Abstract
Telocytes (TCs), a novel type of interstitial cells, were identified in various animals. Since TCs have not observed in avian skin, hence, we carried out immunohistochemistrical and transmission electron microscopical studies in the skin of the silky fowl to investigate the TCs. TCs appear as CD34, c-Kit, and PDGFRα immunopositive. The elongated TCs with 2 long and thin telopodes (Tps) are located in the dermis. Generally, a TC possesses a fusiform, ovoid and polygonal cell body with 2 Tps (lengths = 5.27–21.85 μm), which are uneven in thickness including thick sections – podoms (diameters = 0.40–0.47 μm) and thin sections – podomers (diameters = 0.03–0.04 μm). TCs/Tps are observed frequently in close proximity to neighboring cell types/structures, such as adipocytes, collagen fibers, and capillaries. Under a magnified field, homocellular TCs/Tps contacts are observed through gap junctions (distances = 0.01–0.05 μm), whereas some of TCs/Tps have heterocellular close contacts by point contacts with surrounding cells, including stem cells and melanocytes. The multivisicular bodies, especially exosomes (diameters = 0.09–0.23 μm) releasing from TCs/Tps are observed in close proximity to TCs/Tps. Our results illustrated that the novel type of interstitial cells – TCs are present in the dermis of the silky fowl, and they have special structural relationships with surrounding cell types. The study provides histological evidence for TCs involvement in intercellular communication, skin regeneration, and pigmentogenesis in avian skin.
Key words: chicken, skin, telocytes, immunohistochemistry, ultrastructure
INTRODUCTION
A type of interstitial cells, called interstitial Cajal-like cells (ICLC) because of their apparent similarity with interstitial cells of Cajal (ICC) in morphology, were found by Popescu's team in 2005 (Hinescu and Popescu, 2005; Faussone Pellegrini and Popescu, 2011). In the later researches, it gradually showed that ICLC has different morphological characteristics and immunophenotypes from ICC. ICLC represent a distinct (novel) type of interstitial cells. To make a clear distinction from ICC, in 2010, the new type of interstitial cells was termed “telocyte” (Faussone Pellegrini and Popescu, 2011; Popescu, 2010). Telocytes (TCs) appear as various immunophenotypes in different animals and organs, but the specific immunocytochemical markers of TCs have not yet been confirmed. The morphological structures and immunophenotypes of TCs are heterogeneous (Cretoiu et al., 2017). The most common immunophenotypes are generally suggested to be CD34 and platelet-derived growth factor receptor α (PDGFRα). Two recent studies have demonstrated that TCs express also winged-helix transcription factor forkhead box l1 (FOXL1) and leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) in the intestine (Shoshkes-Carmel et al., 2018; Bahar Halpern et al., 2020). The most typical morphological features of canonical TCs are that they possess several long and thin cell body prolongations called telopodes (Tps), which are hard to observe at the resolution of an optical microscopy. Therefore, electron microscopy is currently the only accurate method for identifying TCs (Roatesi et al., 2015). In tissues, TCs are suggested to play various roles in intercellular communication, immune response and regulation of neighboring cell types functions and homeostasis maintenance (Cretoiu et al., 2012; Kondo and Kaestner, 2019). Notably, TCs are considered to regulate the proliferation and differentiation of stem cells, and participate in cell renewal and tissue repair (Popescu, 2010; Shoshkes-Carmel et al., 2018). Therefore, TCs are recognized as a promising cell for regenerative medicine. TCs are the only new cell type found in humans and animals in the past 40 y. The discovery of TCs is considered to be a milestone in the field of cell biology (Wang, 2015).
As a new type of interstitial cell, TCs have attracted less attention. The information about TCs is still very limited in the last 10 y since TCs discovery. However, recently, various international teams began to pay attention to TCs. These studies further confirmed the central role of TCs in the regulation of proliferation and differentiation of stem cells, and constitute the stem cell niche (Shoshkes-Carmel et al., 2018; Bahar Halpern et al., 2020; McCarthy et al., 2020; Moisan et al., 2021). Therefore, TCs participate in tissue regeneration through regulating and cooperating with stem cells. On the basis of vital role of TCs in tissue repair and renewal, TCs are widely concerned and recognized by scientists in the field of cell biology, translational, and regenerative medicine. It will lead TCs researches to a new era.
TCs were mostly identified in human and rodents in previous studies. The research data of TCs in avian organs are still very limited compared as mammals. Moreover, it became increasingly evident that TCs are difficult to characterize in terms of immunophenotypes and that their phenotypes are different depending on the location and needs of the tissue at one time (Cretoiu et al., 2017). In this work, we aimed to provide a detailed description for structural characteristics of cutaneous TCs and the particular spatial relationships with neighboring cell types/structures in a Chinese unique poultry – the silky fowl by immunohistochemistry (IHC), transmission electron microscopy (TEM), and morphometry. These results will help us to better understand the physiological role of TCs in avian skin. In particular, the study will illustrate the structural relationships between TCs and melanocytes and provide a morphological basis for revealing the role of TCs in melanogenesis.
MATERIALS AND METHODS
Animals
The commercial adult silky fowls were purchased in a farm product market. After the chickens were temporarily raised for 24 h, they were executed and the skin was sampled. This work was approved by the Ethical Committee for Animal Care and Use of Jiangxi Agricultural University after relevant ethical review according to the Code of Practice for the Housing and Care of Animals Used in Scientific Procedures. All measures were made to minimize the suffering of the chickens.
