Key Points
Question
Does a sedation and ventilator liberation protocol intervention reduce duration of invasive mechanical ventilation in infants and children anticipated to require prolonged mechanical ventilation compared with usual care?
Findings
In this stepped-wedge, cluster randomized trial that included 8843 infants and children anticipated to require prolonged mechanical ventilation, the unadjusted median time to successful extubation was 64.8 hours for those receiving the protocol intervention compared with 66.2 hours for those receiving usual care. This difference was statistically significant but smaller than had been anticipated.
Meaning
Among infants and children anticipated to require prolonged mechanical ventilation, a sedation and ventilator liberation protocol intervention resulted in a reduction in time to first successful extubation; however, the clinical importance of the effect size is uncertain.
Abstract
Importance
There is limited evidence on the optimal strategy for liberating infants and children from invasive mechanical ventilation in the pediatric intensive care unit.
Objective
To determine if a sedation and ventilator liberation protocol intervention reduces the duration of invasive mechanical ventilation in infants and children anticipated to require prolonged mechanical ventilation.
Design, Setting, and Participants
A pragmatic multicenter, stepped-wedge, cluster randomized clinical trial was conducted that included 17 hospital sites (18 pediatric intensive care units) in the UK sequentially randomized from usual care to the protocol intervention. From February 2018 to October 2019, 8843 critically ill infants and children anticipated to require prolonged mechanical ventilation were recruited. The last date of follow-up was November 11, 2019.
Interventions
Pediatric intensive care units provided usual care (n = 4155 infants and children) or a sedation and ventilator liberation protocol intervention (n = 4688 infants and children) that consisted of assessment of sedation level, daily screening for readiness to undertake a spontaneous breathing trial, a spontaneous breathing trial to test ventilator liberation potential, and daily rounds to review sedation and readiness screening and set patient-relevant targets.
Main Outcomes and Measures
The primary outcome was the duration of invasive mechanical ventilation from initiation of ventilation until the first successful extubation. The primary estimate of the treatment effect was a hazard ratio (with a 95% CI) adjusted for calendar time and cluster (hospital site) for infants and children anticipated to require prolonged mechanical ventilation.
Results
There were a total of 8843 infants and children (median age, 8 months [interquartile range, 1 to 46 months]; 42% were female) who completed the trial. There was a significantly shorter median time to successful extubation for the protocol intervention compared with usual care (64.8 hours vs 66.2 hours, respectively; adjusted median difference, −6.1 hours [interquartile range, −8.2 to −5.3 hours]; adjusted hazard ratio, 1.11 [95% CI, 1.02 to 1.20], P = .02). The serious adverse event of hypoxia occurred in 9 (0.2%) infants and children for the protocol intervention vs 11 (0.3%) for usual care; nonvascular device dislodgement occurred in 2 (0.04%) vs 7 (0.1%), respectively.
Conclusions and Relevance
Among infants and children anticipated to require prolonged mechanical ventilation, a sedation and ventilator liberation protocol intervention compared with usual care resulted in a statistically significant reduction in time to first successful extubation. However, the clinical importance of the effect size is uncertain.
Trial Registration
isrctn.org Identifier: ISRCTN16998143
This cluster randomized clinical trial compares a sedation and ventilator liberation protocol intervention for the duration of invasive mechanical ventilation vs usual care in infants and children anticipated to require prolonged mechanical ventilation.
Introduction
The majority of infants and children admitted to pediatric intensive care units (ICUs) require invasive mechanical ventilation (IMV).1,2,3,4 Despite its benefits, IMV is associated with complications, including ventilator-associated pneumonia and ventilator-induced lung injury, and requires sedation that is associated with complications, which may prolong duration of IMV.5
Weaning protocols are widely used in adult ICUs. The practice of testing readiness for ventilator liberation with a spontaneous breathing trial (SBT) is well established.6 A meta-analysis7 of protocolized weaning (14 trials including 2205 participants) reported moderate certainty in the evidence for a reduction by 26% (95% CI, 13%-37%) in IMV duration, with 11 trials evaluating SBT. A systematic review8 of protocolized weaning in children (3 trials including 321 participants) concluded that the evidence was insufficient to determine net benefit or harm.
Across the UK, there is variation in pediatric ICU sedation and ventilator weaning practices, and minimal involvement of junior medical and nursing clinicians.9 Furthermore, approximately two-thirds of nurses employed in UK pediatric ICUs are junior staff nurses.4 It was hypothesized that engagement of the existing multiprofessional ICU team in a sedation and ventilation liberation intervention would reduce time to successful liberation from IMV.
Methods
Trial Design and Oversight
This was a pragmatic multicenter, stepped-wedge, cluster randomized clinical trial (Figure 1 and eFigure 1 in Supplement 1).10 The cost-effectiveness and process evaluations are not reported. The pragmatic domains appear in eFigure 2 in Supplement 1. The National East Midlands research ethics committee approved the protocol (17/EM/0301) on September 12, 2017. An opt-out consent approach was used with distribution of study leaflets to parents. There was no requirement for written or oral informed consent. The Northern Ireland Clinical Trials Unit managed the trial. Data collection was managed through the mandatory national registry (Paediatric Intensive Care and Audit Network4) of pediatric ICU admissions with additional items recorded on electronic case report forms. Independent oversight was provided through the steering and data and safety monitoring committees convened by the UK National Institute of Health Research. The trial protocol was published11 (the trial protocol and statistical analysis plan appear in Supplement 2).
