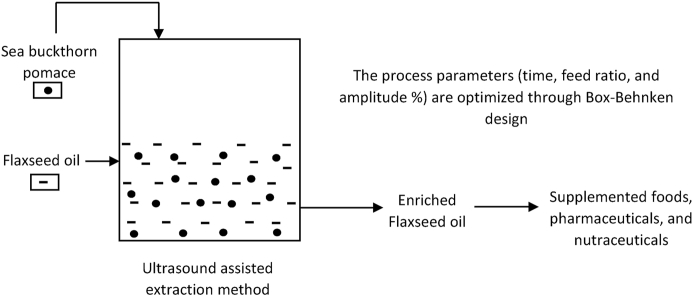
Abstract
Currently, flaxseed oil is used as an important functional food constituent owing to its large content of omega-3 fatty acids. However, flaxseed oil does not contain carotenoids that could enhance the oxidative stability of the oil. In this study, carotenoids extracted from sea buckthorn pomace were used to enrich cold-pressed flaxseed oil via an ultrasound-assisted extraction technique (UAE). The process parameters were optimized through Box-Behnken design to maximize the carotenoid content in the flaxseed oil. The results obtained by statistical analysis indicated that the yield of 14.02 mg/L of carotenoid content was found in the enriched flaxseed oil at 75.6 min, feed to oil ratio of 19.9 (wt. basis), and amplitude 80.81%. Further, UAE at optimum process parameters was compared with the conventional extraction (CE) method, and it was found that UAE had ~ 49 wt% of higher carotenoid content relative to CE. The physicochemical properties of the enriched flaxseed oil were determined to evaluate the effects of carotenoid enrichment in the flaxseed oil. Based on the outcomes of the present investigation, enriched flaxseed oil could be the potential source for the pharmaceuticals and nutraceuticals industry.
Keywords: Antioxidant stability, Carotenoid content, Enrichment, Flaxseed oil, Sea buckthorn pomace, Ultrasound-assisted extraction
Graphical abstract
Highlights
-
➢
Cold-pressed flaxseed oil is enriched by carotenoids of sea buckthorn pomace.
-
➢
The ultrasound-assisted extraction technique is used to extract bioactive components.
-
➢
The use of solvent is eliminated to enhance the value of the Cold-pressed flaxseed oil in a short time.
-
➢
Enriched flaxseed oil had higher free radical scavenging activity and carotenoid content.
-
➢
Enrichment of flaxseed oil with value-added compounds potentially increases the nutritive value of edible oils.
1. Introduction
Over a few decades, flaxseed oil appears as an essential functional food constituent due to its high nutrient and biologically active compounds. It contains omega-3 fatty acids such as α-linolenic acid (ALA), soluble and insoluble fibers, polyunsaturated fatty acid (PUFA), protein, and antioxidants (Oomah et al., 1997; Gebauer et al., 2006; Liang et al., 2017). In general, flaxseed oil is used for human consumption in cooking and baking. Further, flaxseed by-product such as de-oiled cake is used in animal feed formulation (Oomah, 2001; Coelho et al., 2007; Singh et al., 2011). Flaxseed oil has a short shelf life as it can get rancid faster due to the presence of PUFA. The presence of the double bonds in flaxseed oil interacts with oxygen in the atmosphere causing oxidative rancidity (Goyal et al., 2014). Enrichment of flaxseed oil with value-added compounds has either potential to increase the nutritive value of oils, making them more valuable for edible applications, or extend the shelf life of edible oils (Obermeyer et al., 1995).
The enrichment of foods/edible oil with compounds capable of acting as antioxidants either to extend their shelf life or to incorporate compounds with healthy properties has become standard practice in recent years (Kaderides et al., 2015). Currently, the enrichment of edible oils is carried out by three different techniques; the first process involves the extraction of plant biomass through the conventional solvent extraction process and adding it to edible oils in recommended proportions (Hashempour-Baltork et al., 2016; Nde and Foncha, 2020). The drawbacks of this process are the use of commercial solvents, which are not recommended due to their cost and toxicity. Removal of the solvent from the extract by an evaporation process and purification of oil is a time-consuming process. The second method is through steam distillation of plant biomass and the addition of essential oil extracts to edible oils. The drawback of this process is that it involves the consumption of a large amount of water to generate steam. The third method involves the traditional maceration process for the enrichment of edible oils. The bottleneck of this process is that it consumes excessive time, and yield is low as compared to other methods (Chemat et al., 2019, Krishnaswamy et al., 2018).
The ultrasound-assisted extraction (UAE) technique is an innovative technology in the food processing industry because of its advantages over other extraction processes (Cañizares-Macías et al., 2004; Chemat et al., 2004; Khan et al., 2010). UAE is considered as an effective and quick method for improving the nutritional value of vegetable oils (Chemat et al., 2004; Zhang et al., 2009; Zou et al., 2010). It is used to extract bioactive components directly from the plant biomass in a short period by eliminating the use of the solvent During UAE, ultrasound energy generated through the sonicator probe allows the breakdown/dilation of the biomass cell walls; thereby, extractives (carotenoids and tocopherols) are extracted from the biomass and diffuses into the oils that are intended for enrichment (Li et al., 2004, Rostagno et al., 2003). Besides, via the UAE, the contact surface between solid biomass and oil increases; thus, bioactive compounds directly get penetrated the oil via the cavitation technique (Patist and Bates, 2008; Zhang et al., 2008). Several studies are reported on the utilization of different plant biomass to enhance the properties of edible oils through the enrichment process (Borguini et al., 2021). The extraction and application of bioactive compounds from plant biomass (Table 1) can be helpful to the food industry for various health benefits. Bioactive compounds enhance the stability, nutritional values, pharmaceuticals, and nutraceuticals values of the products; further, they are used for the preparation of drugs and health supplements (Vilkhu et al., 2008, Ninčević Grassino et al., 2020).
Table 1.
Enrichment of different edible oils with plant biomass extract.
| Name of the oil | Plant biomass | Targeted bioactive compound in biomass | Experimental conditions | Reference |
|---|---|---|---|---|
| Sunflower | Dry Lamiaceae plants | Antioxidants | Enriched oil contained 0.1–0.5% solvent extracts (ethanol) used in oil stabilization | Marinova and Yanishlieva, 1997 |
| Olive | Rosemary | Pheophytin, chlorophyll, polar phenol content | Total phenolic content is increased 3.5 times with improved quality and higher oxidative stability of oil | Damechki et al. (2001) |
| Virgin olive | Garlic, hot pepper, oregano, rosemary | Flavor extraction from garlic, hot pepper, oregano, rosemary | Various flavored oils obtained with higher stability of oil optimized conditions are: 40 g/L of dried hot pepper, 30 g/L of garlic, 40 g/L of oregano, 20 g/L of rosemary | Gambacorta et al. (2007) |
| Refined corn | Citrus aurantium peel | Aroma extraction from citrus peel to corn oil | Flavored oil with conditions 1 h, 20°C (100 rpm) had suitable volatile compounds with unchanged fatty acid composition of oil | Karoui et al. (2010) |
| Virgin olive | Tunisian aromatic plant | Flavor extraction from rosemary, lavender, sage, menthe, basil, lemon to extra virgin olive oil | The maceration process is used for the enrichment of oil. After enrichment, thermal resistance and stability of the oil is significantly increased | Ayadi et al. (2009) |
| Olive | Olive cake | Total phenol content, Flavonoids | Oil is enriched, and oil with vegetative water and solid residue had better oil quality as compared to other freeze-dried olive cakes | Suárez et al. (2010) |
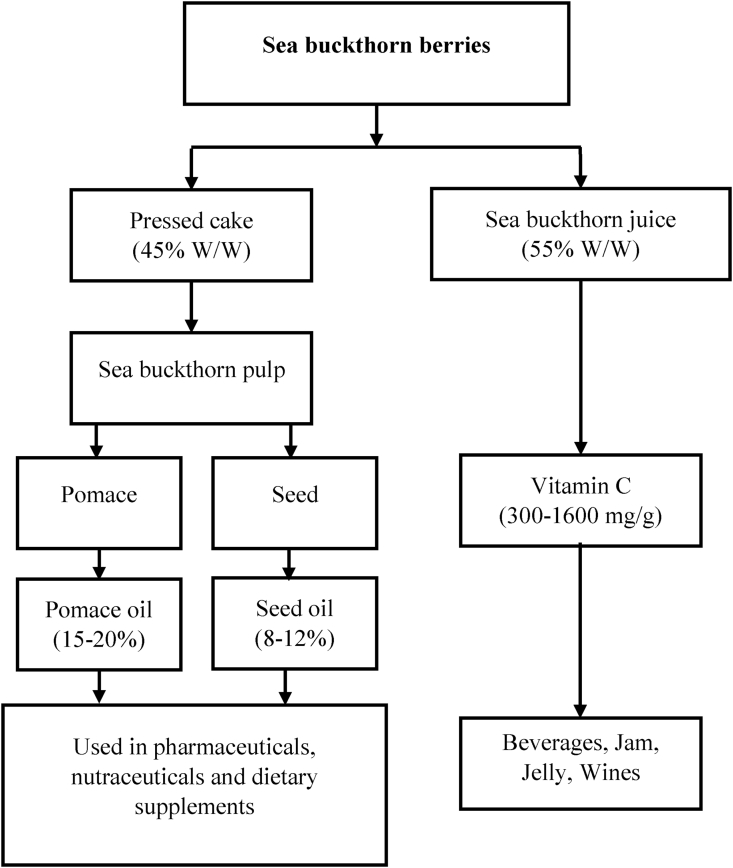
In the current study, sea buckthorn pomace is used for the enrichment of flaxseed oil. Sea buckthorn (Hippophae rhamnoides L.) is a native plant of cold temperature regions of Europe and Asia. Worldwide, various parts of sea buckthorn plants, berries, juice, pomace, and seeds are used in traditional medicine preparation, food, and the cosmetic industry (Beveridge et al., 1999). The processing of sea buckthorn berries is shown in Fig. 1. As sea buckthorn juice is primarily consumed due to its health benefits, the industry produces a large amount of sea buckthorn by-products such as seeds and pomace. Due to the high content of carotenoids (determined at 24.56 ± 1.55 mg/100 g and 16.67 mg/100 g by spectrophotometric and HPLC methods, respectively) in sea buckthorn pomace, it is used in the extraction of bioactive compounds for value addition of edible oils (Corbu et al., 2020; Vilas-Franquesa et al., 2020).
