Abstract
Hepatitis is one of earlier, but serious, signs of liver damage. High doses of statins for a long time can induce hepatitis. This study aimed to evaluate and compare the therapeutic potential of thymoquinone (TQ) and bee pollen (BP) on fluvastatin (F)-induced hepatitis in rats. Rats were randomly divided into: group 1 (G1, control), G2 (F, hepatitis), G3 (F + TQ), G4 (F + BP), and G5 (F + TQ + BP). Single treatment with TQ or BP relieved fluvastatin-induced hepatitis, with best effect for the combined therapy. TQ and/or BP treatment significantly (1) reduced serum levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma glutamyl transpeptidase, and total bilirubin, (2) decreased malondialdehyde levels and increased level of reduced glutathione, and activities of glutathione peroxidase and catalase in the liver, (3) improved liver histology with mild deposition of type I collagen, (4) increased mRNA levels of transforming growth factor beta 1, nuclear factor Kappa B, and cyclooxygenase 1 and 2, and (5) decreased tumor necrosis factor alpha and upregulated interleukin 10 protein in the liver. These data clearly highlight the ability of TQ and BP combined therapy to cause better ameliorative effects on fluvastatin-induced hepatitis than individual treatment by each alone.
Subject terms: Biochemistry, Histocytochemistry
Introduction
Liver damage constitutes one of the main causes of hepatocellular carcinoma (HCC) that leads to death worldwide1–3. Hepatitis is one of earlier signs of liver damage, which is characterized by inflammatory cells infiltration, congestion of blood vessels, and cellular degenerative changes. Hepatitis could be induced by a viral infection, microbial metabolites, metabolic and autoimmune diseases, environmental toxicants, and alcohol and drug abuse1,3,4. If hepatitis was left for a long time without suitable treatments, other complicated liver disorders such as fibrosis and cirrhosis would be developed and in advanced cases become risk factors for HCC5.
Lipid-lowering drugs statins, including fluvastatin, prevents cholesterol biosynthesis by inhibiting hydroxyl-methyl-glutaryl co-enzyme A (HMG-CoA) reductase activity6. Higher doses of statins were reported to induce hepatotoxicity and myotoxicity7,8. Rats administrated fluvastatin (24 mg/kg/day) for 7 days suffered from notable hepatotoxicity7. Treatment with high doses of fluvastatin disturbs liver function as evidenced by elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) serum levels and induces myopathy as revealed by high serum AST and creatine kinase levels in rats8.
Alternative therapeutic strategies using natural products have been traditionally utilized in the treatment of liver damage and cancer9–13. Among these medicinal natural products, Nigella sativa and its main phytochemical component thymoquinone (TQ) have several health beneficial properties, such as antioxidant, anti-inflammatory, immune-stimulant, anti-bacterial, hypoglycemic, and anti-arthritic activities14–18. Due to their potent antioxidant, anti-inflammatory properties, TQ relieved hepatotoxicity induced by carbon tetrachloride (CCl4) in mice19, and by 2,3,7,8-tetrachlorodibenzo-p-dioxin20, ethanol21 and gentamicin22 in rats by inhibiting oxidative stress, inflammation, and apoptosis. TQ also attenuated chemically-induced liver fibrosis in mice23.
The honeybees gather bee pollen (BP) as a supply of nutrients for their hives. Male flowers' reproductive cells produce pollen grains, that contain large amounts of phenolic compounds, phytochemicals, flavonoids, carotenoids, amino acids, minerals, and vitamins24,25. However, the exact ingredients of BP differ based on plant types and growth conditions26. BP was used for many years as a food supplement, or even as a medicine, to improve health especially for people with cardiovascular diseases27. Similar to TQ, BP also had many health-promoting properties including anti-oxidant, anti-inflammatory, anti-cancer, anti-bacterial, and anti-atherosclerotic activities28. The potent BP anti-oxidant properties were mainly attributed to its higher content of phenolic compounds29. Consumption of BP significantly reduced oxidative stress and increased the activities of antioxidant enzymes in rats25. Previous studies reported potent hepatoprotective effect for BP and other apitherapy products with an effect similar to silymarin, the most commonly used drug in the treatment of and/or protection against many liver injuries30–32. For this reason, BP and other apitherapy products utilized to attenuate hepatotoxicity induced by CCl433,34, carbaryl24, and aluminum35 in rats.
The scientific gaps of previous studies included a lack of investigations on the therapeutic potential of either TQ or BP on the hepatitis model induced by fluvastatin or any other statins. Searching the available published data, we also did not find researches evaluating the combined effect of TQ and BP on hepatitis or liver injury animal models. It is also important to know whether single or combined treatment of these two natural products can give better improvement for fluvastatin-induced hepatitis. To fill in these scientific gaps, herein we evaluated and compared the efficacy of TQ and BP treatment on hepatitis induced by fluvastatin in rats using biochemical, structural, and molecular approaches.
Results
Effect of thymoquinone and/or bee pollen on liver function
Rats administered fluvastatin (hepatitis group) showed significantly (p < 0.05) higher levels of liver damage parameters (AST, ALT, ALP, γ-GTP, and total bilirubin) and significantly lower levels of albumin than the control group (Table 1). Treatment with TQ and/or BP restored these parameters to levels comparable to the control group. The highest improvement was observed in the co-treated group (TQ + BP) followed by the TQ-treated group. However, BP-treated group showed less improvement in these parameters, but still significantly different from the hepatitis group. These findings indicate that treatment with TQ and/or BP could ameliorate fluvastatin-induced liver damage with best effect for the co-treated group followed by TQ and then BP group.
Table 1.
Effect of TQ and/or BP treatment on serum levels of liver damage parameters in fluvastatin-induced hepatitis in rats.
| Parameters | Control (G1) | F (G2) | F + TQ (G3) | F + BP (G4) | F + TQ + BP (G5) |
|---|---|---|---|---|---|
| ALT (U/L) | 39.54 ± 3.64e | 180.11 ± 7.30a | 81.2 ± 6.83c | 109.66 ± 6.57b | 62.48 ± 4.23d |
| AST (U/L) | 137.49 ± 6.71e | 375.45 ± 10.49a | 216.06 ± 8.69c | 252.58 ± 9.52b | 171.54 ± 7.13d |
| ALP (U/L) | 89.37 ± 5.27e | 281.34 ± 13.01a | 162.14 ± 7.01c | 208.26 ± 8.17b | 111.36 ± 6.11d |
| γ-GTP (U/L) | 7.06 ± 0.62d | 25.33 ± 1.20a | 15.85 ± 0.64b | 17.76 ± 0.81b | 10.36 ± 0.54c |
| Albumin (g/dl) | 4.12 ± 0.23a | 2.05 ± 0.12d | 3.00 ± 0.13c | 2.71 ± 0.16c | 3.59 ± 0.14b |
| Total bilirubin (mg/dl) | 0.95 ± 0.07d | 3.32 ± 0.14a | 2.12 ± 0.13bc | 2.44 ± 0.11b | 1.63 ± 0.09c |
Data are expressed as mean ± SEM (n = 7/group). Mean values with different superscript letters [a (the highest values) – e (the lowest value)] in the same row are significantly different at (p ≤ 0.05). All groups were compared to each other. BP, Bee pollen; G1-5, Groups 1–5; F, Fluvastatin; TQ, Thymoquinone.
