Abstract
Nerves are commonly injured in case of blunt or penetrating trauma to the extremities. Patients with nerve injuries have profound consequences and thus a timely decision for operative management is a very important. Conventionally, management decisions have been based on clinical findings, patient course and electrophysiological studies. However, imaging modalities have an enormous role not only in localizing and grading of the nerve injuries but also in the follow-up of the nerve recovery. High-resolution ultrasound (HUS) is the modality of choice for evaluation of peripheral nerves. Magnetic resonance neurography (MRN) plays a complementary role, enabling better assessment of muscle changes and deeper nerves. Corresponding to the injured layer of the cross-section of the nerve, imaging manifestations differ in different grades of injury. Since imaging cannot detect ultrastructural changes at the microscopic level, thus there may be overlap in the imaging findings. Herewith, we discuss the imaging findings in different grades of nerve injury and propose a simple 3-tier grading for imaging (HUS and MRN) assessment of peripheral nerve injuries.
Keywords: Peripheral nerve injuries, High-resolution ultrasound (HUS), Magnetic resonance, Neurography (MRN)
1. Introduction
The global prevalence of traumatic peripheral nerve injuries is approximately 5%, including brachial and lumbar plexus injuries.1 These are an important cause of morbidity and disability in young individuals. The patients can present acutely (in case of trauma) or have a chronic course (due to chronic stretching or overuse microtrauma). The clinical presentation is in the form of either motor weakness/sensory impairment or tingling/numbness involving the nerve. Penetrating trauma is the most direct mechanism of nerve injury and may not carry a poor prognosis due to clean-cut ends. Sometimes, the nerves may get injured inadvertently during surgery or any other intervention e.g. secondary to interposition of the nerve during reduction of fractured bones, stretching injuries or due to impingement by screws or other implants. The radial, ulnar, and median nerves are reported to be the most commonly affected following trauma of the upper limbs; while the sciatic, peroneal, tibial, and femoral nerves are most often affected in the lower limbs.2 Upper limb nerves are more commonly affected because of higher chance of trauma to upper limbs.
2. Mechanism of injury
Traumatic peripheral nerve injuries can result from varied mechanisms. They can be due to blunt trauma, penetrating injuries, chronic traction/acute stretch injuries and less commonly due to local chemical injury, electric shock or freeze injury. Acute severe injury can result from direct compression of the nerve against rigid structures like bone or by penetrating trauma. Indirect compression of the nerve by hematoma, fracture fragment, aneurysm or scar usually results in subacute to chronic presentation. Chronic stretch injuries may be due to overuse microtrauma (profession and sports-related), dislocations or iatrogenic injuries and are usually mild. The injuries caused by explosions are associated with poor prognosis. It may be noted that the nerves are usually affected in combination with the adjacent tissues; and the management of the associated bony injuries, vascular injury or soft tissue loss usually takes priority.
3. Patient evaluation and role of imaging
Evaluation of peripheral nerve trauma and its postsurgical outcome has classically been based on clinical and electrophysiological methods.3,4 In addition to the initial clinical symptoms, the course of the neurological manifestations is also important. Sometimes the patients present late; because of missed initial diagnosis or lack of referral of the patients from peripheral centers. Electrophysiological studies (EPS) include measuring nerve conduction velocity (NCV) and electromyography (EMG). The function of the peripheral nerve is assessed by electrical stimulation of the nerve and recording the response at the muscle or the nerve.5,6 It can be used for sensory, motor as well as mixed nerves. The response elicited gives valuable information regarding demyelination/axonal loss of the testing nerve. The motor NCV is performed by giving a supramaximal stimulus to the testing nerve proximally and evaluating the response distally through an electrode placed inside the muscle supplied by testing nerve. The compound muscle action potential (CMAP) so generated is recorded by the electrode and NCV is calculated, using formula; NCV = distance/latency, where distance is the length between stimulating and response recording electrode and latency is the time taken from onset of stimulation to onset of response. EMG is performed to record the intrinsic electrical activity of the muscle, by placing an electrode into the muscle.5,6 Observations are made at rest, minimal voluntary contraction and after maximal contraction of the muscle. The MUP (Motor unit potential), so recorded is assessed for amplitude & duration, which are relatively constant for different muscle fibers. Abnormality in EMG is manifested by fibrillation or fasciculation. Similarly, sensory NCV is performed by electrical stimulation of the peripheral nerve and recording from a purely sensory portion of the nerve.
Limitations of EPS and Clinical features: The major decision in nerve injuries is whether the patient requires surgery or is likely to improve by conservative management. Up to half of all traumatic peripheral nerve injuries may require surgical intervention.1 Sometimes, the site and extent of injury however remains uncertain after physical examination and EMG due to unequivocal findings.7 EPS cannot reliably differentiate between axonotmesis and neurotmesis.7 EPS findings vary as per the timing since injury and EMG muscle changes become apparent after 3 weeks from injury. It has been found that a certain group of patients with high grade nerve injury may actually benefit from early surgery and thus this group cannot be detected based on EPS.8 The precise extent of nerve damage and whether to proceed conservatively or with surgical repair, thus may not be optimally decided with the combination of neurological examination and EMG.7
High-resolution ultrasound (HUS) and magnetic resonance neurography (MRN) are the main imaging modalities for evaluation of nerve injuries.9 HUS and MRN are considered adjunct to each other and both have their advantages and limitations. HUS is an easy, effective, and economical modality in evaluating peripheral nerve injuries. Obtaining preoperative, anatomical information of the injured nerve is crucial for surgical planning. Table 1 lists the role of imaging in evaluation of nerve injuries. Before imaging, it is better to know the nature of trauma, identify the exact anatomical region injured, look for scar corresponding to penetrating injuries, review the radiographs for fractures and implants and analyze the surgical details, if any. The imaging findings must be interpreted in context of the clinical course and EPS tests.
