Abstract
Myxosporean infection of Indian major carps (rohu, Labeo rohita; catla, Gibelion catla; mrigal, Cirrhinus mrigala) was examined from two fish farms and two fish markets in West Bengal, India. One Thelohanellus and four Myxobolus species were detected from the fins and scales of the investigated species. Comprehensive morphological and molecular biological studies revealed four already known species, Thelohanellus caudatus from the fins of rohu, Myxobolus dermiscalis from the scales of rohu, Myxobolus chakravartyi from the fins of catla, and Myxobolus rewensis from the fins of mrigal. This study complemented the species description of M. chakravartyi and M. rewensis with the missing molecular data. Moreover, based on morphometrics and ssrDNA sequence data, a new species was documented from the scales of rohu, and named Myxobolus bandyopadhyayi n. sp.
Keywords: Myxozoa, Thelohanellus, Myxobolus, New species description, Indian major carps, Molecular data
Graphical abstract
Highlights
-
•
Myxozoan infections were recognised from Indian major carps in West Bengal, India.
-
•
A new Myxobolus species was discovered from the scales of rohu: Myxobolus bandiopadhyayi n. sp.
-
•
Redescription of four known Indian Myxobolus and Thelohanellus spp.
-
•
The first molecular data were obtained on Myxobolus chakravartyi and Myxobolus rewensis.
-
•
ssrDNA was used to support the morphological identification of taxa.
1. Introduction
Myxozoans are an abundant and diverse group of fish parasites and, due to their complicated life cycle, their occurrence, development, and pathology are intensively studied (Okamura et al., 2015). The genera Myxobolus Bütschli, 1882 and Thelohanellus Kudo, 1933 are among the most studied fish parasites. In the synopsis written by Eiras et al. (2014), 856 Myxobolus spp. were recorded and their number has grown by about 120 new species since then. The genus Thelohanellus is also rich in species: Zhang et al. (2013) reported 108 nominal species, but several new species have been described in the last couple of years as well (Eiras et al., 2021). The myxozoan parasites of Indian fishes are relatively well studied: Kalavati and Nandi (2007) reported the presence of 103 Myxobolus and 32 Thelohanellus species. Most of the species have been described from Indian cyprinids belonging to the genera Labeo, Gibelion (syn. Catla) and Cirrhinus. These major carps belong to the most important food fishes of India. According to the synopses by Kaur and Singh (2012) and Kaur et al. (2017), 131 and 52 Myxobolus and Thelohanellus spp. were recorded from Indian fish species, respectively. Since then, the number of newly described myxosporean species has increased significantly (Eiras et al., 2021). However, the description of only a part of these species has been complemented with molecular data, therefore it might be possible that several synonymous species exist among them. Gupta et al. (2021) mention 40 species of myxobolids with sequence data based on only a limited number of research studies (Singh and Kaur, 2012; Kaur, 2014; Rajesh et al., 2014; Abraham et al., 2015; Székely et al., 2015; Chaudhary et al., 2018, 2019; Gupta et al., 2018). Myxozoan samples were obtained from the genera Labeo, Gibelion and Cirrhinus collected from West Bengal fish farms and local fish markets which showed signs of myxosporean infections. After dissection, myxosporean cysts and spores isolated from the scales and fins were fixed and transported to Hungary for further morphological and molecular investigations.
In this paper a new species, Myxobolus bandyopadhyayi n. sp. is described from Labeo rohita and the occurrence of three other Myxobolus and one Thelohanellus species is recorded from Indian major carps, with their identity supported by ssrDNA sequences.
2. Materials and methods
Altogether 13 rohu (Labeo rohita Hamilton), 10 mrigal [Cirrhinus mrigala (Hamilton)], 8 catla [Gibelion catla (Hamilton)] specimens were obtained from Diara Fish pond (Hooghly District) and hatchery (22°28′31″N 88°09′54″E), East Kolkata Wetlands 22.5263°N 88.4716°E (District South 24 parganas, Baranagar Block). Furthermore, two markets near Kalyani, Naihati, Battala area (22.89°N 88.42°E) and Kakinara, Bhatpara area (22.51°N 88.23°E), were chosen and 12 rohu, 11 mrigal and 9 catla specimens were purchased in total. The fish individuals ranged in size from 26 to 33 cm, 32–46 cm and 38–46 cm with an average weight of 400 g, 1.75 kg and 2 kg for rohu, mrigal and catla, respectively. In the area of these farms, fishes are cultivated mainly in large pond systems and they were captured using drag net and cast net systems. On the fish markets, we directly purchased the fish from the fish farmers of that area and brought them in live condition to the laboratory for further investigation. The fish were sedated with clove oil and killed by a cervical cut. Only the fins and scales were studied extensively. Plasmodia from the scales were removed with a needle. Fins were cut from the fish body and checked for the presence of plasmodia. A portion of plasmodia was preserved in toto, while other plasmodia were opened and spores obtained. Both plasmodia and spore samples were divided into two parts and fixed in 80% ethanol or in 10% formalin. Fresh spores were photographed with an Olympus DP 74 digital camera and measurements were taken from digitised photos. Measurements were taken from 25 spores, and all morphometric parameters are given in μm.
2.1. Genomic DNA isolation, PCR and sequencing
Isolated plasmodia or myxospores preserved in 80% ethanol were centrifuged at 8,000×g for 10 min. Genomic DNA was isolated from the obtained pellet using the Geneaid Genomic DNA Mini Kit (Tissue), following the manufacturer's recommended protocol for animal tissue. Small subunit ribosomal DNA (SSU rDNA) was amplified in a nested polymerase chain reaction (PCR) described in detail in Cech et al. (2015). After the second round of PCR, amplified products were analysed by electrophoresis in a 1% agarose gel. The PCR products were purified from the gel, with the Gel/PCR DNA Fragments Extraction Kit (Geneaid Tawai City, Taiwan) and sequenced directly using the BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies) with an ABI PRISM 3100 Genetic Analyser (Life Technologies), using the amplification and inner primers for the sequencing reaction.
2.2. Phylogenetic analysis
The obtained sequences were assembled using MEGA X (Kumar et al., 2018) and manual adjustments were performed to correct the alignment and remove ambiguous positions from the dataset. Assembled sequences were verified as myxozoan by GenBank BLAST search. The sequences were aligned using Clustal W implemented in the MEGA X. The evolutionary history was inferred by using the Maximum Likelihood method and GTR + G model. The best evolutionary model of nucleotide substitution using the Akaike Information Criterion (AIC) was determined with the program MEGA X. Initial tree(s) for the heuristic search were obtained automatically by applying the Neighbour Joining method. A discrete Gamma distribution was used to model evolutionary rate differences among sites [5 categories (+G, parameter = 0.4311)]. The analysis involved 44 nucleotide sequences. There were a total of 1,820 positions in the final dataset. Evolutionary analyses were also conducted in MEGA X.
3. Results
Five myxosporean species, one Thelohanellus and four Myxobolus, were found among the collected samples.
Thelohanellus plasmodia occurred on the fins of rohu specimens that originated from the Diara fish farm and the Naihati fish market. In the case of the fish farm, 4 fish out of the 13 examined rohu specimens were infected, while 3 rohu specimens were infected with Thelohanellus plasmodia among 12 fish purchased from the fish market. One to 14 cysts were observed on the infected specimens. This species has been identified as Thelohanellus caudatus Pagarkar and Das (1993).
Besides T. caudatus, two more myxosporeans belonging to the genus Myxobolus were found in the rohu individuals. Both species parasitised the scales; however, based on the morphometrics and molecular results, they represent two different species. One of them, described as a new species, Myxobolus bandyopadhyayi n. sp., formed small round plasmodia with different-sized polar capsules on the external surface of the scales. It was found on fish specimens purchased from the local fish market. One to 4 cysts were found on a single scale and 3 of the examined fish proved to be infected.
The other Myxobolus species infecting the scales was found in 7 rohu specimens sampled in the Diara fish farm. The spores of this species had equal polar capsules. It was identified as Myxobolus dermiscalis Kaur et al. (2016) based on its morphological and molecular characteristics. In the plasmodia from catla and mrigal, Myxobolus spp. with differing spores were found in the fins. The species found in 4 specimens of catla is redescribed and identified with Myxobolus chakravartyi Haldar et al. (1983), while the other Myxobolus species found in 3 mrigal specimens was identified as Myxobolus rewensis Srivastava (1979).