Effect of thymoquinone and/or bee pollen on oxidative stress and antioxidant biomarkers
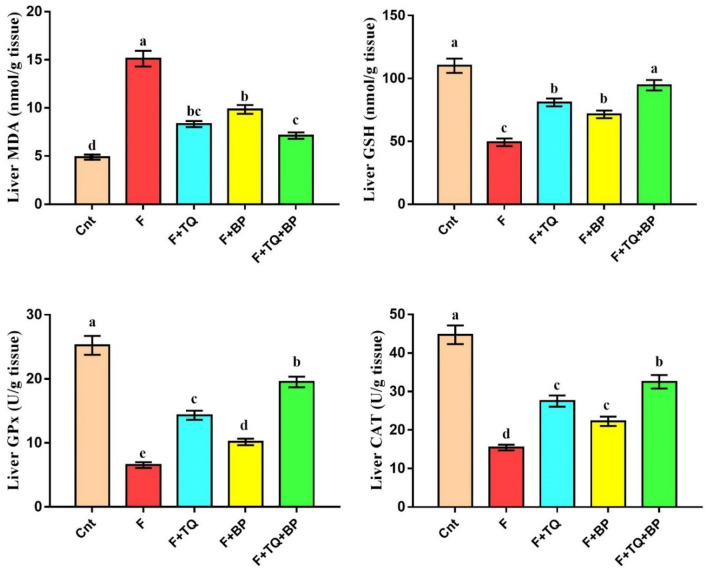
Animals treated with fluvastatin (hepatitis group) had a significantly higher MDA level and a significantly lower GSH level and antioxidant activities of GPx and CAT in their livers than control animals (Fig. 1). Administration of TQ and/or BP restored these oxidant/antioxidant biomarkers to levels comparable to the control. Again, among the three treated groups, the co-treated group showed best improvement (lowest MDA and highest GSH, GPx, and CAT) followed by the TQ group (Fig. 1). Also, rats administrated BP had a significant lower MDA and higher GSH, GPx, and CAT than the hepatitis group. These data imply that TQ and/or BP could attenuate oxidative stress damage in the liver induced by fluvastatin through inhibition of the lipid peroxidation MDA biomarker and activation of antioxidant biomarkers with the best effect in the co-treated group.
Figure 1.
Effect of TQ and/or BP treatment on oxidative stress (MDA) and antioxidant status (GSH, GPx, and CAT) in rat liver. Values are expressed as mean ± SEM (n = 7/group). Columns carrying different letters [a (the highest value) – e (the lowest value)] are significantly different at p < 0.05. All groups were compared to each other. Cnt: control group (G1); F, Fluvastatin-treated group (G2); F + TQ, Thymoquinone-treated group (G3); F + BP, Bee pollen-treated group (G4); F + TQ + BP, co-treated group (G5). (GraphPad Prism 8, https://www.graphpad.com/scientific-software/prism/).
Effect of thymoquinone and/or bee pollen on liver histology
No histopathological changes were noticed in the liver of the control group. Livers of these animals showed normal structure with hepatocytes arranged in cords radiating from the central vein (cv) within the parenchyma (Fig. 2A). However, fluvastatin-treated rats exhibited typical histological alterations associated with hepatitis in form of severe congestion in the portal vein (pv), aggregation of numerous inflammatory mononuclear cells (red arrowhead) in the vicinity of the portal vein (Fig. 2B). Some hepatocytes had early signs of necroptosis such as pyknotic nuclei that contained condensed chromatin (black arrowhead, Fig. 2B). Fibrosis in form of aggregation of fibroblasts (arrow) and collagen fibers (yellow arrowhead), as proved later by immunohistochemistry, was also noticed in liver parenchyma especially in the portal area and adjacent to the area of mononuclear cells infiltration (Fig. 2B). Moreover, few extravasated red blood cells were seen in parenchyma around the portal vein (green arrowhead, Fig. 2B). On the other hand, liver sections in TQ and BP treated groups showed partial improvement in liver architecture with better regeneration in the TQ group. The TQ-treated group showed a mild degree of inflammation with moderate congestion in the portal vein (pv) and a very few inflammatory cells infiltration (red arrowhead, Fig. 2C). Also, nuclear pyknosis (arrow) in few hepatocytes and few extravasated blood (green arrowheads) were noticed (Fig. 2C). The BP-treated group exhibited mild congestion in the portal vein (pv) with moderate inflammatory cells infiltration (red arrowhead), slight perivascular edema and fibrosis (yellow arrowhead), and few hepatocytes nuclear pyknosis (arrow, Fig. 2D). The co-treated group showed the best signs of tissue regeneration with nearly similar tissue architecture to the control group, except for the presence of slightly congested central veins (cv, Fig. 2E). The highest liver damage score was found in the F group and was significantly reduced following treatment with the TQ and/or BP with best improvement in the combined treated group (Fig. 2F). We could infer that treatment with TQ and/or BP ameliorated liver histological damage induced by fluvastatin, with best improvement for the co-treated group.
Figure 2.
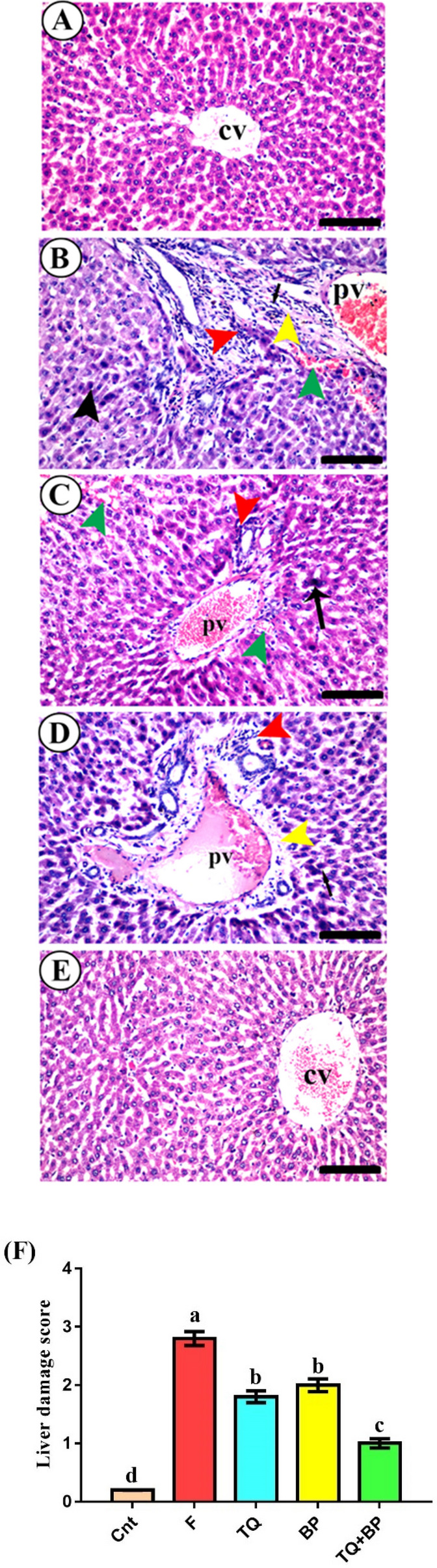
Photomicrographs of rat liver sections stained with H&E in control group (A), fluvastatin-treated group (B), thymoquinone-treated group (C), bee pollen-treated group (D); TQ + BP co-treated group (E). All labels were explained in the main text. Scale bars = 40 µm (A, E) and 30 µm (B–D). (F) Liver damage score, values are the mean ± SEM (n = 5/group). Columns carrying different letters (a–d) are significantly different at p < 0.05.
Effect of thymoquinone and/or bee pollen on COLI immunostaining
Liver parenchyma of the control group showed very little deposition of collagen I (COLI, Fig. 3A). In contrast, liver sections of the fluvastatin-treated group exhibited an abundant positive COL1 immuno-stained area in the portal area (Fig. 3B). COLI positive area was significantly higher in the fluvastatin-treated group (25.20 ± 2.03%) than the control group (1.20 ± 0.11%, Fig. 3F). This positive area was significantly reduced in livers of rats treated with either TQ (8.36 ± 0.62%, Fig. 3C,F) or BP (14.36 ± 1.15%, Fig. 3D,F). The co-treated animals showed very little COLI immunostaining (1.73 ± 0.13%) with no significant difference from the control group (Fig. 3E,F). These results suggest induction of liver fibrosis by fluvastatin and a probable anti-fibrotic effect for TQ and BP.
