Abstract
Background:
In animal models of inflammatory diseases, Mst1 facilitates the programmed cell death as a novel pro-apoptotic kinase. This research aimed to determine the expression level of Mst1 gene in a rat model of SCI treated with VPA.
Methods:
Severe rat model contusion was used for evaluation of the neuroprotective effect of valproic acid. The BBB test, was performed to determine locomotor functions. H&E staining and TUNEL assay were performed to detect cavity formation and apoptosis, respectively. The mRNA levels of the genes Mst1, Nrf2, and Bcl-2 were evaluated, using quantitative RT-PCR.
Results:
The results revealed that Mst1 gene expression and TUNEL-positive cells in the VPA-treated group were significantly reduced as compared to the untreated group (p ≤ 0.05).
Conclusion:
Our findings indicate that VPA has therapeutic potential and can be a candidate for the treatment of neurodegenerative disorders and traumatic injury as a promising drug.
Key Words: Bcl-2, Contusion, Mst1, Nrf2, Valproic acid
INTRODUCTION
Evidence has shown that tissue damage and cell death through necrosis and apoptosis continue in the second phase of SCI. However, apoptosis begins in the early hours after injury and continues for weeks, and caspase-3 activation is one of the essential factors in apoptosis. Mst1 is a key pro-apoptotic gene stimulating caspase-3 activation[1]. In addition, the inhibition of the upper and lower cascading events of caspase-3 can be used as a therapeutic target in treating SCI[2]. Using anti-inflammatory agents such as VPA, as an inhibitor of histone deacetylase, can hinder apoptosis[3]. Mst1 is a serine/threonine protein kinase participates in apoptotic induction. It enters the nucleus after a caspase-mediated cleavage and induces condensation of chromatin, followed by fragmentation of DNA[1]. Mst1 protein has been reported to be able to induce the mitochondrial-dependent apoptosis pathway through caspase-dependent and independent pathways. The Nrf2 gene influences cell proliferation, cell growth, and cell metabolism control via the phosphatidylinositol-3-kinase/ protein kinase pathway and increases the expression of the anti-apoptotic protein Bcl-2[4].
Acute SCI is resulted from secondary progressive tissue destruction and caused by inflammatory response. Therefore, to reduce secondary tissue damage, a logical approach is to restrict this response. Ischemia-related inflammation induces tissue and cellular edema, oxidative damage, and apoptosis depending on the size of the lesion. One strategy to reduce neuronal cell death (apoptosis/necrosis) is the use of biomolecules or protective agents that reduce the function of pro-apoptotic genes.
Herein, we decided to investigate the effect of VPA on Mst1 expression in a rat model of SCI due to the efficacy of VPA in enhancing motor function and decreasing the cell death after SCI, The important role of Mst1 in apoptosis and neuronal damage has also been explored in this study.
MATERIALS AND METHODS
Contusion model
Wistar female rats (n = 32, weighing 200-250 g, aged 8-12 weeks old) were assigned to four experimental groups (n = 8 per group): Severe contused animals without any treatment (untreated), laminectomy (control), contused animals treated with normal saline (0.5 ml; vehicle) and contused animals treated with VPA (400 mg/kg; Sigma, USA). All injections were given intraperitoneally with a final volume of 0.5 ml and continued once daily for seven days, starting three hours after the procedure. The optimal dose of VPA was chosen in accordance with a previous study[5]. Sever contusion SCI model was applied using previously mentioned weight-drop procedure[6,7]. The BBB open-field test was used to evaluate locomotor activity between experimental groups for four minutes. Rats videotaped with a digital video camera (Canon EOS 80D; japan) at 3, 7, 14, 21, and 28 days post SCI. Repeated tests of variance analysis (ANOVA) accompanied by Tukey's post-hoc test reported the significant differences in scores.
Histological assessments
Rats were anesthetized 28 days after SCI and then transcardially perfused with heparin-containing saline solution (1 unit/ml), followed by a fixative solution (4% paraformaldehyde). The lesion area was removed and post fixed in a same solution for next 12 hours. Tissues passed through an automated processor (Leica TP 1020, Germany) and were embedded in paraffin blocks. Serial sections of spinal cords were prepared and then dewaxed with chloroform for cavity evaluation and stained with H&E. The percentage volume of the cavity in the 3000-μm length of the spinal cord (a total of 30 sections, including the rostral, central and caudal regions) was evaluated using the software Image J and the Cavalieri method (equation 1; Vsp, volume spheroid; a, measured area; d, intersection distance)[8].