Seven myxosporean samples were analysed for the ssrDNA. The amplified fragment of the samples was more than 1,600 bps, with the alignment being 1,820 bps, and containing 696 conservative and 1,027 variable (736 of them parsimony informative) sites. The ssrDNA sequences were submitted to the Genbank and published under the accession numbers MZ230375–MZ230381. Maximum likelihood analysis resulted in a robust phylogenetic tree, and high bootstrap values at the terminal nodes confirmed the identity of the studied species (Fig. 1)
Fig. 1.
Phylogenetic analysis of the studied myxozoan species based on ssrDNA sequences with Maximum Likelihood algorithm. Chloromyxum fluviatile was used as the outgroup. Bootstrap values are given at the nodes. The scale-bar indicates the number of expected substitutions per site.
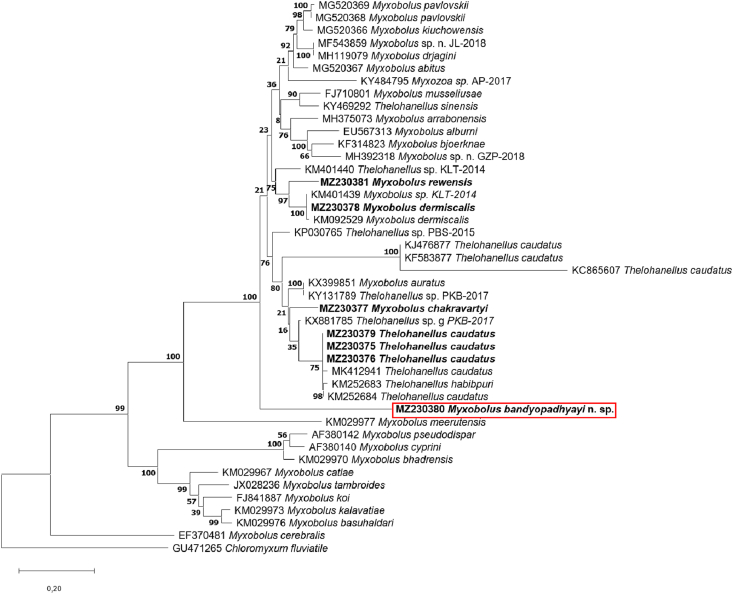
3.1. Thelohanellus caudatus infection of rohu (Fig. 2, Fig. 3, Fig. 4)
Fig. 2.
A plasmodium (P) of Thelohanellus caudatus in the fin of a rohu in close contact with the cartilaginous fin ray (Fr). Mount picture. Bar = 200 μm.
Fig. 3.
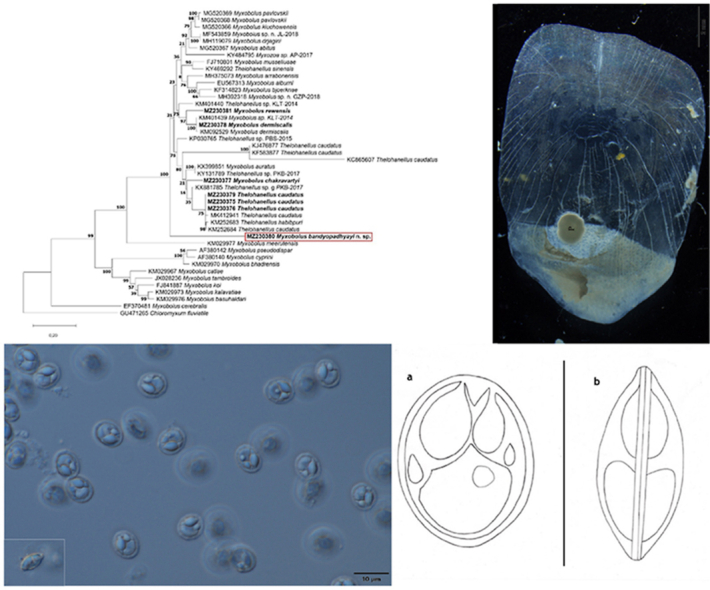
Spores of T. caudatus. Mount picture. Bar = 10 μm.
Fig. 4.
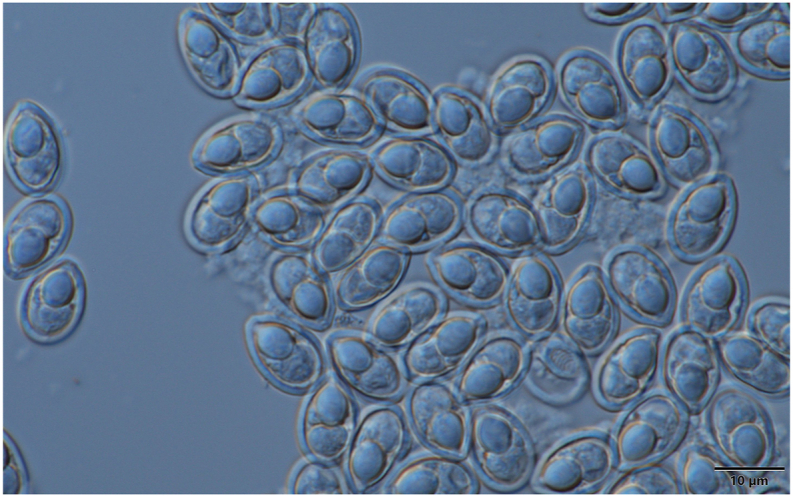
Schematic drawing of spore of T. caudatus, a: frontal view, b: sutural view. Bar = 10 μm.
Thelohanellus infection on the fins was found in 4 rohu specimens from 13 fish obtained from the Diara fish farm, while 3 infected specimens were observed among the 12 fish purchased on the fish market. Plasmodia (1–14 cysts) were recovered from the tail fins and the anal fins. Relatively small plasmodia of elliptical shape (Fig. 2), 1–2 mm long and 0.5–1 mm thick, locating at the proximal parts of the fins, close to the body, attached to the cartilaginous fin rays.
Mature spore (Fig. 3, Fig. 4) pyriform, with slightly tapering anterior and round posterior end, 13.7 ± 0.93 (13.3–14.6) long (n = 25), 9 ± 0.82 (7.5–9) wide (n = 25) and 6.8 ± 0.41 (6.2–7.3) thick (n = 15). The wall relatively thick 1 ± 0.2 (0.7–1.1). Single short ellipsoidal polar capsule present close to apex of spore, 6.5 ± 0.4 (6.2–6.9) (n = 25) long, 4.7 ± 0.47 (4.2–5) (n = 25) thick. Polar tubules not seen. At one side between the polar capsule and the sporoplasm a bright nucleus of the capsulogenic cell, about 1 × 1.5 present. No iodinophilous vacuole or mucous envelope seen.
Host: Rohu, Labeo rohita Hamilton.
Locality: Diara Fish Farm (Hooghly District) fish pond and hatchery (22°28′31″N 88°09′54″E) and fish market Naihati, Battala market (22.89°N 88.42°E) (on the Kalyani expressway)
Site of infection: Fins.
Material: Photo-types were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNHM-PAR-72077. The ssrDNA sequence of T. caudatus was deposited in the GenBank under accession numbers MZ230375, MZ230376 and MZ230379.
Prevalence of infection: 7 specimens from 25 fish.
Molecular data: ssrDNA sequences of T. caudatus (MZ230375-6 and MZ230379) collected from the fins of rohu were identical with each other and showed a 99.8% similarity with the T. caudatus sample deposited in the GenBank as KM252684. However, with the other deposited sequences (MK412941) only 98.8% similarity was found. Interestingly enough, a 99.0% similarity was also obtained with sequence (KM252683) of T. habibpuri Acharia and Dutta (2007). Interestingly, there is also another T. caudatus clade (KC865607, KF583877, KJ476877) with a maximum bootstrap distinct from the above-mentioned sequences. Genetic similarities of our samples are 87.3% in the case of two sequences (KF583877, KJ476877) and 71.1% in the case of KC865607.
Remarks: Based on the similarity in morphology and size of the present species and the one described by Pagarkar and Das (1993), we regard the species found by us as T. caudatus. The identification is strongly supported by the similarity between the sequences reported by Mondal et al. (2014) and those presented in this study. According to the morphological similarity of T. caudatus and T. avijity Basu and Haldar, 2003, we regard the latter species as a synonym of T. caudatus. Likewise, Thelohanellus habibpuri Acharia and Dutta (2007) is considered also a junior synonym of T. caudatus. Acharia and Dutta (2007) described T. habibpuri from the fins of rohu and studied its ultrastructure, but provided only an incomplete morphological description. The spore morphology and measurements given for M. habibpuri correspond to those of T. caudatus sp. (Table 1).
Table 1.
