Highlights
-
•
Thirty percent reduction in population salt intake by 2025 is recommended by WHO.
-
•
South Africa introduced mandatory maximum sodium limits in processed foods in 2016.
-
•
A countrywide impact evaluation assessed change in salt intake after two years.
-
•
Salt intake measured using 24hr Na excretion dropped by 1.15 g per day.
-
•
Ongoing evaluation is necessary as more stringent targets were implemented in 2019.
Keywords: Salt reduction, South Africa, Legislation, Blood pressure, Potassium, Food policy
Abstract
South Africa implemented legislation in June 2016 mandating maximum sodium (Na) levels in processed foods. A pre-post impact evaluation assessed whether the interim legislative approach reduced salt intake and blood pressure. Baseline Na intake was assessed in a nested cohort of the WHO Study on global AGEing and adult health (WHO-SAGE) Wave 2 (Aug-Dec 2015). 24-hour urine samples were collected in a random subsample (n = 1,299; of which n = 750 were considered valid (volume ≥ 300 mL and creatinine ≥ 4 mmol/day (women) or ≥ 6 mmol/day (men))). Follow-up urine samples were collected in Wave 3 (Jun 2018-Jun 2019), with replacements included for those lost to follow-up (n = 1,189; n = 548 valid). In those aged 18 − 49y, median salt intake was 7.8 (4.7, 12.0) g/day in W2 (n = 274), remaining similar in the W3 sample (7.7 (4.9, 11.3) g salt/day (n = 92); P = 0.569). In older adults (50 + y), median salt intake was 5.8 (4.0, 8.5) g/day (n = 467) in W2, and 6.0 (4.0, 8.6) g/day (n = 455) in W3 (P = 0.721). Controlling for differences in background characteristics, overall salt intake dropped by 1.15 g/day (P = 0.028). 24hr urinary Na concentrations from a countrywide South African sample suggest that salt intakes have dropped during the interim phase of mandatory sodium legislation. Further measurement of population level salt intake following stricter Na targets, enforced from June 2019, is necessary.
1. Introduction
In low-income and middle-income countries (LMIC), a disproportionately rapid increase in hypertension is occurring with little evidence of adequate strategies to halt this growth or mitigate the impact on CVDs and death (Yusuf et al., 2014). South Africa is a country with a particularly high prevalence of hypertension, with an estimated age standardised prevalence of 35.1% in those aged 15 years and older (Berry et al., 2017), and poor management of the condition once diagnosed (Lloyd-Sherlock et al., 2014, Day et al., 2014).
In order to reduce the burden of hypertension, the World Health Organization (WHO) and World Health Assembly recommend 30% reduction in population salt/sodium intake by 2025 (WHO, 2020). Population salt reduction efforts are underway in many countries, with a major focus on sodium reduction in processed foods within national salt reduction strategies (Trieu et al., 2015). The most common interventions include engaging with the food industry for product reformulation, more informative front‐of‐pack nutrition labelling, and setting voluntary sodium reduction targets (Charlton et al., 2015).
Few countries have opted for legislative approaches but, in June 2016, the South African government was one of the first to implement legislation for mandatory maximum sodium levels permitted in a wide range of processed food categories (bread; breakfast cereal; butter and margarine; potato crisps; salty snacks; raw sausage; processed meat; instant noodle mix; dry soup powder; and stock cube concentrate) (South African Department of Health, 1972). The sodium targets were introduced in two phases, with further reductions in sodium levels required by June 2019. Modelling of salt reductions achieved in bread, margarine, stock cubes and seasoning in a randomised clinical trial conducted in South African hypertensives (Charlton et al., 2008) was predicted to decrease salt intake by 0.85 g per person per day; and thereby reduce annual CVD deaths by 11% (Bertram et al., 2012).
The effectiveness of legislative approaches to population salt reduction has not yet been widely demonstrated (Charlton et al., 2014), and mechanisms to monitor and enforce such legislation remain challenging (Cappuccio and Capewell, 2015, He et al., 2014, Webster et al., 2014). A pre-mid impact evaluation was undertaken to assess the effectiveness of the legislative approach adopted in South Africa. The primary aim of this study was to determine whether population level sodium intake and blood pressure (BP) reduced in South Africa using 24hr urinary sodium (Na) collections from a nested sample in the nationally representative WHO Study on global AGEing and adult health (WHO-SAGE) in Wave 2 (Pre: 2015) and again in Wave 3 (Post: 2017/18), according to age (young, 18 − 49y; older 50 + y).
2. Methods
2.1. Study population
WHO-SAGE is a multinational longitudinal study examining the health and well-being of adult populations and the ageing process in China, Ghana, India, Mexico, Russia and South Africa (Kowal et al., 2012) (See http://www.who. int/healthinfo/sage/cohorts/en/). Evaluation of the health effects of South Africa’s sodium policy on adults is conducted using a nested study design in Waves 2 and 3.
In Wave 1 (W1; 2007–2010), 4,223 respondents were recruited in South Africa (9% 18–49 years; 40% 50–59 years; 51% 60 + years) using probability sampled enumeration areas (EAs) according to a multistage cluster sampling strategy, with stratification by province, residence and race. (Kowal et al., 2012) The Wave 2 (W2; 2015) sampling strategy was designed to account for expected attrition as a result of participants having moved house or died since W1. Replacements for sample attrition used a systematic sampling approach to randomly select new households using EA aerial photographic maps. The sampling method used in SAGE Wave 3 (W3; 2018–2019) adopted the same follow-up and random systematic sampling method as in W2. The replacement method was the same as for W2, the intention being to include 8 households per EA, at least 1 of which should comprise 1 or more adults aged < 50 years.
2.2. Selection and data collection in the nested cohort
The SAGE South Africa main survey sample in W2 included 2,971 individuals whose systolic (SBP) and diastolic BP (DBP) were measured. Of these, a random subsample of n = 1,200 was targeted for urine collection, as described in the study protocol (Charlton et al., 2016). Adults aged 18 + years were eligible for inclusion in the sub-study, with the final distribution in the main and nested studies reflecting the weighting towards recruiting more adults aged 50 + years. The nested study respondents were sampled from among the first W2 households visited within each probability sampled EA (day 1 in the EA) in order to prioritise the freight of all collected urine samples to a central laboratory (Global Clinical and Viral Laboratory, Durban) within 3 days of collection while maintaining a cold chain regardless of where urine collection took place. Twenty survey teams, comprising one nurse and three interviewers per team, simultaneously collected data and urine from respondents across all provinces in the country over a 5-month period (August to December 2015). W3 fieldwork was conducted by 8 teams in the northern provinces from August to December 2018 and by 4 teams in the south of the country from October 2018 to March 2019.
All survey teams were trained with support from WHO Geneva, with survey teams using standardised household, individual and proxy questionnaires, anthropometry, blood sampling, BP and physical function tests as described previously in SAGE W1. Interviewers were fluent in the respondents’ home languages with consent forms available in the most widely spoken languages for each area. Wrist-worn Omron BP devices (R6) were used to record three sequential measures on the left arm (1 min between each measure), with positional sensors ensuring that the measurement was taken at the level of the heart while the respondent was seated with legs uncrossed. These wrist BP devices are validated to the European Society of Hypertension International Protocol. Valid BP was defined if: SBP and DBP were plausible; and if SBP > DBP with a pulse pressure ≥ 13 mmHg. Minimum acceptable pulse pressure was based on frequency distributions reported by other population studies (Chung et al., 2010) (Nakano et al., 2005). Implausible systolic and diastolic pressures were set using experience and expert consultation and participants with implausible values (defined as SBP < 80 mmHg or SBP > 270 mmHg; or DBP < 40 mmHg or DBP > 180 mmHg) were dropped from the data analysis. This follows the same approach as the NCD-RISC group and others (Cheng et al., 2016) (Collaboration NCDRF, 1975, Hermann et al., 2016) (Arku et al., 2018), with no consistent definition of plausible values within or across populations or age ranges.
The mean of the second and third readings were used to generate the final BP value. Pulse Pressure (PP) was defined as average SBP minus average DBP, while Mean Arterial Pressure (MAP) as (systolic + 2*diastolic)/3. Hypertension was determined by measured blood pressure (SBP ≥ 140 and/or DBP ≥ 90 mmHg) or previous diagnosis and on antihypertensive medication in the last two weeks.
2.3. Urine collection and analysis in the nested study (Waves 2 and 3)
Inclusion criteria for urine collection were: no urinary incontinence or other condition that could impede 24-hour urine collection; and if female, not menstruating, pregnant or breast feeding on the day of collection. In W3, attempts were made to reach all respondents who had provided urine samples in W2, with procedures as described earlier for replacement and refreshment of the sample. Collection of 24-hour urine samples was conducted according to the WHO/Pan American Health Organization (PAHO) guidelines (WHO/PAHO, 2010). After excluding the first pass urine on day 1, all urine passed over the next 24 h was collected up to, and including, the first urine of the following morning (day 2), in a 5 L plastic container that included 1 g thymol as preservative. Thymol is easier and safer to transport than commonly used liquid acids, and does not cause changes in urinary creatinine, sodium and potassium concentrations for up to 5 days after collection (Nicar et al., 1987). Incomplete 24-hour urine collections were assumed if: total volume < 300 mL; or creatinine excretion < 4 mmol/day (women) or < 6 mmol/day (men) (Stolarz-Skrzypek et al., 2011). Para-amino benzoic acid (PABA) was not used to validate 24-hour urine collection completeness due to its declining recovery rate with age in respondents older than 30 years (Jakobsen et al., 2003) and the additional burden of remembering to take the PABA pill 3 days before the urine collection, as discussed in the WHO/PAHO guidelines (WHO/PAHO, 2010).
