Abstract
The near-globally distributed ecto-parasitic mite of the Apis mellifera honeybee, Varroa destructor, has formed a lethal association with Deformed wing virus, a once rare and benign RNA virus. In concert, the two have killed millions of wild and managed colonies, particularly across the Northern Hemisphere, forcing the need for regular acaricide application to ensure colony survival. However, despite the short association (in evolutionary terms), a small but increasing number of A. mellifera populations across the globe have been surviving many years without any mite control methods. This long-term survival, or Varroa resistance, is consistently associated with the same suite of traits (recapping, brood removal and reduced mite reproduction) irrespective of location. Here we conduct an analysis of data extracted from 60 papers to illustrate how these traits connect together to explain decades of mite resistance data. We have potentially a unified understanding of natural Varroa resistance that will help the global industry achieve widespread miticide-free beekeeping and indicate how different honeybee populations across four continents have resolved a recent threat using the same suite of behaviours.
Keywords: Varroa destructor, Apis mellifera, Varroa resistance, recapping, brood removal, mite infertility
1. Introduction
Throughout the world the western honeybee, Apis mellifera, is an irreplaceable species, particularly in terms of their pollination services that contribute to food security and wider ecosystem health [1,2]. Despite the huge reliance on and commercialization of honeybees, their populations have for many years suffered high losses, particularly over the winter period [3,4]. While it is apparent that numerous stressors such as intensive agriculture and diseases are owing to this decline, it is well established that during the past 70 years the synergy between Deformed wing virus (DWV) and its vector Varroa destructor has become a critical global threat to honeybee health [5].
After Varroa jumped the species barrier around the 1950s, from its native host Apis cerana (Asian honeybee) onto A. mellifera, it spread globally along with DWV [6–8]. Currently only Australia and a few small, isolated islands are free of both DWV and Varroa [9,10]. As A. mellifera was completely naive to the mite, Varroa typically increased uncontrollably, which, coupled with a new viral transmission route (during mite feeding), led to the catastrophic collapse of both managed and feral populations across the globe [11]. As a result, particularly in the Northern Hemisphere, the constant use of acaricides is necessary for beekeeping to survive [12]. However, while acaricides help reduce the Varroa and DWV burden, they also remove the selective pressure from A. mellifera hampering any adaptation to the parasite [13–18]. Only three Varroa-infested A. mellifera populations exist without DWV and hence have never been treated with acaricides. These exist in the highlands of Papua New Guinea, the Solomon Islands [19] and on the island of Fernando de Noronha, Brazil [20]. Although the mechanism is unknown, Varroa resistance arose quickly, caused no colony losses and resulted in high levels of infertile mites in the Fernando de Noronha population [20].
In the presence of DWV and the absence of treatment, A. mellifera populations are able to gradually develop Varroa resistance, typically after an initial period of colony losses [21]. Resistance is the ability of a population to survive long term without any treatment for Varroa within a given environment [16]. Thus, we do not view resistance as a fixed trait but the product of adaptive traits and adaptation to the local environment [17,22] in terms of the surrounding managed and feral colonies. Varroa-resistant colonies first appeared in Africa [23,24] and Africanized honeybees (African × European hybrid) in South America [25], and were associated with widespread lack of control due to acaricide cost and the general resilience of the bee populations. These populations, unlike in developed countries, are not frequently treated or medicated against a range of pathogens and pests [26]. Despite this, a small but increasing number of beekeepers in Europe [27], the UK [28,29] and the USA [30,31] have stopped all regular acaricide treatment and often establish their managed colonies from feral swarms [31,32].
Independently, each Varroa-resistant honeybee population previously studied across seven countries has developed the same traits to control the mite. These are: (i) brood removal, in which Varroa-infested pupae are removed; (ii) recapping, where holes are created allowing direct access to the pupa and then resealed; and (iii) mite infertility, where female mites are unable to produce viable (mated) female offspring.
Unlike many maladies the Varroa–DWV assoication is a new problem especially in evolutionary terms, since Varroa has only been in A. mellifera populations between 15 and 70 years, depending on the location [6]. However, three studies [27,33,34] using the same methods found two traits (increased recapping and mite infertility) in Varroa-resistant populations in South Africa, Brazil, France, UK, Norway and Sweden, countries with different environmental conditions (tropical to sub-artic). This indicates that Varroa resistance has arisen in multiple locations, irrespective of honeybee variety or environment, especially since recapping behaviour is rarely seen in Varroa-naive populations in Australia, Isle of Man and Isle of Colonsay, UK [33,34].
This study's aim is to bring together data from 60 publications over the past 40 years combined with a recent breakthrough study [27] to compare the expression of brood removal, recapping and mite infertility in resistant colonies and susceptible colonies. We then construct a potential framework that links these three traits and use modelling to explore various aspects of the framework.
2. Methods
(a) . Data collection
We searched published literature using Scopus, Web of Science and Google Scholar to collect data on the three key traits namely brood removal, recapping and Varroa non-reproduction in worker brood from susceptible and resistant A. mellifera populations. We define resistant populations as those that have survived five or more years without any form of mite treatment, although many populations studied have survived untreated more than 10 years and some for decades. Despite many studies used to collate the data, the methods employed are all basically the same. Furthermore, a study was only included if a minimal sample size of 50 cells was recorded, used natural comb, and only included cells infested with a single foundress.
We extracted information from 60 key data-rich papers. Where possible single colony data were extracted. For example, all recapping data (n = 163) came from single colonies; for brood removal nine of the 86 data points are colony averages and for mite infertility 75 of the 99 data points are colony averages, due to sample size limitations (see electronic supplementary material data for all source data). No susceptible colonies are known from where Africanized and African bees occur hence comparisons with resistant colonies in these locations are not possible. Almost all the data collected concerns the Korean ‘K’ haplotype of Varroa (see electronic supplementary material data for more information).
(b) . Brood removal
We used the standard bee search string (Apis mellifera OR honeybee OR honeybee) AND (removal OR brood removal OR hygienic behaviour OR VSH OR varroa-sensitive hygiene OR varroa-specific hygiene) AND varroa. We looked for studies that measured the removal of brood that had been artificially or naturally infested (one study [35]) with Varroa. Studies using artificial infestation all had to follow the same basic protocol outlined in [33]. In brief, a frame of freshly capped brood is taken from a colony and mites are inserted carefully into the capped cells containing recently capped cells. After around 10 days in the colony the frame is inspected, and the number of infested cells removed is recorded.
(c) . Recapping
We used the standard bee string AND (re-capping OR recapping) AND varroa. To be included, studies had to have measured the recapping of Varroa-infested cells following the correct protocol outlined in [36,37].
(d) . Mite infertility
We used the standard bee search string AND (varroa OR varroa mite OR mite) AND (reproduction OR non-reproduction OR fertility OR infertility). Here we define infertility as the inability to produce a viable (mated) female offspring and so we collected data following this definition. Importantly, some data used were collected from papers that used the definition of no egg laying. The justification for this is that non-egg laying also falls within the definition, and at worst provides an underestimate of the reduced reproductive rate of mites. To calculate the effect of brood removal on offspring production by Varroa, a simple equation was formulated,
where a is proportion of infested cells removed, b is maximum number of viable offspring produced per cycle and c is average number of viable female offspring produced per reproductive cycle.
(e) . Data analysis
The sample sizes (in cells) were used to calculated weighted averages for each of the traits for resistant and susceptible populations. Statistical analyses were conducted in Minitab version 18 on unweighted data [38]. Mann–Whitney U-tests were used to compare the removal abilities, recapping abilities and infertile mite proportions of resistant and susceptible populations. Statistical significance for all tests was p < 0.05.
