Abstract
Aims
The value of core decompression (CD) in the treatment of osteonecrosis of the femoral head (ONFH) remains controversial. We conducted a systematic review and meta-analysis to evaluate whether CD combined with other treatments could improve the clinical and radiological outcomes of ONFH patients compared with CD alone.
Methods
We searched the PubMed, Embase, Web of Science, and Cochrane Library databases until June 2020. All randomized controlled trials (RCTs) and clinical controlled trials (CCTs) comparing CD alone and CD combined with other measures (CD + cell therapy, CD + bone grafting, CD + porous tantalum rod, etc.) for the treatment of ONFH were considered eligible for inclusion. The primary outcomes of interest were Harris Hip Score (HHS), ONFH stage progression, structural failure (collapse) of the femoral head, and conversion to total hip arthroplasty (THA). The pooled data were analyzed using Review Manager 5.3 software.
Results
A total of 20 studies with 2,123 hips were included (CD alone = 768, CD combined with other treatments = 1,355). The combination of CD with other therapeutic interventions resulted in a higher HHS (mean difference (MD) = 6.46, 95% confidence interval (CI) = 2.10 to 10.83, p = 0.004) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score (MD = −10.92, 95% CI = -21.41 to -4.03, p = 0.040) and a lower visual analogue scale (VAS) score (MD = −0.99, 95% CI = -1.56 to -0.42, p < 0.001) than CD alone. For the rates of disease stage progression, 91 (20%) progressed in the intervention group compared to 146 (36%) in the control group (odds ratio (OR) = 0.32, 95% CI = 0.16 to 0.64, p = 0.001). In addition, the intervention group had a more significant advantage in delaying femoral head progression to the collapsed stage (OR = 0.32, 95% CI = 0.17 to 0.61, p < 0.001) and reducing the odds of conversion to THA (OR = 0.35, 95% CI = 0.23 to 0.55, p < 0.001) compared to the control group. There were no serious adverse events in either group. Subgroup analysis showed that the addition of cell therapy significantly improved clinical and radiological outcomes compared to CD alone, and this approach appeared to be more effective than other therapies, particularly in precollapse (stage I to II) ONFH patients.
Conclusion
There was marked heterogeneity in the studies. There is a trend towards improved clinical outcomes with the addition of stem cell therapy to CD.
Cite this article: Bone Joint Res 2021;10(7):445–458.
Keywords: Osteonecrosis of the femoral head, Core decompression, Hip-preserving therapy, Cell therapy, Meta-analysis
Article focus
Evaluate whether core decompression (CD) combined with other treatments would improve the clinical and radiological outcomes of osteonecrosis of the femoral head (ONFH) patients compared with CD alone.
Investigate which hip-preserving surgery is the best for precollapse (stage I to II) ONFH patients.
The primary outcomes of interest were Harris Hip Score (HHS), ONFH stage progression, structural failure (collapse) of the femoral head, and conversion to total hip arthroplasty (THA).
Key messages
Compared with CD alone, the combination of CD with other therapeutic interventions resulted in better clinical and radiological outcomes.
Cell therapy showed a greater advantage compared with other treatments for precollapse (stage I to II) ONFH patients.
The safety of CD combined with other treatment measures is acceptable for ONFH.
Strengths and limitations
A comprehensive systematic search and rigorous screening were conducted, including 20 controlled trials that met the inclusion criteria for a total of 1,379 records, involving 2,123 hips. We provided results based on a relatively large sample size to overcome disadvantages of previous studies.
Subgroup analysis was conducted to fully compare whether four surgical methods can improve the outcome of ONFH patients when compared with CD alone, and to explore the impact of ONFH stages on the results of the study.
There is heterogeneity in some outcome indicators. Although the subgroup and sensitivity analyses were conducted, which may affect the final decision of orthopaedic surgeons, the results of a statistical test did not indicate otherwise.
Introduction
Osteonecrosis of the femoral head (ONFH) is a debilitating disease that may result in collapse of the femoral head and progressive hip joint degeneration.1 The total number of ONFH cases in the world is estimated to be 20 million.2,3 In China, there are 8.12 million patients with nontraumatic ONFH alone.4 The disease affects a relatively young population, and many patients undergo surgical treatment (i.e. arthroplasty) before their conditions degenerate into hip arthritis (stage III disease). Although total hip arthroplasty (THA) has achieved satisfactory results for the treatment of advanced ONFH, it is not the best treatment for patients in the early stage of collapse. Considering the higher risk for THA arthroplasty failure in younger patients, it is important to optimize joint preservation approaches.5
As the most commonly used hip-preservation treatment, core decompression (CD) can delay the process of ONFH to some extent.6 However, its efficacy in the treatment of ONFH is still controversial.7-9 Due to the lack of effective mechanical support in the necrotic area after decompression, the collapse of the bearing surface may be accelerated. In addition, this method does not address the problems of angiogenesis, bone reconstruction, and articular surface repair in the necrotic area.10 Therefore, most joint surgeons only use CD as the basic treatment combined with internal fixation support such as tantalum rods,11 nonvascularized or vascularized bone grafting,12,13 various artificial materials for tissue engineering, cytokines,14,15 and the application of stem cell therapy.16-18 However, these approaches have limitations. Free vascularized fibular grafting (FVFG) requires microsurgical technology and produces great surgical trauma, while fibula-related complications, availability of sufficient transplantable bone, and implantation survival rate will affect the final treatment effect.19-21 Tantalum rods produced by Zimmer Biomet (USA) have problems such as lack of bone ingrowth (only 1.9%) and insufficient support. In addition, the decrease in the strength of the greater trochanter leads to stress fracture after this procedure.22-25
To date, it is still unclear whether CD combined with other treatments has a better efficacy for ONFH patients than CD alone, and many studies have reached inconsistent conclusions. To this end, we conducted a meta-analysis to evaluate whether the combination of CD with other treatments would improve the clinical and radiological outcomes of ONFH patients compared to classical CD alone.
Methods
This meta-analysis was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.26,27
Search strategy
The original papers were primarily retrieved from PubMed, Embase, Web of Science, and Cochrane Library. The search used terms and Boolean operators as follows: osteonecrosis of the femoral head OR femoral head necrosis OR necrosis of femoral head OR avascular necrosis of femoral head AND core decompression OR centre decompression. The search was performed on 10 June 2020, the language was limited to English, and there was no time limit for publication. In addition, we manually searched reference lists of review articles and included studies to identify other potentially eligible studies.
Eligibility criteria
Clinical trials were included if they met the PICOS criteria as follows: Populations: ONFH patients aged 15 to 70 years; Intervention: combination of CD with other treatments such as bone grafting, stem cell therapy, etc.; Comparator: classical CD alone; Outcomes: studies can provide any of the four primary outcomes of interest (Harris Hip Score (HHS),28 stage progression of disease, structural failure of the femoral head, and conversion to THA); Study design: randomized controlled trials (RCTs) or clinical controlled trials (CCTs).
Literature selection and data extraction
The search records were managed via Endnote (Clarivate Analytics, USA), where two reviewers (SZ, YW) independently assessed the titles and abstracts of the retrieved articles to exclude obviously irrelevant literature, after which all potentially eligible articles were obtained in full text and evaluated according to the inclusion criteria. Any discrepancy between the two reviewers was resolved through discussion or consensus with a third reviewer (WQ). The extracted data included: basic information about the included studies: study title, first author, time of publication, etc.; baseline characteristics of included subjects and intervention measures, etc.; key elements of the risk of bias evaluation; and outcome indicators of interest and relevant data.
Risk of bias assessment in the studies
The methodological bias and quality of the included studies were independently assessed by two reviewers according to the Cochrane Collaboration tool,29 including the following domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessments; incomplete outcome data; selective outcome reporting; and other bias. Any discrepancy between them was resolved through discussion. The assessments were classified into three levels: low risk, high risk, and unclear risk.