Fig. 1.
Schematic representation of the processing of sea buckthorn berries.
The main objective of this study is to increase the nutritive value of flaxseed oil by using sea buckthorn pomace. Process parameters (time, feed to oil ratio, and ultrasonic power) are optimized through Box-Behnken design to maximize the carotenoids content in the enriched flaxseed oil. Further, the physicochemical analysis of the flaxseed oil before and after enrichment is carried out to determine the quality and oxidative stability of the flaxseed oils. In addition, UAE is compared with conventional extraction (CE) at similar process parameters for three consecutive cycles to determine the total carotenoids content present in sea buckthorn pomace and extraction efficiency of the UAE technique.
2. Material and methods
2.1. Collection and processing of sea buckthorn pomace, flaxseed oil
Sea buckthorn pomace was collected from local farmers of the Northeast region, Leh, India, and was cleaned manually to remove all foreign particles such as leaves, stones, etc. The separation of sea buckthorn seed from pomace was performed manually using a lab-scale sieve (number 10) with a sieve opening of 2 mm in size. After passing sea buckthorn biomass manually through this sieve, seeds and pomace were separated, and pomace was further dried in a solar dryer. Initially, the moisture content of pomace was found to be 4–4.5 wt% (before drying) which reduced to 1 wt% after drying. Before drying, the average diameter of sea buckthorn pomace was 5–8 mm, which was to <2 mm in size after drying.
Dried sea buckthorn pomace was stored at room temperature in an air-tight container before its use. Cold-pressed unrefined flaxseed oil (Omega Nutrition, Organic Omega Flaxseed oil) was collected from the local markets of Saskatoon, Canada. The flaxseed oil used in the experiment did not contain any additives, preservatives, bleaching agents, or hexane.
All the reagents used for the experiment were of analytical grade. Ethanol, n-hexane, methanol, acetonitrile, chloroform, acetic acid, potassium hydroxide, sodium thiosulphate, potassium iodide, and Standard β-carotene (HPLC grade, 99% purity) is purchased from Sigma Millipore, Oakville, Ontario, Canada.
2.2. Design of experiment
During the flaxseed oil enrichment, the RSM approach was used through a 3-layer 3-factor Box-Behnken design to optimize the extraction of carotenoids in flaxseed oil. An experiment was designed using a Box-Behnken model by varying process parameters i.e., time (15–18 min), feed to oil ratio (5–20, weight basis), and amplitude (50–100%). The final result were obtained in the form of total carotenoid content in ppm (mg/L). Table 2 shows the factors and their levels of the experimental process parameters, the center points selected for the experiments are time (47.5 min), feed ratio (12.5), and amplitude (75%). The number of experiments required for the model was designed by equation (1).
| (1) |
Where N- Number of experiments, K- number of factors, and C- number of center points.
Table 2.
Box-Behnken experimental design factors and their levels.
| Factor | Low Level (−1) | Medium level (0) | High level (+1) |
|---|---|---|---|
| Time (min) | 15 | 47.5 | 80 |
| Feed ratio | 5 | 12.5 | 20 |
| Amplitude (%) | 50 | 75 | 100 |
For optimization of the carotenoid content in flaxseed oil, sixteen experiments were carried out in duplicate with four center points. Design experiment software (version 12) was used to study the effects of process parameters (i.e., time, feed to oil ratio, amplitude) on the total carotenoid content of enriched flaxseed oil. The total carotenoid content of enriched flaxseed oil was determined by spectrophotometer analysis, and the best model was selected using the R2 value. The equation obtained from the design expert was used to analyze the relationship between process parameters and carotenoids extracted into flaxseed oil.
2.3. Ultrasound-assisted extraction technique
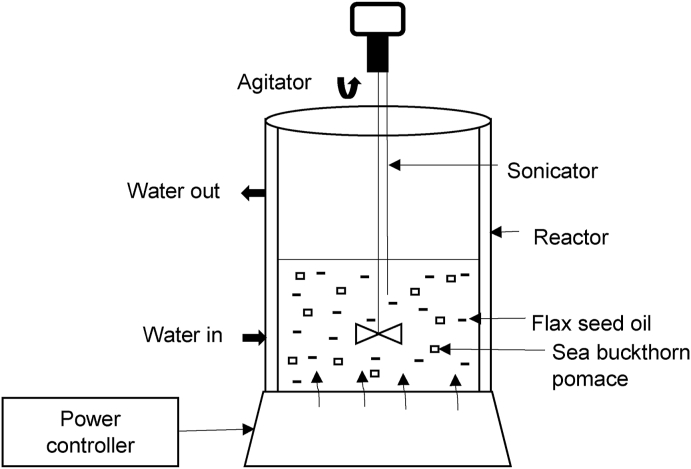
Ultrasound-assisted enrichment of flaxseed oil with sea buckthorn pomace was carried out by the sonicator apparatus (160 W) consisting of a probe and controller, as shown in Fig. 2. During the extraction, the operating frequency of the probe is 20 kHz; the temperature of the reaction vessel was controlled with cold water circulation and is monitored continuously by a thermometer. During all the experiments, the temperature of the reaction mixture did not exceed 20 ± 2°C.
Fig. 2.
Schematic representation of the sonication apparatus used in the present study.
After UAE, pomace was removed manually from flaxseed oil by using filter paper, and enriched flaxseed oil was stored in the refrigerator for further analysis.
2.4. Physicochemical characterization
The moisture content of the cold-pressed flaxseed oil and enriched flaxseed oils (Table 3) was determined by Karl Fischer Method. Acid value (2*FFA), peroxide value, and iodine value of flaxseed oils was estimated by AOCS official methods (Cd 3d-63, Cd 8–53, and Cd 1–25, respectively). The oxidative stability of oils was determined with the 743 Rancimat apparatus (Metrohm, Herisau, Switzerland) according to the AOCS Cd 12b-92 standard method. Each analysis was carried out in triplicate for accurate results (AOAC International, 1999)
Table 3.
Physicochemical properties of flaxseed oils before and after enrichment.
| Oil properties | Flaxseed oil | Enriched flaxseed oil |
|---|---|---|
| Moisture content (wt.%) | 0.067 ± 0.01 | 0.053 ± 0.01 |
| Acid Value (AV, mg KOH/g) | 0.90 ± 0.10 | 0.96 ± 0.10 |
| Free Fatty Acid (FFA, mg KOH/g) | 0.45 ± 0.05 | 0.48 ± 0.05 |
| Peroxide Value (PV, meq O2/kg) | 1.41 ± 0.20 | 1.58 ± 0.18 |
| Iodine Value (IV, g I2/100 g oil) | 184 ± 9.00 | 192 ± 10.00 |
Flaxseed oils before and after enrichment were analyzed for their fatty acid composition. Besides identifying the fatty acid composition, this study was conducted to observe any significant changes in the fatty acid composition after enrichment via 1H-NMR spectroscopy. 1H-NMR spectra were acquired at 10,330 HZ spectral width, 3 s acquisition time, 16 scans. Before processing the 1H-NMR spectra through Topspin 4.0.5, the baseline was corrected, and the spectra were referenced to δ 7.26 ppm in CDCl3. The intensity of each peak in the 1H-NMR spectra was measured at respective ppm that reflected the number of protons. The sum of the saturated fatty acids and the quantity (wt.%) of oleic acid, linoleic acid, and linolenic fatty acids were determined according to the method reported by Guillén and Ruiz (2003).
2.5. Quantification of carotenoid by spectrophotometer
The spectrophotometric analysis was carried out by ultraviolet–visible (UV-VIS) spectrophotometer at 450 nm using the British standard method of analysis (B.S-684, 1977), and the carotenoid content was calculated in parts per million (ppm).
| (2) |
Where V- Volume used for analysis, 383- The extinction coefficient of carotenoids, As- Absorbance of a sample at 450 nm, Ab- Absorbance of Blank at 450 nm, and W- Weight of sample in g.