The effect of brood removal on mite and honeybee population growth was modelled using the BEEHAVE model [39]. Increasing worker pupal mortality rates were used to simulate brood removal (as dead brood is removed in the simulation). The mortality was independent of mite infestation as the effect of DWV was removed from the equation for simplicity since within the BEEHAVE model DWV also affects pupa mortality confounding the observation of the effect of brood removal. This simplification was deemed acceptable as the result would only provide an underrepresentation. In actuality, as bees target infested cells it would likely take less removal to achieve the same outcome.
(f) . Framework construction
After collecting and analysing the data, we constructed a hypothetical framework to explain how many of the various traits are connected. Data from this study or findings from related studies were used to justify the proposed link between each trait.
3. Results
(a) . Honeybee behaviour
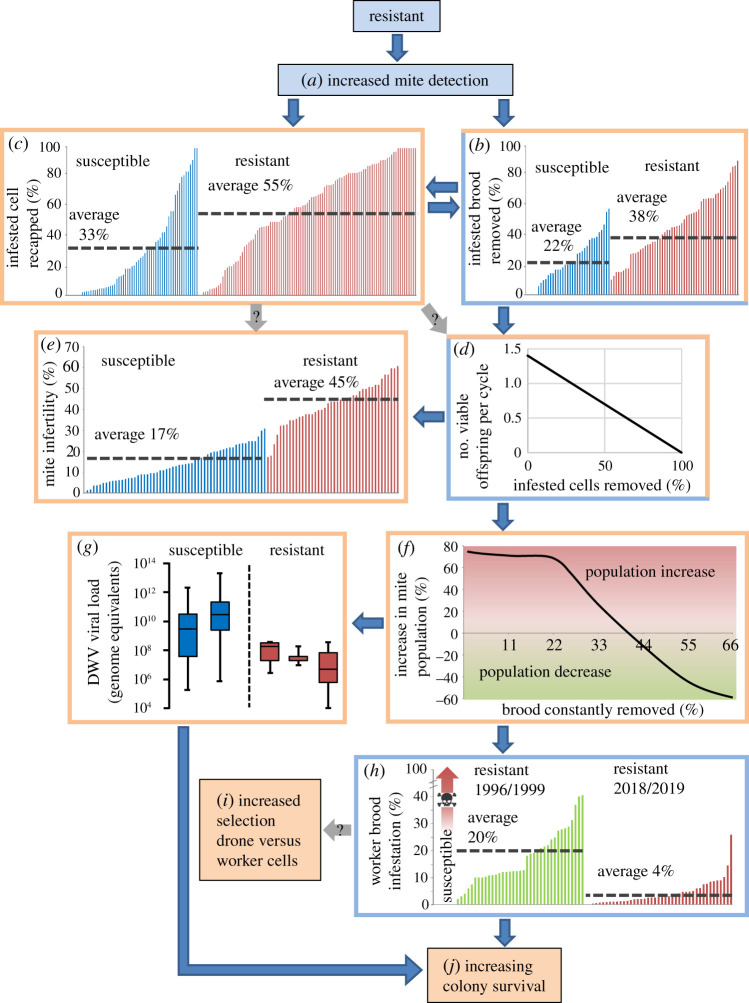
Recapping behaviour is the resealing of holes made in the cap that covers the developing worker pupa, holes allow better access to the signal(s) that trigger hygienic behaviour [33,40]. We collected data from 163 colonies from five studies that took place across seven countries (figure 1c). This showed that in resistant colonies significantly more infested cells are recapped than in susceptible colonies (55% versus 33%) (U = 1280, p < 0.00001).
Figure 1.
A proposed framework for the development of Varroa resistance. Boxes in blue (a) or with a blue border (b,d,h) are ‘causes’ of the ‘effects’ that are indicated by boxes in orange (i,j) or with orange borders (b–g). All source data for each chart are available in the electronic supplementary material, tables S1–S8 and figure S1. Arrows with a question mark indicate possible links suggested in the literature. In box h, the red arrow indicates that in untreated, susceptible colonies Varroa infestations continuously rise until colony death. Deformed wing virus data in box g are adapted from [41] and discussed below. (Online version in colour.)
Brood removal is a trait of honeybees where diseased or dead pupae are removed. It defends the colony against the spread of several diseases including chalkbrood, American foul brood and Varroa infestation. Data from mite-infestation experiments from 403 colonies (86 data points) across 10 studies conducted in seven countries demonstrate that resistant colonies are significantly (U = 341.5, p < 0.0001) better at removing mite-infested brood than susceptible colonies (38% versus 22%) (figure 1b). When separated into populations both Africanized bees and their African relatives (A. m scutellata and A. m capensis) have significantly greater (U = 83, p < 0.0001 and U = 207.5, p = 0.002) removal abilities than susceptible colonies in Europe.
(b) . Varroa reproduction
We used the equation ‘(1 − a) × b = c' (see Methods), which generates a linear relationship between brood removal and reproductive output (figure 1d). The removal of 38% and 22% infested brood in resistant or susceptible colonies (figure 1b) predicts 0.87 (resistant) and 1.09 (susceptible) viable female offspring are produced per reproductive cycle when no removal allows 1.4 viable female offspring to be produced [42]. If a maximum value of 1.6 (56) is used, values of 0.99 (resistant) and 1.25 (susceptible) are obtained. These values are independent of the total number of reproductive cycles performed, which varies between two and three [43–45]. The decrease in reproductive output increases the proportion of infertile mites (see Discussion for details). Data from 786 colonies (99 data points) across 40 studies in 14 countries showed that resistant populations had significantly (U = 28, p < 0.0001) greater proportions of infertile mites than susceptible colonies (45% versus 17%) (figure 1e).
(c) . Colony level effects
The BEEHAVE model predicted that removing greater than 40% of infested pupae results in negative mite population growth (figure 1f). Additionally, it predicted that, irrespective of infestation status, if the brood removal rate were to exceed 40% in spring, 55% in summer or 60% in winter, the colony would collapse (electronic supplementary material, figure S2). However, resistant colonies now typically only have worker-brood infestation rates of around 4% (figure 1h).
(d) . Decreasing worker-brood infestation levels
In the Africanized colonies, which are all resistant, average worker-brood infestation rates have fallen from 20% during 1996–1998 to 4% in 2018–2019 (figure 1h). Additional preliminary data from UK-resistant colonies (n = 44) collected by the authors and [46] found that brood infestation averaged at 6% and was not significantly different to Africanized colonies in 2018/2019 (U = 460, p = 0.052).
(e) . Framework
Using the data and analyses presented above, we constructed a framework to link them together to explain how Varroa resistance may develop in A. mellifera (figure 1). Our interpretation centres on the idea that an existing trait, hygienic behaviour, when adapted to detecting and removing mite-infested pupae, can explain all other traits. Given the data and the models used as well as the findings of other studies, we believe our framework to be the most plausible interpretation of the results we have presented here. Further justifications for the framework are presented in the discussion.
4. Discussion
The proposed framework attempts to explain how Varroa resistance may develop in honeybee (A. mellifera) populations. The framework suggests that resistance is a sequence of events that generate the key traits (increased recapping, brood removal and mite infertility) rather than a single trait [21,47]. Here we found that the enhanced expression of these three key traits is common among resistant populations. This independent occurrence of the key traits within colonies across the world could be an example of parallel evolution [27], because while the recapping and removal behaviours predate Varroa, they have been co-opted to control Varroa, recapping is rare trait in mite-naive colonies, but occurs at low and high levels in susceptible and resistant colonies respectively [33,40]. Similarly, other traits such as brood suppression of mite reproduction [48], or DWV tolerance [49,50] may complement those within the framework. There is also likely to be a mite element to resistance which could be illuminated by further studies into the coevolution of A. mellifera and Varroa [51,52]. As resistance is a population level trait rather than a single colony trait, a resistant colony becomes vulnerable if moved out of its population and could collapse if a sudden influx of mites occurs due to excessive (40–60%) brood removal (electronic supplementary material, figure S2). This may explain why resistant colonies moved out of their population typically do not survive [53] (S.J.M. 2017, 2019, personal observation).