Comparative measurements of Thelohanellus caudatus syn. T. habibpuri and T. avijiti described from the fin of rohu, Labeo rohita.
| Data given by | Length of the spore | Width of the spore | Thickness of the spore | Length of the polar capsule. | Width of the polar capsule. |
|---|---|---|---|---|---|
| T. caudatusPagarkar and Das 1993 | 13.8 ± 0.37 13-14 | 9 ± 0.16 8.5–9.5 | – | 7.02 ± 0.11 7–7.5 | 5.07 ± 0.1813.9 |
| T. caudatus byMondal et al. (2014) | 15.41 14.8–16.6 | 10.33 9.2–12.9 | 6.99 6.2–8.3 | 5.32 6.2–8.3 | |
| Present data | 13,7 ± 0,93 (12,3–14,6) | 9 ± 0.82 (8–10) | 6,8 ± 0,41 (6,2–7,3) | 6,5 ± 0,4 (6,2–6,9) | 4,7 ± 0,47 (4,2–5) |
| T. habibpuriAcharia and Dutta, 2007 | 13.8 ± 0.53 (13–14.3) | 8.5 ± 0.036 (8–9) | 6 ± 0.025 (6–6.5) | 4.9 ± 0.062 (4.1–5) | |
| T. avijiti Basu and Haldar, 2003 | 14 (13.0–14.7) | 9.7 (9.1–10.2 | 6.0 (5.6–6.4) | 4.0 (3.8–4.2) |
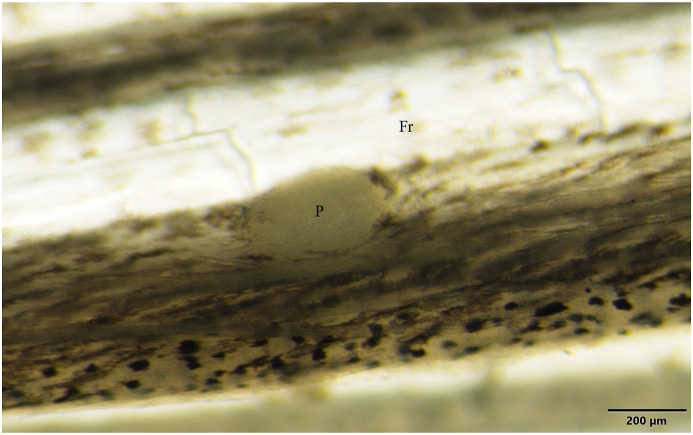
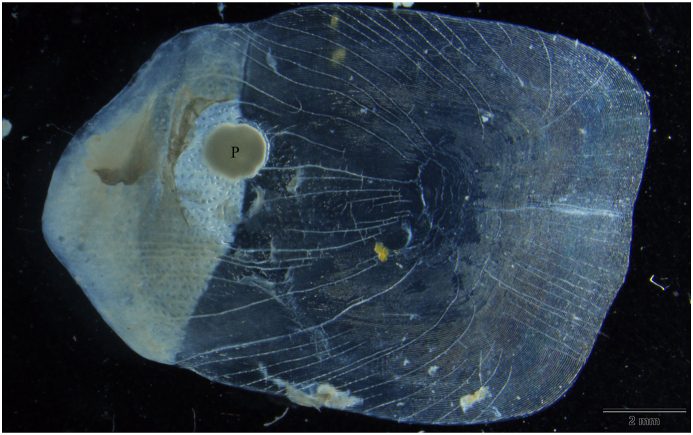
3.2. Description of Myxobolus bandyopadhyayi n. sp. (Fig. 5, Fig. 6, Fig. 7)
Fig. 5.
Myxobolus bandyopadhyayi n. sp. plasmodium (P) in the scale of a rohu. Mount picture. Bar = 2 mm.
Fig. 6.
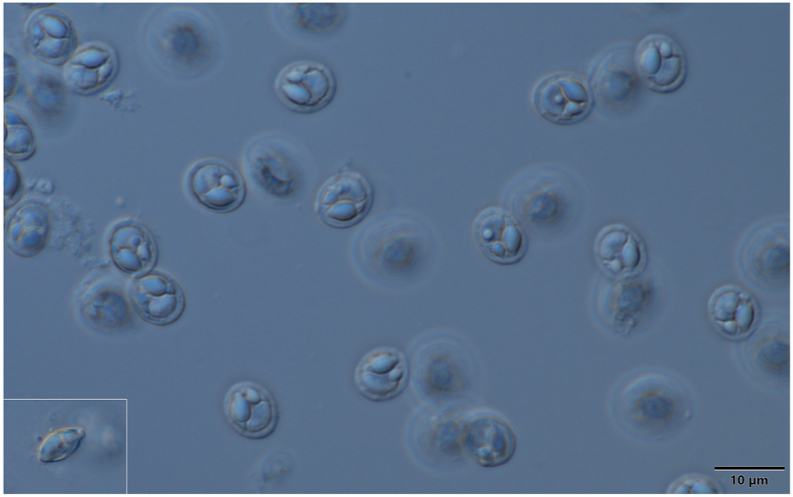
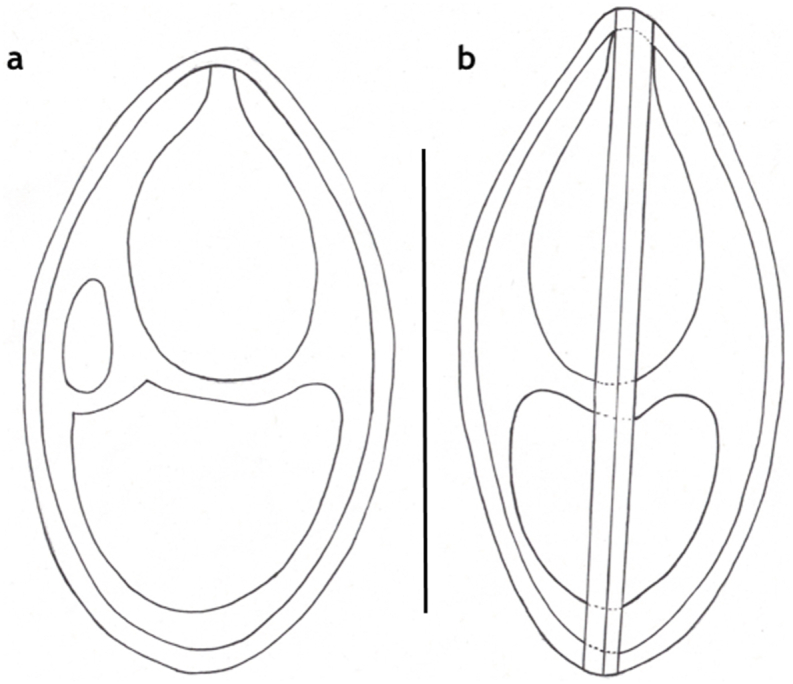
M. bandyopadhyayi n. sp. spores of a rohu in frontal view. Inset: A spore in sutural view. Mount picture. Bar = 10 μm.
Fig. 7.
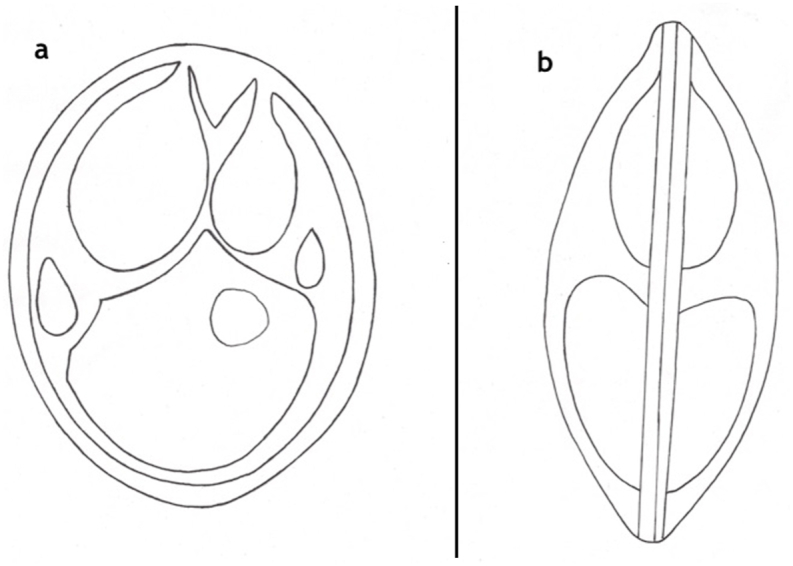
Schematic drawing of spore of M. bandyopadhyayi, a: frontal view, b: sutural view. Bar = 10 μm.