2.6. Quantification of β-carotene by HPLC methods
HPLC analysis was performed using the method adopted from Munasinghe and Wansapala (2015), with slight modification. One gram of oil sample was measured in 25 mL of an amber volumetric flask and was filled with acetonitrile up to the mark. Then it was kept in the dark for 12 h for further use in HPLC analysis. Further, the β-carotene standard stock solution was prepared freshly (10 mg/100 mL) and stored in the dark to arrest the degradation.
HPLC analysis was performed in the HPLC instrument (Agilent Technologies 1100 series), equipped with a C18 column (4.5 × 150 mm) connected in series for analysis. The injection volume was 20 μL, and the flow rate was kept at 1.2 mL/min. The mobile phase was acetonitrile: Methanol (70:30), UV-VIS Detector (λ = 450 nm) was used for the analysis, detector temperature, and the column temperature was kept at 30 °C. Standard β-carotene solution of known concentration was run, and the obtained results were compared with the spectra of β-carotene of flaxseed oils in parts per million (mg/L). All the samples were prepared in amber bottles to prevent the degradation of β-carotene.
2.7. Electro paramagnetic resonance (EPR) spectroscopy
Free radical scavenging activity of the flaxseed oils before and after enrichment was analyzed by measuring DPPH radical scavenging potential through Electro Paramagnetic Resonance (EPR) spectrophotometer (BRUKER EMX EPR; Bremen, Germany). The following operating parameters were used for the DPPH radical quenching assay: microwave power 5.27 mW, center field 3516.85 G, sweep width 100 G, operating frequency 9.8 GHz, modulation amplitude 2 dB, receiver gain 30 dB, attenuation 16 dB, g-factor 2, number of scans 2. Free radical quenching activity of the flaxseed oils was determined by a change in the intensity of the DPPH when the oils were mixed (1 mg/mL) with a standard DPPH stock solution prepared in methanol (0.11 g DPPH in 500 mL methanol). The test specimens were prepared by dissolving 0.2 g of flaxseed oils in 1 mL of DPPH stock solution. Each test specimen was filled in melting point tubes and sealed with Teflon tape for analysis in triplicates at room temperature. The antioxidant activity of the flaxseed oils was evaluated every 6 h during 24 h; thereby, slow-reacting antioxidants could quench the free radicals with time. After each analysis, spectra were baseline corrected and double integrated using Win-EPR software to determine the intensities to compare with the control specimen (DPPH stock solution) without flaxseed oils. Free radical scavenging activity was calculated based on the following equation (3).
| Activity = [Ac–As / Ac] *100 | (3) |
Where Ac: Double integrated average value of the control, and As: Double integrated average value of the specimen.
2.8. Comparison between UAE and CE
To study the effects of UAE on carotenoids of enriched flaxseed oil, a conventional experiment was performed with the same process parameters as UAE (with same process parameters of optimum condition), eliminating ultrasound energy. This experiment was carried out to understand the effect of ultrasound energy on carotenoid yield. After extraction, the carotenoid content of UAE and CE was compared.
Furthermore, to ensure the quantity of total carotenoids extracted in the process of UAE, an experiment was carried out using the same sea buckthorn biomass at sequential extraction with fresh flaxseed oil. The experiment was carried out in three stages, with the same sea buckthorn biomass and fresh flaxseed oil keeping similar experimental conditions (time - 47.5 min, feed to oil ratio - 12.5, amplitude - 75%) for extraction. The same batch of biomass was extracted with a fresh batch of flaxseed oil to ensure carotenoids yield in different stages with the same biomass.
2.9. Statistical evaluation of the model
In this study, a total of sixteen experiments were carried out (Table 4), and the responses of the model were evaluated by carotenoids content, analyzed by the spectrophotometer. To obtain the optimum conditions for extraction of carotenoids and to study the effects of process parameters, the experiments performed according to Box-Behnken design are shown in Table 4. The acceptability of the model was determined by a summary of the model statistics as shown in Table 5.
Table 4.
Experiments performed to establish the optimum process conditions for maximizing carotenoid content.
| Factor 1 | Factor 2 | Factor 3 | Response (actual Value) | Predicted Values | |
|---|---|---|---|---|---|
| Run | A: Time | B: Feed ratio | C: Amplitude | Carotenoids | Carotenoids |
| Unit | Min | NA | % | mg/L | mg/L |
| 1 | 80.0 | 20 | 50 | 11.9 | 12.1 |
| 2 | 47.5 | 20 | 75 | 14.0 | 13.6 |
| 3 | 15.0 | 20 | 50 | 11.3 | 11.6 |
| 4 | 80.0 | 5 | 50 | 11.2 | 11.0 |
| 5 | 47.5 | 12.5 | 100 | 12.0 | 12.3 |
| 6 | 47.5 | 5 | 75 | 12.1 | 12.5 |
| 7 | 80.0 | 12.5 | 75 | 12.7 | 13.3 |
| 8 | 47.5 | 12.5 | 50 | 12.5 | 12.1 |
| 9 | 80.0 | 5 | 100 | 11.9 | 11.7 |
| 10 | 15.0 | 5 | 50 | 11.2 | 11.5 |
| 11 | 15.0 | 20 | 100 | 10.8 | 11.1 |
| 12 | 47.5 | 12.5 | 75 | 13.1 | 13.2 |
| 13 | 15.0 | 12.5 | 75 | 13.0 | 12.3 |
| 14 | 80.0 | 20 | 100 | 13.9 | 13.7 |
| 15 | 15.0 | 5 | 100 | 10.3 | 10.1 |
| 16 | 47.5 | 12.5 | 75 | 13.2 | 13.2 |
Table 5.
Model summary statistics.
| Source | Sequential p-value | Lack of Fit p-value | Adjusted R2 | Predicted R2 | |
|---|---|---|---|---|---|
| Linear | 0.20 | 0.09 | 0.14 | −0.34 | |
| *2FI | 0.43 | 0.09 | 0.15 | −2.34 | |
| Quadratic | 0.02 | 0.15 | 0.74 | 0.07 | Suggested |
| Cubic | 0.04 | 0.40 | 0.98 | −1.89 | Aliased |
*2FI: Two Factor Interaction.
3. Results and discussions
3.1. Physicochemical properties of flaxseed oils before and after enrichment
The physicochemical properties of flaxseed oils before and after enrichment (Fig. 3) are shown in Table 3. The acid value of flaxseed oil and enriched flaxseed oil is 0.90 and 0.96 mg KOH/g oil, respectively. It is found that there is not much change in the free fatty acid content of flaxseed oil; these results are identical to the previous study by Gambacorta et al. (2007) on the flavoring of extra virgin olive oil. It is found that there is no significant difference in acidity between enriched flaxseed oil and control (flaxseed) oil samples. An insignificant difference between the acid value of flaxseed oil before and after enrichment indicates that diffusion of carotenoids from sea buckthorn pomace into flaxseed oil does not affect the free fatty acid content of the enriched flaxseed oil (Gambacorta et al., 2007).
Fig. 3.
Flaxseed oils (a) before enrichment (b) after enrichment.
Peroxide value measures the primary products of oxidative degradation, and it is found to be 1.41 and 1.58 meq O2/kg of flaxseed oil and enriched flaxseed oils respectively. Iodine number indicates the amount of unsaturation in fatty acids; the iodine value of flaxseed oil and enriched flaxseed oils are found to be 184, 192 g I2/100 g oil, respectively.
From the physicochemical characterization results, it is observed that there is a minor increment in the peroxide and iodine values in enriched flaxseed oil. It may be due to the diffusion of carotenoids in flaxseed oil, which acts as an antioxidant, suppressing the formation of hydroperoxides in flaxseed oil. Results are identical to the experimental outcomes of Gambacorta et al. (2007), which explains the slight changes in peroxide value of extra virgin olive oil after the addition of spices and herbs. The maceration process of virgin olive oil with spices and herbs also enhances antioxidant compounds in the oil, limiting the formation of hydroperoxides in oil, which can decelerate the oxidation process in edible oils (Gambacorta et al., 2007).
3.2. Statistical evaluation of the model
In the statistical evaluation of the model, the highest yield of total carotenoids is observed in Run 2 (Time - 47.5 min. Feed ratio - 20, Amplitude – 75%) with 13.6 mg/L of carotenoids. The yield of carotenoid content is increased with an increase in feed to oil ratio and moderately affected by time and amplitude.
The retrieved values from the design are compared with the actual values of carotenoids determined by spectrophotometer analysis. Adequacy of the model is verified by the sum of the squares; a summary of the statistical model is presented in Table 5, Table 6. It is observed from the literature that high F-value and low p-value (probability of getting an error) plays a significant role in the acceptability of the statistical model (Okolie et al., 2019). From Table 7, F-value is found to be 5.8, and the p-value obtained is 0.02, which proved that the model is significant and acceptable (p-value < 0.05 makes the model significant). Lack of fit is an additional factor to be considered to make the regression of the model acceptable (Manorach et al., 2015). In a lack of fit, the p-value should be higher than 0.05, according to Table 5, it is observed that the p-value is 0.15, which indicates that the model is significant.
