Abstract
Purpose
Neoadjuvant chemotherapy (NACT) is increasingly adopted in the therapy of breast cancer (BC) patients with positive axillary nodes (cN+), but the reliability and feasibility of sentinel lymph node biopsy (SLNB) following NACT are still controversial. The objective of the present study is to conduct an updated meta-analysis on this issue.
Methods
A literature search was performed using PubMed, Cochrane, Embase, and Web of Science to identify papers published from January 1, 2000 to October 22, 2020 to research SLNB after NACT in BC patients. Studies that met the quality standard were enrolled for this meta-analysis.
Results
A total of 3578 participants from 27 trials were included in this meta-analysis. The pooled estimate of the identification rate (IR) for SLNB was 91 %, and the false negative rate (FNR) was 15 %. The pooled negative prediction value (NPV), accuracy, specificity, and sensitivity were 82 %, 89 %, 97 %, and 85 %, respectively. In subgroup analysis, the application of dual mapping could clearly decrease the FNR. The FNR was significantly high in the luminal types, and it declined as more sentinel lymph nodes (SLNs) were removed.
Conclusion
SLNB following NACT is now technically feasible for BC with cN+. However, it must be emphasized that the FNR is unacceptable high.
Keywords: Sentinel lymph node biopsy, Breast cancer, Preoperative chemotherapy
Highlights
-
•
We performed a meta-analysis to provide a consensus regarding the application of SLNB post-NACT in cN + patients.
-
•
One comprehensive database search yielded 27 studies (3578 patients).
-
•
The pooled estimate of IR for SLNB was 91 %, and FNR was 15 %.
-
•
The application of dual mapping could clearly decrease the FNR.
1. Introduction
Breast cancer (BC) is the most common malignancy among women, and its incidence has remained persistently high for years [1]. Globally, BC has become a severe threaten to women's physical and mental health. However, the widespread development of primary systemic therapy has revolutionized the management of BC. Under the premise of a guaranteed overall therapeutic effect, it is the major concern and future direction for breast surgery to narrow the extent of operation and enhance patients' quality of life.
Nowadays, neoadjuvant chemotherapy (NACT) is frequently administered to patients with operable BCs in an effort to shrink the tumor size, lower the tumor clinical stage, or increase the rate of breast-conserving surgery [2,3]. Since the number of lymph node positive (cN+) BCs initially treated with NACT has increased over the years, a higher pathological complete response (pCR) rate of axillary nodes has arisen [4]. Two multi-center retrospective studies revealed that axillary lymph node dissection (ALND) after effective NACT plays a limited role in preventing axillary relapse and improving long-term survival [5,6]. Thus, it is of great significance to explore the feasibility and reliability of sentinel lymph node biopsy (SLNB) instead of ALND after NACT for cN + patients. However, the lower identification rate (IR) and higher false negative rate (FNR) severely restrict its clinical application [[6], [7], [8], [9], [10], [11]]. It is generally thought that chemotherapy may give rise to changes in the lymphatic drainage channels [[12], [13], [14]]. In light of this, specific evaluation of the lymph node proven to contain metastases at the time of diagnosis will significantly contribute to the accuracy of nodal assessment after NACT and which is a logical addition to surgical staging. Targeted axillary dissection (TAD) is a procedure that involves SLND with removal of the marked node identified pretherapy as containing metastatic disease [7]. Multiple clinical trials have shown that TAD is one feasible option to decrease the FNR among BC patients with cN+ [7,[15], [16], [17], [18], [19], [20], [21]]. However, TAD is often impractical in general institutions, especially in most developing countries or areas, due to the costs, expertise and technological resources required. For the present, SLNB may be a more realistic and mature method.
Recently, Galimberti et al. reported recurrence and survival figures after received SLNB independent of TAD in initially cN + patients [22]. Data from their study implied that the application of standard SLNB alone is acceptable in cN + patients who become cN0 after NACT and even in the context of a high FNR, overt axillary disease and poorer prognosis are not necessarily increased.
The feasibility and safety of SLNB after NACT for BC are still controversial. We conducted an updated meta-analysis of the feasibility and reliability of SLNB after NACT in BC patients with cN + at initial diagnosis.
2. Methods
This meta-analysis was registered on International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY). The registration number is INPLASY2020110019; https://doi.org/10.37766/inplasy2020.11.0019, and the protocol is available in full on inplasy.com (https://doi.org/10.37766/inplasy00000000).
2.1. Search strategy
PubMed, Cochrane, Embase, and Web of Science were searched systematically for papers published from January 1, 2000 to October 22, 2020. We used a combination of Medical Subject Heading (MeSH) terms and free words as follows: (“breast neoplasms” OR “breast cancer” OR “mammary cancer” OR “breast carcinoma”) AND (“neoadjuvant chemotherapy” OR “preoperative chemotherapy” OR “new supplementary chemotherapy”) AND (“sentinel lymph node biopsy” OR “sentinel node biopsy”) AND (“axillary lymph node dissection” OR “axillary lymphadenectomy” OR “axillary lymph node excision”). The search was restricted to original English-language research papers. Two adjudicators separately scrutinized the titles and abstracts of all retrieved papers and then determined final eligibility.
2.2. Selection criteria
The inclusion criteria were as follows: First, BC patients were diagnosed with cN + via palpation or medical imaging, with or without histopathological diagnosis. In the meantime, all the patients received NACT. In addition, patients had to undergo SLNB subsequent to NACT, followed by ALND. Finally, included studies were required to report at least the IR of SLNs and the FNR of SLNB. The exclusion criteria were as follows: ALND was not performed subsequent to SLNB; radiotherapy or endocrine therapy was received before the operation; the full text could not be accessed; fewer than 20 cases were included; patients with distant metastasis; and the papers were reviews, letters, conference papers, meta-analyses or commentaries.
2.3. Outcome measures
Our core outcomes for this study were the IR of SLNs and the FNR of SLNB after NACT. Secondary outcomes included factors associated with the IR and FNR.
2.4. Quality assessment
Two researchers individually executed the literature quality evaluation, employing the Review Manager software risk assessment tool (RevMan 5.3; Cochrane Collaboration, Oxford, UK), in accordance with the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2); when opinions were inconsistent, a consensus was reached via discussion or consultation with a third party [23]. This meta-analysis was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24].
2.5. Data collection and definition
Data were extracted by one reviewer and audited independently for accuracy by another reviewer. Any discrepancies were resolved by way of team consensus. Data extraction included the author, year, research type, study design, number of cases, and outcome indicators. The IR was identified as the number of patients with smooth detection of SLNs divided by the overall number of patients. The FNR was computed as the number of false negative (FN) cases divided by the number of cases with any axillary nodal metastasis [ie, true positive (TP) cases plus FN cases]. Histopathological results of lymph nodes removed by ALND were considered to be the gold standard. We used the standard formulas as follows: NPV = TN/(FN + TN), sensitivity = TP/(TP + FN), specificity = TN/(TN + FP), and accuracy = (TP + TN)/all patients (where NPV = negative prediction value, TN = true negative, and FP = false positive).
2.6. Data analysis
Statistical analyses were performed by applying STATA version 15.0. For every study included, the effect size and overall pooled estimates were calculated [with the 95 % confidence interval (CI)]. A random-effects model and sensitivity analysis were used if there was heterogeneity among studies; otherwise, a fixed-effects model was used. Heterogeneity of effect sizes was computed using the I [2] index, which evaluated the degree of heterogeneity of individual results. An I [2] statistic greater than 75 % suggested considerable heterogeneity among the studies. Forest plots were used to visually display the results of the individual studies and pooled estimates. Publication bias was estimated via funnel plots. Values were considered statistically significant when the p-value was less than 0.05 (p < 0.05).