Round-shaped plasmodia (Fig. 5) with a diameter of 700 to 1,500 were located in the superficial tissues of the scale. They occurred in 3 out of the 12 rohu specimens from the fish market. Plasmodia contained 4,000 to 10,000 spores.
Myxospores (Fig. 6, Fig. 7) small sized, roundish or short ellipsoidal in frontal view and lemon shaped in sutural view. Spores 8.4 ± 0.19 (8.3–8.8) long, 6.6 ± 0.54 (5.2–7.5) wide and 4.6 ± 0.4 (4.3–5.4) thick. Two polar capsules short pyriform, different in size. The larger 4.2 ± 0.38 (3.7–4.5) long and 2.5 ± 0.18 (2.2–2.7) wide, the smaller 2.3 ± 0.4 (1.7–2.8) long and 1.65 ± 0.05 (1.6–1.7) wide. Polar tubules not seen. Spore with approximately 1.2 long, triangular, eccentrically located intercapsular appendix. Sporoplasm nuclei indiscernible. A small distinct iodinophilous vacuole present in the sporoplasm. Mucous envelope not found. The thickness of the spore wall 0.65 ± 0.05 (0.6–0.7). Nuclei of the polar capsules distinct.
Type host: Rohu Labeo rohita Hamilton.
Type locality: Naihati, Battala market (22.89°N 88.42°E) (on the Kalyani expressway)
Site of development: Scale.
Type material: Photo-types and histological preparations were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNHM-PAR-72078. The ssrDNA sequence of M. bandyopadhyayi was deposited in the GenBank under accession number MZ230380.
Prevalence of infection: 3 specimens from 12 fish.
Molecular data: ssrDNA sequences of M. bandyopadhyayi (MZ230380) collected from the scales of rohu significantly differed from any myxosporean species deposited in the GenBank. This species was located as a single species in a distinct clade, the highest similarity to other myxosporean species was 83.9% in relation to Myxobolus chakravartyi.
Remarks: By the short oval shape of its spores, by the different sized but short ellipsoidal polar capsules, this species differed from most species known from major Indian carps. Of the Myxobolus species with two different-sized polar capsules M. buccoroofus Basu and Haldar, 2004 had dissimilar spore shape. Polar capsules of M. calbasui Chakravarty, 1939, M. chilkensis Kalavati et al., 1992 and M. bhadrensis Seenappa and Manohar, 1980 had elongated shape. Spores of M. edellae Sarkar, 1999 are also nearly spherical, but the difference between its two polar capsules is only moderate.
Etymology: The species is named after Prof. Probir K. Bandyopadhyay, the well-known fish parasitologist, who managed this recent co-operation from the Indian side and worked actively until his decease in January 2021.
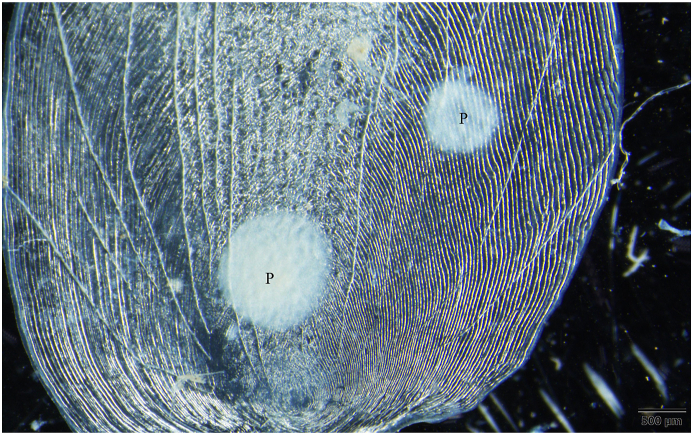
3.3. Myxobolus dermiscalisKaur et al. (2016) (Fig. 8, Fig. 9, Fig. 10)
Fig. 8.
Myxobolus dermiscalis plasmodia (P) in the scale of a rohu. Mount picture. Bar = 500 μm.
Fig. 9.
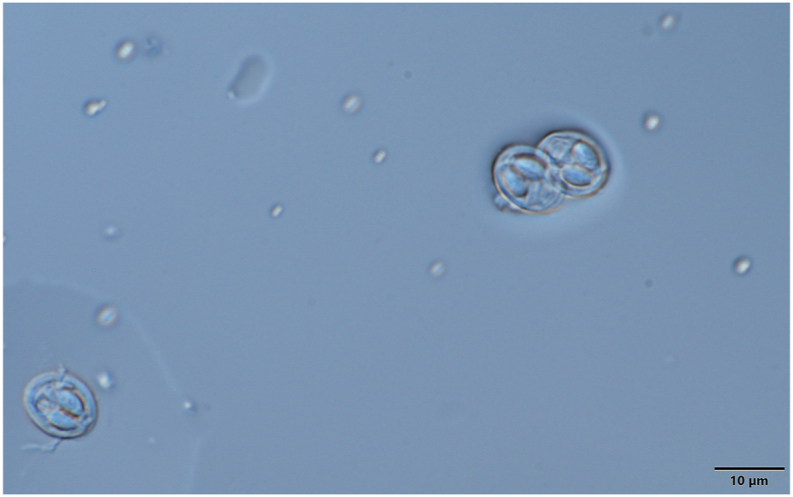
M. dermiscalis spores in frontal view. Mount picture. Bar = 10 μm.
Fig. 10.
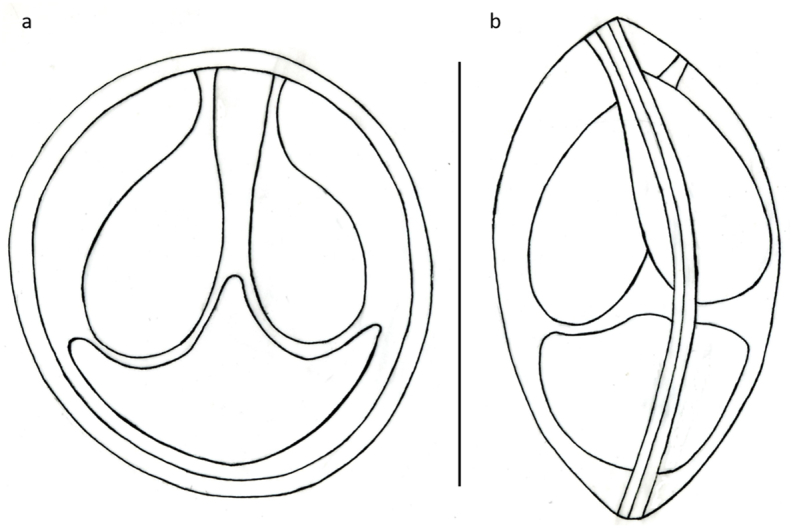
Schematic drawing of spore of M. dermiscalis, a: frontal view, b: sutural view. Bar = 10 μm.
Round-shaped plasmodia (Fig. 8) with a diameter of 700 to 1,500 were located in the superficial tissues of the scale. They occurred in 4 out of 13 rohu specimens from the fish farm. Plasmodia contained 800 to 2,000 spores.
Spores (Fig. 9, Fig. 10) short ellipsoidal in frontal view and lemon shaped in sutural view. Spores 10.6 ± 0.44 (9.9–11.2) long, 8.5 ± 0.3 (8–8.8) wide and 6–6.2 thick. Two polar capsules elongated pyriform, uniform in size, 5.4 ± 0.3 (5–5.8) long and 2.5 ± 0.22 (2.2–2.7) wide. Polar tubules not seen. The proximal end of the spore has a short-flattened plate. Nuclei of the sporoplasm and polar capsules not seen. Iodinophilous vacuole and mucous envelope not found. The thickness of the spore wall 0.7–0.8.
Host: Rohu Labeo rohita Hamilton.
Locality: Naihati, Battala market (22.89°N 88.42°E) (on the Kalyani expressway)
Site of infection: scales.
Prevalence of infection: 4 specimens from 13 fish.
Type material: Photo-types and histological preparations were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNHM-PAR-72079. The ssrDNA sequence of M. bandyopadhyayi was deposited in the GenBank under accession number MZ230378.
Molecular data: ssrDNA sequence (GenBank accession number: MZ230378) of the parasite was 99.4% similar to Myxobolus dermiscalis (KM092529) and 98.8% similar to a Myxobolus sp. sample (KM401439) which was also isolated from the scales of rohu. These three sequences formed a monophyletic clade with maximum bootstrap. As the closest relatives on the phylogenetic tree, the sequences of Myxobolus rewensis (MZ230381, this paper) and Thelohanellus sp. (KM401440) showed 91.8% and 91.6% similarity, respectively.