Table 1.
Goals of Imaging in evaluation of peripheral nerve injuries.
|
|
|
|
|
|
|
|
|
HUS has the highest spatial resolution to evaluate the morphologic appearance of the injured nerve, giving information on location, extent, nerve continuity or transection, presence of impingement or foreign body. Though a detailed discussion on optimal positioning of the patient for HUS evaluation of nerves is beyond the scope of the current article, we have shown some of these in Fig. 1. It can also be used for surface marking before the surgical repair of the nerve. However, HUS shows limited reproducibility compared with MRI, as it depends on operator's skills, requiring expertise to achieve an accurate diagnosis of peripheral nerve injury.10 MRN provides better assessment of secondary denervation changes in the muscles. Table 2 provides an overview of advantages and limitations of both modalities for nerve evaluation. It may be noted here that areas of dense scarring/architectural distortion may complicate imaging interpretation and thus both HUS and MRN may complement each other in accurate assessment. Radiographs and CT enable assessment of fracture site, extruded fragments, and metallic implants.
Fig. 1.
Examples of patient positioning while evaluating peripheral nerves. (a) and (b) show the arm and elbow positioning while evaluating the median nerve. (c) depicts abducted arm for examination of medially located ulnar nerve while (d) shows the positioning for examination of the ulnar nerve at the elbow (patient back towards the examiner). (e) demonstrates the examination of radial nerve in the posterior arm while (f) shows examination of radial nerve in the lateral aspect of distal arm. (g) depicts prone positioning of the patient for assessing sciatic nerve and its divisions. (h) demonstrates evaluation of the tibial nerve posterior to the medial malleolus.
Table 2.
Strengths and weaknesses of HUS and MRN in evaluation of trauma to the peripheral nerves.
| Ultrasound | MRI | |
|---|---|---|
| Strengths |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
| ||
| ||
| ||
| ||
| Limitations |
|
|
|
|
|
|
|
|
|
4. Imaging protocol
First, the nerve in question is focused on the HUS corresponding to anatomical landmarks. Once the nerve is localized in the short axis it is traced cranially and caudally to see for echogenicity, fascicular pattern, contour, internal heterogeneity, and its course. If any abnormality is encountered, then the attention is focused on that particular segment. It is advisable to use the highest possible frequency for visualization of nerves. We can obtain good quality images using a linear high-frequency probe (up to 15 MHz) in majority cases, using a good amount of transmission jelly. Hockey stick probe, with superficial MSK preset, has frequency up to 20 MHz and can be used to better visualize the nerves in their superficial course, like the ulnar nerve behind the medial epicondyle. A Lower-frequency probe may be required for nerves located in deep locations (like the sciatic nerve in the gluteal region).
The anatomical localization site for the ulnar nerve is behind the medial epicondyle or ulnar aspect of the wrist; for the median nerve is the carpal tunnel at the wrist or cubital fossa (where the nerve lies medial to the brachial artery); for the radial nerve is the spiral groove in the arm (posteriorly) or anterolaterally in cubital fossa (where it lies between brachioradialis and brachialis and then divides into 2 branches). Similarly, for the lower limb, the sciatic nerve can be localized in the subgluteal region and tibial & peroneal nerves at the popliteal fossa. Both short-axis and long-axis views of the nerve should be carefully assessed, and cine loops may be recorded while scanning the nerve. Muscle echotexture and bulk should also be assessed. Acute edema is echogenic and gives rise to heterogeneous echotexture of muscles. Chronic changes are characterized by echogenic muscle atrophy.
Suggested protocol for MR neurography is shown in Table 3. Sequences which are most useful include a coronal T2-weighted (T2W)/proton density (PD) sequence, thin-section (2–3 mm) T2W, T2W FS and T1W images. Additional sequences may include a sagittal T2W sequence or a three-dimensional T2W/STIR isotropic sequence. Another sequence which has been suggested is 3D diffusion-weighted (DW) Reversed steady state in free precession (PSIF) with low b-values.11 This provides T2 dominant contrast with suppression of soft-tissue edema and hyperintense signal of vessels. This sequence has been found to be better than 2D T2W FS images for conspicuity of nerves.12 Functional imaging of the nerves can be done by using Volume Diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI). The nerves can be followed in the axial plane while scrolling on a workstation, keeping the key anatomical relations in mind. Fat suppressed fluid-sensitive sequences (STIR/T2W FS) are important to comment on the signal intensity while nonfat-suppressed sequences better provide the anatomical information.
Table 3.
MR Neurography (MRN) sequences.
|
|
|
|
|
|
|
|
|
|
|
DWI is a functional imaging which can detect and quantify movement of water molecules in biological tissues.13,14 The degree of water displacement can be quantified using apparent diffusion coefficient (ADC). ADC is a measure of degree of water displacement (in square millimeters) per time unit (seconds). In peripheral nerves, the movement of water molecules is along the long axis of nerve, due to which these nerves appear bright on high b-value images and dark on ADC maps, reflecting restricted diffusion along short axis.15, 16, 17 The recommended maximum b value for DWI neurography ranges from 600 to 800 s/mm2, enabling adequate visualization of peripheral nerves with an acceptable SNR.