(a) . Honeybee behaviour
The framework begins with the increased detection of Varroa-infested cells, an ability that has been linked to resistant bees by numerous studies [33,54–57] (figure 1a). Unlike most brood diseases Varroa-DWV is a chronic condition that does not kill the developing host pupae but shortens its lifespan as an adult [43,58,59]. Bees already have a well-developed hygienic behaviour response but it typically deals with diseases that cause dead brood [60]. Despite this, clear evidence exists for the detection of infested cells, directly from six mite insertion experiments and one natural infestation experiment (figure 1b), and indirectly from the behaviour known as recapping (figure 1c).
The fact that on average resistant colonies remove and recap significantly greater proportions of infested cells than susceptible colonies (figure 1b,c) indicates that increased detection of infested cells causes these traits to increase. Additionally, recapping has been shown to be positively correlated with brood removal [27,33] further suggesting a common trigger. Increased recapping may occur because more sensitive adults [55–57] investigate sealed brood around infested cells either due to a diffuse signal emanating from infested cells or increased cursory checking near infested cells [33,40].
Typically, hygienic behaviour tests use the freeze-killed brood method [61], and this does not correlate with removal of mite-infested brood [33,46,62–66]. However, this does not negate the contribution of hygienic behaviour to mite resistance, since the cues are different (living versus dead pupae) [47] and freezing kills many brood at the same time in the same location, thus generating an abnormally high concentration of cues. Therefore, if colonies perform exceptionally well (remove greater than 95% dead brood within 24 h) they may remove a reasonable amount (average of 66%) of Varroa-infested brood and have high recapping rates [65].
It is unclear whether the cues involved are emanating from the mites or pupae [55,56,67–69] or both [54], since parasitization by Varroa and DWV infection causes changes to the chemical profile of pupae [68,69,70–73]. Six compounds (four ketones and two acetates) have been detected on both infested pupae and mites, and although all adult workers can detect these compounds only workers from resistant colonies can distinguish the mix of six compounds from healthy brood [54]. Other studies [71,74] have detected different compounds that could also stimulate a hygienic response. The general consensus is that multiple chemical cues are involved in hygienic behaviour, which may prevent the loss of healthy brood, if a cell is wrongly opened the subsequent lack of the secondary cue could trigger resealing or ‘recapping’ [40]. Indeed, recapping of both non-infested and infested cells is consistently elevated in all resistant populations [33]. The hole made in the cell cap is generally less than 1 mm in non-infested cells, but significantly larger (up to 5 mm) in infested cells [33,46], which may increase the detection of less volatile cues such as those described [54].
(b) . Varroa reproduction
In our framework, we link increased removal of mite-infested to reduced reproductive output and thus increased mite infertility (figure 1b,d,e). Previous studies have also suggested links between increased brood removal, potentially recapping [27,75] and reduced mite reproductive success [76]. In agreement, we found that resistant colonies had a significantly greater percentage of infertile mites (figure 1e). A simple explanation is that disrupting the very uniform sequence of mite-reproduction leads to foundress-mites producing less offspring and depleting their finite supply of 18–30 eggs [77–80] and limited supply of spermatozoa [80,81]. Infertile mites have fewer spermatozoa [82], and the number of laid eggs steadily declines in mites preforming more than two reproductive cycles [77]. Using the simple equation (figure 1d), the estimated reproductive values for resistant and susceptible colonies of between 0.87–0.99 and 1.09–1.25, respectively, were similar to actual values from resistant and susceptible colonies [27,33,83]. Whatever the reason, the reproductive asynchrony caused by the removal of infested pupa causes less mites to contribute to the next generation, thus population growth slows and there is a reduced proportion of new fertile mites compared to older infertile mites [76,84]. In addition to brood removal, reductions in mite fertility may be the result of similar interruptions by recapping [75] and/or brood effects [48], but more data is needed.
(c) . Colony-level effects
Reduced fertility we then linked to reduced population growth because our BEEHAVE model predicted that infested brood removal above 40% caused negative mite population growth (figure 1f). Thus, in our framework the detection and removal via cannibalization of infested worker-brood leads to reduced mite population growth, a commonly occurring outcome in surviving populations [47]. Additionally, because brood removal varies within a population (figure 1b) the BEEHAVE model helps explain the fluctuating mite populations observed in long-term studies of resistant colonies [83,85,86]. Other studies also found an association between increased mite infertility and a reduced mite burden [24,63,87–89], again suggesting it may link brood removal and population growth.
Furthermore, reduced mite burden also reduces the number of viral vectors [22], causing lower viral titres (figure 1g) [41,90–92] and a reduced number of deformed bees [93–95]. One study [96] found that removal above 95% of freeze-killed pupae lowered mite population growth and significantly lowered DWV titres in workers than colonies below 95% removal. However, cannibalism of infested pupae allows DWV prevalence to remain high [97] even in resistant populations [98], but titres fall since oral (natural) viral transmission is much less infective than via injection [93,97].
(d) . Decreasing worker-brood infestation levels
In non-resistant untreated colonies, mite populations increase until colony collapse with increasing brood infestation levels from 30% to 100% at colony collapse [99], whereas in resistant colonies worker-brood infestation rate is maintained below 20% (figure 1h). Interestingly, we found that worker-brood infestation has fallen significantly (U = 123, p < 0.0001) from 20% to just 4% over the past two decades in resistant colonies in South America (figure 1h) currently the only location with long-term data.
We speculate that this is because mites are increasingly waiting for drone brood, which is not targeted by hygienic behaviour in either A. mellifera nor A. cerana [100]. Furthermore, the proportion of mites on adult bees decreased when drone brood was plentiful and increased when it was scarce [101]. Similarly, in resistant colonies from Uruguay the ratio of the mites' distribution between worker and drone cells was much greater (1 : 12.6) than in susceptible colonies (1 : 5.7) [102]. Heavily infested drone brood has also been observed in resistant populations in Mexico, Brazil and South Africa (30% [33]); however, much of the evidence is anecdotal and needs studying further.
In fact, the evolutionary reason why V. jacobsoni avoids worker brood in its natural host A. cerana remains unclear. It is well established in A. cerana that V. jacobsoni rarely reproduces in worker brood [103–106], and the drone pupa dies if infested by multiple mite families and becomes entombed within the cell rather than removed [107]. When V. destructor mites are artificially inserted into incubated A. cerana worker brood 30–50% of the pupae die [108], potentially due to a saliva toxin protein from V. destructor, but no mortality occurs in A. mellifera [108,109]. This implies that hygienic behaviour in A. cerana relies on detecting dead brood, making the ability to detect living infested pupa and mites [54] in A. mellifera even more unique. However, further studies in A. cerana are required to differentiate between or link together (i) the detection and removal of living mite-infested brood, (ii) social apoptosis and removal of dead brood, and (iii) any coevolution by Varroa or worker brood that prevents mite reproduction.
Finally, in a small resistant A. mellifera population on the remote Fernando de Noronha Island, Brazil, adult mite infestation levels fell from 26% in 1991 to 1–2% in 2016. However, in worker and drone brood infestation levels have stabilized around 20% and 40%, respectively (electronic supplementary material, figure S3) [20,110], despite very high infertility rates [20]. This may be explained by the very rare absence of DWV from this population that allows high brood infestation levels to persist without the negative impacts of DWV. Confirmatory studies from the other two DWV-free Varroa-infested populations [19] are needed.