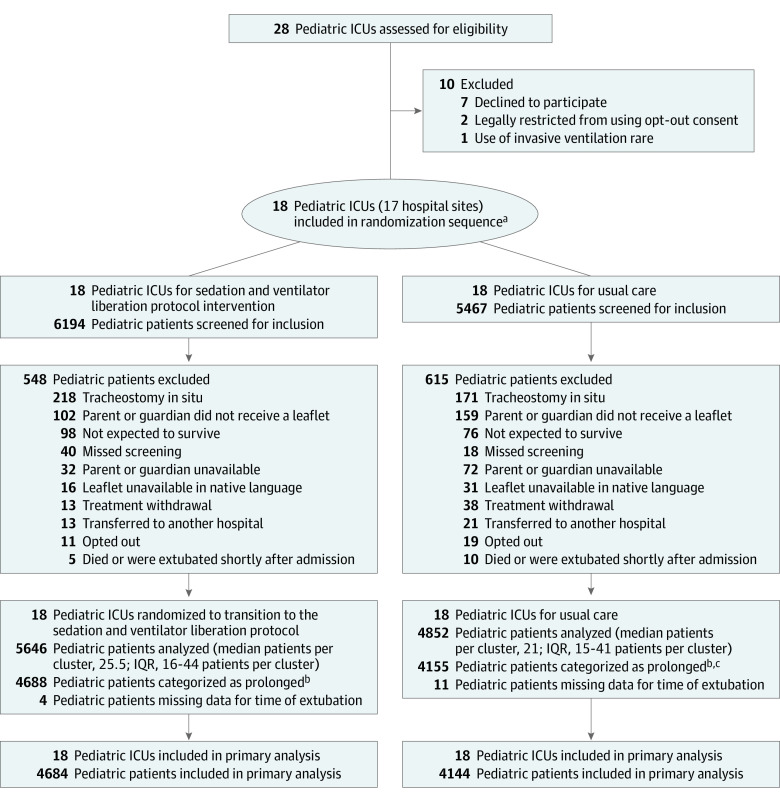
Figure 1. Selection of Pediatric Intensive Care Units (ICUs) and Enrollment of Patients in a Stepped-Wedge, Cluster Randomized Trial of a Sedation and Ventilator Liberation Protocol Intervention in Infants and Children.
IQR indicates interquartile range.
aOne hospital site had 2 pediatric ICUs that were randomized together to avoid contamination of the intervention.
bDiagnostic codes associated with a short duration of ventilation (<24 hours) were categorized as short and all others were categorized as prolonged.
cThere were 3 patients excluded from the analysis because they could not be linked to the Paediatric Intensive Care Audit Network data set.
The primary objective was to determine the effect of the protocol intervention on the duration of IMV in infants and children anticipated to require prolonged IMV, which was defined a priori and determined by the diagnostic codes used. The diagnostic codes associated with IMV duration of less than 24 hours were categorized as short, and all other diagnostic codes were categorized as prolonged (additional details appear in the eMethods in Supplement 1). As a secondary objective, we determined the effect of the protocol intervention on the duration of IMV for all infants and children irrespective of the short or prolonged categorization.
Trial Sites and Participants
All UK hospital sites with 1 or more pediatric ICUs were eligible for the trial. Infants and children (aged <16 years) were eligible if they required IMV and were excluded if they were admitted with a tracheostomy in situ, were not immediately expected to survive, were expected to undergo treatment withdrawal, or if the parent or guardian opted out.
Randomization
The cluster (hospital site) was the unit of randomization. One cluster contained 2 pediatric ICUs that were randomized together to prevent intervention contamination. All clusters started data collection simultaneously. At each 4-week period, starting from period 3 to period 18, 1 cluster transitioned to training and subsequently continued in the protocol intervention. The transition order was randomly determined using a computer-generated algorithm and was restricted to ensure the trial was balanced in terms of a control and an intervention with respect to the cluster size (small or large) determined by published numbers of ICU admissions12 (eMethods in Supplement 1).
Description of Protocol Intervention and Training
A description of the protocol intervention appears in the eMethods in Supplement 1 and the training resources are available on the trial’s website.13 The protocol intervention incorporated education and training for the multiprofessional pediatric ICU team to deliver 4 key components. The components included assessment of sedation levels using COMFORT scale scores, daily screening for readiness to undertake an SBT, initiation of an SBT when screening criteria were satisfied, and a daily multiprofessional round (Box). The protocol intervention training included online and face-to-face education. The trial implementation manager trained the local research team and multiprofessional champions to roll out training. The protocol intervention was delivered to all infants and children requiring IMV in the pediatric ICU.
Box. Components of the Sedation and Ventilator Liberation Protocol Intervention.
Assessment of sedation levels by the bedside nurse using the COMFORT scalea score (every 6 hours as a minimum time interval).
Assessment of readiness to undertake a spontaneous breathing trial by the bedside nurse using the following screening criteria at a minimum of twice daily: fraction of inspired oxygen level ≤0.45; oxygen saturation as measured by pulse oximetry ≥95% (or as appropriate); PEEP level ≤8 cm H2O; peak inspiratory pressure level ≤22 cm H2O; or cough present.
Use of a spontaneous breathing trial to assess readiness for noninvasive ventilator. Decision made by a nurse or physician (with the appropriate experience and authority) to begin spontaneous breathing trial. Spontaneous breathing trial (maximum ≥2 hours) conducted with monitoring of the following outcomes by the bedside nurse: spontaneous breathing mode (continuous positive airway pressure); PEEP level of 5 cm H2O; or pressure support of at least 5 cm H2O in addition to PEEP.
Multidisciplinary review of the child’s COMFORT scale scores during rounds and spontaneous breathing trial assessments with feedback to the bedside nurse on sedation level and ventilation parameter targets (minimum daily assessment).
Adherence was measured by the proportion of (1) 4 protocol intervention components performed and captured daily; (2) staff trained by the end of the transition period; and (3) protocol intervention reach14 (admissions screened divided by IMV admissions during the trial period). The mean adherence proportions for each ICU were ranked and divided into tertiles.
Usual care is described elsewhere9 and a description of the type of usual care provided in participating ICUs at the start of the trial appears in eTable 1 in Supplement 1. Typically, usual care was medically led, involved a slow reduction in ventilator support to low levels of support prior to extubation, and was provided in pediatric ICUs that did not have sedation or ventilator liberation protocols.