DTI can detect and quantify movement of water molecule in a particular direction. It is widely used in CNS for white matter fiber tracking.18 Minimum six diffusion directions are required to obtain the diffusion tensor matrix, which enables to determine the main direction of water molecules through three eigenvectors with their respective eigenvalues. Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD), are some of the quantitative measures that can be calculated to characterize the pathological nerve.11 FA is the most important parameter derived from DTI and allows creation of 3D neurographic reconstructions. FA indicates the degree of fiber organization, ranging from 0 (minimum anisotropy) to 1 (maximum anisotropy).19,20 Higher field strength yields images with better signal and contrast resolution, thus 3 T is preferable. Contrast administration is not required in evaluation of peripheral nerve trauma.
5. Imaging anatomy
The peripheral nerve is composed of myelinated axons, which are covered by thin connective tissue called endoneurium. These myelinated axons (surrounded by endoneurium) are bundled together to form fascicles, which in turn are covered by thin connective tissue layer called perineurium. Fascicles are interspersed with connective tissue of the inner epineurium and then wrapped by outer epineurium.
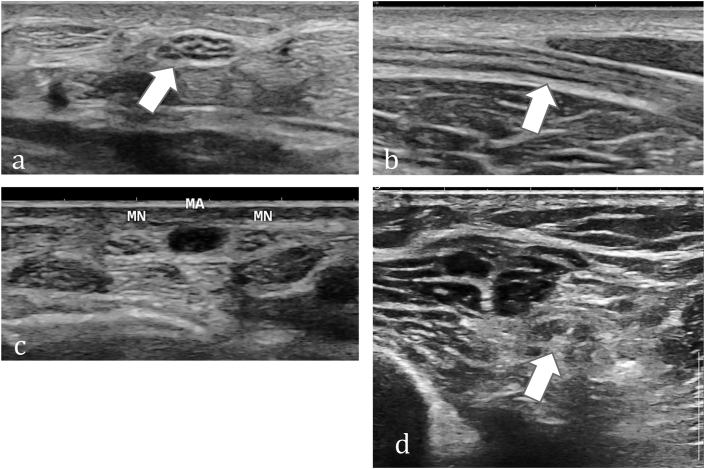
Normal peripheral nerves have a typical sonographic appearance (Fig. 2), showing multiple alternating hypoechoic & hyperechoic bands. This is referred to as ‘speckled’ or ‘honeycombed’ appearance in cross-section (axial image) and fascicular appearance on longitudinal image. This is distinct from ‘fibrillary’ appearance of tendons in longitudinally and broom-end appearance in cross-section.21 Each of these longitudinal hypoechoic bands represent fascicular bundles, surrounded by perineurium, and separated by discontinuous bands of increased echogenicity, corresponding to the epineurial connective tissue. The outer epineurium is represented by the well-defined echogenic rim of the nerve in cross-section. On MRN images, the fascicles appear isointense to muscle and the surrounding perineural and epineural fat appears hyperintense (Fig. 3). On fat-suppressed images, nerve is isointense or slightly hyperintense on T2W images (Fig. 3). Muscles are hypointense on all MRI sequences.
Fig. 2.
Imaging appearance of normal peripheral Nerves on ultrasound. (a) Axial ultrasound image shows speckled appearance in cross-section of the median nerve with hypoechoic fascicles and echogenic connective tissue. (b) Shows fascicular pattern in the longitudinal section of the median nerve.(c) Axial ultrasound image in a different patient depicts duplicated median nerve (MN) with persistent median artery (MA) in between.(d) Axial ultrasound image in a different patient depicts speckled appearance in cross-section of the sciatic nerve.
Fig. 3.
Imaging appearance of Normal peripheral Nerves on MRI (a) Axial T1W, (b) T2W (c) T1W fat-suppressed (FS) and (d) T2W FS turbo-spin-echo (TSE) images show the fascicular pattern of normal tibial (block arrow) and peroneal (thin arrow) nerves. (e) Diffusion-weighted color-coded fiber tractography image depicts the fibers of these nerves after delineating the corresponding regions of interest (ROI). (f) Diffusion tensor imaging (DTI) image shows tibial nerve overlapped with the T2W FS image shows corresponding color-coded FA map (inset in f) (FA value – 0.52 and ADC value 1.39 × 10−3 mm2/s).(g) Coronal thick maximal intensity projection (MIP) image from volume diffusion-weighted imaging in a different patient with old right sciatic nerve injury depicts neuroma-in-continuity in the gluteal region (block arrow) with atrophy and reduced signal intensity of RT sciatic nerve compared to the left (thin arrows).
6. Classification of nerve injuries
In 1943 Sir Herbert Seddon22 classified the nerve injuries into three types: neurapraxia, axonotmesis and neurotmesis (in increasing order of severity) (Table 4). Neuropraxia is characterized by demyelination, leading to transient conduction block along the nerve. No anatomical disruption is seen. Axonotmesis is characterized by axonal injury and Wallerian degeneration but endoneurial tubes and surrounding connective tissue elements remain intact, allowing axonal regeneration. Neurotmesis is the most severe, characterized by discontinuity of the nerve, which can be partial or complete. EPS tests are usually normal in neuropraxia, show reduced conduction in axonotmesis and loss of conduction in neurotmesis, though there may be overlap in latter two. Though simple to comprehend, this classification is limited in utility because it does not give any information regarding differential involvement of various connective tissue layers, which is important from a surgical perspective and nerve recovery potential.
Table 4.