3. Results
3.1. Description of included studies
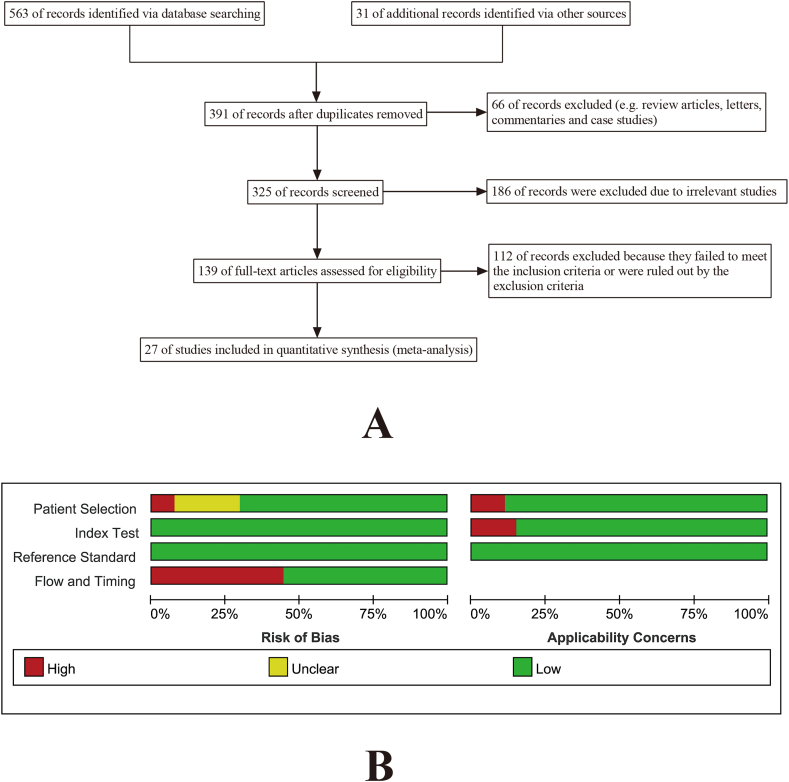
In accordance with the search strategy and study selection criteria, a total of 3578 participants (mean 133 per study, range: 26–637) in 27 trials were identified for inclusion in this meta-analysis (Fig. 1a) [5,[9], [10], [11],16,[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [47]]. The publication dates of these studies ranged from 2004 to 2020. The primary features of the included studies are recorded in Table 1 and Table 2. The reporting quality of the selected studies was generally good. Quality appraisal results are detailed in Fig. 1b.
Fig. 1.
(A)Flowchart of the systematic literature search and article selection. (B)Quality appraisal results according to QUADAS-2 for the included studies.
Table 1.
Features of included studies: design, patients, and tumor characteristics.
| Author (year) | Center | Design | Origin | T/N stage before NACT |
Method of pre-NAC node status assessment | |
|---|---|---|---|---|---|---|
| T stage | N stage | |||||
| [25] | Single | Prospective | USA | T1–T4 | N1–N3 | FNA |
| [26] | Single | Retrospective | Turkey | T0–T4 | N1–N2 | NR |
| [27] | Multiple | Prospective | Canada | T0–T3 | N1–N2 | FNA or CNB |
| [16] | Multiple | Prospective | USA | T0–T4 | N1–N2 | FNA or CNB |
| [28] | Single | Retrospective | USA | T1–T3 | N1–N3 | FNA |
| [29] | Single | Prospective | Italy | T2–T4 | N1–N3 | Palpation & Ultrasound |
| [30] | Single | Retrospective | India | T1–T4 | N1–N2 | MRI & Ultrasound |
| [31] | Single | Prospective | Korea | T1–T4 | N1–N3 | FNA |
| [5] | Single | Prospective | France | T1–T4 | N1–N2 | FNA |
| [32] | Multiple | Prospective | Japan | T1–T3 | N1 | FNA |
| [33] | Single | Retrospective | China | T1–T4 | N1–N3 | FNA |
| [34] | Single | Prospective | Korea | T1–T4 | N+ | NR |
| [9] | Single | Retrospective | Korea | T0–T3 | N+ | FNA |
| [10] | Multiple | Prospective | Germany | T1–T4 | N1–N2 | Palpation & Ultrasound |
| [36] | Single | Prospective | Korea | T1–T4 | N+ | FNA |
| [35] | Single | Prospective | Korea | NR | N1–N3 | PET/CT & Ultrasound |
| [11] | Single | Retrospective | USA | T1–T4 | N+ | FNA |
| [37] | Single | Retrospective | Turkey | T1–T4 | N1–N2 | FNA |
| [39] | Single | Retrospective | Korea | T1–T3 | N+ | FNA |
| [38] | Single | Retrospective | Korea | T1–T4 | N1–N3 | FNA |
| [40] | Single | Prospective | USA | T1–T4 | N1–N3 | FNA |
| [41] | Single | Retrospective | Japan | T1–T4 | N1–N3 | FNA |
| [42] | Single | Prospective | India | T2–T4 | N+ | FNA |
| [43] | Single | Prospective | China | T1–T3 | N1 | FNA |
| [44] | Single | Prospective | Japan | T0–T4 | N+ | FNA |
| [45] | Single | Prospective | China | NR | N+ | FNA |
| [47] | Multiple | Prospective | Sweden | T1–T4 | N+ | FNA |
Abbreviations: NACT, neoadjuvant chemotherapy; NR, not reported; FNA, fine needle aspiration; CNB, core needle biopsy; MRI, magnetic resonance imaging; PET/CT, positron emission tomography/computed tomography.
Table 2.
Features of included studies: sentinel lymph node biopsy technique results.
| Author (year) | Technique of SLNB |
Mean/median number of SLNs removed | ||||
|---|---|---|---|---|---|---|
| BD (1) | RI (2) | Dual (1 + 2) | CEUS (3) | Dual (1 + 3) | ||
| [25] | 15 | 19 | 116 | 0 | 0 | 2.60 |
| [26] | 0 | 94 | 0 | 0 | 0 | 1.60 |
| [27] | 0 | Mixed | 0 | 0 | 2.70 | |
| [16] | 28 | 116 | 545 | 0 | 0 | NR |
| [28] | Mixed | 0 | 0 | 2.00 | ||
| [29] | 0 | 64 | 0 | 0 | 0 | 1.70 |
| [30] | 30 | 0 | 0 | 0 | 0 | NR |
| [31] | Mixed | 0 | 0 | 5.00 | ||
| [5] | 0 | 0 | 307 | 0 | 0 | 2.00 |
| [32] | 7 | 0 | 136 | 0 | 0 | 1.60 |
| [33] | 8 | 7 | 36 | 0 | 0 | 2.50 |
| [34] | 9 | 33 | 12 | 0 | 0 | NR |
| [9] | 0 | 0 | 89 | 0 | 0 | 7.00 |
| [10] | 7 | 389 | 164 | 0 | 0 | 2.70 |
| [36] | Mixed | 0 | 0 | NR | ||
| [35] | 0 | 55 | 0 | 0 | 0 | 2.00 |
| [11] | 5 | 11 | 38 | 0 | 0 | 3.00 |
| [37] | 0 | 0 | 77 | 0 | 0 | 2.10 |
| [39] | 0 | 178 | 0 | 0 | 0 | 2.10 |
| [38] | Mixed | 0 | 0 | 4.00 | ||
| [40] | 5 | 7 | 69 | 0 | 0 | 2.00 |
| [41] | 1 | 0 | 106 | 0 | 0 | 1.50 |
| [42] | 30 | 0 | 0 | 0 | 0 | 1.57 |
| [43] | 0 | 0 | 0 | 0 | 66 | 3.13 |
| [44] | 0 | 0 | 95 | 0 | 0 | 2.00 |
| [45] | 48 | 0 | 0 | 0 | 0 | 1.48 |
| [47] | 7 | 10 | 168 | 0 | 0 | 2.00 |
Abbreviations: BD, blue dye; RI, radioisotopes; CEUS, contrast-enhanced ultrasound; NR, not reported; SLNB, sentinel lymph node biopsy; SLNs, sentinel lymph nodes.
Twenty-two studies were single-center studies [5,9,11,25,26,[28], [29], [30], [31],[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]], and five other studies were multi-center studies [10,16,27,32,47]. Seventeen of 27 studies were prospective studies [5,10,16,25,27,29,31,32,[34], [35], [36],40,[42], [43], [44], [45],47]. The others were retrospective studies [9,11,26,28,30,33,[37], [38], [39],41]. Seven studies were from Korea [9,31,[34], [35], [36],38,39], five were from the United States [11,16,25,28,40], three were from China [33,43,45], three were from Japan [32,41,44], two were from India [30,42], two were from Turkey [26,37], and one each was from France, Germany, Italy, Canada, and Sweden. Four out of 27 studies did not confirm the axillary status of patients by fine needle aspiration (FNA) or core needle biopsy (CNB) [10,29,30,35]. In terms of mapping agents, blue dye (BD) alone was applied across three studies [30,42,45], radioisotopes (RI) alone were used in four studies [26,29,35,39], both tracers were used in four studies [5,9,36,44], and mixed means were used in the remaining studies. The mean or median number of SLNs retrieved in each study is displayed in Table 2.