Outcome Measures
The primary outcome was the duration of IMV from initiation of ventilation until the first successful extubation. Success was defined as an individual who was still breathing spontaneously for 48 hours after extubation. The majority of the prespecified secondary outcomes (as defined in the trial protocol; Supplement 2) are reported; however, cost per complication avoided at 28 days is not reported. Outcomes were measured from patient admission up to 90 days or pediatric ICU discharge (whichever was earlier). At the end of the enrollment period, data collection continued for a maximum of 28 days.
Statistical Analysis
The planned sample size was between 11 024 and 14 310 patients (dependent on the intracluster correlation coefficient). After the internal pilot program, reestimation of the mean duration of IMV was 5.8 days (SD, 9.6 days) and the intracluster correlation coefficient was 0.005 (95% CI, 0.001-0.01). A revised sample size calculation estimated that 9520 patient admissions would provide 80% to 87% power to detect a 1-day target effect size. The 1-day difference was considered by the study team as clinically important and plausible for patients managed with a sedation and ventilator liberation protocol intervention following discussions with ICU staff during the pretrial feasibility work. Sample size calculations assumed a simple exchangeable correlation structure, which was the convention at the time.15
The ICUs were analyzed according to the sequence they were randomized so that all participants were analyzed according to their randomized group. In this way, the ICUs were assumed to have been exposed to the protocol intervention following their training periods. Patients admitted during training periods were not included. For the primary analysis, observations with missing outcome data were excluded. For the secondary analysis, adjusting for individual-level covariates, observations with missing outcome or covariate data were excluded. Missing data were minimal and there was no requirement for multiple imputation. The proportion of missing data for the primary analysis for the primary outcome was 0.17% and was 0.18% for the secondary analysis. The primary estimate of the treatment effect was a time- and cluster-adjusted hazard ratio (HR) with 95% CIs. Because of the potential for type I error due to multiple comparisons, findings for the analyses of the secondary outcomes should be interpreted as exploratory.
For the time-to-event primary and secondary outcomes, Cox proportional hazards models were used with a frailty term for clustering by ICU (which accounts for random cluster effects). Time-to-event outcomes were censored at the point of transitioning from usual care to the protocol intervention training periods, discharge to another hospital, at 90 days, death, and point of receiving a tracheostomy. Checks of the appropriateness of the proportional hazards assumption indicated no evident departures from proportionality on Schoenfeld residuals plots. For time-to-event outcomes, an absolute measure of effect was derived by computing the median of the model-based prediction of survival duration at all 22 periods for both the protocol intervention and usual care and the difference between the 2 and by summarizing the extent of variability using the interquartile range (IQR) over the 22 periods.
Binary secondary outcomes were analyzed using mixed-effects binomial regression with a log link to estimate the adjusted relative risk (RR). A binomial model with identity link was used to estimate the adjusted risk difference using the restricted maximum likelihood approach. All mixed models included cluster as a random effect (assuming an exchangeable correlation structure) and used the Kenward and Roger small sample correction16 to correct the potential inflation of the type I error rate due to the small number of clusters. In the case of nonconvergence of binomial linear mixed models to estimate risk differences, marginal estimates of risk differences are reported that used generalized estimating equations (assuming an independent correlation structure) and a Fay and Graubard small sample correction on standard errors with 95% CIs derived from z distribution.17 In the case of nonconvergence of the binomial model with a log link, a Poisson model with robust standard errors was fitted. For continuous outcomes, similar models were used with an identity link and assuming a normal distribution, but also checking for normality assumptions and making transformations when necessary.
A prespecified secondary analysis of the primary outcome was conducted that adjusted for additional covariates of age, severity of illness (Paediatric Index of Mortality 3 score), respiratory vs other diagnostic grouping, type of admission (planned or unplanned), and reason for admission (surgical or medical). A prespecified exploratory subgroup analysis was conducted for the primary outcome using a global test for interaction and 99% CIs for (1) ICU size (large or small based on annual admissions), (2) adherence to the protocol intervention (tertiles of ranked averages), (3) the type of admission to the ICU (planned or unplanned), and (4) the reason for admission (surgical, medical respiratory, or other medical). To assess sensitivity for the assumptions made about the nature of time effects and correlations, an extensive series of sensitivity analyses for the secondary binary outcomes was conducted (eMethods in Supplement 1). This series of sensitivity analyses showed little difference between the more complex correlation structures and the exchangeable correlation structures that were assumed in the primary analysis.
Variance components (intracluster correlation coefficients) are reported. A 2-sided significance threshold of P < .05 was used for all analyses. The analyses were conducted using Stata/SE version 16.1 (StataCorp) and SAS version 9.4 (SAS Institute Inc).
Results
Trial Sites and Participants
All 18 ICUs opened simultaneously to recruitment on February 5, 2018, and closed on October 14, 2019. The last date of follow-up was November 11, 2019. Participating ICUs had a greater number of beds, a greater number of annual patient admissions, and included more sites in London, England, than nonparticipating ICUs (Table 1 and eTable 2 in Supplement 1). The trial included 10 495 admissions, of which 8843 infants and children (median age, 8 months [IQR, 1-46 months]; 42% were female) were in diagnostic groups identified as anticipated to require prolonged ventilation (Figure 1). Patient characteristics were well-balanced across the protocol intervention and usual care (Table 2; data on all pediatric patient admissions appear in eTable 3 in Supplement 1).
Table 1. Characteristics of Pediatric Intensive Care Units (ICUs).
| Characteristics | No. (%) |
|---|---|
| No. of pediatric ICUs | 18 |
| Beds | |
| 6-11 | 9 (50) |
| 12-30 | 9 (50) |
| Fellowship training provision | 15 (72.2) |
| Intensivist coverage | 18 (100) |
| Unit type | |
| General | 11 (61.1) |
| General and cardiac mixed | 5 (27.8) |
| Cardiac | 2 (11.1) |
| Sedation assessment validated tool in place prior to studya | 13 (72.2) |
| Sedation protocol in place prior to studyb | 4 (22.2) |
| Ventilation weaning protocol in place prior to studyc | 3 (16.7) |
Either the COMFORT original scale or the COMFORT behavioral scale was used prior to the study and during the protocol intervention. The COMFORT scale assesses pain and sedation to determine if the child is adequately comfortable or in need of more or less medication to maintain adequate ventilation.
