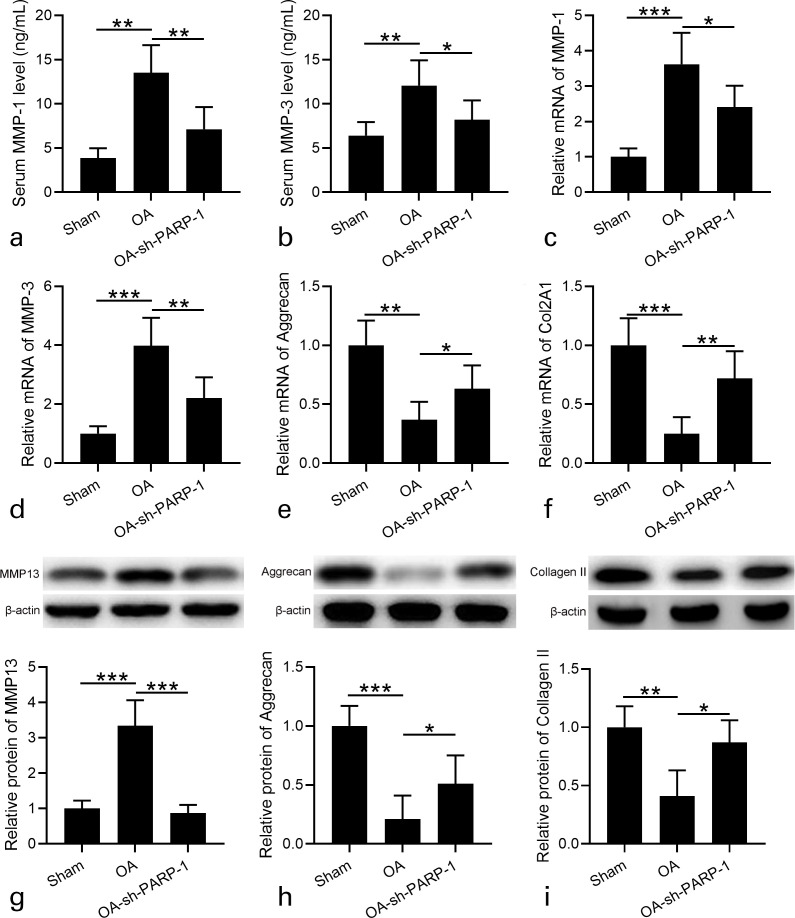
We next determined the effects of sh-PARP-1 treatment on OA cartilage inflammation. The serum MMP-1 and MMP-3 concentrations of OA rats were much higher than those of Sham rats, and sh-PARP-1 treatment clearly suppressed MMP-1 and MMP-3 concentrations in serum (Figures 4a and 4b). Similarly, the increased mRNA levels of MMP-1 and MMP-3 in the cartilage of OA rats were significantly attenuated after sh-PARP-1 treatment (Figures 4c and 4d).
Fig. 4.
Effects of intra-articular injection of poly (ADP-ribose) polymerase 1 (PARP-1) shRNA (short hairpin (sh)-PARP-1) on the expression of cartilage matrix catabolic enzymes in the cartilage and ameliorated osteoarthritis (OA) cartilage degradation in rats 12 weeks after anterior cruciate ligament transection (ACLT) plus medial meniscus removal (MMx). a) and b) Serum matrix metalloproteinase (MMP)-1 and MMP-3 were measured by enzyme-linked immunosorbent assay (ELISA). c) and d) Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) was used to analyze the messenger RNA (mRNA) expression levels of MMP-1 and MMP-3 in cartilage. Relative expression was normalized to sham group. e) and f) RT-qPCR was used to analyze the mRNA expression levels of aggrecan and collagen type II alpha 1 chain (Col2A1) in cartilage. Relative expression was normalized to sham group. g) to i) Western blotting was used to assay the protein expression of g) MMP-13, h) aggrecan, and i) type II collagen in cartilage. Relative expression was normalized to sham group. N = 12 in each group. Data are presented as mean (standard deviation (SD)). *p < 0.05, **p < 0.01, ***p < 0.001 between the indicated groups. One-way analysis of variance (ANOVA) followed by Dunn's multiple comparisons test.