Statistical analysis
Review Manager 5.3 (Cochrane Community, UK) software was used to perform the meta-analysis. We used mean difference (MD) and odds ratio (OR) to assess continuous variable outcomes and dichotomous data, respectively, both with 95% confidence intervals (CIs). Heterogeneity between included studies was assessed by the I2 and chi-squared (χ2) tests. For the former, heterogeneity was considered significant at p < 0.1. For the latter, an I2 value of greater than 50% was taken to represent significant heterogeneity. A fixed-effects model was applied to analyze data if there was low heterogeneity, and a random effects model was used if there was high heterogeneity. In the subgroup analysis, we decided to explore the effect of different treatment methods and stages of ONFH on the final outcome. In addition, sensitivity analysis was performed by omitting each study to explore the source of heterogeneity and evaluate the stability of the results when heterogeneity existed. Statistical significance was set at p < 0.05. Funnel plots were used to assess publication bias for the primary outcomes of interest (HHS, progression of ONFH stage, collapse of femoral head, and conversion of THA).
Results
Search results
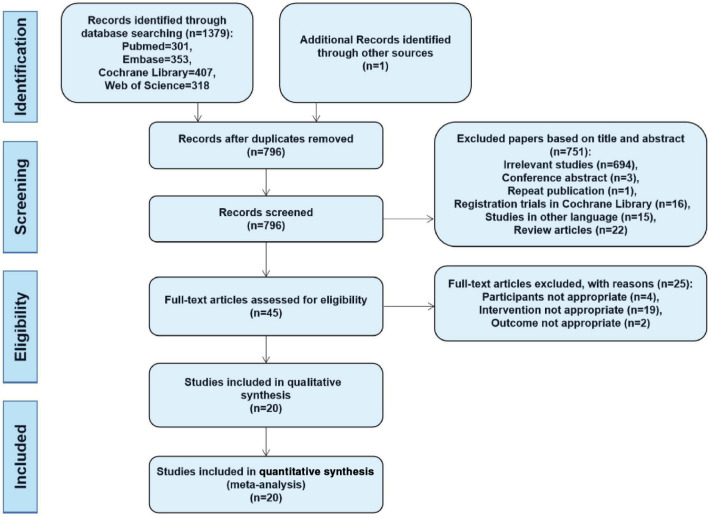
We preliminarily identified 1,379 records, not including additional studies from the reference lists of relevant studies, and removed 584 duplicates. From the remaining 795 records, we excluded 751 by screening titles and abstracts, leaving 44 potentially relevant papers for full-text review. After this stage, 25 studies were eliminated because they failed to meet the inclusion criteria, leaving 19 studies30-48 after the primary search. One additional study49 that met the inclusion criteria was retained from the secondary search of reference lists of relevant studies. Finally, 20 studies (eight RCTs30-36,49 and 12 CCTs37-48 with 2,123 hips (CD alone = 768, CD combined with other treatments = 1,355)) were included in our meta-analysis (Figure 1).
Fig. 1.
Flow diagram of the study selection procedure.
Characteristics of included studies
The included studies were published from 1996 to 2019. Eight of the studies were RCTs,30-36,49 seven were retrospective case-control studies,37,39-43,47 and five were prospective control studies.38,44-48,48 In each of the included studies, the baseline difference between the intervention group and the control group revealed no statistical significance. Cell therapy and bone grafting were the most common interventions in the combined treatment group. The characteristics of the included trials are summarized in Table I.
Table I.
General characteristics of the included studies.
| Study | Author, yr | Country | Study design | Groups compared | Number of hips | Mean age, yrs (SD) | Stage of ONFH | Mean follow-up | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (drill diameter) |
Intervention | Control | Intervention | Total | Control | Intervention | ||||||
| 1 | Hu 201830 | China | RCT | CD (2.5 mm) | CD + NVFG | 65 | 65 | 130 | 40.38 (6.63) | 40.83 (6.73) | N/A | 48 mths |
| 2 | Hauzeur 201831 | Belgium | RCT | CD (4 mm) | CD + BMAC | 23 | 23 | 46 | 49.7 (3.2) | 48.0 (2.8) | ARCO IIIA, IIIB | 24 mths |
| 3 | Cao 201732 | China | RCT | CD (2.5 mm) | CD + FVFG | 21 | 21 | 42 | 31 (6) | 31 (6) | ARCO I to IIIB | 36 mths |
| 4 | Pepke 201633 | Germany | RCT | CD (5 mm) | CD + BMAC | 14 | 11 | 25 | 44.5 (3.3) | 44.3 (3.4) | ARCO II | 24 mths |
| 5 | Tabatabaee 201534 | USA | RCT | CD (2.7 mm) | CD + BMMNCs | 14 | 14 | 28 | 26.8 (5.8) | 31 (11.4) | ARCO I to III | 24 mths |
| 6 | Miao 201549 | China | RCT | CD (3.2 mm) | CD + PTR | 34 | 36 | 70 | 35.2 (5.8) | 32.6 (6.3) | Steinberg I to II | 12 to 28 mths |
| 7 | Zhao 201235 | China | RCT | CD | CD + BMMSCs | 44 | 53 | 97 | 33.8 (7.70) | 32.7 (10.5) | ARCO IC to IIC | 60 mths |
| 8 | Sen 201236 | India | RCT | CD (4 mm) | CD + BMMNCs | 25 | 26 | 51 | 65.72 (15.241) | 66.19 (13.042) | ARCO I, II | 24 mths |
| 9 | Ou 201937 | China | CCT | CD | CD + NVFG | 60 | 62 | 122 | 55.1 (5.8) | 55.0 (6.5) | ARCO II | 41.5 mths (SD 8.6; 22 to 63) |
| 10 | Hernigou 201838 | France | CCT | CD (4 mm) | CD + BMMSCs | 125 | 125 | 250 | 36 (18 to 51) | 36 (18 to 51) | Steinberg I, II | 25 yrs (20 to 30) |
| 11 | Kang 201839 | South Korea | CCT | CD | CD + BMMSCs | 53 | 53 | 106 | 47.3 (9.7) | 46.0 (9.3) | ARCO I to IV | 4.28 yrs (3 to 10) |
| 12 | Sallam 201740 | Egypt | CCT | CD (8 mm) | CD + IFHG | 38 | 33 | 71 | 33.21 (8.79) | 32.67 (8.16) | Ficat I to III | 7.86 yrs (3 to 14) |
| 13 | Cruz-Pardos 201641 | Spain | CCT | CD (4 mm) | CD + BMC | 19 | 41 | 60 | 36.74 | 42.56 | Ficat I, II | 45 mths (24 to 171) |
| 14 | Mohanty 201742 | India | CCT | CD (3.5 to 4.5 mm) | CD + FSG | 33 | 35 | 68 | 36.67 (7.8) | 34.1 (7.3) | Ficat I to III | N/A |
| 15 | Yan 201543 | China | CCT | CD (4.5 mm) | CD + BMMSCs | 42 | 44 | 86 | 37.24 (10.54) | 39.62 (11.83) | ARCO I to II | 26 mths (24 to 43) |
| 16 | Gangji 201144 | Belgium | CCT | CD | CD + ABMCs | 11 | 13 | 24 | 45.7 (2.8) | 42.2 (2.6) | ARCO I, II | 60 mths |
| 17 | Yang 201045 | China | CCT | CD (3 mm) | CD + BL-ATC | 22 | 56 | 78 | 36.5 | 38.6 | Steinberg I to IIIA | 36 to 78 mths |
| 18 | Gangji 200546 | Belgium | CCT | CD (3 mm) | CD + BMMNCs | 8 | 10 | 18 | 48.8 (11.2) | 40.9 (9.8) | ARCO I, II | 24 mths |
| 19 | Scully 199847 | USA | CCT | CD | CD + FVFG | 98 | 614 | 712 | 41 | 35 | Ficat I to III | 50 mths |
| 20 | Kane 199648 | USA | CCT | CD | CD + FVFG | 19 | 20 | 39 | 42 | 42 | Ficat II to III | 24 mths |
ABMC, autologous bone marrow cell; ARCO, Association Research Circulation Osseous; BL-ATC, biomaterial-loaded allograft threaded cage; BMAC, bone marrow aspirate concentrate; BMC, bone marrow concentration; BMMNC, bone marrow mononuclear cell; BMMSC, bone marrow mesenchymal stem cell; CCT, clinical controlled trial; CD, core decompression; FSG, fibular strut grafting; FVFG, free vascularized fibular grafting; IFHG, inverted femoral head grafting; N/A, not available; NVFG, non-vascularized fibular graft; ONFH, osteonecrosis of the femoral head; PTR, porous tantalum rod; RCT, randomized controlled trial; SD, standard deviation.
