Abstract
Several clinical studies have reported a double dissociation between abstract and concrete concepts, suggesting that they are processed by at least partly different networks in the brain. However, neuroimaging data seem not in line with neuropsychological reports. Using the ALE method, we run a meta-analysis on 32 brain-activation imaging studies that considered only nouns and verbs. Five clusters were associated with concrete words, four clusters with abstract words. When only nouns were selected three left activation clusters were found to be associated with concrete stimuli and only one with abstract nouns (left IFG). These results confirm that concrete and abstract words processing involves at least partially segregated brain areas, the IFG being relevant for abstract nouns and verbs while more posterior temporoparietal-occipital regions seem to be crucial for processing concrete words, in contrast with the neuropsychological literature that suggests a temporal anterior involvement for concrete words. We investigated the possible reasons that produce different outcomes in neuroimaging and clinical studies.
Subject terms: Cognitive neuroscience, Neuroscience
Introduction
An advantage for concrete words as compared to abstract words has been demonstrated in a series of psycholinguistic studies. Neurologically unimpaired participants perform better on concrete than abstract words in free recall, cued recall, paired-associate learning and recognition; their reaction times in visual lexical decision are shorter with concrete than abstract words1. This effect is known as “concreteness effect”, and it increases in aphasic patients. This is especially evident in non-fluent aphasia, for example in patients with agrammatism2, where it has been found in spontaneous speech3, reading4, writing5, repetition6, naming7, and comprehension8. Several theories9–12 have been proposed to explain this advantage of concrete words but they share a common feature, namely a quantitative distinction between concrete and abstract concepts, with concrete items more strongly represented than abstract ones, either because they benefit from a verbal and visuo-perceptual representation10 or thanks to a larger contextual support12 or a larger number of semantic features9,11. For instance, the Dual Coding Theory10 postulates that concrete concepts are supported by both perceptual and verbal representations while abstract words are based exclusively on linguistic information. From the Dual Coding Theory perspective, the advantage of concrete compared to abstract concepts is attributed to the additional contribution of the sensory-motor systems triggered by imagery-based richer representations, presumably involving both hemispheres (not only the left hemisphere) and to a greater number of units activated in the semantic system for concrete words10. The hub-and-spokes model assumes that words are processed in a neural network containing one or more amodal hubs, sensorimotor modality-specific regions, and connections between them (cross-modal conjunctive representations13,14. Initially, it was hypothesized that the anterior temporal lobes (ATLs) were the main hub, but, later on, other potential high-order and low-order hubs have been introduced (e.g., left posterior cingulate cortex, dorsomedial pre-frontal cortex, inferior frontal gyrus, inferior parietal cortex, precuneus)15,16. From the Dual Hub Theory17,18 perspective the ATL processes taxonomic knowledge (shared features, e.g., dog → wolf) while the temporo-parietal areas, including the posterior middle temporal gyrus (pMTG), are involved in thematic knowledge (contiguity relations based on co-occurrence in events or scenarios, e.g., dog—leash).
However, these theories cannot explain the reversal of concreteness effect that has been documented in a number of brain-damaged patients, both single cases19–29, and group studies30–34, who consistently show better performance on abstract as compared to concrete words.
To account for the reversed concreteness effect, it has been proposed that abstract and concrete concepts are distinguished by the manner in which they are acquired, and by the relative weight of sensory-perceptual features in their representation20. An alternative explanation by Crutch and Warrington35, points to a fundamental difference in the architecture of concrete and abstract word representations: the primary organization of concrete concepts is categorical, whereas abstract concepts are predominantly represented by association to other items. In this framework, a reversed concreteness effect might result from selective damage to categorical information (which would selectively affect conceptual representations of concrete words).
The selective impairment of concrete and abstract concepts suggests different anatomical correlates. In aphasic patients, an increase of the concreteness effect has been associated to vascular damage in the territory of the left middle cerebral artery, involving the prefrontal cortex. Cases of reversed concreteness effect, in contrast, are associated to herpes simplex encephalitis26,29 and semantic dementia both in single cases20–22,25, and group studies30–32,34, that typically affect anterior temporal regions. These results have been confirmed in patients after left temporal pole resection33 and during direct electrical stimulation in awake surgery36. All these data seem to suggest a role of the left prefrontal cortex and the anterior temporal lobe, in processing abstract and concrete concepts, respectively. Notably, with the exception of Yi et al.’s34 and Bonner et al.’s30 studies, the reversal of concreteness effect has been found for nouns but not for verbs.
Neuroimaging data, however, do not totally match clinical evidence. Indeed, while the role of the left inferior frontal gyrus (IFG) for abstract words is confirmed, a previous meta-analysis37, based on 19 fMRI and PET studies, also showed an activation of the middle temporal gyrus (MTG), and, crucially, concrete concepts compared to abstract ones seem to activate the left posterior cingulate, precuneus, fusiform gyrus, parahippocampal cortex, therefore, posterior regions. However, Wang et al.37 took into consideration not only nouns and verbs, but also sentences and fixed expressions, such as idioms. Thus, we hypothesized that this incongruence between clinical and neuroimaging studies could partly depend on the use of very different type of stimuli.
The present systematic review and meta-analysis aimed at addressing which regions are consistently activated across experiments that require participants to process abstract and concrete words, trying to adopt more stringent criteria considering type of stimuli and modality of presentation (visual or auditory). The rationale of these sub-analyses is based on the fMRI literature suggesting that stimulus type, presentation modality, but also tasks could impact on the pattern of activation38,39. Accordingly, we did not include studies using complex stimuli as sentences, or short stories since these publications might tap on different cognitive processes including for example attention and working memory.
Consequently, our study differs from previous meta-analyses37,40 in two aspects:
We exclusively selected papers that used only words stimuli and presented specific contrasts (concrete > abstract and abstract > concrete stimuli).
We used a different method, choosing the more popular Activation Likelihood Estimation41–43 (ALE) as compared to the multilevel kernel density analysis (MKDA)44 applied by Wang et al.37. MKDA and ALE produce similar results, both using the location (xyz-coordinates) of local maxima reported by the individual studies, but MKDA uses a spherical kernel whose radius is determined by the analyst45 while ALE applies a Gaussian kernel whose FWHM is empirically determined. Moreover, our analyses are conducted on the last version of the GingerAle software, which managed to rectify some of the previous limitations of this instrument, e.g., the frequently used FDR correction is no longer supported43 and proposes new best-practice ALE recommendations like the cluster-level family-wise error (FWE) corrected threshold of p < 0.0546.
Finally, this is also an update of the previous reviews, including publications from the last 10 years.
Materials and methods
The present systematic review was conducted under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines47.
Studies selection
Our meta-analysis is based on 32 neuroimaging studies exploring the neural basis of concrete and abstract words processing, using either PET or fMRI on adult participants, published between January 1996 and February 2021. Studies were selected using four electronic databases: MEDLINE (accessed by PubMed, https://www.ncbi.nlm.nih.gov/pubmed), PsycARTICLES (via EBSCOHost, https://search.ebscohost.com), PsycINFO (via EBSCOHost) and Web of Science (https://webofknowledge.com/). The search terms used were: (1) “semantic decision”, “semantic judgment”; “abstract words”, “concrete words “, “abstract concepts”, “concrete concepts”, “lexical decision” AND (2) “imaging”, “MRI”, “PET”. Additional sources such as reference lists of included studies and relevant systematic reviews were also checked.
Titles, abstracts, and full-text articles were screened and evaluated for eligibility based on the following criteria:
Inclusion criteria:
Imaging technique: PET or fMRI,
Reported stereotaxic coordinates (in the MNI or Talairach atlases),
Whole-brain voxel-based data analyses,
More than 5 participants in each study,
Sample population of healthy, adult participants,
Reported concrete > abstract or abstract > concrete contrast,
Word stimuli,
Published in English,
Exclusion criteria:
Region-of-interest analyses,
Multiple single-case analyses,
Sample population of minors,
Sample population of neurological, brain-damaged, cognitively impaired or psychiatric patients,
Only concrete > baseline or abstract > baseline contrasts,
Articles from the gray literature (i.e., literature that is not formally published in sources such as books or journal articles, e.g. unpublished Ph.D. thesis),
Presentations from international meetings with no specific data provided, perspective and opinion publications, case reports, series of cases, previous reviews or meta-analyses,
Studies not published in, or translated into English,
Phrases or sentences stimuli,
Studies without adequate information (e.g., stereotaxic coordinates) to analyze the concrete vs. abstract contrasts and no reply from the authors after asking for the missing data.
We used a general and broad initial search. We looked for publications that reported word stimuli and concrete > abstract or abstract > concrete MRI and PET contrasts. In a second time, we distinguished the included papers taking into consideration the type of knowledge, type of task, type of stimuli, and type of investigation method. These classifications led to exploratory sub-analyses, with the purpose of controlling as much as possible confounding factors. As previously specified, we looked for publications that reported concrete > abstract words or abstract > concrete words contrast, without analyzing the exact strategies that the authors applied to divide word stimuli into the two categories. Often, the abstractness/concreteness constructs are operationalized in the papers based on two rating methods: (1) asking participants to classify a word as concrete taking into consideration the degree to which it refers to a tangible entity in the world (it has clear references to material objects); (2) or by evaluating its imageability, i.e., the ease with which the word elicits a mental image. Generally speaking, words referring to something that exists in reality, and one can have an immediate experience of it through the senses are considered concrete (e.g., animals, tools); while words whose meaning cannot be experienced directly but can be defined by other words, internal sensory experience, and linguistic information, are classified as abstract (e.g., emotions, morality, social interaction, time).
After removing duplicates, research papers which did not satisfy the above criteria were excluded. For example, several studies focused on sentences or phrases48,49; or reported only words > baseline contrasts50. The more conservative concrete > abstract and abstract > concrete contrast (as opposed to concrete > baseline or abstract > baseline contrasts) was chosen in order to avoid a variety of baselines that could range from resting state, fixation cross51 to pseudowords52 and number or letters53 and could affect the interpretation of the results, since subtractions from different baselines create different activation patterns. We acknowledge that this type of contrast does not reveal which brain regions equally support the processing of both concrete and abstract words. The question that this meta-analysis can answer is which are the most replicated data in the literature (in terms of brain activation and words representation) when contrasting abstract and concrete words. Moreover, since concrete and abstract words can dissociate, we aimed at assessing in which anatomical correlates they differ, and not the common ones.
If the same data were reported in different publications, we chose the most recent one and with the highest number of participants54,55.
Uncertainties regarding some inclusions were solved by the authors through discussion.
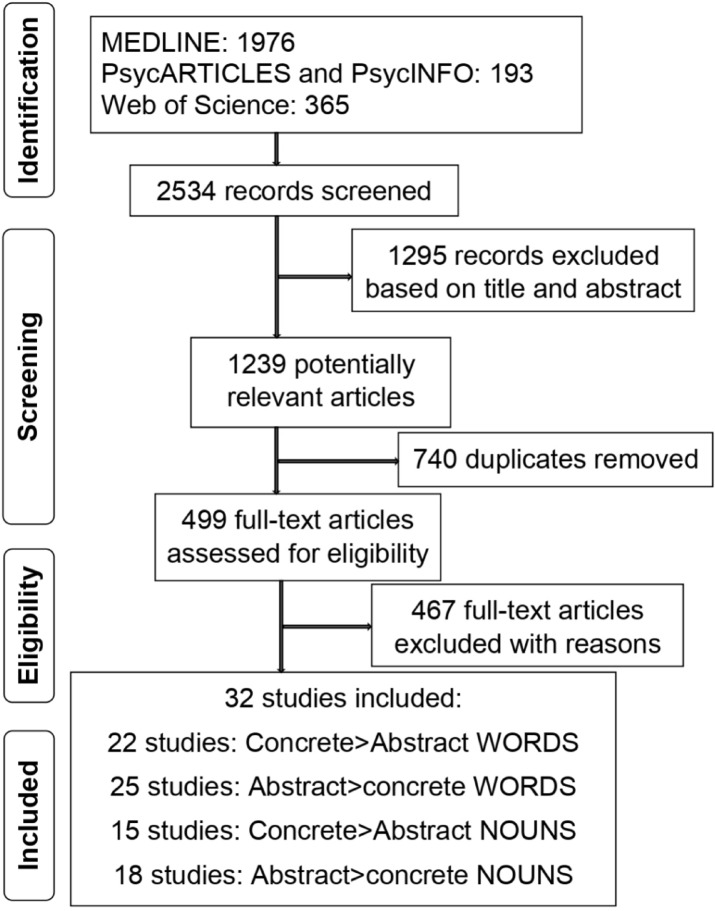
The PRISMA flow of information diagram was used to track the search process as presented in Fig. 1 and the main characteristics of the studies included in this meta-analysis are reported in Table 1.
