Abstract
Purpose
To examine clinicodemographic determinants associated with breast cancer survivorship follow-up during COVID-19.
Methods
We performed a retrospective, population-based cohort study including early stage (Stage I-II) breast cancer patients who underwent resection between 2006 and 2018 in a New York City hospital system. The primary outcome was oncologic follow-up prior to and during the COVID-19 pandemic. Secondary analyses compared differences in follow-up by COVID-19 case rates stratified by ZIP code.
Results
A total of 2942 patients with early-stage breast cancer were available for analysis. 1588 (54%) of patients had attended follow-up in the year prior to the COVID-19 period but failed to continue to follow-up during the pandemic, either in-person or via telemedicine. 1242 (42%) patients attended a follow-up appointment during the COVID-19 pandemic.
Compared with patients who did not present for follow-up during COVID-19, patients who continued their oncologic follow-up during the pandemic were younger (p = 0.049) more likely to have received adjuvant radiation therapy (p = 0.025), and have lower household income (p = 0.031) on multivariate modeling. When patients who live in Bronx, New York, were stratified by ZIP code, there was a modest negative association (r = −0.56) between COVID-19 cases and proportion of patients who continued to follow-up during the COVID-19 period.
Conclusion
We observed a dramatic disruption in routine breast cancer follow-up during the COVID-19 pandemic. Providers and health systems should emphasize reintegrating patients who missed appointments during COVID-19 back into regular surveillance programs to avoid significant morbidity and mortality from missed breast cancer recurrences.
Keywords: COVID-19, Breast cancer, Cancer survivorship
Highlights
-
•
A dramatic disruption in routine oncologic follow-up was observed during the COVID-19 period.
-
•
Over half of patients with breast cancer at our center did not attend routine oncologic follow-up during COVID-19.
-
•
Patients who were younger, had lower SES, and who received radiotherapy were more likely to follow-up during the pandemic.
-
•
A modest negative association was observed between local ZIP code COVID-19 infection rates and follow-up attendance rate.
1. Introduction
In 2019, the 17 million American cancer survivors constituted approximately 5 % of the population. This number is expected to exceed 22 million by 2030, even with declining cancer incidence [1]. Among women, breast cancer remains the most prevalent cancer, accounting for over 3.8 million survivors in 2019 [2].
Improved survival rates with modern cancer therapies has led to an emphasis on managing long-term complications, such as from radiation and chemotherapy [[3], [4], [5]]. Current guidelines for follow-up care of breast cancer patients by the American Cancer Society (ACS) and American Society of Clinical Oncology (ASCO) recommend at-least annual lifelong clinical follow-up and mammography to manage complications and assess for recurrent or new breast cancer [6]. Despite such guidelines, mounting evidence suggests recent substantial and increasing rates of inadequate long-term follow-up among breast cancer survivors [7].
The novel Coronavirus Disease 2019 (COVID-19) has affected all aspects of healthcare [8]. Cancer treatment during COVID-19 has been triaged [9] with modifications to systematic therapy scheduling [10] and radiation delivery [11]. A recent single institutional study demonstrated that over 40 % of breast cancer patients undergoing treatment experienced a delay or change in therapy during the early COVID-19 pandemic [12]. Cancer patients also experience increased case fatality rate from COVID-19 infection [[13], [14], [15], [16]], with evidence suggesting that this may also apply to cancer survivors [17].
This study aimed to estimate disruption of long-term follow-up (beyond 2 years after resection) among early-stage breast cancer survivors during the COVID-19 pandemic at Montefiore Medical Center (MMC), an urban, academic, tertiary-care hospital system in Bronx, New York, which was an early epicenter of the pandemic in the United States. We hypothesized that patients would be less likely to attend follow-up appointments during the pandemic. We also examined associations between follow-up attendance and clinical and demographic risk factors during and prior to the pandemic period.
2. Patients and methods
2.1. Patient selection
Eligible patients had a new diagnosis of stage I or II breast cancer, defined by American Joint Committee on Cancer (AJCC) 7th edition staging guidelines, and underwent surgical resection between January 1, 2006, and June 4, 2018, within the MMC system. Data were obtained from the Montefiore Medical Center, Albert Einstein Cancer Center Cancer Registry (“Cancer Registry”). These dates were chosen so that at least 2 years have passed from initial resection to allow us to evaluate the effect of COVID-19 on long-term follow-up (rather than shorter term follow-up within 2 years after resection).
For patients who underwent adjuvant chemotherapy and/or radiation therapy, the patient's medical and radiation oncologists were also required to be part of the MMC system (e.g. if resection was performed at MMC but chemotherapy was given outside MMC, this patient was excluded from analysis). This study was approved by the Institutional Review Board at Montefiore Medical Center/Albert Einstein College of Medicine (2020–11141) and conducted in accordance with STROBE guidelines.