Figure 3.
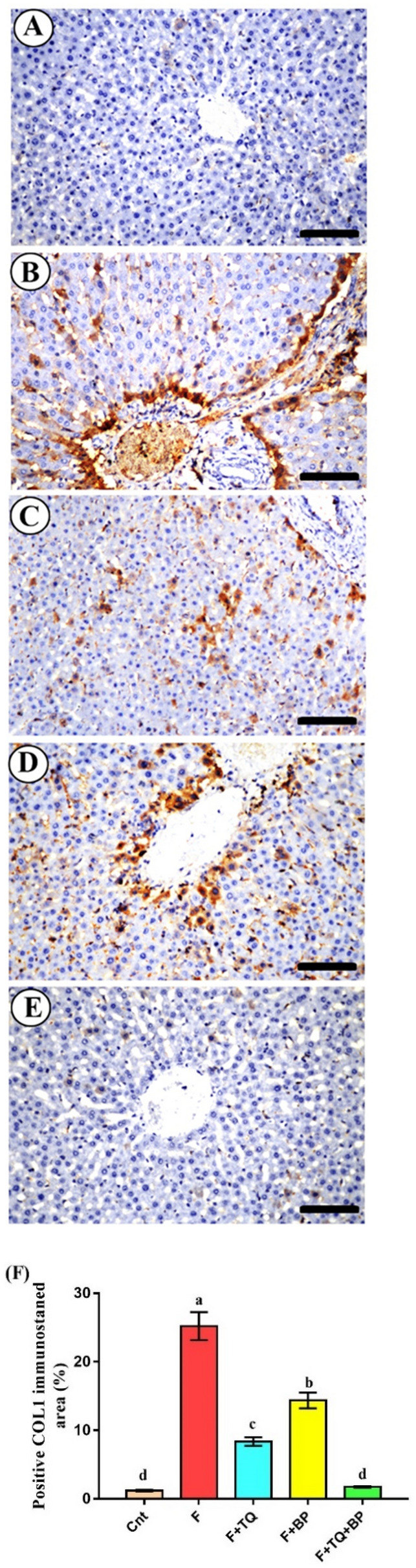
(A–E) Photomicrographs of liver sections immune-stained with COL1 in control group (A), fluvastatin-treated group (B), thymoquinone-treated group (C), bee pollen-treated group (D); TQ + BP co-treated group (E). Scale bars = 40 µm. (F) Quantification of COL1 positive immune staining. Data were presented as mean positive stained area (%) ± SEM (n = 7/group). Columns with different lower-case letters [a (highest value) – d (lowest value)] are significantly different at p < 0.01. All groups were compared to each other.
Effect of thymoquinone and/or bee pollen on expression of inflammatory genes/proteins
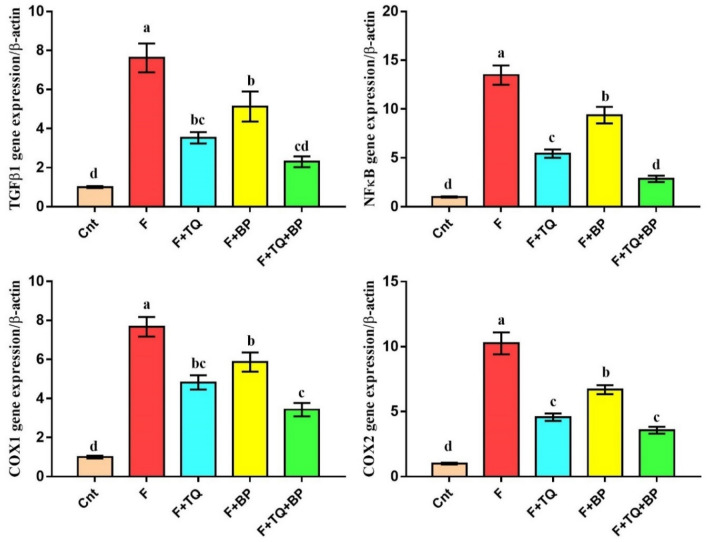
Real-time PCR (qPCR) was applied to determine inflammatory genes, TGFβ1, NFkB, COX1, and COX2 expression in rat liver following different treatments. The obtained results revealed a significant increase in the expression of these genes in the fluvastatin-treated group as compared to the control group (Fig. 4). Treatment with TQ and/or BP significantly downregulated these genes, with lowest expression in the co-treated group, followed by the TQ group, as compared to the fluvastatin-treated group (Fig. 4).
Figure 4.
Real-time PCR analysis shows changes in the relative expression of TGFβ1, NFκB, COX1, and COX2 genes relative to the housekeeping β-actin gene. Gene expression fold changes were presented as mean ± SEM (n = 7/group). Columns carrying different letters [a (the highest value) – d (the lowest value)] are significantly different at p < 0.05. All groups were compared to each other.
Western blot analysis was performed to evaluate changes in the expression of the inflammatory protein TNFα and the anti-inflammatory protein IL10 in the liver. Fluvastatin-treated rats had a significantly (p < 0.001) higher TNFα expression and a significantly (p < 0.001) lower IL10 expression relative to the control group (Fig. 5 and Fig. S1). Treatment with TQ and/or BP restored TNFα and IL10 to levels comparable to the control group, with best results for the co-treated group (which showed lowest TNFα and highest IL10 expression) followed by TQ and BP group (Fig. 5). These molecular-based data confirm the induction of hepatitis by fluvastatin and the ameliorative anti-inflammatory effects of TQ and BP.
Figure 5.
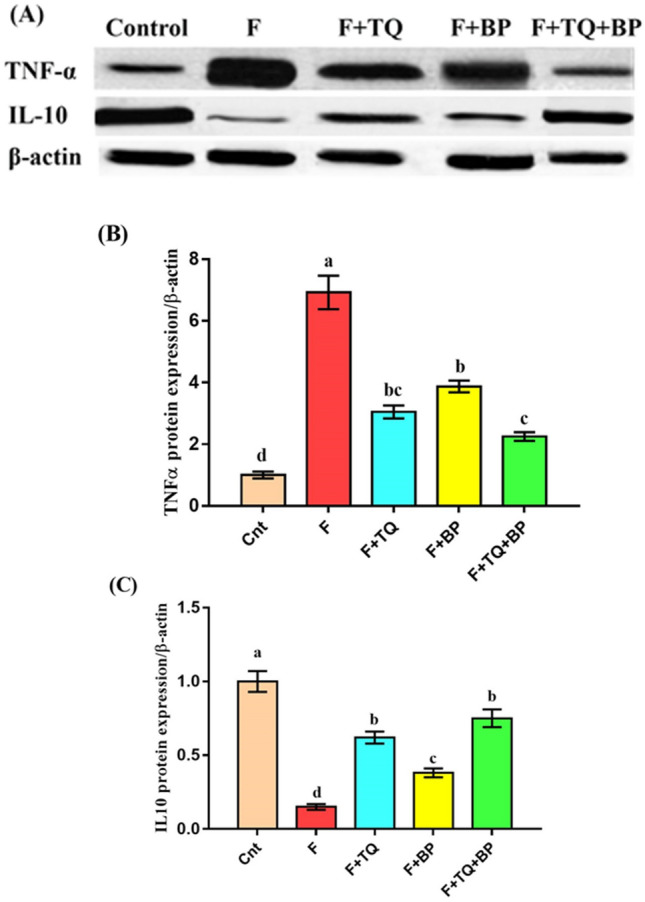
Western blot analysis shows changes in TNFα and IL10 protein expression relative to β-actin protein (control). (A) TNFα and IL10 protein bands. (B and C) Band quantification of TNFα and IL10 protein (ImageJ, https://imagej.nih.gov/ij/index.html). Data presented as fold change (mean) ± SEM (n = 7/group). Columns carrying different letters [a (the highest value) – d (the lowest value)] are significantly different at p < 0.05. All groups were compared to each other.