Figure 1.
PRISMA flowchart of the selection process for included articles.
Table 1.
Descriptive information of the 32 experiments included in the meta-analysis.
| Paper | Technique | Sample size | Age of subjects (years) | Stimuli | Stimuli presentation modality | Experimental task | Design | Random or fixed effect | Contrasts p value* |
|
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | D'Esposito et al., 1997 | MRI, 1.5 Tesla | 7 | Range 18–37 | English nouns (concrete vs abstract) | Auditory | Mental image generation (concrete) and passive listening (abstract) | blocks | Fixed | p < .001 corrected voxel-wise |
| 2 | Mellet et al., 1998 | PET | 8 | Range 20–25 | French nouns (concrete vs abstract) | Auditory | Mental image generation (concrete) and passive listening (abstract) | blocks | Fixed | p = 0.001 uncorrected |
| 3 | Perani et al., 1999 | PET | 14 | Range 22–26 |
Italian words: (1) Concrete verbs, (2) Abstract verbs, (3) Concrete nouns, (4) Abstract nouns |
Visual | Lexical decision (classify stimuli as words or nonwords) | Blocks | Fixed | p < 0.001 uncorrected |
| 4 | Kiehl et al., 1999 | MRI, 1.5 Tesla | 6 | Range 22–26 | English words: concrete or abstract | Visual | Lexical decision (classify stimuli as words or nonwords) | Blocks | Fixed | p < 0.05 corrected voxel-wise |
| 5 | Jessen et al., 2000 | MRI, 1.5 Tesla | 14 | Range 20–44 (31.5 ± 6.3) | German nouns: concrete or abstract | Visual | Memory encoding task | Blocks | Fixed | p < 0.001 uncorrected |
| 6 | Tyler et al., 2001 | PET | 9 |
Range 21–34 26 ± 5 |
English words: concrete or abstract | Visual | Lexical decision (classify stimuli as words or nonwords) | Block | Fixed-effect | p < 0.05 corrected voxel-wise |
| 7 | Grossman et al., 2002 | MRI, 1.5 Tesla | 16 | Mean age 23.4 | English nouns: animals, implement, abstract | Visual | Semantic judgment (pleasant or not) | Blocks | Fixed | p < 0.05 corrected voxel-wise |
| 8 | Kounios et al., 2003 | MRI, 1.5 Tesla | 16 | Mean age 73.9 | English nouns: animal, implement, and abstract | Visual | Semantic judgement (pleasant or not) | Block | Fixed-effect | p < 0.05 corrected |
| 9 | Whatmough et al., 2004 | PET | 15 |
Range 69–90 74.3 ± 5.6 |
English nouns: two pairs (concrete or abstract) | Visual | Semantic similarity decision (read aloud if the pairs are similar in meanings) | ns | Ns | p < 0.05 corrected voxel-wise |
| 10 | Noppeney and Price, 2004 | MRI, 2 Tesla | 15 |
Range 21–46 mean age 30 |
English (1) Abstract concepts, (2) Hand movements, (3) visual attributes (4) Sounds |
Visual | Semantic similarity decision | Blocks | Random | p < 0.001 uncorrected |
| 11 | Fiebach and Friederici, 2004 | MRI, 3 Tesla | 12 | Mean age 25 | German nouns: abstract and concrete | Visual | Lexical decision (classify stimuli as words or nonwords) | Event-related | Ns | p < 0.05 corrected cluster-wise |
| 12 | Giesbrecht et al., 2004 | MRI, 1.5 Tesla | 10 | ns |
English words: high imageable and low imageable |
Visual | Semantic judgement (words pairs related or unrelated) | Event-related | Random | p < 0.005 uncorrected |
| 13 | Sabsevitz et al., 2005 | MRI, 1.5 Tesla | 28 |
Range 18–33 22.8 ± 3.6 |
English nouns: concrete and abstract triads |
Visual | Semantic similarity decision | Event-related | Random | p < .001, uncorrected |
| 14 | Binder et al., 2005 | MRI, 1.5 Tesla | 24 |
Range 20–50 |
English nouns: abstract and concrete |
Visual | Lexical decision (classify stimuli as words or nonwords) | Event-related | Random | p < .005 uncorrected |
| 15 | Harris et al., 2006 | MRI, 1.5 Tesla | 20 |
Range 19–50 31 ± 9 |
English nouns: abstract and concrete |
Visual | Semantic judgment (positive or negative) | Block | Random |
p < 0.05 corrected cluster-wise |
| 16 | Fliessbach et al., 2006 | MRI, 1.5 Tesla | 21 |
Range 19–43 27.4 ± 6.2 |
German nouns: abstract and concrete |
Visual | Recognition task (old/new-decision) | Event-related | Random |
p < 0.05 corrected cluster-wise |
| 17 | Rüschemeyer et al., 2007 | MRI, 3 Tesla | 20 |
Range 22–33 27 ± 3 |
German verbs: simple, complex, motor, abstract | Visual | Lexical decision (classify stimuli as words or nonwords) | Block | Random | p < .001, uncorrected |
| 18 | Pexman et al., 2007 | MRI, 3 Tesla | 20 | 26.5 ± 4.5 |
English nouns: abstract and concrete |
Visual | Semantic categorization (consumable or not) | Event-related | Random |
p < 0.05 ns |
| 19 | Van Dam et al., 2010 | MRI, 3 Tesla | 16 |
range 18–38 24 ± 4.63 |
Dutch verbs denoting (1) Actions that you perform mostly with your arms/hands/ mouth or (2) Abstract events |
Visual | Semantic categorization task (go–no go) | Event-related | Random | p < 0.05 corrected |
| 20 | Zhuang et al., 2011 | MRI, 3 Tesla | 14 |
Range 19–33 |
British English nouns manipulating (cohort competition and imageability) | Auditory | Lexical decision (classify stimuli as words or nonwords) | Event-related | Random |
p < 0.05 corrected cluster-wise |
| 21 | Rodríguez-Ferreiro et al., 2011 | MRI, 3 Tesla | 14 |
Range 23–35 mean 29 |
Spanish verbs: concrete and abstract |
Visual | Passive reading | Block | Mixed effects | p < .001, uncorrected |
| 22 | van Dam et al., 2012 | MRI, 3 Tesla | 20 |
Range 18–24 20.5 ± 2.2 |
Dutch (1) Action color (2) Action nouns (3) Color (4) Abstract nouns |
Auditory | Semantic categorization (action or color characteristics) | Block | Random |
p < 0.005 ns |
| 23 | Wilson-Mendenhall et al., 2013 | MRI, 3 Tesla | 13 |
Range 18–24 |
English words: two abstract (convince, arithmetic) two concrete (rolling, red) |
Visual | Semantic task (concept–scene match) | Block | Random |
p < 0.05 corrected voxel-wise |
| 24 | Vigliocco et al., 2013 | MRI, 3 Tesla | 20 |
Range 18–33 21.9 ± 4.4 |
English nouns: abstract and concrete |
Visual | Lexical decision (classify stimuli as words or nonwords) | Block | Random | p < 0.05 FWE-cluster-wise |
| 25 | Hayashi et al., 2014 | MRI, 1.5 Tesla | 16 |
Range 20–36 26.1 ± 5.9 |
Japanese kanji nouns: concrete and abstract |
Visual | Generate visual imagery | Block | Random | p < .001, uncorrected |
| 26 | Roxbury et al., 2014 | MRI, 3 Tesla | 17 | 27 ± 5.1 | English nouns: concrete, abstract and pseudowords | Auditory | Lexical decision (classify stimuli as words or nonwords) | Event-related | Random | p < .001, uncorrected |
| 27 | Skipper and Olson, 2014 | MRI, 3 Tesla | 19 | Mean age 23 |
English nouns: concrete and abstract |
Visual | Semantic task (answer to question in reference to the 3 words in the block) | Block | Ns |
p < 0.001 FDR corrected cluster-wise |
| 28 | Hoffman et al., 2015 | MRI, 3 Tesla | 20 |
Range 20–39 mean: 25 |
English words: concrete and abstract |
Visual | Semantic task (synonym judgement) | Block | Random |
p < 0.05 corrected cluster-wise |
| 29 | Kumar, 2016 | MRI, 3 Tesla | 20 | 28.3 ± 3 | Hindi nouns: abstract, concrete and non-words | Visual | Perceptual task (orthography judgment) | Block | Fixed | p < 0.05 corrected |
| 30 | Wang et al., 2019 | MRI, 3 Tesla | 23 |
Range 19–29 mean 22.17 |
Chinese nouns: abstract, concrete | Visual | Semantic task (which of the choices was more semantically related to the probe) | Block | Ns |
p < 0.05 FWE corrected cluster-level |
| 31 | Pauligk et al., 2019 | MRI, 3 Tesla | 21 | 23.3 ± 1.9 |
German nouns: abstract and concrete |
Visual |
Delayed lexical decision task (classify stimuli as words or nonwords) |
Block | Ns |
p = 0.001 corrected voxel-wise |
| 32 | Meersmans et al., 2020 | MRI, 3 Tesla | 26 |
Range 18–34 22.9 ± 3.7 |
Dutch nouns: abstract and concrete |
Visual and auditory | Overt repetition task | Event-related | Random |
p < 0.001 uncorrected p < 0.05 FWE-corrected |
Age is reported in years and when it was specified means and standard deviations are presented.
p values (the statistical threshold for the neuroimaging univariate analysis conducted in the included papers) are reported as they were presented in the original articles; the exact value and the correction procedure was not always specified.
ns, not specified.
Classification of the raw data before clustering analyses
From the selected papers, only the stereotactic coordinates representing the concrete > abstract or abstract > concrete contrasts were extracted. Following this procedure, we obtained 295 foci from a total sample of 535 participants. The stereotaxic coordinates reported in terms of the Talairach and Tournoux atlas56 were transformed into the MNI (Montreal Neurological Institute) stereotaxic space57 using the tal2icbm transforms implemented in the GingerALE software41,43,58.
For all the stereotaxic coordinates we extracted the relevant information about the statistical comparisons that generated them. More explicitly, we reported the MNI coordinates (MNI x,y,z), the name of the first author, the journal and the year of publication of the paper, the technique (PET or fMRI) and the stereotactic space used, the age of participants, the type of task, the nature of the contrast from which the peak was extracted, the statistical thresholds, the stimulus type (nouns or verbs) and the presentation modality (auditory or visual).
Clustering procedure
Once obtained the set of MNI coordinates, the meta-analyses were carried out using the revised ALE algorithm41,43 implemented into GingerALE software Version 3.0.258 (http://brainmap.org/ale). The ALE algorithm aims to identify areas with a convergence of reported coordinates across experiments that are higher than expected from a random spatial association. The logic behind this approach implies a spatial probability distribution modeled for each activation peak included in the dataset of interest. Reported foci are treated as centers of 3D Gaussian probability distributions capturing the spatial uncertainty associated with each focus58. The between-subject variance is weighted by the number of participants per study, since larger sample sizes should provide more reliable approximations of the “true” activation effect. The voxel-by-voxel union of these distributions is used as an activation likelihood map, subsequently tested for statistical significance against randomly generated sets of foci. ALE was proven to be a reliable way of blending evidence from multiple studies43 and was used successfully in different fields e.g.,59.
More specifically we used the following procedure:
Anatomical filtering—we applied a first filtering of the coordinates using the most conservative (smallest) mask available in the GingerALE software and 17 foci from the total of 295 fell out of the mask.