Included patients were categorized into three groups:
-
1)
“Continued follow-up” denotes patients who had a last documented follow-up appointment with any MMC oncologist during a nine-month period accounting for the initial wave of COVID-19, March 1, 2020, to December 1, 2020.
-
2)
“Pending follow-up” denotes patients who had a documented last follow-up appointment with any MMC oncologist between March 1, 2019, and March 1, 2020 (i.e. during the year immediately preceding the COVID-19 pandemic) but did not follow-up during the COVID-19 period. These are patients who are most at risk of having their follow-up disrupted by COVID-19.
-
3)
“Lost to follow-up” denotes patients whose last known follow-up appointment was prior to March 1, 2019. These are patients who had gone at least 1 year without MMC oncology follow-up at the beginning of the COVID-19 pandemic.
Both in-person and telehealth visits with any MMC oncology clinic (surgical, medical, or radiation oncology) were counted as follow-up appointments. Appointments with both physicians and nurse practitioners were counted, based on the standard practices of each clinic. Information regarding patients' follow-up, MMC appointment dates, clinical course, and death were obtained via several sources: MMC electronic medical record (EMR) (EPIC Systems), Bronx RHIO (Regional Health Information Organization), CLG (Clinical Looking Glass, a computerized decision support tool), death certificates, and letters from outside facilities and physicians. Date of last contact complied with rules from the Commission on Cancer data standards manual [18]. Data in the Cancer Registry (including date of last contact) is updated monthly. Clinical and demographic information were extracted from the patient's EMR.
Both clinical stage and pathologic stage were evaluated with the higher stage used for analysis for a conservative estimate of stage. The date of collection for this data was December 4, 2020. Patient income was estimated by ZIP code at time of diagnosis.
2.2. COVID-19 case rate estimation
Data for COVID-19 case rates were extracted from the New York City Department of Health for the week of highest COVID-19 rates in New York City (April 5, 2020, to April 11, 2020). For case rates outside of New York City, rates were estimated by county using the New York State Forward County Dashboard. Percentage of COVID-19 positive cases were calculated for all available ZIP codes over the course of the week, and then recoded as quartiles.
2.3. Median income by ZIP code
Median household income data was estimated for each patient using ZIP code at time of diagnosis and compared with 2010 US Census data. Annual household income was grouped into five ranges (<$25,000, $25,001–50,000, $50,001–75,000, $75,001–100,000, $100,001+).
2.4. Covariates
The following covariates were evaluated: age (18–34, 34–44, 45–54, 55–64, 65–74, 75+), sex (male, female), race/ethnicity (Asian, Black, Hispanic, non-Hispanic (NH)-White, NH-other), smoking history (Never, Former, Current, Unknown), household income, stage (I, II), type of breast surgery (lumpectomy or mastectomy), year of diagnosis, and COVID-19 case rate quartile (based on patient ZIP code of residence). Receipt of adjuvant radiation therapy, chemotherapy, and/or endocrine therapy was also recorded.
2.5. Statistical analysis
Descriptive statistics are displayed as percentages of all patients unless otherwise labelled and include a margin of error at the 95 % confidence intervals (CI) among all adults. χ [2] tests were used for bivariate comparisons, unless Fisher's exact test was more appropriate in situations where expected frequencies were less than 5. To estimate determinants of follow-up, we calculated univariate comparisons. A criterion of p value < 0.05 was used as a cutoff to include in the multivariate logistic regression model. A global F test was used to assess significant of all predicts in multivariate regression. Collinearity was assessed to ensure no strong linear relationship among independent variables included in the model was present. For correlation analysis, coefficient of determination, R [2], was used to assess linear association. In addition, p < 0.05 was used as the level of significance. Due to the exploratory nature of this analysis, we did not make adjustments for multiple comparisons. All statistical analyses were conducted using SPSS (IBM Version 24).
3. Results
2942 patients who underwent resection at MMC for early-stage breast cancer at MMC between 2006 and 2018 were included in the analysis. Table 1 summarizes the descriptive baseline characteristics of patients who continued to attend to follow-up during COVID-19 compared to those that had a last known follow-up within the year prior to March 1, 2020. 1242 (42 %) of patients had a follow-up during the COVID-19 period. 1588 patients (54 %) were categorized as having pending follow-up, meaning they attended a follow-up appointment in the year prior to COVID-19 (March 2019–March 2020) but did not follow-up during the COVID-19 period.
Table 1.