Discussion
A large body of studies investigated the ameliorative effect of TQ and BP each alone on different animal models of hepatotoxicity. However, to date little, or even no, information is available in the literature addressed whether TQ and/or BP could relieve fluvastatin-induced hepatitis. To the best of our knowledge, this may be the first study to report that the administration of TQ and/or BP ameliorated hepatitis induced by fluvastatin with best improvement when the two natural products were given together. This overall conclusion was reached depending on our obtained data that animals treated with TQ and/or BP had lower serum levels of liver damage parameters, decreased oxidative stress and histological damage degree, mild deposition of COLI, and decreased expression of inflammatory genes/proteins in their livers.
In the present study, rats orally administrated fluvastatin had a significant elevation in serum levels of liver damage parameters (ALT, AST, ALP, γ-GTP, and total bilirubin) with a significant reduction in the albumin level. This infers that treatment with fluvastatin can induce liver damage. Consistent with our findings, other studies also reported that fluvastatin administration caused hepatocellular damage with a significant increase in AST, ALT, ALP and γ-GTP activities7,8,36. Statins are metabolized by the liver and this could be the cause of hepato-toxicity and elevation of liver damage parameters following long-lasting treatment37. In humans with familiar hypercholesterolemia, statins, including fluvastatin, are very important hypocholesterolemic drugs that also decrease the severity of the associated cardiovascular diseases, thereby reducing the death rate. However, unlike animals which showed clear hepatitis, the most common side effect for statins in humans is the myopathy, which is characterized by rhabdomyolysis38. Cokça, et al.39 reported a case of female suffers from severe liver and muscle injury following taken an overdose of statins. Some patients taken the allowed dose also showed mild symptoms of liver injury following long-lasting treatment with statins40,41. Thus, it is likely that statins dose and duration is more essential determinants of hepato- and myo-toxicity than hypocholesterolemia42. Our results also showed that TQ and/or BP treatment could ameliorate hepatitis induced by fluvastatin and restored liver function. The two natural products restored the elevated liver enzymes (ALT, AST, γ-GTP, and ALP) to levels comparable to the control group, with best improvement for the combined therapy. Additionally, the two natural products elevated albumin serum levels, which proved the recovery of liver function and reverted bilirubin to normal levels. Similarly, other studies showed that supplementation with TQ4,22,43 or BP33 induced hepatoprotective and therapeutic effects against different models of liver injury.
Oxidative stress is one of the most common causes of liver injury. Our findings revealed that fluvastatin administration induced a significant elevation in hepatic MDA level and a significant reduction in hepatic GSH concentration and activities of GPx and CAT antioxidant enzymes. Increased MDA levels in the fluvastatin-treated group could increase lipid peroxidation which damaged lipid in cellular components, especially the cell membrane. This could explain leakage of liver damage parameter from liver to the circulation1. Free radicals overproduction and reactive oxygen species (ROS) are the main causes for oxidative stress and although hepatocytes have a more potent endogenous antioxidant system, they are prone to damage by these harmful elements when they exceed cells response to stress. Treatment with TQ and/or BP decreased hepatic oxidative stress induced by fluvastatin (as indicated by lower MDA level) and increased hepatic antioxidant status (higher GSH level and GPx and CAT activities), with best effect for the co-treated group. TQ has promising radicals scavenger activities enabling it to reduce oxidative damage and improve activities of liver endogenous antioxidant enzymes in animal models of liver injury induced by CCl419, ethanol21, or gentamicin22. BP has potent antioxidant activities due to high contents of phenolic compounds, phytochemicals, and flavonoids, especially quercetin, that prevent free radical production and increase the activity of antioxidant enzymes24,25,29,44. Consistent with our results, other studies also found an ameliorative effect for BP, through induction of antioxidant status and inhibition of oxidative stress, on hepatotoxicity induced by CCl433,34 and carbaryl24 in rats. Moreover, BP induced a more potent hepatoprotective effect than propolis on cisplatin-induced hepatotoxicity in mice45.
At a histological level, fluvastatin also induced typical signs of inflammation as evidenced by the portal and central veins congestion and inflammatory mononuclear cells infiltration. Similar histopathological alterations following treatment with fluvastatin46 or rosuvastatin47 were also observed. However, unlike this study, we did not find vacuolar degeneration in hepatocytes but rather we found a slight degree of hemorrhage in the liver parenchyma. We also found an early sign of necroptosis where nuclei of some hepatocytes become pyknotic. Chromatin condensation was considered as an early sign of necroptosis in cells48. Necrosis was not reported in the liver of rat treated with fluvastatin46, however, it was noticed in both the liver of rat administrated rosuvastatin47 and in skeletal muscles of HMG-CoA reductase (the main target for statins) knockout mice49. Animals treated with TQ or BP showed partial regenerative changes in the liver with slightly better improvement in the TQ group. However, those treated with TQ and BP together had normal liver histology. Thus, treatment with TQ and/or BP restored liver histological damage induced by fluvastatin to level comparable to that of the normal animals, with best results for the combined group. Similarly, administration of either TQ19,21,22 or BP33,34,45 improved liver histology in other chemically-induced liver damage models.
One of the current hot topic in the setting of liver diseases is the diagnosis of drug-induced liver damage (DILI) and, of particular interest, is the differential diagnosis between DILI and autoimmune hepatitis (AIH), especially in the form of DILI with autoimmune features named as DILI-AIH. This has relevant clinical and therapeutic implications since the specific treatment of DILI-AIH like is based on corticosteroid treatment50. The typical histological changes associated with AIH included interface hepatitis, lobular hepatitis and centrolobular necrosis50. Since histological evaluation described in the present study clearly excludes histological changes consistent with autoimmune hepatitis, the fluvastatin (F)- induced hepatitis is not a DILI-AIH like and therefore does not require corticosteroid treatments.
Our histological findings supported by results of COLI immunostaining revealed induction of fibrosis in the liver following treatment with fluvastatin. A significantly higher positive COLI immunostaining was detected in the portal area. Most cases of acute hepatitis induced by chemicals or toxins would be transformed into chronic hepatitis, which further resulted in hepatic fibrosis if left without treatment51. Treatment with TQ reduced liver fibrosis as revealed by a reduction of COLI immunostaining52. The antifibrotic effect of TQ was also reported by Ghazwani, et al.52 who attributed this effect to TQ ability to downregulate fibrosis-related genes COL1, TGFß1, and α-smooth muscle actin (α-SMA) in a mouse model of CCl4-induced liver fibrosis. Bai et al.23 also showed that TQ could decrease liver fibrosis triggered by thioacetamide by targeting COLI expression in the liver. Unlike TQ, PB exerted less antifibrotic effect, however, fibrosis was significantly reduced as compared to the fluvastatin-treated group. A similar mild antifibrotic effect of BP on a rat model of CCl4-induced liver fibrosis was also reported33. However, another study reported potent anti-fibrotic and anti-necrotic effects for BP against cisplatin-induced liver injury in mice45.