ALE maps (quantify the degree of overlap in peak activation across experiments) were calculated using the modified ALE algorithm and the random-effects model41,43;
Thresholding procedure—for each ALE calculation described below significance was tested using 1000 permutations with a cluster forming threshold of p < 0.001 (uncorrected). In order to increase test sensitivity to false positives significance was corrected with a cluster-level family-wise error threshold of p < 0.0546 as used by other meta-analytic studies60.
Unfortunately, ALE cannot deal with multiple independent variables designs, and in this paper we intended to consider the role of different variables like (1) stimulus type (nouns only, verbs only or all word stimuli), (2) modality of presentation (visual only, auditory only or both visual and auditory), and (3) task specificity (e.g., lexical, semantic tasks or all tasks). The ALE strategy we choose in this case was to consider separate sets of foci for each variable and run one meta-analysis for each of these sets when the number of papers was large enough. To this purpose, the overall dataset was divided a-posteriori into several subsets, which automatically implied running meta-analyses on a low number of foci (lowering the power). An important limitation of this approach is that we are not able to statistically assess the interaction between variables like stimuli type and task.
The analyses were based on the following contrasts:
- An analysis included the activation peaks associated with word processing independently of the stimulus type and task
- An analysis with peaks associated with noun processing only (because the number of studies including verbs only was too small (4 studies) for a specific analysis on this type of stimuli70,76,82,83)
- concrete nouns > abstract nouns included 107 stereotactic activation loci from 15 studies (5 foci out of mask), 251 participants;
- abstract nouns > concrete nouns included 99 stereotactic activation loci from 18 studies (8 foci out of mask), 324 participants;
- An analysis included the activation peaks associated with word processing independently of the stimulus type (verbs, names or adjectives), but taking into consideration only visually presented stimuli
- concrete words > abstract words visual stimuli only included 121 stereotactic activation loci from 18 studies, 301 participants
- abstract words > concrete words visual stimuli only included 135 stereotactic activation loci from 22 studies, 374 participants
Since only 5 studies included auditory stimuli we could not perform a specific analysis for this category51,54,62,79,89.
-
(4)An analysis on peaks associated with lexical (words or non-words classification task), or semantic decision tasks (e.g., pleasantness decision task, answering a question about the stimuli), excluding all the studies based on: memory tasks (2 studies), perceptual decision task (1 study), mental image generation (3 studies), passive reading (2 studies).
- concrete > abstract word (only lexical and semantic tasks) included 114 stereotactic activation loci from 16 studies, 273 participants
- abstract > concrete word (only lexical and semantic tasks) included 116 stereotactic activation loci from 17 studies, 289 participants
We explored a-posteriori the role of the (1) stimulus type (nouns), (2) modality of presentation (visual stimuli), and (3) task specificity (lexical and semantic tasks) in order to control as much as possible for each of these variables, i.e., to increase the results accuracy.
It might be argued against the inclusion of PET and fMRI studies in the same meta-analysis due to the substantial methodological differences between the two techniques in terms of experimental design, processing, spatial localization and cluster accuracy. Because the number of studies investigating concrete vs. abstract words is small, we decided to include data from both techniques in order to increase power in the analyses. Nevertheless, in Appendix A (supplementary materials), we present the data analysis after excluding all PET studies (figures and tables are numbered as e.g., Fig. 1A and Table 1A).
For anatomical labeling and figures, we capitalized on the Automatic Anatomical Labeling (AAL) template available in the MRIcron visualization Software (https://www.nitrc.org/projects/mricron).
Results
Once the appropriate studies were collected, we used activation likelihood estimation (ALE) to meta-analytically remodel available neuroimaging data.
CONCRETE > ABSTRACT meta-analysis
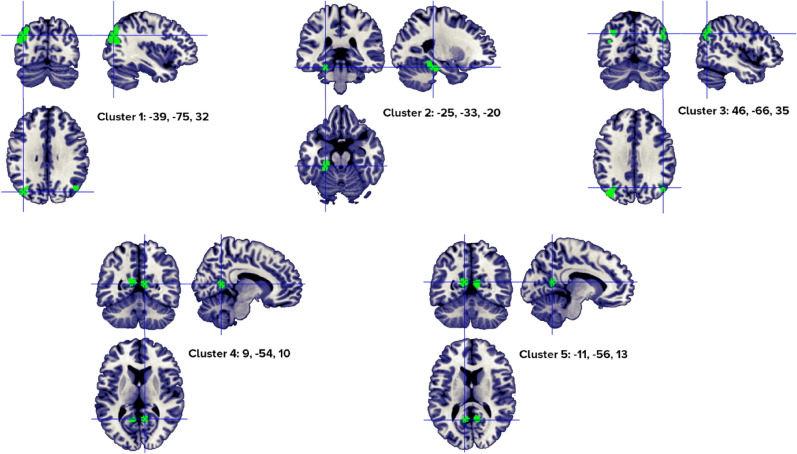
The GingerALE procedure run over the concrete words > abstract words set of coordinates identified a total of 5 clusters, with 1–4 individual peaks each, from 4 to 11 different studies (Fig. 2). Regions that were consistently activated across experiments were localized in the bilateral middle temporal gyrus and posterior cingulate, the left parahippocampal gyrus, left fusiform gyrus, bilateral precuneus and angular gyri, left superior occipital gyrus and left cerebellum culmen. The peaks distribution for each significant cluster is reported in Table 2.
Figure 2.
Clusters activated by the concrete > abstract words contrast. The crosses are centered in the areas correspond to stereotactic coordinates reported in Table 2. The images are presented in neurological convention.
Table 2.
Concrete > abstract word clusters.
| H | Cluster | Macroanatomical location | Cytoarchitectonic Label | Weighted center (MNI; mm) | Vol. (mm3) | Peaks: MNI coordinates (mm) | ALE score | Z Score | Nr | Contributors to cluster | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Gyrus | x | y | Z | x | y | z | Studies | |||||||
| L | 1 |
Temporal Occipital Parietal |
Superior Occipital Middle Temporal Precuneus, Angular Cuneus |
BA 39, BA 19 | − 38.6 | − 74.2 | 31.8 | 4680 | − 40 | − 74 | 34 | 0.025 | 5.859 | 11 | Jessen, 2000 (1); Sabsevitz, 2005 (3); Binder, 2005 (1); Harris, 2006 (1); van Dam, 2010 (1); Zhuang, 2011 (1); Rodríguez-Ferreiro, 2011 (2); van Dam, 2012 (1); Roxbury, 2014 (1); Skipper, 2014 (5); Hoffman, 2015 (2)** |
| − 44 | − 78 | 24 | 0.020 | 4.978 | |||||||||||
| − 40 | − 70 | 22 | 0.015 | 4.117 | |||||||||||
| − 38 | − 72 | 46 | 0.014 | 4.048 | |||||||||||
| L | 2 |
Cerebellum Anterior Lobe, Limbic Lobe Temporal |
Culmen (cerebellum), Parahippocampal Fusiform |
BA 35, BA 36 | − 25.9 | − 34.3 | − 19.8 | 2584 | − 24 | − 36 | − 18 | 0.021 | 5.262 | 7 | Sabsevitz, 2005 (2); Harris, 2006 (1); Rodríguez-Ferreiro, 2011 (2); van Dam, 2012 (1); Hayashi, 2014 (1); Roxbury, 2014 (1); Hoffman, 2015 (2)** |
| − 24 | − 30 | − 22 | 0.019 | 4.928 | |||||||||||
| − 34 | − 36 | − 24 | 0.013 | 3.764 | |||||||||||
| R | 3 |
Parietal Temporal |
Inferior Parietal Angular Precuneus, Middle Temporal |
BA 39 | 44.8 | − 65.6 | 33.4 | 1648 | 44 | − 68 | 32 | 0.017 | 4.496 | 4 | Sabsevitz, 2005 (2); van Dam, 2010 (1); Roxbury, 2014 (1); Hoffman, 2015 (2)** |
| 42 | − 60 | 36 | 0.012 | 3.640 | |||||||||||
| 48 | − 66 | 44 | 0.012 | 3.547 | |||||||||||
| L | 4 | Limbic Occipital |
Posterior Cingulate Lingual Gyrus Cuneus |
BA 30, BA 18 | − 10.0 | − 56.1 | 11.3 | 1184 | − 10 | − 56 | 12 | 0.018 | 4.756 | 5 | Sabsevitz, 2005 (1); Binder, 2005(1); Harris, 2006(1); Rüschemeyer, 2007(1); Roxbury, 2014(1)** |
| R | 5 | Limbic Lobe | Posterior Cingulate | BA 30 | 8.5 | − 54.0 | 10.0 | 840 | 8 | − 54 | 10 | 0.019 | 4.891 | 4 | Sabsevitz, 2005 (1); Harris, 2006 (1); Rodríguez-Ferreiro, 2011 (1);Hoffman, 2015 (1)** |
Included 149 stereotactic activation loci from 22 studies, 353 participants, Chosen min. cluster size 736 mm3.
All the values and labels were extracted from the GingerALE output files. Clusters are ordered for decreasing volume size. Coordinates (x, y, z) are in the MNI space.
H = Hemisphere; ALE = activation likelihood estimation; Nr. = number of studies that contributed to each cluster; L = left; BA = Brodmann area; ** = between brackets are the number of foci from each study that contributed to that specific cluster; R = right.
A similar activation pattern, except for the right hemisphere involvement, was observed when only studies reporting exclusively noun stimuli were taken into consideration (concrete nouns > abstract nouns). We observed three left activation clusters (Fig. 3, Table 3) situated in the middle temporal gyrus, parahippocampal gyrus, posterior cingulate, precuneus, superior occipital gyrus, and culmen (left cerebellum anterior lobe).
Figure 3.
Clusters activated by the concrete > abstract nouns contrast. The crosses are centered in the areas correspond to stereotactic coordinates reported in Table 3. The images are presented in neurological convention.
Table 3.
Concrete > abstract nouns clusters.
| H | Cluster | Macroanatomical location | Cytoarchitectonic Label | Weighted center (MNI; mm) |
Vol. (mm3) | Peaks: MNI coordinates (mm) | ALE score | Z Score | No | Contributors to cluster | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Gyrus | x | Y | z | x | y | z | Studies | |||||||
| L | 1 |
Occipital, Parietal, Temporal |
Superior Occipital, Precuneus, Middle Temporal, Angular |
BA 39, BA 19 | − 37.1 | − 73.8 | 35.2 | 3376 | − 34 | − 68 | 36 | 0.019 | 5.112 | 8 | Jessen, 2000 (1); Sabsevitz, 2005 (3); Binder, 2005 (1); Harris, 2006 (1); Zhuang, 2011 (1); van Dam, 2012 (1); Roxbury, 2014 (1); Skipper, 2014 (4)** |
| − 38 | − 74 | 32 | 0.019 | 5.051 | |||||||||||
| − 34 | − 78 | 38 | 0.017 | 4.784 | |||||||||||
| − 46 | − 76 | 28 | 0.015 | 4.320 | |||||||||||
| − 38 | − 72 | 46 | 0.014 | 4.240 | |||||||||||
| L | 2 | Anterior, Limbic | Culmen, Parahippocampal | BA 36, BA 35 | − 23.7 | − 34.2 | − 18.3 | 1432 | − 24 | − 36 | − 18 | 0.015 | 4.392 | 4 | Hayashi, 2004 (1); Sabsevitz, 2005 (2); Harris, 2006 (2); Roxbury, 2014 (1)** |
| L | 3 | Limbic, Occipital | Posterior Cingulate, Cuneus | BA 30, BA 29 | − 9.5 | − 55.7 | 12.5 | 1040 | − 10 | − 56 | 12 | 0.017 | 4.745 | 4 | Sabsevitz, 2005 (1); Binder, 2005 (2); Harris, 2006 (1); Roxbury, 2014 (1)** |
| − 8 | − 46 | 14 | 0.009 | 3.338 | |||||||||||
Included 107 stereotactic activation loci from 15 studies, 251 participants, Chosen min. cluster size 720 mm3.