3.2. Performance of post-NACT SLNB
3.2.1. IR of SLNB
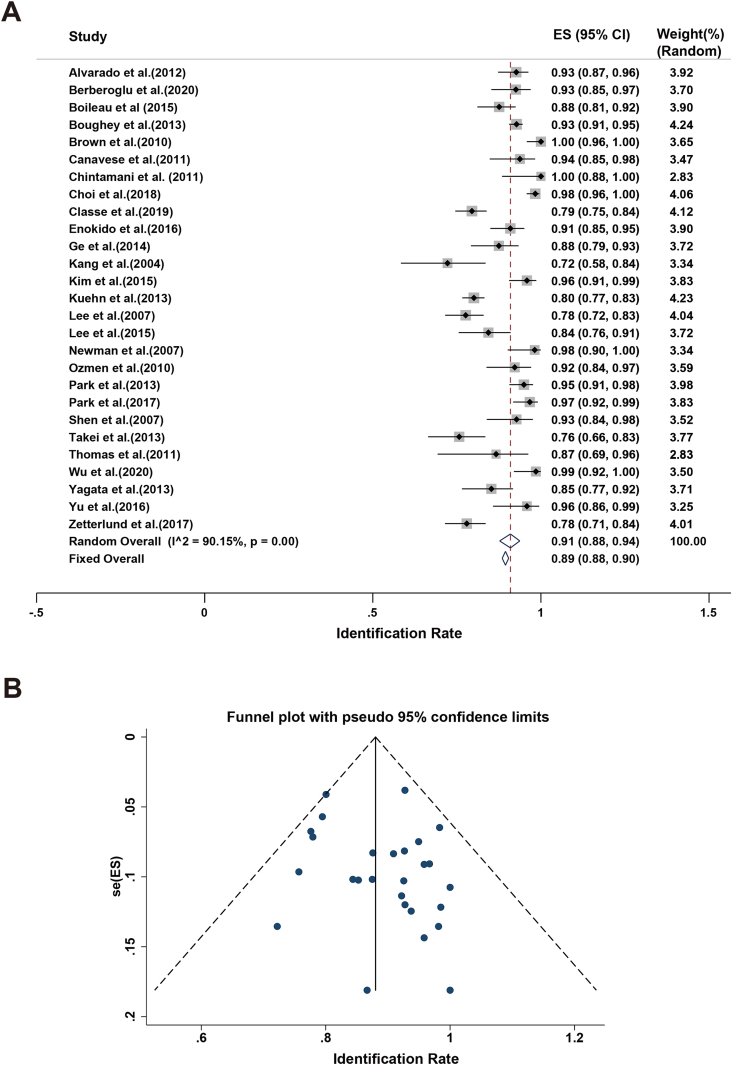
In the studies presented here, the IR of SLNB fluctuated from 72 % to 100 %. The minimal rate was reported by Kang and coworkers [34]. The highest rate was reported by Brown and Chintamani and their research teams [28,30]. The sample sizes were relatively small in the three studies mentioned above. A high level of heterogeneity (I2 = 90.15 % and p-value for Q test < 0.1) was found. A random-effects model was employed to estimate the pooled IR, with a result of 91 % (95%CI 88%–94 %; Fig. 2a). In addition, the sensitivity analysis showed that the results of each study were stable. No indication of substantial publication bias (Fig. 2b) was observed.
Fig. 2.
Meta-analysis of the IR: (A) Forest plot of the IR. The width of the horizontal line represents the 95%CI of individual studies. The vertical dotted line represents the overall expected IR. The combined estimate of IR was 91 % (95%CI: 88%–94 %, I2 = 90.15 %). (B) Funnel plot to assess the publication bias effect on the IR. Each dot represents a separate study. The funnel plot revealed no apparent evidence of publication bias.
3.2.2. FNR of SLNB
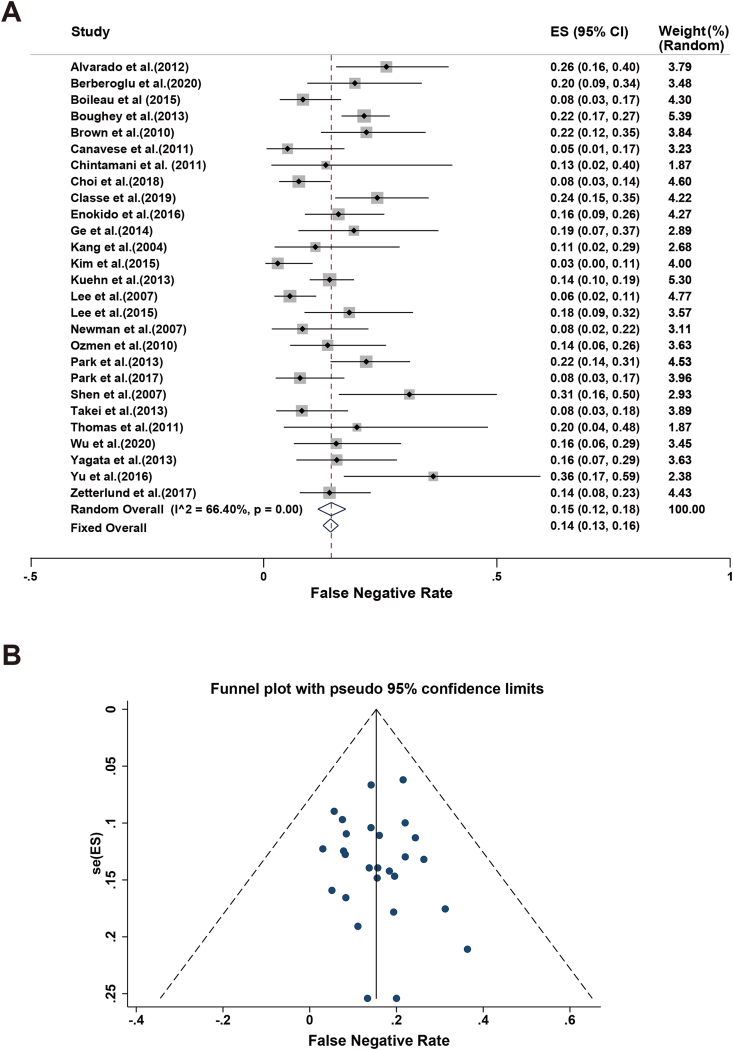
The FNR of SLNB in the 27 included studies varied from 3 % to 36 %. The lowest FNR was reported by researchers from Korea [9], whereas the highest was reported by a Chinese study [45]. Because of high heterogeneity among the included studies (I2 = 66.40 % and p-value for Q test < 0.1), a random-effects model was adopted to assess the FNR. The combined FNR estimate was found to be 15 % (95%CI 12%–18 %; Fig. 3a), with no evidence of publication bias (Fig. 3b). Meanwhile, we conducted sensitivity analyses for the 27 studies mentioned above. No interference of literature with the outcomes was identified, meaning that the meta-analysis had good reliability.
Fig. 3.
Meta-analysis of the FNR: (A) Forest plot of the FNR. The width of the horizontal line represents the 95%CI of individual studies. The vertical dotted line represents the overall expected IR. The combined estimate of FNR was 15 % (95%CI: 12%–18 %, I2 = 66.40 %). (B) Funnel plot to assess the publication bias effect on the FNR. Each dot represents a separate study. The funnel plot revealed no apparent evidence of publication bias.
