Abstract
Background
Catheter ablation for atrial fibrillation (AF) treatment provides effective and durable pulmonary vein isolation (PVI) and is associated with encouraging clinical outcome. A novel CF sensing temperature-controlled radiofrequency (RF) ablation catheter allows for very high-power short-duration (vHP-SD, 90 W/4 s) ablation aiming a potentially safer, more effective and faster ablation. We thought to evaluate preliminary safety and efficacy of vHP-SD ablation for PVI utilizing a novel vHP-SD catheter. The data was compared to conventional power-controlled ablation index (AI) guided PVI utilizing conventional contact force (CF) sensing catheters.
Methods and Results
Fifty-six patients with paroxysmal or persistent AF were prospectively enrolled in this study. Twenty-eight consecutive patients underwent vHP-SD based PVI (vHP-SD group) and were compared to 28 consecutive patients treated with conventional CF-sensing catheters utilizing the AI (control group). All PVs were successfully isolated using vHP-SD. The median RF ablation time for vHP-SD was 338 (IQR 286, 367) seconds vs control 1580 (IQR 1350, 1848) seconds (p < 0.0001), the median procedure duration was vHP-SD 55 (IQR 48–60) minutes vs. control 105 (IQR 92–120) minutes (p < 0.0001). No differences in periprocedural complications were observed.
Conclusions
This preliminary data of the novel vHP-SD ablation mode provides safe and effective PVI. Procedure duration and RF ablation time were substantially shorter in the vHP-SD group in comparison to the control group.
Keywords: Atrial fibrillation; High-power short duration, pulmonary vein isolation; Radiofrequency; Acute efficacy
1. Introduction
Pulmonary vein isolation (PVI) has shown high procedural success rates and encouraging long-term follow-up rates for patients with paroxysmal (PAF) and persistent atrial fibrillation (PersAF) [1], [2], [3]. Catheter improvement implementing contact force (CF) sensing and ablation index (AI) guided radiofrequency (RF) energy ablation shortened procedure time and improved patients outcomes [4], [5]. Nevertheless, balloon based PVI has been shown to decrease procedure time as well as reduce periprocedural complications when compared to standard RF based power-controlled PVI [6], [7]. To further improve safety and efficacy and decrease procedure time of RF based ablation, high-power short-duration (HP-SD) concepts with a maximum of up to 70 W have been evaluated [8], [9]. In CF guided AF ablation procedures power is limited to 50 W [10], [11], while in a power-controlled ablation mode without CF sensing catheter power is limited to 70 W [8], [9]. Although this concept seemed to be safe and effective, no real time temperature monitoring was possible because conventional catheters were utilized in those studies [8], [9], [10], [11]. A novel CF-sensing catheter (QDOT Micro, Biosense Webster, Inc. Diamond Bar, CA, USA) has been developed allowing real-time assessment of catheter-to-tissue interface temperature and therefore temperature-controlled ablation. This catheter incorporates three microelectrodes and six thermocouples at its tip for precise temperature monitoring [12], [13]. In the very high-power short-duration mode (vHP-SD, 90 W/4 s, QMODE+) only power is adapted to adjust the target temperature [14]. This strategy aims to create shallower but wider lesions in a very short time by reducing conductive heating and increasing resistive heating at the same time. Additionally, collateral tissue damage might be reduced [12]. Previous analyses provided evidence for reduced RF ablation time and procedure duration while showing a good safety profile [14], yet no direct comparison of this concept to conventional PVI has been performed up to date. We sought to evaluate the preliminary safety and efficacy of the QMODE+ ablation mode for RF based PVI and compared the data to conventional AI guided ablation.
2. Methods
2.1. Inclusion and exclusion criteria
Since September 2020, 28 consecutive patients with symptomatic, drug-refractory PAF or short-standing PersAF (duration ≤ 3 months) presented for PVI and were treated with the QDOT Micro catheter utilizing the QMODE+ (vHP-SD group). A total of 28 consecutive previous patients treated with conventional CF-sensing AI guided PVI served as control (control group). The patients were prospectively and consecutively enrolled but not randomized. Exclusion criteria were prior left atrial (LA) ablation attempts, LA diameter > 60 mm, severe valvular heart disease or contraindications to post-interventional oral anticoagulation. Transesophageal echocardiography was performed in all patients prior to PVI to rule out intracardiac thrombi and to assess the LA diameter. No further pre-procedural imaging was performed. In patients on vitamin K antagonists the procedure was performed under therapeutic INR values of 2–3. In patients on new oral anticoagulants the morning dose on the day of the procedure was omitted. All patients gave written informed consent and all patient information was anonymized. The study was approved by the local ethics board (Lübeck ablation registry ethical review board number: WF-028/15) and performed in accordance to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
2.2. Intraprocedural management
The detailed intraprocedural management for 3D mapping and AI guided PVI has been described in previous studies from our group [15]. In brief, the procedure was performed under deep sedation using midazolam, fentanyl and propofol. Three ultrasound guided right femoral vein punctures were performed and three 8F short sheaths were inserted. Prior to transseptal puncture one diagnostic catheter was introduced and positioned inside the coronary sinus. Double transseptal puncture (TSP) was performed under fluoroscopic guidance using a modified Brockenbrough technique with 8.5F transseptal sheaths and puncture needle (SL1 sheath and BRK-1 TSP needle, St. Jude Medical, Inc., St. Paul, MN, USA). After TSP, injection of contrast medium via the needle was performed to confirm LA access. Pulmonary vein angiography was performed to identify the pulmonary vein (PV) ostia utilizing a 7F multipurpose catheter or directly via the transseptal sheath. Both sheaths were continuously flushed with heparinized saline (10 ml/h). After TSP heparin boluses were administered targeting an activated clotting time of > 300 s.