Seddon classification.
| Severity | Seddon type | Clinical feature | Due to | Recovery |
|---|---|---|---|---|
| Mild | Neuropraxia | Temporary sensory loss | Demyelination | Complete, in 6–8 weeks |
| Moderate | Axonotmesis | Motor & sensory loss | Axonal loss | Complete, in few months |
| Severe | Neurotmesis | Motor & sensory loss | Physical discontinuity | Needs surgery |
In 1951 Sunderland23 classified peripheral nerve injuries into five degrees (Table 5). The first and second degree correspond to Seddon's neuropraxia and axonotmesis respectively. Third to fifth degrees are based on extent of injury to the connective tissue. The third degree refers to injury to the endoneurium, fourth to the endoneurium and perineurium and the fifth degree corresponds to injury to all three connective tissue layers and reflects complete transection. There is ambiguity in literature regarding whether the Sunderland grade 3 and 4 injuries correspond to severe axonotmesis, neurotmesis or are distinct.24,25
Table 5.
Sunderland Classification of nerve injuries.
| Seddon type | Grade | Structures injured | Key features | Management |
|---|---|---|---|---|
| Neuropraxia | 1 | Demyelination/conduction defect | Normal | Conservative |
| Axonotmesis | 2 | Axonal loss | Diffuse swelling | |
| 3 | Endoneurium | Intact but edematous fascicles | Slow & incomplete recovery; surgery may be needed, especially to relieve external compression | |
| 4 | Perineurium | Fascicular discontinuity (Neuroma-in-continuity) | Surgery | |
| Neurotmesis | 5 | Epineurium | Nerve transection with end-bulb neuromas |
Both these classification systems are not imaging-based; rather correlation with electrophysiological studies and clinical course has been described along with. A Grade 6 (mixed-type) injury has been described by Mackinnon and Dellon and refers to involvement of various layers across the cross-section of the nerve (not necessarily traditional inside-outside as per Sunderland).26
7. Imaging findings of nerve injury
Majority of the literature is on imaging findings of nerve injury, rather than listing the findings as per grade of injury. The imaging findings can be direct changes in the nerve or secondary changes in the muscles (Table 6). Signal intensity similar to vessel or fluid on T2W image is a definitive sign of nerve abnormality. Discontinuity of the nerve with end-neuroma formation is diagnostic of complete tear of the nerve. Compression of the nerve by fractured bone/metallic implant can also be seen.27 Chronic Wallerian degeneration happens in distal segment of the injured nerve and may lead to echogenic atrophic nerve with decreased caliber & increased intraneural fat. Acute denervation edema manifests as increased signal on T2W images and may become apparent as early as within 48 h of injury. However due to associated direct soft tissue injury, this finding may be overlooked. The edema becomes less apparent after few months and later there is progressive atrophy and fatty replacement.
Table 6.
Imaging findings of peripheral nerve injuries.
| US | MRI |
|---|---|
| Direct signs | |
|
|
|
|
|
|
|
|
|
|
| |
| Indirect signs (Muscle denervation changes) | |
|
|
|
|
In 2014, Chhabra et al.25 delineated imaging features on MR neurography, corresponding to each grade of injury. Corresponding to Sunderland classification, the MRI features were described as: Grade I injury – T2 hyperintensity, Grade-II injuries cause diffuse nerve swelling and T2 hyperintensity, Grade III produces fascicular thickening, Grade IV manifests as focal nerve enlargement with fascicular discontinuity and heterogeneous signal intensity while Grade V injury manifests as nerve transection, with clear gap and intervening hemorrhage and/or fibrosis. The proximal nerve segment shows end-bulb neuroma. Muscle denervation changes are usually consistently seen from grade 3 onwards, though may be seen in grade 2 as well.
Few other researchers24,28, 29, 30, 31 have also tried to categorize the imaging findings as per severity but there is some overlap amongst the different grades. We hereby propose a simplified imaging classification based on literature and our experience, which is helpful in deciding management (Table 7, Fig. 4). This categorizes imaging findings on both ultrasound and MRI. Edema is labelled when there is hypoechogenicity on HUS or T2 hyperintensity (compared to proximal segment of nerve).32, 33, 34 Bulkiness refers to increased cross-sectional area (at least 20%) compared to the immediate proximal segment.34 In the presence of edema, it may be difficult to appreciate the fascicles separate from the (inner) epineural fat, but attention must be paid to any disruption of the internal architecture. While sometimes it may be possible to suggest the exact Sunderland grade of injury (based on clinico-radiologic-electrophysiological findings), at times it may be difficult and thus this simplified classification may be used to decide management. Presence of mild diffuse changes suggest grade 1–2 injury ((Fig. 5, Fig. 6) while focal fascicular changes in the nerve suggests a moderate grade (Fig. 7, Fig. 8, Fig. 9). Disruption of the fascicles/loss of internal architecture with intact epineurium is hallmark of grade 4 injury and better appreciated on longitudinal ultrasound images. In cross-section, this may be inferred from the loss of speckled pattern and may involve part or whole of the thickness of the nerve. There may be overlap in the imaging findings of grade 3 and 4 injury because of difficulty in differentiating fascicular thickening from disruption. In grade 5 injury, it is important to look for the status of interposed tissues between retracted nerve ends (Fig. 10, Fig. 11). Size of neuroma is also important because these are usually resected during surgery and thus this needs to be added to the size of the gap between the retracted ends.
Table 7.