Tissue Materials Collection and Treatment
After executing, the skin was separated and taken. The skin was divided into many small pieces. Some of them were fixed in 4% paraformaldehyde/PBS overnight for immunohistochemical method, and then they were washed with 0.01 M PBS at pH 7.4, dehydrated in a series of graded concentration ethanol, and embedded in paraffin for sectioning. The serial sections (5 µm) were cut with a RM2245 microtome (Leica, Germany). Other skin samples were fixed in 2.5% glutaraldehyde/PBS overnight for transmission electron microscopy method.
CD34, c-Kit, and PDGFRα IHC for TCs Identification
After deparaffination in xylene and rehydration, the serial skin sections were incubated in 3% H2O2/PBS for 15 min at 40°C, and then they were pretreated using heat mediated antigen retrieval with sodium citrate buffer (0.01 M, pH = 6.0) for 15 min. All following steps were performed in a moist chamber in a dark environment. The sections were incubated in goat serum for 20 min at room temperature. After discarding goat serum, the serial skin sections were incubated in the anti-c-Kit (ab228036), anti-CD34 (ab150060) and PDGFRα (ab118514) antibodies (Abcam, Cambridge, UK), respectively, at 4°C in a refrigerator overnight. The control skin sections were conducted as the above protocol except that the primary antibodies were not added. The next day, after washing by PBS, the sections were incubated with biotinylated anti-rabbit IgG (ZSGB-Bio, China) at 37°C for 30 min, and they were incubated with Streptavidin-Horseradish Peroxidase working fluid (SP-9001, ZSGB-Bio, China) at 37°C for 30 min. The 3,3-diaminobenzidin (DAB) (Sigma-Aldrich, Darmstadt, Germany) was used as the chromogen. The coloration was terminated with PBS when positive cells were clearly visible. The sections were then counterstained with hematoxylin and mounted. Finally, the sections were photographed by a BX 53 light microscopy with DP73 microscopiccal imaging system (Olympus, Japan).
TEM for Ultrastructure of TCs
TEM was conducted according to our previous study (Ge et al., 2019). After taking from 2.5% glutaraldehyde/PBS, small pieces of skin tissue were washed in PBS. The samples were postfixed in 1% OsO4 for 1 h, dehydrated in a concentration series of ethanol, infiltrated with propylene oxide – Araldite mixture and embedded in Araldite. The sample blocks were sectioned at 50 nm with an ultramicrotome (Wien, Austria), and then the sections were mounted on cooper coated grids. Finally, these sections were stained with 1% uranyl acetate and Reynold′s lead citrate for 20 min. The stained sections were observed and photographed by a high-resolution digital camera connected to a Hitachi TEM 7800 (Tokyo, Japan).
Morphometric Analysis
Diameters and distances were measured by ImageJ (FIJI) software (NIH, Bethesda, MD). At first, opened a micrograph for distance measure by the software. Set scale using the original scale of respective micrograph by the tool “Set Scale” of “Analyze” in the menu. Converted scale distance pixel unit to length unit (μm). The straight distances, diameters of podomers and podoms, and lengths of Tps by scaled by the “Straight Line” and “Segmented Line”, and then they were measured in the tool “Measure” of “Analyze” in the menu. The data were outputted and analyzed by Office Excel software (Microsoft, Redmond, WA).
RESULTS
TCs in the Dermis of Silky Fowl by IHC
Cutaneous TCs showed CD34, c-Kit and PDGFRα immunopositive (Figure 1, Figure 2). These immunopositive TCs appeared as complete slender cell outlines (Figures 1A–1C and 2) and sections/telopodes (Tps) (Figure 1D). All TCs were located in dermis. TCs were observed in the dermis of superficial skin, and close to stratum basale of epidermis (Figures 1A, 1C, 1D and Figure 2B), but were not present in the epidermis. TCs were also observed in stratum spongiosum of dermis (Figures 1B and 2A). Others TCs were present in stratum compactum of dermis (Figures 1A, 1C, 1D), and some of them were located in the deep layer of skin near adipocytes (Figure 2C).
Figure 1.
Light microscopy microphotographs showing telocytes (TCs) by immunohistochemistry in the skin of the silky fowl. (A) and (B) are serial sections from the identical skin sample. (C) and (D) are also serial sections from another identical skin sample. (A), (B), (C) and (D) showing slender CD34+, c-Kit+, CD34+ and PDGFRα+ TCs/telopodes (Tps) (arrows), respectively.
Figure 2.
Light microscopy microphotographs showing telocytes (TCs) by immunohistochemistry in the skin of the silky fowl. (A) A CD34+ TC (arrow) with two long telopodes (Tps) locates stratum spongiosum of dermis. (B) c-Kit+ TC (arrows) with long Tps are present in stratum basale of epidermis. (C) PDGFRα+ TCs with long Tps in stratum compactum of dermis. Abbreviations: AC, subcutaneous adipocyte; De, dermis; Ep, epidermis.