Moreover, the expression levels of aggrecan and collagen type II alpha 1 chain (Col2A1) were determined in the cartilage. As shown in Figures 4e and 4f, the mRNA levels of aggrecan and Col2A1 significantly decreased in OA rats compared with Sham rats, while sh-PARP-1 treatment enhanced aggrecan and Col2A1 expression levels in OA rats. MMP-13 is considered the major collagenase in OA.27 Therefore, we detected MMP-13 expression in the cartilage. MMP-13 protein levels significantly increased in OA rats, while sh-PARP-1 treatment suppressed MMP-13 expression in OA rats (Figure 4g). Consistently, the protein levels of aggrecan and type II collagen in OA rats were significantly decreased compared with in Sham rats, while sh-PARP-1 treatment increased their expression in OA rats (Figures 4h and 4i). These results suggest that sh-PARP-1 treatment ameliorates OA cartilage inflammation.
sh-PARP-1 attenuates inflammatory response of OA rats
Inflammation was generally believed to be crucial for OA.28 Therefore, we detected whether sh-PARP-1 treatment would attenuate the inflammatory response of OA rats. IL-6, IL-1β, and TNF-α were the proinflammatory cytokines and their expression levels were detected. As shown in Figures 5a to 5c, the serum concentrations of IL-6, IL-1β, and TNF-α significantly increased in OA rats compared with in Sham rats, and sh-PARP-1 treatment dramatically decreased their serum concentrations.
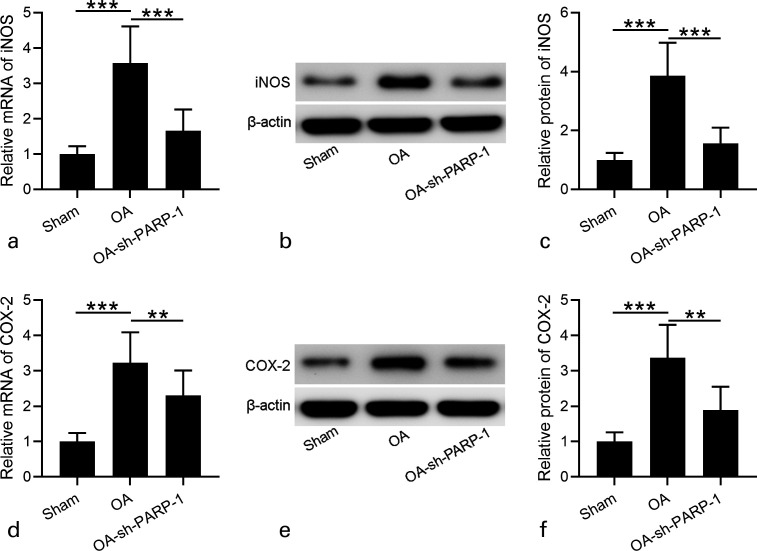
Consistently, the increased mRNA expression levels of IL-6, IL-1β, and TNF-α in OA rats were decreased after sh-PARP-1 treatment (Figures 5d to 5f). These data suggest that sh-PARP-1 attenuated the inflammatory response of OA rats. sh-PARP-1 suppressed iNOS and COX-2 expression in OA rats; iNOS and COX-2 are crucial for the inflammatory response,29 and we finally detected iNOS and COX-2 expression in cartilage at both mRNA and protein levels. The mRNA levels of iNOS and COX-2 in cartilage of OA rats were much higher than in Sham rats, and sh-PARP-1 significantly suppressed their mRNA levels (Figures 6a and 6d). At the protein levels, we also found that iNOS and COX-2 levels in the cartilage of OA rats significantly increased compared with in Sham rats, and sh-PARP-1 treatment significantly decreased iNOS and COX-2 expression levels (Figures 6b to 6f). These results demonstrate that sh-PARP-1 suppresses iNOS and COX-2 expression in OA rats.
Fig. 6.
Effects of intra-articular injection of poly (ADP-ribose) polymerase 1 (PARP-1) shRNA (short hairpin (sh)-PARP-1) on the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in cartilage of rats 12 weeks after anterior cruciate ligament transection (ACLT) plus medial meniscus removal (MMx). a) and d) Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) was used to analyze the messenger RNA (mRNA) expression levels of iNOS and COX-2 in cartilage. b) and e) Western blotting was used to assay the protein expression of iNOS and COX-2 in the cartilage. c) and f) Relative expression levels of iNOS and COX-2 are shown, which were normalized to the corresponding sham. N = 12 in each group. Data are presented as mean (standard deviation (SD)). **p < 0.01, ***p < 0.001 between the indicated groups. One-way analysis of variance (ANOVA) followed by Dunn's multiple comparisons test. OA, osteoarthritis.