Remarks: The collected spores were in ethanol-fixed preserved state and showed only the most important figures for identification. The horizontal flattened plate at the anterior end of the spores resembled closely to the figures presented by Kaur et al. (2016). However, the authors made some mistakes in measuring oocysts. These authors measured the size of spores as 5.84–7.84 × 3.98–5.98 μm; however, the bar shown on spore photos and line drawings prove that the spores were larger than 10 μm. Molecular data deposited to the GenBank by the latter authors showed a close similarity to our data; moreover, sequences in the GenBank (KM401439, Myxobolus sp. KLT, 2014) from the scale of the rohu differed from our sequences only in 0.01%.
3.4. Myxobolus chakravartyiHaldar et al. (1983) (Fig. 11, Fig. 12, Fig. 13)
Fig. 11.
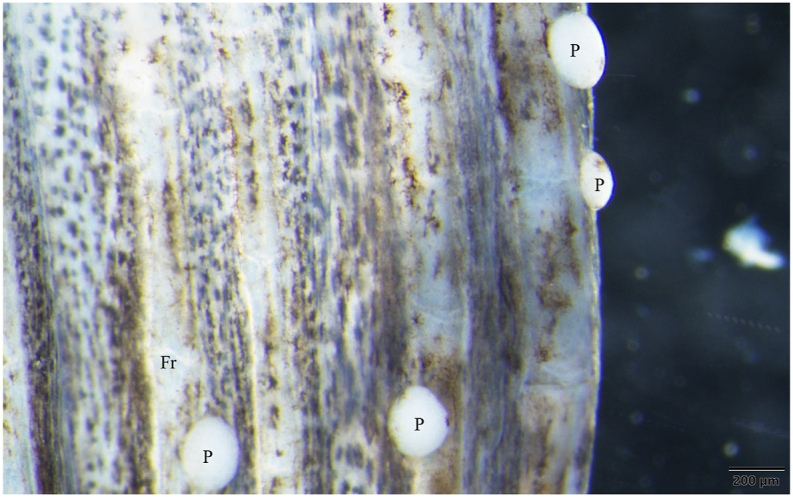
Myxobolus chakravartyi plasmodia (P) in the fin of a catla in close contact with fin rays (Fr). Mount picture. Bar = 200 μm.
Fig. 12.
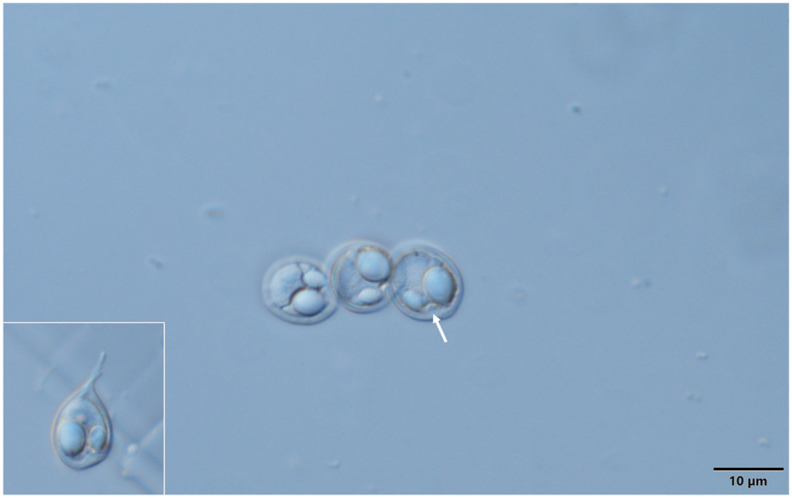
M. chakravartyi spores from the fin of a catla. Arrow is indicating the charasteristic intercapsular appendix. Inset: deformed spore with the Henneguya like caudal extension. Mount picture. Bar = 10 μm.
Fig. 13.
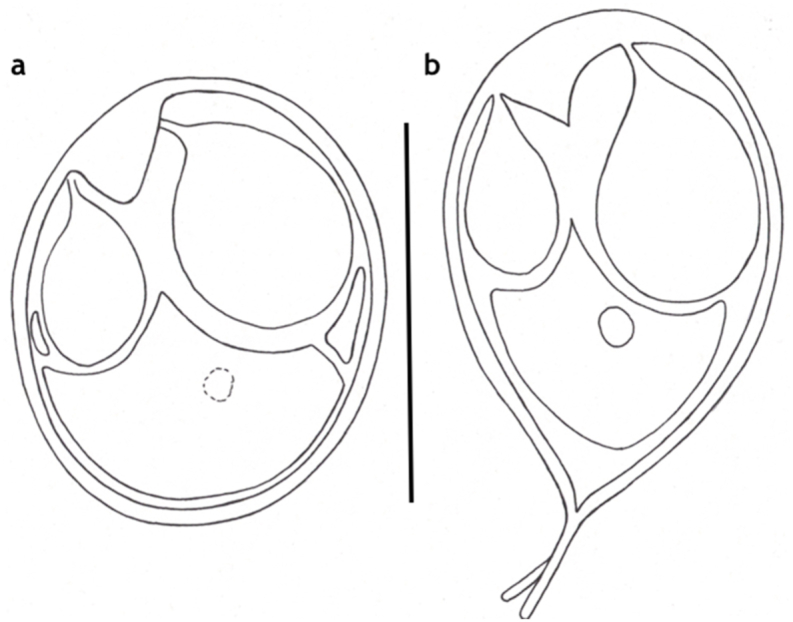
Schematic drawing of spore of M. chakravartyi, a: frontal view, b: frontal view of deformed spore with caudal extension. Bar = 10 μm.
Four out of the 9 catla specimens purchased on the fish market were infected with small ellipsoidal-shaped plasmodia reaching a size of 200–300 in length and 150–200 in width and attaching to the cartilaginous fin rays (Fig. 11). The plasmodia contained 2,000 to 8,000 spores.
Spores (Fig. 12, Fig. 13) medium sized, roundish or short ellipsoidal in frontal view and lemon shaped in sutural view having a small conical, 0.5–1 long protuberance at the anterior end. Spores 12 ± 0.38 (11.6–12.6) long, 10 ± 0.45 (9.4–10.7) wide and 6 ± 0.36 (4–6.2) thick. Two polar capsules short pyriform, different in size. The larger 5.7 ± 0.36 (5.4–6.2) long and 4.1 ± 0.61 (3.5–5) wide, the smaller 3.6 ± 0.15 (3.5–3.8) long and 2.2 ± 0.1 (2.1–2.3) wide. Polar tubules not seen. Spore with a large, triangular, eccentrically located intercapsular appendix. Sporoplasm nuclei indiscernible. A small iodinophilous vacuole found in the sporoplasm. Mucous envelope not found. The thickness of the spore wall (which corresponds to the emerging collar of the suture) 1 ± 0.16 (0.8–1.2). Some crippled spores (Fig. 12 inset) bear Henneguya like tails of 4–5.
Host: Catla Gibelion catla (Hamilton)
Locality: Kakinara, Bhatpara market (22.51°N 88.23°E), on the Kalyani expressway.
Site of infection: fins.
Prevalence of infection: 4 specimens from 9 fish.
Type material: Photo-types and histological preparations were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNHM-PAR-72080. The ssrDNA sequence of M. bandyopadhyayi was deposited in the GenBank under accession number MZ230377.
Molecular data: The ssrDNA sequence of Myxobolus chakravartyi (GenBank accession number: MZ230377) was closest to the sequences of Myxobolus auratus (KX399851) and several Thelohanellus sp. samples (KP030765, KX881785, KY131789). The sequence similarities fell within the range between 93.4 and 94.7%. Sequences of Thelohanellus caudatus showed a similarity between 92.9 and 94.1%.
Remarks: Spores found in catla specimens corresponded both in measurements and shape to Myxobolus chakravartyi Haldar et al. (1983) species. They differed, however, in three features. Although Haldar et al. (1983) mentioned a triangular thickening at the anterior end of the spores in their description, they failed to present the prominent intercapsular appendix in their figures, which was present in our morphological analysis. On the other hand, the streak of the protuberance in their figures was visible. These authors found their species in the internal musculature inside the eye of a catla specimen. In our case, plasmodia were found only in the fins, most probably in the connective tissue. Based on the strict tissue specificity of Myxobolus spp. we assume that the plasmodia in the eye were rather in contact with connective tissue elements.
3.5. Myxobolus rewensisSrivastava (1979) (Fig. 14, Fig. 15, Fig. 16)
Fig. 14.