Vsp = a × d. (Eq. 1)
The TUNEL assay was conducted according to the manufacturer's protocol (Roche, Germany). Using an Olympus BX61 fluorescence microscope, five non-overlapping fields around the injury site were randomly chosen.
Quantitative RT-PCR
In all the groups, 1,000 ng of purified RNA (DNA free) was applied to synthesize 20 µl of cDNA, using a cDNA synthesis kit (Fermentas, USA). All quantitative PCR reactions were performed in triplicate, and the final volume for each reaction was 12.5 µl, containing 6 µl of RealQ Plus 2× Master Mix Green (Ampliqon; Denmark), 0.76 µl of each forward and reverse primer (0.3 µM; Table 1), 4.5 µl of RNase-free water, and 0.5 µl of cDNA (final concentration of 25 ng per quantitative PCR). The PCR reaction (Applied Biosystems 7500, USA) was conducted for 40 cycles. We used the Pfaffl method (equation 2) for evaluating relative changes in mRNA levels[9]. Mst1, Nrf2, and Bcl-2 mRNA were normalized against GAPDH, and the samples of laminectomy SCI group were employed as a calibrator.
Table 1.
Primer sequences and PCR parameters
| Gene | Gene Accession no. | Sense 5 → 3 | Anti-sense 5 → 3 |
|---|---|---|---|
| Mst1 | NM_001107800.1 | GCTAAAGTGAAGTGGACGGATACC | GGAACAGTTGCTACCAGAGTGTCAG |
| Nrf2 | NM_031789.2 | CACCAGTGGATCTGTCAGCTACTC | GTGGTGAAGACTGAGCTCTCAACG |
| Bcl-2 | NM_016993.1 | GTGGCCTTCTTTGAGTTCGGTG | ATCCCAGCCTCCGTTATCCTG |
| GAPDH | NG_028301.2 | AACCCATCACCATCTTCCAG | GTGGTTCACACCCATCACAA |
(Eq.2)
Statistical analysis
Using SPSS15 software, the statistical analysis was carried out. All data were presented as mean ± SEM. A one-way ANOVA accompanied by Tukey's post hoc comparison and student's t-test analysis was employed to compare different means in the groups. Values of p ≤ 0.05 were considered as statistically significant.
Ethical statement
All the experimental procedures were carried out in compliance with Zanjan University's (ZUMS) ethical guidelines (ethical code: A-12-973-5).
RESULTS
BBB score
Recorded BBB scores showed a significant increase at 21 and 28 days post SCI (10.75 ± 1.06 and 9.37 ± 1.08, respectively) in the VPA-treated group compared to the contusion group. The numerical difference (delta number) between days 3 and 28 post SCI were significantly different between VPA-treated (10.68 ± 1.1) and contusion (1.58 ± 0.49) groups.
Histological assessment
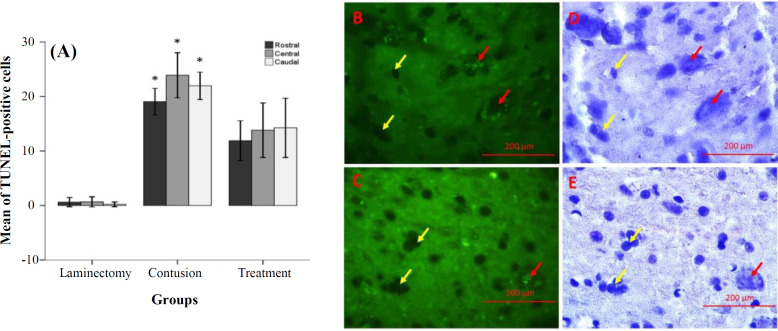
Analysis of cavity percentage revealed a significant difference between VPA-treated (5.35 ± 0.3) and contusion (19.26 ± 2.04) groups. A small population of apoptotic cells was observed in the laminectomy group (rostral: 0.61 ± 0.41, central: 0.65 ± 0.45, and caudal: 0.21±0.21); however, a large number of apoptotic cells were observed in the contusion group (rostra: 19.05 ± 1.21, central: 23.86 ± 2.05, and caudal: 21.95 ± 1.25), as demonstrated by TUNEL assay. The number of apoptotic cells significantly decreased in the VPA-treated group (rostral: 11.88±1.82, central: 13.8±2.45, and caudal: 14.24±2.71) compared to the contusion group. These results are demonstrated in Figure 1.