Ultrastructural Characteristics of TCs/Tps in the Skin of Silky Fowl by TEM
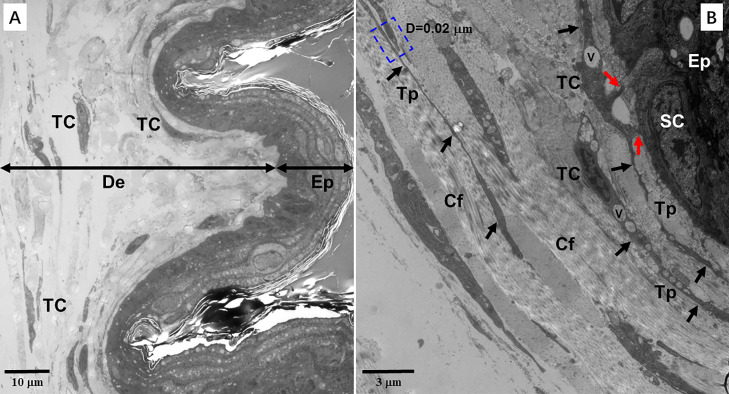
TCs were located in the dermis with characteristic cellular processes – Tps (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9). Some of TCs were close to the stratum basale of epidermis, but they were not observed in epidermis (Figure 3). TCs were also located in the dermis near adipocytes (Figures 4 and 9). TCs were present frequently between collagen fibers of dermis. TCs appeared as fusiform, ovoid and polygonal cell bodies with long and thin Tps (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9). The shapes of the nuclei of TCs were indented and consistent with the outlines of the cell bodies. The clusters of electron dense heterochromatin attached to the nuclear envelope were observed (Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9). Some of TCs had scarce cytoplasm and encompassed few organelles (Figure 4). The electron-loose vesicles and rounded clathrin-coated pits were observed in the cytoplasm of TCs (Figures 6B, 7C, 7D). The caveolae were present in the Tps (Figure 7B). The Tps were straight and thin (Figure 3B). Other Tps were convoluted. By the morphometry, the lengths of Tps were about 5.27 to 21.85 μm (Figures 5, 6, and 9). The Tps exhibited labyrinth-like (Figures 4, 6, 7, and 8), and some sections of Tps appeared as zigzag-like and overlapping processes (Figure 5C). The Tps were uneven in thickness, including the thick podoms (diameters = 0.40–0.47 μm) and thin podomers (diameters = 0.03–0.04 μm) (Figures 5 and 6). The podoms contained rough endoplasmic reticulum (Figure 6C). The vesicles were also observed in the Tps (Figures 3B, 4B, 6B, 7C, 7D). The Tps contained 2 to 6 branches (Figures 6C, 7B and 8B), and formed a ditch-like structure. The branches had no free ends, and they finally came together. No basement membranes were observed in all TCs.
Figure 3.
Transmission electron microscopy (TEM) microphotographs showing telocytes (TCs) in the skin of the silky fowl. (A) TCs with long telopodes (Tps) are observed in the dermis (De). (B) A TEM microphotograph shows the details of TCs and Tps (arrows) in the dermis close to epidermis (Ep). Tps establish heterocellular contacts with a stem cell (SC) of stratum basale in epidermis by point contacts (red arrows). The SC has electron-loose cytoplasm and few organelles. Overlapping telopodes establish homocellular contacts by gap junctions (blue dashed line boxed areas). The distance between two Tps is 0.02 μm. The electron-loose vesicles (V) were observed the cytoplasm of TCs and Tps. Abbreviations: Cf, collagen fiber; Sc, Stratum corneum.
Figure 4.
Transmission electron microscopy (TEM) microphotographs showing telocytes (TCs) in the skin of the silky fowl. (A) A TC with a long and labyrinth-like telopode (Tp) in the dermis and close to an adipocyte (AC). The distance (D) between the TC and AC is 0.28 μm. (B) A TEM microphotograph of the white dashed line boxed areas shown in (A) with details of the TC. The TC contains a rounded nucleus (N) and a small amount of cytoplasm. The labyrinth-like telopode is convoluted and uneven in thickness. The electron-loose vesicles (V) are located the Tps. The exosomes (black arrowheads) are observed near the TCs/Tps. The diameters (Di) of exosomes are 0.10–0.19 μm. Abbreviation: Cf, collagen fiber.
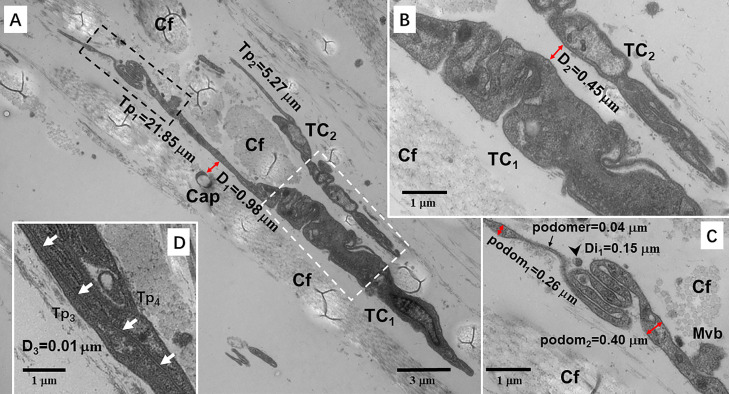
Figure 5.
Transmission electron microscopy (TEM) microphotographs showing telocytes (TCs) in the skin of the silky fowl. (A) Two TCs (TC1 and TC2) with long telopodes (Tp1 = 21.85 μm and Tp2 = 5.27 μm) in the dermis. A capillary (Cap) is present close to TC (Distance = 0.98 μm). (B) A TEM microphotograph of the white dashed line boxed areas shown in (A) with details of TC. The distance (D2) between TC1 and TC2 is 0.45 μm. (C) A TEM microphotograph of the black dashed line boxed areas shown in (A) with details of Tp1. A zigzag-like and overlapping Tp segment is observed. Tps are uneven in thickness. The thickness of thick podom is 0.26–0.40 μm. The thickness of thin podomer is about 0.04 μm. An exosome (black arrowhead) close to Tp and its diameter (Di) is 0.15 μm. A multivisicular body (Mvb) is also observed in close vicinity to the Tp. (D) Two TCs cling to each other tightly. Overlapping Tps establish homocellular contacts by gap junctions (white arrows). The distance is 0.01 μm. Abbreviation: Cf, collagen fiber.