Sodium and potassium were determined using the indirect ion-selective electrode method and creatinine analysed using the standardised urinary Jaffe kinetic method (Beckman Coulter Synchron DXC600/800 System). The WHO population target for salt intake is 5 g salt (NaCl) per day, equivalent to urinary sodium excretion of 85 mmol/24 h. Urinary potassium is recommended to be > 90 mmol/24 h, with a sodium-to-potassium ratio ≤ 1 shown to be protective for all-cause, cardiovascular and ischaemic heart disease mortality (WHO, 2012aa, WHO, 2012bb). Discretionary salt intake was assessed using a validated salt behaviour questionnaire (Menyanu et al., 2017) included in the main survey sample.
2.4. Data capture and statistical analysis
All questionnaire data were captured using an electronic Computer Assisted Personal Interviews (CAPI) data capture system and uploaded to a secure central server. Cleaning and analysis of survey data was coordinated by WHO. Quantitative data are presented as medians and interquartile range (1st and 3rd quartiles), while categorical data as absolute numbers and percentages. Differences between W2 and W3 quantitative data were assessed by Mann-Whitney test when comparing independent subjects, while by Wilcoxon signed-rank test when comparing paired (follow-up) subjects. Differences in categorical data were inspected by Pearson Chi-Square test or Fisher Exact test when appropriate. Correlation coefficients were calculated between change in urinary sodium excretion and BP measures. Multivariable regression analyses were performed in order to assess predictors of change in sodium and potassium and the BP profiles in the follow-up sample, taking into account age, sex, BMI, ethnicity and location (urban/rural). Analogue multivariable regression analyses were performed on the independent samples, but given the higher sample size, the inclusion of additional socio-demographic (marital status and education level), health risk factors (alcohol use, smoking status, waist-hip ratio) and urine measurements (sodium, potassium, creatinine and iodine) was possible and these were included in the multivariable regression analysis. Changes in urinary sodium and potassium were also analysed in this way. All statistical analyses were performed using STATA SE, version 15.1 (Stata Statistical Software: Release 15. College Station, TX: StataCorp LP).
SAGE was approved by the WHO Ethics Review Committee (reference number RPC149) with local approval for the South African salt sub study from the North-West University Human Research Ethics Committee and University of the Witwatersrand Human Research Ethics Committee. All respondents provided written informed consent prior to taking part in the study. The study complied with the ethical principles for medical research involving human participants as per the Declaration of Helsinki (General Assembly of the World Medical Association., 2014).
3. Results
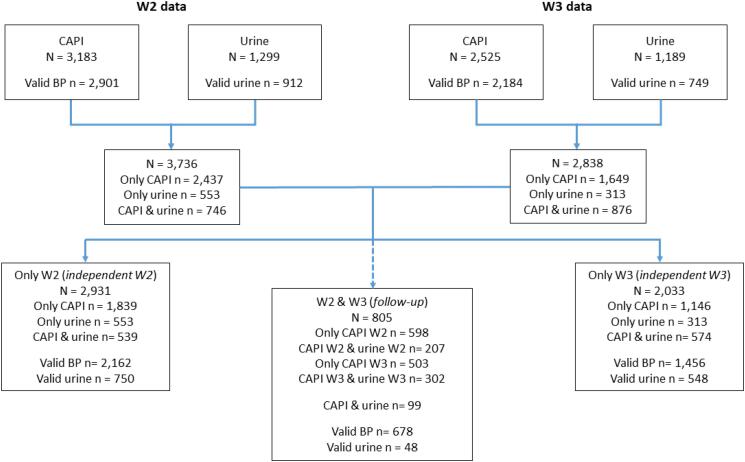
In W2, questionnaire data (CAPI) was collected from 3,183 respondents, while urine samples were provided by n = 1,299 subjects (n = 912 were considered as valid urine samples). CAPI and urine identifiers were matched in n = 746 subjects (n = 570 of these were considered to be valid urine collections). In W3, CAPI data was collected from 2,525 respondents, while n = 1,189 provided urine samples (n = 749 were considered as valid urine samples). CAPI and urine identifiers were matched in n = 876 subjects (n = 593 of these were considered to be valid urine collections). The 24 h urine collection was a nested sub-study within the larger WHO-SAGE endeavour. While the study questions and measurements (including salt intake behaviour questions and BP measurements) were asked of all respondents – the sample size calculations for urine sample collections were done independently as nested sub-sample. The main reasons for an inability to match survey and laboratory urine results were due to failures in the CAPI programming. The digital data collection platform for interviews were meant to be available to the laboratory team, with paper-based back-ups for the urine sample collections. However, the failures in the CAPI system were found only after pursuing the CAPI programmer and after the data collection was completed. Matching via paper records was checked in triplicate by the laboratory and two members of the research team to try locate any unmatched records. Because of CAPI errors, data from W2 matched with that collected in W3, producing two type of samples: independent samples (subjects present in W2 but not in W3, and vice versa) and follow-up sample (individuals present in both waves). Fig. 1 shows participant selection for the study.
Fig. 1.
Flowchart of recruitment and data available from Waves 2 and 3: Urine samples, blood pressure and questionnaire (CAPI) data.
The demographic profile, BP and urine measurements for Waves 2 and 3 are shown for individuals with valid urine in the independent samples for the total sample (Table 1) and for age groups 18 −49y (Table 2), and 50 + y (Table 3). Overall, median salt intake was 6.3 (4.2, 9.5) g salt/day in W2 and 6.2 (4.1, 9.2) g salt/day in W3 (P = 0.266), with proportion of individuals meeting the salt target of < 5 g/day remaining similar (35.8 vs 36.1%, respectively; P = 0.897).
Table 1.
Demographic profile and urine results for individuals with valid urine* regardless of CAPI data: W2 vs W3 independent samples.
| only W2 |
only W3 |
||
|---|---|---|---|
| (N = 750) | (N = 548) | p-value | |
| Age (years) | <0.001 | ||
| Median (Q1, Q3) | 54.0 (41.0, 65.0) | 59.0 (53.0, 67.0) | |
| N (% Missing) | 741 (1.2%) | 547 (0.2%) | |
| Age group | <0.001 | ||
| 18–49 | 274 (37.0%) | 92 (16.8%) | |
| 50+ | 467 (63.0%) | 455 (83.2%) | |
| Sex | 0.033 | ||
| male | 177 (23.6%) | 158 (28.8%) | |
| female | 573 (76.4%) | 390 (71.2%) | |
| Ethnicity | <0.001 | ||
| African/black | 277 (68.1%) | 273 (69.8%) | |
| White | 10 (2.5%) | 14 (3.6%) | |
| Coloured | 78 (19.2%) | 93 (23.8%) | |
| Indian/Asian | 42 (10.3%) | 11 (2.8%) | |
| Location | 0.003 | ||
| urban | 298 (73.2%) | 249 (63.5%) | |
| rural | 109 (26.8%) | 143 (36.5%) | |
| Marital status | 0.002 | ||
| never married | 181 (44.5%) | 127 (32.4%) | |
| married/cohabiting | 113 (27.8%) | 124 (31.6%) | |
| separate/divorced | 18 (4.4%) | 32 (8.2%) | |
| widowed | 95 (23.3%) | 109 (27.8%) | |
| Ever school | 0.001 | ||
| yes | 337 (82.8%) | 355 (90.6%) | |
| no | 70 (17.2%) | 37 (9.4%) | |
| Education level | 0.424 | ||
| low | 253 (75.1%) | 257 (72.4%) | |
| high | 84 (24.9%) | 98 (27.6%) | |
| Alcohol | <0.001 | ||
| yes | 69 (17.1%) | 110 (28.1%) | |
| no, never | 335 (82.9%) | 282 (71.9%) | |
| Smoking | 0.004 | ||
| no | 365 (90.3%) | 327 (83.4%) | |
| yes | 39 (9.7%) | 65 (16.6%) | |
| Waist to Height ratio < 0.5 | |||
| no | 215 (73.6%) | 266 (77.6%) | 0.251 |
| yes | 77 (26.4%) | 77 (22.4%) | |
| Body Mass Index (kg/m2) | |||
| Median (Q1, Q3) | 29.3 (24.6, 35.3) | 29.6 (25.0, 35.5) | 0.401 |