Fig. 1.
Effects of VPA on the inhibition of apoptosis 28 days post SCI (TUNEL assay, fluorescence microscope). (A) Bar graphs indicate the mean percentage of apoptotic cells in the experimental groups. Apoptotic cells were calculated in three (rostral, central and caudal) regions; (B and C) representative images of TUNEL staining (B, contusion and C, VPA); (D and E) counterstaining of the same field with hematoxylin. Red and yellow arrows show positive and negative cells, respectively. The bars indicate the mean ± SEM (*p ≤ 0.05 vs. VPA group; magnification 400×).
Gene expression
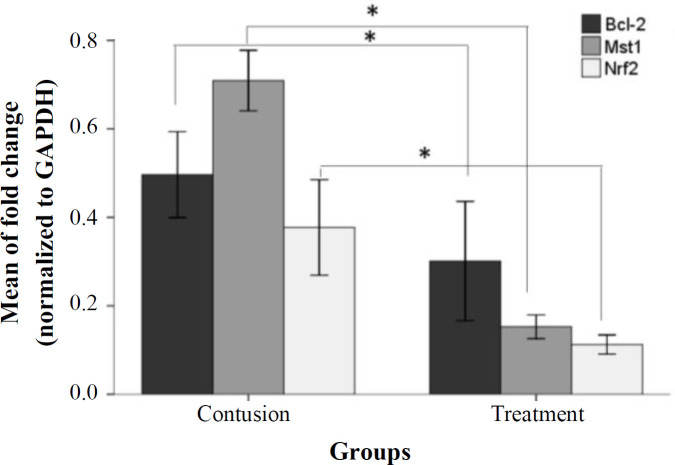
Using quantitative RT-PCR, improvements in the expression of Mst1, Nrf2, and Bcl-2 mRNA levels inall experimental groups were analyzed. Data relating to the laminectomy community were presented. In the VPA-treated group, Mst1, Nrf2, and Bcl-2 mRNA expression (0.15 ± 0.01, 0.11 ± 0.01, and 0.3 ± 0.06, respectively) significantly decreased compared to the control group (0.7 ± 0.03, 0.37 ± 0.03, and 0.49 ± 0.04, respectively), as depicted in Figure 2 (p ≤ 0.05).
Fig. 2.
Quantitative RT-PCR results relative to the laminectomy group. The mRNA level of Mst1, Nrf2, and Bcl-2 is presented as the relative expression normalized to GAPDH mRNA amplification. The bars indicate the mean ± SEM (*p ≤ 0.05 vs. VPA group).
DISCUSSION
In the chronic phase of SCI, cell death continues due to apoptosis. In this study, we have shown that VPA reduced Mst1 gene expression and apoptosis rate after SCI. There are extensive findings regarding the activation of the mechanisms of apoptosis following SCI[10]. Mst1 plays an important role in mediating apoptosis, but its precise role has not been well known[1]. Studies have indicated that apoptosis happens in the models of SCI and is followed by caspase-3 activation[11,12]. Lee et al.[13] have suggested that VPA prevents the cell death and caspase 3 activation, reduces spinal cord lesions and improves locomotor function after SCI. For the advancement of novel approaches for the prevention and treatment of SCI, discovering pathways that can inhibit the progression of inflammation and apoptosis is important. MST1 is regarded as one of the proteins directly and indirectly associated with caspase-3[13].
Results from this study indicated that SCI can activate apoptosis in the lesion site and surrounding regions. Mst1 apparently activates downstream targets such as JNK/p38, histone H2B, and FOXO[14]. Mst1 also induces apoptosis in cardiac myocytes by phosphorylation and Bcl-xLL inhibition[15]. Another group of regulatory proteins, such as anti-apoptotic Bcl-2, Bcl-XL and Nrf2, can protect apoptotic cell death. Several apoptotic signals converge on caspase activation, and this pathway is regulated by Bcl-2, Bcl-XL and Nrf2, as well[16]. Regarding the role of apoptosis in SCI, new therapies, i.e. the inhibition of genes involved in apoptosis signaling pathway such as Mst1, could result in the reduced cell death[17].