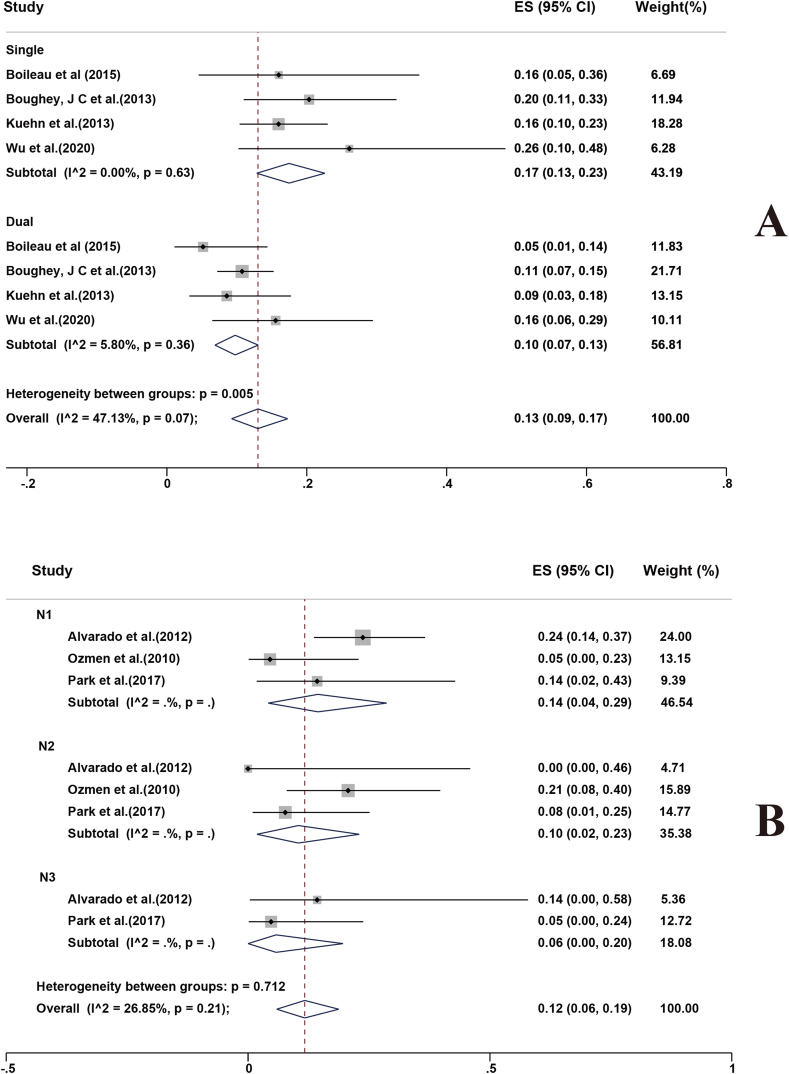
Four studies analyzed the FNRs of single and dual tracers for SLNB following NACT [10,16,27,43]. The combined estimate of FNR for a single tracer was 17 % (95%CI 13%–23 %), compared with 10 % (95%CI 7%–13 %) with dual mapping (Fig. 4a). The study results were homogeneous (I2 = 47.13 %, p = 0.07). Moreover, it has been reported that the FNR of the amalgamation of utilizing BD and contrast-enhanced ultrasonography (CEUS) was considerably lower than that of BD alone (15.6 % vs. 26.1 %) [45].
Fig. 4.
Forest plots displaying FNRs: (A) single and dual mapping techniques for sentinel lymph node biopsy, and (B) N stages for patients. Rates are shown with 95%CIs.
Three studies reported the FNRs of different N-stages of BC patients with cN + at initial diagnosis post-NACT [25,37,38]. For stages N1, N2, and N3, the combined FNR estimates were 14 % (95%CI 4%–29 %), 10 % (95%CI 2%–23 %), and 6 % (95%CI 0%–20 %), respectively (Fig. 4b). The study results were homogeneous (I2 = 26.85 %, p = 0.21).
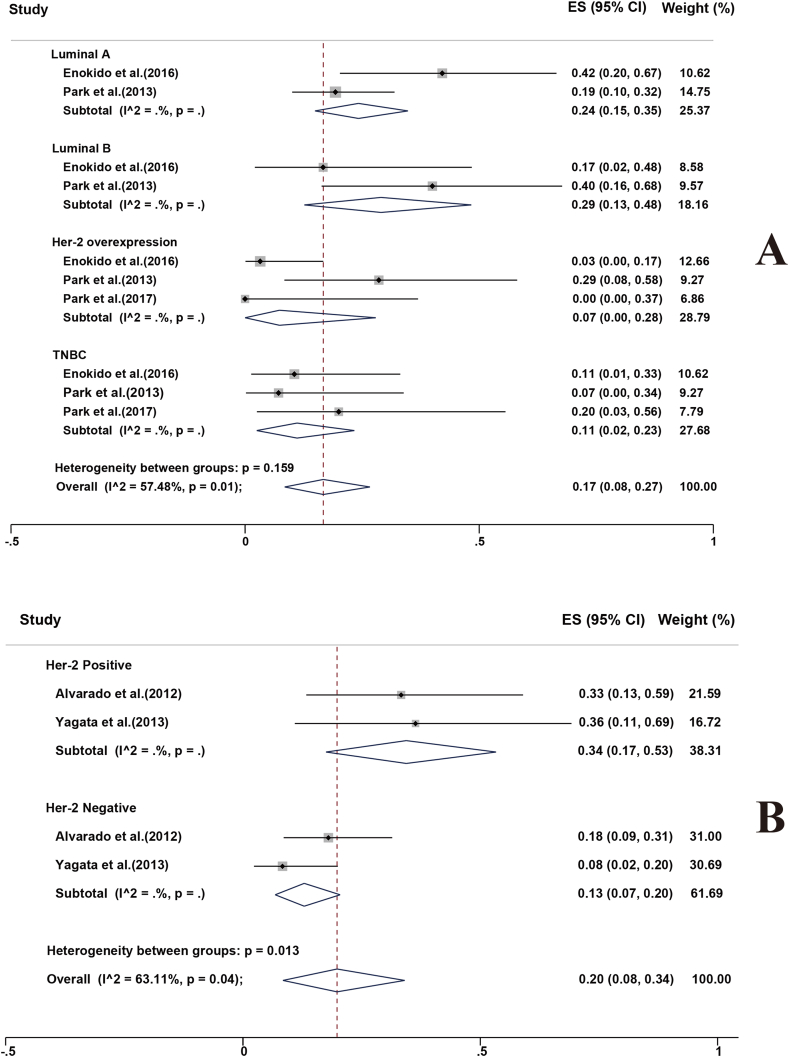
Several studies analyzed the FNRs of different molecular subtypes of BC patients with cN + at initial diagnosis post-NACT [32,38,39]. The combined estimates were determined as follows: Luminal A, 24 % (95%CI 15%–35 %); Luminal B, 29 % (95%CI 13%–48 %); Her-2 overexpression, 7 % (95%CI 0%–28 %); and triple negative breast cancer (TNBC), 11 % (95%CI 2%–23 %; Fig. 5a). The FNR was significantly higher in the luminal type than that in the non-luminal type.
Fig. 5.
Forest plots displaying FNRs: (A) molecular subtypes for patients, and (B) Her-2 status for patients. Rates are shown with 95%CIs.
Two studies reported the FNRs of different human epidermal growth factor receptor 2 (Her-2) statuses of BC patients with cN + at initial diagnosis post-NACT [25,44]. The combined estimate of FNR for Her-2 positive breast cancer was 34 % (95%CI 17%–53 %), compared with 13 % (95%CI 7%–20 %) for Her-2 negative breast cancer (Fig. 5b).
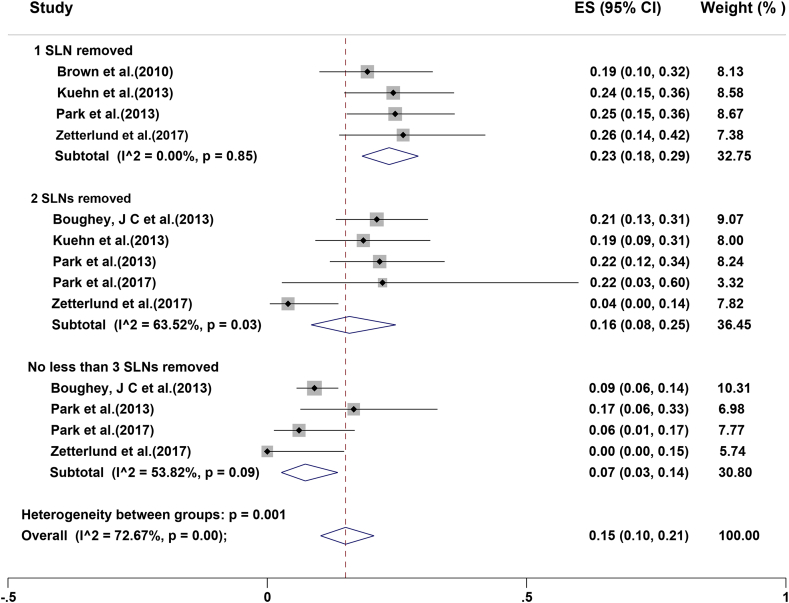
Five studies analyzed the correlation of FNR and the number of SLNs retrieved after NACT (Fig. 6) [7,28,38,39,47]. If one SLN was resected, the combined estimate of FNR was 23 % (95%CI 18%–29 %), while it was 16 % (95%CI 8%–22 %) when two SLNs were dissected and 7 % (95%CI 3%–14 %) with removal of no less than three SLNs.