| N (% Missing) | 302 (59.7%) | 364 (33.6%) | |
| Systolic BP (mm Hg) | |||
| Median (Q1, Q3) | 128.0 (118.0, 141.5) | 131.5 (119.5, 150.0) | 0.012 |
| N (% Missing) | 386 (48.5%) | 371 (32.3%) | |
| Diastolic BP (mm Hg) | <0.001 | ||
| Median (Q1, Q3) | 79.0 (71.5, 86.5) | 83.5 (72.5, 95.0) | |
| N (% Missing) | 386 (48.5%) | 371 (32.3%) | |
| Hypertension from measurement | 0.001 | ||
| no | 253 (65.5%) | 200 (53.9%) | |
| yes | 133 (34.5%) | 171 (46.1%) | |
| Hypertension Self-reported | <0.001 | ||
| no | 289 (77.9%) | 256 (65.5%) | |
| yes | 82 (22.1%) | 135 (34.5%) | |
| Hypertension BP/SR/med | <0.001 | ||
| no | 195 (53.1%) | 144 (38.1%) | |
| yes | 172 (46.9%) | 234 (61.9%) | |
| Sodium, mmol/24hr | 0.266 | ||
| Median (Q1, Q3) | 106.6 (71.2, 160.1) | 105.5 (68.8, 155.0) | |
| N (% Missing) | 749 (0.1%) | 548 (0.0%) | |
| Calculated salt excretion, g/day | 0.266 | ||
| Median (Q1, Q3) | 6.3 (4.2, 9.5) | 6.2 (4.1, 9.2) | |
| N (% Missing) | 749 (0.1%) | 548 (0.0%) | |
| Achieving salt target (<5g/day) | 0.897 | ||
| <5g/day | 268 (35.8%) | 198 (36.1%) | |
| >=5g/day | 481 (64.2%) | 350 (63.9%) | |
| High salt intake (≥10 g/day) | 0.526 | ||
| <10 g/day | 580 (77.5%) | 433 (79.0%) | |
| >=10 g/day | 168 (22.5%) | 115 (21.0%) | |
| Potassium (K), mmol/24hr | 0.138 | ||
| Median (Q1, Q3) | 30.8 (19.5, 52.7) | 30.6 (20.1, 43.2) | |
| N (% Missing) | 750 (0.0%) | 548 (0.0%) | |
| Achieving K target (≥90 mmol/day) | <0.001 | ||
| <90 mmoll/24 h | 689 (91.9%) | 531 (96.9%) | |
| >=90 mmoll/24 h | 61 (8.1%) | 17 (3.1%) | |
| Sodium-to-potassium ratio | 0.953 | ||
| Median (Q1, Q3) | 3.5 (2.4, 4.6) | 3.4 (2.4, 4.7) | |
| N (% Missing) | 749 (0.1%) | 548 (0.0%) | |
| Achieving Na:K ratio (≤1.0) | 0.214 | ||
| <=1 | 20 (2.7%) | 9 (1.6%) | |
| >1 | 727 (97.3%) | 539 (98.4%) | |
| Creatinine, mmol/24 h | <0.001 | ||
| Median (Q1, Q3) | 9.4 (6.7, 14.6) | 8.5 (6.2, 12.2) | |
| N (% Missing) | 750 (0.0%) | 548 (0.0%) | |
| Iodine, µg/l | 0.685 | ||
| Median (Q1, Q3) | 94.1 (52.1, 162.2) | 93.8 (52.9, 162.4) | |
| N (% Missing) | 468 (37.6%) | 483 (11.9%) |
Data are presented as median (Q1:1st quartile, Q3:3rd quartile) and number of valid cases (N) for quantitative data and as absolute number (%) for categorical data. P-value obtained by: Mann-Whitney test when comparing medians, while Pearson Chi-Square test or Fisher Exact test when comparing categorical data. *Valid urine defined as: volume ≥ 300 mL and creatinine excretion ≥ 4 mmol/day (women) or ≥ 6 mmol/day (men). The CAPI data could not always be matched with the urine data thus there is missing data for some variables. BP: Blood Pressure, SR: Self-Reported, med: on medication for hypertension in the last two weeks.
Table 2.
Demographic profile and urine results for individuals 18–49 years with valid urine* regardless of CAPI data: W2 vs W3 independent samples.
| only W2 |
only W3 |
||
|---|---|---|---|
| (N = 274) | (N = 92) | p-value | |
| Age (years) | 0.385 | ||
| Median (Q1, Q3) | 35.0 (26.0, 43.0) | 37.0 (27.5, 44.0) | |
| N (% Missing) | 274 (0.0%) | 92 (0.0%) | |
| Sex | 0.045 | ||
| male | 74 (27.0%) | 35 (38.0%) | |
| female | 200 (73.0%) | 57 (62.0%) | |
| Ethnicity | 0.288 | ||
| African/black | 119 (79.9%) | 51 (78.5%) | |
| White | 0 (0.0%) | 1 (1.5%) | |
| Coloured | 23 (15.4%) | 12 (18.5%) | |
| Indian/asian | 7 (4.7%) | 1 (1.5%) | |
| Location | 0.428 | ||
| urban | 103 (69.1%) | 42 (63.6%) | |
| rural | 46 (30.9%) | 24 (36.4%) | |
| Marital status | 0.105 | ||
| never married | 117 (78.5%) | 44 (66.7%) | |
| married/cohabiting | 28 (18.8%) | 18 (27.3%) | |
| separate/divorced | 1 (0.7%) | 3 (4.5%) | |
| widowed | 3 (2.0%) | 1 (1.5%) | |
| Ever school | 0.453 | ||
| yes | 141 (94.6%) | 64 (97.0%) | |
| no | 8 (5.4%) | 2 (3.0%) | |
| Education level | 0.974 | ||
| low | 90 (63.8%) | 41 (64.1%) | |
| high | 51 (36.2%) | 23 (35.9%) | |
| Alcohol | 0.083 | ||
| yes | 33 (22.1%) | 22 (33.3%) | |
| no, never | 116 (77.9%) | 44 (66.7%) | |
| Smoking | 0.023 | ||
| no | 136 (91.3%) | 53 (80.3%) | |
| yes | 13 (8.7%) | 13 (19.7%) | |
| Waist to Height ratio < 0.5 | 0.650 | ||
| no | 68 (63.6%) | 36 (60.0%) | |
| yes | 39 (36.4%) | 24 (40.0%) | |
| Body Mass Index (kg/m2) | 0.855 | ||
| Median (Q1, Q3) | 28.0 (23.5, 33.9) | 28.2 (24.2, 34.2) | |
| N (% Missing) | 112 (59.1%) | 62 (32.6%) | |
| Systolic BP (mm Hg) | 0.458 | ||
| Median (Q1, Q3) | 122.5 (112.5, 134.0) | 120.5 (111.0, 131.0) | |
| N (% Missing) | 141 (48.5%) | 61 (33.7%) | |
| Diastolic BP (mm Hg) | 0.839 | ||
| Median (Q1, Q3) | 77.0 (71.0, 83.5) | 77.0 (70.5, 86.5) | |
| N (% Missing) | 141 (48.5%) | 61 (33.7%) | |
| Hypertension from measurement | 0.770 | ||
| no | 109 (77.3%) | 46 (75.4%) | |
| yes | 32 (22.7%) | 15 (24.6%) | |
| Hypertension Self-reported | 0.672 | ||
| no | 135 (91.2%) | 59 (89.4%) | |
| yes | 13 (8.8%) | 7 (10.6%) | |
| Hypertension BP/SR/med | 0.358 | ||
| no | 105 (74.5%) | 43 (68.3%) | |
| yes | 36 (25.5%) | 20 (31.7%) | |
| Sodium, mmol/24hr | 0.569 | ||
| Median (Q1, Q3) | 131.2 (79.8, 202.3) | 129.5 (82.0, 190.0) | |
| N (% Missing) | 274 (0.0%) | 92 (0.0%) | |
| Calculated salt excretion, g/day | 0.569 | ||
| Median (Q1, Q3) | 7.8 (4.7, 12.0) | 7.7 (4.9, 11.3) | |
| N (% Missing) | 274 (0.0%) | 92 (0.0%) | |
| Achieving salt target (<5g/day) | 0.660 | ||
| <5g/day | 78 (28.5%) | 24 (26.1%) | |
| >=5g/day | 196 (71.5%) | 68 (73.9%) | |
| High salt intake (≥10 g/day) | 0.799 | ||
| <10 g/day | 182 (66.7%) | 60 (65.2%) | |
| >=10 g/day | 91 (33.3%) | 32 (34.8%) | |
| Potassium (K), mmol/24hr | 0.124 | ||
| Median (Q1, Q3) | 36.0 (21.8, 63.8) | 35.3 (19.3, 47.7) | |
| N (% Missing) | 274 (0.0%) | 92 (0.0%) | |
| Achieving K target (≥90 mmol/day) | 0.006 | ||
| <90 mmoll/24 h | 232 (84.7%) | 88 (95.7%) | |
| >=90 mmoll/24 h | 42 (15.3%) | 4 (4.3%) | |
| Sodium-to-potassium ratio | 0.476 | ||
| Median (Q1, Q3) | 3.7 (2.6, 4.8) | 3.8 (2.9, 4.9) | |
| N (% Missing) | 274 (0.0%) | 92 (0.0%) | |
| Achieving Na:K ratio (≤1.0) | 0.723 | ||
| <=1 | 7 (2.6%) | 3 (3.3%) | |
| >1 | 266 (97.4%) | 89 (96.7%) | |
| Creatinine, mmol/24 h | 0.048 | ||
| Median (Q1, Q3) | 11.9 (7.7, 18.8) | 10.6 (7.5, 14.0) | |
| N (% Missing) | 274 (0.0%) | 92 (0.0%) | |
| Iodine, µg/l | 0.230 | ||
| Median (Q1, Q3) | 90.1 (48.0, 173.0) | 98.7 (57.0, 198.8) | |
| N (% Missing) | 166 (39.4%) | 82 (10.9%) |
Data are presented as median (Q1:1st quartile, Q3:3rd quartile) and number of valid cases (N) for quantitative data and as absolute number (%) for categorical data. P-value obtained by: Mann-Whitney test when comparing medians, while Pearson Chi-Square test or Fisher Exact test when comparing categorical data. *Valid urine defined as: volume ≥ 300 mL and creatinine excretion ≥ 4 mmol/day (women) or ≥ 6 mmol/day (men). The CAPI data could not always be matched with the urine data thus there is missing data for some variables. BP: Blood Pressure, SR: Self-Reported, med: on medication for hypertension in the last two weeks
Table 3.