Risk of bias assessment
Of the 20 articles included in the meta-analysis, eight were RCTs and the remaining 12 were CCTs. Due to a lack of random generation and concealment of the allocation sequence, selective bias may be present in most trials. Although all RCTs reported randomization, only five adequately described the randomization method (randomization list generated by using random permuted blocks of two letters,31 randomization sequence created by a third party,32,35 randomization method based upon sequential patient allocation,33 and envelope technique34), whereas three RCTs exhibited adequate allocation concealment method (sealed envelope,31,34 randomization sequence created by a third party).32 Due to poor blinding of participants, personnel, or outcome assessors, performance bias and detection bias may be present in most trials. Five trials31,34,44-46 described double blinding of subjects and participants, and six trials31,34,42,44,46,48 mentioned that they were blinded to outcome assessment. Finally, most of the studies reported patient follow-up or drop-out,31,32,35,36,38,40-46,49 and no other biases were found in these trials (data not shown).
Primary outcome measures
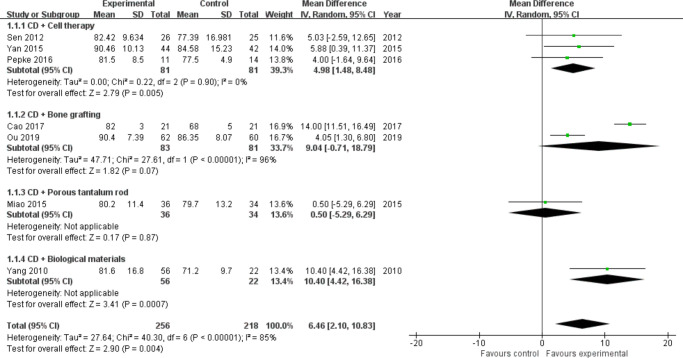
Harris Hip Score: A total of 11 trials29,31,32,35-39,41,42,44,48,48 reported HHS postoperatively during follow-up. Due to the lack of standard deviation (SD) data for four studies,30,38,40,42 we only analyzed seven studies32,33,36,37,43,45,49 involving 474 hips (Intervention = 256, Control = 218). According to the different intervention measures, we divided the studies into four subgroups (cell therapy = 3,33,36,43 bone grafting = 2,32,37 porous tantalum rod = 1,49 and biological materials = 145). Heterogeneity existed between studies of bone grafting (I2 = 96%, p < 0.001, chi-squared test) (Figure 2), and a random effects model was used. The results showed that the addition of stem cells (MD = 4.98, 95% CI = 1.48 to 8.48, Z = 2.79, p = 0.005, chi-squared test) or biomaterials (Z = 0.17, p < 0.001, chi-squared test) markedly improved functional scores in patients with ONFH treated with CD, and no significant differences were seen in the bone grafting (MD = 9.04, 95% CI = -0.71 to 18.79, Z = 1.82, p = 0.070) and porous tantalum rod groups (Z = 0.17, p = 0.870, chi-squared test) (Figure 2). Of the four studies excluded (Figure 2) due to missing SDs, three involved bone grafting30,40,42 and the remaining one was cell therapy.38 All reported that the HHS was better in the intervention group than in the control group.
Fig. 2.
Forest plot of Harris Hip Score. CD, core decompression; CI, confidence interval; IV, instrumental variable. Statistical analysis, chi-squared test.
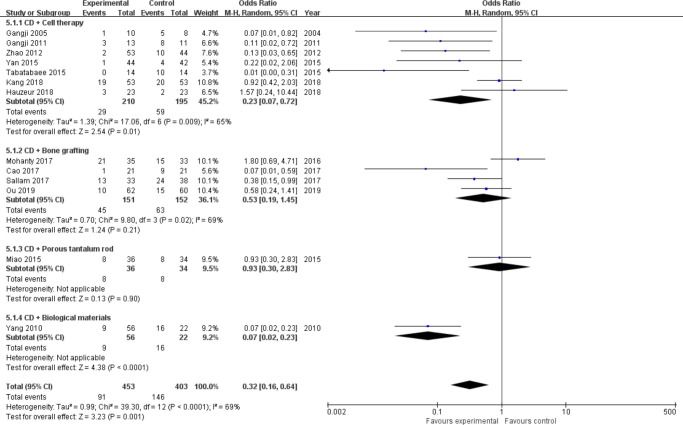
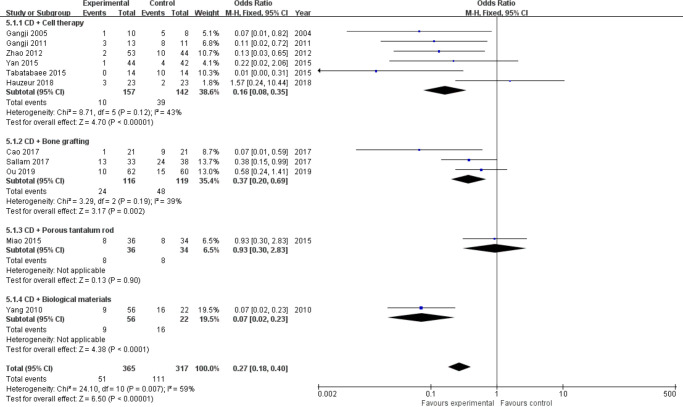
Progression of ONFH stage: A total of 13 trials31,32,34,35,37,39,40,42-46,49 assessed radiological progression, including 856 hips (Intervention = 453, Control = 403). According to the different intervention measures, we divided the studies into four subgroups (cell therapy = 7,31,34,35,39,43,44,46 bone grafting = 4,32,37,40,42 porous tantalum rod = 1,49 and biological materials = 145). Heterogeneity existed between studies of cell therapy (I2 = 65%, p = 0.009, chi-squared test) and bone grafting (I2 = 69%, p = 0.020, chi-squared test) (Figure 3 and Figure 4); therefore, we performed sensitivity analysis by omitting each study to explore the source of heterogeneity. Finally, we removed the study from Kang et al39 in the cell therapy group and Mohanty et al42 in the bone grafting group, and heterogeneity was not observed (I2 = 43%, p = 0.120; and I2 = 39%, p = 0.190, chi-squared test) (Figure 4). A fixed-effects model was used. Meta-analysis results showed that CD combined with cell therapy (OR = 0.16, 95% CI = 0.08 to 0.35, Z = 4.70, p < 0.001, chi-squared test), bone grafting (OR = 0.37, 95% CI = 0.20 to 0.69, Z = 3.17, p = 0.002, chi-squared test), or biomaterials (p < 0.001, chi-squared test) can significantly delay the progression of disease in patients with ONFH compared with CD alone. In particular, the odds of ONFH stage progression in the cell therapy group decreased by more than six-fold (OR = 0.16) (Figure 4).
Fig. 3.