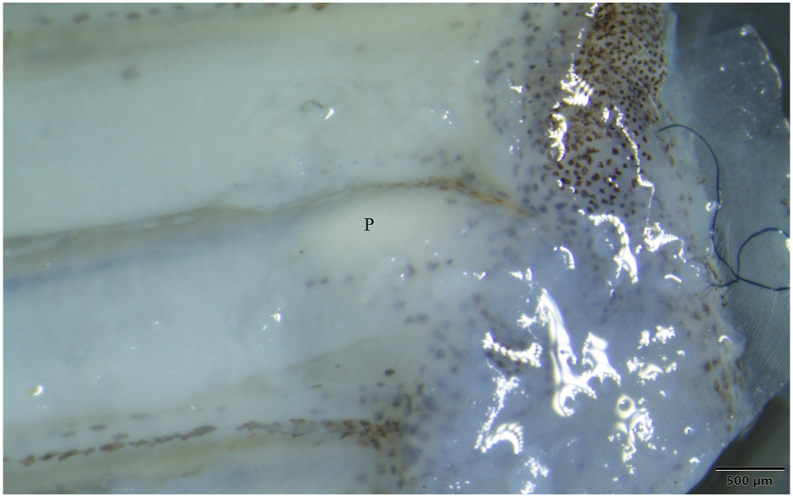
Myxobolus rewensis plasmodium (P) in the fin of a mrigal. Mount picture. Bar = 500 μm.
Fig. 15.
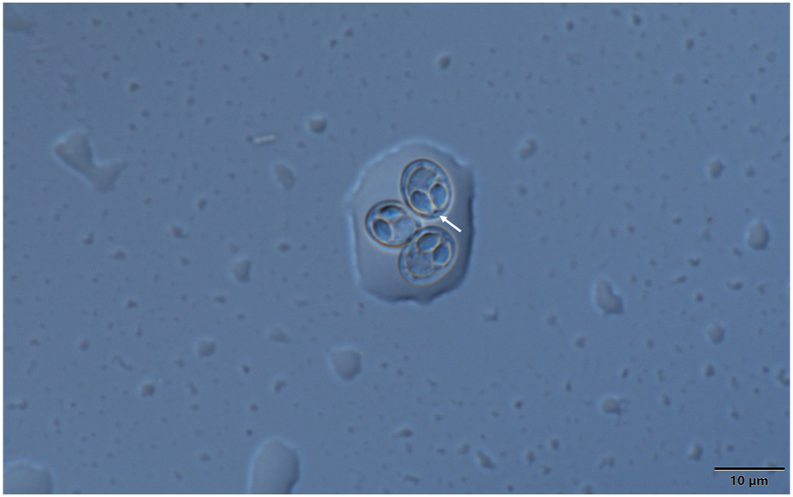
Spores of M. rewensis from the fin of mrigal. Arrow is indicating the characteristic globule inside the intercapsular appendix. Mount picture. Bar = 10 μm.
Fig. 16.
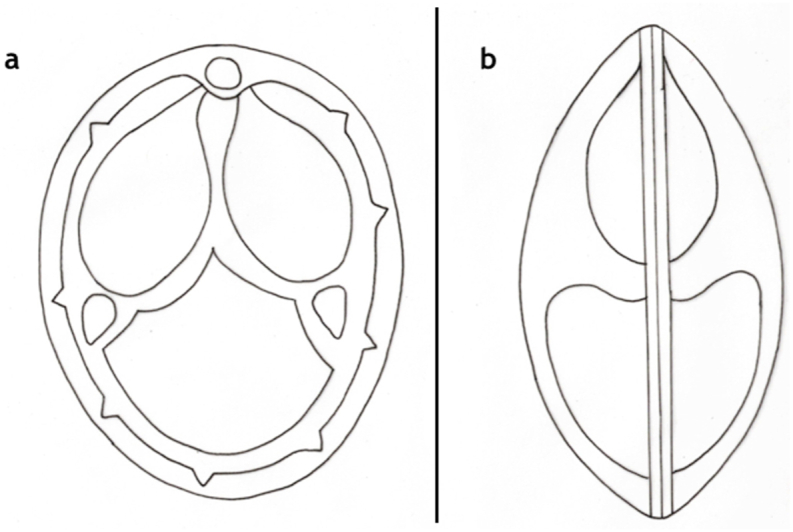
Schematic drawing of spore of M. rewensis, a: frontal view, b: sutural view. Bar = 10 μm.
Three out of the 11 mrigal specimens purchased on the fish market were infected with small ellipsoidal-shaped plasmodia reaching a size of 1–2.2 mm in length and 0.5–0.7 mm in width, which were located in the fins between two fin rays close to the base of the fin (Fig. 14). The plasmodia contained 2,000 to 10,000 spores.
Spores (Fig. 15, Fig. 16) small sized, ellipsoidal in frontal view and lemon shaped in sutural view, 8.8 ± 0.16 (8.5–9.1) long, 7.3 ± 0.27 (6.8–9.7) wide and 5.2 ± 0.22 (5–5.4) thick. Two oval polar capsules equal, 3.8 ± 0.19 (3.4–4) long, 2.1 ± 0.14 (2.1–2.3) wide. Polar tubules not seen. Nuclei of the polar capsules distinct, measuring 1.7 × 0.6 on average. Spore with a rounded, about 0.9–1 thick intercapsular appendix in which a bright globule with a diameter of 0.8 is located. Sporoplasm nuclei indiscernible. A small iodinophilous vacuole present in the sporoplasm. Mucous envelope not found. The spore wall (which corresponds to the emerging collar of the suture) has 8 sutural edge markings. Its thickness 0.66 ± 0.05 (0.6–0.7).
Host: Mrigal Cyrrhinus mrigala Hamilton.
Locality: East Kolkata Wetlands 22.5263°N 88.4716°E (District South 24 parganas, Baranagar Block).
Site of infection: Fins.
Type material: Photo-types and histological preparations were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNHM-PAR-72081 . The ssrDNA sequence of M. rewensis was deposited in the GenBank under accession number MZ230381.
Prevalence of infection: 3 specimens from 10 fish.
Molecular data: ssrDNA sequence of Myxobolus rewensis was a sister group of Myxobolus dermiscalis showing a 91.9% similarity. There was also a 90.1% similarity to a Thelohanellus sp. sample (KM401440) from the skin and gill arch of rohu.
Remarks: The Myxobolus species found by us resembles Myxobolus rewensis, differing only in two characteristics. Plasmodia found by us were located in the fin of mrigal, while Srivastava (1979) described the infection from the scales. Both spores found by us and spores of M. rewensis have a relatively large intercapsular appendix, but we were able to observe a characteristic bright nodule inside the appendix. Four other morphologically similar species (Table 2, M. shetti Seenappa and Manohar, 1981 and M. vanivilasae Seenappa and Manohar, 1980 from mrigal; M. dermatis Haldar et al., 1981 and M. rohitae Haldar et al., 1983 from rohu) have been described from labeonid fishes (Seenappa and Manohar, 1980, 1981; Haldar et al., 1981, 1983). However, the plasmodia of the latter species develop either in the scales or in the gills. Myxobolus dermatis and M. rohitae were described from rohu which is phylogenetically relatively far from mrigal (Khedkar et al., 2014; Chakraborty and Ghosh, 2014). Among the species described from mrigal, M. vanivilasae differed from M. rewensis in spore morphology having relatively small polar capsules, while the spores of M. shetti have only a small indistinct intercapsular appendix. Despite the different location and the small globule found in the intercapsular appendix, we regard the spores found by us as belonging to M. rewensis Srivastava (1979) until organ and tissue specificity can be studied in more detail.
Table 2.