Figure 6.
Transmission electron microscopy (TEM) microphotographs showing telocytes (TCs) in the skin of the silky fowl. (A) A TC with convoluted and a long telopode (Tp1 = 16.78 μm). Another convoluted and labyrinth-like Tp2 is observed near Tp1. (B) A TEM microphotograph of the white dashed line boxed areas shown in (A) with details of the TC. The electron-loose vesicles (V) and rounded clathrin-coated pits (yellow arrows) locate the cytoplasm of TCs. Three exosomes (black arrowheads) are observed in close vicinity to the TC. The diameters (Di) of exosomes are 0.15–0.19 μm. (C) A TEM microphotograph of the white dashed line boxed areas shown in (A) with details of Tp1. Tp1 has two branches in a segment (B1 and B2). Tp is uneven in thickness. The thickness of thick podom is 0.47 μm. The podom contain rough endoplasmic reticulum (rER). The thickness of thin podomer is 0.03 μm. Many exosomes (black arrowheads) are present in close proximity to Tp1. The diameters (Di) of exosomes are 0.09–0.23 μm. The shedding exosomes (black double arrowheads) from Tp1 are observed. A capillary (Cap) is present close to Tp1 (Distance = 0.26 μm). Abbreviation: Cf, collagen fiber.
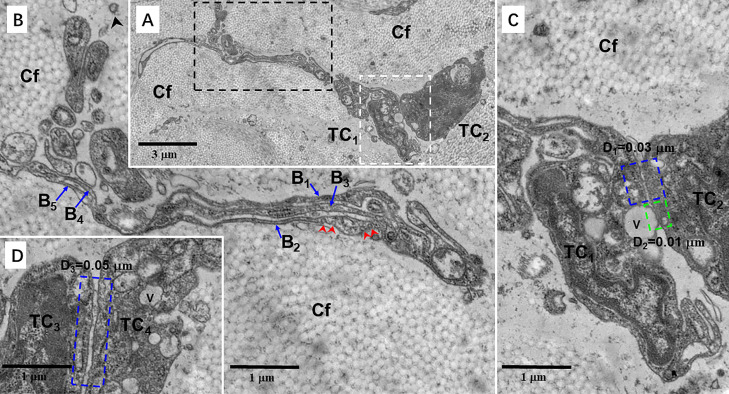
Figure 7.
Transmission electron microscopy (TEM) microphotographs showing telocytes (TCs) in the skin of the silky fowl. (A) Two TCs between collagen fibers (Cf) in the dermis. (B) A TEM microphotograph of the black-dashed line boxed areas shown in (A) with details of a telopode (Tp) of TC1. The Tp of TC1 contains 5 branches (B1–B5) in different segments. The caveolae were observed in the Tp (red arrowheads). (C) A TEM microphotograph of the white dashed line boxed areas shown in (A) with details of TC1 and TC2. Two TCs close together and establish homocellular contacts by gap junctions (blue dashed line boxed areas). The distance (D1) between TC1 and TC2 is about 0.03 μm. The shortest distance (D2, green dashed line boxed areas) between the cytoplasmic vesicle region of TC1 and TC2 is 0.01 μm. (D) Two TCs (TC3 and TC4) establish also homocellular contacts by gap junctions (blue dashed line boxed areas). The distance (D3) between two TCs is 0.05 μm. Black arrowhead showing exosome. Abbreviation: V, vesicle.
Figure 8.
Transmission electron microscopy (TEM) microphotographs showing telocytes (TCs) in the skin of the silky fowl. (A) A TC with a telopode (Tp) between collagen fibers (Cf) in the dermis. (B) A TEM microphotograph shows the details of Tp of the TC. The Tp has 6 branches (B1–B6) in different segments. Two adjacent branches form a ditch-like structure. These branches eventually merge into one Tp segment.
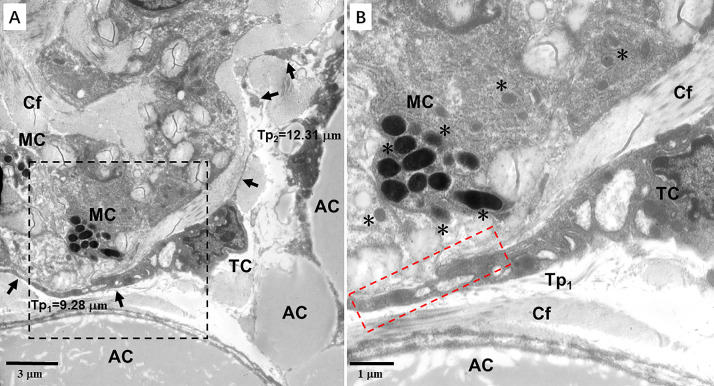
Figure 9.
Transmission electron microscopy (TEM) microphotographs showing telocytes (TCs) in the skin of the silky fowl. (A) A TC with two thin and long telopodes (arrows, Tp1 = 9.28 μm, Tp2 = 12.31 μm) are observed in close proximity to melanocytes (MC) in the dermis near adipocytes (AC). (B) A TEM microphotograph of the black dashed line boxed areas shown in (A) with details of MC and Tp. MC contain many melanosomes (asterisks) with different electron density and developmental stages. The mature melanosomes show rounded/oval and electron-dense. A heterocellular contact (red dashed line boxed areas) is observed between Tp1 and MC. Abbreviation: Cf, collagen fibers.