Demographic profile and urine results for individuals 50 years or older with valid urine* regardless of CAPI data: W2 vs W3 independent samples.
| only W2 |
only W3 |
||
|---|---|---|---|
| (N = 467) | (N = 455) | p-value | |
| Age (years) | 0.947 | ||
| Median (Q1, Q3) | 62.0 (55.0, 71.0) | 62.0 (56.0, 69.0) | |
| N (% Missing) | 467 (0.0%) | 455 (0.0%) | |
| Sex | 0.079 | ||
| male | 103 (22.1%) | 123 (27.0%) | |
| female | 364 (77.9%) | 332 (73.0%) | |
| Ethnicity | <0.001 | ||
| African/black | 158 (61.2%) | 222 (68.1%) | |
| White | 10 (3.9%) | 13 (4.0%) | |
| Coloured | 55 (21.3%) | 81 (24.8%) | |
| Indian/asian | 35 (13.6%) | 10 (3.1%) | |
| Location | 0.002 | ||
| urban | 195 (75.6%) | 207 (63.5%) | |
| rural | 63 (24.4%) | 119 (36.5%) | |
| Marital status | 0.735 | ||
| never married | 64 (24.8%) | 83 (25.5%) | |
| married/cohabiting | 85 (32.9%) | 106 (32.5%) | |
| separate/divorced | 17 (6.6%) | 29 (8.9%) | |
| widowed | 92 (35.7%) | 108 (33.1%) | |
| Ever school | <0.001 | ||
| yes | 196 (76.0%) | 291 (89.3%) | |
| no | 62 (24.0%) | 35 (10.7%) | |
| Education level | 0.020 | ||
| low | 163 (83.2%) | 216 (74.2%) | |
| high | 33 (16.8%) | 75 (25.8%) | |
| Alcohol | <0.001 | ||
| yes | 36 (14.1%) | 88 (27.0%) | |
| no, never | 219 (85.9%) | 238 (73.0%) | |
| Smoking | 0.043 | ||
| no | 229 (89.8%) | 274 (84.0%) | |
| yes | 26 (10.2%) | 52 (16.0%) | |
| Waist to Height ratio < 0.5 | 0.628 | ||
| no | 147 (79.5%) | 230 (81.3%) | |
| yes | 38 (20.5%) | 53 (18.7%) | |
| Body Mass Index (kg/m2) | 0.769 | ||
| Median (Q1, Q3) | 30.1 (25.4, 35.9) | 29.9 (25.3, 35.8) | |
| N (% Missing) | 190 (59.3%) | 302 (33.6%) | |
| Systolic BP (mm Hg) | 0.119 | ||
| Median (Q1, Q3) | 133.5 (121.5, 144.5) | 135.5 (122.0, 153.5) | |
| N (% Missing) | 245 (47.5%) | 310 (31.9%) | |
| Diastolic BP (mm Hg) | 0.001 | ||
| Median (Q1, Q3) | 80.5 (72.0, 88.5) | 84.0 (73.5, 97.0) | |
| N (% Missing) | 245 (47.5%) | 310 (31.9%) | |
| Hypertension from measurement | 0.033 | ||
| no | 144 (58.8%) | 154 (49.7%) | |
| yes | 101 (41.2%) | 156 (50.3%) | |
| Hypertension Self-reported | 0.043 | ||
| no | 154 (69.1%) | 197 (60.6%) | |
| yes | 69 (30.9%) | 128 (39.4%) | |
| Hypertension BP/SR/med | 0.063 | ||
| no | 90 (39.8%) | 101 (32.1%) | |
| yes | 136 (60.2%) | 214 (67.9%) | |
| Sodium, mmol/24hr | 0.721 | ||
| Median (Q1, Q3) | 97.8 (68.1, 143.6) | 101.0 (68.0, 144.9) | |
| N (% Missing) | 466 (0.2%) | 455 (0.0%) | |
| Calculated salt excretion, g/day | 0.721 | ||
| Median (Q1, Q3) | 5.8 (4.0, 8.5) | 6.0 (4.0, 8.6) | |
| N (% Missing) | 466 (0.2%) | 455 (0.0%) | |
| Achieving salt target (<5g/day) | 0.650 | ||
| <5g/day | 185 (39.7%) | 174 (38.2%) | |
| >=5g/day | 281 (60.3%) | 281 (61.8%) | |
| High salt intake (≥10 g/day) | 0.547 | ||
| <10 g/day | 389 (83.5%) | 373 (82.0%) | |
| >=10 g/day | 77 (16.5%) | 82 (18.0%) | |
| Potassium (K), mmol/24hr | 0.875 | ||
| Median (Q1, Q3) | 29.6 (19.0, 45.9) | 30.0 (20.2, 41.5) | |
| N (% Missing) | 467 (0.0%) | 455 (0.0%) | |
| Achieving K target (≥90 mmol/day) | 0.315 | ||
| <90 mmoll/24 h | 448 (95.9%) | 442 (97.1%) | |
| >=90 mmoll/24 h | 19 (4.1%) | 13 (2.9%) | |
| Sodium-to-potassium ratio | 0.676 | ||
| Median (Q1, Q3) | 3.3 (2.3, 4.5) | 3.3 (2.3, 4.6) | |
| N (% Missing) | 466 (0.2%) | 455 (0.0%) | |
| Achieving Na:K ratio (≤1.0) | 0.115 | ||
| <=1 | 13 (2.8%) | 6 (1.3%) | |
| >1 | 452 (97.2%) | 449 (98.7%) | |
| Creatinine, mmol/24 h | 0.057 | ||
| Median (Q1, Q3) | 8.5 (6.3, 13.0) | 8.2 (6.1, 11.6) | |
| N (% Missing) | 467 (0.0%) | 455 (0.0%) | |
| Iodine, µg/l | 0.942 | ||
| Median (Q1, Q3) | 96.3 (53.9, 160.9) | 93.8 (52.9, 159.0) | |
| N (% Missing) | 293 (37.3%) | 400 (12.1%) |
Data are presented as median (Q1:1st quartile, Q3:3rd quartile) and number of valid cases (N) for quantitative data and as absolute number (%) for categorical data. P-value obtained by: Mann-Whitney test when comparing medians, while Pearson Chi-Square test or Fisher Exact test when comparing categorical data. *Valid urine defined as: volume ≥ 300 mL and creatinine excretion ≥ 4 mmol/day (women) or ≥ 6 mmol/day (men). The CAPI data could not always be matched with the urine data thus there is missing data for some variables. BP: Blood Pressure, SR: Self-Reported, med: on medication for hypertension in the last two weeks
Younger people had substantially higher salt intakes than older adults in both waves (P < 0.001). In those aged 18 − 49y, median salt intake was estimated to be 7.8 (4.7, 12.0) g/day in W2, remaining the same in the W3 sample (7.7 (4.9, 11.3) g salt/day; P = 0.569) (Table 2). The proportion meeting the salt target of < 5 g/day remained similar (28.5 vs 26.1%, respectively; P = 0.660).
In older adults (50 + y), median salt intake was 5.8 (4.0, 8.5) g/day in W2, and 6.0 (4.0, 8.6) g/day in W3 (P = 0.721) (Table 3). The proportion meeting the salt target of < 5 g/day remained similar (39.7 vs 38.2%, respectively; P = 0.650).
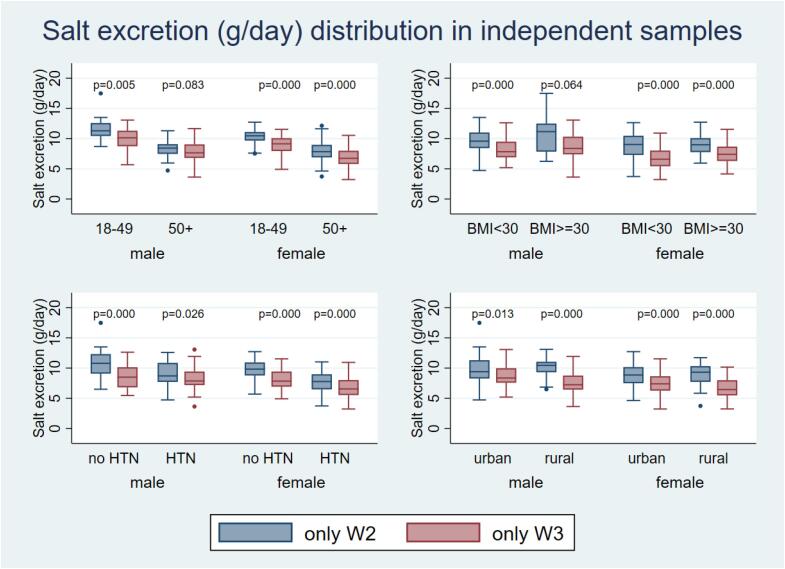
Salt intake in the independent samples differed between W2 and W3 for men and women, once adjusted for covariates, according to age groups (except for men aged 50 + y; P = 0.083), BMI categories (except for men with BMI ≥ 30; P = 0.064), and hypertensive status, as shown in Fig. 2.