– Baseline characteristics of COVID-19 follow-up cohort.
| Baseline Characteristics | Continued Follow-up (n = 1242) | Pending Follow-up (n = 1588) | Total (n = 2830) | P value |
|---|---|---|---|---|
| Age, y | 0.022 | |||
| 18-34 | 17 (1.4) | 22 (1.4) | 39 (1.4) | |
| 35-44 | 97 (7.8) | 92 (5.8) | 189 (6.7) | |
| 45-54 | 276 (22.2) | 92 (22.8) | 638 (22.5) | |
| 55-64 | 389 (31.3) | 452 (28.5) | 841 (29.7) | |
| 65-74 | 313 (25.2) | 409 (25.8) | 722 (25.5) | |
| 75+ | 150 (12.1) | 251 (15.8) | 401 (14.2) | |
| Gender | 0.600 | |||
| Female | 1233 (99.3) | 1579 (99.4) | 2812 (99.4) | |
| Male | 9 (0.7) | 9 (0.6) | 18 (0.6) | |
| Race/ethnicity | 0.003 | |||
| White | 221 (17.8) | 362 (22.8) | 583 (20.6) | |
| Asian | 30 (2.4) | 37 (2.3) | 67 (2.4) | |
| Black | 504 (40.6) | 608 (38.3) | 1112 (39.3) | |
| Hispanic | 443 (35.7) | 502 (31.6) | 250 (33.4) | |
| Other | 44 (3.5) | 79 (5.0) | 32 (4.3) | |
| Smoking | 0.130 | |||
| Never | 783 (63.0) | 1053 (66.3) | 1836 (64.9) | |
| Former | 275 (22.1) | 313 (19.7) | 588 (20.8) | |
| Current | 157 (12.6) | 177 (11.1) | 334 (11.8) | |
| Unknown | 27 (2.2) | 45 (2.8) | 72 (2.5) | |
| Household Income | 0.041 | |||
| <25,000 | 164 (13.2) | 179 (11.3) | 343 (12.1) | |
| 25,001–50,000 | 789 (63.5) | 962 (60.6) | 1751 (61.9) | |
| 50,001–75,000 | 229 (18.4) | 347 (21.9) | 576 (20.4) | |
| 75,001–100,000 | 27 (2.2) | 49 (3.1) | 76 (2.7) | |
| 100,001+ | 33 (2.7) | 51 (3.2) | 84 (3.0) | |
| Stage | 0.925 | |||
| I | 910 (73.3) | 1166 (73.4) | 2076 (73.4) | |
| II | 332 (26.7) | 422 (26.6) | 754 (26.6) | |
| Surgery | 0.672 | |||
| Lumpectomy | 870 (70.0) | 1124 (70.8) | 1994 (70.5) | |
| Mastectomy | 372 (30.0) | 464 (29.2) | 836 (29.5) | |
| Radiation | 0.009 | |||
| No | 412 (33.2) | 602 (37.9) | 1014 (35.8) | |
| Yes | 830 (66.8) | 986 (62.1) | 1816 (64.2) | |
| Chemotherapy | 0.050 | |||
| No | 798 (64.3) | 1076 (67.8) | 1874 (66.2) | |
| Yes | 444 (35.7) | 512 (32.2) | 956 (33.8) | |
| Endocrine Therapy | 0.066 | |||
| No | 410 (33.0) | 577 (36.3) | 987 (34.9) | |
| Yes | 832 (67.0) | 1011 (63.7) | 1843 (65.1) | |
| Community COVID-19 Case rate by ZIP code | 0.072 | |||
| 21.8–48 % | 198 (15.9) | 294 (18.5) | 492 (17.4) | |
| 48–57 % | 214 (17.2) | 305 (19.2) | 519 (18.3) | |
| 57.1–61.9 % | 647 (52.1) | 786 (49.5) | 1433 (50.6) | |
| 62–77.8 % | 183 (14.7) | 203 (12.8) | 386 (13.6) |
Patients who continued to follow-up with their oncology providers during COVID-19 tended to be younger (p = 0.022), non-white in racial identification (p = 0.003), have a lower median household income (p = 0.041), and have undergone adjuvant therapy with either radiation (p = 0.009) or chemotherapy (p = 0.050). When patient ZIP codes were grouped into quartiles based on local COVID-19 case rate at the height of the NYC pandemic in April 2020, local case rate was not a predictor of continued follow-up.
On multivariable modelling, the difference in groups observed by age, income and radiation remained significant, while chemotherapy (p=0.392) and race/ethnic identification were no longer significant (p = 0.128). Results of the multivariable analysis are shown in Table 2.
Table 2.