Location Relationships Between TCs/Tps and Neighboring Cell Types/Structures
All TCs were tightly wrapped by collagen fibers of dermis (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9). TCs adhered to collagen fibers. TCs/Tps were very close to each other (distance = 0.45 μm) (Figures 3B, 5) and established homocellular close contacts by gap junctions (Distance = 0.01–0.05 μm) (Figures 3B, 5, 7). In addition, TCs closely bordered other types of cells/structures, including collagen fibers (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9), adipocytes (distance = 0.28 μm) (Figure 4A) and capillary (distances = 0.98 μm; 0.26 μm) (Figures 5A, 6C). TCs that located dermis established heterocellular close contacts with stem cells (Figure 3B) and melanocytes (Figure 9) by point contacts in stratum basale of epidermis. A multivisicular body was present near the Tps (Figure 5C). The exosomes were observed in close proximity to TCs/Tps (Figures 4B, 5C, 6B, 6C, 7B). In particular, the shedding exosomes from Tps were also found (Figure 6C). The diameters of exosomes were 0.09 to 0.23 μm.
DISCUSSION
In previous studies, TCs were identified as a novel type of interstitial cells and have been described in various organs of different vertebrates, especially in the human and rodents (Cretoiu and Popescu, 2014). In birds, TCs were also observed in several organs, such as ileum, spleen, trachea, oviduct, and syrinx (Yang et al., 2015, 2017; Ibrahim et al., 2020; Mokhtar and Hussien, 2020; Mustafa and El-Desoky, 2020). These studies have demonstrated that the morphological characteristics of avian TCs are similar to those of other animal organs. Avian TCs made up frequently close contacts with neighboring cell types/structures, such as immune cells (including mast cells and macrophages), smooth muscle cells, nerve fibers and blood vessels. Based on the appearance of direct homocellular contacts between Tps (Tps with Tps, Tps with cell bodies), heterocellular contacts between Tps and neighboring cell types/structures, and the release of shedding vesicles (including exosomes), which act as molecular complex intercellular signaling organelles, avian TCs may be implicated in intercellular signaling (Ibrahim et al., 2020). Subsequently, avian TCs were suggested to regulate the physiological functions of surrounding cell types by direct/indirect contacts and shedding vesicles. Moreover, TCs might play a potential role in the pathogenesis of avian organ diseases (Yang et al., 2017).
TCs have been described in the skin of the human and mammals (Ceafalan et al., 2012; Rusu et al., 2012; Cretoiu et al., 2015; Kang et al., 2015; Arafat, 2016; Wang et al., 2020), but TCs not were identified in avian skin. In the present study, TCs were first recorded in the skin of a special poultry – the silky fowl. Avian cutaneous TCs were only observed in the dermis, but not in epidermis. The location of TCs was the same as that of TCs in human and rodent skin (Rusu et al., 2012; Arafat, 2016). Likewise, avian cutaneous TCs were closely related to or contacting adipocytes, microvessels, collagenous fibers. Moreover, TCs were observed to close to the melanocytes with melanosomes. Particularly, the TCs established heterocellular close contacts with stem cells by point contacts in stratum basale of epidermis. Avian cutaneous TCs do not have basal lamina as the TCs in the human skin (Rusu et al., 2012). In morphology, avian cutaneous TCs appeared as TCs in other animals with long Tps (including thick podoms and thin podomers segments) and the shedding exosomes released from Tps. Additionally, avian cutaneous TCs exhibited CD34, c-Kit, and PDGFRα immunopositive by IHC.
In general, avian cutaneous TCs are consistent with the most typical structural characteristics of canonical TCs. There are still some differences between avian and mammalian cutaneous TCs. In human skin, TCs were found in the dermis, around blood vessels, in the perifollicular sheath, outside the glassy membrane, and surrounding hair follicle and sebaceous glands, arrector pili muscles and both the secretory and excretory portions of eccrine sweat glands (Ceafalan et al., 2012; Wang et al., 2020). However, in avian skin, the sweat glands were absent. Moreover, avian cutaneous TCs were only observed among collagen fibers, but no elastic fibres. Avian cutaneous TCs were positive for c-Kit, CD34, and vimentin. In human skin, TCs were immunophenotypically characterized by CD34, but negative for c-Kit (Manetti et al., 2013). However, another study exhibited that TCs were both CD34 and CD117 (c-Kit) immunopositve in human scalp (Wang et al., 2020). Also, in the skin of 1-day-old newborn rats, TCs showed positive reaction for both CD34 and CD117 immunopositves (Arafat, 2016). These findings support that the immunophenotypes of TCs are heterogeneous, even in the same animals and organs.
In the present study, avian cutaneous TCs expressed CD34, c-Kit, and PDGFRα. TCs expressed receptors and contained molecules might participate in the control of the organ functions (Vannucchi, 2020). TCs expressed CD34, which is frequently expressed in hematopoietic stem/progenitor cells. It is suggested that TCs have the stemness and property of undifferentiated cells. TCs may be a subpopulation of mesenchymal stem/progenitor cells (Vannucchi, 2020), and have the potential to differentiate into other cell types. Furthermore, it is demonstrated that c-Kit-positive TCs have stemness capacity (Marini et al., 2017). TCs expressed also PDGFRα, which play a key role in regulating cell proliferation and differentiation (Vannucchi et al., 2013). TCs represent adult stromal mesenchymal stem cells. Therefore, TCs may not only be the nurse of progenitor cells but could also have a progenitor capacity. TCs had the potential to differentiate into other cell types as undifferentiated cells (Díaz-Flores et al., 2014). The immunophenotypes of TCs define their stemness capacity. It may be an important reason for their participating in tissue regeneration and repair processes.