Comparative measurements of Myxobolus rewensis and similar spp.
| Data given by | Hosts | Site of infection | Length of the spore | Width of the spore | Thickness of the spore | Length of the polar capsule. | Width of the polar capsule. | Inter- capsular appendix |
|---|---|---|---|---|---|---|---|---|
| Myxobolus rewensisSrivastava, 1979 | Cirrhinus mrigala | scales | 8.0–10.0 | 7.0–9.0 | 3.0–4.0 (3.57) | 2.0–3.0 (2.5) | Distinct | |
| Present data | Cirrhinus mrigala | fins | 8.8 ± 0.16 (8.5–9.1) | 7.3 ± 0.27 (6.8–7.7) | 5.2 ± 0.22 (5–5.4) | 3.8 ± 0.19 (3.4–4) | 2.1 ± 0.14 (2.1–2.3) | present |
| M. dermatisHaldar et al. (1981) | Labeo rohita | scales | 9.0–11.0 (10.3) | 8.0–10.0 (9.4) | 4.0–5.0 (4.4) | 2.0–3.0 (2.2) | present | |
| M. rohitaeHaldar et al. (1983) | Labeo rohita | scales | 9.9–12.1 (10.6) | 8.8–9.9 (9.0) | 6.6 | 3.3 | present | |
| M. shettiSeenappa and Manohar (1981) | Cirrhinus mrigala | gills | 8.0–9.0 (8.8) | 7.0–8.0 (7.4) | 3.0–4.0 (3.4) | 2.0–3.0 (2.3) | present | |
| M. vanivilasaeSeenappa and Manohar (1980) | Cirrhinus mrigala | scales | 8.0–10.0 | 7.0–9.0 | 3.0–4.0 (2.98) | 2.0–3.0 (2.5) | present |
4. Discussion
The occurrence of myxosporean parasites in Indian fishes is relatively well studied: Kaur and Singh (2012) listed 130 species. Especially Myxobolus spp. infecting major carps (Labeo, Cirrhinus and Catla) are well represented by 20 species from the genera Labeo and Cirrhinus and by 10 species from the genus Gibelion (Catla). Since their synopsis from 2012, at least 20 new species have been described (Eiras et al., 2021). Unfortunately, the majority of the Indian myxosporean species have been poorly described: they are characterised only by morphometrical measurements and by the shape of spores and polar capsules, and less information is provided about the location of plasmodia in infected tissues. The validity of several Indian myxosporean species has recently been supported by molecular biological data (Székely et al., 2015; Chaudhary et al., 2018, 2019; Gupta et al., 2018). Presumably, more in-depth investigations supplemented with sequence data might prove several species to be synonyms. Moreover, little is known on the host, organ and tissue specificity of Indian Myxobolus spp. Molnár (1994) stated that myxosporeans are host-, organ- and tissue-specific parasites, which usually infect a single host (e.g. Thelohanellus nikolskii Achmerov, 1955) or only a few closely related fish species (e.g. Myxobolus cerebralis Hofer, 1903), and they prefer a specific organ and develop strictly in a specific tissue.
All of the Indian major carps belong to the Labeoninae subfamily, they are closely related species and have several cultured hybrids in fish farms where different major carp species are frequently cultured together in the same ponds. Chakraborty and Ghosh (2014) and Khedkar et al. (2014) studied the molecular phylogeny of several Indian fish species including the genera Labeo, Catla and Cirrhinus. Labeo rohita and Catla catla show a close relationship in the phylogenetic analysis as being sister species, while Cirrhinus mrigala is positioned relatively far from the Labeo and Catla and shows a closer relation to common carp (Cyprinus carpio). Lots of myxosporean species have been described from more than one Labeoninae fish species. Further molecular analysis can reveal whether the myxosporean parasites of India have a broad host range or these myxosporeans of similar morphology described from different fish species represent distinct species.
Besides host specificity, organ specificity may also facilitate the differentiation and description of myxosporean species. However, in the case of many species, plasmodia may develop in different organs, but in the same type of tissue, e.g. T. nikolskii in the scales and fin rays which contain the same cartilaginous tissues (Moshu and Molnár, 1997), which emphasises the importance of determining the tissue preference, too. In the majority of species descriptions from Indian fishes, no data can be found about host and tissue specificity. Authors frequently compare only the spores of myxosporeans isolated from phylogenetically unrelated fish species, and the location of plasmodia is often characterised only by indicating the organ without mentioning the tissue type.
Recently, Ahmad and Kaur (2018) have studied the prevalence as well as the site and tissue preference of myxosporeans from fingerlings of major carps in the same fish farm and concluded that the 10 investigated Myxobolus species found by them developed in three different fish species (C. mrigala, L. rohita, G. catla) and all of them showed a typical site preference, producing intralamellar gill infections in most cases. The findings of our present survey are in agreement with the results of the above-cited authors, as host, organ and tissue specificity was observable in all of our cases.
Phylogenetic analysis of the ssrDNA positioned our samples in well-defined clades. Some of them were placed next to already sequenced species like Myxobolus dermiscalis or Thelohanellus caudatus. In the case of Myxobolus dermiscalis, the morphology and sequence data from previous studies (Kaur et al., 2016) were consistent with our results, and its identification raised no doubts at all. However, Thelohanellus caudatus samples are grouped into two distinct clades. Unfortunately, the sequences KC865607, KF583877 and KJ476877 forming one clade do not have available publications under the annotation, and therefore the morphology of those samples and their species identity cannot be verified. The other clade included our samples (MZ230375, MZ230376 and MZ230379), Thelohanellus caudatus samples (KM252684, MK412941) and Thelohanellus habibpuri (KM252683). The identity of MK412941 seems to be well supported as the related article by Mondal et al. (2014) includes morphological observations besides the sequence data. The other T. caudatus sample in this clade (KM252684), together with T. habibpuri (KM252683), lacks a published research article; however, KM252684 (T. caudatus) having the same species identity raises no question to be answered. On the other hand, the presence of T. habibpuri (KM252683) can be explained by the assumption that this species can be regarded as a junior synonym of T. caudatus. The incomplete description of T. habibpuri by Acharia and Dutta (2007) provides morphological characteristics highly similar to those of T. caudatus.
Other species, like Myxobolus chakravartyi and M. rewensis, were placed separately from other species, as these species had no sequence data up to this point. Myxobolus chakravartyi is mostly surrounded by Thelohanellus sequences with the exception of Myxobolus auratus (KX399851); however, the annotation of this Myxobolus sample lacks a lot of details like host species, isolation source etc.; moreover, publication data are also missing. The position of M. chakravartyi between Thelohanellus samples is not unusual as Thelohanellus and Myxobolus species do not form distinct groups in phylogenetic analyses, and Thelohanellus species are scattered between the Myxobolus species (Shin et al., 2014).
Myxobolus rewensis was placed next to M. dermiscalis on the phylogenetic tree. Other known parasites of mrigal, like M. catlae and M. kalavatiae, are placed in a different clade. These latter species infect the gill lamellae unlike M. rewensis, which occurs on the fins.
Interestingly, M. bandyopadhyayi n. sp. occupied a distinct position on the phylogenetic tree, and there was no other species in close relation to it. Myxobolus bandyopadhyayi n. sp. could be a representative of a separate lineage inside the myxobolid clade; further molecular data of Indian myxosporeans might reveal closer relatives of this species.
It is supposed that there are still many undescribed myxosporean species in the Indian fauna. Moreover, most of the described species lack sequence information, or simply the original description lacks important characteristics. Therefore, it would be important to obtain more data about the myxosporean fauna of the country and to supplement old, incomplete descriptions in order to get a full picture about the myxosporean diversity of India.
Acknowledgements
The study was supported by the 2017–2.3.7-TÉT-IN-2017-00003 Indo-Hungarian Inter-Governmental Science and Technology Cooperation Program. The author thanks Ms Györgyi Ostoros for the histological slides and Mr Ádám Varga for helping with photo techniques.