In addition to the typical histological lesion of hepatitis seen in the fluvastatin-treated group, hepatitis was confirmed on molecular levels. We found a significantly higher expression of inflammatory genes (TGFβ1, NFkB, COX1, and COX2) and TNFα protein and a significantly lower expression of the anti-inflammatory IL10 protein in the fluvastatin-treated group as compared to the control group. High mRNA levels of the epithelial-mesenchymal transition marker TGFβ1 could explain the occurrence of fibrosis in fluvastatin-treated animals53. Additionally, the elevation of TNFα protein, NFkB and its downstream target COX2 gene could also be associated with chronic inflammation and fibrosis54. Persistent high NFkB mRNA and protein levels in chronic hepatitis is the main inducer of liver cancer55. Our data showed that animals treated with TQ and/or BP had a lower expression of these inflammatory markers and a higher expression of IL10. This suggests an ameliorative effect of these two natural products, with best effect for the combined therapy, on the fluvastatin -induced hepatitis. Similarly, TQ has been reported to decrease TNFα, NFκB, and COX2 levels associated with many inflammatory conditions [Reviewed by56]. Bai et al.23 also demonstrated that TQ anti-fibrotic effect on the liver was mediated, at least in part, via the inhibition of TGFβ. Furthermore, TQ potentially reduced inflammation through targeting COX1,257. The anti-inflammatory activity of BP and its main component quercetin was mediated by targeting COX1,2 and subsequent inhibition of the NFκB pathway58.
Statins are widely used for lowering total cholesterol and reducing the risk of a heart attack or stroke. Introduced 40 years ago, statins rarely cause liver disease in humans, they only induce a mild increase in liver enzymes38,39,41. On rare occasions, they may induce a larger increase and on those occasions a different statin should be used42. Among the seven statins used in clinical practice, fluvastatin is actually one of the least used. However, no data are available in the literature regarding the side effect of fluvastatin overdose for a long time. For this reason, we used an overdose (75 mg/kg) a triple of the dose of previous existing studies7 to evaluate the possible side effect on the liver. If the same adverse effects were clinically proven in humans, hypercholesteremic patients should avoid fluvastatin overdose.
Conclusions
To the best of our knowledge, this is the first report to demonstrate that treatment with TQ and/or BP natural products antagonizes the detrimental effects of fluvastatin on rat liver. TQ and BP co-treatment gave better therapeutic potential than each alone. The findings offer new insight into the mode of fluvastatin-induced hepatitis and propose the use of the two natural products TQ and BP together as a promising feed additive to decrease inflammation and oxidative stress induced by fluvastatin and probably other statins. They also could be used as a suitable alternative in the treatment of hepatitis in patients, however further preclinical and clinical studies are required to warrant their safety and efficacy.
Materials and methods
Chemicals and natural products
Fluvastatin (Lescol 80 mg tablets) was obtained from (Novartis Pharmaceuticals, Spain). TQ (164.20 molecular weight, 99% purity) was purchased from Sigma-Aldrich (Sigma Chemical Co, USA, CAS Number: 490–91-5). Honeybee pollens (BP) were obtained from local beekeepers in a powder form. The BP source in a particular area is determined by the type of vegetation available (which also varied according to optimal temperatures, and soil texture, pH, and drainage), growing degrees, and the duration of its flowering period. These pollens were gathered by Egyptian bees from plant flowers growing nearby the hives in Tanta, in the north of Delta, Egypt. A suspension of 280 g BP dissolved in 10 ml distilled water was prepared as previously described45. Following overnight storage, centrifugation (10,000 rpm/10 °C/45 min), and filtration of supernatant, the obtained filtrate was frozen (− 20 °C) until use. All other chemicals and reagents were of the highest purity available.
Experimental design
Animals were handled and treated according to guidelines for experimental animals uses in research which was approved by Ethical committee at the Faculty of Science, Tanta University, with ethical approval license (Rec-Sci-Tu-0417). All methods are reported in accordance with ARRIVE guidelines. Fifty adult female Sprague Dawley rats (200 ± 20 g) were obtained from the National Research Center (NRC, Cairo, Egypt), housed under standard environmental conditions (temperature 23 ± 2 °C, humidity of 54 ± 2% and 12 h/12 h light/dark cycle). Rats were randomly divided into five groups (n = 10). In group 1 (G1, control), rats received distilled water. In G2-G5, animals were orally administered with fluvastatin (75 mg/kg body weight/day) for 10 days. The dose of fluvastatin was chosen based on a previous study46 and was also confirmed by a pilot study using four different doses (25, 50, 75, and 100 mg/kg/day) for 5, 10, and 20 days. Even, another study used a lower dose of fluvastatin at 24 mg/Kg/day for a shorter time (7 days), we did not find a notable hepatotoxic effect before our selected dose and duration. In G2, (hepatitis group), animals were treated only with fluvastatin for 10 days and left without treatment for a further 10 days. In G3, animals were orally administered with fluvastatin for 10 days and then were orally treated with TQ at a dose of 20 mg/kg/day59 from D11 to D20. In G4, animals were treated with fluvastatin for 10 days and then were orally administered with BP at a dose of 400 mg/kg body weight/day34 from D11 to D20. In G5, animals treated with fluvastatin for 10 days and then treated with both TQ and BP with similar doses and duration as in G3 and G4. All groups were treated by gavage.
At the end of the experiment (D21), rats were anesthetized by 500 µL of Ketamine-Xylazine intraperitoneal injection (100 and 20 mg/kg body weight, respectively) and blood samples were collected from eye venous plexus in plain tubes. Sera were obtained by centrifugation for the determination of biochemical parameters as previously detailed2. After euthanization by exsanguination, livers were immediately excised and divided into three portions; the first portion was stored in − 80 °C freezer (for qPCR and western blot assays), the second portion was homogenized (for biochemical assay), and the third portion was fixed in 10% formalin (for histological investigation).
Biochemical analysis
Liver damage enzymes [aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP)] were determined in serum using commercially available kits (Diamond- Diagnostics, Egypt). Gamma glutamyl transpeptidase (γ-GTP), albumin, and total bilirubin were determined in serum using commercially available kits (BioMed-Diagnostics, EGY-CHEM, Egypt). Liver homogenates were prepared as previously described35. The levels of lipid peroxidation marker malondialdehyde (MDA) and reduced glutathione (GSH) as well as the activities of the antioxidant enzymes glutathione peroxidase (GPx) and catalase (CAT) were determined in liver homogenates using commercially available kits (Biodiagnostics, Egypt) and as previously detailed60,61.
Histopathological examination
Fixed liver specimens were dehydrated in alcohol, cleared in xylene, embedded in paraffin, sectioned at 5 µm thickness, and stained by hematoxylin and eosin. The liver damage score involved portal and central veins congestion, mononuclear cells infiltration, blood extravasation, hepatocyte nuclear pyknosis, and fibrosis. These histopathological alterations were determined in 10 randomly chosen, non-overlapping fields at a magnification power of 400 X per each specimen. The damage score was set as follows: 0 (no lesion), 1 (mild), 2 (moderate), and 3 (severe)20.
Immunohistochemical examination
Immunostaining of liver specimens was performed as previously described62,63. In brief, sections were first incubated with 5% fetal calf serum (to block nonspecific binding), and then with anti-collagen I (COLI) primary mouse monoclonal antibody (1:300 dilution, Santa Cruz Biotechnology, USA, Cat # sc-59772) overnight at 4 °C. Subsequently, sections were incubated with secondary biotinylated mouse antibodies (1:1000, Dako, CA, # K4003) for 1 h, followed by incubation with streptavidin–biotin peroxidase conjugate (1:100, Vector Laboratories, Burlingame, CA) and with diaminobenzidine chromogen to initiate color development. Finally, sections were counterstained with Harris 's modified hematoxylin. COLI positive staining area (%) was quantified at magnifications of X400 by an image analyzer (Leica Q 500 DMLB, Leica, UK). We examined 50 specimens (n = 10/group). The number of fields per each specimen was 20 fields (4 different non-overlapping fields X 5 different sections).