TCs were observed in close vicinity to capillaries in human skin (Rusu et al., 2012). Here, the same results were also observed in the avian skin. Moreover, the multivesicular bodies were observed in the vicinity of TCs/Tps. In particular, the exosomes were observed frequently in close proximity to TCs/Tps in the skin of the silky fowl. Noteworthy, the releasing exosomes from Tps were observed by TEM. These findings supported again that TCs do release the exosomes. Exosomes are extracellular vesicles released by the vast majority of cell types, upon the fusion of multivesicular bodies with the cellular plasma membrane (Aheget et al., 2020). They load various biomolecules, including DNA, mRNAs, miRNAs, proteins, and lipids (and other substances such as vitamins and trace elements), as cargos (Asgarpour et al., 2020). The exosomes as mediators have been implicated cellular cross talk. Therefore, TCs may transfer signals for intercellular communication by their releasing multivesicular bodies and/or exosomes (Cretoiu et al., 2015). It is suggested that TCs may affect the activity of surrounding cells through paracrine and/or juxtacrine signaling (Popescu et al., 2011; Ceafalan et al., 2012). In addition, TCs extended alongside the capillaries. The extracellular multivesicular bodies were present near Tps with functional importance in intercellular communication. The releasing extracellular vesicles from TCs may enter nearby capillaries and perform endocrine function. TCs play a role in regulating the local microenvironment by cell-to-cell contacts and extracellular shedding vesicles (Díaz-Flores et al., 2020). A recent study confirmed that TCs inhibit microvascular endothelial cell apoptosis through exosomal miRNA to improve angiogenesis (Liao et al., 2021). It is suggested that TCs participate in the repair of injury through their exosomes.
In the present study, avian cutaneous TCs established homocellular close contacts by gap junctions. Furthermore, TCs established heterocellular close contacts by point contacts with stem cells in the stratum basale of the epidermis. Homocellular interactions between TCs/Tps suggested that TCs form an interstitial meshwork to serve as skeleton, and play a role in mechanical support in tissue. Heterocellular interactions supported the notion that TCs may perform intercellular communication (Ceafalan et al., 2012). TCs are likely involved in controlling the movement, proliferation and differentiation of stem cells behaving as nurse/supporting cells in stem cell niches (Vannucchi, 2020; Zhang et al., 2020). Thus, TCs can regulate stem cells and melanocytes in avian skin. TCs strategic positioning surrounding stem cells suggests their possible involvement in regeneration and repair, remodeling and homeostasis in the skin (Ceafalan et al., 2012; Wang et al., 2020). Clinically, TCs and stem cells co-transplantation may be better than single cell type transplantation for repairing skin lesions. In addition, in the present study, TCs were observed surrounding collagen fibbers. TCs might play apart in increasing the efficiency of collagen fibbers in the process of repair and regeneration (Xu et al., 2016). The presence of TCs was suggested to potential direct (intercellular interactions) and/or indirect (multivesicular bodies and exosomes) contacts with resident cell types (Ceafalan et al., 2012).
In the present study, TCs that located dermis established heterocellular close contacts with melanocytes by point contacts in the stratum basale of the epidermis. Moreover, avian TCs expressed c-Kit. It has been demonstrated that c-Kit plays a pivotal role in melanogenesis (Hachiya et al., 2009). These results suggested that TCs might be involved in melanogenesis by the c-Kit signal. In addition, it has been demonstrated that TCs in skin had the phagocytic-like capacity to uptake and store pigments (hemosiderin and melanin) (Díaz-Flores et al., 2014). TCs may be related to the transfer of macromolecules (e.g., melanin) in some cell type, including melanocytes. This function (the ability to internalize small particles), together with the capacity of these cells to release extracellular vesicles with macromolecules, could close the cellular bidirectional cooperative circle of informative exchange and intercellular interactions. In general, the melanocytes producing melanin are located in dermis but no in epidermis. However, the melanin is observed frequently in the epidermis. It is suggested that TCs surrounding melanocytes in the dermis can uptake the melanin releasing from melanocytes and transfer melanin to keratinocytes into epidermis by their Tps. Melanin in the epidermis participate in ray protection. These data suggested that TCs play a role in pigmentation of skin. To summarize, there may be 2 reasons why TCs are involved in regeneration. On the one hand, TCs that expressed CD34, c-Kit and PDGFRα are directly involved in tissue regeneration and repair as an undifferentiated cell by their stemness. On the other hand, TCs regulate proliferation and differentiation of stem cells by heterocellular close contacts, and constitute the stem cell niche to participate in tissue regeneration and renewal (Shoshkes-Carmel et al., 2018; Bahar Halpern et al., 2020; Vannucchi, 2020).
In conclusion, our findings confirm that the presence of TCs with special structural features in the dermis of the silky fowl. Avian cutaneous TCs expressed CD34, c-Kit and PDGFRα, which be suggested to be the markers of stemness capacity. In addition, the nanostructural relationships between TCs/Tps and neighboring cell types/structures were demonstrated by the morphometry. TCs/Tps established homocellular contacts each other, and heterocellular close contacts with stem cells and melanocytes. The multivisicular bodies, more exosomes were observed frequently in close proximity to TCs/Tps. These results provide histological evidences for the notion that TCs are involved in intercellular communication, skin regeneration and pigmentogenesis in avian skin.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No.31760716; 31560681), the Project of Jiangxi Province (No. 20151BBF60007; 20171ACB21028).
The manuscript represents original material that has not been submitted for publication nor has been published in whole or in part elsewhere. This work was approved by the Ethical Committee for Animal Care and Use of Jiangxi Agricultural University after relevant ethical review according to the Code of Practice for the Housing and Care of Animals Used in Scientific Procedures.