Forest plot of progression of osteonecrosis of the femoral head (ONFH) stage (heterogeneity existed). CD, core decompression; CI, confidence interval; M-H, Mantel-Haenszel. Statistical analysis, chi-squared test.
Fig. 4.
Forest plot of progression of osteonecrosis of the femoral head (ONFH) stage (sensitivity analysis). CD, core decompression; CI, confidence interval; M-H, Mantel-Haenszel. Statistical analysis, chi-squared test.
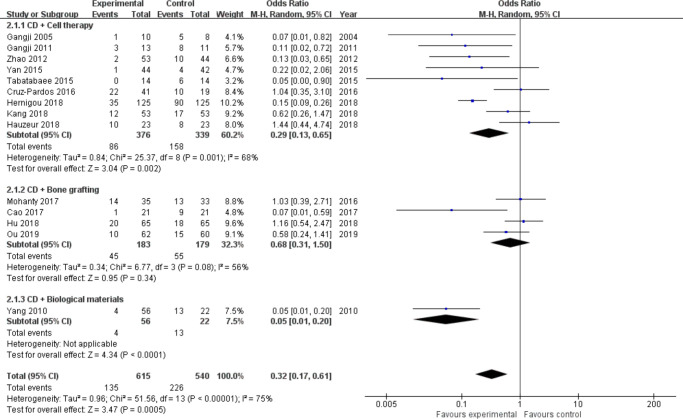
Collapse of the femoral head: A total of 14 trials30-34,35,37–39-46,41-46 enrolling a total of 1,155 hips (Intervention = 615, Control = 540) mentioned the number of collapse cases of the femoral head. According to the different intervention measures, we divided the studies into three subgroups (cell therapy = 9,31,34,35,38,39,41,43,44,46 bone grafting = 4,30,32,37,42 and biological materials = 145). Heterogeneity existed between studies of cell therapy (I2 = 68%, p = 0.001, chi-squared test) and the bone grafting group (I2 = 56%, p = 0.080, chi-squared test) (Figure 5); therefore, we performed sensitivity analysis by omitting each study but did not identify the source of heterogeneity. We then used a random effects model. The results showed that the addition of stem cells (OR = 0.29, 95% CI 0.13 to 0.65, Z = 3.04, p = 0.002, chi-squared test) or biomaterial therapy (Z = 4.34, p < 0.001, chi-squared test) reduced the risk of femoral head collapse in patients with ONFH treated with CD (Figure 5). However, CD combined with bone grafting did not reduce the risk of femoral head collapse compared to CD alone (OR = 0.68, 95% CI 0.31 to 1.50, Z = 0.95, p = 0.340, chi-squared test) (Figure 5).
Fig. 5.
Forest plot of collapse of femoral head. CD, core decompression; CI, confidence interval. Statistical analysis, chi-squared test.
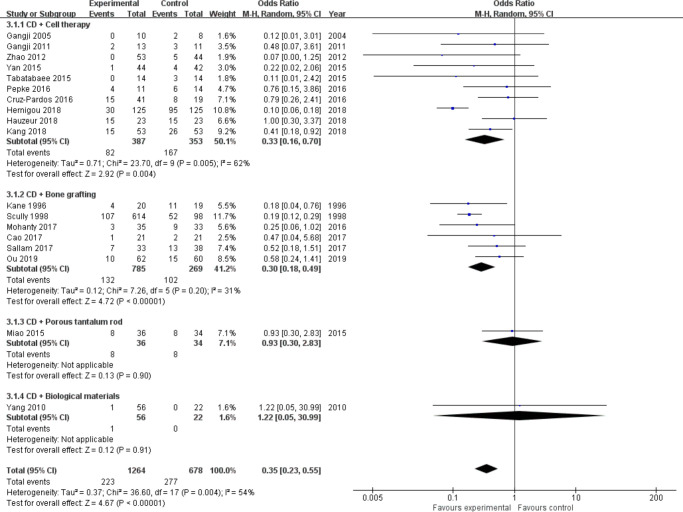
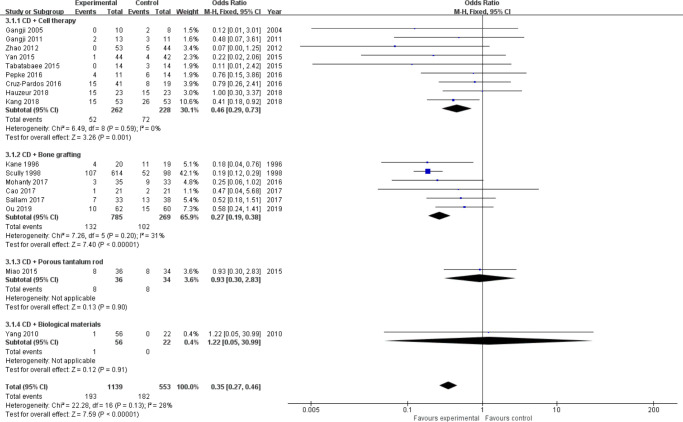
Conversion to THA: Almost all studies31-35,37-49 have reported this outcome of interest, including a total of 1,942 hips (Intervention = 1,264, Control = 678). We divided them into four subgroups (cell therapy = 10,30,32-37,38,40,42,43,45,45 bone grafting = 6,32,37,40,42,47,48 porous tantalum rod = 1,49 biological materials = 145) according to the different intervention measures. There was mild heterogeneity between studies in the cell therapy group (I2 = 62%, p = 0.005, chi-squared test; Figure 6 and Figure 7); therefore, we performed sensitivity analysis by omitting each study to explore the source of heterogeneity. Finally, we removed the study from Hernigou et al,38 and heterogeneity was not observed (I2 = 0, p = 0.590, chi-squared test) (Figure 7). A fixed-effects model was used. Results showed that the odds for conversion to THA in the cell therapy group (OR = 0.46, 95% CI 0.29 to 0.73, Z = 3.26, p = 0.001, chi-squared test) and bone grafting group (OR = 0.27, 95% CI 0.19 to 0.38, Z = 7.40, p < 0.001, chi-squared test) were two and four times lower than in the control group (Figure 7). However, porous tantalum rods (Z = 0.13, p = 0.900, chi-squared test) and biomaterials (Z = 0.12, p = 0.910, chi-squared test) did not reduce the number of patients who subsequently required THA surgery compared to CD alone (Figure 6b).
Fig. 6.
Forest plot of conversion to total hip arthroplasty (THA) (heterogeneity existed). b) Forest plot of conversion to THA (sensitivity analysis). CD, core decompression; CI, confidence interval; M-H, Mantel-Haenszel. Statistical analysis, chi-squared test.
Fig. 7.
Forest plot of conversion to total hip arthroplasty (THA) (sensitivity analysis). CD, core decompression; CI, confidence interval; M-H, Mantel-Haenszel. Statistical analysis, chi-squared test.
Secondary outcome measures
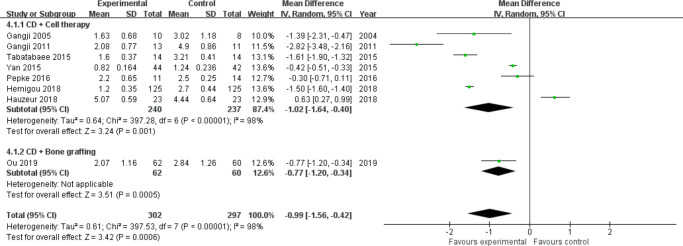
Visual analogue scale score: Seven studies involving cell therapy31,33,34,38,43,44,46 and one study involving bone grafting37 assessed visual analogue scale (VAS) scores postoperatively during follow-up (excluding one study39 due to the lack of SDs). Significant statistical heterogeneity was observed between studies of cell therapy (I2 = 98%, p < 0.001, chi-squared test) (Figure 8), so we performed sensitivity analysis by omitting each study but did not identify the source of heterogeneity. Then, a random effects model was used. Results showed that CD combined with cell therapy (MD = −1.02, 95% CI = -1.64 to -0.40, Z = 3.24, p = 0.001) or bone grafting (Z = 3.51, p < 0.001, chi-squared test) markedly reduced pain in ONFH patients compared with CD alone (Figure 8). The study by Kang et al39 was excluded due to missing SDs, but their results showed that CD + bone marrow mesenchymal stem cells (BMMSCs) did not reduce VAS scores in patients with ONFH compared to CD alone (p > 0.05).