Real-time PCR
Relative hepatic expression of inflammation related genes (TGFβ1, NFκB, COX1, and COX2) was determined using real-time PCR (qPCR). Total RNA was extracted from liver tissues using a commercially available kit (GeneJET RNA Purification Kit, Thermo Scientific, # K0731, USA) as previously detailed64. Before reverse transcription, all isolated RNA samples were quantified by a Nanodrop (Quawell, USA). Reverse transcription of RNA into cDNA was achieved using a commercially available kit (Thermo Scientific, #EP0451, USA). A total qPCR reaction mixture with a volume of 20 µl was prepared. This mixture contained cDNA, QuantiTect SYBR Green qPCR Master Mix, and specific primers designed by the Primer 3 web-based tool based on published rat sequences and previous publications (Table 2). Thermal conditions in StepOnePlus qPCR thermal cycler (Applied Biosystem, USA) included one cycle of initial denaturation at 94 °C for 4 min, followed by 40 cycles of amplification each cycle contained denaturation at 94 °C for 40 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. β actin was used as an internal control. Fold changes of relative expression were determined and the melting curve condition was set as previously described65–67.
Table 2.
Forward and reverse primers sequence for real time PCR.
| Gene | Forward primer (/5–/3) | Reverse primer (/5–/3) |
|---|---|---|
| TGFβ1 | AAGAAGTCACCCGCGTGCTA | TGTGTGATGTCTTTGGTTTTGTCA |
| NFκB | CCTAGCTTTCTCTGAACTGCAAA | GGGTCAGAGGCCAATAGAGA |
| COX1 | CCCAGAGTCATGAGTCGAAGGAG | CAGGCGCATGAGTACTTCTCGG |
| COX2 | GATTGACAGCCCACCAACTT | CGGGATGAACTCTCTCCTCA |
| β-actin | ACCCACACTGTGCCCATCTA | CGTCACACTTCATGATG |
Western blot
The expression of both TNFα and IL10 proteins were detected in liver tissues of all groups using western blot assay. This assay was done as previously described66. Briefly, liver specimens were lysed using RIPA lysis buffer. The obtained proteins were separated on SDS-PAGE gels and transferred to a polyvinylidene difluoride membrane (PVDF). PVDF membranes were separately incubated with the following mouse monoclonal primary antibodies: anti-TNFα (1:100 dilution, Santa Cruz Biotechnology, Cat # sc-52746), anti-IL10 (1:200 dilution, Santa Cruz Biotechnology, Cat # sc-365858), and anti-β-actin (housekeeping, 1:200 dilution, Cat # sc-47778). PVDF membranes were then incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:2000 dilution, Cell Signaling Technology, Beverly, MA, USA). The color was developed using tetramethylbenzidine (Sigma) and the band density was quantified by ImageJ software.
Statistical analysis
Normal distribution of data was tested by the Kolmogorov–Smirnov normality test, followed by the Levene test for variance equivalent. One-way analysis of variance was used to assess significant differences among groups using GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA). Tukey test was used to compare all groups with each other and to show the significant effect of different treatments. Data were expressed as mean ± standard error of mean (SEM). Significance was set at p < 0.05.
Institutional Review Board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by Ethical committee at the Faculty of Science, Tanta University, with ethical approval license (Rec-Sci-Tu-0417).
Supplementary Information
Acknowledgements
None.
Author contributions
A.F.S., M.E.-S., M.A.E.-E., E.M.T. participated in research design. A.E.M. Conducted experiments. A.E.M., M.E.-S., K.S.E.-S. Performed data analysis. All authors.wrote or contributed to the writing of the manuscript.
Funding
This work was partially funded by Taif University Researches Supporting Project number (TURSP-2020/139), Taif University, Taif, Saudi Arabia.
Data availability
The data presented in this study are available on request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95342-7.
References
- 1.Abdelhady D, El-Abasy M, Abou-Asa S, Elbialy Z, Shukry M, Hussein A, Saleh A, El-Magd M. The ameliorative effect of aspergillus awamori on aflatoxin b1-induced hepatic damage in rabbits. World Mycotoxin J. 2017;10:363–373. doi: 10.3920/WMJ2017.2188. [DOI] [Google Scholar]
- 2.Alzahrani FA, El-Magd MA, Abdelfattah-Hassan A, Saleh AA, Saadeldin IM, El-Shetry ES, Badawy AA, Alkarim S. Potential effect of exosomes derived from cancer stem cells and mscs on progression of den-induced hcc in rats. Stem Cells Int. 2018;2018:17. doi: 10.1155/2018/8058979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelkawy KS, El-Haggar SM, Ziada DH, Ebaid NF, El-Magd MA, Elbarbry FA. The effect of genetic variations on ribavirin pharmacokinetics and treatment response in hcv-4 egyptian patients receiving sofosbuvir/daclatasvir and ribavirin. Biomed. Pharmacother. 2020;121:109657. doi: 10.1016/j.biopha.2019.109657. [DOI] [PubMed] [Google Scholar]
- 4.Sayed-Ahmed MM, Aleisa AM, Al-Rejaie SS, Al-Yahya AA, Al-Shabanah OA, Hafez MM, Nagi MN. Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid. Med. Cell. Longev. 2010;3:254–261. doi: 10.4161/oxim.3.4.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muriel P. Role of free radicals in liver diseases. Hep. Intl. 2009;3:526–536. doi: 10.1007/s12072-009-9158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson TA. The safety of aggressive statin therapy: How much can low-density lipoprotein cholesterol be lowered? Mayo Clin Proc. 2006;81:1225–1231. doi: 10.4065/81.9.1225. [DOI] [PubMed] [Google Scholar]
- 7.Steiner S, Gatlin CL, Lennon JJ, McGrath AM, Seonarain MD, Makusky AJ, Aponte AM, Esquer-Blasco R, Anderson NL. Cholesterol biosynthesis regulation and protein changes in rat liver following treatment with fluvastatin. Toxicol. Lett. 2001;120:369–377. doi: 10.1016/S0378-4274(01)00268-5. [DOI] [PubMed] [Google Scholar]
- 8.Sugatani J, Sadamitsu S, Kurosawa M, Ikushiro S, Sakaki T, Ikari A, Miwa M. Nutritional status affects fluvastatin-induced hepatotoxicity and myopathy in rats. Drug Metab. Dispos. 2010;38:1655–1664. doi: 10.1124/dmd.110.034090. [DOI] [PubMed] [Google Scholar]
- 9.El-Magd MA, Mohamed Y, El-Shetry ES, Elsayed SA, Abo Gazia M, Abdel-Aleem GA, Shafik NM, Abdo WS, El-Desouki NI, Basyony MA. Melatonin maximizes the therapeutic potential of non-preconditioned mscs in a den-induced rat model of hcc. Biomed. Pharmacother. 2019;114:108732. doi: 10.1016/j.biopha.2019.108732. [DOI] [PubMed] [Google Scholar]
- 10.Badawy A, Hassanean H, Ibrahim AK, Habib ES, El- Magd MA, Ahmed SA. Isolates from thymelaea hirsuta inhibit progression of hepatocellular carcinoma in vitro and in vivo. Nat. Prod. Res. 2019 doi: 10.1080/1478641920191643859. [DOI] [PubMed] [Google Scholar]
- 11.Mohamed Y, Basyony MA, El-Desouki NI, Abdo WS, El-Magd MA. The potential therapeutic effect for melatonin and mesenchymal stem cells on hepatocellular carcinoma. Biomedicine. 2019;9:23–29. doi: 10.1051/bmdcn/2019090424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Upadhyay G, Singh AK, Kumar A, Prakash O, Singh MP. Resveratrol modulates pyrogallol-induced changes in hepatic toxicity markers, xenobiotic metabolizing enzymes and oxidative stress. Eur. J. Pharmacol. 2008;596:146–152. doi: 10.1016/j.ejphar.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 13.El-Mahalaway AM, Selim AA, Mahboub FAR. The potential protective effect of propolis on experimentally induced hepatitis in adult male albino rats. Histological and immunohistochemical study. J. Histol. Histopathol. 2015;2:14–20. doi: 10.7243/2055-091X-2-14. [DOI] [Google Scholar]
- 14.Ahmad S, Beg ZH. Hypolipidemic and antioxidant activities of thymoquinone and limonene in atherogenic suspension fed rats. Food Chem. 2013;138:1116–1124. doi: 10.1016/j.foodchem.2012.11.109. [DOI] [PubMed] [Google Scholar]