(e) . Reduced colony losses
The final link in our framework is that reduced mite and virus burden will lead to enhanced colony survival [43]. Indeed, the reduction of mite burden and associated enhanced survival is the primary function of acaricides. Enhanced survival is hard to measure as susceptible colonies are usually treated with acaricides. However, the annual loss rates of treated colonies are higher than resistant populations in Le Mans and Avignon (France) [111]. Additionally, over 100 beekeepers across a 2500 km² region of north Wales (UK) have maintained 499 colonies treatment free for 11 years [32], and in Swindon (UK) a small beekeeper group have kept treatment-free colonies since 1995 [112], and neither group have report increased losses. In South Africa, after an initial period of high losses, annual colony losses stabilized at around 5% between 1998 and 2004, which is similar to pre-Varroa levels [23]. Also, in Algeria, Tunisia and Morocco, initial colony losses were high, although short-lived [113]. Across most of Africa [23,113–116] and in Africanized colonies throughout Latin America no widespread losses were reported where lack of acaracide use, due to cost and availability, may have helped resistance develop. Instead, widespread colony losses occurred in the Northern Hemisphere as Varroa spread from Asia throughout Europe and into the Americas, where acaracides were quickly adopted.
(f) . Variability of data
A substantial issue when it comes to measuring resistance traits is the inherent variability within colonies and thus across populations. Within a colony, traits themselves are not static and fluctuate with the changing season along with the associated availability of worker and drone brood and the infestation level [52,117–123]. Variability is also likely due to temporal changes in the composition of the different hygienic workers. To elaborate, the three main stages of brood removal (the initial detection and opening of the cell cap, the full uncapping of the cell and finally removing or cannibalizing the pupae or recapping the cell [124]) are conducted by bees of different ages and sensory acuity, a division of labour further affected by genetic, neural, social and environmental conditions [55,125–128]. For example, an imbalance of ‘uncapper’ versus ‘recapper’ bees may cause many brood cells to be left open [55]. Consequently, it can be very hard to accurately measure resistance-associated traits [117,118,129], resulting in a high degree of variability within colonies and across colony-level datasets (figure 1b,c,e). Ultimately, variability severely affects selection programmes (reviewed in [130]), whereas in natural selection-based experiments such as bond experiments [15] and black box experiments [13,131], assumptions on the importance of traits are not made.
5. Conclusion
This study shows that the resistance traits of recapping, brood removal and mite infertility are expressed at significantly higher levels in resistant colonies than susceptible ones, and we present a framework to potentially explain how these common traits shared by resistant colonies can link together. Although many local sub-species exist, A. mellifera remains a single species and environmental conditions within the colony (i.e. those that Varroa are subject to) remain remarkably constant irrespective of location, which has aided its semi-domestication and global distribution. Natural bee-driven resistance to Varroa is a sustainable, long-term solution, prevents the constant usage of acaricides, will not weaken bees to any other maladies should they arise and may provide an example of parallel evolution with the same three traits arising in populations in several different continents.
Supplementary Material
Acknowledgements
We would like to thank four reviewers and the editor for helping to improve the manuscript, and M. Spivak and M. Oddie for initial discussions.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h18931zk6p [132]. The data are provided in electronic supplementary material [133].
Authors' contributions
I.G.: conceptualization, data curation, formal analysis, methodology, writing-original draft, writing-review and editing; S.J.M.: conceptualization, supervision, writing-original draft, writing-review and editing.
Both authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
The authors declare no competing interests.
Funding
Funding for I.G. was provided by Bee Diseases Insurance Ltd, UK.
References
- 1.Gallai N, Salles J-M, Settele J, Vaissière BE. 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68, 810-821. ( 10.1016/j.ecolecon.2008.06.014) [DOI] [Google Scholar]
- 2.Hung KJ, Kingston JM, Albrecht M, Holway DA, Kohn JR. 2018. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B 285, 20172140. ( 10.1098/rspb.2017.2140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345-353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 4.Gray A, et al. 2019. Loss rates of honey bee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J. Api Res. 58, 479-485. ( 10.1080/00218839.2019.1615661) [DOI] [Google Scholar]
- 5.Nazzi F, Le Conte Y. 2016. Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu. Rev. Entomol. 61, 417-432. ( 10.1146/annurev-ento-010715-023731) [DOI] [PubMed] [Google Scholar]
- 6.Oldroyd BP. 1999. Coevolution while you wait: Varroa jacobsoni, a new parasite of western honeybees. Trends Ecol. Evol. 14, 312-315. ( 10.1016/S0169-5347(99)01613-4) [DOI] [PubMed] [Google Scholar]
- 7.Wilfert L, Long G, Leggett HC, Schmid-Hempel P, Butlin R, Martin SJM, Boots M. 2016. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 351, 594. ( 10.1126/science.aac9976) [DOI] [PubMed] [Google Scholar]
- 8.Martin SJ, Brettell LE. 2019. Deformed wing virus in honeybees and other insects. Annu. Rev. Virol. 6, 49-69. ( 10.1146/annurev-virology-092818-015700) [DOI] [PubMed] [Google Scholar]
- 9.Roberts JMK, Anderson DL, Durr PA. 2017. Absence of deformed wing virus and Varroa destructor in Australia provides unique perspectives on honeybee viral landscapes and colony losses. Sci. Rep. 7, 6925. ( 10.1038/s41598-017-07290-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shutler D, Head K, Burgher-MacLellan KL, Colwell MJ, Levitt AL, Ostiguy N, Williams GR. 2014. Honey bee Apis mellifera parasites in the absence of Nosema ceranae fungi and Varroa destructor mites. PLoS ONE 9, e98599. ( 10.1371/journal.pone.0098599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliash N, Mikheyev A. 2020. Varroa mite evolution: a neglected aspect of worldwide bee collapses? Curr. Opin. Insect Sci. 39, 21-26. ( 10.1016/j.cois.2019.11.004) [DOI] [PubMed] [Google Scholar]
- 12.Boecking O, Genersch E. 2008. Varroosis: the ongoing crisis in bee keeping. J. Verbraucherschutz und Lebensmittelsicherheit 3, 221-228. ( 10.1007/s00003-008-0331-y) [DOI] [Google Scholar]
- 13.Neumann P, Blacquiere T. 2017. The Darwin cure for apiculture? Natural selection and managed honeybee health. Evol. Appl. 10, 226-230. ( 10.1111/eva.12448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fries I, Bommarco R. 2007. Possible host-parasite adaptations in honey bees infested by Varroa destructor mites. Apidologie 38, 525-533. ( 10.1051/apido:2007039) [DOI] [Google Scholar]
- 15.Fries I, Imdorf A, Rosenkranz P. 2006. Survival of mite infested (Varroa destructor) honey bee (Apis mellifera) colonies in a Nordic climate. Apidologie 37, 564-570. ( 10.1051/apido:2006031) [DOI] [Google Scholar]
- 16.Büchler R, Berg S, Le Conte Y. 2010. Breeding for resistance to Varroa destructor in Europe. Apidologie 41, 393-408. ( 10.1051/apido/2010011) [DOI] [Google Scholar]