Table 6.
Sequential model sum of squares.
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Linear | 12.03 | 11 | 1.09 | 80.32 | 0.0868 | |
| *2FI | 8.96 | 8 | 1.12 | 82.31 | 0.0851 | |
| Quadratic | 1.79 | 5 | 0.36 | 26.34 | 0.1468 | Suggested |
| Cubic | 0.03 | 1 | 0.03 | 1.91 | 0.3984 | Aliased |
| Pure Error | 0.01 | 1 | 0.014 |
Table 7.
Analysis of variance (ANOVA).
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 15.72 | 9 | 1.75 | 5.80 | 0.02 | significant |
| T-Time | 2.67 | 1 | 2.67 | 8.85 | 0.03 | |
| F-Feed ratio | 2.76 | 1 | 2.76 | 9.16 | 0.02 | |
| A- Amplitude | 0.06 | 1 | 0.06 | 0.20 | 0.67 | |
| TF | 0.52 | 1 | 0.52 | 1.72 | 0.24 | |
| TA | 2.15 | 1 | 2.15 | 7.14 | 0.04 | |
| FA | 0.40 | 1 | 0.40 | 1.32 | 0.29 | |
| T2 | 0.46 | 1 | 0.46 | 1.53 | 0.26 | |
| F2 | 0.11 | 1 | 0.11 | 0.35 | 0.57 | |
| A2 | 2.72 | 1 | 2.72 | 9.04 | 0.02 | |
| Residual | 1.81 | 6 | 0.30 | |||
| Lack of Fit | 1.79 | 5 | 0.36 | 26.34 | 0.15 | not significant |
| Pure Error | 0.02 | 1 | 0.01 | |||
| Corrected Total | 17.53 | 15 | ||||
The summary of the model has a considerable impact on the adequacy of the mathematical model, which is dependent upon the value of R2 (coefficient of determination), adjusted R2 value, predicted R2 value, and standard deviation (Okolie et al., 2019). As per Table 5, the quadratic model has an adjusted R2 value of 0.74 and a predicted R2 value of 0.07. The values of the model do not exist in the cubic and linear models; so, a quadratic model is the best model to study the correlation between carotenoid content and process parameters (time, feed ratio, and amplitude %).
A second-order Box-Behnken equation obtained using multiple regression analysis:
| Total carotenoids (mg/L) = 4.78–0.0074*T + 0.0205*F + 0.201*A + 0.001*TF + 0.0006*TA + 0.0011*FA -0.0003*T2 - 0.0035*F2 - 0.0016*A2 | (4) |
Where T indicates the time of reaction (min), F indicates feed to oil ratio, and A indicates % of amplitude in the sonicator apparatus. TF, TA, and FA indicate the interaction between time and feed ratio, time and amplitude; feed ratio, and amplitude respectively. After analyzing the significance of the model, the two basic experiments are performed to check the adaptability of the theoretical equation. To analyze the reliability of the model, an experiment is performed at optimum conditions, and the yield of carotenoids obtained from the experiment is coordinating with values obtained using the theoretical equation, with an error of 0.2 mg/L. Table 4 shows the predicted and actual values of carotenoids, which are identical except for a few runs.
3.3. Assessment of experimental factors and their interactions on carotenoid content
A design experiment model analyzes the effect of different experimental factors on carotenoids extraction from sea buckthorn pomace to flaxseed oil. It also clarifies the relation between different factors and their impact on the extraction of carotenoids. From Table 7, it is observed that various factors, such as time, feed ratio, and amplitude %, have a significant effect on the yield of carotenoids. The average time of the extraction is found to be 47.5 min, with an extraction yield of 13.6 mg/L. Further, with the increase in time up to 80 min, only 0.16 mg/L of increment of carotenoids is found. This slight increment in carotenoids concentration is observed due to prolong contact (32.5 min) of Sea buckthorn pomace with flaxseeds oil. Therefore, 47.5 min is kept as the optimum time for a better yield of carotenoids (Okolie et al., 2019).
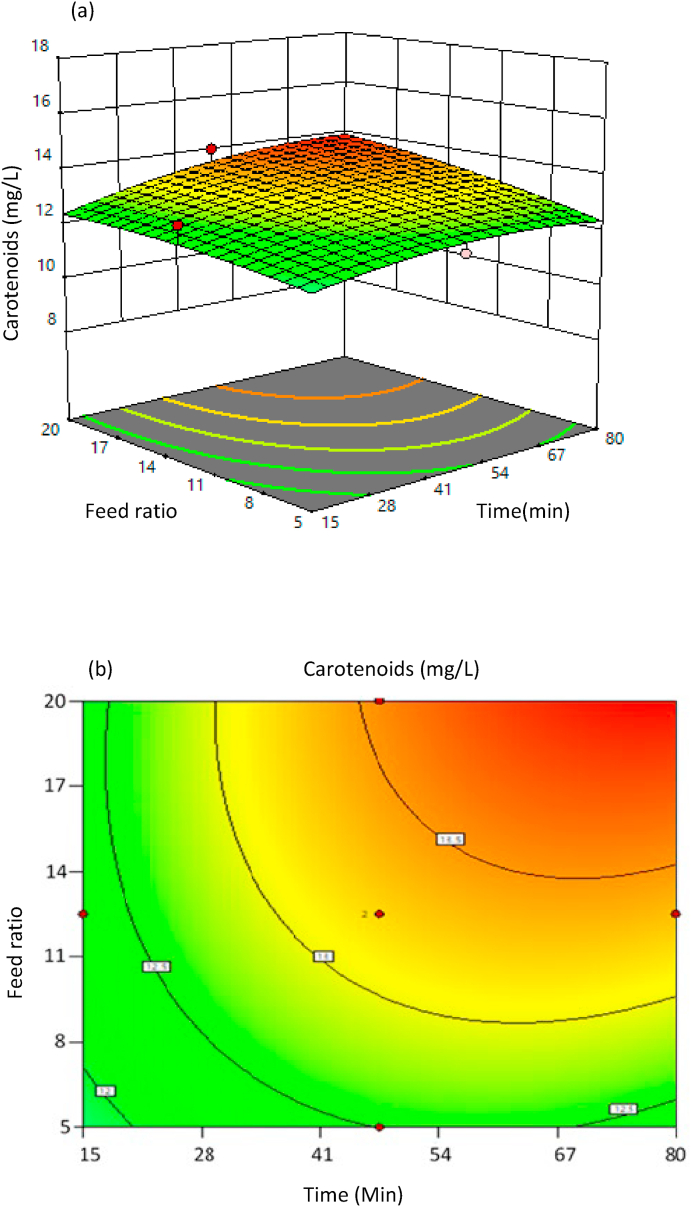
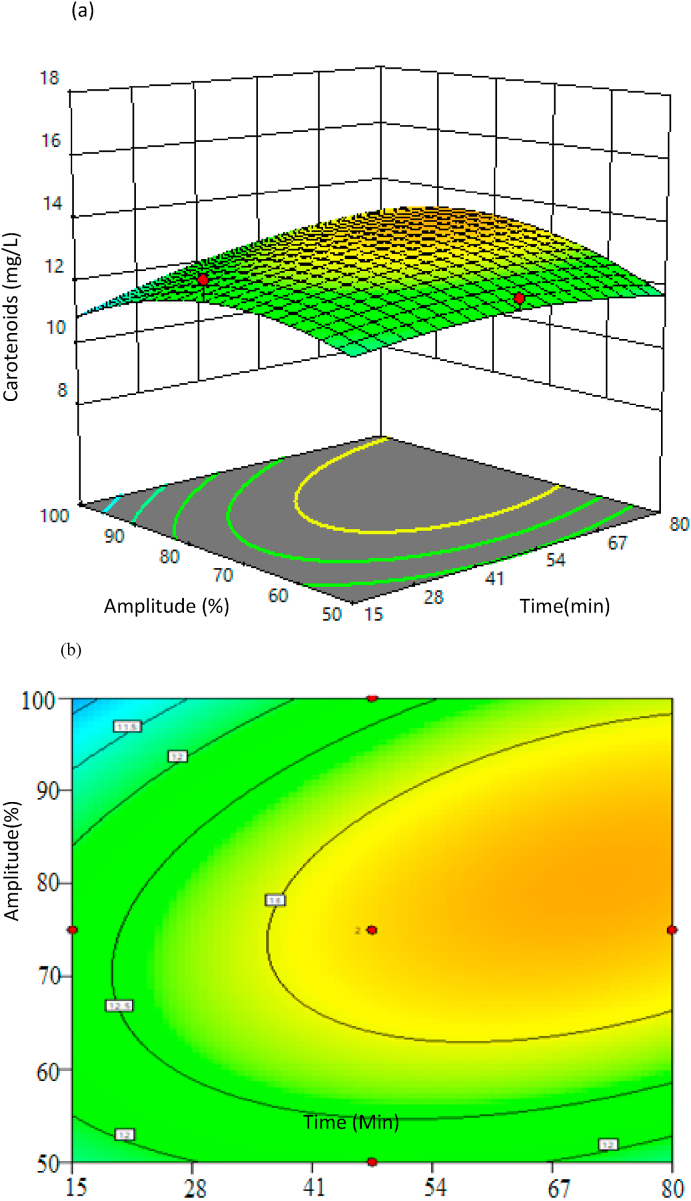
It is clear from Table 7 that other than the time, other process parameters are insignificant in the statistical model. The 2D contour graphs and the 3D response surface plots show better clarity of the model. The statistical 2D counterplots and 3D response surface plots specify the relation between process parameters and the response of increase in carotenoid content. In these plots, one of the process parameters is at a constant level, and the other process parameters are varied in the design model. Fig. 4, Fig. 5 represent 3D response surface and 2D contour plots of the interaction between the time and feed to oil ratio and interaction between time and amplitude %, respectively. These plots indicated that an increase in feed ratio has significantly less effect on the response of carotenoids yield.