2.3. Ablation procedure
Three-dimensional electroanatomic LA reconstruction (CARTO 3, Biosense Webster) was performed via fast anatomical mapping (FAM) with a multi-electrode spiral mapping catheter. For the LA voltage map, the bipolar voltage reference interval was set between 0.05 and 0.5 mV. After PV angiography the ipsilateral PVs were tagged according to 3D mapping and PV angiography. During PVI a multi-electrode spiral mapping catheter was positioned inside the ipsilateral PVs. All procedures in both groups have been performed by two highly experienced operators only.
2.3.1. The vHP-SD ablation group
In the vHP-SD group an open-irrigated tip catheter (QDOT Micro, Biosense Webster) was utilized. For all applications vHP-SD ablation (90 W, 4sec; QMODE+ mode) was performed. The target temperature of the temperature-controlled ablation was 60 °C based on the hottest surface thermocouple. The QDOT Micro catheter also allows for conventional ablation (QMODE) [14]. In the conventional mode the system adjusts 1) the irrigation flow rate and 2) power based on the measured temperature to stabilize the catheter tip temperature.
In the QMODE+ mode only power is adapted to adjust the target temperature (Fig. 1) [14]. The irrigation flow rate delays the energy application for a minimum of 2 sec before and 4 sec after each RF application. A touch-up change to conventional QMODE is always possible by changing the ablation mode. For anterior lesions an inter-lesion distance of 3–4 mm and for posterior lesions an inter-lesion distance of 5–6 mm was used. An S-shaped temperature probe (CIRCA S-CATH, Circa Scientific, Englewood, CO, USA) was advanced into the esophagus to monitor the esophageal temperature (Teso) in all cases of the vHP-SD group. The intraluminal Teso cut-off was set at 38.5 °C. During the procedures special attention was drawn for audible pops and all ablation were checked after removal for charring.
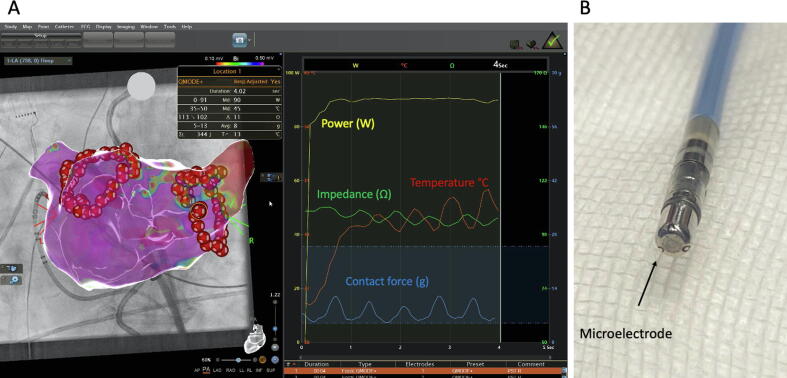
Fig. 1.
QDOT Micro ablation catheter and QMODE+ A: Three-dimensional electroanatomic reconstruction (CARTO 3, UNIVIEW module, Biosense Webster) of the left atrium of case #2 in PA view. Please note the two circles depicted through red-white tags created by radiofrequency ablation utilizing the QDOT Micro catheter in the QMODE+ ablation mode. The data of location 1 ablation point is depicted in the right sided diagram of the figure and shows the biophysics parameters of a very-high power short duration ablation by 90 W/4 s. The parameters of power (W) Impedance (Ω), temperature (°C) and contact force (g) are shown. B: Picture of the QDOT Micro catheter tip showing the three micro-electrodes on top of the tip. The black arrow highlights one micro-electrode. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.3.2. Conventional ablation
In the control group conventional AI guided ablation was used. Ablation was performed with an open-irrigated tip catheter (Thermocool Smart-touch SF, Biosense Webster). Ablation was performed in a power-controlled mode. Energy application was limited to 40 W at the anterior and 25 W at the roof and posterior segments. Target range for CF was 10–40 g. Target AIs were 550, 450 and 380 for the anterior, roof and posterior segments of the LA, respectively [15]. The inter-lesion distance was set to 5–6 mm. In case of previously known or periprocedural typical atrial flutter, cavotricuspid isthmus ablation was performed in both groups.