All persons listed as authors have read, contributed to preparing the manuscript and attest to the validity and legitimacy of the data and its interpretation, and agree to its submission to Poultry Science.
DISCLOSURES
The authors declared that they have no conflicts of interest in this work.
REFERENCES
- Aheget H., Tristán-Manzano M., Mazini L., Cortijo-Gutierrez M., Galindo-Moreno P., Herrera C., Martin F., Marchal J.A., Benabdellah K. Exosome: a new player in translational nanomedicine. J. Clin. Med. 2020;9:2380. doi: 10.3390/jcm9082380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafat E.A. Ultrastructural and immunohistochemical characteristics of telocytes in the skin and skeletal muscle of newborn rats. Acta Histochem. 2016;118:574–580. doi: 10.1016/j.acthis.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Asgarpour K., Shojaei Z., Amiri F., Ai J., Mahjoubin-Tehran M., Ghasemi F., ArefNezhad R., Hamblin M.R., Mirzaei H. Exosomal microRNAs derived from mesenchymal stem cells: cell-to-cell messages. Cell Commun. Signal. 2020;18:149. doi: 10.1186/s12964-020-00650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar Halpern K., Massalha H., Zwick R.K., Moor A.E., Castillo-Azofeifa D., Rozenberg M., Farack L., Egozi A., Miller D.R., Averbukh I., Harnik Y., Weinberg-Corem N., de Sauvage F.J., Amit I., Klein O.D., Shoshkes-Carmel M., Itzkovitz S. Lgr5+ telocytes are a signaling source at the intestinal villus tip. Nat. Commun. 2020;11:1936. doi: 10.1038/s41467-020-15714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceafalan L., Gherghiceanu M., Popescu L.M., Simionescu O. Telocytes in human skin–are they involved in skin regeneration? J. Cell Mol. Med. 2012;16:1405–1420. doi: 10.1111/j.1582-4934.2012.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretoiu D., Cretoiu S.M., Simionescu A.A., Popescu L.M. Telocytes, a distinct type of cell among the stromal cells present in the lamina propria of jejunum. Histol. Histopathol. 2012;27:1067–1078. doi: 10.14670/HH-27.1067. [DOI] [PubMed] [Google Scholar]
- Cretoiu D., Gherghicean M., Hummel E., Zimmermann H., Simionescu O., Popescu L.M. FIB-SEM tomography of human skin telocytes and their extracellular vesicles. J. Cell Mol. Med. 2015;19:714–722. doi: 10.1111/jcmm.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretoiu D., Radu B.M., Banciu A., Banciu D.D., Cretoiu S.M. Telocytes heterogeneity: from cellular morphology to functional evidence. Semin. Cell Dev. Biol. 2017;64:26–39. doi: 10.1016/j.semcdb.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Cretoiu S.M., Popescu L.M. Telocytes revisited. Biomol. Concepts. 2014;5:353–569. doi: 10.1515/bmc-2014-0029. [DOI] [PubMed] [Google Scholar]
- Díaz-Flores L., Gutiérrez R., García M.P., González-Gómez M., Carrasco J.L., Alvarez-Argüelles H., Díaz-Flores L., Jr Telocytes/CD34+ stromal cells in pathologically affected white adipose tissue. Int. J. Mol. Sci. 2020;21:9694. doi: 10.3390/ijms21249694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Flores L., Gutiérrez R., García M.P., Sáez F.J., Aparicio F., Díaz-Flores L., Jr, Madrid J.F. Uptake and intracytoplasmic storage of pigmented particles by human CD34+ stromal cells/telocytes: endocytic property of telocytes. J. Cell Mol. Med. 2014;18:2478–2487. doi: 10.1111/jcmm.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faussone Pellegrini M.S., Popescu L.M. Telocytes. Biomol. Concepts. 2011;2:481–489. doi: 10.1515/BMC.2011.039. [DOI] [PubMed] [Google Scholar]
- Ge T., Ye Y., Zhang H. Ultrastructure of telocytes, a new type of interstitial cells in the myocardium of the Chinese giant salamander (Andrias davidianus) Eur. J. Histochem. 2019;63:3021. doi: 10.4081/ejh.2019.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya A., Sriwiriyanont P., Kobayashi T., Nagasawa A., Yoshida H., Ohuchi A., Kitahara T., Visscher M.O., Takema Y., Tsuboi R., Boissy R.E. Stem cell factor-KIT signalling plays a pivotal role in regulating pigmentation in mammalian hair. J. Pathol. 2009;218:30–39. doi: 10.1002/path.2503. [DOI] [PubMed] [Google Scholar]
- Hinescu M.E., Popescu L.M. Interstitial Cajal-like cells (ICLC) in human atrial myocardium. J. Cell Mol. Med. 2005;9:972–975. doi: 10.1111/j.1582-4934.2005.tb00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim I.A., Hussein M.M., Hamdy A., Abdel-Maksoud F.M. Comparative morphological features of syrinx in male domestic fowl Gallus gallus domesticus and male domestic pigeon Columba livia domestica: a histochemical, ultrastructural, scanning electron microscopic and morphometrical study. Microsc. Microanal. 2020;26:326–347. doi: 10.1017/S1431927620000021. [DOI] [PubMed] [Google Scholar]