– Multivariate modeling of determinants of follow-up during COVID-19.
| Variable | Adjusted Odds Ratio | 95 % CI | P value |
|---|---|---|---|
| Age, y | 0.049 | ||
| 18-34 | ref | ||
| 35-44 | 1.4 | 0.7–2.8 | |
| 45-54 | 1.0 | 0.5–2.0 | |
| 55-64 | 1.1 | 0.6–2.2 | |
| 65-74 | 1.0 | 0.5–2.0 | |
| 75+ | 0.9 | 0.4–1.7 | |
| Race/ethnicity | 0.128 | ||
| White | ref | ||
| Asian | 1.2 | 0.7–2.1 | |
| Black | 1.3 | 1.0–1.6 | |
| Hispanic | 1.3 | 1.1–1.7 | |
| Other | 0.9 | 0.6–1.3 | |
| Household Income | 0.031 | ||
| <25,000 | ref | ||
| 25,001–50,000 | 0.9 | 0.7–1.2 | |
| 50,001–75,000 | 0.8 | 0.6–1.0 | |
| 75,001–100,000 | 0.6 | 0.4–1.1 | |
| 100,001+ | 0.8 | 0.5–1.3 | |
| Radiation | 0.025 | ||
| No | ref | ||
| Yes | 1.2 | 1.0–1.4 | |
| Chemotherapy | 0.392 | ||
| No | ref | ||
| Yes | 1.1 | 0.9–1.3 |
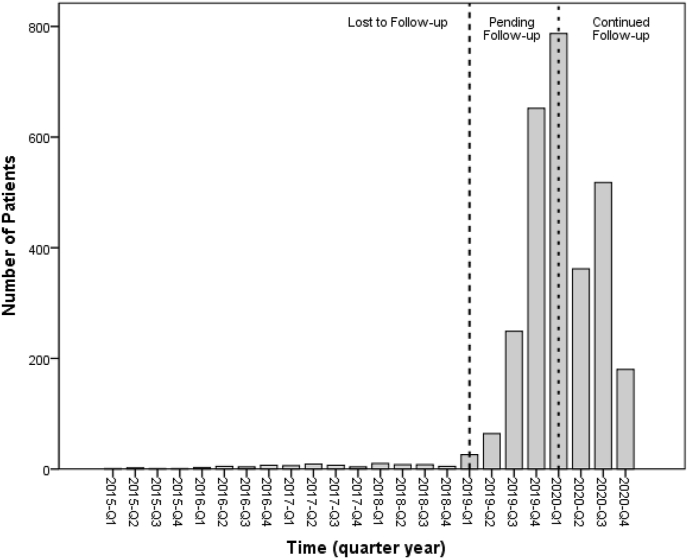
There is also an observed decline in the number of last follow-up visits in the months immediately after the start of the COVID-19 period (March and April 2020) compared with the months immediately prior to the pandemic start. Fig. 1 shows the number of patients with last known follow-up by month leading up to and during the COVID-19 period.
Fig. 1.
Number of patients with last follow-up in each quarter year, 2015 to present. Data prior to 2015 not shown.
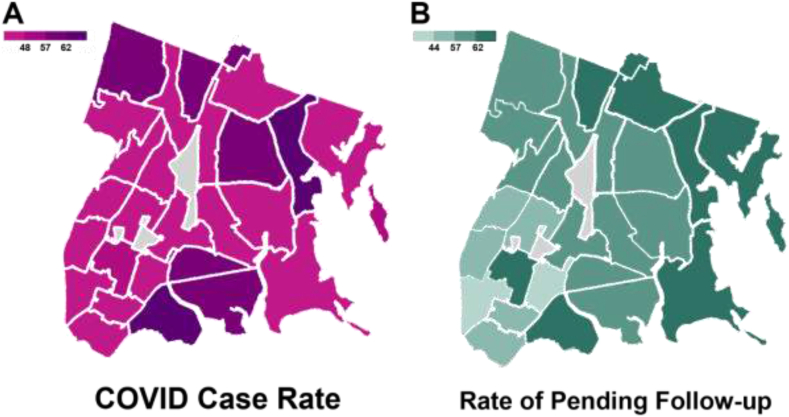
3.1. Geographic distribution within The Bronx
Fig. 2 depicts the geographical distribution of COVID-19 case percentages within each ZIP code in The Bronx (Fig. 2A), along with the proportion of Bronx resident patients with pending follow-up prior to COVID-19 as a percentage of the total number of breast cancer patients (including those who continued follow-up during COVID-19), grouped by ZIP code (Fig. 2B). There was a modest negative association (r = −0.56) between COVID-19 case percentage within ZIP code and proportion of patients who continued to follow-up during the COVID-19 period.
Fig. 2.
A, Case rate of COVID-19 in Bronx, New York, by ZIP code during April 5–11, 2020; B, Proportion of Bronx resident patients with last follow-up prior to COVID-19 as a percentage of the total number of breast cancer patients (including those who continued follow-up during COVID-19), grouped by ZIP code of residence.