Fig. 6.
Forest plots displaying FNR by number of sentinel lymph nodes removed after neoadjuvant chemotherapy. Rates are shown with 95%CIs.
3.2.3. NPV, accuracy, specificity, and sensitivity of SLNB
In addition to the IR and FNR, four other performance indices of SLNB were analyzed, which were as follows: NPV, accuracy, specificity, and sensitivity (Table 3). Overall, the NPV, accuracy, specificity, and sensitivity differed in the 27 studies, and they were found in the ranges of 62%–94 %, 59%–98 %, 51%–100 %, and 64%–97 %, respectively. The combined NPV, accuracy, specificity, and sensitivity were 82 % (95%CI 79%–85 %), 89 % (95%CI 84%–92 %), 97 % (95%CI 92%–100 %), and 85 % (95%CI 82%–88 %), respectively.
Table 3.
Test performance of SLNB after NAC in individual studies.
| Author (year) | Number of Patients | IR | FNR | NPV | Accuracy | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|
| [25] | 111 | 93 % | 26 % | 72 % | 73 % | 72 % | 74 % |
| [26] | 87 | 93 % | 20 % | 82 % | 90 % | 100 % | 80 % |
| [27] | 127 | 88 % | 8 % | 86 % | 94 % | 100 % | 92 % |
| [16] | 637 | 93 % | 22 % | 82 % | 72 % | 68 % | 78 % |
| [28] | 86 | 100 % | 22 % | 67 % | 84 % | 96 % | 78 % |
| [29] | 60 | 94 % | 5 % | 91 % | 97 % | 100 % | 95 % |
| [30] | 30 | 100 % | 13 % | 88 % | 93 % | 100 % | 87 % |
| [31] | 234 | 98 % | 8 % | 94 % | 94 % | 95 % | 92 % |
| [5] | 244 | 79 % | 24 % | 82 % | 59 % | 51 % | 76 % |
| [32] | 130 | 91 % | 16 % | 79 % | 90 % | 100 % | 84 % |
| [33] | 43 | 88 % | 19 % | 67 % | 86 % | 100 % | 81 % |
| [34] | 39 | 72 % | 11 % | 80 % | 92 % | 100 % | 89 % |
| [9] | 84 | 96 % | 3 % | 90 % | 98 % | 100 % | 97 % |
| [10] | 474 | 80 % | 14 % | 89 % | 93 % | 100 % | 86 % |
| [36] | 170 | 78 % | 6 % | 87 % | 96 % | 100 % | 94 % |
| [35] | 81 | 84 % | 18 % | 78 % | 89 % | 100 % | 82 % |
| [11] | 53 | 98 % | 8 % | 85 % | 94 % | 100 % | 92 % |
| [37] | 71 | 92 % | 14 % | 74 % | 90 % | 100 % | 86 % |
| [39] | 169 | 95 % | 22 % | 76 % | 87 % | 100 % | 78 % |
| [38] | 117 | 97 % | 8 % | 91 % | 96 % | 100 % | 92 % |
| [40] | 56 | 93 % | 31 % | 62 % | 68 % | 67 % | 69 % |
| [41] | 103 | 76 % | 8 % | 89 % | 95 % | 100 % | 92 % |
| [42] | 26 | 87 % | 20 % | 73 % | 77 % | 73 % | 80 % |
| [43] | 67 | 99 % | 16 % | 75 % | 89 % | 100 % | 84 % |
| [44] | 81 | 85 % | 16 % | 79 % | 90 % | 100 % | 84 % |
| [45] | 46 | 96 % | 36 % | 75 % | 83 % | 100 % | 64 % |
| [47] | 152 | 78 % | 14 % | 82 % | 91 % | 100 % | 86 % |
Abbreviations: IR: identification rate, FNR: false negative rate; NPV: negative predictive value.
4. Discussion
It has been a longstanding consensus that patients with cN + typically undergo ALND, and patients with clinically negative (cN−) nodes may undergo SLNB. Nearly 40%–50 % of cN + patients can achieve complete pathological response of axillary nodes after NACT [16,39,48]. Supposing that such patients receive SLNB rather than ALND, related complications may be avoided. As a result, it is necessary for us to validate the feasibility and reliability of SLNB in BC patients with cN + after NACT.
In our latest meta-analysis, we pooled the largest series of studies (N = 27) and patients (N = 3578) ever reviewed in the feasibility of SLNB in BC patients with cN + after NACT. Generally speaking, we performed one comprehensive database search using a strict, standardized method to identify all studies meeting the inclusion criteria.
This study detailed the two essential test performance parameters in SLNB—IR and FNR. The IR of SLNB determines whether ALND can be replaced effectively. The reliability of SLNB is reflected in whether FNR can be controlled within acceptable limits. The combined estimate for IR was found to be 91 %, which is close to similar prior research (89 %, 90.9 %, 90 %, 89.6 %) [8,[49], [50], [51]], but it is below the rate recorded for node-negative BC patients post-NACT (96 %, 94 %) and cN− patients without NACT (95 %) [[52], [53], [54]]. A pooled estimate of 15 % for FNR can be found in our results, which is in general agreement with previous meta-analyses (13 %, 14 %,14 %, 14.2 %) [8,[49], [50], [51]]. However, our FNR is still inferior to that of cN− patients following NACT (6 %, 7 %) or early-stage BC without NACT (7.3 %) [[52], [53], [54]]. Overall, SLNB seems feasible for patients with node-positive BC after NACT. However, the reliability of this procedure remains to be verified. Below, we systematically discuss the two indexes mentioned above.
4.1. IR of SLNB
The success of SLNB depends strongly on the ability to identify the “true” SLNs. Hence, IR is a crucial determining factor for SLNB. In light of significant heterogeneity identified among the included trials, we must consider the mapping method. From four studies that used both BD and RI, the pooled IR was found to be 89 % (95%CI 79%–96 %) [5,9,37,44]. The pooled IR from three studies that only used BD was 96 % (95%CI 86%–100 %) [30,42,45]. For another four studies that employed RI only, the pooled IR was 92 % (95%CI 87%–96 %) [26,29,35,39]. No significant difference in IR of SLN was noted between the different mapping means used (p = 0.55, Table 4). However, in the American College of Surgeons Oncology Group (ACOSOG) Z1071 trial, the IR was remarkably influenced by the mapping technique [16]. The utilization of dual tracers may improve not only the IR but also the FNR. In our study, no consensus was reached concerning the mapping approach. This finding is in line with the results of one meta-analysis [54]. Accordingly, we cannot appraise its impact on the IR.
Table 4.
IR of SLN according to mapping technique.
| Method | Number of studies | Number of patients SLN identified | Number of patients SLNB attempted | IR (95 % CI) |
|---|---|---|---|---|
| BD | 3 | 102 | 108 | 89 % (79%–96 %) |
| RI | 4 | 397 | 432 | 96 % (86%–100 %) |
| BD + RI | 4 | 511 | 599 | 92 % (87%–96 %) |
Abbreviation: IR: identification rate; BD: blue dye; RI, radioisotopes; SLNB: sentinel lymph node biopsy; SLN, sentinel lymph nodes.
It is noteworthy that a team from China made the first attempt to combine the use of BD and CEUS [43]. This study shows that the IR of the combination method (98.5 %) was much better than that of using BD alone (84.6 %). As mentioned in the introduction, chemotherapy could bring about the fibrosis of lymphatic drainage channels, which then induces difficulty in identifying SLNs. With the aid of CEUS, real-time displays for lymphatic pathways are achieved. On top of this, SLNs may be accurately located. This study affords a novel idea for further work.
On the one hand, tumor lymphatic metastasis may modify or block the lymphatic pathway. On the other, NACT can reroute the lymphatic reflux system by virtue of atrophy and fibrosis of lymphatics, as well as by impeding lymphatic vessels. Given that both factors can trigger a lower IR of SLNB, the application of dual tracers probably helping to guarantee the successful identification of SLNs.
4.2. FNR of SLNB
The FNR is a fundamental indicator of reliability for SLNB. The American Society of Clinical Oncology Clinical Practice Guideline Update Committee considers the FNR of SLNB after NACT in cN + BC patients (from 10 % to 30 %) is unacceptable [55]. Potential risk from omitting node metastases involve under-staging the patient and an increased hazard of carcinoma recurrence [56]. Consequently, it is necessary to analyze the potential factors that may have affected the FNR as closely as possible.