Fig. 4.
Interaction between time, feed ratio, and their effects on carotenoids yield; (a) 3D response surface plot (b) 2D counter plot.
Fig. 5.
Interactions between time and amplitude % and their effects on carotenoid yield; (a) 3D response surface plot, (b) 2D counter plot.
Although the feed ratio of sea buckthorn pomace to flaxseed oil did not show a significant effect on the carotenoid yield, the mild impact of this can be observed from Fig. 4, the addition of sea buckthorn pomace increases the extraction of carotenoids in flaxseed oil. This phenomenon is observed due to an increase in contact time between sea buckthorn pomace and flaxseed oil in the reaction vessel. This leads to an increase in the yield of carotenoids, i.e., feed ratio of 20 showed 13.6 mg/L of carotenoids content. Experimental results indicated that an increase in feed ratio has a minor effect on the response of carotenoid content, as shown in Table 4 (Manorach et al., 2015).
Increasing amplitude % in ultrasound-assisted extraction significantly increases the temperature of extraction vessels in the UAE. This is due to the mechanism of the sonication process; it converts the electric signal into physical vibration through the sonication probe, which causes breaking/dilating pomace. The energy in the form of sound waves generates friction in the mixture of pomace and flaxseed oil that creates heat in flaxseed oil (Yara-Varón et al., 2017). From Table 4, it can be observed that the increase in amplitude % (power of sonication), yield decreases from 13.2% (Amplitude - 75%) to 12.3% (Amplitude – 100%). These results are in agreement with a study by Chemat et al. (2012), which explains that sonication power significantly affects the extraction of carotenoids in different edible oils (sunflower, olive, rapeseed). From Fig. 5, it can be observed that amplitude plays a significant role in the extraction of carotenoids, and 75% of amplitude led to a good yield of carotenoids as compared to other experimental conditions. However, increment in amplitude % is not favorable for edible oil owing to heat generation during the UAE which leads to degradation of the quality of the flaxseed oil.
3.4. Optimization of the extraction process
After verifying the regression model using different process parameters from the design expert, optimization condition for carotenoids yield is found to be time - 75.5 min, feed ratio – 19.9, and amplitude - 80.8%; at this condition, the predicted yield of carotenoids is 14.0 mg/L. To verify the experimental error along with optimized values, an experiment is performed using the above condition and the experimental error is found to be 3.4%.
3.5. Fatty acid profile of flaxseed oil
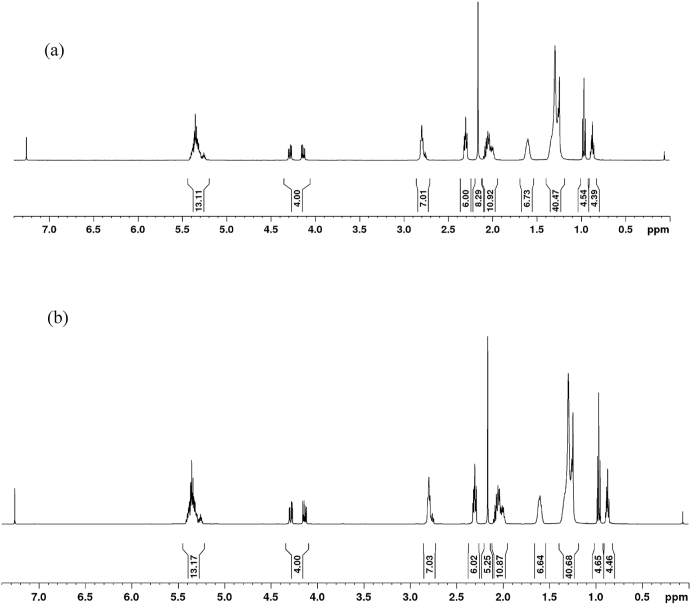
1H-NMR spectra of the flaxseed oils before and after enrichment are shown in Fig. 6, indicating the number of protons at each chemical shift with different acyl groups. Spectral signals are attributed to the presence of the protons in the triglycerols, and the area of each chemical shift belongs to the flaxseed oils before and after enrichment. From Fig. 6, it can be seen that the proton count at each chemical shift is identical except in the unsaturation region (5.2–5.4 ppm) and the chemical shift at 2.2. ppm, which indicated that the fatty acid composition of the flaxseed oil before and after enrichment is identical. Similar observations are noticed in section 3.1, i.e., no significant difference in free fatty acids content in the enriched oil. As reported in the materials and methods section, the fatty acid composition is calculated according to Guillén and Ruiz (2003) using 1H-NMR spectra and found to be linolenic fatty acids (ꞷ-3) – 50 wt%, linoleic fatty acid (ꞷ-6) – 16.7 wt%, oleic acid – 25 wt%, and saturated fatty acids – 8.3 wt%. Reported fatty acid composition in this study is in line with the previous studies by Bean and Leeson (2002), an extensive study on the fatty acid composition of the 23 different flaxseed oils collected from the commercial feed mills in Ontario (Bean and Leeson, 2002). Similar experimental outcomes are noticed in the fatty acid composition of the corn oil before and after enrichment with citrus peel essential oil (Karoui et al., 2010). Iodine value is also calculated from the 1H-NMR analysis by the Hunus method (IV = 10.54 + 13.39*% of olefinic protons in flaxseed oils), and it is found to be 168.5 g I2/100 g of flaxseed oil and 173.9 g I2/100 g of enriched flaxseed oil. From the outcomes of the study, it is noticed that the difference in the iodine value is ascribed to the addition of β-carotene from the sea buckthorn pomace with a molecular formula of C40H56 that has an unsaturation. This observation further confirms that the carotenoid from sea buckthorn pomace is diffused into the flaxseed oil and enriched the oxidative and antioxidant stability, reported in section 3.6. Therefore, enriched flaxseed oil could be a possible supplement for edible, pharmaceutical, and nutraceutical applications.
Fig. 6.
1H-NMR spectra for (a). Flaxseed oil, and (b). Enriched flaxseed oil.
3.6. Oxidative stability assessment
Rancimat analysis monitored the deterioration of flaxseed oils before and after enrichment. The data obtained is measured in terms of the induction period (IP, Table 8) to evaluate the resistance against oil oxidations at high temperatures (100/110 °C). Higher induction time indicates that it would take a longer time to oxidize the oils; thus, they would have better stability of the oils. It is observed that the induction period at temperatures 100 and 110 °C of enriched flaxseed oil (6.07 h and 2.92 h) is higher as compared to that of non-enriched flaxseed oil (4.11 h and 1.58 h), respectively. The results of the oxidative stability of enriched flaxseed oil before and after enrichment found an overall increase in IP at 100 °C–110 °C. At both temperatures, higher stability is observed in enriched flaxseed oil. A similar pattern of Rancimat relative time is observed by another study of enriched edible oils (Sánchez De Medina et al., 2011). The stability of edible oils increased after the enrichment of oil with phenolic extracts from an olive tree. Enriched oils have shown better stability relative to pure oils (Gordon and Mursi, 1994; Sharav et al., 2014; Bilska et al., 2018). So, it can be concluded that the increase in the stability of flaxseed oil is due to the enrichment of flaxseed oil with carotenoids of sea buckthorn pomace.
Table 8.
Induction period of flaxseed oils (before and after enrichment).
| Temperature (°C) | Induction period (h) |
|
|---|---|---|
| Flaxseed Oil | Enriched Flaxseed Oil | |
| 100 | 4.11 ± 0.8 | 6.07 ± 1.2 |
| 110 | 1.58 ± 0.9 | 2.92 ± 1.1 |
3.7. Carotenoids and β-carotene content (ppm) by spectrophotometer and HPLC method
The composition of carotenoids and β-carotene in flaxseed oil and enriched flaxseed oils are analyzed using a spectrophotometer and HPLC analysis (Table 9). Initially, the carotenoids, β carotene content of flaxseed oil before enrichment is studied by spectrophotometer and HPLC methods. It is found that there are no traces of β carotene present in the cold-pressed flaxseed oil. After enrichment, it is found that the carotenoid of flaxseed oil is 14.02 mg/L by spectrophotometer analysis. To ensure the quantity of β-carotene content, HPLC analysis is performed in comparison with standard β-carotene, and it is found to be 11.26 mg/L in enriched flaxseed oil. Comparative analysis of spectrophotometric methods showed that the results are slightly different, and the variation between the two different quantities is attributed to the sensitivity of the instruments. The above results are in line with the study of β-carotene of M. Longifolia seed oil (Munasinghe and Wansapala, 2015), which shows the difference between carotenoid content of the same oil by two methods may be due to the sensitivity of the instruments used to measure (de Carvalho et al., 2012; Tesfaye et al., 2017).