All the values and labels were extracted from the GingerALE output files. Clusters are ordered for decreasing volume size. Coordinates (x, y, z) are in the MNI space.
H = Hemisphere; ALE = activation likelihood estimation; Nr. = number of studies that contributed to each cluster; L = left; BA = Brodmann area; ** = between brackets are the number of foci from each study that contributed to that specific cluster.
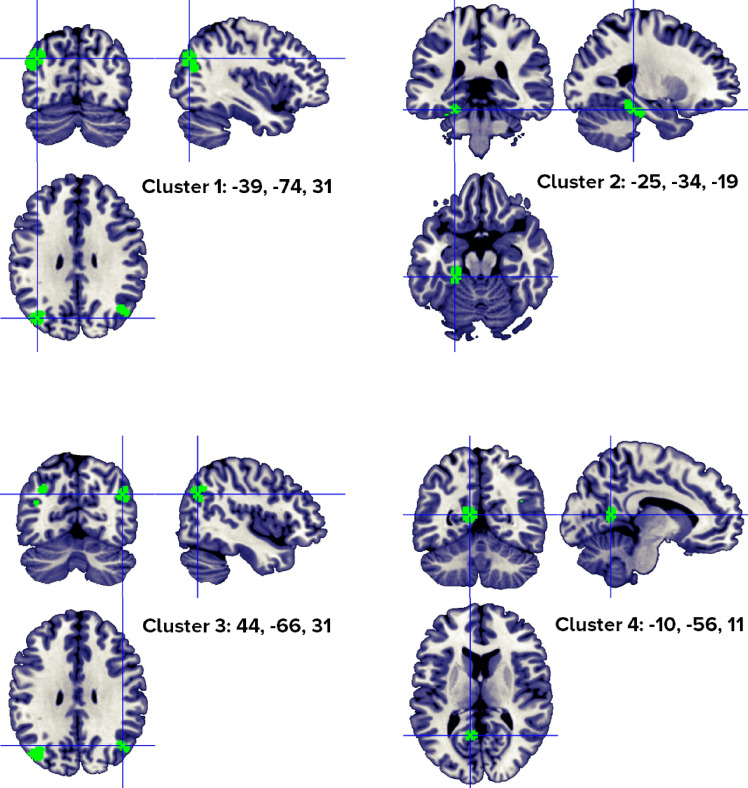
The ALE procedure run over the concrete words > abstract words, visual stimuli only set of coordinates, identified a total of 5 clusters, with 1–6 individual peaks each, from 4 to 8 different studies (Fig. 4). Regions that were consistently activated across experiments were localized in the left middle temporal gyrus, bilateral posterior cingulate, and parahippocampal gyrus, left fusiform gyrus, bilateral precuneus and angular gyri, left superior occipital gyrus and left cerebellum culmen. The peaks distribution for each significant cluster is reported in Table 4.
Figure 4.
Clusters activated by the concrete > abstract words—visual stimuli—contrast. The crosses are centered in the areas correspond to stereotactic coordinates reported in Table 4. The images are presented in neurological convention.
Table 4.
Concrete > abstract words—visual stimuli- clusters.
| H | Cluster | Macroanatomical location | Cytoarchitectonic Label | Weighted center (MNI; mm) | Vol. (mm3) | Peaks: MNI coordinates (mm) | ALE score | Z Score | No | Contributors to cluster | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Gyrus | x | Y | z | x | y | z | Studies | |||||||
| L | 1 | Temporal, Occipital, Parietal |
Superior Occipital, Middle Temporal, Precuneus, Angular |
BA 19, BA 39 | − 38.5 | − 74.6 | 31.9 | 4360 | − 40 | − 76 | 34 | 0.0212 | 5.3445 | 8 | Sabsevitz, 2005 (1); Binder, 2005 (3); Harris, 2006 (1); van Dam, 2010 (1); Zhuang, 2011 (1); Rodríguez-Ferreiro, 2011 (2); Skipper, 2014 (5); Hoffman, 2015 (2)** |
| − 44 | − 78 | 24 | 0.0174 | 4.7222 | |||||||||||
| − 36 | − 78 | 38 | 0.0172 | 4.6952 | |||||||||||
| − 34 | − 68 | 36 | 0.0169 | 4.6447 | |||||||||||
| − 40 | − 70 | 22 | 0.0145 | 4.1705 | |||||||||||
| − 38 | − 72 | 46 | 0.0143 | 4.1460 | |||||||||||
| L | 2 | Limbic Lobe, Anterior, Temporal |
Parahippocampal, Culmen, Fusiform |
BA 35, BA 36 | − 25 | − 33.2 | − 19.5 | 1752 | − 24 | − 30 | − 22 | 0.0180 | 4.8145 | 4 | Sabsevitz, 2005 (2); Harris, 2006 (1); Hayashi, 2014 (1); Hoffman, 2015 (2)** |
| − 26 | − 38 | − 16 | 0.0169 | 4.6525 | |||||||||||
| R | 3 | Parietal |
Inferior Parietal, Angular, Precuneus |
BA 39 | 45.9 | − 65.9 | 35 | 1336 | 46 | − 68 | 34 | 0.0151 | 4.2998 | 4 | Jessen, 2000 (1); Sabsevitz, 2005 (2); van Dam, 2010 (1); Hoffman, 2015 (2)** |
| 48 | − 66 | 44 | 0.0118 | 3.6536 | |||||||||||
| 42 | − 60 | 36 | 0.0116 | 3.6238 | |||||||||||
| R | 4 | Limbic |
Posterior Cingulate, Parahippocampal |
BA 30, BA 29 | 8.7 | − 54 | 10 | 992 | 8 | − 54 | 10 | 0.0192 | 5.0153 | 4 | Sabsevitz, 2005 (1); Harris, 2006 (1); Rodríguez-Ferreiro, 2011 (1); Hoffman, 2015 (1)** |
| L | 5 | Limbic, Occipital |
Posterior Cingulate, Lingual |
BA 30, BA 18 | − 11 | − 55.6 | 12.8 | 888 | − 12 | − 56 | 14 | 0.0164 | 4.5739 | 4 | Sabsevitz, 2005 (1); Binder, 2005 (1); Harris, 2006 (1); Rüschemeyer, 2007 (1)** |
Included 121 stereotactic activation loci from 18 studies, 301 participants, chosen min. cluster size 616 mm3.
All the values and labels were extracted from the GingerALE output files. Clusters are ordered for decreasing volume size. Coordinates (x, y, z) are in the MNI space.
H = Hemisphere; ALE = activation likelihood estimation; Nr. = number of studies that contributed to each cluster; L = left; BA = Brodmann area; ** = between brackets are the number of foci from each study that contributed to that specific cluster; R = right.
A comparable activation pattern was observed when only studies based on lexical and semantic tasks were taken into consideration. The analysis indicated 4 activation clusters correlated with concrete words > abstract words—lexical and semantic tasks: bilateral middle temporal gyrus, left posterior cingulate and the left parahippocampal gyri, bilateral precuneus, left angular, left superior occipital gyrus and left cerebellum culmen (Fig. 5, Table 5).
Figure 5.
Clusters activated by the concrete > abstract words -semantic and lexical tasks—contrast. The crosses are centered in the areas correspond to stereotactic coordinates reported in Table 5. The images are presented in neurological convention.
Table 5.
Concrete > abstract words—semantic and lexical tasks only- clusters.
| H | Cluster | Macroanatomical location | Cytoarchitectonic label | Weighted center (MNI; mm) | Vol. (mm3) | Peaks: MNI coordinates (mm) | ALE score | Z Score | Nr | Contributors to cluster | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Gyrus | X | y | z | x | y | z | Studies | |||||||
| L | 1 | Occipital, temporal, parietal |
Superior occipital, middle temporal, precuneus, angular |
BA 19, BA 39 | − 38.6 | − 73.9 | 31.3 | 3856 | − 40 | − 74 | 34 | 0.0247 | 5.9191 | 9 | Sabsevitz, 2005 (2); Binder, 2005 (1); Harris, 2006 (1); van Dam, 2010 (1); Zhuang, 2011 (1); van Dam, 2012 (1); Roxbury, 2014 (1), Skipper, 2014 (5); Hoffman, 2015 (2)** |
| − 46 | − 78 | 26 | 0.0183 | 4.8754 | |||||||||||
| − 40 | − 70 | 22 | 0.0144 | 4.1581 | |||||||||||
| L | 2 |
Limbic Lobe, Anterior lobe, Temporal |
Parahippocampal, Culmen |
BA 35, BA 36 | − 24.9 | − 33.8 | − 18.6 | 1792 | − 24 | − 38 | − 16 | 0.0193 | 5.0320 | 5 | Sabsevitz, 2005 (2); Harris, 2006 (1); van Dam, 2012 (1); Roxbury, 2014 (1); Hoffman, 2015 (2)** |
| − 24 | − 28 | − 22 | 0.0156 | 4.4119 | |||||||||||
| R | 3 | Temporal, Parietal |
Middle Temporal, Precuneus |
BA 39 | 44.3 | − 65.6 | 31.4 | 1696 | 44 | − 68 | 32 | 0.0167 | 4.6298 | 4 | Sabsevitz, 2005 (2); van Dam, 2010 (1); Roxbury, 2014 (1); Hoffman, 2015 (3)** |
| 42 | − 60 | 36 | 0.0122 | 3.7327 | |||||||||||
| 40 | − 56 | 24 | 0.0091 | 3.1937 | |||||||||||
| L | 4 | Limbic Lobe, Occipital |
Posterior Cingulate, Lingual |
BA 30, BA 18 | − 10.1 | − 55.9 | 11.4 | 1392 | − 10 | − 56 | 12 | 0.0184 | 4.8883 | 5 | Sabsevitz, 2005 (1); Binder, 2005 (2); Harris, 2006 (1); Rüschemeyer, 2007 (1); Roxbury, 2014 (1)** |
| − 8 | − 46 | 14 | 0.0095 | 3.2622 | |||||||||||
Included 114 stereotactic activation loci from 16 studies, 273 participants, chosen min. cluster size 656 mm3.
All the values and labels were extracted from the GingerALE output files. Clusters are ordered for decreasing volume size. Coordinates (x, y, z) are in the MNI space.
H = Hemisphere; ALE = activation likelihood estimation; Nr. = number of studies that contributed to each cluster; L = left; BA = Brodmann area; ** = between brackets are the number of foci from each study that contributed to that specific cluster; R = right.
Abstract > concrete meta-analysis
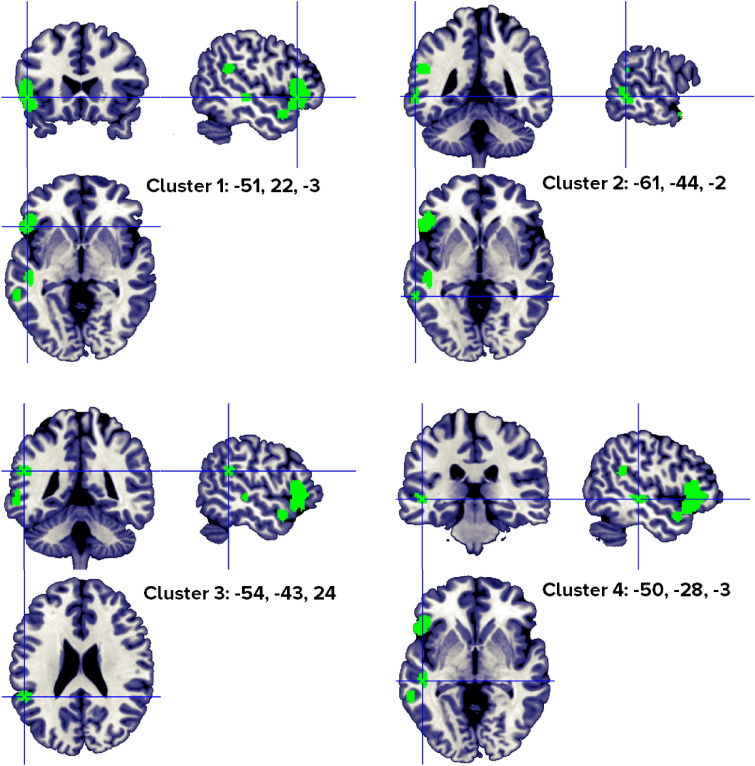
The revised ALE algorithm discriminated four clusters that correlated with abstract word processing in a healthy population (Fig. 6), from four to 12 different papers (Table 6). Our analyses identified a robust neural pattern of activity in the left frontal and temporal lobes, specifically, the inferior frontal gyrus, the superior and middle temporal gyri and left inferior parietal.