Fig. 8.
Forest plot of visual analogue scale (VAS) score. CD, core decompression; CI, confidence interval; SD, standard deviation. Statistical analysis, chi-squared test.
Western Ontario and McMaster Universities Osteoarthritis Index score: Only three studies34,38,46 including 296 hips (Intervention = 149, Control = 147) reported the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score postoperatively. The intervention groups in all three studies were treated with CD + cell therapy. Large heterogeneity was observed (I2 = 99%, p < 0.001, chi-squared test; Figure 9); therefore, a random effects model was used. The results showed that CD combined with cell therapy was more effective than CD alone in decreasing the WOMAC score (MD = −10.92, 95% CI = -21.41 to -4.03, Z = 2.04, p = 0.040, chi-squared test) (Figure 9).
Fig. 9.
Forest plot of Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score. CD, core decompression; CI, confidence interval; SD, standard deviation. Statistical analysis, chi-squared test.
The volume of femoral head necrosis: Seven studies31,33,35,36,38,44,46 provided relevant data but not in a consistent way to allow us to obtain a summarized estimate of the effect size of any functional outcome. Most studies35,36,38,44,46 have shown these data to be more favourable in the intervention group by MRI during follow-up (p < 0.05, chi-squared test), but the study from Hauzeur et al31 and Pepke et al33 showed that there was no statistically significant difference (p > 0.05, chi-squared test) between the mean volumes of osteonecrosis in either group.
Adverse events: Six31,40,42,44,46,48 of 20 studies described adverse events or perioperative complications (Table II), and seven35,36,39,41,43,45,49 studies reported no adverse effects in either group. The remaining seven30,32-37,38,47,47 studies did not mention whether or not there were adverse events. Overall, most of the included studies indicated that the survey of potential side effects did not reveal any serious adverse events in either group, and the safety of CD combined with other treatment measures is acceptable for ONFH.
Table II.
Adverse events.
| Adverse events or complications | Control | Intervention | Reported study |
|---|---|---|---|
| Surgical site-related pain | 3 | 9 | Hauzeur31 , Gangji44,46 |
| Surgical site haematoma | 0 | 2 | Gangji44,46 |
| Nausea and vomiting | 1 | 1 | Hauzeur31 |
| Deep vein thrombosis | 1 | 1 | Sallam40 |
| Painless limp | 1 | 3 | Sallam40 |
| Transient lateral popliteal nerve paralysis | 0 | 1 | Mohanty42 |
| Fractures of the proximal femur | 2 | 2 | Kane48 |
| Discomfort at the ankle | 0 | 6 | Kane48 |
| Superficial wound infection without debridement | 1 | 2 | Sallam40 |
| Fever with negative bacteriological investigations | 0 | 2 | Hauzeur31 |
| Positive bone marrow bacteriology culture without clinical symptoms of sepsis | 0 | 2 | Gangji44,46 |
Subgroup analysis
Studies have shown that once a patient has any collapse or a crescent sign, CD is not effective. Therefore, we reanalyzed the outcome of interest, including only studies reporting on precollapse (stage I to II) ONFH patients. Ten studies30,33,35-43,44,46,44,46 met this criterion, eight were CD + cell therapy33,35,36,38,41,43,44,46 in the intervention group, and the other two were CD + non-vascularized fibular graft (NVFG).30,37
The clinical outcomes are listed below:
HHS: HHS was reported in three studies33,36,43 in which the intervention group was CD + cell therapy and one study37 in which the intervention group was CD + NVFG, and the results showed that both groups improved HHS in ONFH patients compared to CD alone (cell therapy: MD = 4.93, 95% CI = 1.52 to 8.35, Z = 2.83, p = 0.005, chi-squared test) (NVFG: Z = 2.89, p = 0.004, chi-squared test) (Supplementary Figure a).
VAS scores: VAS scores were reported in five studies33,38,43,44,46 with an intervention group of CD + cell therapy and one study37 with an intervention group of CD + NVFG. The results showed that both groups were able to reduce VAS scores in ONFH patients compared to CD alone (cell therapy: MD = −1.22, 95% CI = -2.00 to -0.45, Z = 3.09, p = 0.002, chi-squared test) (NVFG: Z = 3.51, p < 0.001, chi-squared test). However, greater heterogeneity existed between studies of cell therapy (I2 = 99%, p < 0.001, chi-squared test); therefore, we performed sensitivity analysis by omitting each study, but did not identify the source of heterogeneity (Supplementary Figure b).
WOMAC score: Two studies38,46 reported WOMAC scores, and their intervention group was CD + cell therapy. The overall estimate of effect size for WOMAC favoured the cell therapy group, although it reached only borderline significance levels in the presence of a huge degree of statistical heterogeneity (I2 = 85%, p = 0.009, chi-squared test) (MD = −7.15, 95% CI = -14.52 to 0.02, Z = 1.90, p = 0.06, chi-squared test) (Supplementary Figure c).
The radiological outcomes are listed below:
Progression of ONFH stage: Four studies35,43,44,46 with an intervention group of CD + cell therapy and one study37 with an intervention group of CD + NVFG reported the progression of ONFH stage. The results showed that CD + cell therapy significantly delayed the progression of ONFH stage compared to CD alone (OR = 0.13, 95% CI = 0.05 to 0.34, Z = 4.09, p < 0.001, chi-squared test). There was no significant difference between the CD + NVFG group and the control group (Z = 1.21, p = 0.230, chi-squared test) (Supplementary Figure d).
Collapse of the femoral head: Six studies35,38,41,43,44,46 in which the intervention group was CD + cell therapy and two studies30,37 in which the intervention group was CD + NVFG reported collapse of the femoral head. Due to the presence of slight heterogeneity between studies in the cell therapy group (I2 = 55%, p = 0.050, chi-squared test) (Supplementary Figure ea), we performed sensitivity analysis by omitting each study to explore the source of heterogeneity and ultimately excluded the study by Cruz-Pardos et al.41 Then, a fixed-effects model was used. Results showed that CD + cell therapy could significantly reduce the risk of femoral head collapse compared with CD alone (OR = 0.14, 95% CI = 0.09 to 0.23, Z = 7.87, p < 0.001, chi-squared test), while there was no significant difference between the CD + NVFG group and the control group (Z = 0.50, p = 0.620, chi-squared test) (Supplementary Figure eb).
Conversion to THA: Seven studies33,35,38,41,43,44,46 with an intervention group of CD + cell therapy and one study37 with an intervention group of CD + NVFG reported the number of hips converted to THA. Results showed that CD + cell therapy reduced the odds of conversion to THA by more than two-fold compared to CD alone (OR = 0.43, 95% CI = 0.22 to 0.85, Z = 2.41, p = 0.020, chi-squared test), while there was no significant difference between the CD + NVFG group and the control group (Z = 1.21, p = 0.230, chi-squared test) (Supplementary Figure f).
Sensitivity analysis
Sensitivity analysis was performed by omitting each study to explore the source of heterogeneity. The results of the meta-analysis did not change, indicating that the results were reliable. Unfortunately, however, for most of the outcome indicators, we did not explore the sources of statistical heterogeneity.