Simplified imaging-based classification of nerve injuries with corresponding findings on ultrasound and MRI.
| Severity | Sunderland Grade | Injured structures | Imaging findings | Management | |
|---|---|---|---|---|---|
| Mild | 1 | Demyelination | Nerve is Normal or shows mild edema | Nerve is Normal or shows Diffuse changes | Non-operative management |
| 2 | Axonal loss (intact connective tissue) | Edema and Bulkiness∗ (distal to site of injury) |
|||
| Moderate | 3 | Endoneurium | Focally enlarged nerve (thickened fascicles) with altered echogenicity (Heteroechoic/hypoechoic) and heterogeneous signal intensity | Focal changes [Fascicles echogenic/enlarged (thickened)/effaced/disrupted] | Usually needs surgery, especially with grade 4 injury |
| 4 | Perineurium | Disruption of the fascicles/Focal loss of internal architecture with intact epineurium (Neuroma-in-continuity) |
|||
| Severe | 5 | Epineurium | Epineurium discontinuity (in addition to fascicular disruption) | Surgery | |
| +/− Nerve gap with end neuromas | |||||
•Edema and bulkiness are most apparent acutely and may resolve with time.
Fig. 4.
Schematic representation of different grades of nerve injuries (Sunderland). (a) Normal appearing Nerve in grade 1 injury, (b) mild bulkiness (nerve edema) in grade II injury, (c) Focal bulkiness and fascicular thickening in grade III injury, (d) Focal fascicular disruption in grade 4 injury and (e) nerve transection with discontinuity of epineurium in grade 5 injury. The individual nerve fascicles are covered by perineurium (arrows in a) while the white area between the fascicles represents the connective tissue of the inner epineurium. Outer epineurium covers the entire nerve (arrowheads in a).
Fig. 5.
Imaging findings in Neuropraxia (Sunderland Grade 1 injury).(a) Axial ultrasound image in a patient with sensory loss in radial nerve distribution after humeral shaft fracture shows normal appearing radial nerve adjacent to the bony fragment. The sensory loss significantly improved spontaneously within two months. Findings are consistent with neuropraxia with normal ultrasound appearance. (b) Axial T2W FS image in a different patient shows abnormal T2 hyperintensity in the right radial nerve (c) Caudal section shows absence of any muscle edema. Diffuse T2 hyperintensity with absent denervation changes suggested Grade 1 injury.
Fig. 6.
Imaging findings in Axonotmesis (Sunderland Grade 2 injury). (a) Longitudinal ultrasound image shows diffuse hypoechogenicity of ulnar nerve in cubital fossa (rectangle). Distal part shows preserved fascicular pattern. (b) Axial US image shows normal honeycombed appearance in the distal part. (c) Focused assessment of the abnormal part longitudinal image shows diffuse hypoechogenicity and bulkiness. (d) Axial US image better demonstrates the bulkiness of the hypoechoic ulnar nerve along with thickening of perineural soft tissues, suggesting grade 2 injury. (e) Axial T2W FS image in a different patient with left wrist drop after humeral shaft fracture shows mildly bulky and hyperintense radial nerve. (f) Caudal section shows edema in supinator muscle. Findings are consistent with at least grade 2 injury.
Fig. 7.
Imaging findings in Sunderland Grade 3 injury. (a) Axial ultrasound image in a patient with ulnar nerve injury shows normal appearing ulnar nerve proximal to the cubital tunnel. (b) Axial ultrasound image at the injury site shows focal bulkiness and hypoechogenicity with perineural thickening at the site of injury. (c) Longitudinal US image in a different patient shows focal effacement of fascicles (block arrow) in median nerve, corresponding to grade 3 or 4 injury. (d) Oblique coronal T1W MR Image shows focal bulkiness of peroneal nerve (arrow) at the site of injury adjacent to the fibular head (asterisk), suggesting grade 3 injury. (e) Axial US image in a different patient shows focal changes in the radial nerve (arrow) with loss of speckled pattern corresponding to the hypoechoic scar tract of penetrating injury. (f) Axial T2W FS image in a different patient shows abnormal focal T2 hyperintensity in the ulnar nerve with thickened fascicles and surrounding perineural edema, suggesting grade 3 ulnar nerve injury behind the medial epicondyle.
Fig. 8.
Imaging findings in Sunderland Grade 4 injury. (a) Longitudinal ultrasound image in a patient with ulnar claw hand after trauma shows focal bulkiness and disruption of the nerve fascicles (block arrow, area between the 2 cursors), suggesting grade 4 injury. (b) Longitudinal ultrasound image in a patient with peroneal nerve injury after knee replacement surgery, shows focal bulkiness and disruption of the nerve fascicles (block arrows) suggesting grade 4 injury. (c) Corresponding axial section shows hypoechogenicity, bulkiness and loss of speckled pattern, concordant with fascicular disruption.
Fig. 9.