- 15.Schneider-Stock R, Fakhoury IH, Zaki AM, El-Baba CO, Gali-Muhtasib HU. Thymoquinone: Fifty years of success in the battle against cancer models. Drug Discov. Today. 2014;19:18–30. doi: 10.1016/j.drudis.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Saadat S, Aslani MR, Ghorani V, Keyhanmanesh R, Boskabady MH. The effects of nigella sativa on respiratory, allergic and immunologic disorders, evidence from experimental and clinical studies, a comprehensive and updated review. Phytother. Res. 2021;35:2968–2996. doi: 10.1002/ptr.7003. [DOI] [PubMed] [Google Scholar]
- 17.Boskabady M, Khazdair MR, Bargi R, Saadat S, Memarzia A, Mohammadian Roshan N, Hosseini M, Askari VR, Boskabady MH. Thymoquinone ameliorates lung inflammation and pathological changes observed in lipopolysaccharide-induced lung injury. Evid. Based Complement. Altern. Med. 2021;2021:6681729. doi: 10.1155/2021/6681729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gholamnezhad Z, Shakeri F, Saadat S, Ghorani V, Boskabady MH. Clinical and experimental effects of nigella sativa and its constituents on respiratory and allergic disorders. Avicenna J. Phytomed. 2019;9:195–212. [PMC free article] [PubMed] [Google Scholar]
- 19.Mansour M. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci. 2000;2000:2583–2591. doi: 10.1016/S0024-3205(00)00592-0. [DOI] [PubMed] [Google Scholar]
- 20.Erdemli ME, Yigitcan B, Gul M, Gozukara Bag H, Gul S, Aksungur Z. Thymoquinone is protective against 2,3,7,8-tetrachlorodibenzo-p-dioxin induced hepatotoxicity. Biotech. Histochem. 2018;93:1–10. doi: 10.1080/10520295.2018.1453549. [DOI] [PubMed] [Google Scholar]
- 21.Alsaif MA. Effect of thymoquinone on ethanol-induced hepatotoxicity in wistar rats. J. Med. Sci. 2007;7:1164–1170. doi: 10.3923/jms.2007.1164.1170. [DOI] [Google Scholar]
- 22.Galaly SR, Ahmed OM, Mahmoud AM. Thymoquinone and curcumin prevent gentamicin-induced liver injury by attenuating oxidative stress, inflammation and apoptosis. J. Physiol. Pharmacol. 2014;65:823–832. [PubMed] [Google Scholar]
- 23.Bai T, Lian L-H, Wu Y-L, Wan Y, Nan J-X. Thymoquinone attenuates liver fibrosis via pi3k and tlr4 signaling pathways in activated hepatic stellate cells. Int. Immunopharmacol. 2013;15:275–281. doi: 10.1016/j.intimp.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Eraslan G, Kanbur M, Silici S. Effect of carbaryl on some biochemical changes in rats: The ameliorative effect of bee pollen. Food Chem. Toxicol. 2009;47:86–91. doi: 10.1016/j.fct.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Sarić A, Balog T, Sobocanec S, Kusić B, Sverko V, Rusak G, Likić S, Bubalo D, Pinto B, Reali D, Marotti T. Antioxidant effects of flavonoid from croatian cystus incanus l. Rich bee pollen. Food Chem. Toxicol. 2009;47:547–554. doi: 10.1016/j.fct.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima Y, Tsuruma K, Shimazawa M, Mishima S, Hara H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement. Altern. Med. 2009;9:4. doi: 10.1186/1472-6882-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nogueira C, Iglesias A, Feás X, Estevinho LM. Commercial bee pollen with different geographical origins: A comprehensive approach. Int. J. Mol. Sci. 2012;13:11173–11187. doi: 10.3390/ijms130911173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denisow B, Denisow-Pietrzyk M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016;96:4303–4309. doi: 10.1002/jsfa.7729. [DOI] [PubMed] [Google Scholar]
- 29.Mărghitaş LA, Stanciu OG, Dezmirean DS, Bobiş O, Popescu O, Bogdanov S, Campos MG. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from romania. Food Chem. 2009;115:878–883. doi: 10.1016/j.foodchem.2009.01.014. [DOI] [Google Scholar]
- 30.Bogdanov S. Quality and standards of pollen and beeswax. APIACTA. 2004;38:334–341. [Google Scholar]
- 31.Hegazi A. Medical importance of bee products. Uludag Bee J. 2012;12:136–146. [Google Scholar]
- 32.D'Andrea V, Pérez LM, Sánchez Pozzi EJ. Inhibition of rat liver udp-glucuronosyltransferase by silymarin and the metabolite silibinin-glucuronide. Life Sci. 2005;77:683–692. doi: 10.1016/j.lfs.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Andriţoiu CV, Andriţoiu V, Cuciureanu M, Nica-Badea D, Bibire N, Popa M. Effect of apitherapy products against carbon tetrachloride-induced toxicity in wistar rats. Rom J. Morphol. Embryol. 2014;55:835–847. [PubMed] [Google Scholar]
- 34.Yıldız O, Can Z, Saral O, Yuluğ E, Oztürk F, Aliyazıcıoğlu R, Canpolat S, Kolaylı S. Hepatoprotective potential of chestnut bee pollen on carbon tetrachloride-induced hepatic damages in rats. Evid. Based Complement. Altern. Med. eCAM. 2013;2013:461478. doi: 10.1155/2013/461478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Türkez H, Yousef MI, Geyikoglu F. Propolis prevents aluminium-induced genetic and hepatic damages in rat liver. Food Chem. Toxicol. 2010;48:2741–2746. doi: 10.1016/j.fct.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 36.Hwang SJ, Luo JC, Lai CR, Chu CW, Tsay SH, Lu CL, Wu JC, Chang FY, Lee SD. Clinical, virologic and pathologic significance of elevated serum gamma-glutamyl transpeptidase in patients with chronic hepatitis c. Zhonghua Yi Xue Za Zhi (Taipei) 2000;63:527–535. [PubMed] [Google Scholar]
- 37.Clarke AT, Mills PR. Atorvastatin associated liver disease. Dig. Liver Dis. 2006;38:772–777. doi: 10.1016/j.dld.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Bitzur R, Cohen H, Kamari Y, Harats D. Intolerance to statins: Mechanisms and management. Diabetes Care. 2013;36(Suppl 2):S325–330. doi: 10.2337/dcS13-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cokça F, Ozkan S, Nergisoglu G, Memikoglu O, Azap A. Statin toxicity: A situation that mimics viral hepatitis. Int. J. Clin. Pharmacol. Ther. 2005;43:543–545. doi: 10.5414/CPP43543. [DOI] [PubMed] [Google Scholar]
- 40.Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ. Res. 2019;124:328–350. doi: 10.1161/CIRCRESAHA.118.312782. [DOI] [PubMed] [Google Scholar]
- 41.Swislocki AL, Lin K, Cogburn D, Fann KY, Khuu DT, Noth RH. Postmarketing analysis of lovastatin use in the va northern california system of clinics: A retrospective, computer-based study. Am. J. Manag. Care. 1997;3:1537–1545. [PubMed] [Google Scholar]
- 42.Alsheikh-Ali AA, Maddukuri PV, Han H, Karas RH. Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: Insights from large randomized statin trials. J. Am. Coll. Cardiol. 2007;50:409–418. doi: 10.1016/j.jacc.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 43.Rahmani AH, Almatroudi A, Babiker AY, Khan AA, Alsahli MA. Thymoquinone, an active constituent of black seed attenuates ccl4 induced liver injury in mice via modulation of antioxidant enzymes, pten, p53 and vegf protein. Open Access Maced. J. Med. Sci. 2019;7:311–317. doi: 10.3889/oamjms.2019.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandey KB, Rizvi SI. Protective effect of resveratrol on markers of oxidative stress in human erythrocytes subjected to in vitro oxidative insult. Phytother. Res. 2010;24(Suppl 1):S11–14. doi: 10.1002/ptr.2853. [DOI] [PubMed] [Google Scholar]
- 45.Tohamy AA, Abdella EM, Ahmed RR, Ahmed YK. Assessment of anti-mutagenic, anti-histopathologic and antioxidant capacities of egyptian bee pollen and propolis extracts. Cytotechnology. 2014;66:283–297. doi: 10.1007/s10616-013-9568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdel Kader R. Influence of hepatotoxic compounds on the molecular changes as well as DNA repairing mechanisms in hepatocytes. PhD Thesis in Pharmaceutical Sciences, Faculty of Pharmacy, Jagiellonian University Collegium Medicum 2012.