Figure 6.
Clusters activated by the abstract > concrete words contrast. The crosses are centered in the areas correspond to stereotactic coordinates reported in Table 6. The images are presented in neurological convention.
Table 6.
Abstract > concrete word clusters.
| H | Cluster | Macroanatomical location | Cytoarchitectonic Label | Weighted center (MNI; mm) | Vol. (mm3) | Peaks: MNI coordinates (mm) | ALE score | Z Score | Nr | Contributors to cluster | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Gyrus | x | y | Z | x | y | z | Studies | |||||||
| L | 1 |
Frontal, Temporal |
Inferior Frontal, Superior Temporal |
BA 45, BA 47, BA 44 | − 50.8 | 21.7 | − 3.4 | 6680 | − 52 | 24 | 4 | 0.029 | 6.369 | 12 | Perani, 1999 (2); Fiebach, 2004 (1); Sabsevitz, 2005 (3); Binder, 2005 (5); Fliessbach, 2006 (2); Pexman, 2007 (1); Rodríguez-Ferreiro, 2011 (2); Hayashi, 2014 (1); Hoffman, 2015 (3); Skipper, 2014 (2); Wang, 2019 (1); Pauligk, 2019 (2)** |
| − 48 | 20 | − 10 | 0.025 | 5.835 | |||||||||||
| − 54 | 8 | − 18 | 0.016 | 4.346 | |||||||||||
| L | 2 | Temporal | Middle Temporal | BA 22, BA 21 | − 60.5 | − 44.4 | − 1.6 | 1048 | − 60 | − 42 | − 6 | 0.016 | 4.369 | 5 | Noppeney, 2004 (1); Sabsevitz, 2005 (1); Pexman, 2007 (2); Rodríguez-Ferreiro, 2011 (2); Wang, 2019 (1)** |
| − 60 | − 48 | 4 | 0.015 | 4.219 | |||||||||||
| L | 3 |
Temporal Parietal |
Superior Temporal Inferior Parietal, |
BA 13, BA 40 | − 53.5 | − 42.9 | 24.1 | 960 | − 54 | − 42 | 24 | 0.021 | 5.112 | 4 | Hayashi, 2014 (1); Hoffman, 2015 (2); Wang, 2019 (1); Meersmans, 2020 (1)** |
| L | 4 | Temporal | Superior and Middle Temporal | BA 22, BA 21 | − 49.5 | − 27.5 | − 2.6 | 840 | − 50 | − 28 | − 4 | 0.016 | 4.350 | 4 | Sabsevitz, 2005 (1); Hoffman, 2015 (2); Kumar, 2016 (1); Wang, 2019 (1)** |
Included 146 stereotactic activation loci from 25 studies, 415 participants, chosen min. cluster size 704 mm3.
All the values and labels were extracted from the GingerALE output files. Clusters are ordered for decreasing volume size. Coordinates (x, y, z) are in the MNI space.
H = Hemisphere; ALE = activation likelihood estimation; Nr. = number of studies that contributed to each cluster; L = left; BA = Brodmann area; ** = between brackets are the number of foci from each study that contributed to that specific cluster.
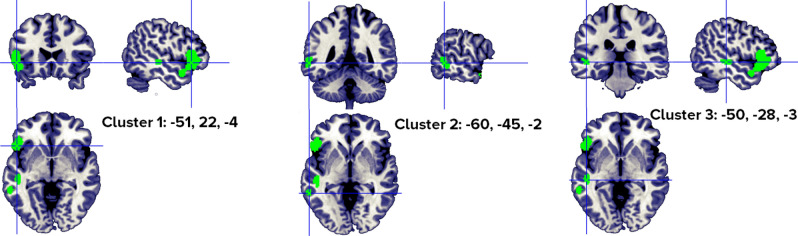
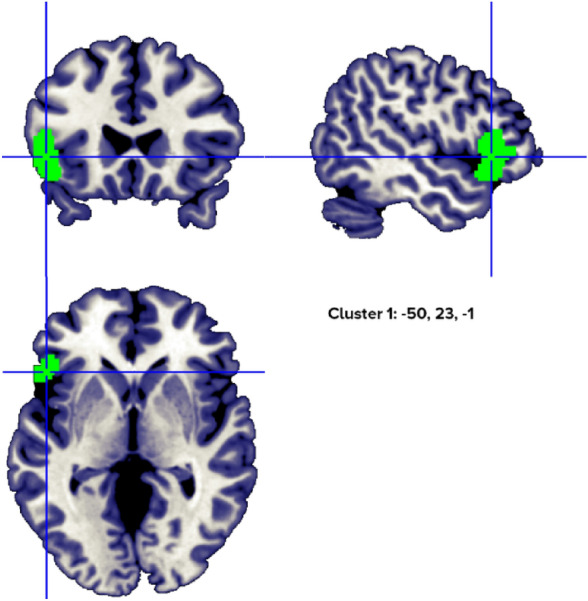
When only abstract nouns (abstract nouns > concrete nouns) were analyzed, the results indicated a single cluster with two peaks, from 9 studies, in the left inferior frontal gyrus (Fig. 7, Table 7).
Figure 7.
Clusters activated by the abstract > concrete nouns contrast. The crosses are centered in the areas correspond to stereotactic coordinates reported in Table 7. The images are presented in neurological convention.
Table 7.
Abstract > concrete nouns clusters.
| H | Cluster | Macroanatomical location | Cytoarchitectonic label | Weighted Center (MNI; mm) | Vol. (mm3) | Peaks: MNI Coordinates (mm) | ALE score | Z score | Nr | Contributors to cluster | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Gyrus | X | y | z | x | y | z | Studies | |||||||
| L | 1 | Frontal |
Inferior Frontal, Precentral |
BA 47, BA 45, BA 44 | − 50.1 | 23.4 | − 1.3 | 4520 | − 52 | 22 | 6 | 0.0253 | 6.118 | 9 | Fiebach, 2004 (1); Sabsevitz, 2005 (2); Binder, 2005 (3); Fliessbach, 2006 (2); Pexman, 2007 (1); Hayashi, 2004 (1); Skipper, 2014 (2); Wang, 2019 (1); Pauligk, 2019 (2)** |
| − 48 | 22 | − 10 | 0.0227 | 5.712 | |||||||||||
Included 99 stereotactic activation loci from 18 studies, 324 participants, chosen min. cluster size 728 mm3.
All the values and labels were extracted from the GingerALE output files. Clusters are ordered for decreasing volume size. Coordinates (x, y, z) are in the MNI space.
H = Hemisphere; ALE = activation likelihood estimation; Nr. = number of studies that contributed to each cluster; L = left; BA = Brodmann area; ** = between brackets are the number of foci from each study that contributed to that specific cluster.
We identified three clusters associated with abstract words processing in a healthy population when only studies reporting abstract visual stimuli were included (Fig. 8), from 4 to 12 different papers (Table 8). Our analyses revealed a robust neural pattern of activity in the frontal and temporal lobes, specifically, the inferior frontal gyrus and the superior and middle temporal gyri.
Figure 8.
Clusters activated by the abstract > concrete words—visual stimuli—contrast. The crosses are centered in the areas correspond to stereotactic coordinates reported in Table 8. The images are presented in neurological convention.
Table 8.
Abstract > concrete words—visual stimuli- clusters.
| H | Cluster | Macroanatomical location | Cytoarchitectonic label | Weighted Center (MNI; mm) | Vol. (mm3) | Peaks: MNI coordinates (mm) | ALE score | Z score | No | Contributors to cluster | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Gyrus | x | y | z | x | y | z | Studies | |||||||
| L | 1 |
Frontal, temporal |
Inferior frontal, superior temporal |
BA 47, BA 45, BA 38 |
− 50.7 | 21.7 | − 3.5 | 6992 | − 52 | 24 | 4 | 0.029 | 6.440 | 12 | Perani, 1999 (2); Fiebach, 2004 (1); Sabsevitz, 2005 (3); Binder, 2005 (5); Fliessbach, 2006 (2); Pexman, 2007 (1); Rodríguez-Ferreiro, 2011 (2); Hayashi, 2014 (1); Hoffman, 2015 (3); Skipper, 2014 (2); Wang, 2019 (1); Pauligk, 2019 (2)** |
| − 48 | 20 | − 10 | 0.025 | 5.900 | |||||||||||
| − 54 | 8 | − 18 | 0.016 | 4.403 | |||||||||||
| L | 2 | Temporal | Middle temporal | BA 22, BA 21 | − 60.4 | − 44.5 | − 1.7 | 1160 | − 60 | − 42 | − 6 | 0.016 | 4.425 | 5 | Noppeney, 2004 (1); Sabsevitz, 2005 (1); Pexman, 2007 (2); Rodríguez-Ferreiro, 2011 (2); Wang, 2019 (1)** |
| − 60 | − 48 | 4 | 0.015 | 4.273 | |||||||||||
| L | 3 | Temporal | Superior temporal | BA 22, BA 21 | − 49.5 | − 27.5 | − 2.6 | 904 | − 50 | − 28 | − 4 | 0.016 | 4.407 | 4 | Sabsevitz, 2005 (1); Hoffman, 2015 (2); Kumar, 2016 (1); Wang, 2019 (1)** |
Included 135 stereotactic activation loci from 22 studies, 374 participants, chosen min. cluster size 688 mm3.
All the values and labels were extracted from the GingerALE output files. Clusters are ordered for decreasing volume size. Coordinates (x, y, z) are in the MNI space.
H = Hemisphere; ALE = activation likelihood estimation; Nr. = number of studies that contributed to each cluster; L = left; BA = Brodmann area; ** = between brackets are the number of foci from each study that contributed to that specific cluster.
When only foci from lexical and semantic tasks were analyzed, the results indicated 2 clusters (with 1–4 individual peaks each, from 3 to 9 different studies), in the left inferior frontal gyrus, superior and middle temporal gyrus (Fig. 9, Table 9).
Figure 9.
Clusters activated by the abstract > concrete words -semantic and lexical task- contrast. The crosses are centered in the areas correspond to stereotactic coordinates reported in Table 9. The images are presented in neurological convention.
Table 9.
Abstract > concrete words—semantic and lexical task only- clusters.
| H | Cluster | Macroanatomical location | Cytoarchitectonic label | Weighted center (MNI; mm) | Vol. (mm3) | Peaks: MNI Coordinates (mm) | ALE score | Z Score | Nr | Contributors to cluster | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Gyrus | x | y | z | x | Y | z | Studies | |||||||
| L | 1 | Frontal, temporal |
Inferior frontal, Superior temporal |
BA 47, BA 38 | − 50.3 | 20.7 | − 6.7 | 6080 | − 48 | 20 | − 10 | 0.025 | 6.014 | 9 | Perani, 1999 (2); Fiebach, 2004 (1); Sabsevitz, 2005 (3); Binder, 2005 (4); Pexman, 2007 (1); Hoffman, 2015 (3); Skipper, 2014 (2); Wang, 2019 (1); Pauligk, 2019 (2)** |
| − 48 | 30 | − 4 | 0.017 | 4.737 | |||||||||||
| − 54 | 8 | − 18 | 0.016 | 4.497 | |||||||||||
| − 52 | 24 | 6 | 0.016 | 4.484 | |||||||||||
| L | 2 | Temporal |
Superior Temporal Middle Temporal |
BA 22, BA 21 | − 50 | − 29.2 | − 2.6 | 688 | − 50 | − 28 | − 4 | 0.015 | 4.340 | 3 | Sabsevitz, 2005 (1); Hoffman, 2015 (2); Wang, 2019 (1)** |
Included 116 stereotactic activation loci from 17 studies, 289 participants, Chosen min. cluster size 680 mm3
.