Table 9.
Carotenoid content (ppm) by spectrophotometer and HPLC methods.
| Name of the method | Carotenoid content (mg/L) |
|
|---|---|---|
| Flaxseed Oil | Enriched Flaxseed Oil | |
| Spectrophotometer | Nil | 14.02 ± 0.63 |
| HPLC | Nil | 11. 26 ± 0.36 |
3.8. Comparison between UAE and CE
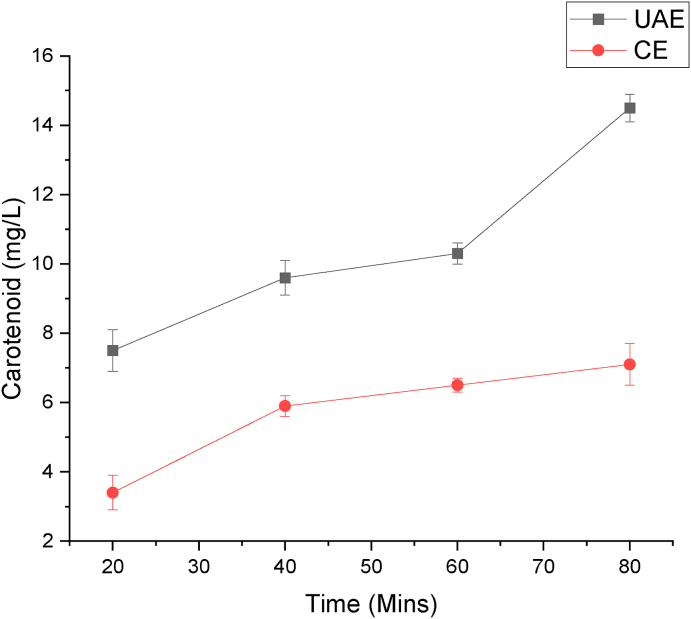
In Comparison with CE, UAE showed a higher yield after 80 min of extraction, as shown in Fig. 7. The carotenoid content of the enriched flaxseed oil via the UAE technique is 14.5 mg/L, which is 49% more relative to CE, which is 7.1 mg/L. The increase in the yield could be attributed to the application of ultrasound-assisted extraction. This indicates that the UAE significantly enhances the extraction of carotenoid content in less time compared to CE.
Fig. 7.
Comparision between ultra-sound assisted extraction and conventional extraction for maximum carotenoid yield.
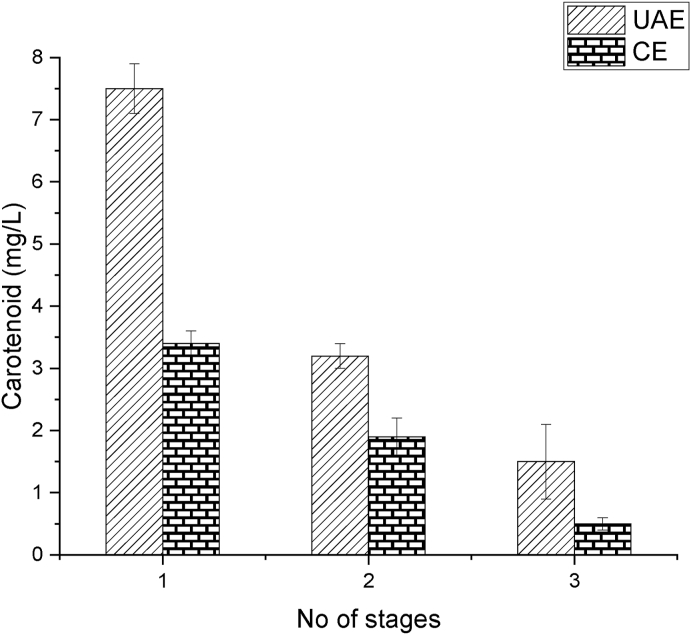
Besides, a three-stage experiment was performed to ensure the (maximum) quantity of carotenoids extracted in flaxseed oil, keeping identical experimental conditions (time - 47.5 min, feed to oil ratio - 12.5, amplitude - 75%). Experimental outcomes revealed that UAE yielded higher carotenoids in each stage (7.5, 3.2, 1.5 mg/L) relative to CE (3.4, 1.9,0.5 mg/L) respectively. This specifies that the UAE enhances the yield of carotenoid compared with CE, and UAE extracted a higher amount of carotenoid in the first stage of extraction. Multi-stage extraction also revealed that the total carotenoid content in sea buckthorn pomace is around 12.1 mg/L in three series of extractions (Fig. 8). Via UAE, more than half of the carotenoid (7.5 mg/L) is extracted into the flaxseed oil in first stage of extraction, which is higher than the carotenoid content by CE in three stages of extraction (5.6 mg/L).
Fig. 8.
Comparative analysis of the carotenoid extraction through three series of extraction stages via ultrasound-assisted extraction and conventional extraction for maximum carotenoid yield.
3.9. Free radical scavenging activity of flaxseed oils before and after enrichment
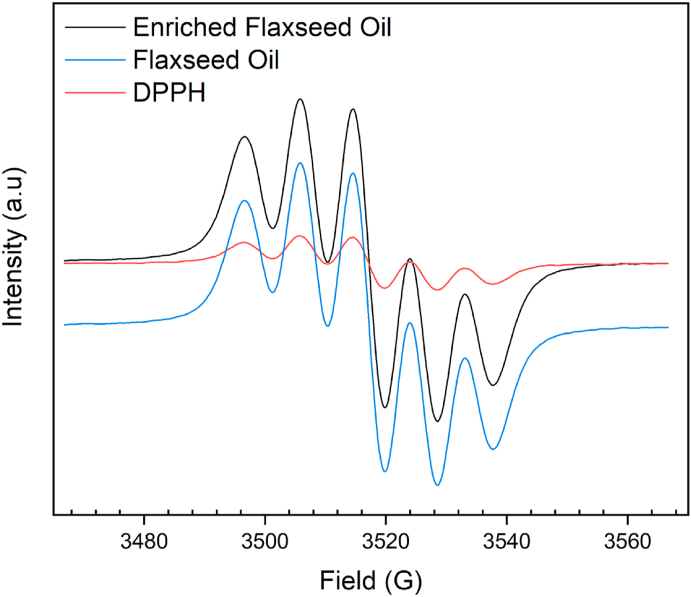
Consumption of a wide variety of fruits, vegetables, edible oils with rich antioxidant compounds renders defense against diseases such as cardiovascular and cancer. Such protection can be understood and explained by the capacity of the antioxidants to scavenge free radicals (via electro paramagnetic resonance spectroscopy) that are responsible for the oxidative destruction of lipids, nucleic acids, and proteins (Chemat et al., 2000, Espín et al., 2000). Electro paramagnetic resonance spectroscopy has found a wide variety of applications in detecting free radicals owing to its high sensitivity to unpaired electrons Martínez-Yusta and Guillén (2019). The close interaction of DPPH with the free radical scavengers present in the enriched flaxseed oil presented a reduced signal intensity relative to pure flaxseed oil and DPPH stock solution EPR spectra (Fig. 9). The initial scavenging activity of the flaxseed oil is found to be 84%; this value is in agreement with the results reported by Espín et al. (2000). Upon addition of 0.2 g of enriched flaxseed oil to 1 mL of DPPH stock solution quenched 93% of free radicals that are present DPPH in the first 2 h. The declining trend in the spectral intensity for the enriched flaxseed oil is observed relative to the flaxseed oil and DPPH spectra (Blank). Further, the antioxidant activity of the enriched flaxseed oil is evaluated after every 6 h (up to 20 h); therefore, the DPPH stock solution allows the dissolution of both DPPH and lipid-associated antioxidants that are present in the flaxseed oils before and after enrichment. Along with the decrease in the absorption intensity of the DPPH, a change in the color of the flaxseed oils are noticed from purple to pale yellow further confirms that the quantity of the synthetic-free radicals present in the DPPH is quenched by the presence of flaxseed oil that is enriched with sea buckthorn pomace. Upon analyzing the spectra at 8, 14, and 20 h, enhanced scavenging activity is observed by the antioxidant compounds present in the enriched flaxseed (97%) oil. After 8 h, there is no significant difference in the antioxidant activity, i.e., within 8 h of reaction with DPPH, enriched flaxseed oil quenched or neutralized the synthetic-free radicals present in the DPPH; primary and secondary oxidation products from the enriched flaxseed oil. As discussed in the previous sections (3.6 and 3.7), HPLC analysis of the enriched flaxseed oil revealed the presence of β-carotene and other antioxidant compounds such as sterols, phenolics, and tocopherols that are primarily responsible antioxidants present in the flaxseed oil (Śpitalniak-Bajerska et al., 2018), Free radical scavenging activity of the enriched flaxseed oil (98%) has shown that it could quench the reactive oxygen species and their derivatives in a shorter time. Therefore, it is anticipated that the presence of antioxidants in the flaxseed oil could be a value-added feedstock for the production of pharmaceuticals, nutraceuticals, and edible oils (salad dressing), as reported by Polovka et al. (2007).