Sedation protocols already in place prescribed the reduction of sedatives, whereas the protocol intervention recommended that sedatives should be adjusted to achieve an appropriate score on the COMFORT scale.
Weaning protocols already in place prescribed stepwise reductions in ventilator support, whereas the protocol intervention prescribed daily screening and a spontaneous breathing trial.
Table 2. Baseline Characteristics of Pediatric Patient Admissions.
| Characteristics | No. (%) | |
|---|---|---|
| Protocol intervention | Usual care | |
| No. of pediatric patient admissions | 4688 | 4155 |
| Sex | ||
| Female | 1970 (42.0) | 1744 (42.0) |
| Male | 2716 (57.9) | 2410 (58.0) |
| Age at admission, median (IQR), mo | 7 (1-45) | 9 (1-47) |
| <1 | 1042 (22.2) | 772 (18.6) |
| 1-<24 | 2077 (44.3) | 1937 (46.6) |
| 24-<72 | 710 (15.2) | 665 (16.0) |
| ≥72 | 859 (18.3) | 780 (18.8) |
| Previous ICU admission | 1429 (30.5) | 1102 (26.5) |
| Pediatric Index of Mortality 3, median (IQR)a | 0.02 (0.01-0.05) | 0.02 (0.01-0.05) |
| Primary diagnostic group | ||
| Cardiovascular | 1613 (34.4) | 1226 (29.5) |
| Respiratory | 1410 (30.1) | 1289 (31.0) |
| Other | 602 (12.8) | 484 (11.7) |
| Neurology | 385 (8.2) | 431 (10.4) |
| Gastroenterology | 316 (6.7) | 294 (7.1) |
| Infection | 253 (5.4) | 307 (7.4) |
| Oncology | 109 (2.3) | 124 (3.0) |
| Type of admission | ||
| Unplanned medical | 2659 (56.7) | 2624 (63.2) |
| Planned medical | 265 (5.7) | 153 (3.7) |
| Planned postsurgical | 1532 (32.7) | 1128 (27.2) |
| Unplanned postsurgical | 232 (5.0) | 250 (6.0) |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
A predictive model based on 10 explanatory variables collected at admission to the ICU to estimate the probability of death. Ranges from 0 to 1; a higher index is associated with a higher estimated probability of death.
Delivery of the Protocol Intervention
A total of 1865 of 2247 (median, 85%; IQR, 80%-90%) eligible clinical staff completed training within the 8-week training period. By 12 weeks, 1955 of 2247 (median, 88%; IQR, 80%-90%) eligible clinical staff completed training (eTable 4 in Supplement 1). Across ICUs, adherence was high for protocol intervention reach (median, 82%; IQR, 77%-89%), sedation assessment (median, 83%; IQR, 82%-91%), and setting targets for sedation level (median, 85%; IQR, 63%-89%) and ventilation parameters (median, 90%; IQR, 81%-96%). Adherence was moderate for SBT screening (median, 74%; IQR, 66%-83%) and lower for proceeding to SBT when screening criteria were met (median, 40%; IQR, 31%-51%) (eTable 5 in Supplement 1). Documented reasons for not progressing to SBT and extubation appear in eTables 6 and 7 in Supplement 1.
Primary Outcome
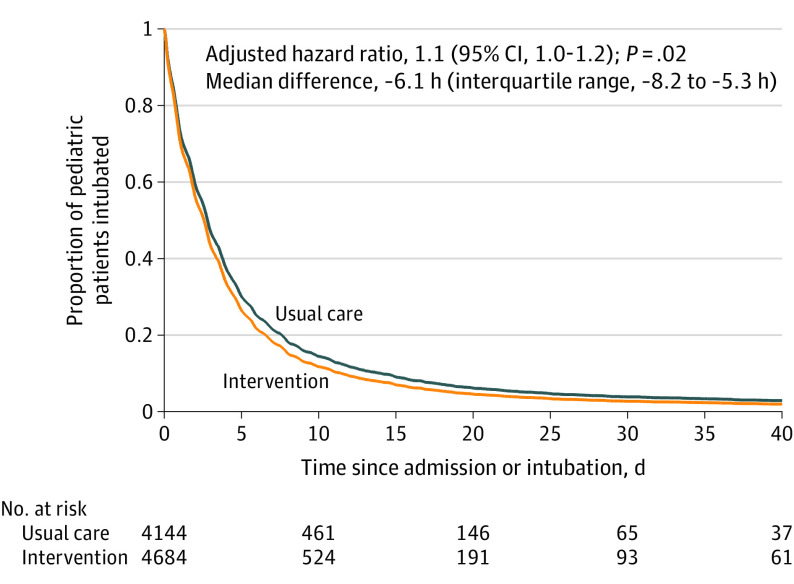
After adjustment for cluster and calendar time, implementation of the protocol intervention resulted in a significantly shorter median duration of IMV before successful extubation of 64.8 hours (IQR, 22.1 to 141.4 hours) compared with a median duration of IMV of 66.2 hours (IQR, 21.8 to 138.0 hours) for usual care. The adjusted median difference was −6.1 hours (IQR, −8.2 to −5.3 hours) across all calendar periods and the adjusted HR was 1.11 (95% CI, 1.02 to 1.20, P = .02; Table 3). The outcomes for all infants and children appear in eTable 8 in Supplement 1. The probability and time to successful extubation by observation period appear in Figure 2 and the data for all infants and children appear in eFigure 3 in Supplement 1.