Discussion
OA incidence has been reported to be increased in older people, and even in young people with joint injury.30 However, the treatment for OA is still unsatisfactory. In this study, we focused on the effect of PARP inhibition using lentiviral vector-delivered shRNA on cartilage inflammatory response in the OA rat model, based on the fact that PARP was demonstrated to be important for the inflammatory response and PARP inhibition suppressed inflammation in OA animal models.31
We first successfully established OA rat model by ACLT with medial meniscectomy surgery, which is evidenced by increased Δ Force (the difference between the weight on ipsilateral limb and contralateral limb), enhanced knee width, elevated serum concentrations of inflammatory collagenase (MMP-1 and MMP-3), protein expression levels of MMP-13, inflammatory cytokines (IL-6, IL-1β, and TNF-α), and decreased protein expression levels of aggrecan and type II collagen. In the OA rat model, we performed intra-articular injection of sh-PARP-1 once a week to stably suppress both mRNA and protein expressions of PARP-1 in the cartilage, which is the basis of the present study, to investigate the role of sh-PARP-1 in cartilage inflammatory response of OA rats.
Cartilage inflammation is important for the development of OA.28 The strategies for preventing cartilage inflammation have been reported to attenuate OA.32 In the postmenopausal OA rat model, Labisia pumila, a tropical herb, suppressed concentrations of serum collagenases and type II collagen degradation, accompanying reduced cartilage erosions and osteophytes,33 indicating that Labisia pumila attenuates OA at least partly by suppressing cartilage inflammation. Calcitonin administration could suppress cartilage inflammation, improve the nociceptive test results in the OA rat model, and attenuate OA development possibly through enhancing TGF-β1 expression level in chondrocytes.34 In ACLT with medial meniscectomy-induced rat OA model, shea nut oil triterpene concentrates suppress cartilage inflammation and knee joint matrix loss.35 Consistently, in our study we found that sh-PARP-1 treatment decreased the expression of cartilage matrix catabolic enzymes and ameliorated OA cartilage inflammation in OA rat model, indicating the protecting effect of sh-PARP-1 on OA.
PARP is one type of nuclear enzyme that was reported to be crucial in the inflammatory response.31 In the zymosan-induced multiple organ failure animal model and adjuvant-induced arthritis animal model, PARP inhibitor suppressed the inflammatory response, indicating that PARP inhibitor exerts anti-inflammatory effects, which is partly due to the decreased neutrophil infiltration.25 In lipopolysaccharide (LPS)-induced pulmonary inflammation animal models, pharmacological inhibition of PARP (PARP inhibitor PJ-34) or genetic deletion of PARP (PARP-/- animal) attenuate alveolar neutrophil accumulation, cytokine induction, nitric oxide production, lung hyperpermeability, and lipid peroxidation. Histologically, the lung injury was also attenuated in animal models with PARP dysfunction,36 indicating that pharmacological PARP inhibition may be a potential choice for patients with lung inflammation. In trinitrobenzene sulfonic acid-induced rat colitis model, the PARP inhibitors 1,5-dihydroxyisoquinoline and nicotinamide reduced colon injury, and decreased myeloperoxidase activity and prostaglandin E2 level in the colon,37 demonstrating that PARP inhibition is effective in an experimental colitis model and may be useful for ulcerative colitis treatment. In an ischaemia/reperfusion injury rat model, PARP inhibitor 3-aminobenzamide administration suppressed the expression levels of inflammation-associated genes (CD11b, intercellular cell adhesion molecule-1 (ICAM-1), and COX-2), accompanied by decreased water content and decreased infarct volume,38 suggesting the neuroprotective effect of PARP inhibition in ischaemic stroke. In human osteoarthritic chondrocytes, PARP inhibition inhibited IL-1β-induced inflammation.39 Summarily, in different inflammation-related animal models and in human osteoarthritic chondrocytes, PARP inhibition was clearly demonstrated to attenuate inflammation, indicating the common effect of PARP inhibition on suppressing the inflammatory response.
In this study, we inhibited PARP-1 expression by shRNA. Functional assay indicated that sh-PARP-1 treatment inhibited serum concentrations of inflammatory cytokines (IL-6, IL-1β, and TNF-α) and their mRNA expression levels. Moreover, the expression levels of iNOS and COX-2 dramatically decreased after sh-PARP-1 treatment. Besides the effect of sh-PARP-1 treatment on inflammation, we also found that this significantly suppressed the Δ Force and enhanced knee width, indicating its protecting role in OA animal model. However, we did not detect the effect of sh-PARP-1 treatment on CD11b and ICAM-1 expression level, which were indicated to be altered after PARP inhibition treatment.38 To the best of our knowledge, this is the first study to investigate the effect of sh-PARP-1 on inflammatory cytokines on the OA rat model. Based on the results in animal models, sh-PARP-1 may be potentially used in a clinical setting only after more clear evidence has been gathered of the toxicity and tolerance of sh-PARP-1 on OA models and cell lines.