3.2. Pre-COVID-19 follow-up cohort
Among the 2942 patients initially evaluated, 112 (4 %) had their last follow-up prior to March 2019 and were deemed lost to follow-up. Characteristics of these patients are shown in Table S1. Therefore, we observed an approximately 14-fold increase in patients not attending follow-up during COVID-19 compared with the annual rate of patients lost to follow-up (112 patients over the past 10 years).
An analysis was then performed to compare those patients who historically were lost to follow-up in the pre-COVID era (i.e. had last follow-up prior to March 2019) and individuals who had pending follow-up during the COVID-19 pandemic.
Compared with patients who had pending follow-up, patients who were lost to follow-up were more likely to be older (p = 0.030), White in racial identification (p = 0.009), former smokers (p = 0.031), have a higher median house hold income (p < 0.001) and tended to not have received adjuvant therapy including RT (p = 0.048), or endocrine therapy (p < 0.001). On multivariable modelling, the difference in groups observed by age, smoking, income, and adjuvant hormonal therapy remained significant, while racial identification (p = 0.758) and radiation therapy (p = 0.962) were no longer significant. Results of the multivariable analysis are shown in Table S2.
4. Discussion
Cancer survivors have been an understudied population [19]. Post-treatment follow-up and survivorship care seeks to reduce adverse effects associated with cancer and its treatment and includes multi-disciplinary domains that improve physiologic, psychosocial, and functional outcomes for cancer survivors and their families. However, in order to implement strategies to enhance health after cancer treatment, optimal follow-up care and surveillance strategies must be achieved.
In this study, we observed a disruption in guideline-recommended, annual, long-term clinical follow-up during the COVID-19 pandemic period among survivors of early-stage breast cancer living in Bronx, New York, the initial North American epicenter of the COVID-19 pandemic. Prior to the pandemic period, approximately 4 % of patients treated since 2006 were lost to follow-up, suggesting that this represents a baseline rate of attrition in the absence of novel factors such as the COVID-19 pandemic. Over half of patients who had continued follow-up prior to COVID did not present for an appointment during the pandemic period (these patients were classified as “pending follow-up”). These patients may be at elevated risk of being lost to follow-up if efforts are not made by providers and institutions to re-establish follow-up care for them after the pandemic.
Approximately forty percent of patients continued to follow-up with MMC oncology services during the COVID-19 pandemic. Some patients who did not may have perceived breast cancer survivorship care as non-essential and opted to defer these visits per recommendations by the organizations such as the Centers for Disease Control and Prevention (CDC) [20] or European Society for Medical Oncology, which often recommended risk-adapted follow-up programs [21,22]. A recent survey study found that more than three-quarters of cancer survivors were worried about both potential COVID-19 risks associated with in-person appointments and the potential of recurrence due to delays in routine care [23]. Patients’ lack of awareness of government or other guidelines regarding the relative risks of missing follow-up appointments compared with COVID-19 exposure risks also may have contributed to the observed decline in follow-up appointments.
Continued follow-up during COVID-19 was more likely among younger patients, those who had received adjuvant radiotherapy and those with lower socioeconomic status. We also observed a modest negative association between local COVID-19 prevalence and the proportion of patients who continued to follow-up. To our knowledge, this is the first study to assess the potential effect of COVID-19 on follow-up care of cancer survivors within the United States and includes a large cohort of patients from New York City, the early North American epicenter of the COVID-19 pandemic.
To facilitate ongoing care during the pandemic, guidelines issued by several medical societies [[24], [25], [26]] recommend transitioning to telemedicine visits to minimize the risk of COVID-19 exposure among patients and providers [27,28]. Montefiore's operational response throughout the pandemic was guided by recommendations from the New York State Department of Health and CDC. During the initial two-month peak of the COVID-19 period (approximately March and April 2020), non-emergent outpatient visits were canceled at MMC, and a large subset of medical staff were redistributed to help with inpatient non-cancer care. After this initial phase, patients were encouraged to transition to telemedicine in order to avoid risks of COVID exposure among patients and providers from in-person visits. Although our data did not contain patient-level data regarding type of insurance, approximately 80% of patients at MMC are covered by public insurance (i.e. Medicare and/or Medicaid). All insurance providers, public and private, allowed (and reimbursed for) telemedicine visits during the pandemic period.