VPA is an anticonvulsant and a mood-stabilizing drug with proven neuroprotective and anti-apoptotic effects in rat SCI model and other neurological diseases[18]. In this study, we have displayed that VPA significantly reduces the expression of Mst1 and subsequently decreases apoptosis compared to the untreated contused group. VPA is recognized as a strong histone deacetylase inhibitor[3]. Histone acetylation is a key mechanism for modification of chromatin structure and genes expression[19]. In our previous research, we have demonstrated that VPA would decreases the production of secondary damage in rat spinal cord trauma dependently on the dosage, resulting in improved locomotor score and recovery time[20]. To date, very limited study has been carried out on the function of VPA in the expression of the Mst1 gene. Lee et al.[21] have found that in a rat model SCI, 300 mg/kg of VPA increases the expression levels of Bcl-2 and Bax mRNAs. In the current study, VPA treatment was performed in the acute phase of injury, but apoptosis rate and gene expressions participating in apoptosis were evaluated in the chronic phase.
In vivo documents have demonstrated that the lack of the Mst1 increases spinal motor neuron survival after trauma, locomotor scores, and synapse survival[22]. MST1 proteins are mainly located within the cytoplasm, but during stress, they can be cleaved by caspase-3 and relocated into the nucleus[23]. One of the key factors involved in the inhibition of apoptosis proteins is Nrf2. This transcription factor, along with the increased expression of enzymes, is related to antioxidant and detoxification, prevents cell death and is considered as an anti-apoptotic factor[24]. Evidence has revealed that in the cytoplasm, Keap1 preserves Nrf2[25]. Our results indicated that the expression of Nrf2 reduced in the valproic acid-treated group. This reduction may be due to decreased inflammation and apoptosis in the lesion areas. We can conclude that with the injection of valproic acid, the amount of cellular stress declines, resulting in the reduced expression of Nrf2 and Bcl-2 genes.
In conclusion, VPA may be seen as a potential drug candidate for the treatment of neurodegenerative conditions. Moreover, pharmacological inhibition of Mst1 can be used as a form of therapy for neurodegenerative disorders.
ACKNOWLEDGEMENTS
The current study was funded by Zanjan University of Medical Sciences, Zanjan, Iran (grant no. A-12-973-5).
CONFLICT OF INTEREST.
None declared.
References
- 1.Rawat SJ, Chernoff J. Regulation of mammalian Ste20 (Mst) kinases. Trends in biochemical sciences. 2015;40(3):149–156. doi: 10.1016/j.tibs.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. Journal of neuroscience. 1997;17(7):2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. The EMBO journal. 2001;20(24):6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar S, Raymick J, Imam S. Neuroprotective and therapeutic strategies against Parkinson's disease: Recent perspectives. International journal of molecular sciences. 2016;17(6):904. doi: 10.3390/ijms17060904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rattanarojsakul P, Thawesaengskulthai N. A medication safety model: a case study in Thai hospital. Global journal of health science. 2013;5(5):89–101. doi: 10.5539/gjhs.v5n5p89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozçelik T, Rosenthal A, Francke U. Chromosomal mapping of brain-derived neurotrophic factor and neurotrophin-3 genes in man and mouse. Genomics. 1991;10(3):569–575. doi: 10.1016/0888-7543(91)90437-j. [DOI] [PubMed] [Google Scholar]
- 7.Kjell J, Finn A, Hao J, Wellfelt K, Josephson A, Svensson CI, Wiesenfeld-Hallin Z, Eriksson U, Abrams M, Olson L. Delayed imatinib treatment for acute spinal cord injury: Functional recovery and serum biomarkers. Journalof neurotrauma. 2015;32(21):1645–1657. doi: 10.1089/neu.2014.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michel RP, Cruz-Orive LM. Application of the Cavalieri principle and vertical sections method to lung: estimation of volume and pleural surface area. Journal of microscopy. 1988;150(Pt2):117–136. doi: 10.1111/j.1365-2818.1988.tb04603.x. [DOI] [PubMed] [Google Scholar]