Fig. 2.
Salt intake between Wave 2 and Wave 3, according to age, sex, obesity and hypertension status. Box plots represent median and IQR. Independent samples with valid 24hr urine collections: Wave 2 (n = 750, blue) and Wave 3 (n = 548, red). P-values are from Mann–Whitney tests for differences between the waves. Analyses were adjusted for: age, ethnicity, location, marital status, education level, alcohol use, smoking status, waist-hip ratio, hypertension status at W3, SBP and DBP except if the variable was the categorizing factor. BMI: Body Mass Index; HTN: hypertensive; no HTN: not hypertensive. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In both waves (Table 1), median urinary potassium excretion remained very low (30.8 (19.5, 52.7) vs 30.6 (20.1, 43.2) mmol/day in W2 and W3, respectively; P = 0.138). In W3, 97% of respondents had urinary concentrations less than the recommended potassium intake of 90 mmol/day and urinary molar sodium:potassium ratio (Na:K) of 3.5 (2.4, 4.6) vs 3.4 (2.4, 4.7) for W2 and W3, respectively (P = 0.963). Only 1.6% were achieving a Na:K ratio of 1 or below in W3, which is considered to be protective for all-cause, cardiovascular and ischaemic heart disease mortality. (WHO, 2012aa, WHO, 2012bb).
Of the same 805 subjects from W2 that also were followed up in W3, only 48 had valid urine measurements, based on volume and creatinine reference cut-offs (See Table 4) and had accompanying CAPI data. In this under-powered sub-sample, median salt intake was 6.7 (4.3) g/day in W2 and 6.1 (5.4) g/day) in W3 (P = 0.6444). The percentage meeting the < 5 g/day salt target increased from 27.1% to 33.3%, but again lacked statistical significance (P = 0.735). Potassium excretion remained similarly low over time (32.8 (26.2) vs 29.7 (15.2) mmol/day; P = 0.6371).
Table 4.
Demographic profile and urine results for individuals with valid urine*: W2 vs W3 follow-up sample.
| W2 | W3 (N = 48) | p-value | |
|---|---|---|---|
| (N = 48) | |||
| Age (years) | 57.0 (51.0, 64.5) | 61.0 (53.0, 69.5) | <0.0001 |
| Median (Q1, Q3) | 48 (0.0%) | 48 (0.0%) | |
| N (% Missing) | |||
| Sex | 10 (20.8%) | ||
| male | 38 (79.2%) | ||
| female | |||
| Ethnicity | 40 (83.3%) | ||
| African/black | 1 (2.1%) | ||
| White | 7 (14.6%) | ||
| Coloured | 0 (0.0%) | ||
| Indian/Asian | |||
| Location | 26 (54.2%) | ||
| urban | 22 (45.8%) | ||
| rural | |||
| Marital status | 19 (39.6%) | 12 (25.0%) | <0.0001§ |
| never married | 13 (27.1%) | 18 (37.5%) | |
| married/cohabiting | 3 (6.3%) | 5 (10.4%) | |
| separate/divorced | 13 (27.1%) | 13 (27.1%) | |
| widowed | |||
| Ever school | 32 (66.7%) | 40 (83.3%) | 0.012§ |
| yes | 16 (33.3%) | 8 (16.7%) | |
| no | |||
| Alcohol | 3 (6.3%) | 12 (25.0%) | 0.587 |
| yes | 45 (93.8%) | 36 (75.0%) | |
| no, never | |||
| Smoking | 42 (87.5%) | 41 (85.4%) | 0.206§ |
| no | 6 (12.5%) | 7 (14.6%) | |
| yes | |||
| Waist to Height ratio < 0.5 | 29 (82.9%) | 37 (84.1%) | 0.310§ |
| no | 6 (17.1%) | 7 (15.9%) | |
| yes | |||
| Body Mass Index (kg/m2) | 29.7 (24.4, 33.0) | 30.9 (26.0, 37.6) | 0.1392 |
| Median (Q1, Q3) | 36 (25.0%) | 45 (6.3%) | |
| N (% Missing) | |||
| Systolic BP (mm Hg) | 135.3 (123.0, 151.5) | 132.5 (126.0, 147.0) | 0.2232 |
| Median (Q1, Q3) | 44 (8.3%) | 45 (6.3%) | |
| N (% Missing) | |||
| Diastolic BP (mm Hg) | 85.0 (75.8, 96.8) | 83.5 (77.5, 97.0) | 0.6455 |
| Median (Q1, Q3) | 44 (8.3%) | 45 (6.3%) | |
| N (% Missing) | |||
| Hypertension from measurement | 20 (45.5%) | 23 (51.1%) | 0.89 |
| no | 24 (54.5%) | 22 (48.9%) | |
| yes | |||
| Hypertension Self-reported | 37 (82.2%) | 24 (50.0%) | 0.022§ |
| no | 8 (17.8%) | 24 (50.0%) | |
| yes | |||
| Hypertension BP/SR/med | 15 (34.1%) | 14 (29.8%) | 0.162§ |
| no | 29 (65.9%) | 33 (70.2%) | |
| yes | |||
| Sodium, mmol/24hr | 112.5 (78.3, 150.6) | 103.0 (71.0, 161.5) | 0.6444 |
| Median (Q1, Q3) | 48 (0.0%) | 48 (0.0%) | |
| N (% Missing) | |||
| Calculated salt excretion, g/day | 6.7 (4.6, 8.9) | 6.1 (4.2, 9.6) | 0.6444 |
| Median (Q1, Q3) | 48 (0.0%) | 48 (0.0%) | |
| N (% Missing) | |||
| Achieving salt target (<5g/day) | 13 (27.1%) | 16 (33.3%) | 0.735§ |
| <5g/day | 35 (72.9%) | 32 (66.7%) | |
| >=5g/day | |||
| High salt intake (≥10 g/day) | 39 (81.3%) | 40 (83.3%) | 0.322§ |
| <10 g/day | 9 (18.8%) | 8 (16.7%) | |
| >=10 g/day | |||
| Potassium (K), mmol/24hr | 32.8 (19.4, 45.6) | 29.6 (22.3, 37.5) | 0.6371 |
| Median (Q1, Q3) | 48 (0.0%) | 48 (0.0%) | |
| N (% Missing) | |||
| Achieving K target (≥90 mmol/day) | 44 (91.7%) | 48 (100.0%) | – |
| <90 mmoll/24 h | 4 (8.3%) | 0 (0.0%) | |
| >=90 mmoll/24 h | |||
| Sodium-to-potassium ratio | 3.5 (2.6, 4.6) | 3.3 (2.5, 4.4) | 0.356 |
| Median (Q1, Q3) | 48 (0.0%) | 48 (0.0%) | |
| N (% Missing) | |||
| Achieving Na:K ratio (≤1.0) | 4 (8.3%) | 2 (4.2%) | – |
| <=1 | 44 (91.7%) | 46 (95.8%) | |
| >1 | |||
| Creatinine, mmol/24 h | 10.3 (7.3, 13.8) | 8.2 (6.4, 10.8) | 0.0619 |
| Median (Q1, Q3) | 48 (0.0%) | 48 (0.0%) | |
| N (% Missing) | |||
| Iodine, µg/l** | 108.9 (63.0, 172.9) | 91.3 (63.2, 202.0) | 0.7317 |
| Median (Q1, Q3) | 26 (45.8%) | 129.1 (63.5, 198.0) 48 (0.0%)*** | |
| N (% Missing) |
Data are presented as median (Q1:1st quartile, Q3:3rd quartile) and number of valid cases (N) for quantitative data and as absolute number (%) for categorical data. P-value obtained by: Wilcoxon signed-rank test when comparing medians, while Pearson Chi-Square test or Fisher Exact test (§) when comparing categorical data. *Valid urine defined as: volume ≥ 300 mL and creatinine excretion ≥ 4 mmol/day (women) or ≥ 6 mmol/day (men). BP: Blood Pressure, SR: Self-Reported, med: on medication for hypertension in the last two weeks. ** Available on n = 26 subjects in W2; *** Calculated on the total n = 48 subjects for W3.
3.1. Change in BP and hypertension over time, and association with salt intake
In W2, for independent samples with CAPI, hypertension assessed by measured BP (≥140/90 mmHg) or previous diagnosis and on medication indicated an overall prevalence of 53% (Supplementary Table 1), rising to 70% for those aged 60-69y and 70-79y. In W3, overall hypertension prevalence was 62% (Supplementary Table 1), rising to 70% for those aged 60-69y and over 80% in 70-79y and 80-89y. A statistically significant increase of 3.5 mmHg in DBP between W2 and W3 was found in the independent samples by multivariable regression analysis when controlling for socio-demographic and risk behaviours (P < 0.0001) (Table 5). This increase remained statistically significant when hypertension status in W3 was added into the model (β = 2.2 mmHg; P < 0.0001), and also when controlling for urinary Na, K, creatinine and iodine (β = 3.3 mmHg; P = 0.017). No statistically significant difference was found for SBP. In the W2 and W3 independent samples, SBP and DBP are shown according to age, sex, BMI and hypertension status in Supplementary Figures 1a and b, respectively.