However, the significant decrease in follow-up that we observed during the COVID-19 period occurred despite our center allowing telemedicine visits in lieu of in-person visits. This finding may suggest that telemedicine is not sufficient to maintain proper follow-up for all patients and that re-establishing in-person visits once the COVID-19 pandemic abates is paramount. As well, our result showing that younger patients were more likely to continue follow-up during the pandemic may reflect greater facility with technology among younger patients, allowing them to better access telemedicine services for follow-up appointments. Expanded telemedicine use may continue to increase in the future as providers and patients become more familiar with the technology, allowing telemedicine to become an important tool for long-term follow-up of cancer patients. Future work should assess facility with telemedicine among patients and providers in order to determine best practices for telemedicine and in-person visits to re-integrate patients of all ages and demographics into follow-up care.
There has also been increasing availability of COVID-19 vaccines and testing for the general public, with over 50 % of New York City residents fully vaccinated by the end of July 2021 (although the proportion of Bronx residents is lower than city-wide, at approximately 45 %). Given the low single-digit rate of COVID-19 positivity in New York in summer 2021, routine testing for COVID-19 among outpatient cancer patients is currently not recommended within our institution, but all patients are screened for symptoms and possible exposure prior to entering the hospital. Among our cancer patient population, vaccination is highly encouraged, with demonstrated similar efficacy of IgG production compared to non-cancer healthy individuals [29]. However, there may be slight variations in efficacy especially among patients on endocrine therapy or individuals undergoing cytotoxic chemotherapy or monoclonal antibody directed treatment [30,31].
Whether long term follow-up returns to pre-COVID levels after the pandemic subsides remains to be seen. If patients with pending follow-up due to COVID-19 are not reintegrated into regular follow-up, we estimate that the effect on breast cancer outcomes could be substantial, even in the context of low-risk breast cancer survivors. There were approximately 3.9 million breast cancer survivors in the United States in 2019, of which 52 % (approximately 2 million) were within the first 10 years since diagnosis [32]. In our cohort, approximately 1600 patients had pending follow-up due to the COVID-19 pandemic. Estimating a recurrence risk of 3 % over 5 years for these patients suggests that not re-integrating these patients into follow-up could result in up to 10 missed breast cancer recurrences among these patients alone. If our observed trends were to hold country-wide in the approximately 4 million current breast cancer survivors, up to 2 million patients may have missed follow-up during COVID which could result in 12000 or more missed recurrences (even without accounting for higher recurrence risk among patients with later-stage disease). Although further work will elucidate the extent to which our findings are generalizable and how many patients with delayed follow-up during COVID return to their recommended follow-up patterns, these estimates demonstrate the potential risks to patient and public health if these patients are not sought out and reestablished to follow-up. We propose that providers and health systems specifically emphasize efforts to re-establish proper follow-up care with patients as the COVID-19 situation improves.
To date, few studies have assessed the direct effect of COVID-19 on cancer survivor follow-up [33,34]. Jammu et al., performed a literature review of COVID-19 on cancer survivors and found limited definitive evidence assessing impacts, although preliminary indications predicted detrimental effects on physical, psychosocial, and economic wellbeing. One report from Italy identified a significant increase in lymph node positive and stage III breast cancer after a two-month interruption in routine breast cancer screening [35]. Even prior to the COVID-19 pandemic, previous work on breast cancer survivorship care had also suggested an increasing rate of inadequate follow-up in the United States among breast cancer survivors over the past decade [7,36]. Ruddy et al. reported that non-adherence was associated with older age, no radiation, chemotherapy, endocrine, and increasing time after surgery [7]. In our historic long-term follow-up analysis, we observed that increasing age, prior smoking history, higher median house hold income, and lack of adjuvant hormonal therapy were associated with patients who were lost to follow-up. This latter observation is similar to increased rates of continued follow-up among patients who received adjuvant radiation. The association of adjuvant therapies with follow-up can likely be explained by having more oncologists involved in their care and, thus, more providers ensuring that follow-up guidelines are adhered to.
We observed a moderate negative association between ZIP code case rate and patients who continued to follow-up during COVID-19. This finding could reflect the high overall COVID-19 numbers throughout The Bronx borough with pervasive awareness and concern throughout all geographical regions of risks of contracting the disease, with a small contribution of concern related to local disease rates. In addition, the lack of difference in follow-up between patients belonging to different ethnic groups was similar to previously reported clinical outcomes at our institution [37,38]. In these prior studies, which evaluated risk factors for missed daily radiotherapy treatments, low socioeconomic status was an independent predictor for missed appointments while racial/ethnic identification was not. We observed similar results on multivariate analysis in our study.