Table 3. Primary and Secondary Outcomes.
| Observation period | Adjusted analysesa | |||||||
|---|---|---|---|---|---|---|---|---|
| Protocol intervention | Usual care | Median difference (IQR)b | P value | Hazard ratio (95% CI) | P value | |||
| Median (IQR) | No. of patients | Median (IQR) | No. of patients | |||||
| Primary outcome | ||||||||
| Duration of invasive mechanical ventilation until first successful extubation, hc | 64.8 (22.1 to 141.4) | 4684 | 66.2 (21.8 to 138.0) | 4144 | −6.1 (−8.2 to −5.3) | .02 | 1.11 (1.02 to 1.20) | .02 |
| Secondary outcomes | ||||||||
| Total duration of invasive mechanical ventilation, dc | 2.7 (0.9 to 6.3) | 4684 | 2.8 (0.9 to 5.9) | 4144 | −0.20 (−0.25 to −0.18) | .06 | 1.09 (1.00 to 1.18) | .06 |
| Duration of noninvasive ventilation after extubation, dc | 1.8 (0.7 to 6.8) | 805 | 2.1 (0.7 to 6.6) | 556 | 0.22 (0.18 to 0.29) | .43 | 0.91 (0.72 to 1.15) | .43 |
| Length of stay, d | ||||||||
| Pediatric ICU | 5.0 (3.0 to 10.0) | 4688 | 5.0 (3.0 to 9.0) | 4155 | 0 (0 to 0) | .53 | 0.97 (0.90 to 1.06) | .53 |
| Hospital | 9.6 (5.0 to 19.8) | 4010 | 9.1 (5.0 to 18.9) | 3581 | 0.91 (0.84 to 0.97) | .01 | 0.89 (0.81 to 0.97) | .01 |
| No. (%) | No. (%) |
Percentage point difference (95% CI)d |
Relative risk (95% CI)e | |||||
| Extubation | ||||||||
| Successfulf | 4161 (98.6) | 4222 | 3788 (98.4) | 3849 | 0.95 (−0.07 to 1.97) | .07 | 1.01 (1.00 to 1.02) | .03 |
| Unplanned | 142 (3.0) | 4688 | 107 (2.6) | 4155 | 0.98 (−0.32 to 2.27) | .14 | 1.62 (1.05 to 2.51) | .03 |
| Reintubation | 544 (11.6) | 4688 | 507 (12.2) | 4155 | 0.83 (−1.70 to 3.37)g | .52 | 1.10 (0.89 to 1.36)g | .38 |
| Noninvasive ventilation after extubation | 810 (18.9) | 4285 | 558 (14.4) | 3886 | 9.42 (4.30 to 14.54) | <.001 | 1.22 (1.01 to 1.49) | .04 |
| Tracheostomy during the study | 46 (1.0) | 4688 | 33 (0.8) | 4155 | −0.03 (−0.49 to 0.43)h | .89 | 0.88 (0.36 to 2.17) | .79 |
| Stridor after extubationi | 419 (8.9) | 4688 | 356 (8.6) | 4155 | 3.05 (−1.71 to 7.80) | .21 | 0.94 (0.73 to 1.22) | .66 |
| Mortality | ||||||||
| Pediatric ICU | 220 (4.7) | 4682 | 173 (4.2) | 4154 | 0.25 (−1.98 to 2.49) | .82 | 1.06 (0.73 to 1.54) | .75 |
| Hospital or ICU | 268 (6.3) | 4278 | 200 (5.3) | 3785 | 0.82 (−1.96 to 3.61) | .56 | 1.15 (0.82 to 1.63) | .41 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
All outcomes were adjusted for cluster (pediatric ICU) and calendar time (period categorical effect).
Calculated across the 22 periods.
Time-to-event outcomes were censored at the point of transitioning from usual care to the training period, discharge to another hospital, at 90 days, death, and at the point of receiving a tracheostomy.
Estimated using generalized estimating equations.
Poisson regression with robust standard errors (to correct for misspecification of Poisson distribution for binomial distribution) was used to estimate the relative risk.
An extubation that did not require reintubation within a 48-hour period.
Estimated using a mixed-effects binomial model with identity link.
Due to the lack of convergence, marginal estimates of risk difference were developed without using a small sample correction.
Laryngeal edema resulting in stridor.
Figure 2. Probability and Time to Successful Extubation by Observation Period in the Prolonged Invasive Ventilation Cohort.
The hazard ratio (95% CI) and the median difference (interquartile range) were adjusted for cluster and calendar time. Patients were observed from initiation of ventilation until the first successful extubation (defined as still breathing spontaneously for 48 hours after extubation). The curves on the graph were created using adjusted data. The No. at risk are the absolute patient numbers and therefore will not match precisely with the covariate-adjusted curve.
In a prespecified secondary analysis that adjusted for additional covariates, the findings were not statistically significant for the prolonged ventilation cohort (adjusted HR, 1.07 [95% CI, 0.98-1.16]; P = .13) or for all infants and children (adjusted HR, 1.06 [95% CI, 0.98-1.14]; P = .17).
Secondary Outcomes
There was a significantly higher incidence of successful extubation for the protocol intervention (adjusted RR, 1.01 [95% CI, 1.00 to 1.02]; P = .03). There was no significant difference in total duration of IMV for the protocol intervention (a median of 2.7 days [IQR, 0.9 to 6.3 days]) compared with total duration of IMV for usual care (a median of 2.8 days [IQR, 0.9 to 5.9 days]). The adjusted median difference was −0.20 days (IQR, −0.25 to −0.18 days) and the adjusted HR was 1.09 (95% CI, 1.00 to 1.18; P = .06). Use of noninvasive ventilation after extubation was significantly higher for the protocol intervention (adjusted RR, 1.22 [95% CI, 1.01 to 1.49], P = .04). However, there was no significant difference in the duration of noninvasive ventilation with a median of 1.8 days (IQR, 0.7 to 6.8 days) for the protocol intervention compared with a median of 2.1 days (IQR, 0.7 to 6.6 days) for usual care. The adjusted median difference was 0.22 days (IQR, 0.18 to 0.29 days) and the adjusted HR was 0.9 (95% CI, 0.7 to 1.2; P = .43).