- 17.Traynor KS, Mondet F, de Miranda JR, Techer M, Kowallik V, Oddie MAY, Chantawannakul P, Mcafee A. 2020. Varroa destructor: a complex parasite crippling honey bees worldwide. Trends Parasitol. 20, 592-606. ( 10.1016/j.pt.2020.04.004) [DOI] [PubMed] [Google Scholar]
- 18.Råberg L, Graham AL, Read AF. 2009. Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364, 37-49. ( 10.1098/rstb.2008.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts JMK, Simbiken N, Dale C, Armstrong J, Anderson DL. 2020. Tolerance of honey bees to Varroa mite in the absence of deformed wing virus. Viruses 12, 575. ( 10.3390/v12050575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brettell LE, Martin SJ. 2017. Oldest Varroa tolerant honey bee population provides insight into the origins of the global decline of honey bees. Sci. Rep. 7, 45953. ( 10.1038/srep45953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locke B. 2016. Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 47, 467-482. ( 10.1007/s13592-015-0412-8) [DOI] [Google Scholar]
- 22.Le Conte Y, Meixner M, Brandt A, Carreck NL, Costa C, Mondet F. 2020. Geographical distribution and selection of European honey bees resistant to Varroa destructor. Insects 11, 873. ( 10.3390/insects11120873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allsopp M. 2006. Analysis of Varroa destructor infestation of southern African honeybee populations. MRes thesis, University of Pretoria, Pretoria. [Google Scholar]
- 24.Nganso BT, Fombong AT, Yusuf AA, Pirk CWW, Stuhl C, Torto B. 2018. Low fertility, fecundity and numbers of mated female offspring explain the lower reproductive success of the parasitic mite Varroa destructor in African honeybees. Parasitology 145, 1633-1639. ( 10.1017/S0031182018000616) [DOI] [PubMed] [Google Scholar]
- 25.Moretto G, Gonçalves LS, De Jong D, Bichuette MZ. 1991. The effects of climate and bee race on Varroa jacobsoni Oud infestations in Brazil. Apidologie 22, 197-203. ( 10.1051/apido:19910303) [DOI] [Google Scholar]
- 26.Pirk CWW, Crewe RM, Moritz RFA, Nicolson S. 2017. Risks and benefits of the biological interface between managed and wild bee pollinators. Funct. Ecol. 31, 47-55. ( 10.1111/1365-2435.12768) [DOI] [Google Scholar]
- 27.Oddie M, Buchler R, Dahle B, Kovacic M, Le Conte Y, Locke B, De Miranda JR, Mondet F, Neumann P. 2018. Rapid parallel evolution overcomes global honey bee parasite. Sci. Rep. 8, 7704. ( 10.1038/s41598-018-26001-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruitwagen A, van Langevelde F, van Dooremalen C, Blacquière T. 2017. Naturally selected honey bee (Apis mellifera) colonies resistant to Varroa destructor do not groom more intensively. J. Api Res. 56, 354-365. ( 10.1080/00218839.2017.1329797) [DOI] [Google Scholar]
- 29.Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, Vanengelsdorp D, Pettis JS. 2010. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5, e9754. ( 10.1371/journal.pone.0009754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Underwood RM, Traver BE, Lopez-Uribe MM. 2019. Beekeeping management practices are associated with operation size and beekeepers' philosophy towards in-hive chemicals. Insects 10, 10. ( 10.3390/insects10010010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin SJ. 2020. Naturally mite-resistant colonies evolve on Hawaii. Am. Bee J. 160, 649-651. [Google Scholar]
- 32.Hudson C, Shan C. 2020. Treatment-free beekeeping. BBKA News 277, 229-232. [Google Scholar]
- 33.Martin SJ, Hawkins G, Brettell LE, Reece N, Correia-Oliveira M, Allsopp M. 2019. Varroa destructor reproduction and cell re-capping in mite-resistant Apis mellifera populations. Apidologie 51, 1-3. [Google Scholar]
- 34.Hawkins GP, Martin SJ. 2021. Elevated recapping behaviour and reduced Varroa destructor reproduction in natural Varroa resistant Apis mellifera honey bees from the UK. Apidologie 52, 647-657. ( 10.1007/s13592-021-00852-y) [DOI] [Google Scholar]
- 35.Vandame R, Morand S, Colin M, Belzunces L. 2002. Parasitism in the social bee Apis mellifera: quantifying costs and benefits of behavioral resistance to Varroa destructor mites. Apidologie 33, 433-445. ( 10.1051/apido:2002025) [DOI] [Google Scholar]
- 36.Harris J, Danka R, Villa JD. 2012. Changes in infestation, cell cap condition, and reproductive status of Varroa destructor (Mesostigmata: Varroidae) in brood exposed to honey bees with Varroa sensitive hygiene. Ann. Entomol. Soc. Am. 105, 512-518. ( 10.1603/AN11188) [DOI] [Google Scholar]
- 37.Boecking O, Spivak M. 1999. Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 30, 141-158. ( 10.1051/apido:19990205) [DOI] [Google Scholar]
- 38.Software MS. 2017. Minitab statistical software, 18th edn. State College, PA: Minitab. [Google Scholar]
- 39.Becher MA, Grimm V, Thorbek P, Horn J, Kennedy PJ, Osborne JL. 2014. BEEHAVE: a systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. J. Appl. Ecol. 51, 470-482. ( 10.1111/1365-2664.12222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grindrod I, Martin SJ. In press. Spatial distribution of recapping behaviour indicates clustering around Varroa infested cells. J. Api Res. ( 10.1080/00218839.2021.1890419) [DOI] [Google Scholar]
- 41.de Souza FS, Allsopp M, Martin SJ. 2020. Deformed wing virus prevalence and load in honeybees in South Africa. Arch. Virol. 166, 237-241. ( 10.1007/s00705-020-04863-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin SJ. 1994. Ontogenesis of the mite Varroa jacobsoni Oud. in worker brood of the honeybee Apis mellifera L. under natural conditions. Exp. Appl. Acarol. 18, 87-100. ( 10.1007/BF00055033) [DOI] [Google Scholar]
- 43.Martin SJ. 2001. The role of Varroa and viral pathogens in the collapse of honeybee colonies: a modelling approach. J. Appl. Ecol. 38, 1082-1093. ( 10.1046/j.1365-2664.2001.00662.x) [DOI] [Google Scholar]
- 44.Rosenkranz P, Aumeier P, Ziegelmann B. 2010. Biology and control of Varroa destructor. J. Invertebr. Pathol. 103, 96-119. ( 10.1016/j.jip.2009.07.016) [DOI] [PubMed] [Google Scholar]
- 45.Martin SJ, Kemp D. 1997. Average number of reproductive cycles performed by Varroa jacobsoni in honey bee (Apis mellifera) colonies. J. Api Res. 36, 113-123. ( 10.1080/00218839.1997.11100937) [DOI] [Google Scholar]
- 46.Hawkins G. 2020. Investigating naturally evolved Varroa destructor resistance in Apis mellifera honey bees: host behavioural traits and parasite reproductive biology. MRes thesis, The University of Salford, Salford. [Google Scholar]
- 47.Mondet F, Beaurepaire A, McAfee A, Locke B, Alaux C, Blanchard S, Danka B, Le Conte Y. 2020. Honey bee survival mechanisms against the parasite Varroa destructor: a systematic review of phenotypic and genomic research efforts. Int. J. Parasitol. 50, 433-447. ( 10.1016/j.ijpara.2020.03.005) [DOI] [PubMed] [Google Scholar]
- 48.Conlon BH, Aurori A, Giurgiu A, Kefuss J, Dezmirean DS, Mortiz RFA, Routtu J. 2019. A gene for resistance to the Varroa mite (Acari) in honey bee (Apis mellifera) pupae. Mol. Ecol. 28, 2958-2966. ( 10.1111/mec.15080) [DOI] [PubMed] [Google Scholar]