Our institution serves a large catchment area within the New York City metropolitan area with a diverse population including many patients with low socioeconomic status and/or belonging to potentially marginalized groups such as ethnic or linguistic minorities. Montefiore is the largest hospital network serving the borough of The Bronx, the poorest urban county in the United States with a poverty rate of 31 % (compared with 19 % in the rest of New York City). 54 % of Bronx residents identify as Hispanic and 33 % identify as Black or African American. Patients from such populations are historically underrepresented in medical research and our ability to analyze behavioral patterns from a large cohort of these patients represents a major strength. While we acknowledge that, given the demographics of The Bronx, our findings may not be generalizable to other populations, we believe that our findings likely hold for other potentially marginalized communities within the United States and beyond. Another strength is our ability to make temporal assessments of determinants of follow-up in a large population of cancer survivors.
Limitations of the study include our inability to directly assess the role of other chronic conditions, including prior cancer diagnoses, and co-morbid conditions on long-term cancer follow-up. We were also not able to extract certain tumor characteristics such as grade and receptor status. Patients with receptor positive disease would likely be prescribed adjuvant hormonal therapy (e.g. Tamoxifen or an aromatase-inhibitor) and be followed by a medical oncologist while on this treatment. Thus, patients receiving endocrine therapy may be more likely to have continued follow-up during the pandemic as they would be on active therapy and have more physicians monitoring their condition. However, patients who received adjuvant chemotherapy were not more likely to continue follow-up. Future work may better elucidate the effect of tumor biology on follow-up patterns. Finally, some element of survivorship bias may be present where lack of follow-up may be the result of increased mortality during the COVID period, although we did attempt to verify vital status using death certificates and letters to primary care providers as per Commission on Cancer standards.
In conclusion, COVID-19 has introduced new stressors on our healthcare providers and systems and our study is the first to document a dramatic disruption in follow-up among early-stage breast cancer survivors during the pandemic [39]. If the patients who are pending follow-up during COVID-19 are not reintegrated into evidence-based follow-up patterns after the COVID-19 period, it has the potential to dramatically affect cancer survival outcomes.
Role of funding
There are no conflicts, or funding to report for the study design, collection, analysis, interpretation of data, or writing of the article.
Author contributions
Conception and design: Allen Mo, Jonathan Klein.
Collection and assembly of data: Julie Chung, Allen Mo, Jeremy Eichler, Sarah Yukelis.
All authors assisted in providing critical feedback, discussed the results, and contributed to the final manuscript.
Declaration of competing interest
NO is a consultant for Merck and AstraZeneca. There are no additional conflicts of interests to disclose.
Acknowledgements
We would like to thank the Montefiore Cancer Registry for their assistance with curating and compiling the data for this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.07.018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA A Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Miller K.D., Nogueira L., Mariotto A.B. Cancer treatment and survivorship statistics. CA A Cancer J Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 3.Oeffinger K.C., Hudson M.M. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA A Cancer J Clin. 2004;54:208–236. doi: 10.3322/canjclin.54.4.208. [DOI] [PubMed] [Google Scholar]
- 4.Ng A.K., Kenney L.B., Gilbert E.S. Secondary malignancies across the age spectrum. Semin Radiat Oncol. 2010;20:67–78. doi: 10.1016/j.semradonc.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaworski C., Mariani J.A., Wheeler G. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61:2319–2328. doi: 10.1016/j.jacc.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 6.Runowicz C.D., Leach C.R., Henry N.L. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34:611–635. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 7.Ruddy K.J., Herrin J., Sangaralingham L. Follow-up care for breast cancer survivors. J Natl Cancer Inst. 2020;112:111–113. doi: 10.1093/jnci/djz203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanne J.H., Hayasaki E., Zastrow M. Covid-19: how doctors and healthcare systems are tackling coronavirus worldwide. BMJ. 2020;368:1090. doi: 10.1136/bmj.m1090. [DOI] [PubMed] [Google Scholar]
- 9.Hartman H.E., Sun Y., Devasia T.P. Integrated survival estimates for cancer treatment delay among adults with cancer during the COVID-19 pandemic. JAMA Oncology. 2020;6:1881–1889. doi: 10.1001/jamaoncol.2020.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein D.A., Ratain M.J., Saltz L.B. Weight-based dosing of pembrolizumab every 6 Weeks in the time of COVID-19. JAMA Oncology. 2020;6:1694–1695. doi: 10.1001/jamaoncol.2020.2493. [DOI] [PubMed] [Google Scholar]