The length of stay in the ICU was not significantly different between the protocol intervention (a median of 5.0 days [IQR, 3.0-10.0 days]) compared with usual care (a median of 5.0 days [IQR, 3.0-9.0 days]). The adjusted median difference was 0 days (IQR, 0-0 days) and the adjusted HR was 0.97 (95% CI, 0.90-1.06; P = .53). However, there was a significantly longer hospital length of stay for the protocol intervention (median, 9.6 days [IQR, 5.0-19.8 days]) compared with the hospital length of stay for usual care (median, 9.1 days [IQR, 5.0-18.9 days]). The adjusted median difference was 0.91 days (IQR, 0.84-0.97 days) and the adjusted HR was 0.89 (95% CI, 0.81-0.97; P = .01). The protocol intervention resulted in a significantly higher incidence of unplanned extubation (adjusted RR, 1.62 [95% CI, 1.05-2.51]; P = .03), but there were no significant differences in reintubation (adjusted RR, 1.10 [95% CI, 0.89-1.36]; P = .38) (Table 2). The data for all infants and children appear in eTable 8 in Supplement 1.
In relation to other safety outcomes, there were no statistically significant differences between the protocol intervention and usual care for risk of tracheostomy, stridor after extubation, or mortality in the ICU or in the hospital (Table 2). The data for all infants and children appear in eTable 8 in Supplement 1. Variance components (intracluster correlation coefficient) for all secondary binary outcomes are reported in eTable 9 in Supplement 1.
Adverse Events
There were 18 serious adverse events for the protocol intervention and 25 for usual care. Adverse events included hypoxia (9 [0.2%] infants and children for the protocol intervention vs 11 [0.3%] for usual care) and nonvascular device dislodgement (2 [0.04%] vs 7 [0.1%], respectively; eTables 10 and 11 in Supplement 1).
Clinical and Exploratory Outcomes
Baseline ventilation parameters were similar (eTable 12 in Supplement 1). Ventilation parameters were not different in any clinically important extent immediately before the SBT for the protocol intervention and 2 hours before extubation for usual care (eTable 13 in Supplement 1).
Exploratory subgroup analyses for the duration of IMV before successful extubation showed no significant interactions in the prespecified subgroups based on size of ICU, type of admission, reason for admission, or adherence to the protocol intervention (eFigure 4 in Supplement 1).
Discussion
In this stepped-wedge, cluster randomized clinical trial in infants and children anticipated to require prolonged ventilation, the use of a sedation and ventilator liberation protocol intervention significantly reduced the duration of IMV to successful extubation compared with usual care. The effect size was small and thus the clinical significance is uncertain. The significant effect was consistent across all infants and children requiring IMV.
The small effect may have resulted from several factors. First, the trial recruited a broad population and, as a result, there may have been heterogeneity in the treatment effect that could have attenuated the overall effect. A greater effect in a more focused population cannot be excluded. Second, given the historic lack of involvement by bedside nurses in ventilator weaning in the UK,9 engaging the nurses fully in the process may have prompted earlier consideration of extubation, which was a key factor in a previous study.18 Third, observations showed a lower adherence to undertaking an SBT when screening criteria were satisfied and may reflect clinician hesitancy to move swiftly from a high level to a low level of support to test readiness for ventilator liberation. Even though the screening criteria indicated potential to proceed to an SBT, such progression may not have been clinically appropriate. Reluctance and nonadherence may plausibly be a sign of the difficulties clinicians experience in changing long-standing practices.19 Furthermore, the large numbers of staff required to deliver the protocol intervention may have attenuated the effect compared with the effect size seen in other smaller pediatric trials evaluating an SBT as a ventilator liberation intervention.
Few pediatric randomized clinical trials have specifically evaluated a daily screening and SBT strategy. In a 2-center trial recruiting mainly medical patients, Foronda et al20 reported a reduction in duration of IMV by more than 24 hours in the SBT group (n = 294; median, 3.5 days vs 4.7 days, P = .01). A single-site trial of cardiac surgical patients by Ferreira et al21 reported a significant reduction in extubation success in the SBT group (n = 110; 83% vs 68%, P = .02), but a longer difference in duration of IMV in the SBT group that did not meet statistical significance (median, 29.4 hours vs 21.5 hours, P = .29). In both trials, relatively few clinicians delivered the intervention in a controlled manner; thus, the findings may not directly translate when applied to wider clinical practice. In contrast, Curley et al5 evaluated a sedation protocol incorporating an SBT delivered by each site’s multidisciplinary team in a 31-site cluster trial that enrolled medical patients. They reported no significant between-group differences in duration of IMV (n = 2449; median, 6.5 days for both groups), but showed reduced variation in sedation management with interprofessional involvement. In the current study, the median duration of IMV for usual care was less than 3 days and much shorter than that reported in other pediatric studies evaluating SBTs5,20,21 or other weaning protocols.22,23,24 It was also shorter than the pretrial estimations that were based on the mean duration of IMV days. It is possible that the protocol intervention had a reduced absolute effect with a shorter duration of IMV than usual care.
The significantly higher incidence of unplanned extubation may be associated with less sedation and more awake patients. However, the proportion of patients with unplanned extubation was lower than the 4% to 8% reported elsewhere,5,25 and did not result in a higher rate of reintubation. This may be an indication that some patients might be ready to breathe without assistance sooner than previously expected, a point raised in previous adult and pediatric studies.26,27 Thus in some respects, usual care may be a conservative approach. The greater use of noninvasive ventilation after extubation with the protocol intervention may reflect the need for continued ventilator support because of earlier extubation. Alternatively, it could also reflect clinician discomfort with a more accelerated weaning and extubation approach in contrast to a conservative approach.