- 49.Thaduri S, Stephan JG, de Miranda JR, Locke B. 2019. Disentangling host-parasite-pathogen interactions in a varroa-resistant honeybee population reveals virus tolerance as an independent, naturally adapted survival mechanism. Sci. Rep. 9, 1-10. ( 10.1038/s41598-019-42741-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Locke B, Thaduri S, Stephan JG, Low M, Blacquière T, Dahle B, Le Conte Y, Neumann P, De Miranda JR. 2021. Adapted tolerance to virus infections in four geographically distinct Varroa destructor-resistant honeybee populations. Sci. Rep. 11, 12359. ( 10.1038/s41598-021-91686-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beaurepaire A, Moro A, Mondet F, Le Conte Y, Neumann P, Locke B. 2019. Population genetics of ectoparasitic mites suggest arms race with honeybee hosts. Sci. Rep. 9, 1-9. ( 10.1038/s41598-019-47801-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moro A, Blacquière T, Panziera D, Dietemann V, Neumann P. 2021. Host-parasite co-evolution in real-time: changes in honey bee resistance mechanisms and mite reproductive strategies. Insects. 12, 120. ( 10.3390/insects12020120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Büchler R, et al. 2015. The influence of genetic origin and its interaction with environmental effects on the survival of Apis mellifera L. colonies in Europe. J. Api Res. 53, 205-214. ( 10.3896/IBRA.1.53.2.03) [DOI] [Google Scholar]
- 54.Mondet F, et al. 2021. Chemical detection triggers honey bee defense against a destructive parasitic threat. Nat. Chem. Biol. 17, 524-530. ( 10.1038/s41589-020-00720-3) [DOI] [PubMed] [Google Scholar]
- 55.Gramacho KP, Spivak M. 2003. Differences in olfactory sensitivity and behavioral responses among honey bees bred for hygienic behavior. Behav. Ecol. Sociobiol. 54, 472-479. ( 10.1007/s00265-003-0643-y) [DOI] [Google Scholar]
- 56.Masterman R, Ross R, Mesce K, Spivak M. 2001. Olfactory and behavioral response thresholds to odors of diseased blood differ between hygienic and non-hygienic honey bees (Apis mellifera L.). J. Comp. Physiol. A 187, 441-452. ( 10.1007/s003590100216) [DOI] [PubMed] [Google Scholar]
- 57.Mondet F, Alaux C, Severac D, Rohmer M, Mercer AR, Le Conte Y. 2015. Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci. Rep. 5, 10454. ( 10.1038/srep10454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benaets K, et al. 2017. Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proc. R. Soc. B 284, 20162149. ( 10.1098/rspb.2016.2149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dainat B, Evans J, Chen Y-P, Gauthier L, Neumann P. 2011. Dead or alive: deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl. Environ. Microbiol. 78, 981-987. ( 10.1128/AEM.06537-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spivak M, Gilliam M. 1993. Facultative expression of hygienic behavior of honey bees in relation to disease resistance. J. Api Res. 32, 147-157. ( 10.1080/00218839.1993.11101300) [DOI] [Google Scholar]
- 61.Spivak M, Gilliam M. 1998. Hygienic behaviour of honey bees and its application for control of brood diseases and varroa. Part I. Hygienic behaviour and resistance to American foulbrood. Bee World. 79, 124-134. ( 10.1080/0005772X.1998.11099394) [DOI] [Google Scholar]
- 62.Boecking O, Drescher W. 1992. The removal response of Apis mellifera L. colonies to brood in wax and plastic cells after artificial and natural infestation with Varroa jacobsoni Oud and to freeze-killed brood. Exp. Appl. Acarol. 16, 321-329. ( 10.1007/BF01218574) [DOI] [Google Scholar]
- 63.Oddie M, Dahle B, Neumann P. 2017. Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. PeerJ. 5, e3956. ( 10.7717/peerj.3956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leclercq G, Pannebakker B, Gengler N, Nguyen BK, Francis F. 2017. Drawbacks and benefits of hygienic behavior in honey bees (Apis mellifera L.): a review. J. Apicult. Res. 56, 366-375. ( 10.1080/00218839.2017.1327938) [DOI] [Google Scholar]
- 65.Leclercq G, Blacquière T, Gengler N, Francis F. 2018. Hygienic removal of freeze-killed brood does not predict Varroa-resistance traits in unselected stocks. J. Apicult. Res. 57, 292-299. ( 10.1080/00218839.2018.1426350) [DOI] [Google Scholar]
- 66.Danka RG, Harris J, Villa JD, Dodds GE. 2013. Varying congruence of hygienic responses to Varroa destructor and freeze-killed brood among different types of honeybees. Apidologie 44, 447-457. ( 10.1007/s13592-013-0195-8) [DOI] [Google Scholar]
- 67.Mondet F, Kim SH, de Miranda JR, Beslay D, Le Conte Y, Mercer AR. 2016. Specific cues associated with honey bee social defence against varroa destructor infested brood. Sci. Rep. 6, 25444. ( 10.1038/srep25444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagoner KM, Spivak M, Rueppell O. 2018. Brood affects hygienic behavior in the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 111, 2520-2530. ( 10.1093/jee/toy266) [DOI] [PubMed] [Google Scholar]
- 69.Wagoner K, Spivak M, Hefetz A, Reams T, Rueppell O. 2019. Stock-specific chemical brood signals are induced by Varroa and Deformed Wing Virus, and elicit hygienic response in the honey bee. Sci. Rep. 9, 8753. ( 10.1038/s41598-019-45008-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baracchi D, Fadda A, Turillazzi S. 2012. Evidence for antiseptic behaviour towards sick adult bees in honey bee colonies. J. Insect. Physiol. 58, 1589-1596. ( 10.1016/j.jinsphys.2012.09.014) [DOI] [PubMed] [Google Scholar]
- 71.Wagoner KM, Millar JG, Schal C, Rueppell O. 2020. Cuticular pheromones stimulate hygienic behavior in the honey bee (Apis mellifera). Sci. Rep. 10, 7132. ( 10.1038/s41598-020-64144-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schoning C, Gisder S, Geiselhardt S, Kretschmann I, Bienefeld K, Hilker M, Genersch E. 2012. Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. J. Exp. Biol. 215, 264-271. ( 10.1242/jeb.062562) [DOI] [PubMed] [Google Scholar]
- 73.Salvy M, Martin C, Bagnères AG, Provost E, Roux M, Le Conte Y. 2001. Modifications of the cuticular hydrocarbon profile of Apis mellifera worker bees in the presence of the ectoparasitic mite Varroa jacobsoni in brood cells. Parasitology 122, 145-159. ( 10.1017/S0031182001007181) [DOI] [PubMed] [Google Scholar]
- 74.Nazzi F, Della Vedova G, D'Agaro M. 2004. A semiochemical from brood cells infested by Varroa destructor triggers hygienic behaviour in Apis mellifera. Apidologie 35, 65-70. ( 10.1051/apido:2003065) [DOI] [Google Scholar]
- 75.Oddie MAY, Burke A, Dahle B, Le Conte Y, Mondet F, Locke B. 2021. Reproductive success of the parasitic mite (Varroa destructor) is lower in honeybee colonies that target infested cells with recapping. Sci. Rep. 11, 9133. ( 10.1038/s41598-021-88592-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirrane MJ, De Guzman LI, Rinderer TE, Frake AM, Wagnitz J, Whelan PM. 2011. Asynchronous development of honey bee host and Varroa destructor (Mesostigmata: Varroidae) influences reproductive potential of mites. J. Econ. Entomol. 104, 1146-1152. ( 10.1603/EC11035) [DOI] [PubMed] [Google Scholar]
- 77.Ruijter A. 1987. Reproduction of Varroa jacobsoni during successive brood cycles of the honeybee. Apidologie 18, 321-326. ( 10.1051/apido:19870403) [DOI] [Google Scholar]
- 78.Akimov IA, Yastrebtsov AV. 1984. Reproductive system of Varroa jacobsoni I. Female reproductive system and oogenesis. Vestnik Zoologii. 6, 61-68. [Google Scholar]
- 79.Mikityuk. 1979. Reproductive ability of Varroa females. Pchelovodstvo 9, 2. [Google Scholar]
- 80.Alberti G, Hänel H. 1986. Fine structure of the genital system in the bee parasite, Varroa jacobsoni (Gamasida: Dermanyssina) with remarks on spermiogenesis, spermatozoa and capacitation. Exp. Appl. Acarol. 2, 63-104. ( 10.1007/BF01193355) [DOI] [Google Scholar]
- 81.Donzé G, Herrmann M, Bachofen B, Guerin P. 1996. Effect of mating freqiency and brood cell infestation rate on the reproductive success of the honeybee parasite Varroa jacobsoni. Ecol. Entomol. 21, 17-26. ( 10.1111/j.1365-2311.1996.tb00261.x) [DOI] [Google Scholar]