Fig. 9.
EPR spectra for DPPH (standard), flaxseed oil, and enriched flaxseed oil.
4. Conclusions
In this study, UAE is investigated for the enrichment of flaxseed oil. It is found that UAE significantly enhanced the extraction of carotenoids in less time compared to CE. From the assessment of design expert results, optimized conditions are found to be time - 75.5 min, feed ratio - 19.9, and amplitude - 80.8%; at these conditions, the yield of carotenoids is 14.02 mg/L. The studies indicated that the UAE significantly decreased the extraction time. Carotenoid of enriched flaxseed oil is found to be 14.02 mg/L by spectrophotometer analysis, likewise, β-carotene content is found to be 11.26 mg/L.
The results of this study determined the application of sea buckthorn pomace (a by-product of the sea buckthorn berry processing industry) for the enrichment of edible oils. UAE technique is found to be an effective method for extraction and direct enrichment of oils. It improved the nutritional value of edible oil, eliminating the use of hazardous solvents in the process. All the parameters of enriched flaxseed oil indicate the improvement of stability and quality of flaxseed oil. β-carotene enriched flaxseed oil can be used in the nutraceutical and pharmaceutical industry as a dietary supplement. It can also be used in salad dressing oil, taste enhancer instead of butter or margarine in the food industry.
CRediT authorship contribution statement
Vidhi H. Bhimjiyani: Conceptualization, Experiment, Formal analysis, Writing, Writing – original draft. Venu Babu Borugadda: Conceptualization, Experiment, Formal analysis, Investigation, Writing, Writing – original draft, Writing – review & editing. Satyanarayan Naik: Conceptualization, Investigation, Writing – review & editing, Supervision. Ajay K. Dalai: Conceptualization, Investigation, Writing – review & editing, Supervision.
Declaration of competing interest
The authors have declared no conflict of interest.
Acknowledgments
The authors thank the Saskatchewan Structural Sciences Center for providing EPR experimental facilities.
References
- AOAC International . sixteenth ed. 1999. Official Methods of Analysis of AOAC International. Gaithersburg. [Google Scholar]
- Ayadi M.A., Grati-Kamoun N., Attia H. Physico-chemical change and heat stability of extra virgin olive oils flavoured by selected Tunisian aromatic plants. Food Chem. Toxicol. 2009;47(10):2613–2619. doi: 10.1016/j.fct.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Bean L.D., Leeson S. Fatty acid profiles of 23 samples of flaxseed collected from commercial feed mills in Ontario in 2001. J. Appl. Poultry Res. 2002;11(2):209–211. doi: 10.1093/japr/11.2.209. [DOI] [Google Scholar]
- Beveridge T., Li T.S.C., Oomah B.D., Smith A. Sea buckthorn products: manufacture and composition. J. Agric. Food Chem. 1999;47(9):3480–3488. doi: 10.1021/jf981331m. [DOI] [PubMed] [Google Scholar]
- Bilska A., Iszkowiak K., Błaszyk M., Rudzińska M., Kowalski R. Effect of liver pâté enrichment with flaxseed oil and flaxseed extract on lipid composition and stability. J. Sci. Food Agric. 2018;98(11):4112–4120. doi: 10.1002/jsfa.8928. [DOI] [PubMed] [Google Scholar]
- Borguini R.G., Pacheco S., Chávez D.W.H., Couto G.A., Wilhelm A.E., Santiago M.C. P. de A. Carotenoid extraction using edible vegetable oil: an enriched provitamin A product. Sci. Agric. 2021;78(5) doi: 10.1590/1678-992x-2019-0332. [DOI] [Google Scholar]
- Cañizares-Macías M.P., García-Mesa J.A., Luque De Castro M.D. Fast ultrasound-assisted method for the determination of the oxidative stability of virgin olive oil. Anal. Chim. Acta. 2004;502(2):161–166. doi: 10.1016/j.aca.2003.10.022. [DOI] [Google Scholar]
- Chemat F., Périno-Issartier S., Loucif L., Elmaataoui M., Mason T.J., Grondin I., Costes P., Moutoussamy L., Sing A.S.C., Smadja J., Espín J.C., Soler-Rivas C., Wichers H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000;114(3):453–460. doi: 10.1016/j.ultsonch.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Chemat S., Lagha A., AitAmar H., Bartels P.V., Chemat F. Comparison of conventional and ultrasound-assissted extraction of carvone and limonene from caraway seeds. Flavour Fragrance J. 2004;19(3):188–195. doi: 10.1002/ffj.1339. [DOI] [Google Scholar]
- Chemat F., Périno-Issartier S., Loucif L., Elmaataoui M., Mason T.J. Enrichment of edible oil with sea buckthorn by products using ultrasound-assisted extraction. Eur. J. Lipid Sci. Technol. 2012;114(4):453–460. doi: 10.1002/ejlt.201100349. [DOI] [Google Scholar]
- Chemat F., Vian M.A., Ravi H.K., Khadhraoui B., Hilali S., Perino S., Tixier A.S.F. Review of alternative solvents for green extraction of food and natural products: panorama, principles, applications and prospects. Molecules. 2019;24(16) doi: 10.3390/molecules24163007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho C.M.M., Bellato C. de M., Santos J.C.P., Ortega E.M.M., Tsai S.M. Effect of phytate and storage conditions on the development of the ‘ hard-to-cook. J. Sci. Food Agric. 2007;1243:1237–1243. doi: 10.1002/jsfa. July 2006. [DOI] [Google Scholar]
- Corbu A.R., Rotaru A., Nour V. Edible vegetable oils enriched with carotenoids extractedfrom by-products of sea buckthorn (Hippophae rhamnoides ssp. sinensis): the investigation of some characteristic properties, oxidative stability and the effect on thermal behaviour. J. Therm. Anal. Calorim. 2020;142(2):735–747. doi: 10.1007/s10973-019-08875-5. [DOI] [Google Scholar]
- Damechki M., Sotiropoulou S., Tsimidou M. Antioxidant and pro-oxidant factors in oregano and rosemary gourmet olive oils. Grasas Aceites. 2001;52(3–4):207–213. doi: 10.3989/gya.2001.v52.i3-4.359. [DOI] [Google Scholar]
- de Carvalho L.M.J., Gomes P.B., Godoy R.L. de O., Pacheco S., do Monte P.H.F., de Carvalho J.L.V., Nutti M.R., Neves A.C.L., Vieira A.C.R.A., Ramos S.R.R. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): a preliminary study. Food Res. Int. 2012;47(2):337–340. doi: 10.1016/j.foodres.2011.07.040. [DOI] [Google Scholar]
- Espín J.C., Soler-Rivas C., Wichers H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000;48(3):648–656. doi: 10.1021/jf9908188. [DOI] [PubMed] [Google Scholar]
- Gambacorta G., Faccia M., Pati S., Lamacchia C., Baiano A., La Notte E. Changes in the chemical and sensorial profile of extra virgin olive oils flavored with herbs and spices during storage. J. Food Lipids. 2007;14(2):202–215. doi: 10.1111/j.1745-4522.2007.00080.x. [DOI] [Google Scholar]
- Gebauer S.K., Psota T.L., Harris W.S., Kris-Etherton P.M. n-3 Fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 2006;83(6):1526–1535. doi: 10.1093/ajcn/83.6.1526s. [DOI] [PubMed] [Google Scholar]
- Gordon M.H., Mursi E. A comparison of oil stability based on the Metrohm Rancimat with storage at 20 degrees C. J. Am. Oil Chem. Soc. 1994 Jun;71(6):649–651. doi: 10.1007/bf02540595. [DOI] [Google Scholar]
- Goyal A., Sharma V., Upadhyay N., Gill S., Sihag M. Flax and flaxseed oil: an ancient medicine & modern functional food. J. Food Sci. Technol. 2014;51(9):1633–1653. doi: 10.1007/s13197-013-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén M.D., Ruiz A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. Eur. J. Lipid Sci. Technol. 2003;105:688–696. doi: 10.1002/ejlt.200300866. [DOI] [Google Scholar]
- Hashempour-Baltork F., Torbati M., Azadmard-Damirchi S., Savage G.P. Vegetable oil blending: a review of physicochemical, nutritional and health effects. Trends Food Sci. Technol. 2016;57:52–58. doi: 10.1016/j.tifs.2016.09.007. [DOI] [Google Scholar]