Publication bias
Publication bias was assessed by generating funnel plots for the primary outcomes of interest (HHS, progression of ONFH stage, collapse of femoral head, and conversion to THA). Symmetrical scatters were observed in the funnel plot, which show that the publication bias is low (Supplementary Figures ga to gd).
Discussion
Increasing intramedullary pressure is considered to be a major factor in the inadequate blood supply to the femoral head,50 making CD the most commonly used hip-preserving therapy for the treatment of ONFH.51 It was first described by Ficat52 and used as a method to obtain biopsy specimens to establish the diagnosis of osteonecrosis. CD is a simple procedure that effectively reduces the pressure in the medullary cavity while removing necrotic bone, which provides a new blood supply for the necrotic area. A systematic review of 42 studies (2,025 hips, CD = 1,206, conservative treatment = 819) showed that the excellent and good rate of the CD group was much better than that of the nonoperative treatment group (71.0% vs 34.5%).53
Nonetheless, there are still some studies suggesting that the efficacy of CD can be unreliable with a notable proportion of patients, even with early-stage disease requiring THA.7-9 First, the lack of effective mechanical support in the necrotic area after CD reduces the mechanical properties of the already weak subchondral bone, which may accelerate the collapse of the weight-bearing surface of the femoral head. Second, this method also does not address the issues of angiogenesis, bone reconstruction, and articular surface repair in the necrotic area of the femoral head.10,49 Therefore, most joint surgeons only use CD as the basic treatment, combining it with internal fixation support such as tantalum rods, non-vascularized or vascularized bone grafting, various artificial materials for tissue engineering, cytokines, and the application of stem cell therapy.19
Despite the heterogeneity of some outcome indicators and the low quality of some of the studies included in the meta-analysis, our results still suggest that the combination of other therapeutic measures in addition to CD appears to result in better clinical and radiological outcomes. In addition, there were no serious complications or adverse events in either group. In the subgroup analysis (Supplementary Figures a to f), stages I to II were compared, and we found that the addition of cell therapy to CD was more definitive than CD alone in the precollapse stage (I to II). However, due to the limited number of included studies, more studies are needed to prove whether other treatments are better than CD alone.
Many meta-analyses have been published on this topic,6,50,54-57 most of which explore whether the addition of cell therapy to CD can result in better clinical outcomes and lower rates of disease progression than core decompression alone. Compared with earlier studies, our research has the following advantages.
First, we conducted a comprehensive systematic search and included 20 controlled trials that met the inclusion criteria in a total of 1,379 records, involving a total of 2,123 hips.
Second, we conducted a rigorous screening. First, patients in the control group must only use core decompression without additional treatment. However, not all control groups in previously published meta-analyses similar to this study only used core decompression, which can introduce other confounding variables and bias. For example, the control group of the studies included in some meta-analyses also included CD + bone grafts,7,50,54,55 CD + biomaterials,7,54 CD + porous tantalum rod,54 and CD + unprocessed bone marrow injection.50,55 Second, we limited the language to English, thus excluding many low-quality studies. In addition, we also excluded some studies in which baseline characteristics of the two groups of patients were not consistent. For example, although the study from Lakshminarayana et al58 is a controlled trial, it uses CD for stage I and CD + bone grafting for stage II patients with ONFH.
Third, we conducted a subgroup analysis to fully compare whether four surgical methods (CD + cell therapy, CD + bone grafting, CD + porous tantalum rod, and CD + biological materials) can improve the outcome of ONFH patients when compared with core decompression alone, and to explore the impact of different ONFH stages on the results of the study.
Fourth, we also performed sensitivity analyses to further increase the robustness of our meta-analysis.
Our meta-analysis shows that the addition of cell therapy to CD markedly improved function scores (MD = 4.98, Z = 2.79, p = 0.005), reduced pain (MD = −1.02, Z = 3.24, p = 0.001), delayed the progression of ONFH (OR = 0.23, Z = 2.54, p = 0.010), decreased collapse of the femoral head (OR = 0.29, Z = 3.04, p = 0.002), and decreased conversion to THA (OR = 0.33, Z = 2.92, p = 0.004). This approach appears to be more effective than bone grafting, as the latter does not show significant differences from controls in the evaluation of many outcome indicators, especially in precollapse patients (progression of ONFH stage: p = 0.230; collapse: p = 0.620; THA: p = 0.230). However, this result should be interpreted with caution, as there are relatively few controlled trials involving bone grafting included in this meta-analysis.
The application of this method can be traced back nearly three decades. Many studies have shown that the number and quality of mesenchymal stem cells (MSCs) in the femoral head of patients with ONFH are defective, which leads to a lack of angiogenesis and bone remodelling after CD.59 In 1993, Hernigou and Beaujean18 first proposed injecting concentrated bone marrow aspirate containing autologous bone marrow mononuclear cells (BMMCs) through the CD channel to solve this problem. This method can theoretically increase the number of osteogenic active stem cells.60 The first mid-term results were reported by Hernigou and Beaujean17 in 2002; 116 patients (189 hips) were followed up for a mean of seven years, and the success rate of hip preservation for early ONFH was as high as 94%. In another long-term follow-up RCT by Hernigou et al,38 a total of 125 patients with bilateral ONFH were included (Steinberg I to II). After 25 years of follow-up, the collapse rate of the femoral head in the stem cell group was only 28%, which was far superior to CD alone (72%). MSCs are especially suitable for the treatment of ONFH because they exist in BMMCs and have strong self-proliferation and multidirectional differentiation abilities. As they provide the source of osteoblasts for the sites of interest, these cells can also participate in osteogenesis and repair of necrotic bone defects. In addition, secreted bone marrow MSCs, such as bone morphogenetic protein-2 and vascular endothelial growth factor, can also be used to stimulate the local repair process to prevent ONFH.16,61
However, there are also many questions that remain unanswered by stem cell therapy, such as whether the clinical therapeutic effects of MSCs from different sources (bone marrow, fat, and periosteum) are the same. How does the response to autologous stem cell transplantation differ in patients with ONFH of different aetiologies (steroid-induced, alcoholic and traumatic ONFH)? Will the function of stem cells decrease after repeated culture? In addition, the optimal concentration or number of transplanted stem cells and the risk of cancer formation at the implantation site should also be evaluated.16,44,61
In a recently published study, it was shown that between 2009 and 2015, more than 200,000 patients in the USA were diagnosed with ONFH, but only 6% of patients were treated with joint-preserving procedures.62 This is a strange phenomenon, as CD, bone grafting, and stem cell therapy have all been shown to be reliable options for patients with early-stage femoral head necrosis. Although this meta-analysis suggests that CD combined with cell therapy may be the most promising treatment in the precollapse stage of ONFH, well-designed randomized controlled trials with long-term follow-up are needed to confirm the efficacy of various surgical procedures for patients at different stages of the disease, in order to maximize efforts to save the hip joint.
This meta-analysis has the following limitations. First, the overall quality of the evidence was heterogeneous and poor, and included trials that failed to detail information about randomization, allocation concealment, and blinding. These omissions contributed to bias. Second, although we conducted subgroup analyses of different methods and different stages of ONFH, different aetiologies of ONFH may also pose risks for bias. Third, although we conducted a subgroup analysis to explore whether the addition of cell therapy achieves better clinical and radiological outcomes than CD alone, the processing, quality, and number of stem cells harvested for implantation were not standardized, thus adding to the heterogeneity of the data. Fourth, different classification systems for ONFH were used in studies (Ficat and Arlet; Association Research Circulation Osseous (ARCO); Steinberg), which may affect the final meta-analysis results to some extent. In addition, several recent studies have reported that Japanese Investigation Committee (JIC) classification based on the size and location of ONFH lesions involving the acetabular head may provide better assistance in the selection of treatment for femoral head necrosis.63,64 This of course requires further research. Fifth, although we conducted the subgroup analysis and sensitivity analysis, there is still heterogeneity in some outcome indicators. This may affect the final decision of orthopaedic surgeons, although the results of a statistical test did not indicate otherwise. Finally, the sample size was small in some of the trials, which weakened validity of the statistical analysis and may overestimate the therapeutic effects of certain methods. Therefore, we should be cautious about the results of the meta-analysis.