- 82.Harris J, Harbo JR. 1999. Low sperm counts and reduced fecundity of mites in colonies of honey bees (Hymenoptera: Apidae) resistant to Varroa jacobsoni (Mesostigmata: Varroidae). J. Econ. Entomol. 92, 83-90. ( 10.1093/jee/92.1.83) [DOI] [Google Scholar]
- 83.Medina LM, Martin SJ. 1999. A comparative study of Varroa jacobsoni reproduction in worker cells of honey bees (Apis Mellifera) in England and Africanized bees in Yucatan, Mexico. Exp. Appl. Acarol. 23, 659-667. ( 10.1023/A:1006275525463) [DOI] [Google Scholar]
- 84.Harris J, Danka RG, Villa JD. 2010. Honey Bees (Hymenoptera: Apidae) with the trait of Varroa sensitive hygiene remove brood with all reproductive stages of Varroa mites (Mesostigmata: Varroidae). Ann. Entomol. Soc. Am. 103, 146-152. ( 10.1603/AN09138) [DOI] [Google Scholar]
- 85.Souza LS. 2019. Varroa destructor mite infestation and virus detection in Africanized bees. PhD thesis, Universidade Federal do Recôncavo da Bahia, Brazil. [Google Scholar]
- 86.Mondragón L, Martin S, Vandame R. 2006. Mortality of mite offspring: a major component of Varroa destructor resistance in a population of Africanized bees. Apidologie 37, 67-74. ( 10.1051/apido:2005053) [DOI] [Google Scholar]
- 87.Locke B, Le Conte Y, Crauser D, Fries I. 2012. Host adaptations reduce the reproductive success of Varroa destructor in two distinct European honey bee populations. Ecol. Evol. 2, 1144-1150. ( 10.1002/ece3.248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kefuss J, Vanpoucke J, Bolt M, Kefuss C. 2015. Selection for resistance to Varroa destructor under commercial beekeeping conditions. J. Apicult. Res. 54, 563-576. ( 10.1080/00218839.2016.1160709) [DOI] [Google Scholar]
- 89.Strauss AS, Dietemann V, Human H, Crewe RM, Pirk CWW. 2015. Resistance rather than tolerance explains survival of savannah honeybees (Apis mellifera scutellata) to infestation by the parasitic mite Varroa destructor. Parasitology 143, 374-387. ( 10.1017/S0031182015001754) [DOI] [PubMed] [Google Scholar]
- 90.Kevill JL, de Souza FS, Sharples C, Oliver R, Schroeder DC, Martin SJ. 2019. DWV-a lethal to honey bees (Apis mellifera): a colony level survey of DWV variants (A, B, and C) in England, Wales, and 32 States across the US. Viruses 11, 426. ( 10.3390/v11050426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Souza FS, Kevill JL, Correia-Oliveira ME, de Carvalho CAL, Martin SJ. 2019. Occurrence of deformed wing virus variants in the stingless bee Melipona subnitida and honey bee Apis mellifera populations in Brazil. J. Gen. Virol. 100, 289-294. ( 10.1099/jgv.0.001206) [DOI] [PubMed] [Google Scholar]
- 92.Ryabov EV, Childers AK, Chen Y, Madella S, Nessa A, vanEngelsdorp D, Evans JD. 2017. Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States. Sci. Rep. 7, 17447. ( 10.1038/s41598-017-17802-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gusachenko ON, Woodford L, Balbirnie-Cumming K, Campbell EM, Christie CR, Bowman AS, Evans DJ. 2020. Green bees: reverse genetic analysis of deformed wing virus transmission, replication, and tropism. Viruses 12, 532. ( 10.3390/v12050532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dainat B, Neumann P. 2013. Clinical signs of deformed wing virus infection are predictive markers for honey bee colony losses. J. Invertebr. Pathol. 112, 278-280. ( 10.1016/j.jip.2012.12.009) [DOI] [PubMed] [Google Scholar]
- 95.Francis RM, Nielsen SL, Kryger P. 2013. Varroa-virus interaction in collapsing honey bee colonies. PLoS ONE 8, e57540. ( 10.1371/journal.pone.0057540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Toufailia HMA, Amiri E, Scandian L, Kryger P, Ratnieks FLW. 2014. Towards integrated control of Varroa: effect of variation in hygienic behaviour among honey bee colonies on mite population increase and deformed wing virus incidence. J. Api Res. 53, 555-562. ( 10.3896/IBRA.1.53.5.10) [DOI] [Google Scholar]
- 97.Posada-Florez F, Lamas Z, Hawthorne D, Chen Y, Evans DJ, Ryabov EV. 2020. Pupal cannibalism by worker honey bees contributes to the spread of Deformed wing virus. bioRxiv. ( 10.1101/2020.11.25.396259) [DOI] [PMC free article] [PubMed]
- 98.Kevill JL, Highfield A, Mordecai GJ, Martin SJ, Schroeder DC. 2017. ABC assay: method development and application to quantify the role of three DWV master variants in overwinter colony losses of European honey bees. Viruses 9, 314. ( 10.3390/v9110314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin SJ, Ball BV, Carreck NL. 2010. Prevalence and persistence of deformed wing virus (DWV) in untreated or acaricide-treated Varroa destructor infested honey bee (Apis mellifera) colonies. J. Api Res. 49, 72-79. ( 10.3896/IBRA.1.49.1.10) [DOI] [Google Scholar]
- 100.Harris JW. 2008. Effect of brood type on Varroa-sensitive hygiene by worker honey bees (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 101, 1137-1144. ( 10.1603/0013-8746-101.6.1137) [DOI] [Google Scholar]
- 101.Medina LM, Martin SJ, Espinosa-Montaño L, Ratnieks FLW. 2002. Reproduction of Varroa destructor in worker brood of Africanized honey bees (Apis mellifera). Exp. Appl. Acarol. 27, 79-88. ( 10.1023/A:1021579113907) [DOI] [PubMed] [Google Scholar]
- 102.Mendoza Y, Tomasco I, Antunez K, Castelli L, Branchiccela B, Santos E, Invernizzi C. 2020. Unraveling honey bee–Varroa destructor interaction: multiple factors involved in differential resistance between two Uruguayan populations. Vet. Sci. 7, 116. ( 10.3390/vetsci7030116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anderson DL. 1994. Non-reproduction of Varroa jacobsoni in Apis mellifera colonies in Papua New Guinea and Indonesia. Apidologie 25, 412-421. ( 10.1051/apido:19940408) [DOI] [Google Scholar]
- 104.Koeniger N, Koeniger G. 1983. Observations on mites of the asian honeybee species. Apidologie 14, 197-204. ( 10.1051/apido:19830305) [DOI] [Google Scholar]
- 105.Tewarson NC, Singh A, Engels W. 1992. Reproduction of Varroa jacobsoni in colonies of Apis cerana indica under natural and experimental conditions. Apidologie 23, 161-171. ( 10.1051/apido:19920209) [DOI] [Google Scholar]
- 106.Boot W, Tan NQ, Dien PC, Van Huan L, Van Dung N, Long LT, Beetsma J. 1997. Reproductive success of Varroa jacobsoni in brood of its original host, Apis cerana, in comparison to that of its new host, A. mellifera (Hymenoptera: Apidae). Bull. Entomol. Res. 87, 119-126. ( 10.1017/S0007485300027255) [DOI] [Google Scholar]
- 107.Rath W. 1999. Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie 30, 97-110. ( 10.1051/apido:19990202) [DOI] [Google Scholar]
- 108.Page P, Lin Z, Buawangpong N, Zheng H, Hu F, Neumann P, Chantawannakul P, Dietemann V. 2016. Social apoptosis in honey bee superorganisms. Sci. Rep. 6, 27210. ( 10.1038/srep27210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y, Han R. 2018. A saliva protein of Varroa mites contributes to the toxicity toward Apis cerana and the DWV elevation in A. mellifera. Sci. Rep. 8, 3387. ( 10.1038/s41598-018-21736-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Mattos IM, De Jong D, Soares AEE. 2016. Island population of European honey bees in Northeastern Brazil that have survived Varroa infestations for over 30 years. Apidologie 47, 818-827. ( 10.1007/s13592-016-0439-5) [DOI] [Google Scholar]
- 111.Le Conte Y, de Vaublanc G, Crauser D, Jeanne F, Rousselle J-C, Bécard J-M. 2007. Honey bee colonies that have survived Varroa destructor. Apidologie 38, 566-572. ( 10.1051/apido:2007040) [DOI] [Google Scholar]
- 112.Hoskins R. 2014. Swindon Honeybee Conservation Group. See http://www.swindonhoneybeeconservation.org.uk/.