There were several limitations in this study. First, the effect of sh-PARP-1 was not studied in other OA animal models. Second, we did not perform a histological analysis; the histological sections of the cartilages and the histological score may help to intuitively show the effect of sh-PARP-1 on OA development. Third, no immunofluorescence was performed to identify whether sh-PARP-1 is just limited to the superficial layer of the cartilage or if it penetrates deeper. Fourth, the toxicity and tolerance of sh-PARP-1 on OA models would supply evidence for the potential use of sh-PARP-1 for OA treatment in the clinic. Finally, gene expression within the synovium of rat knees was not examined, while this examination would answer an important question: whether the reported protective actions on cartilage could be at least partly attributed to sh-PARP-1 effects on synovial cells.
In conclusion, using the ACLT with medial meniscectomy-induced rat OA model, we have shown for the first time that sh-PARP-1 treatment attenuates OA cartilage inflammatory response.
Author contributions
Z. Liu: Conducted the experiments, Analyzed the data, Wrote the manuscript.
H. Wang: Conducted the experiments, Analyzed the data, Wrote the manuscript.
S. Wang: Conducted the experiments, Analyzed the data, Wrote the manuscript.
J. Gao: Conducted the experiments, Analyzed the data, Wrote the manuscript.
L. Niu: Conceived the study, Wrote the manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical review statement
All animal studies were approved by Hefei Affiliated Hospital of Anhui Medical University.
Open access funding
The authors report that the open access funding was provided by their department, and could be considered as self-funded according to the department's policy.
Supplementary material
Individual values for Figure 3 and original western blot films for Figures 2, 4, and 6. ARRIVE checklist also provided to show that the ARRIVE guidelines were adhered to in the study.
References
- 1. Glyn-Jones S, Palmer AJR, Agricola R, et al. . Osteoarthritis. Lancet. 2015;386(9991):376–387. [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang X, Bu Y, Zhu B, et al. . Global transcriptome analysis to identify critical genes involved in the pathology of osteoarthritis. Bone Joint Res. 2018;7(4):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Che Ahmad Tantowi NA, Hussin P, Lau SF, Mohamed S. Mistletoe fig (Ficus deltoidea jack) leaf extract prevented postmenopausal osteoarthritis by attenuating inflammation and cartilage degradation in rat model. Menopause. 2017;24(9):1071–1080. [DOI] [PubMed] [Google Scholar]
- 5. Li H, Yang HH, Sun ZG, Tang HB, Min JK. Whole-transcriptome sequencing of knee joint cartilage from osteoarthritis patients. Bone Joint Res. 2019;8(7):290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bajaj P, Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain. 2001;93(2):107–114. [DOI] [PubMed] [Google Scholar]
- 7. Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012;85(1):49–56. [PubMed] [Google Scholar]
- 8. Anandacoomarasamy A, March L. Current evidence for osteoarthritis treatments. Ther Adv Musculoskelet Dis. 2010;2(1):17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White RH, Henderson MC. Risk factors for venous thromboembolism after total hip and knee replacement surgery. Curr Opin Pulm Med. 2002;8(5):365–371. [DOI] [PubMed] [Google Scholar]
- 11. Man GS, Mologhianu G. Osteoarthritis pathogenesis - a complex process that involves the entire joint. J Med Life. 2014;7(1):37–41. [PMC free article] [PubMed] [Google Scholar]
- 12. He A, Ning Y, Wen Y, et al. . Use of integrative epigenetic and mRNA expression analyses to identify significantly changed genes and functional pathways in osteoarthritic cartilage. Bone Joint Res. 2018;7(5):343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3(2):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schnabel M, Marlovits S, Eckhoff G, et al. . Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage. 2002;10(1):62–70. [DOI] [PubMed] [Google Scholar]