- 11.Thomson D.J., Yom S.S., Saeed H. Radiation fractionation schedules published during the COVID-19 pandemic: a systematic review of the quality of evidence and recommendations for future development. Int J Radiat Oncol Biol Phys. 2020;108:379–389. doi: 10.1016/j.ijrobp.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satish T., Raghunathan R., Prigoff J.G. Care delivery impact of the COVID-19 pandemic on breast cancer care. JCO Oncol Pract. 2021 Mar 19;OP2001062 doi: 10.1200/OP.20.01062. [DOI] [PubMed] [Google Scholar]
- 13.Lee L.Y.W., Cazier J.B., Starkey T. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jee J., Foote M.B., Lumish M. Chemotherapy and COVID-19 outcomes in patients with cancer. J Clin Oncol. 2020;38:3538–3546. doi: 10.1200/JCO.20.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinato D.J., Zambelli A., Aguilar-Company J. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Canc Discov. 2020;10:1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuderer N.M., Choueiri T.K., Shah D.P. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carreira H, Strongman H, Peppa M, et al: Prevalence of COVID-19-related risk factors and risk of severe influenza outcomes in cancer survivors: a matched cohort study using linked English electronic health records data. EClinicalMedicine 29, 2020. [DOI] [PMC free article] [PubMed]
- 18.Surgeons ACo . 2018. Standards for oncology Registry Entry. [Google Scholar]
- 19.Aziz N.M. Cancer survivorship research: state of knowledge, challenges and opportunities. Acta Oncol. 2007;46:417–432. doi: 10.1080/02841860701367878. [DOI] [PubMed] [Google Scholar]
- 20.Thronson L.R., Jackson S.L., Chew L.D. The pandemic of health care inequity. JAMA Network Open. 2020;3:e2021767. doi: 10.1001/jamanetworkopen.2020.21767. [DOI] [PubMed] [Google Scholar]
- 21.de Azambuja E., Trapani D., Loibl S. ESMO Management and treatment adapted recommendations in the COVID-19 era: breast Cancer. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanna T.P., Evans G.A., Booth C.M. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020;17:268–270. doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leach CR, Kirkland EG, Masters M, et al: Cancer survivor worries about treatment disruption and detrimental health outcomes due to the COVID-19 pandemic. J Psychosoc Oncol1–16, 2021. [DOI] [PubMed]
- 24.Battisti N.M.L., Mislang A.R., Cooper L. Adapting care for older cancer patients during the COVID-19 pandemic: recommendations from the international society of geriatric oncology (SIOG) COVID-19 working group. J Geriatr Oncol. 2020;11:1190–1198. doi: 10.1016/j.jgo.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones J.M., Saeed H., Katz M.S. J Natl Cancer Inst; 2020. Re-addressing the needs of cancer survivors during COVID-19: a path forward. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbruggen L.C., Wang Y., Armenian S.H. Guidance regarding COVID-19 for survivors of childhood, adolescent, and young adult cancer: a statement from the international late effects of childhood cancer guideline harmonization group. Pediatr Blood Canc. 2020;67 doi: 10.1002/pbc.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wosik J., Fudim M., Cameron B. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inf Assoc. 2020;27:957–962. doi: 10.1093/jamia/ocaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portnoy J., Waller M., Elliott T. Telemedicine in the era of COVID-19. J Allergy Clin Immunol Pract. 2020;8:1489–1491. doi: 10.1016/j.jaip.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marra A., Generali D., Zagami P. Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2. Ann Oncol. 2021;32:113–119. doi: 10.1016/j.annonc.2020.10.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Addeo A., Shah P.K., Bordry N. Cancer Cell; 2021. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thakkar A., Gonzalez-Lugo J.D., Goradia N. Cancer Cell; 2021. Seroconversion rates following COVID-19 vaccination among patients with cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Society A.C. American Cancer Society; Atlanta: 2019. Cancer treatment & survivorship Facts & Figures 2019-2021. [Google Scholar]
- 33.Jammu A.S., Chasen M.R., Lofters A.K. Systematic rapid living review of the impact of the COVID-19 pandemic on cancer survivors: update to August 27, 2020. Support Care Canc. 2020;1–10 doi: 10.1007/s00520-020-05908-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koczwara B. Cancer survivorship care at the time of the COVID-19 pandemic. Med J Aust. 2020;213:107–108. doi: 10.5694/mja2.50684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toss A., Isca C., Venturelli M. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill D.A., Friend S., Lomo L. Breast cancer survival, survival disparities, and guideline-based treatment. Breast Canc Res Treat. 2018;170:405–414. doi: 10.1007/s10549-018-4761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohri N., Rapkin B.D., Guha C. Radiation therapy noncompliance and clinical outcomes in an urban academic cancer center. Int J Radiat Oncol Biol Phys. 2016;95:563–570. doi: 10.1016/j.ijrobp.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 38.Ohri N., Rapkin B.D., Guha D. Predictors of radiation therapy noncompliance in an urban academic cancer center. Int J Radiat Oncol Biol Phys. 2015;91:232–238. doi: 10.1016/j.ijrobp.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Burki T.K. Cancer care in the time of COVID-19. Lancet Oncol. 2020;21(628) doi: 10.1016/S1470-2045(20)30201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.