2.4. Postprocedural care
A figure-of-eight suture and a pressure bandage were used to prevent femoral bleeding. The pressure bandage was removed after 4 h and the figure-of-eight suture on the next day. Following ablation, all patients underwent transthoracic echocardiography immediately post procedure, after 2 h and at day 1 after the procedure to rule out a pericardial effusion. New oral anticoagulants were re-initiated 6 h post ablation. Anticoagulation was continued for at least 3 months and continued thereafter based on the individual CHA2DS2-VASc score. Previously ineffective antiarrhythmic drugs or a new antiarrhythmic drug were prescribed and continued for 3 months post ablation. All patients were treated with proton-pump inhibitors for 6 weeks.
2.5. Statistical analysis
Continuous variables are presented as median with interquartile range (first quartile [Q1], third quartile [Q3]); they were compared using the Wilcoxon-Mann Whitney test. Categorical variables are presented as absolute and relative frequencies; they were compared using the chi-square test or Fisher’s exact test (in case of small expected cell frequencies). All p-values are two-sided and a p-value < 0.05 was considered significant. All calculations were performed with the statistical analysis software SAS (SAS Institute Inc., version 9.3, Cary, NC, USA).
3. Results
3.1. Patient characteristics
Fifty-six patients with paroxysmal or persistent AF were prospectively enrolled in this study. A total of 28 consecutive patients underwent vHP-SD based PVI utilizing the QMODE+ ablation mode. The data was compared to 28 consecutive patients with PVI by conventional CF-sensing AI guided ablation. Patient baseline characteristics are shown in Table 1. No demographic differences were detected between the groups.
Table 1.
Baseline patient characteristics.
| Variable | V-HP-SD | Control | P |
|---|---|---|---|
| Patients | 28 | 28 | |
| Age, years | 69 (61, 73) | 69 (62, 75) | 0.815 |
| LA volume, ml/m2* | 26 (25, 35) | 32 (26, 39) | 0.070 |
| Female gender | 7 (25) | 9 (32) | 0.554 |
| Paroxysmal AF | 11 (39) | 14 (50) | 0.420 |
| Congestive heart failure | 3 (11) | 8 (29) | 0.093 |
| Arterial hypertension | 16 (57) | 22 (79) | 0.086 |
| Diabetes mellitus type 2 | 5 (18) | 3 (11) | 0.445 |
| Coronary artery disease | 6 (22) | 9 (32) | 0.365 |
| CHA2DS2-VASc score | 0.504 | ||
| 0 | 4 (14) | 2 (7) | |
| 1 | 6 (22) | 3 (11) | |
| 2 | 8 (29) | 8 (29) | |
| 3 | 5 (18) | 5 (18) | |
| ≥4 | 5 (18) | 10 (36) |
Values are counts, n (%) or median (first quartile, third quartile). *per body surface area AF = atrial fibrillation, LA = left atrium.
3.2. Procedural characteristics
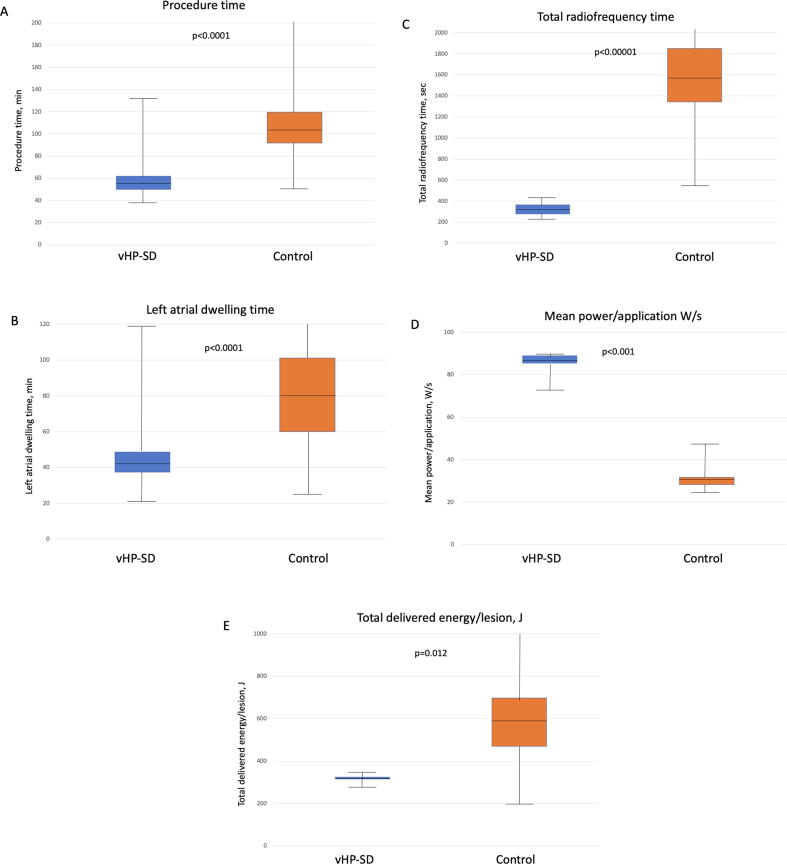