Infants and children had a significantly longer hospital stay with the protocol intervention. Whether this finding represents an association with the protocol intervention or is a consequence of greater use of noninvasive ventilation or other factors cannot be ascertained within the present study.
The stepped-wedge design had several strengths. It helped (1) overcome the risk of intervention contamination with usual care; (2) maximize power to detect an effect; (3) facilitate protocol intervention training; and (4) increase ICU participation by guaranteeing receipt of the protocol intervention.28 The pragmatic recruitment facilitated testing in a broader population of patients who would potentially benefit from the protocol intervention.
Limitations
This study has several limitations. First, assignment of the protocol intervention was unblinded. This may have led to performance or detection bias. Second, hospital sites were the unit of randomization and the infants and children enrolled were a heterogeneous group with a variety of respiratory, cardiac, and other impairments. Whether the protocol intervention would perform differently in a more homogenous group remains to be determined.
Third, the protocol intervention included several components and adherence to all components was not uniformly observed. It was not possible to determine which components were primarily responsible for the observed effect. Fourth, data on sedatives, analgesics, and sedation levels were not collected; rather it was recommended that ICU teams consider the sedation needs of the infant or child based on COMFORT scores and SBT readiness screenings.
Fifth, the categorization of diagnostic codes to define prolonged ventilation was based on diagnoses that typically require more than 24 hours of ventilation. Stratification based on codes requiring more prolonged ventilation (eg, >48 hours) may have shown different effects.
Conclusions
Among infants and children anticipated to require prolonged mechanical ventilation, a sedation and ventilator liberation protocol intervention compared with usual care resulted in a statistically significant reduction in time to first successful extubation. However, the clinical importance of the effect size is uncertain.
eFigure 1. Study schematic
eFigure 2. PRagmatic-Explanatory Continuum Indicator Summary (PRECIS-2)
eFigure 3. Probability and time to successful extubation by observation period in all children
eFigure 4. Subgroup analyses for time to successful extubation
eMethods
eTable 1. Usual care in participating pediatric ICUs
eTable 2. Characteristics of UK pediatric ICUs
eTable 3. Characteristics of all patient admissions at baseline
eTable 4. Proportion (%) of staff trained within 8 and 12 weeks at each hospital site
eTable 5. Proportion (%) of intervention adherence at each hospital site
eTable 6. Reasons provided for not progressing to conduct a spontaneous breathing trial when the screening criteria were satisfied
eTable 7. Reasons provided for not progressing to extubation when the spontaneous trial was successful
eTable 8. Outcomes for all children
eTable 9. Intra-cluster correlation coefficient variance components
eTable 10. Adverse and serious adverse events (prolonged ventilation cohort)
eTable 11. Adverse and serious adverse events (all children)
eTable 12. Baseline ventilation parameters
eTable 13. Comparison of ventilation parameters two hours prior to extubation (control period) and prior to the start of SBT (intervention period)
eReferences
Trial protocol and statistical analysis plan
Nonauthor Collaborators. The SANDWICH Collaborators nonauthor collaborators
Data sharing statement
Footnotes
Abbreviation: PEEP, positive end-expiratory pressure.
Assesses pain and sedation to determine if the child is adequately comfortable or in need of more or less medication to maintain adequate ventilation.
References
- 1.Balcells Ramírez J, López-Herce Cid J, Modesto Alapont V; Grupo de Respiratorio de la Sociedad Española de Cuidados Intensivos Pediátricos . Prevalence of mechanical ventilation in pediatric intensive care units in Spain. Published in Spanish. An Pediatr (Barc). 2004;61(6):533-541. [DOI] [PubMed] [Google Scholar]
- 2.Wolfler A, Calderoni E, Ottonello G, et al. ; SISPE Study Group . Daily practice of mechanical ventilation in Italian pediatric intensive care units: a prospective survey. Pediatr Crit Care Med. 2011;12(2):141-146. doi: 10.1097/PCC.0b013e3181dbaeb3 [DOI] [PubMed] [Google Scholar]
- 3.Farias JA, Fernández A, Monteverde E, et al. ; Latin-American Group for Mechanical Ventilation in Children . Mechanical ventilation in pediatric intensive care units during the season for acute lower respiratory infection: a multicenter study. Pediatr Crit Care Med. 2012;13(2):158-164. doi: 10.1097/PCC.0b013e3182257b82 [DOI] [PubMed] [Google Scholar]
- 4.Paediatric Intensive Care Audit Network . 2019 annual report. Accessed July 16, 2020. https://www.picanet.org.uk/annual-reporting-and-publications/
- 5.Curley MA, Wypij D, Watson RS, et al. ; RESTORE Study Investigators and the Pediatric Acute Lung Injury and Sepsis Investigators Network . Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313(4):379-389. doi: 10.1001/jama.2014.18399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns KEA, Raptis S, Nisenbaum R, et al. International practice variation in weaning critically ill adults from invasive mechanical ventilation. Ann Am Thorac Soc. 2018;15(4):494-502. doi: 10.1513/AnnalsATS.201705-410OC [DOI] [PubMed] [Google Scholar]
- 7.Blackwood B, Burns KE, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev. 2014;2014(11):CD006904. doi: 10.1002/14651858.CD006904.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackwood B, Murray M, Chisakuta A, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of invasive mechanical ventilation in critically ill paediatric patients. Cochrane Database Syst Rev. 2013;2013(7):CD009082. doi: 10.1002/14651858.CD009082.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackwood B, Tume L. The implausibility of ‘usual care’ in an open system: sedation and weaning practices in paediatric intensive care units (PICUs) in the United Kingdom (UK). Trials. 2015;16:325. doi: 10.1186/s13063-015-0846-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackwood B. Pragmatic-Explanatory Continuum Indicator Summary (PRECIS-2) for the SANDWICH trial. Accessed February 1, 2021. http://www.precis-2.org/Trials