- 15. Zhao X, Huang P, Li G, Lv Z, Hu G, Xu Q. Activation of the leptin pathway by high expression of the long form of the leptin receptor (Ob-Rb) accelerates chondrocyte senescence in osteoarthritis. Bone Joint Res. 2019;8(9):425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tawonsawatruk T, Sriwatananukulkit O, Himakhun W, Hemstapat W. Comparison of pain behaviour and osteoarthritis progression between anterior cruciate ligament transection and osteochondral injury in rat models. Bone Joint Res. 2018;7(3):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gentili C, Cancedda R. Cartilage and bone extracellular matrix. Curr Pharm Des. 2009;15(12):1334–1348. [DOI] [PubMed] [Google Scholar]
- 18. Wojdasiewicz P, Poniatowski Łukasz A, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40(1):1–11. [DOI] [PubMed] [Google Scholar]
- 20. Lee AS, Ellman MB, Yan D, et al. . A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527(2):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saito T, Kawaguchi H. Transcriptional regulation of osteoarthritis. Clin Calcium. 2011;21(6):853–859. (Article in Japanese) [PubMed] [Google Scholar]
- 22. Kawaguchi H. Progress of research in osteoarthritis. Molecular targeting for osteoarthritis treatment. Clin Calcium. 2009;19(11):1608–1614. (Article in Japanese) [PubMed] [Google Scholar]
- 23. Dantzer F, Amé J-C, Schreiber V, Nakamura J, Ménissier-de Murcia J, de Murcia G. Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol. 2006;409:493–510. [DOI] [PubMed] [Google Scholar]
- 24. Cuzzocrea S. Shock, inflammation and PARP. Pharmacol Res. 2005;52(1):72–82. [DOI] [PubMed] [Google Scholar]
- 25. Mazzon E, Serraino I, Li JH, et al. . GPI 6150, a poly (adp-ribose) polymerase inhibitor, exhibits an anti-inflammatory effect in rat models of inflammation. Eur J Pharmacol. 2001;415(1):85–94. [DOI] [PubMed] [Google Scholar]
- 26. Dai L, Zhang X, Hu X, et al. . Silencing of miR-101 prevents cartilage degradation by regulating extracellular matrix-related genes in a rat model of osteoarthritis. Mol Ther. 2015;23(8):1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moita E, Gil-Izquierdo A, Sousa C, et al. . Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoS One. 2013;8(3):e59131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rahmati M, Nalesso G, Mobasheri A, Mozafari M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res Rev. 2017;40:20–30. [DOI] [PubMed] [Google Scholar]
- 31. Ke Y, Wang C, Zhang J, et al. . The role of PARPs in Inflammation-and metabolic-related diseases: molecular mechanisms and beyond. Cells. 2019;8(9):E1047:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Y, Pizzute T, Pei M. Anti-inflammatory strategies in cartilage repair. Tissue Eng Part B Rev. 2014;20(6):655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Madzuki IN, Lau SF, Che Ahmad Tantowi NA, Mohd Ishak NI, Mohamed S. Labisia pumila prevented osteoarthritis cartilage degeneration by attenuating joint inflammation and collagen breakdown in postmenopausal rat model. Inflammopharmacology. 2018;26(5):1207–1217. [DOI] [PubMed] [Google Scholar]
- 34. Wen Z-H, Tang C-C, Chang Y-C, et al. . Calcitonin attenuates cartilage degeneration and nociception in an experimental rat model of osteoarthritis: role of TGF-β in chondrocytes. Sci Rep. 2016;6:28862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kao J-H, Lin S-H, Lai C-F, Lin Y-C, Kong Z-L, Wong C-S. Shea nut oil triterpene concentrate attenuates knee osteoarthritis development in rats: evidence from knee joint histology. PLoS One. 2016;11(9):e0162022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liaudet L, Pacher P, Mabley JG, et al. . Activation of poly(ADP-Ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am J Respir Crit Care Med. 2002;165(3):372–377. [DOI] [PubMed] [Google Scholar]
- 37. Sánchez-Fidalgo S, Villegas I, Martín A, Sánchez-Hidalgo M, Alarcón de la Lastra C. PARP inhibition reduces acute colonic inflammation in rats. Eur J Pharmacol. 2007;563(1-3):216–223. [DOI] [PubMed] [Google Scholar]
- 38. Koh S-H, Park Y, Song CW, et al. . The effect of PARP inhibitor on ischaemic cell death, its related inflammation and survival signals. Eur J Neurosci. 2004;20(6):1461–1472. [DOI] [PubMed] [Google Scholar]
- 39. Sun Y, Zhou L, Lv D, Liu H, He T, Wang X. Poly(ADP-ribose) polymerase 1 inhibition prevents interleukin-1β-induced inflammation in human osteoarthritic chondrocytes. Acta Biochim Biophys Sin. 2015;47(6):422–430. [DOI] [PubMed] [Google Scholar]