Procedural data are summarized in Table 2, Table 3 as well as Fig. 2. All PVs were successfully isolated in either group. Apart from PVI, additional ablation strategies were performed in both groups, yet no differences were observed for the amount of additional ablation strategies. With 61% (vHP-SD) and 50% (control) an equal rate (p = 0.420) of first pass isolations were observed in both groups (first attempt all veins isolated, FAAVI). For right PVs the rate of first pass isolation (first attempt vein isolated, FAVI) was significantly higher in the vHP-SD group (96%) than in control patients (57%), p = 0.005. For left PVs no difference in FAVI was observed (64% vs. 71%; p = 0.571). Significantly shorter procedure times (55 [IQR 51, 62) vs. 105 [IQR 92, 120] minutes, p < 0.0001), LA dwelling times (43 [IQR 37, 48] vs. 80 [IQR 60, 104] minutes, p < 0.0001) and fluoroscopy times (7 [IQR 4, 8] vs. 13 [IQR 10, 17] minutes, p < 0.001) were observed in the vHP-SD group.
Table 2.
Procedural details.
| Variable | vHP-SD | Control | P |
|---|---|---|---|
| Number of patients | 28 | 28 | |
| Number of PVs | 112 | 112 | |
| Total number of isolated PVs | 112 (100) | 112 (100) | 0.999 |
| FAAVI | 17 (61) | 14 (50) | 0.420 |
| Total procedure time, min | 55 (51, 62) | 105 (92, 120) | <0.0001 |
| Total LA dwelling time, min | 43 (37, 48) | 80 (60, 104) | <0.0001 |
| Total fluoroscopy time, min | 7 (4,8) | 13 (10, 17) | <0.001 |
| Total amount of contrast agent, ml | 50 (48, 60) | 50 (30, 50) | 0.135 |
| Total ablation time, sec | 338 (286, 367) | 1580 (1350, 1848) | <0.0001 |
| Total number of applications | 85 (72, 92) | 82 (58, 110) | 0.513 |
| Mean application duration, sec | 4 (4, 4) | 21 (15, 24) | <0.0001 |
| Mean contact force, g | 14 (12, 17) | 18 (15, 21) | <0.001 |
| Mean power/application, Watt | 84 (83, 85) | 31 (29, 32) | <0.001 |
| Total delivered power/lesion, Joule | 335 (332, 338) | 594 (460, 698 | 0.012 |
| Teso Temp. > 38,5 °C | 11 (39) | – | – |
| Teso Temp. > 38,5 °C/patient | 0.6 | – | – |
| Max Teso, °C | 42 (41, 43) | – | – |
| Additional ablation strategies | |||
| Cavotricuspid isthmus block | 8 (29) | 9 (32) | 0.771 |
| Roof line | 5 (18) | 3 (11) | 0.275 |
| Anterior line | 2 (7) | 6 (21) | 0.127 |
| Periprocedural complications | |||
| Major complications | 2 (7) | 1 (4) | 0.553 |
| Cardiac tamponade | 0 | 0 | 0.999 |
| Severe bleeding | 1 (4) | 1 (4) | 0.999 |
| Phrenic nerve injury | 0 | 0 | 0.999 |
| Stroke or TIA | 0 | 0 | 0.999 |
| Postprocedural pulmonary edema | 1 (4) | 0 | 0.678 |
| Minor complications | 2 (7) | 1 (4) | 0.553 |
| Minor bleeding | 2 (7) | 1 (4) | 0.553 |
| Pericardial effusion | 0 | 0 | 0.999 |
| Transient air embolism | 0 | 0 | 0.999 |
Values are counts, n (%) or median (first quartile, third quartile). PV(s) = Pulmonary vein(s), FAAVI = first attempt all veins isolated, LA = left atrium, min = minutes, sec = seconds, g = gramms.
Table 3.