- 11.Blackwood B, Agus A, Boyle R, et al. ; Paediatric Intensive Care Society Study Group (PICS-SG) . Sedation and Weaning in Children (SANDWICH): protocol for a cluster randomised stepped wedge trial. BMJ Open. 2019;9(11):e031630. doi: 10.1136/bmjopen-2019-031630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivers NM, Halperin IJ, Barnsley J, et al. Allocation techniques for balance at baseline in cluster randomized trials: a methodological review. Trials. 2012;13:120. doi: 10.1186/1745-6215-13-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIlmurray L. SANDWICH: Sedation and Weaning in Children. Accessed December 1, 2020. https://www.qub.ac.uk/sites/sandwich/
- 14.Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015;350:h1258. doi: 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemming K, Kasza J, Hooper R, Forbes A, Taljaard M. A tutorial on sample size calculation for multiple-period cluster randomized parallel, cross-over and stepped-wedge trials using the shiny CRT calculator. Int J Epidemiol. 2020;49(3):979-995. doi: 10.1093/ije/dyz237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyrat C, Morgan KE, Leurent B, Kahan BC. Cluster randomized trials with a small number of clusters: which analyses should be used? Int J Epidemiol. 2018;47(3):1012. doi: 10.1093/ije/dyy057 [DOI] [PubMed] [Google Scholar]
- 17.Thompson JA, Hemming K, Forbes A, Fielding K, Hayes R. Comparison of small-sample standard-error corrections for generalised estimating equations in stepped wedge cluster randomised trials with a binary outcome: a simulation study. Stat Methods Med Res. 2021;30(2):425-439. doi: 10.1177/0962280220958735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ely EW, Baker AM, Evans GW, Haponik EF. The prognostic significance of passing a daily screen of weaning parameters. Intensive Care Med. 1999;25(6):581-587. doi: 10.1007/s001340050906 [DOI] [PubMed] [Google Scholar]
- 19.Gupta DM, Boland RJ Jr, Aron DC. The physician’s experience of changing clinical practice: a struggle to unlearn. Implement Sci. 2017;12(1):28-28. doi: 10.1186/s13012-017-0555-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foronda FK, Troster EJ, Farias JA, et al. The impact of daily evaluation and spontaneous breathing test on the duration of pediatric mechanical ventilation: a randomized controlled trial. Crit Care Med. 2011;39(11):2526-2533. doi: 10.1097/CCM.0b013e3182257520 [DOI] [PubMed] [Google Scholar]
- 21.Ferreira FV, Sugo EK, Aragon DC, Carmona F, Carlotti APCP. Spontaneous breathing trial for prediction of extubation success in pediatric patients following congenital heart surgery: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(10):940-946. doi: 10.1097/PCC.0000000000002006 [DOI] [PubMed] [Google Scholar]
- 22.Schultz TR, Lin RJ, Watzman HM, et al. Weaning children from mechanical ventilation: a prospective randomized trial of protocol-directed versus physician-directed weaning. Respir Care. 2001;46(8):772-782. [PubMed] [Google Scholar]
- 23.Randolph AG, Wypij D, Venkataraman ST, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network . Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288(20):2561-2568. doi: 10.1001/jama.288.20.2561 [DOI] [PubMed] [Google Scholar]
- 24.Jouvet PA, Payen V, Gauvin F, Emeriaud G, Lacroix J. Weaning children from mechanical ventilation with a computer-driven protocol: a pilot trial. Intensive Care Med. 2013;39(5):919-925. doi: 10.1007/s00134-013-2837-8 [DOI] [PubMed] [Google Scholar]
- 25.Baisch SD, Wheeler WB, Kurachek SC, Cornfield DN. Extubation failure in pediatric intensive care incidence and outcomes. Pediatr Crit Care Med. 2005;6(3):312-318. doi: 10.1097/01.PCC.0000161119.05076.91 [DOI] [PubMed] [Google Scholar]
- 26.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126-134. doi: 10.1016/S0140-6736(08)60105-1 [DOI] [PubMed] [Google Scholar]
- 27.Kurachek SC, Newth CJ, Quasney MW, et al. Extubation failure in pediatric intensive care: a multiple-center study of risk factors and outcomes. Published correction appears in Crit Care Med. 2003;32(7):1632-1633. Crit Care Med. 2003;31(11):2657-2664. doi: 10.1097/01.CCM.0000094228.90557.85 [DOI] [PubMed] [Google Scholar]
- 28.Hemming K, Taljaard M. Reflection on modern methods: when is a stepped-wedge cluster randomized trial a good study design choice? Int J Epidemiol. 2020;49(3):1043-1052. doi: 10.1093/ije/dyaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study schematic
eFigure 2. PRagmatic-Explanatory Continuum Indicator Summary (PRECIS-2)
eFigure 3. Probability and time to successful extubation by observation period in all children
eFigure 4. Subgroup analyses for time to successful extubation
eMethods
eTable 1. Usual care in participating pediatric ICUs
eTable 2. Characteristics of UK pediatric ICUs
eTable 3. Characteristics of all patient admissions at baseline
eTable 4. Proportion (%) of staff trained within 8 and 12 weeks at each hospital site
eTable 5. Proportion (%) of intervention adherence at each hospital site
eTable 6. Reasons provided for not progressing to conduct a spontaneous breathing trial when the screening criteria were satisfied
eTable 7. Reasons provided for not progressing to extubation when the spontaneous trial was successful
eTable 8. Outcomes for all children
eTable 9. Intra-cluster correlation coefficient variance components
eTable 10. Adverse and serious adverse events (prolonged ventilation cohort)
eTable 11. Adverse and serious adverse events (all children)
eTable 12. Baseline ventilation parameters
eTable 13. Comparison of ventilation parameters two hours prior to extubation (control period) and prior to the start of SBT (intervention period)
eReferences
Trial protocol and statistical analysis plan
Nonauthor Collaborators. The SANDWICH Collaborators nonauthor collaborators
Data sharing statement