- 47.Al-Doaiss A, Alarifi S, Jararr B. Hepatic histological and histochemical alterations induced by rosuvastatin therapeutic doses. Pak. J. Zool. 2013;45:149–157. [Google Scholar]
- 48.El-Magd MA, Abdo WS, El-Maddaway M, Nasr NM, Gaber RA, El-Shetry ES, Saleh AA, Alzahrani FAA, Abdelhady DH. High doses of s-methylcysteine cause hypoxia-induced cardiomyocyte apoptosis accompanied by engulfment of mitochondaria by nucleus. Biomed. Pharmacother. 2017;94:589–597. doi: 10.1016/j.biopha.2017.07.100. [DOI] [PubMed] [Google Scholar]
- 49.Osaki Y, Nakagawa Y, Miyahara S, Iwasaki H, Ishii A, Matsuzaka T, Kobayashi K, Yatoh S, Takahashi A, Yahagi N, Suzuki H, Sone H, Ohashi K, Ishibashi S, Yamada N, Shimano H. Skeletal muscle-specific hmg-coa reductase knockout mice exhibit rhabdomyolysis: A model for statin-induced myopathy. Biochem. Biophys. Res. Commun. 2015;466:536–540. doi: 10.1016/j.bbrc.2015.09.065. [DOI] [PubMed] [Google Scholar]
- 50.Granito A, Muratori P, Ferri S, Pappas G, Quarneti C, Lenzi M, Bianchi FB, Muratori L. Diagnosis and therapy of autoimmune hepatitis. Mini. Rev. Med. Chem. 2009;9:847–860. doi: 10.2174/138955709788452676. [DOI] [PubMed] [Google Scholar]
- 51.Sánchez-Valle V, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012;19:4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- 52.Ghazwani M, Zhang Y, Gao X, Fan J, Li J, Li S. Anti-fibrotic effect of thymoquinone on hepatic stellate cells. Phytomedicine. 2014;21:254–260. doi: 10.1016/j.phymed.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karabela SP, Kairi CA, Magkouta S, Psallidas I, Moschos C, Stathopoulos I, Zakynthinos SG, Roussos C, Kalomenidis I, Stathopoulos GT. Neutralization of tumor necrosis factor bioactivity ameliorates urethane-induced pulmonary oncogenesis in mice. Neoplasia. 2011;13:1143–1151. doi: 10.1593/neo.111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sass G, Barikbin R, Tiegs G. The multiple functions of heme oxygenase-1 in the liver. Z. Gastroenterol. 2012;50:34–40. doi: 10.1055/s-0031-1282046. [DOI] [PubMed] [Google Scholar]
- 56.Majdalawieh AF, Fayyad MW. Immunomodulatory and anti-inflammatory action of nigella sativa and thymoquinone: A comprehensive review. Int. Immunopharmacol. 2015;28:295–304. doi: 10.1016/j.intimp.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 57.El-Tawil O, Moussa SZJJEST. Antioxidant and hepatoprotective effects of thymoquinone against carbon tetrachloride-induced hepatotoxicity in isolated rat hepatocyte. J. Egypt. Soc. Toxicol. 2006;34:33–41. [Google Scholar]
- 58.Choi EM. Antinociceptive and antiinflammatory activities of pine (pinus densiflora) pollen extract. Phytother. Res. 2007;21:471–475. doi: 10.1002/ptr.2103. [DOI] [PubMed] [Google Scholar]
- 59.Lebda F. Protective effect of fhymoquinone against d-galactos-amine-induced liver injury in rats. Aust. J. Basic Appl. Sci. 2011;5:49–58. [Google Scholar]
- 60.El-Bayomi KM, Saleh AA, Awad A, El-Tarabany MS, El-Qaliouby HS, Afifi M, El-Komy S, Essawi WM, Almadaly EA, El-Magd MA. Association of cyp19a1 gene polymorphisms with anoestrus in water buffaloes. Reprod. Fertil. Dev. 2018;30:487–497. doi: 10.1071/RD16528. [DOI] [PubMed] [Google Scholar]
- 61.Saleh AA, El-Magd MA. Beneficial effects of dietary silver nanoparticles and silver nitrate on broiler nutrition. Environ. Sci. Pollut. Res. 2018;25:27031–27038. doi: 10.1007/s11356-018-2730-7. [DOI] [PubMed] [Google Scholar]
- 62.Abu Gazia M, El-Magd MA. Effect of pristine and functionalized multiwalled carbon nanotubes on rat renal cortex. Acta Histochem. 2018;121(2):207–217. doi: 10.1016/j.acthis.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Helal MB, Labah DA, El-Magd MA, Sarhan NH, Nagy NB. Thyroidectomy induces thyroglobulin formation by parotid salivary glands in rats. Acta Histochem. 2020;122:151568. doi: 10.1016/j.acthis.2020.151568. [DOI] [PubMed] [Google Scholar]
- 64.Abou-Easa K, El-Magd M, Tousson E, Hassanin A, Shukry M, Salama M. Pineal gland plays a role in gonadal development after eyelids separation in puppies. Int. J. Morphol. 2014;33:7–18. doi: 10.4067/S0717-95022015000100001. [DOI] [Google Scholar]
- 65.Selim NM, Elgazar AA, Abdel-Hamid NM, El-Magd MRA, Yasri A, Hefnawy HME, Sobeh M. Chrysophanol, physcion, hesperidin and curcumin modulate the gene expression of pro-inflammatory mediators induced by lps in hepg2: In silico and molecular studies. Antioxidants. 2019;8:371. doi: 10.3390/antiox8090371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elgazar AA, Selim NM, Abdel-Hamid NM, El-Magd MA, El Hefnawy HM. Isolates from alpinia officinarum hance attenuate lps induced inflammation in hepg2: Evidence from in silico and in vitro studies. Phytother. Res. 2018;32:1273–1288. doi: 10.1002/ptr.6056. [DOI] [PubMed] [Google Scholar]
- 67.Saleh AA, Amber K, El-Magd MA, Atta MS, Mohammed AA, Ragab MM, Abd E-K. Integrative effects of feeding aspergillus awamori and fructooligosaccharide on growth performance and digestibility in broilers: Promotion muscle protein metabolism. BioMed Res.Int. 2014;2014:946859. doi: 10.1155/2014/946859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.