Procedural details - individual pulmonary vein.
| Variable | vHP-SD | Control | P |
|---|---|---|---|
| Right-sided PVs | 28 | 28 | |
| Total ablation time, sec | 154 (124, 176) | 750 (580, 1006) | <0.0001 |
| Total number of applications | 39 (31, 44) | 40 (27, 53) | 0.296 |
| Mean application duration, sec | 4 (4, 4) | 21 (16, 23) | 0.001 |
| Mean contact force, g | 16 (14, 19) | 20 (18, 25) | <0.0001 |
| Mean power/application, Watt | 84 (82, 85) | 31 (29, 32) | <0.001 |
| Total delivered power/lesion, Joule | 338 (328, 339) | 594 (500, 683) | <0.001 |
| FAVI | 27 (96) | 16 (57) | 0.005 |
| Left-sided PVs | 28 | 28 | |
| Total ablation time, sec | 172 (143, 211) | 831 (545, 972) | <0.0001 |
| Total number of applications | 43 (36, 53) | 40 (27, 58) | 0.658 |
| Mean application duration, sec | 4 (4, 4) | 21 (15, 24) | 0.001 |
| Mean contact force, g | 14 (11, 17) | 16 (14, 19) | <0.0001 |
| Mean power/application, Watt | 84 (82, 85) | 31 (29, 31) | <0.001 |
| Total delivered power/lesion, Joule | 336 (328, 338) | 608 (460, 709) | 0.002 |
| FAVI | 18 (64) | 20 (71) | 0.571 |
Values are counts, n (%) or median (first quartile, third quartile). PV(s) = Pulmonary vein(s), FAVI = first attempt vein isolated, sec = seconds, g = grams.
Fig. 2.
Periprocedural data:Periprocedural duration (A): Procedure time, (B): left atrial dwelling time, (C): Total radiofrequency time, (D) total mean power/application and (E) total delivered energy of the QMODE+ (vHP-SD group) compared to the control group. W = Watts, J = Joules.
While the total number of applications was similar in both groups (p = 0.513), the total ablation time (338 [IQR 286, 367] vs. 1580 [IQR 1350, 1848] sec, p < 0.0001) and mean application duration (4 [IQR 4, 4] vs. 21 [IQR 15, 24] sec, p < 0.0001) were significantly shorter in the vHP-SD group. Despite a higher mean power per application in the vHP-SD group (84 [IQR 83, 85] vs. 31 [IQR 29, 32] W/s, p < 0.001, the total delivered energy per lesion was significantly lower (335 [IQR 332, 338] vs. 594 [IQR 460, 698] J, p = 0.012). The mean contact force was significantly lower in the vHP-SD group (14 [IQR 12, 17] vs. 18 [IQR 15, 21] g, p < 0.001). An esophageal temperature probe was utilized only in the vHP-SD group. A Teso > 38.5 °C was detected in 11 (39%) of patients solely at the posterior part of the left PVs. The median maximum Teso was measured at 42 [IQR 41, 43] °C.
The QMODE+ ablation mode was exclusively used for all procedures in the vHP-SD group. No switch to the conventional QMODE was necessary to achieve the ablation goals. No differences were observed between the groups with regard to catheter maneuverability and catheter stability along the targeted PVs. After discharge all patients received antiarrhythmic drugs post ablation for 3 months.
3.3. Safety
There was no difference in terms of major periprocedural complications (Table 3). In each group one patient experienced a major groin bleeding requiring blood transfusion. In the vHP-SD group, one patient, with pre-existing severe LV dysfunction and heart failure, developed cardiogenic shock and pulmonary edema following the procedure, which resolved with cardiac supportive medication. There were no further major complications such as cardiac tamponade, pericardial effusion, stroke, phrenic nerve palsy or atrioesophageal fistula in either group. Two patients in the vHP-SD group and one patient in the control group suffered from minor bleeding of the groin, not requiring blood transfusion. All patients were managed conservatively. There were no documented steam pops and no charring was detected in either group.
4. Discussion
This study is the first clinical evaluation to assess preliminary efficacy, ablation characteristics and safety during PVI utilizing the novel QMODE+ ablation mode of the QDOT Micro catheter in comparison to standard CF-sensing AI guided ablation. The major findings are 1) All PVs could be isolated utilizing vHP-SD, with a higher first pass isolation rate at the right-sided PVs compared to control; 2) RF ablation time was significantly reduced; and 3) overall procedural time was significantly shorter in the vHP-SD group; 4) no differences were observed between the groups concerning periprocedural complications and 5) only the QMODE+ ablation mode was used, with no switch to QMDOE necessary to achieve the ablation goals.
To date, PVI is the cornerstone of catheter ablation for AF therapy [1]. The FIRE AND ICE trial showed noninferiority of cryoballoon-based PVI compared to point-to-point RF [6]. As a consequence, cryoballoon-based PVI is increasingly performed. Cryoballoon-based PVI is associated with shorter learning curves, shorter procedures times as well as high rates of safety and efficacy, compared to current RF-based catheter ablation [6]. However, recent studies are focusing on RF-based PVI with increased power and shorter duration to possibly speed up RF-based PVI utilizing conventional CF-sensing ablation catheters. In this context, an increased power of up to 50 W was suggested and safety and feasibility were shown in previous analyses [10], [11]. Although the first experiences utilizing this protocol showed safety and efficacy, they were limited by the use of a power-controlled ablation mode and no real-time tissue temperature monitoring was possible because conventional ablation catheters were used. The authors reported a steam pop phenomenon in 8% and a Teso rise in 50% of patients [10].