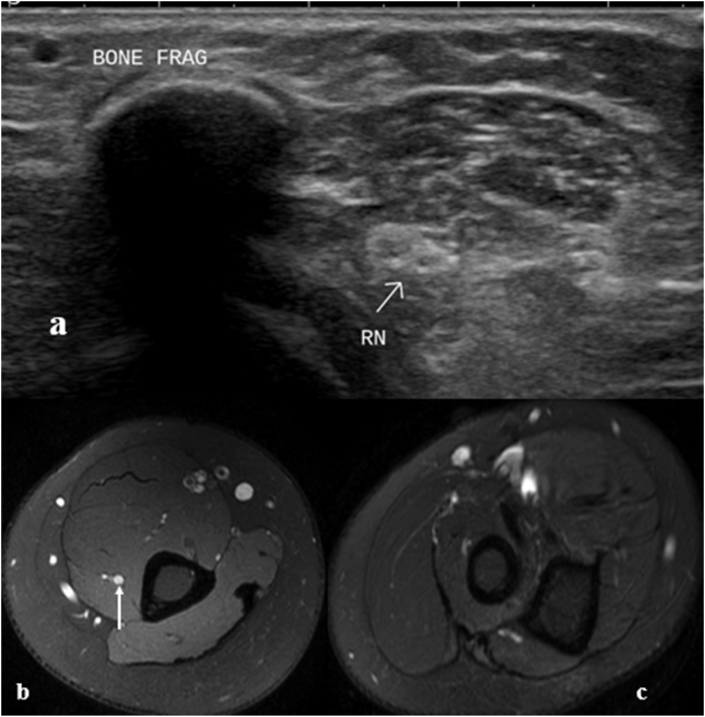
Imaging findings in Sunderland Grade 4 injury at the origin of peroneal nerve. (a) Axial US image at sciatic nerve division and (b) distally show bulky hypoechoic peroneal component (TN: Tibial nerve and CPN: Peroneal nerve). ((c) Longitudinal extended field-of-view panoramic US image shows fascicular sciatic nerve proximally (asterisk) and hypoechoic bulky peroneal nerve (oval), abnormal right from its origin. (d) Focused longitudinal image of peroneal nerve segment shows loss of fascicular pattern (oval), but continuity is preserved and fascicular pattern is seen in distal part (block arrow). (e) Radiograph shows fracture of lateral tibial condyle and fibular head and neck with internal fixation implant in situ.
Fig. 10.
Ultrasound findings in Sunderland Grade 5 injury. (a) Longitudinal US image shows complete transection of ulnar nerve after penetrating injury (tract seen, denoted by asterisk) with retracted neuromas (block arrows) (b) Axial US image shows multiple punctate echogenic foci, suggestive of retained foreign bodies (glass pieces in this case) in the gap between the neuromas(c) Longitudinal ultrasound image shows typical imaging appearance of end neuroma with round bulbous hypoechoic retracted ending. (d) Longitudinal ultrasound image in a different patient shows complete discontinuity of the median nerve with break in epineurium (block arrow) and intervening hematoma.
Fig. 11.
MR Imaging findings in Sunderland Grade 5 injury. (a) Axial T2W FS image shows thickened median (thick arrow) and ulnar nerves (thin arrow). (b) Caudal section shows that the nerves are not visualized, rather there is hyperintense scar tissue (block arrow). (c) Distally, bulky nerves are seen with muscle denervation changes. (d) Oblique Sagittal T2w image depicts the nerve transections with end neuromas (arrows).
8. Imaging follow-up of nerve injuries
The natural process of nerve recovery involves regeneration of axons, occurring at a rate of 1 inch/month, trying to grow down the nerve to re-innervate muscle or skin. It is important to make correct fascicular connection, motor nerve fascicle to corresponding motor fascicle and similarly for the sensory fascicles, which would help to restore adequate function. No recovery will occur if the regenerating nerve fibers do not make a correct connection. While sensation can be regained even after long periods of denervation, muscle re-innervation will not occur after long. Therefore, it is necessary to get nerve to muscle as quickly as possible if it is not going to recover on its own.
Several different types of surgery including nerve repair, nerve graft, nerve transfer or neurolysis. Needle EMG & NCS can also be used to access the muscle re innervation, post-surgery. Morphological imaging like MR and US can be used to access the integrity of the nerve post-surgery.35,36 Functional imaging like DTI/DWI produce susceptibility artefacts, due to the presence of sutures like nylon, fibrin, hemorrhage, nerve guidance conduit and hence careful reading is required in post-surgical period.37
9. Limitations and future directions
It must be borne in mind that imaging findings need to be interpreted in conjunction with clinical course and EPS. Accurate differentiation between grade 3 and grade 4 injuries is difficult. Anisotropy on HUS and magic angle artefacts on MR may further make the evaluation difficult. Moreover, it may be difficult to assess the inhomogeneity in case of smaller nerves and involvement of a part of the nerve (instead of whole cross-section). In such cases, clinical follow-up and assessment of recovery of function may prove useful. Microneurography of peripheral nerve using high strength Magnets (9.4 T), can provide non-invasive insight into the microanatomy, which is currently not available for in vivo imaging.38 Functional imaging like DWI and DTI may play an important role in better characterization of nerve injury in future. DTI has a significant role in the evaluation of peripheral nerve regeneration following nerve repair and/or reconstruction.39,40 However, reproducibility of these methods is not yet established. Nerve specific MR contrast agents may be used to evaluate the functional nature of nerve injury. Gadofluorine M is an experimental MR contrast agent which has been shown to selectively accumulate in the nerves undergoing Wallerian degeneration and in areas of focal demyelination.41 Ultrasound elastography may find application in evaluation of traumatic nerve injuries. The elasticity values on shear-wave elastography have been found to be increased in entrapment neuropathies, suggesting higher stiffness42,43
10. Conclusion
Imaging plays an important role in diagnosis and management of peripheral nerve injuries. High frequency ultrasonography and MRI help in morphological characterization of the injured nerve and grading its severity, which enable optimal management of the patient. Ultrasound allows better assessment of the nerve architecture while MRI fares better in assessing denervation changes. Functional imaging like DTI has the potential to show disruption of the nerve fibers, which may help in better prognostication. It is important to interpret the imaging findings in context of nature of injury and clinical course of the patient.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The Authors declare that there is no conflict of interest.
Acknowledgements
Nil.