Table 5.
Multivariate regression analyses for differences in blood pressure and urinary Na and K excretion between W2 and W3 in the independent sample.
| Outcome | β of change from W2 to W3 | Std.Err. | p-value | n sample |
|---|---|---|---|---|
| SBP | 0.94 | 0.92 | 0.309 | 2,331 |
| SBP* | −1.09 | 0.80 | 0.172 | 2,249 |
| SBP** | 0.94 | 1.95 | 0.628 | 443 |
| DBP | 3.54 | 0.65 | 0.0001 | 2,331 |
| DBP* | 2.23 | 0.57 | 0.0001 | 2,249 |
| DBP** | 3.36 | 1.40 | 0.017 | 443 |
| Calculated urinary salt (NaCl) excretion, g/day§ | −1.16 | 0.53 | 0.028 | 522 |
| Urinary potassium (K), mmol/24hr§ | −8.75 | 2.76 | 0.002 | 522 |
All models are adjusted for: age, gender, ethnicity, location, marital status, education level, alcohol use, smoking status, waist-hip ratio. * including hypertension status as adjusting factor. **including hypertension status, sodium, potassium, creatinine and iodine as adjusting factors. § including hypertension status, SBP and DBP as adjusting factors. SBP: systolic blood pressure, DBP: diastolic blood pressure.
A significant decrease in urinary sodium levels was observed in W3, compared to W2 in independent samples with valid urine (n = 522) in multivariable analysis adjusted for socio-demographic variables (age, gender, ethnicity, location, marital status, education level), health risk factors (alcohol use, smoking status, waist-hip ratio) and hypertension status at W3, SBP and DBP) (β=- 1.16 (SE 0.53) g salt/24hr; P = 0.028) (See Table 5). Urinary potassium levels also decreased in W3 when adjusting for these variables (β = 8.75 (SE 2.8) mmol/24hr; P = 0.002).
In subjects that were followed up in the larger CAPI survey sample and had BP measurements at both W2 and W3 (n = 771), there was a significant increase in median DBP (80.5 (16) vs 84 (18) mmHg, respectively (P = <0.001)) (Supplementary Table 3) and a subsequent increase in hypertension prevalence (53.7 vs 65.8%, P = 0.002).
When investigating predictors for change in SBP and DBP between W2 and W3 in the paired sample for urine (n = 48), in univariate analyses, there were positive and significant correlations between the two waves for change in urinary Na and (1) change in urinary K (r = 0.69, P < 0.0001) and (2) change in SBP (r = 0.32, P = 0.039). Change in Na:K ratio was correlated with change in SBP (r = 0.36, P = 0.020). In multivariable regressions that accounted for age, sex, ethnicity, location and BMI, there were no statistically significant predictors for both SBP and DBP, while adding hypertension status at W3 to the models, it resulted as marginally significant for change in DBP (P = 0.083). However the R2 of the models were 0.2160 (SBP) and 0.2465 (DBP), so other predictors are likely. When change in salt intake between W2 and W3 was added as a predictor to the models, BMI, hypertension and change in salt intake were statistically significant for both change in SBP (β = -1.9, P = 0.051; β = -24.9, P = 0.048 and β = 0.12, P = 0.020, respectively) and change in DBP (β = -1.12, P = 0.060; β = -17.2, P = 0.031 and β = 0.06, P = 0.047, respectively). Adding change in salt intake to the models, the R2 increased to 0.3472 and 0.3409 for SBP and DBP, respectively.
3.2. Reported salt use behaviours
In participants that were followed up in both waves (n = 771), there was a significant reduction in W3 in the proportion that reported frequently adding salt to food at the table (25.1% to 15.4%, P = 0.007 (See Table 6) but no change for other salt use behaviours.
Table 6.
Reported salt use behaviours W2 and W3 respondents, all CAPI data.
| All CAPI data | W2 (n = 3,075) | W3 (n = 2,368) | p-value |
|---|---|---|---|
| Frequently add salt to food at table | 684 (22.2) | 416 (17.6) | |
| Frequently add salt to food during cooking | 1,831 (59.5) | 1,540 (65.0) | |
| Believe they consume too much salt | 234 (7.66) | 234 (10.2) | |
| Believe a high salt diet is bad for health | 2,042 (70.5) | 1,749 (82.8) | |
| Regularly control of salt intake | 896 (30.5) | 861 (41.3) | |
| Independent samples | W2 (n = 2,378) | W3 (n = 1,606) | |
| Frequently add salt to food at table | 483 (21.1) | 294 (18.4) | 0.036 |
| Frequently add salt to food during cooking | 1,346 (58.8) | 1,035 (64.6) | <0.0001 |
| Believe they consume too much salt | 161 (7.1) | 166 (10.8) | <0.0001 |
| Believe a high salt diet is bad for health | 1,497 (69.8) | 1,169 (82.4) | <0.0001 |
| Regularly control of salt intake | 628 (28.6) | 596 (42.7) | <0.0001 |
| Follow-up sample | W2 (n = 771) | W3 (n = 771) | |
| Frequently add salt to food at table | 189 (25.1) | 107 (15.4) | 0.007 |
| Frequently add salt to food during cooking | 468 (62.1) | 452 (64.9) | ns (0.650) |
| Believe they consume too much salt | 71 (9.4) | 59 (8.6) | ns (0.541) |
| Believe a high salt diet is bad for health | 526 (72.6) | 530 (84.1) | ns (0.147) |
| Regularly control of salt intake | 256 (35.6) | 241 (38.7) | ns (0.220) |
Data are presented as absolute number (%) and p-value obtained by Pearson Chi-Square.
4. Discussion
This is the first evaluation of the effectiveness of South Africa’s mandatory salt reduction legislation for salt levels permitted in a range of commonly consumed processed foods. Comparison between W2 and W3 for the nested samples included in the WHO-SAGE South Africa cohort study was conducted for salt intake, potassium excretion, and urinary Na:K ratio. For this purpose, two independent samples were compared and when background characteristics were accounted for in multivariable analyses, a reduction in salt intake by 1.16 g per day was evident. Potassium excretion remained low across both waves and reduced significantly by 9 mmol/day. In both W2 (reported elsewhere (Ware et al., 2017) and W3 (data not shown), the ratio of Na:K was more strongly associated, than urinary Na excretion alone, with the slope of SBP and DBP plotted against age. Other authors have reported that urinary sodium-to-potassium ratio may predict CVD mortality better than sodium excretion alone (Cook et al., 2009). In a smaller paired sub-sample that had both valid urine data and survey data in the same participants in both W2 and W3, change in salt intake over time was a significant predictor of increase in SBP thus confirming the importance of reducing salt intake.
Despite our finding that the magnitude of population salt reduction following introduction of South Africa’s mandatory salt reduction programme exceeded the previously modelled estimate of 0.85 g/day (Bertram et al., 2012), the high reported level of salt being added during cooking and the fact that a third of the sample still had salt intakes greater than the target of 5 g per day indicates that a more multi-pronged approach that includes nutrition education, and possibly improved availability of low sodium salt replacements, is required. It is possible that the food industry had already adopted changes in formulation of processed products prior to the June 2016 implementation date for Phase 1 of the salt targets (Republic of South Africa, 2013). The salt reduction legislation had a two phased approach with the first level of targets set for June 2016 and the second, more stringent targets that were implemented in June 2019. The timing of W2 data collection may have occurred following changes having already been made to the food supply. This is supported by an analysis of food label information collected using the FoodSwitch app in South Africa around the time of W2 (Peters et al., 2017). It is also supported by previously higher estimates of salt intake reported from repeated 24hr urinary Na collections in a survey of South Africans in 2005. In that study, salt intakes were much higher than those reported by W2, namely values equating to a daily salt intake of 7.8, 8.5 and 9.5 g in black, mixed-ancestry and white individuals, respectively (Charlton et al., 2005). Population weighted estimates of salt intake based on that data was 8.1 g/day (Bertram et al., 2012). Similarly, in 2016, Swanepoel and colleagues reported a median salt intake of 7.2 g/d for a multi-ethnic sample of South Africans (Swanepoel et al., 2016). Considering these other sources of information on salt intake, our data suggests that there had already been shifts in salt intake by the time of W2 data collection. In Ghana, a comparative sub Saharan African country that does not have a salt reduction policy, salt intake is estimated to be much higher than in South Africa from data collected in WHO-SAGE W3 in that country and with similar methodology. In Ghana, median salt intake in 2018–19 was 8.3 g/day, and higher in younger participants (18–49 y) compared to older ones (50 + y) (9.7 (IQR 7.9) vs 8.1 (7.1) g/day, respectively; p < 0.01) (Menyanu et al., 2020). While the median salt intake is higher than in South Africa, the pattern of elevated intakes in younger adults appears consistent across the two countries.