An even higher power setting was recently suggested by Kottmaier et al. Utilizing a HP-SD ablation protocol with a power setting of 70 W and a duration of 5–7 sec, an RF time of 12.4 ± 3.4 min and a procedural time of 89.5 ± 23.9 min was reported.8 In this analysis no Teso probe was utilized and the incidence of steam-pops was not mentioned. However, similar to the above mentioned 50 W HP-SD protocol, catheter ablation was performed in a power-controlled mode without real-time temperature measurements. Until today only one atrioesophageal fistula out of 11,436 treated patients (0.009%) was reported during catheter ablation utilizing a HP-SD protocol and the reported complications rates were generally low [16]. The six thermocouples of the QDOT Micro catheter enable precise temperature control and power modulation to potentially avoid tissue overheating, collateral damage, catheter tip charring and steam pops. Since a Teso probe temperature rise was detected in 39% of patients we strongly suggest to use Teso probes and predefined temperature cut off values to avoid esophageal injuries. A recent study utilizing the Qmode of the QDOT Micro catheter with a 50 W HP-SD protocol results in a 16% incidence of ablation‐induced esophageal injuries [17]. In our study no charring, no steam pops and no clinical apparent esophageal injuries occurred, suggesting an excellent safety profile of the QMODE+ ablation mode.
The present study shows that PVI utilizing the QMODE+ ablation mode provides similar acute success and periprocedural complications rates when compared to the standard CF-sensing AI guided PVI – despite the fact that the operators had to pass a certain learning curve. The fact that the application duration and consequently the total RF ablation time was dramatically reduced utilizing the QMODE+ translated into significantly reduced median LA dwelling times and a shorter median procedure times. It is prudent to state that the total delivered energy/lesion was significantly reduced when using vHP-SD (335 vs. 594 J, p = 0.012). A higher first pass isolation rate at the right-sided PVs may reflect a higher lesion quality and potentially more durable lesions. However, for left sided PVs no significant difference for first pass isolation rates was observed. Whether this effect was driven by the relatively low patient number, learning curve effects, thicker tissue on the LA-ridge or by the QMODE+ ablation mode we can not answer in our recent study. Nevertheless, further studies should focus on this specific point.
With a median procedure time of 55 min and despite the fact that further ablation strategies have been performed, the QMODE+ offers short procedures times compared to current balloon based PVI. Recent studies utilizing the cryoballoon reported mean procedure times of 114–140 min when omitting a bonus-freeze cycle [18], and 77–96 min when using individualized energy titration protocols [19], [20], [21]. For the latest generation laser balloon, 77 min of median procedure time where reported [22]. Although a comparable procedure time of 55.6 ± 6.6 min for PVI only was reported for the 50 W power-controlled HP-SD protocol suggested by Chen et al. [10] in our series 29% of patients received additional CTI block, 18% received a roof line and 7% an anterior line ablation. In the first in man study of the QDOT Micro catheter Reddy et al. published a mean procedure time of 105.2 min which is almost twice as the reported procedure time in our study. Yet, due to the fact that a 20 min waiting time plus adenosine or isoproterenol challenge was performed in this trial and not in our study the procedures times might not be adequately comparable [14]. With an impressive 5.4 min of total RF time for PVI this was less than a half compared to the HP-SD power-controlled protocol (11.2 min) [10]. With potentially similar or even faster PVI compared to balloon-based ablation, the ability to set further ablation strategies in the left and right atrium as well as an excellent safety profile, vHP-SD has the potential for an ideal ablation tool. However, the vHP-SD ablation mode will be further evaluated in clinical trials and studies with larger patient numbers.
4.1. Limitations
The current study with a relatively small number of patients reflects a single-center experience. Additionally, this is a non-randomized analysis resulting in potential biases. Yet, consecutive patients where prospectively evaluated and all procedures were performed by two highly experienced operators. Although a Teso probe was provided in all patients of the vHP-SD group, no routine post-ablation endoscopy was performed. Therefore, no data on subclinical esophageal injury is available. The study was designed to exclusively assess acute periprocedural data and does not provide follow-up data. The data concerning safety and efficacy are only preliminary since no follow-up was conducted and especially atrioesophageal fistula typically occur weeks after the procedure. The long-term outcomes as well as PVI durability needs to be assessed in further clinical trials.