- 9.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang N, Yin Y, Xu SJ, Wu YP, Chen WS. Inflammation & apoptosis in spinal cord injury. Indian journal of medical research. 2012;135(3):287–296. [PMC free article] [PubMed] [Google Scholar]
- 11.Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD. Apoptosis after traumatic human spinal cord injury. Journal of neurosurgery. 1998;89(6):911–920. doi: 10.3171/jns.1998.89.6.0911. [DOI] [PubMed] [Google Scholar]
- 12.Ekshyyan O, Aw TY. Apoptosis in acute and chronic neurological disorders. Frontiers in bioscience. 2004;9:1567–1576. doi: 10.2741/1357. [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Kim HS, Choi HY, Oh TH, Ju BG, Yune TY. Valproic acid attenuates blood–spinal cord barrier disruption by inhibiting matrix metalloprotease‐9 activity and improves functional recovery after spinal cord injury. Journal of neurochemistry. 2012;121(5):818–829. doi: 10.1111/j.1471-4159.2012.07731.x. [DOI] [PubMed] [Google Scholar]
- 14.Yuan F, Xie Q, Wu J, Bai Y, Mao B, Dong Y, Bi W, Ji G, Tao W, Wang Y, Yuan Z. MST1 promotes apoptosis through regulating Sirt1-dependent p53 deacetylation. Journal of biological chemistry. 2011;286(9):6940–6945. doi: 10.1074/jbc.M110.182543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Re DP, Matsuda T, Zhai P, Maejima Y, Jain MR, Liu T, Li H, Hsu CP, Sadoshima J. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Molecular cell. 2014;54(4):639–650. doi: 10.1016/j.molcel.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Cheng L, Hou Y, Si M, Zhao YP, Nie L. Plumbagin protects against spinal cord injury-induced oxidative stress and inflammation in Wistar rats through Nrf-2 upregulation. Drug research(Stuttgart) 2015;65(9):495–499. doi: 10.1055/s-0034-1389950. [DOI] [PubMed] [Google Scholar]
- 17.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature reviews molecular cell biology. 2014;15(1):49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 18.Lv L, Sun Y, Han X, Xu CC, Tang YP, Dong Q. Valproic acid improves outcome after rodent spinal cord injury: potential roles of histone deacetylase inhibition. Brain research. 2011;1396:60–68. doi: 10.1016/j.brainres.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 19.Bhavsar P, Ahmad T, Adcock IM. The role of histone deacetylases in asthma and allergic diseases. Journal of allergy and clinical immunology. 2008;121(3):580–584. doi: 10.1016/j.jaci.2007.12.1156. [DOI] [PubMed] [Google Scholar]
- 20.Abdanipour A, Schluesener HJ, Tiraihi T. Effects of valproic acid, a histone deacetylase inhibitor, on improvement of locomotor function in rat spinal cord injury based on epigenetic science. Iranian biomedical journal. 2012;16(2):90–100. doi: 10.6091/ibj.1060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JY, Maeng S, Kang SR, Choi HY, Oh TH, Ju BG, Yune TY. Valproic acid protects motor neuron death by inhibiting oxidative stress and endoplasmic reticulum stress-mediated cytochrome C release after spinal cord injury. Journalof neurotrauma. 2014;31(6):582–594. doi: 10.1089/neu.2013.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M, Tao W, Yuan Z, Liu Y. Mst‐1 deficiency promotes post‐traumatic spinal motor neuron survival via enhancement of autophagy flux. Journal of neurochemistry. 2017;143(2):244–256. doi: 10.1111/jnc.14154. [DOI] [PubMed] [Google Scholar]
- 23.Hao H-H, Wang L, Guo Z-J, Bai L, Zhang RP, Shuang WB, Jia YJ, Wang J, Li XY, Liu Q. Valproic acid reduces autophagy and promotes functional recovery after spinal cord injury in rats. Neuroscience bulletin. 2013;29(4):484–492. doi: 10.1007/s12264-013-1355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tummala KS, Kottakis F, Bardeesy N. NRF2: Translating the redox code. Trends in molecular medicine. 2016;22(10):829–831. doi: 10.1016/j.molmed.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Molecular and cellular biology. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]