- Kaderides K., Goula A.M., Adamopoulos K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innovat. Food Sci. Emerg. Technol. 2015;31:204–215. doi: 10.1016/j.ifset.2015.08.006. [DOI] [Google Scholar]
- Karoui I.J., Wannes W.A., Marzouk B. Refined corn oil aromatization by Citrus aurantium peel essential oil. Ind. Crop. Prod. 2010;32(3):202–207. doi: 10.1016/j.indcrop.2010.04.020. [DOI] [Google Scholar]
- Khan M.K., Abert-Vian M., Fabiano-Tixier A.S., Dangles O., Chemat F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010;119(2):851–858. doi: 10.1016/j.foodchem.2009.08.046. [DOI] [Google Scholar]
- Krishnaswamy Kiruba, Venkatesh Mannar M.G., Hurrell Richard F., Levente László Diosady . vol. 2018. Academic Press; 2018. Chapter 17 - micronutrient fortification of edible oils, editor(s) pp. 167–174. (Food Fortification in a Globalized World). ISBN 9780128028612, [DOI] [Google Scholar]
- Li H., Pordesimo L., Weiss J. High intensity ultrasound-assisted extraction of oil from soybeans. Food Res. Int. 2004;37(7):731–738. doi: 10.1016/j.foodres.2004.02.016. [DOI] [Google Scholar]
- Liang L., Chen F., Wang X., Jin Q., Decker E.A., McClements D.J. Physical and oxidative stability of flaxseed oil-in-water emulsions fabricated from sunflower lecithins: impact of blending lecithins with different phospholipid profiles. J. Agric. Food Chem. 2017;65(23):4755–4765. doi: 10.1021/acs.jafc.7b01469. [DOI] [PubMed] [Google Scholar]
- Manorach K., Poonsrisawat A., Viriya-Empikul N., Laosiripojana N. vol. 79. Elsevier B.V; 2015. Optimization of sub-critical water pretreatment for enzymatic hydrolysis of sugarcane bagasse. (Energy Procedia). [DOI] [Google Scholar]
- Marinova E.M., Yanishlieva N.V. Antioxidative activity of extracts from selected species of the family Lamiaceae in sunflower oil. Food Chem. 1997;58(3):245–248. doi: 10.1016/S0308-8146(96)00223-3. [DOI] [Google Scholar]
- Martínez-Yusta A., Guillén M.D. Enrichment of sunflower oil with γ-tocopherol. Study by 1H NMR of its effect under accelerated storage conditions. Eur. J. Lipid Sci. Technol. 2019;121(6) doi: 10.1002/ejlt.201800457. [DOI] [Google Scholar]
- Munasinghe M., Wansapala J. B-carotene content of M. longifolia seed oil in different agro-climatic zones in Sri Lanka, the effect of heat on its stability and the composition of seed cake. Potravinarstvo. 2015;9(1):474–479. doi: 10.5219/502. [DOI] [Google Scholar]
- Nde D.B., Foncha A.C. Optimization methods for the extraction of vegetable oils: a review. Processes. 2020;8(2) doi: 10.3390/pr8020209. [DOI] [Google Scholar]
- Ninčević Grassino A., Ostojić J., Miletić V., Djaković S., Bosiljkov T., Zorić Z., Ježek D., Rimac Brnčić S., Brnčić M. Application of high hydrostatic pressure and ultrasound-assisted extractions as a novel approach for pectin and polyphenols recovery from tomato peel iste. Innovat. Food Sci. Emerg. Technol. 2020;64 doi: 10.1016/j.ifset.2020.102424. October 2019. [DOI] [Google Scholar]
- Obermeyer W.R., Musser S.M., Betz J.M., Casey R.E., Pohland A.E. Chemical studies of phytoestrogens and related compounds in dietary supplements: flax and chaparral. Proc Soc Exp, Biol. Med. 1995;208(1):6–12. doi: 10.3181/00379727-208-43824. [DOI] [PubMed] [Google Scholar]
- Okolie J.A., Nanda S., Dalai A.K., Kozinski J.A. Optimization and modeling of process parameters during hydrothermal gasification of biomass model compound to generate hydrogen-rich gas products. Int. J. Hydrogen Energy. International Journal of Hydrogen Energy. 2019;45(36):18275–18288. doi: 10.1016/j.ijhydene.2019.05.132. [DOI] [Google Scholar]
- Oomah B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001;81(9):889–894. doi: 10.1002/jsfa.898. [DOI] [Google Scholar]
- Oomah B.D., Kenaschuk E.O., Mazza G. Tocopherols in flaxseed. J. Agric. Food Chem. 1997;45(6):2076–2080. doi: 10.1021/jf960735g. [DOI] [Google Scholar]
- Patist A., Bates D. Ultrasonic innovations in the food industry: from the laboratory to commercial production. Innovat. Food Sci. Emerg. Technol. 2008;9(2):147–154. doi: 10.1016/j.ifset.2007.07.004. [DOI] [Google Scholar]
- Polovka M., Brezová V., Šimko P. EPR spectroscopy: a tool to characterize gamma-Irradiated foods. Journal of Food and Nutrition Research. 2007;46(2):75–83. [Google Scholar]
- Rostagno M.A., Palma M., Barroso C.G. vol. 1012. 2003. pp. 119–128. (docslide.net_ultrasound-assisted-extraction-of-soy-isoflavones - Copy.Pdf). [DOI] [PubMed] [Google Scholar]
- Sánchez De Medina V., Priego-Capote F., Jiménez-Ot C., Luque De Castro M.D. Quality and stability of edible oils enriched with hydrophilic antioxidants from the olive tree: the role of enrichment extracts and lipid composition. J. Agric. Food Chem. 2011;59(21):11432–11441. doi: 10.1021/jf2020528. [DOI] [PubMed] [Google Scholar]
- Sharav O., Shim Y.Y., Okinyo-Owiti D.P., Sammynaiken R., Reaney M.J.T. Effect of cyclolinopeptides on the oxidative stability of flaxseed oil. J. Agric. Food Chem. 2014;62(1):88–96. doi: 10.1021/jf4037744. [DOI] [PubMed] [Google Scholar]
- Singh K.K., Mridula D., Rehal J., Barnwal P. Flaxseed: a potential source of food, feed and fiber. Crit. Rev. Food Sci. Nutr. 2011;51(3):210–222. doi: 10.1080/10408390903537241. [DOI] [PubMed] [Google Scholar]
- Śpitalniak-Bajerska Kinga, Szumny Antoni, Alicja Zofia Kucharska. Kupczyński Robert. Effect of natural antioxidants on the stability of linseed oil and fish stored under anaerobic conditions. J. Chem. 2018;2018:1–8. doi: 10.1155/2018/9375085. [DOI] [Google Scholar]
- Suárez M., Romero M.P., Motilva M.J. Development of a phenol-enriched olive oil with phenolic compounds from olive cake. J. Agric. Food Chem. 2010;58(19):10396–10403. doi: 10.1021/jf102203x. [DOI] [PubMed] [Google Scholar]
- Tesfaye B., Abebaw A., Reddy M.U. Determination of cholesterol and β-carotene content in some selected edible oils. International Journal of Innovative Science and Research Technology. 2017;2(7):14–18. www.ijisrt.com14 [Google Scholar]
- Vilas-Franquesa A., Saldo J., Juan B. Potential of sea buckthorn-based ingredients for the food and feed industry – a review. Food Production, Processing and Nutrition. 2020;2(1) doi: 10.1186/s43014-020-00032-y. [DOI] [Google Scholar]
- Vilkhu K., Mawson R., Simons L., Bates D. Applications and opportunities for ultrasound assisted extraction in the food industry - a review. Innovat. Food Sci. Emerg. Technol. 2008;9(2):161–169. doi: 10.1016/j.ifset.2007.04.014. [DOI] [Google Scholar]
- Yara-Varón E., Li Y., Balcells M., Canela-Garayoa R., Fabiano-Tixier A.S., Chemat F. Vegetable oils as alternative solvents for green oleo-extraction, purification and formulation of food and natural products. Molecules. 2017;22(9):1–24. doi: 10.3390/molecules22091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.S., Wang L.J., Li D., Jiao S.S., Chen X.D., Mao Z.H. Ultrasound-assisted extraction of oil from flaxseed. Separ. Purif. Technol. 2008;62(1):192–198. doi: 10.1016/j.seppur.2008.01.014. [DOI] [Google Scholar]
- Zhang H.F., Yang X.H., Zhao L.D., Wang Y. Ultrasonic-assisted extraction of epimedin C from fresh leaves of Epimedium and extraction mechanism. Innovat. Food Sci. Emerg. Technol. 2009;10(1):54–60. doi: 10.1016/j.ifset.2008.09.007. [DOI] [Google Scholar]
- Zou Y., Xie C., Fan G., Gu Z., Han Y. Optimization of ultrasound-assisted extraction of melanin from Auricularia auricula fruit bodies. Innovat. Food Sci. Emerg. Technol. 2010;11(4):611–615. doi: 10.1016/j.ifset.2010.07.002. [DOI] [Google Scholar]