4.2. Conclusions
To the best of our knowledge this is the first study reporting preliminary data on the acute efficacy and safety of QMODE+ based PVI as compared to standard CF-sensing AI guided PVI. While demonstrating similar acute efficacy for PVI the total ablation time as well as procedural duration were impressively low utilizing vHP-SD.
Funding statement: no funding declared.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CHH received travel grants and research grants by Boston Scientific, Biosense Webster and Cardiofocus and Speakeŕs Honoraria from Boston Scientific, Biosense Webster and Cardiofocus. RRT is a consultant of Boston Scientific, Biotronik and Biosense Webster and received Speakeŕs Honoraria from Biosense Webster, Medtronic, Boston Scientific and Abbot Medical. KHK reports grants and personal fees from Abbott Vascular,. Medtronic, Biosense Webster outside submitted work. All other authors have no relevant disclosures.
Contributor Information
Roland Richard Tilz, Email: roland.tilz@uksh.de.
Christian-Hendrik Heeger, Email: christian.heeger@gmx.net.
References
- 1.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS)The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehab648. 2020;ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang F., Tilz R., Chun J., Schmidt B., Wissner E., Zerm T., Neven K., Köktürk B., Konstantinidou M., Metzner A., Fuernkranz A., Kuck K.-H. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122(23):2368–2377. doi: 10.1161/CIRCULATIONAHA.110.946806. [DOI] [PubMed] [Google Scholar]
- 3.Tilz R.R., Heeger C.-H., Wick A., Saguner A.M., Metzner A., Rillig A., Wohlmuth P., Reissmann B., Lemeš C., Maurer T., Santoro F., Riedl J., Sohns C., Mathew S., Kuck K.-H., Ouyang F. Ten-Year Clinical Outcome After Circumferential Pulmonary Vein Isolation Utilizing the Hamburg Approach in Patients With Symptomatic Drug-Refractory Paroxysmal Atrial Fibrillation. Circulation Arrhythmia and electrophysiology. 2018;11(2) doi: 10.1161/CIRCEP.117.005250. [DOI] [PubMed] [Google Scholar]
- 4.Hussein A., Das M., Chaturvedi V., Asfour I.K., Daryanani N., Morgan M., Ronayne C., Shaw M., Snowdon R., Gupta D. Prospective use of Ablation Index targets improves clinical outcomes following ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2017;28(9):1037–1047. doi: 10.1111/jce.13281. [DOI] [PubMed] [Google Scholar]
- 5.Hussein A., Das M., Riva S., Morgan M., Ronayne C., Sahni A., Shaw M., Todd D., Hall M., Modi S., Natale A., Dello Russo A., Snowdon R., Gupta D. Use of Ablation Index-Guided Ablation Results in High Rates of Durable Pulmonary Vein Isolation and Freedom From Arrhythmia in Persistent Atrial Fibrillation Patients. Circulation Arrhythmia Electrophysiol. 2018;11(9) doi: 10.1161/CIRCEP.118.006576. [DOI] [PubMed] [Google Scholar]
- 6.Kuck K.-H., Brugada J., Fürnkranz A., Metzner A., Ouyang F., Chun K.R.J., Elvan A., Arentz T., Bestehorn K., Pocock S.J., Albenque J.-P., Tondo C. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. The New England journal of medicine. 2016;374(23):2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 7.Kuck K.-H., Fürnkranz A., Chun K.R.J., Metzner A., Ouyang F., Schlüter M., Elvan A., Lim H.W., Kueffer F.J., Arentz T., Albenque J.-P., Tondo C., Kühne M., Sticherling C., Brugada J. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur. Heart J. 2016;37(38):2858–2865. doi: 10.1093/eurheartj/ehw285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kottmaier M, Popa M, Bourier F, Reents T, Cifuentes J, Semmler V, et al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Ep Europace 2019;22:388–393 [DOI] [PubMed]
- 9.Bourier F., Duchateau J., Vlachos K., Lam A., Martin C.A., Takigawa M., Kitamura T., Frontera A., Cheniti G., Pambrun T., Klotz N., Denis A., Derval N., Cochet H., Sacher F., Hocini M., Haïssaguerre M., Jais P. High-power short-duration versus standard radiofrequency ablation: Insights on lesion metrics. J Cardiovasc Electr. 2018;29(11):1570–1575. doi: 10.1111/jce.13724. [DOI] [PubMed] [Google Scholar]
- 10.Chen S., Schmidt B., Bordignon S., Urbanek L., Tohoku S., Bologna F. Ablation index-guided 50 W ablation for pulmonary vein isolation in patients with atrial fibrillation: Procedural data, lesion analysis, and initial results from the FAFA AI High Power Study. J Cardiovasc Electr. 2019;30:2724–2731. doi: 10.1111/jce.14219. [DOI] [PubMed] [Google Scholar]