In summary, there is marked heterogeneity in the studies. There is a trend towards improved clinical outcomes with the addition of other therapies to CD, and this improvement seems to be pronounced for stem cell therapy.
However, more rigorously designed and higher-quality prospective and randomized trials with adequate sample sizes are required to confirm the true efficacy of cell therapy and other treatment measures in the management of ONFH.
Author contributions
S. Zhu: Writing - original draft.
X. Zhang: Writing - review & editing, Investigation.
X. Chen: Data curation.
Y. Wang: Investigation.
S. Li: Investigation.
W. Qian: Conceptualization, Writing - review & editing.
Funding statement
The open access funding was sourced from the National Natural Science Foundation of China and Natural Science Foundation of Beijing Municipality. The funding number is the grant number: 81972046 and 7192173. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
The authors declare that they have no conflict of interest.
Acknowledgements
We thank He Hongmei of Henan University School of Medicine for providing guidance on design and statistical analysis.
Supplementary material
Forest and funnel plots of various outcomes.
References
- 1. Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM, Joaquin MA. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015;6(8):590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsu H, Nallamothu S. Hip Osteonecrosis. Treasure Island:StatPearls. 2020. [Google Scholar]
- 3. Cui L, Zhuang Q, Lin J, et al. . Multicentric epidemiologic study on six thousand three hundred and ninety five cases of femoral head osteonecrosis in China. Int Orthop. 2016;40(2):267–276. [DOI] [PubMed] [Google Scholar]
- 4. Zhao D-W, Yu M, Hu K, et al. . Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: results from a nationally representative survey. Chin Med J. 2015;128(21):2843–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petek D, Hannouche D, Suva D. Osteonecrosis of the femoral head: pathophysiology and current concepts of treatment. EFORT Open Rev. 2019;4(3):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Papakostidis C, Tosounidis TH, Jones E, Giannoudis PV. The role of "cell therapy" in osteonecrosis of the femoral head. A systematic review of the literature and meta-analysis of 7 studies. Acta Orthop. 2016;87(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lieberman JR. Core decompression for osteonecrosis of the hip. Clin Orthop Relat Res. 2004;418:29–33. [DOI] [PubMed] [Google Scholar]
- 8. Yoon TR, Song EK, Rowe SM, Park CH. Failure after core decompression in osteonecrosis of the femoral head. Int Orthop. 2001;24(6):316–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steinberg ME, Larcom PG, Strafford B, et al. . Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;386:71–78. [DOI] [PubMed] [Google Scholar]
- 10. Aurégan J-C, Villain B, Bégué T. What is the rate of patients undergoing a total hip arthroplasty after core decompression and insertion of a tantalum rod in osteonecrosis of the femoral head: a systematic review. Int Orthop. 2018;42(7):1631–1638. [DOI] [PubMed] [Google Scholar]
- 11. Pedersen DR, Brown TD, Poggie RA. Finite element characterization of a porous tantalum material for treatment of avascular necrosis. Trans Orthop Res Soc. 1997;22:598. [Google Scholar]
- 12. Feng B, Qian W, Weng X, Wang W, Zhao L, Jiang C. Outcome of the treatment of osteonecrosis of femoral head using the core decompression with bone impaction grafting. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37(2):133–139. [DOI] [PubMed] [Google Scholar]
- 13. Korompilias AV, Beris AE, Lykissas MG, Kostas-Agnantis IP, Soucacos PN. Femoral head osteonecrosis: why choose free vascularized fibula grafting. Microsurgery. 2011;31(3):223–228. [DOI] [PubMed] [Google Scholar]
- 14. Sun W, Li Z, Gao F, Shi Z, Zhang Q, Guo W. Recombinant human bone morphogenetic protein-2 in debridement and impacted bone graft for the treatment of femoral head osteonecrosis. PLoS One. 2014;9(6):e100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang B-L, Sun W, Shi Z-C, et al. . Treatment of nontraumatic osteonecrosis of the femoral head using bone impaction grafting through a femoral neck window. Int Orthop. 2010;34(5):635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernigou P, Trousselier M, Roubineau F, et al. . Stem cell therapy for the treatment of hip osteonecrosis: a 30-year review of progress. Clin Orthop Surg. 2016;8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. [DOI] [PubMed] [Google Scholar]
- 18. Hernigou P, Beaujean F. Bone marrow activity in the upper femoral extremity in avascular osteonecrosis. Rev Rhum Engl. 1993;60(1):610. [Google Scholar]
- 19. Ye YH, Chen K, Jin KK, Zhang YF, Chen L. Progress on surgical treatment for femoral head-preservering in the precollapse stage of femoral head necrosis. Zhongguo Gu Shang. 2017;30(3):287–292. (Article in Chinese) [DOI] [PubMed] [Google Scholar]
- 20. Yao H, Hu W, Li H, et al. . Allogeneic fibular implantation for the treatment of femoral head necrosis: Clinical observation of 132 hips during 2.5 years follow-up. Zhongguo Zu Zhi Gong Cheng Yan Jiu. 2013;17(18):3311–3317. http://en.cnki.com.cn/Article_en/CJFDTOTAL-XDKF201318020.htm [Google Scholar]
- 21. Korompilias AV, Lykissas MG, Beris AE, Urbaniak JR, Soucacos PN. Vascularised fibular graft in the management of femoral head osteonecrosis: twenty years later. J Bone Joint Surg Br. 2009;91-B(3):287–293. [DOI] [PubMed] [Google Scholar]
- 22. Ma J, Sun W, Guo W, et al. . Retrospective analysis of clinically failed implants following porous tantalum implantation for femoral head osteonecrosis[J]. Chinese Journal of Joint Surgery. 2017;11(04):331–337. [Google Scholar]
- 23. Floerkemeier T, Lutz A, Nackenhorst U, et al. . Core decompression and osteonecrosis intervention rod in osteonecrosis of the femoral head: clinical outcome and finite element analysis. Int Orthop. 2011;35(10):1461–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oh K-J, Pandher DS. A new mode of clinical failure of porous tantalum rod. Indian J Orthop. 2010;44(4):464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanzer M, Bobyn JD, Krygier JJ, Karabasz D. Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants. J Bone Joint Surg Am. 2008;90-A(6):1282–1289. [DOI] [PubMed] [Google Scholar]
- 26. Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: Treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51-A(4):737–755. [PubMed] [Google Scholar]
- 29. Higgins JPT, Altman DG, Gotzsche PC, et al. . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu B, Gao D, He Y. Efficacy of fibula fixation in the early treatment of osteonecrosis of the femoral head and its effects on local microcirculation, articular surface collapse, joint pain and function. J Musculoskelet Neuronal Interact. 2018;18(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- 31. Hauzeur J-P, De Maertelaer V, Baudoux E, Malaise M, Beguin Y, Gangji V. Inefficacy of autologous bone marrow concentrate in stage three osteonecrosis: a randomized controlled double-blind trial. Int Orthop. 2018;42(7):1429–1435. [DOI] [PubMed] [Google Scholar]