For SLNB in early-stage BC, the use of dual tracers is associated with an almost significant trend toward a lower FNR [57]. In our analysis, three studies contrasted the FNR of dual versus single tracers, and the combined estimate was 10 % versus 17 % (Fig. 5a). The FNR with dual-agent mapping reported in this study is similar to that of the ACOSOG Z1071 Clinical Trial [16]. The SENTinel NeoAdjuvant (SENTINA) Trial also reported that the combination of BD and RI enables FNR within an acceptable limit [10]. In the Sentinel Node Biopsy Following Neoadjuvant Chemotherapy (SN FNAC) study, the application of dual tracers (BD + RI) was correlated with a threefold decline in FNR when compared with RI alone [27]. As with the IR, one study from China confirmed that the FNR for the combination method (BD + CEUS) was significantly lower than that for the single BD method (15.6 % vs. 26.1 %) [43]. Because of the high IR and low FNR, BD + CEUS provides a new option for SLNB. There is still much room left for future study.
Based on obtainable data, axillary nodal status before NACT (N1–N3) appears to be negatively associated with FNR, although the confidence interval was substantially wide (Fig. 4b). For stages N1, N2, and N3, the combined estimates of FNR were 14 %, 10 %, and 6 %, respectively. Possible explanations for this are as follows: The higher the N stage, the more severe the lymphatic metastasis will be and the lower the likelihood of false negative results.
According to the hormonal receptor status and Her-2 status, BC is categorized into several molecular subgroups as follows: Luminal A, Luminal B, Her-2 overexpression, and TNBC. Each subtype displays varying sensitivity to different chemotherapeutic agents. In this study, we found that the FNR was significantly lower in TNBC and Her-2 overexpression BC patients. Based on this, we recommend that post-NACT SLNB could be conducted in a chosen group of patients, such as non-luminal-type BCs. Similar recommendations were also suggested by Enokido and Park [32,39]. Larger prospective studies are needed to validate this finding.
In the subgroup analysis, 2 of the 27 teams reported that the FNR was markedly lower in the Her-2 negative breast cancer group than it was in the Her-2 positive breast cancer group [25,44]. Alvarado reported a considerable alteration in FNR depending on the Her-2 condition, with an considerably lower FNR seen in the Her-2-negative group when contrasted with the Her-2-positive group (18 % vs. 33.3 %) [25]. Similar results have been reported by other researchers [44]. Given the relatively small sample size of the two trials discussed above, the results from such studies should be interpreted with caution. Larger studies are warranted to attest to the authenticity of this phenomenon.
The FNR is directly correlated to the number of SLNs resected [58]. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial, removal of two sentinel nodes rather than one almost halved the FNR [59]. Table 2 shows that the mean or median number of SLNs resected in each study fluctuated from 1.48 to 7, and less than three SLNs were dissected in a considerable proportion. The SN FNAC study has demonstrated that, if only one SLN was dissected, the FNR was 18.2 %; however, the FNR dropped to 4.9 % when two or more SLNs were removed [27]. In the present study, the pooled estimate of FNR was 23 % with one SLN removed, 6 % with two SLNs dissected, and 7 % with the removal of no less than three SLNs. All the above results indicated that SLNB is less accurate in the setting of cN + patients who undergo SLNB after NACT, particularly if merely one or two SLNs are identified. However, in real clinical scenarios, because of the number of SLNs cannot be predicted in advance, it is not always possible to identify and resect three or more SLNs. To illustrate this point, 57 % of patients had three or more SLNs in the ACOSOG Z1071 Clinical Trial [16], whereas only 34 % did in the SENTINA Trial [10]. There is also need to recognise the tracer molecules are rather small, and therefore they can quickly diffuse through SLNs. Upon that, a marked node may not be the real SLN, but instead a level II or even level III, non-SLN. Consequently, non-SLNs could incorrectly be identified as SLNs, causing more “innocent” nodes to be excised and false-negative staging. The number of stained lymph nodes is time-dependent [60], and the FNR is directly related to the number of SLNs removed [58]. In order to reduce FNR, surgeons may remove a higher number of non-SLNs and designate them as SLNs. Herein, it must be noted that removing more SLNs only contributes to lower FNR when they are true SLNs; the results should not be an incentive to remove 3 or more nodes when there are only 1–2 identified by the tracer technique.
How can the safety of post-NACT SLNB be further improved? We think that the crux in overcoming this challenge lies in reducing the FNR as much as possible. Usually, axillary nodes are biopsied using preoperative axillary ultrasonography. However, crucially, it is unknown to the surgeons whether such nodes are SLNs. It appears likely that precise localization of suspicious lymph nodes before NACT are of high importance to successful node detection. Currently, several approaches can be used to identify the positioning of confirmed metastatic axillary lymph nodes (ALN), such as a marking clip, ALN tattooing, or radioiodine/magnetic seed localization.
A clip seems to be a good option. So far, the largest amount of papers has been published on clip-based TAD [61]. However, it is difficult to determine the clip's position accurately if imaging equipment is not available in the operating room. Other disadvantages include: 1)Visibility of some clips may decrease over time, 2) Reaction of the node tissue to the clip may be misinterpreted on pathological examination and so on [61]. Pre-NACT tattoo marking may be an alternative method for marking biopsied ALNs. This method is relatively unsophisticated, and the substance used for tattooing is economical. Black-stained nodes can be observed with the naked eye, and no special equipment is required. More importantly, the safety and accuracy have been proven previously [21,62]. The main limitations of this method are the inevitable learning curve and the possible permanent tattooing of local skin. What's more, another disadvantage of this technique is the inability to further interrogate the axillary contents for the marked node if the tattoo is not seen at the time of surgery [7]. Finally, guided by ultrasound, Donker attempted to mark ALN with radioactive iodine seeds (the MARI procedure) for axillary staging after NACT in BC patients [18]. After NACT, marked nodes were selectively removed using a γ-detection probe and subsequent ALND was performed. The results demonstrated that the IR and FNR of this approach were 97 % and 7 %, respectively. A similar study was performed by Caudle [7]. Another research on Radioactive Iodine Seed placement in the Axilla with SLNB(RISAS) after NACT was reported in the 2020 San Antonio Breast Cancer Symposium [63]. This is one prospective, multicenter trial included cT1-4N1,2,3b patients treated with NACT. A total of 227 pathologically proven cN + BC patients underwent the RISAS procedure. The IR of the RISAS procedure is 98 % (223/227). Preliminary analysis shows a FNR of approximately 5 % and a NPV of approximately 91 %. Compared with either the SLNB alone or the excision of the marked lymph node alone, the combination of SLNB with excision of the marked lymph node can improve the IR and the accuracy. The previous work showed that staining and selectively retrieving metastatic lymph nodes after NACT can help to decrease the FNR.
Generally, immunohistochemistry (IHC) analysis is not routinely performed in the pathological examination of SLN for BC, but Boileau reported that a low FNR can be realized by using mandatory IHC for SLN evaluation [27]. In the SN FNAC study, if isolated tumor cell (ITC) clusters were considered negative, the FNR of post-NACT SLNB rose to 13.3 %. By contrast, if ITCs, micrometastases, and macrometastases were considered positive, the FNR dropped to 8.4 %. Similar results were also found in the ACOSOG Z1071 trial [16]. Based on this, SLN metastases of any size are significant and should be considered positive. SLN evaluation with IHC appears to be a feasible, reliable alternative for reducing the FNR of SLNB, and it warrants further research. A similar discovery was formerly reported by two meta-analyses [8,54]. Aside from that, the experience of the operator should never be underrated. Research has shown that the learning curve is strongly linked to the FNR and IR for SLNB following NACT [64].
However, some scholars believe that even FNR is an important measure of accuracy, all efforts to reduce the FNR does not have clinical prognostic significance [22]. These researchers also considered a supposed high FNR should not be used a priori in SLNB decisions. Considering that this is a single instruction ten-year follow-up, further prospective and multicenter trials are needed. Fortunately, the European Breast Cancer Research Association of Surgical Trialists (EUBREAST) has initiated AXSANA (Axillary Surgery After Neoadjuvant Treatment). This multinational prospective cohort study was designed with the aim of assessing the impact of different surgical staging procedures in the axilla on the oncologic outcome and on health-related quality of life [61]. The results of AXSANA trial are awaited with interest.
5. Limitations
The potential drawbacks of this study are as follows: First, this meta-analysis only included studies published in English, possibly resulting in a publication bias. Second, a potential limitation of this study was heterogeneity between the included studies. For instance, several studies did not verify axillary status via histopathological examination. These trials depended on physical or imaging examination [10,29,30,35]. Because diagnostic accuracy varies markedly using different methodologies, an issue of misappraisal of SLNB may exist in this study. Third, we used a random-effects model instead of a fixed-effects model, which may have affected the reliability of our results.