It remains to be seen how the second, stricter phase of the salt reduction targets (2019) impact population level salt intake and blood pressure. Previous modelling for salt reduction predictions (Bertram et al., 2012) were based on sodium reductions achieved in a limited number of foods in an experimental study (Charlton et al., 2008), and included stricter levels in bread, namely 350 mg Na/100 g, compared to those adopted in the interim and final legislation targets (400 and 380 mg/100 g, respectively). (South African Department of Health, 1972)
South Africa is now one of a number of countries with mandatory sodium targets for food reformulation. A review of national salt reduction initiatives around the world in 2019 identified a 28% increase in the number of all initiatives since 2014, to number 96 countries (Santos et al., 2021). Of the 57 countries with salt targets for food reformulation, 19 have mandatory maximum salt limits for foods. Half of these countries set mandatory targets for bread alone (Bahrain, Belgium, Hungary, Netherlands, Palestine, Paraguay, Portugal, Qatar, Spain, and Turkmenistan), whereas the other half covered a wider range of foods, including processed meats, cheeses, crisps and snacks, soups and stocks, canned fish, tomato products, and fruit and vegetables (Argentina, Belarus, Bulgaria, Finland, Greece, Iran, Slovakia, South Africa, and Uzbekistan).
In Argentina, it was found over 90% of foods were compliant, as reported on the nutrition information panels, four years after implementation of the legislation (Allemandi et al., 2019). This high compliance in food sodium content may be attributed to the successful government-led programme ‘Less salt, more life’ introduced in 2011 (Ministerio de Salud, 2012), that encouraged voluntary buy-in from the food industry to lower sodium content of processed foods. However, a learning from Argentina is that sodium discussions often take place between government and the bigger food companies, and an ongoing challenge is to determine compliance with sodium targets in small-sized and medium-sized food producers (Allemandi et al., 2015). In the South African context, foods sold by informal street food vendors, and commonly consumed takeaway foods are important targets for future salt reduction efforts, especially for younger South Africans who are estimated to consume, on average, 8 serves of fast foods per week (Feeley et al., 2009).
Process evaluation did not form part of the current study, with impact evaluation being the main focus (i.e. sodium intake and blood pressure). It is however important to note that South Africa does not have the same stringent monitoring and surveillance systems in place as Argentina (Allemandi et al., 2019), and it is not possible to comment on compliance of the food industry with mandated targets at present which is a limitation. Data on program impact of mandatory salt targets in foods to date has been limited and more rigorous monitoring and evaluation of strategies to assess achievement towards the global salt reduction target is required (Santos et al., 2021).
A strength of the study was the use of a largely countrywide sample of participants aged 50 + years, keeping with the aim of WHO SAGE studies. This design, on the other hand, may limit generalization to the entire population. Women are more likely to volunteer to participate in surveys than men, as was evident in the current study and has been observed in another 24 h urine collection study in South Africa. (General Assembly of the World Medical Association, 2014) Robust methodology and strict quality control was applied in data collection processes across both study waves. Collection of a single 24 h urine collection may be insufficient to assess usual salt intake in individuals (Lerchl et al., 2015) but in larger population studies, multiple days of collection results in more refusals and incomplete samples (Mente et al., 2015), and potential underestimates of salt intake (Wielgosz et al., 2016). We conducted a sensitivity analysis in a random subsample of 48 participants in W3 in order to assess whether correction for intra-individual variability in Na excretion was required (Charlton et al., 2020). Three repeated 24 h urinary Na collections resulted in shrinkage of the population distribution at the upper extremes but did not influence the median value, thereby suggesting that a single 24 h urinary Na collection is appropriate for our use. A study limitation is the inability to follow up many of the same participants in the salt sub-study between W2 (2015) and W3 (2018–19) because of logistical complexities in the South African context. This resulted in some sociodemographic differences between participants in W2 and those in W3 samples which could have introduced bias in ability to compare salt intake directly between the two groups. However, multivariable analyses adjusting for these differences showed that sodium intakes have reduced.
South Africa is known to have a highly mobile population with increased levels of circular migration and individuals moving between households based on fluid family and social relationships, and economic pressures or opportunities (Hosegood et al., 2005, Jinnah, 2020). Such challenges require longitudinal cohorts in the region to have adaptive and flexible methods and designs for follow-up.
5. Conclusion
These first results of salt intake from South Africa following implementation of its mandatory sodium legislation in 2016 use valid 24hr urinary Na concentrations in a countrywide sample. Our data suggest that salt intake reduced by 1.16 g salt per day between 2015 and 2018/early 2019, with older adults having significantly lower salt intakes than younger South Africans. It is likely that salt intake may have already been reduced by the time of baseline measurements, which occurred during the year leading up to implementation of the first phase of the legislation. It remains to be demonstrated how the stricter maximum sodium levels in foods, enforced from June 2019, will further impact population salt reduction and blood pressure change. Systematic monitoring and surveillance of the food system is an integral and essential component to further evaluate the effectiveness of South Africa’s salt reduction strategies.
Author contributions
K.E.C., L.J.W., A.E.S., and P.K. designed the research; L.J.W. and L.W. implemented the research; B.C. and N.M. performed the statistical analyses; N.N. oversaw data quality for the main SAGE survey data collection. K.E.C. wrote the first draft of the manuscript, while all authors contributed to data interpretation and the final write-up. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by an agreement with the CDC Foundation with financial support provided by Bloomberg Philanthropies. SAGE is supported by WHO and the Division of Behavioural and Social Research (BSR) at the National Institute on Aging (NIA), US National Institutes of Health, through Interagency Agreements (OGHA 04034785; YA1323–08-CN-0020; Y1-AG-1005–01) with WHO and a Research Project Grant R01AG034479. Pilot work was supported by the Partnership and Research Development Fund of the Australia Africa Universities Network. L.J.W. is supported by the South African DSI-NRF Centre of Excellence (CoE) in Human Development. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the World Health Organization nor the CoE in Human Development.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all study participants and acknowledge Stephen Rule of the Human Sciences Research Council of South Africa who coordinated the data collection in both waves, and Ezinne Igwe is thanked for editorial assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2021.101469.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Allemandi L, Tiscornia MV, Guarnieri L, et al. Monitoring Sodium Content in Processed Foods in Argentina 2017–2018: Compliance with National Legislation and Regional Targets. Nutrients 2019;11(7):1474. [DOI] [PMC free article] [PubMed]
- Allemandi L., Tiscornia M.V., Ponce M. Sodium content in processed foods in Argentina: Compliance with the national law. Cardiovascular Diagnos. Therapy. 2015;5(3):197. doi: 10.3978/j.issn.2223-3652.2015.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arku R.E., Ezzati M., Baumgartner J., Fink G., Zhou B., Hystad P., Brauer M. Elevated blood pressure and household solid fuel use in premenopausal women: Analysis of 12 Demographic and Health Surveys (DHS) from 10 countries. Environ. Res. 2018;160:499–505. doi: 10.1016/j.envres.2017.10.026. [DOI] [PubMed] [Google Scholar]
- Berry KM, Parker W-a, Mchiza ZJ, et al. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011-2012. BMJ global health 2017;2(3):e000348. [DOI] [PMC free article] [PubMed]
- Bertram M.Y., Steyn K., Wentzel-Viljoen E., Tollman S., Hofman K.J. Reducing the sodium content of high-salt foods: Effect on cardiovascular disease in South Africa. S. Afr. Med. J. 2012;102(9):743–745. doi: 10.7196/samj.5832. [DOI] [PubMed] [Google Scholar]
- Cappuccio F., Capewell S. Facts, issues, and controversies in salt reduction for the prevention of cardiovascular disease. Functional Food Rev. 2015;7(1):41–61. [Google Scholar]
- Charlton KE, Steyn K, Levitt NS, et al. Ethnic differences in intake and excretion of sodium, potassium, calcium and magnesium in South Africans. European Journal of Cardiovascular Prevention & Rehabilitation 2005;12(4):355-62. [DOI] [PubMed]
- Charlton K.E., Steyn K., Levitt N.S., Peer N., Jonathan D., Gogela T., Rossouw K., Gwebushe N., Lombard C.J. A food-based dietary strategy lowers blood pressure in a low socio-economic setting: a randomised study in South Africa. Public Health Nutr. 2008;11(12):1397–1406. doi: 10.1017/S136898000800342X. [DOI] [PubMed] [Google Scholar]
- Charlton K.E., Langford K., Kaldor J. Innovative and collaborative strategies to reduce population-wide sodium intake. Current Nutrition Reports. 2015;4(4):279–289. [Google Scholar]