- Kang Y., Zhu Z., Zheng Y., Wan W., Manole C.G., Zhang Q. Skin telocytes versus fibroblasts: two distinct dermal cell populations. J. Cell Mol. Med. 2015;19:2530–2539. doi: 10.1111/jcmm.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo A., Kaestner K.H. Emerging diverse roles of telocytes. Development. 2019;146 doi: 10.1242/dev.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Chen Y., Duan C., Zhu K., Huang R., Zhao H., Hintze M., Pu Q., Yuan Z., Lv L., Chen H., Lai B., Feng S., Qi X., Cai D. Cardiac telocytes inhibit cardiac microvascular endothelial cell apoptosis through exosomal miRNA-21-5p-targeted cdip1 silencing to improve angiogenesis following myocardial infarction. Theranostics. 2021;11:268–291. doi: 10.7150/thno.47021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti M., Guiducci S., Ruffo M., Rosa I., Faussone-Pellegrini M.S., Matucci-Cerinic M., Ibba-Manneschi L. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. J. Cell Mol. Med. 2013;17:482–496. doi: 10.1111/jcmm.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini M., Mencucci R., Rosa I., Favuzza E., Guasti D., Ibba-Manneschi L. Telocytes in normal and keratoconic human cornea: an immunohistochemical and transmission electron microscopy study. J. Cell Mol. Med. 2017;21:3602–3611. doi: 10.1111/jcmm.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy N., Manieri E., Storm E.E., Saadatpour A., Luoma A.M., Kapoor V.N., Madha S., Gaynor L.T., Cox C., Keerthivasan S., Wucherpfennig K., Yuan G.C., de Sauvage F.J., Turley S.J., Shivdasani R.A. Distinct mesenchymal cell populations generate the essential intestinal BMP signaling gradient. Cell Stem Cell. 2020;26:391–402. doi: 10.1016/j.stem.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan F., Oucherif S., Kaulanjan-Checkmodine P., Prey S., Rousseau B., Bonneu M., Claverol S., Gontier E., Lacomme S., Dousset L., Couffinhal T., Toutain J., Loot M., Cario-André M., Jullié M.L., Léauté-Labrèze C., Taieb A., Rezvani H.R. Critical role of Aquaporin-1 and telocytes in infantile hemangioma response to propranolol beta blockade. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2018690118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtar D.M., Hussien M.M. Cellular elements organization in the trachea of mallard (Anas platyrhynchos) with a special reference to its local immunological role. Protoplasma. 2020;257:407–420. doi: 10.1007/s00709-019-01444-5. [DOI] [PubMed] [Google Scholar]
- Mustafa F.E.A., El-Desoky S.M.M. Architecture and cellular composition of the spleen in the Japanese Quail (Coturnix japonica) Microsc. Microanal. 2020;26:589–598. doi: 10.1017/S143192762000152X. [DOI] [PubMed] [Google Scholar]
- Popescu L.M. TELOCYTES - a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-LikeCells (ICLC) to TELOCYTES. J. Cell Mol. Med. 2010;14:729–740. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu L.M., Gherghiceanu M., Suciu L.C., Manole C.G., Hinescu M.E. Telocytes and putative stem cells in the lungs: electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011;345:391–403. doi: 10.1007/s00441-011-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roatesi I., Radu B.M., Cretoiu D., Cretoiu S.M. Uterine telocytes: a review of current knowledge. Biol. Reprod. 2015;93:10. doi: 10.1095/biolreprod.114.125906. [DOI] [PubMed] [Google Scholar]
- Rusu M.C., Mirancea N., Mănoiu V.S., Vâlcu M., Nicolescu M.I., Păduraru D. Skin telocytes. Ann. Anat. 2012;194:359–367. doi: 10.1016/j.aanat.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Shoshkes-Carmel M., Wang Y.J., Wangensteen K.J., Tóth B., Kondo A., Massasa E.E., Itzkovitz S., Kaestner K.H. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature. 2018;557:242–246. doi: 10.1038/s41586-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi M.G. The telocytes: ten years after their introduction in the scientific literature. an update on their morphology, distribution, and potential roles in the gut. Int. J. Mol. Sci. 2020;21:4478. doi: 10.3390/ijms21124478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi M.G., Traini C., Manetti M., Ibba-Manneschi L., Faussone-Pellegrini M.S. Telocytes express PDGFRα in the human gastrointestinal tract. J. Cell Mol. Med. 2013;17:1099–1108. doi: 10.1111/jcmm.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xiao L., Zhang R., Jin H., Shi H. Ultrastructural and immunohistochemical characteristics of telocytes in human scalp tissue. Sci. Rep. 2020;10:1693. doi: 10.1038/s41598-020-58628-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. In memoriam: Laurentiu Mircea Popescu (1944-2015) J. Cell Mol. Med. 2015;19:2045–2046. doi: 10.1111/jcmm.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Lu S., Zhang H. Transmission electron microscope evidence of telocytes in canine dura mater. J. Cell Mol. Med. 2016;20:188–192. doi: 10.1111/jcmm.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Liu Y., Ahmed N., Ullah S., Liu Y.I., Chen Q. Ultrastructural identification of telocytes in the muscularis of chicken ileum. Exp. Ther. Med. 2015;10:2325–2330. doi: 10.3892/etm.2015.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Zhu X., Wang L., Ahmed N., Huang Y., Chen H., Zhang Q., Ullah S., Liu T., Guo D., Brohi S.A., Chen Q. Cellular evidence of telocytes as novel interstitial cells within the magnum of chicken oviduct. Cell Transplant. 2017;26:135–143. doi: 10.3727/096368916X692942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Song D., Shi L., Sun X., Zheng Y., Zeng Y., Wang X. Mechanisms of interactions between lung-origin telocytes and mesenchymal stem cells to treat experimental acute lung injury. Clin. Transl. Med. 2020;10:e231. doi: 10.1002/ctm2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]