6. Conclusion
Based on the meta-analysis of currently available evidence, we suggest that SLNB is technically viable for BC patients with cN+. However, it must be emphasized that the FNR is unacceptable high.
Author contributions
Siyang Cao: Conceptualization, data curation, investigation, formal analysis and writing original draft. Xia Liu: Data curation, investigation and formal analysis. Junwei Cui: Reviewing and editing. Xiaoling Liu: Methodology. Jieyu Zhong: Supervision.Zijian Yang: Software. Wei Wei & Desheng Sun: Project administration and funding acquisition.
Funding
This study was backed by grants from the Shenzhen San-Ming Project (No. SZSM201612010) and Shenzhen Key Medical Discipline Construction Fund(No. SZXK017)
Declaration of competing interest
The authors confirm that this article content involves no conflicts of interest.
Availability of data and material
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Some or all data, models, or code generated or used during the study are available from the corresponding author by request.
Ethical approval
This study involved a meta-analysis; thus, no ethical approval or informed consent was required.
Consent to participate
This study involved a meta-analysis; thus, no medical informed consent was required.
Consent for publication
This study involved a meta-analysis; thus, no informed consent was required.
Acknowledgments
We are grateful all authors of the papers listed in Table 1 for making their data accessible. And special thanks to Scribendi for the language assistance regarding this study.
Contributor Information
Desheng Sun, Email: szdssun@163.com.
Wei Wei, Email: rxwei1123@163.com.
References
- 1.Siegel R., Miller K., Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Saw S., Lim J., Lim S., Wong M., Lim C., Yap Y. Patterns of relapse after neoadjuvant chemotherapy in breast cancer: implications for surveillance in clinical practice. Breast Canc Res Treat. 2019;177(1):197–206. doi: 10.1007/s10549-019-05290-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhu S., Li Y., Wang Y. Efficacy of neoadjuvant chemotherapy and Annexin A3 expression in breast cancer. Journal of BUON : official journal of the Balkan Union of Oncology. 2019;24(2):522–528. [PubMed] [Google Scholar]
- 4.Fisher B., Brown A., Mamounas E. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol : official journal of the American Society of Clinical Oncology. 1997;15(7):2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 5.Classe J., Loaec C., Gimbergues P. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Canc Res Treat. 2019;173(2):343–352. doi: 10.1007/s10549-018-5004-7. [DOI] [PubMed] [Google Scholar]
- 6.Kang Y., Han W., Park S. Outcome following sentinel lymph node biopsy-guided decisions in breast cancer patients with conversion from positive to negative axillary lymph nodes after neoadjuvant chemotherapy. Breast Canc Res Treat. 2017;166(2):473–480. doi: 10.1007/s10549-017-4423-1. [DOI] [PubMed] [Google Scholar]
- 7.Caudle A., Yang W., Krishnamurthy S. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2016;34(10):1072–1078. doi: 10.1200/JCO.2015.64.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu J., Chen H., Yang J., Yi C., Zheng S. Feasibility and accuracy of sentinel lymph node biopsy in clinically node-positive breast cancer after neoadjuvant chemotherapy: a meta-analysis. PloS One. 2014;9(9) doi: 10.1371/journal.pone.0105316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J., Kim M., Lee J. Sentinel lymph node biopsy alone after neoadjuvant chemotherapy in patients with initial cytology-proven axillary node metastasis. Journal of breast cancer. 2015;18(1):22–28. doi: 10.4048/jbc.2015.18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuehn T., Bauerfeind I., Fehm T. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 11.Newman E., Sabel M., Nees A. Sentinel lymph node biopsy performed after neoadjuvant chemotherapy is accurate in patients with documented node-positive breast cancer at presentation. Ann Surg Oncol. 2007;14(10):2946–2952. doi: 10.1245/s10434-007-9403-y. [DOI] [PubMed] [Google Scholar]
- 12.Sharkey F., Addington S., Fowler L., Page C., Cruz A. Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol : an official journal of the United States and Canadian Academy of Pathology, Inc. 1996;9(9):893–900. [PubMed] [Google Scholar]
- 13.Sugie T., Sawada T., Tagaya N. Comparison of the indocyanine green fluorescence and blue dye methods in detection of sentinel lymph nodes in early-stage breast cancer. Ann Surg Oncol. 2013;20(7):2213–2218. doi: 10.1245/s10434-013-2890-0. [DOI] [PubMed] [Google Scholar]
- 14.Tsuyuki S., Yamaguchi A., Kawata Y., Kawaguchi K. Assessing the effects of neoadjuvant chemotherapy on lymphatic pathways to sentinel lymph nodes in cases of breast cancer: usefulness of the indocyanine green-fluorescence method. Breast. 2015;24(3):298–301. doi: 10.1016/j.breast.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Boughey J., Ballman K., Le-Petross H. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (alliance) Ann Surg. 2016;263(4):802–807. doi: 10.1097/SLA.0000000000001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boughey J., Suman V., Mittendorf E. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. J Am Med Assoc. 2013;310(14):1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caudle A., Yang W., Mittendorf E. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA surgery. 2015;150(2):137–143. doi: 10.1001/jamasurg.2014.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donker M., Straver M., Wesseling J. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378–382. doi: 10.1097/SLA.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 19.Flores-Funes D., Aguilar-Jiménez J., Martínez-Gálvez M. Validation of the targeted axillary dissection technique in the axillary staging of breast cancer after neoadjuvant therapy: preliminary results. Surgical oncology. 2019;30:52–57. doi: 10.1016/j.suronc.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Kanesalingam K., Sriram N., Heilat G. Targeted axillary dissection after neoadjuvant systemic therapy in patients with node-positive breast cancer. ANZ J Surg. 2020;90(3):332–338. doi: 10.1111/ans.15604. [DOI] [PubMed] [Google Scholar]
- 21.Natsiopoulos I., Intzes S., Liappis T. Axillary lymph node tattooing and targeted axillary dissection in breast cancer patients who presented as cN+ before neoadjuvant chemotherapy and became cN0 after treatment. Clin Breast Canc. 2019;19(3):208–215. doi: 10.1016/j.clbc.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Kahler-Ribeiro-Fontana S., Pagan E., Magnoni F. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2021;47(4):804–812. doi: 10.1016/j.ejso.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Whiting P., Rutjes A., Westwood M. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarado R., Yi M., Le-Petross H. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol. 2012;19(10):3177–3184. doi: 10.1245/s10434-012-2484-2. [DOI] [PubMed] [Google Scholar]
- 26.Berberoglu K., Erdemir A., Rasa K., Baloglu H., Cakmakci M. Role of gamma probe-assisted intraoperative sentinel lymph node evaluation in predicting axillary breast cancer metastasis after neoadjuvant chemotherapy. Nucl Med Commun. 2020;41(2):120–125. doi: 10.1097/MNM.0000000000001111. [DOI] [PubMed] [Google Scholar]
- 27.Boileau J., Poirier B., Basik M. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2015;33(3):258–264. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 28.Brown A., Hunt K., Shen J. Histologic changes associated with false-negative sentinel lymph nodes after preoperative chemotherapy in patients with confirmed lymph node-positive breast cancer before treatment. Cancer. 2010;116(12):2878–2883. doi: 10.1002/cncr.25066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canavese G., Dozin B., Vecchio C. Accuracy of sentinel lymph node biopsy after neo-adjuvant chemotherapy in patients with locally advanced breast cancer and clinically positive axillary nodes. Eur J Surg Oncol : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2011;37(8):688–694. doi: 10.1016/j.ejso.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Chintamani Tandon M., Mishra A., Agarwal U., Saxena S. Sentinel lymph node biopsy using dye alone method is reliable and accurate even after neo-adjuvant chemotherapy in locally advanced breast cancer--a prospective study. World J Surg Oncol. 2011;9:19. doi: 10.1186/1477-7819-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi H., Kim I., Alsharif E. Use of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with axillary node-positive breast cancer in diagnosis. Journal of breast cancer. 2018;21(4):433–441. doi: 10.4048/jbc.2018.21.e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enokido K., Watanabe C., Nakamura S. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with an initial diagnosis of cytology-proven lymph node-positive breast cancer. Clin Breast Canc. 2016;16(4):299–304. doi: 10.1016/j.clbc.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Ge W., Yang B., Zuo W. Sentinel lymph node biopsy does not apply to all axillary lymph node-positive breast cancer patients after neoadjuvant chemotherapy. Thoracic cancer. 2014;5(6):550–555. doi: 10.1111/1759-7714.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang S., Kim S., Kwon Y. Decreased identification rate of sentinel lymph node after neoadjuvant chemotherapy. World J Surg. 2004;28(10):1019–1024. doi: 10.1007/s00268-004-7367-7. [DOI] [PubMed] [Google Scholar]