All the values and labels were extracted from the GingerALE output files. Clusters are ordered for decreasing volume size. Coordinates (x, y, z) are in the MNI space.
H = Hemisphere; ALE = activation likelihood estimation; Nr. = number of studies that contributed to each cluster; L = left; BA = Brodmann area; ** = between brackets are the number of foci from each study that contributed to that specific cluster.
As previously specified, due to the very small number of studies we could not conduct sub-analyses based on the (1) verbs only, (2) other types of tasks present in the included publications like mental image generation, memory tasks, or perceptual decision task only; (3) auditory stimuli only.
In Table 10 the descriptive information for each sub-analysis is reported.
Table 10.
Descriptive information for each sub-analysis.
| Contrasts | Inclusion criteria: | Participants | Stimuli | Stimuli presentation modality | Experimental task | ||
|---|---|---|---|---|---|---|---|
| Mean sample size | SD | Min–Max | |||||
| Words |
Imaging technique: PET or fMRI, reported stereotaxic coordinates (in the MNI or Talairach atlases), whole-brain voxel-based data analyses, more than 5 participants in each study, sample population of healthy, adult participants, reported concrete > abstract or abstract > concrete contrast, word stimuli, published in English, |
||||||
| concrete > abstract words | 16 | 5.2 | 7/28 | concrete and abstract words | visual and auditory |
passive listening passive reading perceptual task overt repetition task lexical decision memory encoding task recognition task semantic judgment semantic similarity decision semantic categorization mental image generation |
|
| abstract words > concrete words | 16.7 | 5.8 | 6/28 | ||||
| Nouns |
Imaging technique: PET or fMRI, reported stereotaxic coordinates (in the MNI or Talairach atlases), whole-brain voxel-based data analyses, more than 5 participants in each study, sample population of healthy, adult participants, reported concrete nouns > abstract nouns, or abstract nouns > concrete nouns contrast, nouns stimuli, published in English, |
||||||
| concrete nouns > abstract nouns | 16.7 | 5.6 | 7/28 | Concrete and abstract nouns | Visual and auditory |
Passive listening passive reading perceptual task overt repetition task lexical decision memory encoding task recognition task semantic judgment semantic similarity decision semantic categorization mental image generation |
|
| abstract nouns > concrete nouns | 18.1 | 5.7 | 7/28 | ||||
| visually presented stimuli |
Imaging technique: PET or fMRI, reported stereotaxic coordinates (in the MNI or Talairach atlases), whole-brain voxel-based data analyses, more than 5 participants in each study, sample population of healthy, adult participants, reported concrete > abstract or abstract > concrete contrast, visual presented stimuli, word stimuli, published in English, |
||||||
| concrete words > abstract words visual stimuli only | 16.7 | 4.9 | 9/28 | Concrete and abstract words | Visual |
Passive listening passive reading perceptual task overt repetition task lexical decision memory encoding task recognition task semantic judgment semantic similarity decision semantic categorization mental image generation |
|
| abstract words > concrete words visual stimuli only | 17.1 | 5.1 | 6/28 | ||||
| lexical and semantic tasks |
Imaging technique: PET or fMRI, reported stereotaxic coordinates (in the MNI or Talairach atlases), whole-brain voxel-based data analyses, more than 5 participants in each study, sample population of healthy, adult participants, reported concrete > abstract or abstract > concrete contrast, lexical or semantic decision tasks only, word stimuli, published in English, |
||||||
|
concrete > abstract word (only lexical and semantic tasks) |
17.1 | 5.0 | 9/28 | Concrete and abstract words | Visual and auditory |
Lexical decision semantic judgment semantic similarity decision semantic categorization |
|
|
abstract > concrete word (only lexical and semantic tasks) |
17.1 | 5.6 | 6/28 | ||||
In Appendix A (supplementary materials) we present the analyses without PET data. Except for a few clusters that have a smaller number of voxels and one cluster that fragmented into two smaller ones (for the abstract > concrete contrast), all the other clusters are perfectly overlapped (for detail see from Tables 2A, 3, 4, 5, 6, 7, 8, 9A and from Figs. 1A, 2, 3, 4, 5, 6, 7, 8A). No cluster disappeared and no new clusters were observed, indicating a good data consistency. In Appendix A we also added Table 10. A in which we presented the Brodmann area (BA) for the activated clusters with a brief description.
Discussion
As we pointed out in the introduction, neuropsychological studies suggest a role of the lateral prefrontal cortex in processing abstract words and of the left anterior temporal lobe in processing concrete ones. These data are not confirmed by neuroimaging studies. We run a meta-analysis using more stringent criteria to assess whether imaging data can support not only this segregation but also in which components the two networks differ. There are many variables that could influence our findings concerning the neural correlates, like the type of task, type of stimuli, stimuli presentation modality. We tried to control for all these factors in order to obtain accurate results. Since the number of studies was limited, we could not analyze data according to type of task (e.g., lexical decision task vs. semantic task), but at least we excluded those studies without a semantic or lexical decision task. The task performed during fMRI scan is particularly relevant because the activation observed during passively hearing/reading words might be very different from the one observed during semantic judgments for concrete vs. abstract (e.g., the decision for—which is better associated to a table: a chair or a bench?). Regarding the stimulus type, there is an ongoing debate concerning nouns vs. verbs in general38. This question becomes even more difficult when we try to separate abstract and concrete nouns, and abstract and concrete verbs (we could not analyze verbs separately for the lack of studies). Both, Wang et al.37 and Binder et al.40 combined different types of stimuli, e.g., words, sentences, fixed expressions such as idioms, and short stories without further focusing on the stimulus type. Furthermore, since Binder et al.’s40 objective was to investigate the semantic processing in general and not concrete and abstract distinction (although they run a sub-analysis on these two categories), the activation peaks meta-analyzed were obtained from different contrasts: concrete and abstract stimuli > baseline, concrete > abstract and abstract > concrete stimuli. This choice is comprehensible given their objective but the results could be biased by the type of contrast applied; indeed, discrepancies in the patterns of cortical activation across studies may be attributable, at least in part, to differences in baseline tasks, and hence, reflect the limits of the subtractive logic.
Thirty-two imaging studies were included, which evaluated the activation patterns in response to concrete and abstract concepts. All the data included in the ALEanalysis are based on general linear model, GLM. We also looked for studies that used the more modern multivariate pattern analysis, i.e., a set of methods that analyze neural responses as patterns of activity90, in order to have a separate dataset with this type of methods. Unfortunately, we found a very small number of publications preventing a further meta-analytic procedure48,91,92.
The results of this meta-analysis, consistent with those of previous ones37,40, confirmed that concrete and abstract words processing relies, at least in part, on different brain regions. Based on the currently available data we could not investigate the existence of overlapping networks between concrete and abstract words. The ALE procedure was completely data-driven, without a prior theoretical basis, and the results are constrained only by the nature of our data (e.g., the limited temporal resolution of the neuroimaging techniques, the correlational nature of the data), and by our inclusion/exclusion criteria.
As previously mentioned, experiments testing for greater activation for concrete than abstract words (concrete words > abstract words) converge in the temporo-parieto-occipital regions; namely, the left middle temporal gyrus, left fusiform, left parahippocampal and lingual gyri, bilateral angular gyrus and precuneus, bilateral posterior cingulate, left superior occipital gyrus and left culmen in the cerebellum. The neuroimaging evidence indicates that concrete concept processing is at least partly associated to the perceptual system, and also rely on mental imagery (precuneus, superior occipital gyrus). Binder et al.40 found significant overlapping for concrete stimuli in the angular gyrus bilaterally, left mid-fusiform gyrus, left posterior cingulate, and left dorsomedial prefrontal cortex (DMPFC). With the exception of DMPFC that might be related to the stimuli complexity and/or different baselines, all the other regions are confirmed by our data. At variance with Wang et al.’s meta-analysis37 we found a bilateral involvement of the posterior cingulate cortex (PCC), angular and precuneus gyri. Although involved in many semantic-based tasks, the function of the PCC in semantic cognition is still debated. The following hypothesis are proposed: (1) this region could act as a supramodal convergence zone40, (2) PCC activation could reflect the greater engagement of an imagery-based perceptual system for concrete stimuli, or (3) PCC might be an interface between semantic knowledge and episodic memory91. The precuneus also seems associated with visuospatial imagery, a hypothesis supported by experiments conducted on episodic memory retrieval and linguistic tasks which required the processing of high imagery words or mental image generation83. The same regions were found when only nouns were considered (concrete nouns > abstract nouns contrast) with the difference that the right hemisphere activation disappeared. The two right hemisphere clusters might be specifically correlated with action verbs but this result could also be a consequence of the lack of power due to the limited number of studies (15 studies in the nouns dataset vs. 22 in the noun-and-verb database).
The results on abstract words replicated those reported by Wang and colleagues37 and Binder et al.40; higher activation for abstract compared to concrete words conditions (abstract words > concrete words) is more frequently reported in a left lateralized network, encompassing the inferior frontal gyrus (IFG, Brodmann areas 45, 47), a very small portion of the precentral gyrus, the superior and middle temporal gyri, and inferior parietal. They are also in line with the results observed in brain-damaged patients.
It has been suggested that the ventrolateral prefrontal cortex (VLPFC) implements semantic control in two steps93. Step 1 constitutes controlled access to stored representations when bottom-up input is not sufficient. Step 2 operates at post-retrieval and is thought to bias competition among representations that have been activated during Step 1. According to Badre and Wagner94, both steps recruit VLPFC, though different parts of it, with BA 45 involved in Step 2. In other words, IFG activation could reflect a higher level of semantic control processes (additional resources) since abstract stimuli might require semantic selection, irrelevant cues inhibition, effortful integration, top-down control and working-memory related processes95, in agreement with the context availability theory96. In line with this hypothesis, this region showed greater activation for abstract words when a judgment task was performed following irrelevant cues and reduced activation when semantic decisions were made with contextual help, supporting the idea that this area responds more strongly to abstract words because their meanings are inherently more variable and require more control during linguistic processing as compared to the concrete ones53,97. An alternative explanation is offered by Della Rosa98 using a lexical decision task; they found that the left IFG was particularly active during presentation of words characterized by low imageability and low context availability. The authors’ interpretation was that this area could be a functional convergence zone between imageability and context availability, differentiating abstract from concrete concepts.
In neuroimaging studies, besides the IFG, additional clusters were found in the left superior and middle temporal lobe. However, when only nouns were considered (and not verbs), these clusters lost significance, supporting the idea that the cerebral networks deputed to noun and verb processing might be slightly different.
On the other side, results on concrete words do not support neuropsychological data. Indeed, apart from several single case reports, a study comparing the behavioral variant of frontotemporal dementia (FTD), in which there is a predominant prefrontal atrophy, to the semantic variant, with anterior temporal atrophy showed that while the former group had an increase of the concreteness effect, the reversal was found in the semantic variant group. Similarly, patients with left Anterior Temporal Lobe (ATL) resection show the same pattern of reversal concreteness effect33.
One possibility of this inconsistent results is the type of task; the neuroimaging studies used pleasantness judgments, memory tasks, lexical decision, etc. while, in general, patients are examined by means of naming and comprehension tasks and, occasionally, also semantic judgments. Orena et al.36, for example, using direct electrical stimulation (DES) for brain mapping during awake surgery found no behavioral differences between BA 44 and BA 38 stimulation while patients performed a lexical decision task, but they registered a dissociation between abstract and concrete words during a concreteness judgment task; in particular, abstracts words were impaired during stimulation of BA 44 and concrete words during BA 38 stimulation.
Neuroimaging studies are often hard to compare, and many variables could influence the reported results as the duration of the stimuli presentation, stimuli number, stimulus types. For example, in the same type of experiment a large number of stimuli [e.g., 164 nouns74] were presented while in other studies, only four words were repeated for more than 140 trials78. Moreover, selected stimuli greatly varied among studies encompassing emotions, mind states, living and nonliving things, of different frequency of use, age of acquisition and imageability. In addition, many studies used interchangeably “concreteness” and “imageability” , which are in fact two distinct properties that can differently affect naming and recall99–101.