References
- Abraham T.J., Banerjee S., Patra A., Sarkar A., Adikesavalu H., Dash G. Molecular phylogeny of Myxobolus orissae (Myxosporea: Myxobolidae) infecting the gill lamellae of mrigal carp Cirrhinus mrigala (Actinopterygii: Cyprinidae) Mol. Biol. Res. Commun. 2015;4:15–24. [PMC free article] [PubMed] [Google Scholar]
- Acharia S., Dutta T. Thelohanellus habibpuri sp. n. (Myxozoa: Bivalvulida) from the tropical freshwater fish rohu, Labeo rohita (Hamilton-Buchanan, 1882) in West Bengal, India: light and electron microscope observations. Anim. Biol. Leiden. 2007;57(3):293–300. doi: 10.1163/157075607781753119. [DOI] [Google Scholar]
- Ahmad I., Kaur H. Prevalence, site and tissue preference of myxozoan parasites infecting gills of cultured fingerlings of Indian major carps in District Fatehgarh Sahib, Punjab (India) J. Parasit. Dis. 2018;42(4):559–569. doi: 10.1007/s12639-018-1035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech G., Borzák R., Molnár K., Székely C. Three new species of Myxobolus Bütschli, 1882 (myxozoa: Myxobolidae) infecting the common nase Chondrostoma nasus (L.) in the river Danube. Syst. Parasitol. 2015;92:101–111. doi: 10.1007/s11230-015-9589-5. [DOI] [PubMed] [Google Scholar]
- Chakraborty M., Ghosh S.K. An assessment of the DNA barcodes of Indian freshwater fishes. Gene. 2014;537(1):20–28. doi: 10.1016/j.gene.2013.12.047. [DOI] [PubMed] [Google Scholar]
- Chaudhary A., Goswami U., Gupta A., Cech G., Singh H.S., Molnár K., Szekely C., Sharma B. Morphological, histological, and molecular description of Myx-obolus ompok n. sp. (Myxosporea: Myxobolidae), a kidney myxozoan from Pabdah catfish Ompok pabda (Hamilton, 1822) (Siluriformes: Siluridae) in India. Parasitol. Res. 2018;117:1899–1905. doi: 10.1007/s00436-018-5882-y. [DOI] [PubMed] [Google Scholar]
- Chaudhary A., Gupta A., Goswami U., Cech G., Molnár K., Singh H.S., Székely C. Molecular genetic studies on Myxobolus cylindricus and Henneguya mystasi (Myxosporea: Myxobolidae) infecting two Indian fish species, Channa gachua and Mystus vittatus, respectively. Acta Parasitol. 2019;64:129–137. doi: 10.2478/s11686-018-00014-8. [DOI] [PubMed] [Google Scholar]
- Eiras J.C., Zhang J., Molnár K. Synopsis of the species of Myxobolus Bütschli, 1882 (myxozoa, Myxosporea, Myxobolidae) described between 2005 and 2013. Syst. Parasitol. 2014;81:11–36. doi: 10.1007/s11230-014-9484-5. [DOI] [PubMed] [Google Scholar]
- Eiras J.C., Cruz C.F., Saraiva A., Adriano E. Synopsis of the species of Myxobolus (Cnidaria, myxozoa, Myxosporea) described between 2014 and 2020. Folia Parasitol. 2021;68 doi: 10.14411/fp.2021.012. [DOI] [PubMed] [Google Scholar]
- Gupta A., Chaudhary A., Garg A., Verma C., Singh H.S., Sharma B. First molecular evidence of Thelohanellus wallagoi Sarkar, 1985 (Myxozoa) from economically important food fish, freshwater shark Wallago attu (Siluridae) in India. Acta Parasitol. 2018;63:647–653. doi: 10.1515/ap-2018-0075. [DOI] [PubMed] [Google Scholar]
- Gupta A., Chaudhary A., Tyagi A., Sharma B., Singh H.S. Molecular tools for identification and classification of Myxozoan parasites (Cnidaria: Myxosporea) in India: Current status. J. App. Nat. Sci. 2021;13(1):51–58. doi: 10.31018/jans.v13i1.2451. [DOI] [Google Scholar]
- Haldar D.P., Das M.K., Sharma B.K. Studies on protozoan parasites from fishes. Four new species of the genera Henneguya Thelohan, 1882, Thelohanellus Kudo, 1933, and Myxobolus Butschli, 1882. Arch. Protistenkd. 1983;127(2):283–296. [Google Scholar]
- Haldar D.P., Mukherjee M., Kundu T.K. Observations on two new species of Myxosoma Thelohan, 1892 (Myxozoa : Myxosomatidae) from fresh water teleost fishes. Arch. Protistenkd. 1981;124:244–251. [Google Scholar]
- Kalavati C., Nandi N.C. Zoological Survey of India; Kolkata: 2007. Handbook on Myxosporidean Parasites of Indian Fishes; p. 293. [Google Scholar]
- Kaur H. Myxozoan infestation in freshwater fishes in wetlands and aquaculture in Punjab (India) Adv. Anim. Vet. Sci. 2014;2:488–502. doi: 10.14737/journal.aavs/2014/2.9.488.502. [DOI] [Google Scholar]
- Kaur H., Singh R. A synopsis of the species of Myxobolus Bütschli, 1882 (Myxozoa: Bivalvulida) parasitising Indian fishes and a revised dichotomous key to myxosporean genera. Syst. Parasitol. 2012;81(1):17–37. doi: 10.1007/s11230-011-9321-z. [DOI] [PubMed] [Google Scholar]
- Kaur H., Attri R., Joshi J. Molecular identification of a new myxozoan, Myxobolus dermiscalis n. sp. (Myxosporea) infecting scales of Labeo rohita Hamilton in Harike Wetland, Punjab (India) Int. J. Parasitol. Parasites Wildl. 2016;5:139–144. doi: 10.1016/j.ijppaw.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., Singh R., Katoch A., Attri R., Dar S.A., Gupta A. Species diversity of the genus Thelohanellus Kudo, 1933 (Myxozoa: Bivalvulida) parasitizing fishes in Indian subcontinent. J. Parasit. Dis. 2017;41(2):305–312. doi: 10.1007/s12639-016-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedkar G.D., Jamdade R., Naik S., David L., Haymer D. DNA barcodes for the fishes of the Narmada, one of India's longest rivers. PloS One. 2014;9:1–10. doi: 10.1371/journal.pone.0101460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. Mega X: molecular evolutionary Genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár K. Comments on the host, organ and tissue specificity of fish myxosporeans and on the types of their intrapiscine development. Parasitol. Hung. 1994;27:5–20. [Google Scholar]
- Mondal A., Banerjee S., Patra A., Adikesavalu H., Ramudu K.R., Dash G., Joardar S.N., Abraham T.J. Molecular and morphometric characterization of Thelohanellus caudatus (Myxosporea: Myxobolidae) infecting the caudal fin of Labeo rohita (Hamilton) Protistology. 2014;8:41–52. [Google Scholar]
- Moshu A., Molnár K. Thelohanellus (Myxozoa: Myxosporea) infection of the scales in the European wild carp Cyprinus carpio carpio. Dis. Aquat. Org. 1997;28:115–123. doi: 10.3354/dao028115. [DOI] [Google Scholar]
- Okamura A., Gruhl B., Bartolomew J.L. Springer; Cham Heildelberg, New York, Dordrecht, London: 2015. Myxozoan Evolution, Ecology and Development; p. 441. [Google Scholar]
- Pagarkar A.U., Das M. Two new species of myxozoa, Thelohanellus caudatus n. sp., and Myxobolus serrata n. sp., from the cultural carps. J. Inl. Fish. Soc. India. 1993;25(1):30–35. [Google Scholar]
- Rajesh S.C., Banerjee S., Patra A., Dash G., Abraham T.J. Molecular characterization of Myxobolus cuttacki (Myxozoa, Myxosporea, Bivalvulida) infecting gill lamellae of minor carp Labeo bata (Ham.) Mol. Biol. Res. Commun. 2014;3:231–239. [PMC free article] [PubMed] [Google Scholar]
- Seenappa D., Manohar L. Myxobolus vanivilasae n. sp. parasitic in Cirrhina mrigala (Ham.) Proc. Indian Acad. Sci. 1980;89B:485–491. [Google Scholar]
- Seenappa D., Manohar L. Five new species of Myxobolus (Myxosporea: Protozoa), parasitic in Cirrhina mrigala (Hamilton) and Labeo rohita (Hamilton), with a note on a new host record for M. curmucae Seenappa and Manohar, 1980. J. Protozool. 1981;28:358–360. [Google Scholar]
- Shin S.P., Nguyen V.G., Jeong J.M., Jun J.W., Kim J.H., Han J.E., Baeck G.W., Park S.C. The phylogenetic study on Thelohanellus species (Myxosporea) in relation to host specificity and infection site tropism. Mol. Phylogenet. Evol. 2014;72:31–34. doi: 10.1016/j.ympev.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Singh R., Kaur H. Biodiversity of myxozoan parasites infecting freshwater fishes of three main wetlands of Punjab, India. Protistology. 2012;7:79–89. [Google Scholar]
- Srivastava S.P. A new species of Myxobolus from scales of Cirrhina mrigala (Hamilton) Sci. Cult. 1979;45:444–445. doi: 10.1007/s12639-011-0033-8. [DOI] [Google Scholar]
- Székely C., Cech G., Chaudhary A., Borzák R., Singh H.S., Molnár K. Myxozoan infections of the three Indian major carps in fish ponds around Meerut, UP, India, with descriptions of three new species, Myxobolus basuhaldari sp. n., M. kalavatiae sp. n. and M. meerutensis sp. n., and the redescription of M. catlae and M. bhadrensis. Parasitol. Res. 2015;114:1301–1311. doi: 10.1007/s00436-014-4307-9. [DOI] [PubMed] [Google Scholar]
- Zhang J.Y., Gu Z.M., Kalavati C., Eiras J.C., Liu Y., Guo Q.Y., Molnár K. Synopsis of the species of Thelohanellus Kudo, 1933 (myxozoa: Myxosporea: Bivalvulida. Syst. Parasitol. 2013;86:235–256. doi: 10.1007/s11230-013-9449-0. [DOI] [PubMed] [Google Scholar]