- 113.Fazier M, Muli E, Conklin T, Schmehl D, Torto B, Frazier J, Tumlinson J, Evans JD, Raina S. 2010. A scientific note on Varroa destructor found in East Africa; threat or opportunity? Apidologie 41, 463-465. ( 10.1051/apido/2009073) [DOI] [Google Scholar]
- 114.Dietemann V, Pirk CWW, Crewe R. 2009. Is there a need for conservation of honeybees in Africa? Apidologie 40, 285-295. ( 10.1051/apido/2009013) [DOI] [Google Scholar]
- 115.Muli E, et al. 2014. Evaluation of the distribution and impacts of parasites, pathogens, and pesticides on honey bee (Apis mellifera) populations in East Africa. PLoS ONE 9, e94459. ( 10.1371/journal.pone.0094459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nganso BT, Fombong AT, Yusuf AA, Pirk CWW, Stuhl C, Torto B. 2017. Hygienic and grooming behaviors in African and European honeybees—New damage categories in Varroa destructor. PLoS ONE 12, e0179329. ( 10.1371/journal.pone.0179329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eynard SE, Sann C, Basso B, Guirao AL, Le Conte Y, Servin B, Tison L, Vignal A, Mondet F. 2020. Descriptive analysis of the Varroa non-reproduction trait in honey bee colonies and association with other traits related to Varroa resistance. Insects 11, 492. ( 10.3390/insects11080492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mondet F, et al. 2020. Evaluation of suppressed mite reproduction (SMR) reveals potential for Varroa Resistance in European honey bees (Apis mellifera L.). Insects 11, 595. ( 10.3390/insects11090595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kulinčević JM, Rinderer TE, Urošević DJ. 1988. Seasonality and colony variation of reproducing and non-reproducing Varroa jacobsoni females in western honey bee (Apis mellifera) worker brood. Apidologie 20, 173-180. ( 10.1051/apido:19880207) [DOI] [Google Scholar]
- 120.Marcangeli J, Eguaras M, Fernández N. 1992. Reproduction of Varroa jacobsoni (Acari: Mesostigmata: Varroidae) in temperate climates of Argentina. Apidologie 23, 57-60. ( 10.1051/apido:19920106) [DOI] [Google Scholar]
- 121.Otten C, Fuchs S. 1990. Seasonal variations in the reproductive behavior of Varroa jacobsoni in colonies of Apis mellifera carnica, A. m. ligustica and A. m. mellifera. Apidologie 21, 367-368. ( 10.1051/apido:19900304)) [DOI] [Google Scholar]
- 122.Bienefeld K, Radtke J, Zautke F. 1995. Influence of thermoregulation within honeybee colonies on the reproduction success of Varroa jacobsoni Oud. Apidologie 26, 329-330. [Google Scholar]
- 123.Moretto G, Gonçalves L, De Jong D. 1997. Relationship between food availability and the reproductive ability of the mite Varroa jacobsoni in Africanized bee colonies. Am. Bee J. 137, 67-69. [Google Scholar]
- 124.Palacio MA, Rodriguez E, Goncalves L, Bedascarrasbure E, Spivak M. 2010. Hygienic behaviors of honey bees in response to brood experimentally pin-killed or infected with Ascosphaera apis. Apidologie 41, 602-612. ( 10.1051/apido/2010022) [DOI] [Google Scholar]
- 125.Spivak M, Masterman R, Ross R, Mesce KA. 2003. Hygienic behavior in the honey bee (Apis mellifera L.) and the modulatory role of octopamine. J. Neurobiol. 55, 341-354. ( 10.1002/neu.10219) [DOI] [PubMed] [Google Scholar]
- 126.Scannapieco AC, et al. 2016. Individual precocity, temporal persistence, and task-specialization of hygienic bees from selected colonies of Apis mellifera. J. Apic Sci. 60, 63-74. [Google Scholar]
- 127.Goode K, Huber Z, Mesce KA, Spivak M. 2006. Hygienic behavior of the honey bee (Apis mellifera) is independent of sucrose responsiveness and foraging ontogeny. Horm. Behav. 49, 391-397. ( 10.1016/j.yhbeh.2005.08.007) [DOI] [PubMed] [Google Scholar]
- 128.Page RE, Robinson GE. 1991. The genetics of division of labour in honey bee colonies. In Advances in insect physiology, vol. 23 (ed. Evans PD), pp. 117-169. New York, NY: Academic Press. [Google Scholar]
- 129.Buchler R, Kovacic M, Buchegger M, Puskadija Z, Hoppe A, Brascamp EW. 2020. Evaluation of traits for the selection of Apis mellifera for resistance against Varroa destructor. Insects 11, 618. ( 10.3390/insects11090618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guichard M, Dietemann V, Neuditschko M, Dainat B. 2020. Advances and perspectives in selecting resistance traits against the parasitic mite Varroa destructor in honey bees. Genet. Sel. Evol. 52, 1-12. ( 10.1186/s12711-020-00591-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blacquière T, Boot W, Calis J, Moro A, Neumann P, Panziera D. 2019. Darwinian black box selection for resistance to settled invasive Varroa destructor parasites in honey bees. Biol. Invasions 21, 2519-2528. ( 10.1007/s10530-019-02001-0) [DOI] [Google Scholar]
- 132.Grindrod I, Martin SJ. 2021. Data from: Parallel evolution of Varroa resistance in honey bees: a common mechanism across continents? Dryad Digital Repository. ( 10.5061/dryad.h18931zk6p) [DOI] [PMC free article] [PubMed]
- 133.Grindrod I, Martin SJ. 2021. Parallel evolution of Varroa resistance in honey bees: a common mechanism across continents? Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Grindrod I, Martin SJ. 2021. Data from: Parallel evolution of Varroa resistance in honey bees: a common mechanism across continents? Dryad Digital Repository. ( 10.5061/dryad.h18931zk6p) [DOI] [PMC free article] [PubMed]
- Grindrod I, Martin SJ. 2021. Parallel evolution of Varroa resistance in honey bees: a common mechanism across continents? Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h18931zk6p [132]. The data are provided in electronic supplementary material [133].