- 32. Cao L, Guo C, Chen J, Chen Z, Yan Z. Free vascularized fibular grafting improves vascularity compared with core decompression in femoral head osteonecrosis: a randomized clinical trial. Clin Orthop Relat Res. 2017;475(9):2230–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pepke W, Kasten P, Beckmann N, Janicki P, Egermann M. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev. 2016;8(1):6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tabatabaee RM, Saberi S, Parvizi J, Mortazavi SMJ, Farzan M. Combining concentrated autologous bone marrow stem cells injection with core decompression improves outcome for patients with early-stage osteonecrosis of the femoral head: a comparative study. J Arthroplasty. 2015;30(9 Suppl):11–15. [DOI] [PubMed] [Google Scholar]
- 35. Zhao D, Cui D, Wang B, et al. . Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–330. [DOI] [PubMed] [Google Scholar]
- 36. Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplasty. 2012;27(5):679–686. [DOI] [PubMed] [Google Scholar]
- 37. Ou Z, Zeng P, Zhou Y, et al. . Clinical efficacy of core decompression combined with free fibular graft in the treatment of femoral head necrosis. Int J Clin Exp Med. 2019;12(12):13823–13830. [Google Scholar]
- 38. Hernigou P, Dubory A, Homma Y, et al. . Cell therapy versus simultaneous contralateral decompression in symptomatic corticosteroid osteonecrosis: a thirty year follow-up prospective randomized study of one hundred and twenty five adult patients. Int Orthop. 2018;42(7):1639–1649. [DOI] [PubMed] [Google Scholar]
- 39. Kang JS, Suh YJ, Moon KH, et al. . Clinical efficiency of bone marrow mesenchymal stem cell implantation for osteonecrosis of the femoral head: a matched pair control study with simple core decompression. Stem Cell Res Ther. 2018;9(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sallam AA, Imam MA, Salama KS, Mohamed OA. Inverted femoral head graft versus standard core decompression in nontraumatic hip osteonecrosis at minimum 3 years follow-up. Hip Int. 2017;27(1):74–81. [DOI] [PubMed] [Google Scholar]
- 41. Cruz-Pardos A, Garcia-Rey E, Ortega-Chamarro JA, Duran-Manrique D, Gomez-Barrena E. Mid-term comparative outcomes of autologous bone-marrow concentration to treat osteonecrosis of the femoral head in standard practice. Hip Int. 2016;26(5):432–437. [DOI] [PubMed] [Google Scholar]
- 42. Mohanty SP, Singh KA, Kundangar R, Shankar V. Management of non-traumatic avascular necrosis of the femoral head-a comparative analysis of the outcome of multiple small diameter drilling and core decompression with fibular grafting. Musculoskelet Surg. 2017;101(1):59–66. [DOI] [PubMed] [Google Scholar]
- 43. Yan D, Chen L, Li Z, Guo W, Sun W, et al. . Autologous mesenchymal stem cell implantation in the management of osteonecrosis of the femoral head. Curr Orthop Pract. 2015;26(3):265–268. [Google Scholar]
- 44. Gangji V, De Maertelaer V, Hauzeur J-P. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49(5):1005–1009. [DOI] [PubMed] [Google Scholar]
- 45. Yang S, Wu X, Xu W, Ye S, Liu X, Liu X. Structural augmentation with biomaterial-loaded allograft threaded cage for the treatment of femoral head osteonecrosis. J Arthroplasty. 2010;25(8):1223–1230. [DOI] [PubMed] [Google Scholar]
- 46. Gangji V, Hauzeur J-P. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. surgical technique. J Bone Joint Surg Am. 2005;87 Suppl 1(Pt 1):106–112. [DOI] [PubMed] [Google Scholar]
- 47. Scully SP, Aaron RK, Urbaniak JR. Survival analysis of hips treated with core decompression or vascularized fibular grafting because of avascular necrosis. J Bone Joint Surg Am. 1998;80-A(9):1270–1275. [DOI] [PubMed] [Google Scholar]
- 48. Kane SM, Ward WA, Jordan LC, Guilford WB, Hanley EN. Vascularized fibular grafting compared with core decompression in the treatment of femoral head osteonecrosis. Orthopedics. 1996;19(10):869–872. [DOI] [PubMed] [Google Scholar]
- 49. Miao H, Ye D, Liang W, Yao Y. Effect of Osteonecrosis Intervention Rod Versus Core Decompression Using Multiple Small Drill Holes on Early Stages of Necrosis of the Femoral Head: A Prospective Study on a Series of 60 Patients with a Minimum 1-Year-Follow-Up. Open Orthop J. 2015;9:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu S, Zhang L, Jin H, et al. . Autologous stem cells combined core decompression for treatment of avascular necrosis of the femoral head: a systematic meta-analysis. Biomed Res Int. 2017;2017(418):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lau RL, Perruccio AV, Evans HMK, Mahomed SR, Mahomed NN, Gandhi R. Stem cell therapy for the treatment of early stage avascular necrosis of the femoral head: a systematic review. BMC Musculoskelet Disord. 2014;15(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ficat RP. Idiopathic bone necrosis of the femoral head. early diagnosis and treatment. J Bone Joint Surg Br. 1985;67-B(1):3–9. [DOI] [PubMed] [Google Scholar]
- 53. Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res. 1996;324:169–178. [DOI] [PubMed] [Google Scholar]
- 54. Zhang C, Fang X, Huang Z, Li W, Zhang W, Lee GC. Addition of Bone Marrow Stem Cells Therapy Achieves Better Clinical Outcomes and Lower Rates of Disease Progression Compared With Core Decompression Alone for Early Stage Osteonecrosis of the Femoral Head: A Systematic Review and Meta-Analysis. J Am Acad Orthop Surg. 2020;28(23):973–979. [DOI] [PubMed] [Google Scholar]
- 55. Wang Z, Sun Q-M, Zhang F-Q, Zhang Q-L, Wang L-G, Wang W-J. Core decompression combined with autologous bone marrow stem cells versus core decompression alone for patients with osteonecrosis of the femoral head: a meta-analysis. Int J Surg. 2019;69:23–31. [DOI] [PubMed] [Google Scholar]
- 56. Sadile F, Bernasconi A, Russo S, Maffulli N. Core decompression versus other joint preserving treatments for osteonecrosis of the femoral head: a meta-analysis. Br Med Bull. 2016;118(1):33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li X, Xu X, Wu W. Comparison of bone marrow mesenchymal stem cells and core decompression in treatment of osteonecrosis of the femoral head: a meta-analysis. Int J Clin Exp Pathol. 2014;7(8):5024–5030. [PMC free article] [PubMed] [Google Scholar]
- 58. Lakshminarayana S, Dhammi IK, Jain AK, Bhayana H, Kumar S, Anshuman R. Outcomes of core decompression with or without Nonvascularized fibular grafting in avascular necrosis of femoral head: short term followup study. Indian J Orthop. 2019;53(3):420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Houdek MT, Wyles CC, Packard BD, Terzic A, Behfar A, Sierra RJ. Decreased osteogenic activity of mesenchymal stem cells in patients with corticosteroid-induced osteonecrosis of the femoral head. J Arthroplasty. 2016;31(4):893–898. [DOI] [PubMed] [Google Scholar]
- 60. Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87-B(7):896–902. [DOI] [PubMed] [Google Scholar]
- 61. Larson E, Jones LC, Goodman SB, Koo K-H, Cui Q. Early-stage osteonecrosis of the femoral head: where are we and where are we going in year 2018? Int Orthop. 2018;42(7):1723–1728. [DOI] [PubMed] [Google Scholar]
- 62. Sodhi N, Acuna A, Etcheson J, et al. . Management of osteonecrosis of the femoral head. Bone Joint J. 2020;102-B(7_Supple_B):122–128. [DOI] [PubMed] [Google Scholar]
- 63. Kuroda Y, Tanaka T, Miyagawa T, et al. . Classification of osteonecrosis of the femoral head: who should have surgery? Bone Joint Res. 2019;8(10):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen L, Hong G, Hong Z, et al. . Optimizing indications of impacting bone allograft transplantation in osteonecrosis of the femoral head. Bone Joint J. 2020;102-B(7):838–844. [DOI] [PubMed] [Google Scholar]