We also controlled for presentation modality. When only visually presented words were included in the analysis no relevant differences were observed between auditory and visual stimuli combined, and only visually presented words (see Figs. 5, 9). This can be partially due to the very small number of studies using auditory information (only 5 studies out of 32).
Another relevant element is the participants’ age since aging can modify neural organization due to neuroplasticity102. With two exceptions69,77 in which the participants’ mean age was > 70, all other studies included a young population with a mean age < 30 (see Table 1). Neuropsychological studies (on patients) involve a different population ranging from 55 to 75. Information obtained from healthy young people cannot be optimal to interpret data from elderly, brain-damaged patients.
According to Eickhoff et al.46, the statistical power of the current meta-analysis to detect not only large, but also small- and medium-size effects can be considered acceptable. Nevertheless, meta-analytic power is intrinsically limited by the number of currently available data especially for two sub-analyses: (1) concrete nouns > abstract nouns, only 15 independent experiments, and (2) lexical and semantical task—concrete words > abstract words, 16 studies. This indicates that, in these two cases, we cannot properly control the influence of individual experiments and that we might have failed to detect small effects. Another limitation is related to the sample size of the included experiments that ranged from 6 to 28 participants. We acknowledge the need to consider only well-designed and controlled studies but taking into account the limited number of papers we were forced to include data from studies with uncorrected p values (see Table 1) risking subtle activation differences that may underlie abstract-concrete differences.
This meta-analysis is focused on how representations of abstract and concrete words are processed in the brain. Regarding this last point, future research should better understand the specific role of each region within the semantic network, how they are connected, and specify how task and stimuli characteristics interact and modify activation patterns.
Considering the main question, we can confirm that concrete and abstract words involve at least partially segregated brain areas, the IFG being relevant for abstract nouns and verbs; in contrast, we could not find evidence of the ATL involvement for concrete items. Our data indicate a more posterior activation for concrete words in regions that are often correlated with mental imagery processes. This meta-analysis seems to support the hypothesis that abstract and concrete words have partly separate neural correlates but the specific features that differentiate between these two classes of stimuli are still open to discussion. The cortical regions that are commonly activated in imaging studies investigating concrete and abstract words seem more congruent with the Dual Coding Theory10, i.e., concrete words have richer representations, depending on both hemispheres. Regarding the hub-and-spoke model (hub regions interacting with modality-specific processing areas), we observed activation patterns in areas considered neural crossroads of the semantic network like the posterior cingulate region, the anterior temporal lobe, and the left inferior frontal gyrus91,98,103 , but these data cannot be interpreted in the frame of this theoretical model.
The lack of converging evidence from clinical neuropsychological and neuroimaging data might be explained by several variables like task and stimuli type, differences in terms of age and brain plasticity between the two populations (young vs. elderly people), etc. These discrepancies deserve further investigation, for example by means of balanced groups of healthy and clinical participants, combining different techniques in the same experiment as TMS-EEG, or TMS and fMRI.
Supplementary Information
Acknowledgements
This research was supported by the Center for Mind/Brain Sciences and University of Trento and by Ph.D. program sponsors: the Autonomous Province of Trento, the Fondazione Cassa di Risparmio di Trento e Rovereto and the Municipality of Trento. We are grateful to all the authors that answered our e-mails and offered supplementary data from their studies.
Author contributions
CP wrote the first draft, reviewed it, and defined the final format of the article, MB conducted the meta-analysis and prepared tables and figures. Both authors discussed the inclusion/exclusion criteria, and reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-94506-9.
References
- 1.James CT. The role of semantic information in lexical decisions. J. Exp. Psychol. Hum. Percept. Perform. 1975;1:130–136. doi: 10.1037/0096-1523.1.2.130. [DOI] [Google Scholar]
- 2.Tyler LK, Moss HE, Jennings F. Abstract word deficits in aphasia: Evidence from semantic priming. Neuropsychology. 1995;9:354–363. doi: 10.1037/0894-4105.9.3.354. [DOI] [Google Scholar]
- 3.Howes, D. & Geschwind, N. The brain and disorders of communication. quantitative studies of aphasic language. Res. Publ. Res. Nerv. Ment. Dis.42, 229 (1964). [PubMed]
- 4.Newcombe, F., Marshall, J. C., Coltheart, M. & Patterson, K. E. Deep dyslexia. (1980).
- 5.Bub D, Kertesz A. Deep agraphia. Brain Lang. 1982;17:146–165. doi: 10.1016/0093-934X(82)90011-6. [DOI] [PubMed] [Google Scholar]
- 6.Martin N, Saffran EM. A computational account of deep dysphasia: Evidence from a single case study. Brain Lang. 1992;43:240–274. doi: 10.1016/0093-934X(92)90130-7. [DOI] [PubMed] [Google Scholar]
- 7.Franklin S, Howard D, Patterson K. Abstract word anomia. Cogn. Neuropsychol. 1995;12:549–566. doi: 10.1080/02643299508252007. [DOI] [Google Scholar]
- 8.Franklin S, Howard D, Patterson K. Abstract word meaning deafness. Cogn. Neuropsychol. 1994;11:1–34. doi: 10.1080/02643299408251964. [DOI] [Google Scholar]
- 9.Jones GV. Deep dyslexia, imageability, and ease of predication. Brain Lang. 1985;24:1–19. doi: 10.1016/0093-934X(85)90094-X. [DOI] [PubMed] [Google Scholar]
- 10.Paivio A. Dual coding theory: Retrospect and current status. Can. J. Psychol. Can. Psychol. 1991;45:255–287. doi: 10.1037/h0084295. [DOI] [Google Scholar]
- 11.Plaut, D. C. & Shallice, T. Effects of word abstractness in a connectionist model of deep dyslexia. In Proceedings of the 13th annual meeting of the Cognitive Science Society 73–78 (Erlbaum Hillsdale, NJ, 1991).
- 12.Schwanenflugel PJ, Shoben EJ. Differential context effects in the comprehension of abstract and concrete verbal materials. J. Exp. Psychol. Learn. Mem. Cogn. 1983;9:82. doi: 10.1037/0278-7393.9.1.82. [DOI] [Google Scholar]
- 13.Binder JR. In defense of abstract conceptual representations. Psychon. Bull. Rev. 2016;23:1096–1108. doi: 10.3758/s13423-015-0909-1. [DOI] [PubMed] [Google Scholar]
- 14.Binder JR, et al. Toward a brain-based componential semantic representation. Cogn. Neuropsychol. 2016;33:130–174. doi: 10.1080/02643294.2016.1147426. [DOI] [PubMed] [Google Scholar]
- 15.Reilly J, Peelle JE, Garcia A, Crutch SJ. Linking somatic and symbolic representation in semantic memory: the dynamic multilevel reactivation framework. Psychon. Bull. Rev. 2016;23:1002–1014. doi: 10.3758/s13423-015-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borghesani V, Piazza M. The neuro-cognitive representations of symbols: the case of concrete words. Neuropsychologia. 2017;105:4–17. doi: 10.1016/j.neuropsychologia.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz MF, et al. Neuroanatomical dissociation for taxonomic and thematic knowledge in the human brain. Proc. Natl. Acad. Sci. 2011;108:8520–8524. doi: 10.1073/pnas.1014935108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirman D, Landrigan J-F, Britt AE. Taxonomic and thematic semantic systems. Psychol. Bull. 2017;143:499–520. doi: 10.1037/bul0000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachoud-Lévi A-C, Dupoux E. An influence of syntactic and semantic variables on word form retrieval. Cogn. Neuropsychol. 2003;20:163–188. doi: 10.1080/02643290242000907. [DOI] [PubMed] [Google Scholar]
- 20.Breedin SD, Saffran EM, Coslett HB. Reversal of the concreteness effect in a patient with semantic dementia. Cogn. Neuropsychol. 1994;11:617–660. doi: 10.1080/02643299408251987. [DOI] [Google Scholar]
- 21.Cipolotti L, Warrington EK. Semantic memory and reading abilities: A case report. J. Int. Neuropsychol. Soc. 1995;1:104–110. doi: 10.1017/S1355617700000163. [DOI] [PubMed] [Google Scholar]
- 22.Macoir J. Is a plum a memory problem?: Longitudinal study of the reversal of concreteness effect in a patient with semantic dementia. Neuropsychologia. 2009;47:518–535. doi: 10.1016/j.neuropsychologia.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Marshall, J., Pring, T., Chiat, S. & Robson, J. Calling a salad a federation: An investigation of semantic jargon. Part 1—Nouns. J. Neurolinguistics9, 237–250 (1996).
- 24.Mattioli, F. The reverse of the concreteness effect. in October Talk presented at the 46th annual conference of the academy of Aphasia Turku (Finland) 19–21 (2008).