- Charlton K.E., Schutte A.E., Wepener L., Corso B., Kowal P., Ware L.J. Correcting for intra-individual variability in sodium excretion in spot urine samples does not improve the ability to predict 24 h urinary sodium excretion. Nutrients. 2020;12(7):2026. doi: 10.3390/nu12072026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton K., Webster J., Kowal P. To legislate or not to legislate? A comparison of the UK and South African approaches to the development and implementation of salt reduction programs. Nutrients. 2014;6(9):3672–3695. doi: 10.3390/nu6093672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton K., Ware L.J., Menyanu E., Biritwum R.B., Naidoo N., Pieterse C., Madurai S., Baumgartner J., Asare G.A., Thiele E., Schutte A.E., Kowal P. Leveraging ongoing research to evaluate the health impacts of South Africa's salt reduction strategy: a prospective nested cohort within the WHO-SAGE multicountry, longitudinal study. BMJ open. 2016;6(11) doi: 10.1136/bmjopen-2016-013316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F.W., Gao X., Mitchell D.C., Wood C., Still C.D., Rolston D., Jensen G.L. Body mass index and all-cause mortality among older adults. Obesity. 2016;24(10):2232–2239. doi: 10.1002/oby.21612. [DOI] [PubMed] [Google Scholar]
- Chung J.W., Lee Y.S., Kim J.H., Seong M.J., Kim S.Y., Lee J.B., Ryu J.K., Choi J.Y., Kim K.S., Chang S.G., Lee G.H., Kim S.H. Reference values for the augmentation index and pulse pressure in apparently healthy Korean subjects. Korean Circulat. J. 2010;40(4):165. doi: 10.4070/kcj.2010.40.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaboration NCDRF. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017;389(10064):37-55. doi: 10.1016/S0140-6736(16)31919-5 [published Online First: 2016/11/16]. [DOI] [PMC free article] [PubMed]
- Cook N.R., Obarzanek E., Cutler J.A. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med. 2009;169(1):32–40. doi: 10.1001/archinternmed.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C., Groenewald P., Laubscher R., Chaudhry S., Van Schaik N., Bradshaw D. Monitoring of non-communicable diseases such as hypertension in South Africa: Challenges for the post-2015 global development agenda. S. Afr. Med. J. 2014;104(10):680–687. doi: 10.7196/samj.7868. [DOI] [PubMed] [Google Scholar]
- Feeley A., Pettifor J.M., Norris S.a. Fast-food consumption among 17-year-olds in the Birth to Twenty cohort. South African J. Clinic. Nutrit. 2009;22(3):118–123. [Google Scholar]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. The Journal of the American College of Dentists 2014;81(3):14. [PubMed]
- He F.J., Brinsden H.C., MacGregor G.A. Salt reduction in the United Kingdom: a successful experiment in public health. J. Hum. Hypertens. 2014;28(6):345–352. doi: 10.1038/jhh.2013.105. [DOI] [PubMed] [Google Scholar]
- Hermann J.M., Rosenbauer J., Dost A., Steigleder-Schweiger C., Kiess W., Schöfl C., Holl R.W. Seasonal variation in blood pressure in 162,135 patients with type 1 or type 2 diabetes mellitus. J. Clinic. Hypertens. 2016;18(4):270–278. doi: 10.1111/jch.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosegood V., Benzler J., Solarsh G.C. Population mobility and household dynamics in rural South Africa: implications for demographic and health research. Southern African J. Demography. 2005;10(1/2):43–68. [Google Scholar]
- Jakobsen J., Pedersen A.N., Ovesen L. Para-aminobenzoic acid (PABA) used as a marker for completeness of 24 hour urine: effects of age and dosage scheduling. Eur. J. Clin. Nutr. 2003;57(1):138–142. doi: 10.1038/sj.ejcn.1601505. [DOI] [PubMed] [Google Scholar]
- Jinnah Z. Negotiated precarity in the global south: A case study of migration and domestic work in South Africa. Stud. Soc. Justice. 2020;2020(14):210–227. [Google Scholar]
- Kowal P, Chatterji S, Naidoo N, et al. Data resource profile: the World Health Organization Study on global AGEing and adult health (SAGE). International journal of epidemiology 2012;41(6):1639-49. [DOI] [PMC free article] [PubMed]
- Lerchl K., Rakova N., Dahlmann A., Rauh M., Goller U., Basner M., Dinges D.F., Beck L., Agureev A., Larina I., Baranov V., Morukov B., Eckardt K.-U., Vassilieva G., Wabel P., Vienken J., Kirsch K., Johannes B., Krannich A., Luft F.C., Titze J. Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension. 2015;66(4):850–857. doi: 10.1161/HYPERTENSIONAHA.115.05851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Sherlock P., Beard J., Minicuci N., Ebrahim S., Chatterji S. Hypertension among older adults in low-and middle-income countries: prevalence, awareness and control. Int. J. Epidemiol. 2014;43(1):116–128. doi: 10.1093/ije/dyt215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mente A., O’Donnell M.J., Yusuf S. Measuring sodium intake in populations: simple is best? Am. J. Hypertens. 2015;28(11):1303–1305. doi: 10.1093/ajh/hpv076. [DOI] [PubMed] [Google Scholar]
- Menyanu E., Charlton K., Ware L., Russell J., Biritwum R., Kowal P. Salt use behaviours of Ghanaians and South Africans: a comparative study of knowledge, attitudes and practices. Nutrients. 2017;9(9):939. doi: 10.3390/nu9090939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menyanu E.K., Corso B., Minicuci N., Rocco I., Russell J., Ware L.J., Biritwum R., Kowal P., Schutte A.E., Charlton K.E. Salt and potassium intake among adult Ghanaians: WHO-SAGE Ghana Wave 3. BMC Nutrition. 2020;6(1) doi: 10.1186/s40795-020-00379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministerio de Salud PdlN. Argentina Argentine initiative to reduce sodium consumption “Less Salt, More Life”. . In: Ministerio de Salud, ed. Argentina, 2012.
- Nakano S., Konishi K., Furuya K., Uehara K., Nishizawa M., Nakagawa A., Kigoshi T., Uchida K. A prognostic role of mean 24-h pulse pressure level for cardiovascular events in type 2 diabetic subjects under 60 years of age. Diabetes Care. 2005;28(1):95–100. doi: 10.2337/diacare.28.1.95. [DOI] [PubMed] [Google Scholar]
- Nicar M.J., Hsu M.C., Johnson T., Pak C.Y.C. The preservation of urine samples for determination of renal stone risk factors. Laboratory Med. 1987;18(6):382–384. doi: 10.1093/labmed/18.6.382. [DOI] [PubMed] [Google Scholar]
- Peters S., Dunford E., Ware L., Harris T., Walker A., Wicks M., van Zyl T., Swanepoel B., Charlton K., Woodward M., Webster J., Neal B. The sodium content of processed foods in South Africa during the introduction of mandatory sodium limits. Nutrients. 2017;9(4):404. doi: 10.3390/nu9040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Republic of South Africa. Government Gazette. Regulations relating to the reduction of sodium in certain foodstuffs and related matters. R214. 20 March 2013. . In: Health SADo, ed. South Africa, 2013.
- Santos JA, Tekle D, Rosewarne E, et al. A Systematic Review of Salt Reduction Initiatives Around the World: A Midterm Evaluation of Progress Towards the 2025 Global Non-Communicable Diseases Salt Reduction Target. Advances in Nutrition 2021. nmab008, 10.1093/advances/nmab008. Published 07 March 2021. [DOI] [PMC free article] [PubMed]
- South African Department of Health. Foodstuffs, cosmetics and disinfectants Act, 1972 (Act 54 of 1972). Regulations relating to the reduction of sodium in certain foodstuffs and related matters. In: South African Department of Health, ed. South Africa, 2013.
- Stolarz-Skrzypek K., Kuznetsova T., Thijs L. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305(17):1777–1785. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- Swanepoel B., Schutte A.E., Cockeran M., Steyn K., Wentzel-Viljoen E. Sodium and potassium intake in South Africa: an evaluation of 24-hour urine collections in a white, black, and Indian population. J. Am. Soc. Hypertens. 2016;10(11):829–837. doi: 10.1016/j.jash.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Trieu K., Neal B., Hawkes C., Dunford E., Campbell N., Rodriguez-Fernandez R., Legetic B., McLaren L., Barberio A., Webster J., DeAngelis M.M. Salt reduction initiatives around the world–a systematic review of progress towards the global target. PLoS ONE. 2015;10(7):e0130247. doi: 10.1371/journal.pone.0130247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware LJ, Charlton K, Schutte AE, et al. Associations between dietary salt, potassium and blood pressure in South African adults: WHO SAGE Wave 2 Salt & Tobacco. Nutrition, Metabolism and Cardiovascular Diseases 2017;27(9):784-91. [DOI] [PubMed]
- Webster J., Trieu K., Dunford E., Hawkes C. Target salt 2025: A global overview of national programs to encourage the food industry to reduce salt in foods. Nutrients. 2014;6(8):3274–3287. doi: 10.3390/nu6083274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization (WHO); Geneva: 2012. Guideline: Potassium intake for adults and children. [PubMed] [Google Scholar]
- WHO . World Health Organization (WHO); Geneva: 2012. Guideline: Sodium intake for adults and children. [PubMed] [Google Scholar]
- WHO. Global Targets 2025 2020 [Available from: https://www.who.int/nutrition/global-target-2025/en/ accessed November, 2020 2020.
- WHO/PAHO Regional Expert Group for Cardiovascular Disease Prevention through Population-wide Dietary Salt Reduction. Protocol for population level sodium determination in 24-hour urine samples. Geneva: World Health Organization, 2010 Geneva: World Health Organization; 2010 [Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=21488&Itemid accessed November 2020 2020.
- Wielgosz A., Robinson C., Mao Y., Jiang Y., Campbell N.R.C., Muthuri S., Morrison H. The impact of using different methods to assess completeness of 24-hour urine collection on estimating dietary sodium. J. Clinic. Hypertens. 2016;18(6):581–584. doi: 10.1111/jch.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. New England Journal of Medicine 2014;371(9):818-27. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.