- 11.Vassallo F., Cunha C., Serpa E., Meigre L.L., Carloni H., Simoes A., Hespanhol D., Lovatto C.V., Batista W., Serpa R. Comparison of high-power short-duration (HPSD) ablation of atrial fibrillation using a contact force-sensing catheter and conventional technique: Initial results. J Cardiovasc Electr. 2019;30(10):1877–1883. doi: 10.1111/jce.14110. [DOI] [PubMed] [Google Scholar]
- 12.Barkagan M., Contreras‐Valdes F.M., Leshem E., Buxton A.E., Nakagawa H., Anter E. High-power and short-duration ablation for pulmonary vein isolation: Safety, efficacy, and long-term durability. J Cardiovasc Electr. 2018;29(9):1287–1296. doi: 10.1111/jce.13651. [DOI] [PubMed] [Google Scholar]
- 13.Leshem E., Tschabrunn C.M., Jang J., Whitaker J., Zilberman I., Beeckler C., Govari A., Kautzner J., Peichl P., Nezafat R., Anter E. High-Resolution Mapping of Ventricular Scar Evaluation of a Novel Integrated Multielectrode Mapping and Ablation Catheter. Jacc Clin Electrophysiol. 2017;3(3):220–231. doi: 10.1016/j.jacep.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Reddy VY, Grimaldi M, Potter TD, Vijgen JM, Bulava A, Duytschaever MF, et al. Pulmonary Vein Isolation With Very High Power, Short Duration, Temperature Controlled Lesions | Elsevier Enhanced Reader.pdf. JACC: Clinical Electrophysiology n.d.;7:778-786. [DOI] [PubMed]
- 15.Münkler P, Kröger S, Liosis S, Abdin A, Lyan E, Eitel C, et al. Ablation Index for Catheter Ablation of Atrial Fibrillation– Clinical Applicability and Comparison With Force-Time Integral –. Circ J 2018;82:CJ-18-0361 [DOI] [PubMed]
- 16.Winkle R.A., Mohanty S., Patrawala R.A., Mead R.H., Kong M.H., Engel G., Salcedo J., Trivedi C.G., Gianni C., Jais P., Natale A., Day J.D. Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Heart Rhythm. 2019;16(2):165–169. doi: 10.1016/j.hrthm.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 17.Piringer R., Deneke T., Foldyna B., Sonne K., Nentwich K., Ene E., Barth S., Lüsebrink U., Berkovitz A., Halbfass P. Incidence of ablation-induced esophageal injury associated with high-power short duration temperature-controlled pulmonary vein isolation using a specialized open-irrigated ablation catheter: A retrospective single-center study. J Cardiovasc Electr. 2021;32(3):695–703. doi: 10.1111/jce.14883. [DOI] [PubMed] [Google Scholar]
- 18.Heeger C.-H., Wissner E., Wohlmuth P., Mathew S., Hayashi K., Sohns C., Reißmann B., Lemes C., Maurer T., Saguner A.M., Santoro F., Riedl J., Ouyang F., Kuck K.-H., Metzner A. Bonus-freeze: benefit or risk? Two-year outcome and procedural comparison of a “bonus-freeze” and “no bonus-freeze” protocol using the second-generation cryoballoon for pulmonary vein isolation. Clinical research in cardiology. 2016;105(9):774–782. doi: 10.1007/s00392-016-0987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun K.R.J., Stich M., Fürnkranz A., Bordignon S., Perrotta L., Dugo D., Bologna F., Schmidt B. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Heart rhythm. 2017;14(4):495–500. doi: 10.1016/j.hrthm.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Reissmann B, Wissner E, Deiss S, Heeger C, Schlueter M, Wohlmuth P, et al. First insights into cryoballoon-based pulmonary vein isolation taking the individual time-to-isolation into account. Europace. 2017;19:1676–1680 [DOI] [PubMed]
- 21.Heeger C.-H., Wissner E., Mathew S., Hayashi K., Sohns C., Reißmann B., Lemes C., Maurer T., Fink T., Saguner A.M., Santoro F., Riedl J., Ouyang F., Kuck K.-H., Metzner A. Short tip-big difference? First-in-man experience and procedural efficacy of pulmonary vein isolation using the third-generation cryoballoon. Clinical research in cardiology. 2016;105(6):482–488. doi: 10.1007/s00392-015-0944-y. [DOI] [PubMed] [Google Scholar]
- 22.Heeger C.-H., Tiemeyer C.M., Phan H.-L., Meyer-Saraei R., Fink T., Sciacca V., Liosis S., Brüggemann B., Große N., Fahimi B., Reincke S., Kuck K.-H., Ouyang F., Vogler J., Eitel C., Tilz R.R. Rapid pulmonary vein isolation utilizing the third-generation laserballoon – The PhoeniX registry. Ijc Hear Vasc. 2020;29:100576. doi: 10.1016/j.ijcha.2020.100576. [DOI] [PMC free article] [PubMed] [Google Scholar]