- 35.Lee H., Ahn S., Lee S., Lee H., Jeong J. Prospective evaluation of the feasibility of sentinel lymph node biopsy in breast cancer patients with negative axillary conversion after neoadjuvant chemotherapy. Cancer research and treatment. official journal of Korean Cancer Association. 2015;47(1):26–33. doi: 10.4143/crt.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S., Kim E., Kang S. Sentinel node identification rate, but not accuracy, is significantly decreased after pre-operative chemotherapy in axillary node-positive breast cancer patients. Breast Canc Res Treat. 2007;102(3):283–288. doi: 10.1007/s10549-006-9330-9. [DOI] [PubMed] [Google Scholar]
- 37.Ozmen V., Unal E., Muslumanoglu M. Axillary sentinel node biopsy after neoadjuvant chemotherapy. Eur J Surg Oncol : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2010;36(1):23–29. doi: 10.1016/j.ejso.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Park S., Lee J., Paik H. Feasibility and prognostic effect of sentinel lymph node biopsy after neoadjuvant chemotherapy in cytology-proven, node-positive breast cancer. Clin Breast Canc. 2017;17(1):e19–e29. doi: 10.1016/j.clbc.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Park S., Park J., Cho J., Park H., Kim S., Park B. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with cytologically proven node-positive breast cancer at diagnosis. Ann Surg Oncol. 2013;20(9):2858–2865. doi: 10.1245/s10434-013-2992-8. [DOI] [PubMed] [Google Scholar]
- 40.Shen J., Gilcrease M., Babiera G. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer. 2007;109(7):1255–1263. doi: 10.1002/cncr.22540. [DOI] [PubMed] [Google Scholar]
- 41.Takei H., Yoshida T., Kurosumi M. Sentinel lymph node biopsy after neoadjuvant chemotherapy predicts pathological axillary lymph node status in breast cancer patients with clinically positive axillary lymph nodes at presentation. Int J Clin Oncol. 2013;18(3):547–553. doi: 10.1007/s10147-012-0418-4. [DOI] [PubMed] [Google Scholar]
- 42.Thomas S., Prakash A., Goyal V., Popli M., Agarwal S., Choudhury M. Evaluation of sentinel node biopsy in locally advanced breast cancer patients who become clinically node-negative after neoadjuvant chemotherapy: a preliminary study. International journal of breast cancer. 2011;2011:870263. doi: 10.4061/2011/870263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu X., Tang L., Huang W., Huang S., Peng W., Hu D. Contrast-enhanced ultrasonography and blue dye methods in detection of sentinel lymph nodes following neoadjuvant chemotherapy in initially node positive breast cancer. Arch Gynecol Obstet. 2020;302(3):685–692. doi: 10.1007/s00404-020-05646-8. [DOI] [PubMed] [Google Scholar]
- 44.Yagata H., Yamauchi H., Tsugawa K. Sentinel node biopsy after neoadjuvant chemotherapy in cytologically proven node-positive breast cancer. Clin Breast Canc. 2013;13(6):471–477. doi: 10.1016/j.clbc.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Yu Y., Cui N., Li H. Sentinel lymph node biopsy after neoadjuvant chemotherapy for breast cancer: retrospective comparative evaluation of clinically axillary lymph node positive and negative patients, including those with axillary lymph node metastases confirmed by fine needle aspiration. BMC Canc. 2016;16(1):808. doi: 10.1186/s12885-016-2829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zetterlund L., Frisell J., Zouzos A. Swedish prospective multicenter trial evaluating sentinel lymph node biopsy after neoadjuvant systemic therapy in clinically node-positive breast cancer. Breast Canc Res Treat. 2017;163(1):103–110. doi: 10.1007/s10549-017-4164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galimberti V., Ribeiro Fontana S., Maisonneuve P. Sentinel node biopsy after neoadjuvant treatment in breast cancer: five-year follow-up of patients with clinically node-negative or node-positive disease before treatment. Eur J Surg Oncol : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2016;42(3):361–368. doi: 10.1016/j.ejso.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 49.El Hage Chehade H., Headon H., El Tokhy O., Heeney J., Kasem A., Mokbel K. Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? An updated meta-analysis involving 3,398 patients. Am J Surg. 2016;212(5):969–981. doi: 10.1016/j.amjsurg.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 50.Mocellin S., Goldin E., Marchet A., Nitti D. Sentinel node biopsy performance after neoadjuvant chemotherapy in locally advanced breast cancer: a systematic review and meta-analysis. Int J Canc. 2016;138(2):472–480. doi: 10.1002/ijc.29644. [DOI] [PubMed] [Google Scholar]
- 51.Tee S., Devane L., Evoy D. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br J Surg. 2018;105(12):1541–1552. doi: 10.1002/bjs.10986. [DOI] [PubMed] [Google Scholar]
- 52.Geng C., Chen X., Pan X., Li J. The feasibility and accuracy of sentinel lymph node biopsy in initially clinically node-negative breast cancer after neoadjuvant chemotherapy: a systematic review and meta-analysis. PloS One. 2016;11(9) doi: 10.1371/journal.pone.0162605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim T., Giuliano A., Lyman G. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006;106(1):4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 54.Tan V., Goh B., Fook-Chong S., Khin L., Wong W., Yong W. The feasibility and accuracy of sentinel lymph node biopsy in clinically node-negative patients after neoadjuvant chemotherapy for breast cancer--a systematic review and meta-analysis. J Surg Oncol. 2011;104(1):97–103. doi: 10.1002/jso.21911. [DOI] [PubMed] [Google Scholar]
- 55.Lyman G., Somerfield M., Bosserman L., Perkins C., Weaver D., Giuliano A. Sentinel lymph node biopsy for patients with early-stage breast cancer: American society of clinical Oncology clinical practice guideline update. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2017;35(5):561–564. doi: 10.1200/JCO.2016.71.0947. [DOI] [PubMed] [Google Scholar]
- 56.Pesek S., Ashikaga T., Krag L., Krag D. The false-negative rate of sentinel node biopsy in patients with breast cancer: a meta-analysis. World J Surg. 2012;36(9):2239–2251. doi: 10.1007/s00268-012-1623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyman G., Giuliano A., Somerfield M. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2005;23(30):7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Wong S., Edwards M., Chao C. Sentinel lymph node biopsy for breast cancer: impact of the number of sentinel nodes removed on the false-negative rate. J Am Coll Surg. 2001;192(6):684–689. doi: 10.1016/s1072-7515(01)00858-4. discussion 689-691. [DOI] [PubMed] [Google Scholar]
- 59.Krag D., Anderson S., Julian T. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 60.Li X., Chen S., Duan Y. Identification and preservation of stained non-sentinel lymph nodes in breast cancer. Oncology letters. 2020;20(6):373. doi: 10.3892/ol.2020.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banys-Paluchowski M., Gasparri M., de Boniface J. Surgical management of the axilla in clinically node-positive breast cancer patients converting to clinical node negativity through neoadjuvant chemotherapy: current status, knowledge gaps, and rationale for the EUBREAST-03 AXSANA study. Cancers. 2021;13(7) doi: 10.3390/cancers13071565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel R., MacKerricher W., Tsai J. Pretreatment tattoo marking of suspicious axillary lymph nodes: reliability and correlation with sentinel lymph node. Ann Surg Oncol. 2019;26(8):2452–2458. doi: 10.1245/s10434-019-07419-3. [DOI] [PubMed] [Google Scholar]
- 63.Simons J., Nijnatten T.J.A.V., Koppert L.B. Radioactive Iodine Seed placement in the Axilla with Sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer: results of the prospective multicenter RISAS trial. Canc Res. 2021;81(4 SUPPL) [Google Scholar]
- 64.Patten D., Zacharioudakis K., Chauhan H., Cleator S., Hadjiminas D. Sentinel lymph node biopsy after neo-adjuvant chemotherapy in patients with breast cancer: are the current false negative rates acceptable? Breast. 2015;24(4):318–320. doi: 10.1016/j.breast.2015.02.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.