- 25.Papagno C, Capasso R, Miceli G. Reversed concreteness effect for nouns in a subject with semantic dementia. Neuropsychologia. 2009;47:1138–1148. doi: 10.1016/j.neuropsychologia.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Sirigu A, Duhamel J-R, Poncet M. The role of sensorimotor experience in object recognition: A case of multimodal agnosia. Brain. 1991;114:2555–2573. doi: 10.1093/brain/114.6.2555. [DOI] [PubMed] [Google Scholar]
- 27.Warrington EK. The selective impairment of semantic memory. Q. J. Exp. Psychol. 1975;27:635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- 28.Warrington EK. Concrete word dyslexia. Br. J. Psychol. 1981;72:175–196. doi: 10.1111/j.2044-8295.1981.tb02175.x. [DOI] [PubMed] [Google Scholar]
- 29.Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107:829–853. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- 30.Bonner MF, et al. Reversal of the concreteness effect in semantic dementia. Cogn. Neuropsychol. 2009;26:568–579. doi: 10.1080/02643290903512305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cousins KAQ, York C, Bauer L, Grossman M. Cognitive and anatomic double dissociation in the representation of concrete and abstract words in semantic variant and behavioral variant frontotemporal degeneration. Neuropsychologia. 2016;84:244–251. doi: 10.1016/j.neuropsychologia.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joubert S, et al. Comprehension of concrete and abstract words in semantic variant primary progressive aphasia and Alzheimer’s disease: A behavioral and neuroimaging study. Brain Lang. 2017;170:93–102. doi: 10.1016/j.bandl.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Loiselle M, et al. Comprehension of concrete and abstract words in patients with selective anterior temporal lobe resection and in patients with selective amygdalo-hippocampectomy. Neuropsychologia. 2012;50:630–639. doi: 10.1016/j.neuropsychologia.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 34.Yi H-A, Moore P, Grossman M. Reversal of the concreteness effect for verbs in patients with semantic dementia. Neuropsychology. 2007;21:9. doi: 10.1037/0894-4105.21.1.9. [DOI] [PubMed] [Google Scholar]
- 35.Crutch SJ, Warrington EK. Abstract and concrete concepts have structurally different representational frameworks. Brain. 2005;128:615–627. doi: 10.1093/brain/awh349. [DOI] [PubMed] [Google Scholar]
- 36.Orena EF, Caldiroli D, Acerbi F, Barazzetta I, Papagno C. Investigating the functional neuroanatomy of concrete and abstract word processing through direct electric stimulation (DES) during awake surgery. Cogn. Neuropsychol. 2019;36:167–177. doi: 10.1080/02643294.2018.1477748. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Conder JA, Blitzer DN, Shinkareva SV. Neural representation of abstract and concrete concepts: a meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2010;31:1459–1468. doi: 10.1002/hbm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crepaldi D, et al. Clustering the lexicon in the brain: a meta-analysis of the neurofunctional evidence on noun and verb processing. Front. Hum. Neurosci. 2013;7:303. doi: 10.3389/fnhum.2013.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNorgan C, Chabal S, O’Young D, Lukic S, Booth JR. Task dependent lexicality effects support interactive models of reading: a meta-analytic neuroimaging review. Neuropsychologia. 2015;67:148–158. doi: 10.1016/j.neuropsychologia.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 43.Turkeltaub PE, et al. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc. Cogn. Affect. Neurosci. 2007;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eickhoff SB, et al. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage. 2016;137:70–85. doi: 10.1016/j.neuroimage.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moher D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghio M, Haegert K, Vaghi MM, Tettamanti M. Sentential negation of abstract and concrete conceptual categories: a brain decoding multivariate pattern analysis study. Philos. Trans. R. Soc. B Biol. Sci. 2018;373:20170124. doi: 10.1098/rstb.2017.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero Lauro LJ, Mattavelli G, Papagno C, Tettamanti M. She runs, the road runs, my mind runs, bad blood runs between us: literal and figurative motion verbs: an fMRI study. Neuroimage. 2013;83:361–371. doi: 10.1016/j.neuroimage.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 50.Harpaintner M, Sim E, Trumpp NM, Ulrich M, Kiefer M. The grounding of abstract concepts in the motor and visual system: an fMRI study. Cortex. 2020;124:1–22. doi: 10.1016/j.cortex.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 51.Mellet E, Tzourio N, Denis M, Mazoyer B. Cortical anatomy of mental imagery of concrete nouns based on their dictionary definition. NeuroReport. 1998;9:803–808. doi: 10.1097/00001756-199803300-00007. [DOI] [PubMed] [Google Scholar]
- 52.Kiehl KA, et al. Neural pathways involved in the processing of concrete and abstract words. Hum. Brain Mapp. 1999;7:225–233. doi: 10.1002/(SICI)1097-0193(1999)7:4<225::AID-HBM1>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffman P, Binney RJ, Lambon Ralph MA. Differing contributions of inferior prefrontal and anterior temporal cortex to concrete and abstract conceptual knowledge. Cortex. 2015;63:250–266. doi: 10.1016/j.cortex.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Dam WO, van Dijk M, Bekkering H, Rueschemeyer S-A. Flexibility in embodied lexical-semantic representations. Hum. Brain Mapp. 2012;33:2322–2333. doi: 10.1002/hbm.21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Dam WO, van Dongen EV, Bekkering H, Rueschemeyer S-A. Context-dependent changes in functional connectivity of auditory cortices during the perception of object words. J. Cogn. Neurosci. 2012;24:2108–2119. doi: 10.1162/jocn_a_00264. [DOI] [PubMed] [Google Scholar]
- 56.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 1988. Theime Stuttgart Ger. 1988;270:90125–90128. [Google Scholar]
- 57.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- 58.Eickhoff SB, et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fornara GA, Papagno C, Berlingeri M. A neuroanatomical account of mental time travelling in schizophrenia: a meta-analysis of functional and structural neuroimaging data. Neurosci. Biobehav. Rev. 2017;80:211–222. doi: 10.1016/j.neubiorev.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 60.Papitto G, Friederici AD, Zaccarella E. The topographical organization of motor processing: An ALE meta-analysis on six action domains and the relevance of Broca’s region. Neuroimage. 2020;206:116321. doi: 10.1016/j.neuroimage.2019.116321. [DOI] [PubMed] [Google Scholar]
- 61.Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA. distinct brain systems for processing concrete and abstract concepts. J. Cogn. Neurosci. 2005;17:905–917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- 62.D’Esposito M, et al. A functional MRI study of mental image generation. Neuropsychologia. 1997;35:725–730. doi: 10.1016/S0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- 63.Fiebach CJ, Friederici AD. Processing concrete words: fMRI evidence against a specific right-hemisphere involvement. Neuropsychologia. 2004;42:62–70. doi: 10.1016/S0028-3932(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 64.Fliessbach K, Weis S, Klaver P, Elger CE, Weber B. The effect of word concreteness on recognition memory. Neuroimage. 2006;32:1413–1421. doi: 10.1016/j.neuroimage.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 65.Giesbrecht B. Separable effects of semantic priming and imageability on word processing in human cortex. Cereb. Cortex. 2004;14:521–529. doi: 10.1093/cercor/bhh014. [DOI] [PubMed] [Google Scholar]
- 66.Harris GJ, et al. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 67.Hayashi A, et al. Visual imagery while reading concrete and abstract Japanese kanji words: an fMRI study. Neurosci. Res. 2014;79:61–66. doi: 10.1016/j.neures.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Jessen F, et al. The concreteness effect: evidence for dual coding and context availability. Brain Lang. 2000;74:103–112. doi: 10.1006/brln.2000.2340. [DOI] [PubMed] [Google Scholar]
- 69.Kounios J, et al. Category-specific medial temporal lobe activation and the consolidation of semantic memory: evidence from fMRI. Cogn. Brain Res. 2003;17:484–494. doi: 10.1016/S0926-6410(03)00164-2. [DOI] [PubMed] [Google Scholar]
- 70.Rodríguez-Ferreiro J, Gennari SP, Davies R, Cuetos F. Neural correlates of abstract verb processing. J. Cogn. Neurosci. 2011;23:106–118. doi: 10.1162/jocn.2010.21414. [DOI] [PubMed] [Google Scholar]
- 71.Roxbury T, McMahon K, Copland DA. An fMRI study of concreteness effects in spoken word recognition. Behav. Brain Funct. 2014;10:34. doi: 10.1186/1744-9081-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rüschemeyer SA, Brass M, Friederici AD. Comprehending prehending: Neural correlates of processing verbs with motor stems. J. Cogn. Neurosci. 2007;19:855–865. doi: 10.1162/jocn.2007.19.5.855. [DOI] [PubMed] [Google Scholar]
- 73.Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. Neuroimage. 2005;27:188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 74.Skipper LM, Olson IR. Semantic memory: distinct neural representations for abstractness and valence. Brain Lang. 2014;130:1–10. doi: 10.1016/j.bandl.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tyler LK. The neural representation of nouns and verbs: PET studies. Brain. 2001;124:1619–1634. doi: 10.1093/brain/124.8.1619. [DOI] [PubMed] [Google Scholar]
- 76.van Dam WO, Rueschemeyer S-A, Bekkering H. How specifically are action verbs represented in the neural motor system: an fMRI study. Neuroimage. 2010;53:1318–1325. doi: 10.1016/j.neuroimage.2010.06.071. [DOI] [PubMed] [Google Scholar]
- 77.Whatmough C, Verret L, Fung D, Chertkow H. Common and contrasting areas of activation for abstract and concrete concepts: an H 2 15 O PET study. J. Cogn. Neurosci. 2004;16:1211–1226. doi: 10.1162/0898929041920540. [DOI] [PubMed] [Google Scholar]
- 78.Wilson-Mendenhall CD, Simmons WK, Martin A, Barsalou LW. Contextual processing of abstract concepts reveals neural representations of nonlinguistic semantic content. J. Cogn. Neurosci. 2013;25:920–935. doi: 10.1162/jocn_a_00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhuang J, Randall B, Stamatakis EA, Marslen-Wilson WD, Tyler LK. The Interaction of lexical semantics and cohort competition in spoken word recognition: an fMRI study. J. Cogn. Neurosci. 2011;23:3778–3790. doi: 10.1162/jocn_a_00046. [DOI] [PubMed] [Google Scholar]
- 80.Grossman M, et al. The neural basis for category-specific knowledge: an fMRI study. Neuroimage. 2002;15:936–948. doi: 10.1006/nimg.2001.1028. [DOI] [PubMed] [Google Scholar]
- 81.Kumar U. Neural dichotomy of word concreteness: a view from functional neuroimaging. Cogn. Process. 2016;17:39–48. doi: 10.1007/s10339-015-0738-1. [DOI] [PubMed] [Google Scholar]
- 82.Noppeney U, Price CJ. Retrieval of abstract semantics. Neuroimage. 2004;22:164–170. doi: 10.1016/j.neuroimage.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 83.Perani D, et al. The neural correlates of verb and noun processing. A PET study. Brain. 1999;122:2337–2344. doi: 10.1093/brain/122.12.2337. [DOI] [PubMed] [Google Scholar]
- 84.Pexman PM, Hargreaves IS, Edwards JD, Henry LC, Goodyear BG. Neural correlates of concreteness in semantic categorization. J. Cogn. Neurosci. 2007;19:1407–1419. doi: 10.1162/jocn.2007.19.8.1407. [DOI] [PubMed] [Google Scholar]
- 85.Vigliocco G, et al. The neural representation of abstract words: the role of emotion. Cereb. Cortex. 2014;24:1767–1777. doi: 10.1093/cercor/bht025. [DOI] [PubMed] [Google Scholar]
- 86.Meersmans K, et al. Representation of associative and affective semantic similarity of abstract words in the lateral temporal perisylvian language regions. Neuroimage. 2020;217:116892. doi: 10.1016/j.neuroimage.2020.116892. [DOI] [PubMed] [Google Scholar]
- 87.Pauligk S, Kotz SA, Kanske P. Differential impact of emotion on semantic processing of abstract and concrete words: ERP and fMRI evidence. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-50755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Wang B, Bi Y. Close yet independent: dissociation of social from valence and abstract semantic dimensions in the left anterior temporal lobe. Hum. Brain Mapp. 2019;40:4759–4776. doi: 10.1002/hbm.24735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roxbury T, McMahon K, Coulthard A, Copland DA. An fMRI study of concreteness effects during spoken word recognition in aging. Preservation or attenuation? Front. Aging Neurosci. 2015;7:240. doi: 10.3389/fnagi.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haxby JV, Connolly AC, Guntupalli JS. Decoding neural representational spaces using multivariate pattern analysis. Annu. Rev. Neurosci. 2014;37:435–456. doi: 10.1146/annurev-neuro-062012-170325. [DOI] [PubMed] [Google Scholar]
- 91.Gao C, et al. Distinguishing abstract from concrete concepts in supramodal brain regions. Neuropsychologia. 2019;131:102–110. doi: 10.1016/j.neuropsychologia.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 92.van Dam WO, et al. Distinct neural mechanisms underlying conceptual knowledge of manner and instrument verbs. Neuropsychologia. 2019;133:107183. doi: 10.1016/j.neuropsychologia.2019.107183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nozari N, Thompson-Schill SL. Left ventrolateral prefrontal cortex in processing of words and sentences. In: Hickok G, Small SL, editors. Neurobiology of language. Amsterdam: Elsevier; 2016. pp. 569–584. [Google Scholar]
- 94.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 95.Jefferies E. The neural basis of semantic cognition: Converging evidence from neuropsychology, neuroimaging and TMS. Cortex. 2013;49:611–625. doi: 10.1016/j.cortex.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 96.Schwanenflugel PJ, Kippharnishfeger K, Stowe RW. Context availability and lexical decisions concrete words. J. Mem. Lang. 1988;27:499–520. doi: 10.1016/0749-596X(88)90022-8. [DOI] [Google Scholar]
- 97.Davey J, et al. Shared neural processes support semantic control and action understanding. Brain Lang. 2015;142:24–35. doi: 10.1016/j.bandl.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Della Rosa PA, Catricalà E, Canini M, Vigliocco G, Cappa SF. The left inferior frontal gyrus: A neural crossroads between abstract and concrete knowledge. Neuroimage. 2018;175:449–459. doi: 10.1016/j.neuroimage.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 99.Boles DB. Dissociated imageability, concreteness, and familiarity in lateralized word recognition. Mem. Cognit. 1983;11:511–519. doi: 10.3758/BF03196988. [DOI] [PubMed] [Google Scholar]
- 100.Connell L, Lynott D. Strength of perceptual experience predicts word processing performance better than concreteness or imageability. Cognition. 2012;125:452–465. doi: 10.1016/j.cognition.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 101.Richardson JTE. Concreteness and imageability. Q. J. Exp. Psychol. 1975;27:235–249. doi: 10.1080/14640747508400483. [DOI] [PubMed] [Google Scholar]
- 102.Fei N, Ge J, Wang Y, Gao J-H. Aging-related differences in the cortical network subserving intelligible speech. Brain Lang. 2020;201:104713. doi: 10.1016/j.bandl.2019.104713. [DOI] [PubMed] [Google Scholar]
- 103.Xu Y, Lin Q, Han Z, He Y, Bi Y. Intrinsic functional network architecture of human semantic processing: Modules and hubs. Neuroimage. 2016;132:542–555. doi: 10.1016/j.neuroimage.2016.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.