References
- 1.Noble J., Munro C.A., Prasad V.S., Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998 Jul;45(1):116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Smith P.J. Nerve injuries and their repair—a critical appraisal Sir Sydney Sunderland.560 pages, 158 illustrations. Churchill Livingstone, Edinburgh, 1991. ISBN 0–443–04161–X. Price £79.50. J Hand Surg. 1992;17(1):120–121. [Google Scholar]
- 3.Chung T., Prasad K., Lloyd T.E. Peripheral neuropathy: clinical and electrophysiological considerations. Neuroimaging Clin N Am. 2014;24(1):49–65. doi: 10.1016/j.nic.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman W.A. The electrophysiology of peripheral nerve injuries. Neurosurg Clin N Am. 1991;2(1):43–56. [PubMed] [Google Scholar]
- 5.Oh Sj M.D. first ed. Williams & Wilkins; Baltimore: 1998. Principles of Clinical Electromyography: Case Studies; p. 604. [Google Scholar]
- 6.Hilburn J.W. General principles and use of electrodiagnostic studies in carpal and cubital tunnel syndromes. With special, attention to pitfalls and interpretation. Hand Clin. 1996;12(2):205–221. [PubMed] [Google Scholar]
- 7.Toros T., Karabay N., Ozaksar K., Sugun T.S., Kayalar M., Bal E. Evaluation of peripheral nerves of the upper limb with ultrasonography: a comparison of ultrasonographic examination and the intra-operative findings. J Bone Joint Surg Br. 2009;91(6):762–765. doi: 10.1302/0301-620X.91B6.22284. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg J. EMG: myths and facts. HSS J. 2006 Feb;2(1):19–21. doi: 10.1007/s11420-005-0124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pham M., Bäumer T., Bendszus M. Peripheral nerves and plexus: imaging by MR-neurography and high-resolution ultrasound. Curr Opin Neurol. 2014;27(4):370–379. doi: 10.1097/WCO.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 10.Hollister A.M., Simoncini A., Sciuk A., Jordan J. High frequency ultrasound evaluation of traumatic peripheral nerve injuries. Neurol Res. 2012;34(1):98–103. doi: 10.1179/1743132811Y.0000000048. [DOI] [PubMed] [Google Scholar]
- 11.Budzik J.-F., Balbi V., Verclytte S., Pansini V., Thuc V.L., Cotten A. Diffusion tensor imaging in musculoskeletal disorders. RadioGraphics. 2014;34(3):E56–E72. doi: 10.1148/rg.343125062. [DOI] [PubMed] [Google Scholar]
- 12.Zare M., Faeghi F., Hosseini A., Ardekani M.S., Heidari M.H., Zarei E. Comparison between three-dimensional diffusion-weighted PSIF technique and routine imaging sequences in evaluation of peripheral nerves in healthy people. Basic Clin Neurosci. 2018;9(1):65–71. doi: 10.29252/NIRP.BCN.9.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Figueiredo E.H., Borgonovi A.F., Doring T.M. Basic concepts of MR imaging, diffusion MR imaging, and diffusion tensor imaging. Magn Reson Imag Clin N Am. 2011;19(1):1–22. doi: 10.1016/j.mric.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Huisman T.A. Diffusion-weighted imaging: basic concepts and application in cerebral stroke and head trauma. Eur Radiol. 2003;13(10):2283–2297. doi: 10.1007/s00330-003-1843-6. [DOI] [PubMed] [Google Scholar]
- 15.Takahara T., Hendrikse J., Yamashita T. Diffusion-weighted MR neurography of the brachial plexus: feasibility study. Radiology. 2008;249(2):653–660. doi: 10.1148/radiol.2492071826. [DOI] [PubMed] [Google Scholar]
- 16.Chhabra A., Thakkar R.S., Andreisek G. Anatomic MR imaging and functional diffusion tensor imaging of peripheral nerve tumors and tumorlike conditions. AJNR Am J Neuroradiol. 2013;34(4):802–807. doi: 10.3174/ajnr.A3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita T., Kwee T.C., Takahara T. Whole-body magnetic resonance neurography. N Engl J Med. 2009;361(5):538–539. doi: 10.1056/NEJMc0902318. [DOI] [PubMed] [Google Scholar]
- 18.Hagmann P., Jonasson L., Maeder P., Thiran J.-P., Wedeen V.J., Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. RadioGraphics. 2006;26(suppl_1):S205–S223. doi: 10.1148/rg.26si065510. [DOI] [PubMed] [Google Scholar]
- 19.Westin C.-F., Maier S.E., Mamata H., Nabavi A., Jolesz F.A., Kikinis R. Processing and visualization for diffusion tensor MRI. Med Image Anal. 2002;6(2):93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- 20.Breckwoldt M.O., Stock C., Xia A. Diffusion tensor imaging adds diagnostic accuracy in magnetic resonance neurography. Invest Radiol. 2015;50(8):498–504. doi: 10.1097/RLI.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 21.Kim S.Y., Cheon J.H., Seo W.J., Yang G.Y., Choi Y.M., Kim K.H. A pictorial review of signature patterns living in musculoskeletal ultrasonography. Kor J Pain. 2016;29(4):217–228. doi: 10.3344/kjp.2016.29.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seddon H.J. Three types of nerve injury. Brain. 1943;66(4):237–288. [Google Scholar]
- 23.Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain J Neurol. 1951;74(4):491–516. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- 24.Deniel A., Causeret A., Moser T., Rolland Y., Dréano T., Guillin R. Entrapment and traumatic neuropathies of the elbow and hand: an imaging approach. Diagn Interv Imaging. 2015;96(12):1261–1278. doi: 10.1016/j.diii.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Chhabra A., Ahlawat S., Belzberg A., Andreseik G. Peripheral nerve injury grading simplified on MR neurography: as referenced to Seddon and Sunderland classifications. Indian J Radiol Imag. 2014;24(3):217. doi: 10.4103/0971-3026.137025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackinnon S.E., Dellon A.L. Thieme Medical Publishers; G. Thieme Verlag; New York; Stuttgart; New York: 1988. Surgery of the Peripheral Nerve. [Google Scholar]
- 27.Fornage B.D. Peripheral nerves of the extremities: imaging with US. Radiology. 1988 Apr;167(1):179–182. doi: 10.1148/radiology.167.1.3279453. [DOI] [PubMed] [Google Scholar]
- 28.Marquez Neto O.R., Leite M.S., Freitas T., Mendelovitz P., Villela E.A., Kessler I.M. The role of magnetic resonance imaging in the evaluation of peripheral nerves following traumatic lesion: where do we stand? Acta Neurochir. 2017;159(2):281–290. doi: 10.1007/s00701-016-3055-2. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal A., Chandra A., Jaipal U., Bagarhatta M., Mendiratta K., Goyal A., Kumar R., Mangalhara N. Can imaging be the new yardstick for diagnosing peripheral neuropathy?-a comparison between high resolution ultrasound and MR neurography with an approach to diagnosis. Insights Imag. 2019 Nov 1;10(1):104. doi: 10.1186/s13244-019-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aggarwal A., Jana M., Srivastava D.N. Magnetic resonance neurography and ultrasonogram findings in upper limb peripheral neuropathies. Neurol India. 2019;67(7):125. doi: 10.4103/0028-3886.250701. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal A., Srivastava D.N., Jana M. Comparison of different sequences of magnetic resonance imaging and ultrasonography with nerve conduction studies in peripheral neuropathies. World Neurosurg. 2017;108:185–200. doi: 10.1016/j.wneu.2017.08.054. [DOI] [PubMed] [Google Scholar]
- 32.Petchprapa C.N., Rosenberg Z.S., Sconfienza L.M., Cavalcanti C.F., Vieira R.L., Zember J.S. MR imaging of entrapment neuropathies of the lower extremity. Part 1. The pelvis and hip. Radiographics. 2010 Jul-Aug;30(4):983–1000. doi: 10.1148/rg.304095135. [DOI] [PubMed] [Google Scholar]
- 33.Keen N.N., Chin C.T., Engstrom J.W., Saloner D., Steinbach L.S. Diagnosing ulnar neuropathy at the elbow using magnetic resonance neurography. Skeletal Radiol. 2012;41(4):401–407. doi: 10.1007/s00256-011-1251-y. [DOI] [PubMed] [Google Scholar]
- 34.Bäumer P., Dombert T., Staub F. Ulnar neuropathy at the elbow: MR neurography—nerve T2 signal increase and caliber. Radiology. 2011;260(1):199–206. doi: 10.1148/radiol.11102357. [DOI] [PubMed] [Google Scholar]
- 35.Marquez Neto O.R., Freitas T., Mendelovitz P., Schetchtman N., Kessler I. An initial clinical experience to improve postoperative monitoring of peripheral nerve regeneration following neurotmesis using magnetic resonance imaging at 1.5 Tesla. J Neurosurg Sci. 2014;60(3):329–338. [PubMed] [Google Scholar]
- 36.Thawait S.K., Wang K., Subhawong T.K. Peripheral nerve surgery: the role of high-resolution MR neurography. AJNR Am J Neuroradiol. 2012;33(2):203–210. doi: 10.3174/ajnr.A2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martín Noguerol T., Barousse R., Gómez Cabrera M., Socolovsky M., Bencardino J.T., Luna A. Functional MR neurography in evaluation of peripheral nerve trauma and postsurgical assessment. RadioGraphics. 2019;39(2):427–446. doi: 10.1148/rg.2019180112. [DOI] [PubMed] [Google Scholar]
- 38.Bilgen M., Heddings A., Al-Hafez B. Microneurography of human median nerve. J Magn Reson Imag. 2005;21(6):826–830. doi: 10.1002/jmri.20345. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann H.C., Zhang J., Mori S., Sheikh K.A. Diffusion tensor imaging to assess axonal regeneration in peripheral nerves. Exp Neurol. 2010;223(1):238–244. doi: 10.1016/j.expneurol.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morisaki S., Kawai Y., Umeda M., Nishi M., Oda R., Fujiwara H., Yamada K., Higuchi T., Tanaka C., Kawata M., Kubo T. In vivo assessment of peripheral nerve regeneration by diffusion tensor imaging. J Magn Reson Imag. 2011 Mar;33(3):535–542. doi: 10.1002/jmri.22442. [DOI] [PubMed] [Google Scholar]
- 41.Wessig C., Jestaedt L., Sereda M.W., Bendszus M., Stoll G. Gadofluorine M-enhanced magnetic resonance nerve imaging: comparison between acute inflammatory and chronic degenerative demyelination in rats. Exp Neurol. 2008 Mar 1;210(1):137–143. doi: 10.1016/j.expneurol.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Dąbrowska-Thing A., Zakrzewski J., Nowak O., Nitek Ż. Ultrasound elastography as a potential method to evaluate entrapment neuropathies in elite athletes: a mini-review. Pol J Radiol. 2019;84:625–629. doi: 10.5114/pjr.2019.92422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wee T.C., Simon N.G. Ultrasound elastography for the evaluation of peripheral nerves: a systematic review. Muscle Nerve. 2019;60(5):501–512. doi: 10.1002/mus.26624. [DOI] [PubMed] [Google Scholar]