Abstract
Despite the efforts made to mitigate the consequences of this disease, natural rubber latex allergy (NRLA) continues to be a global health problem and is still considered one of the main worries in the working environment in many countries throughout the world.
Due to thousands of products containing latex, it is not surprising that the current statistics suggest that prevalence remains high among healthcare workers and susceptible patients.
In developed countries, reduction in the prevalence of IgE-mediated allergy to latex proteins from gloves may lead to lax attention by health care personnel. On the other hand, this situation is different in developing countries where there is a lack of epidemiological data associated with a deficit in education and awareness of this issue.
The aim of this review is to provide an update of the current knowledge and practical recommendations regarding NRLA by allergologists from different parts of the world with experience in this field.
Keywords: Latex, IgE mediated hypersensitivity, Management, Wordwide
Introduction
We have now entered the fifth decade of experience with NRLA. The epidemic of type 1 hypersensitivity IgE-mediated reactions to proteins retained in finished rubber products began in earnest in the 1980s and 1990s. This coincided with the introduction of universal precautions (now called standard precautions) to prevent blood-transmitted HIV infection.
The production of gloves with a low allergen content, the reduction or even banning of powdered latex gloves in some countries, and public health campaigns have resulted in a significant decrease in NRLA;1 however, the disease remains worrisome for many individuals and continues to be a global health problem.
There are large differences in the management of NRLA worldwide. While in developed countries warnings are required, in developing countries epidemiologic data are lacking and economic deprivation limits management options which leaves education as the only tool available to decrease risks. The aim of this review is to provide an update of the current knowledge on NRLA shared by allergy specialists from different parts of the world with experience in this field.
Current epidemiology and risk factors
People with frequent and intense contact with latex products were identified as having a higher risk of developing NRLA.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 The prevalence of latex sensitization in studies of the general population range from <1%16 to a high of 7.6%.15 This broad range of prevalence depends on where and how the population was studied. Specific groups with frequent exposure to natural rubber latex (NRL) may be found to have NRLA prevalence from 3% to 64%.15 NRLA prevalence also varies depending on professional activity. Dentists show figures close to 30%, surgeons approximately 50%, and the frequency is 15% in the remaining of the medical staff while it may range from 25 to 50% among nursing staff.17
Patients with history of more than 5 surgeries have a higher risk of presenting latex sensitization.18 On the other hand, prevalence of latex allergy may be higher in patients with spina bifida and cloacal anomalies, children with other congenital anomalies, such as esophageal atresia, gastroschisis, omphalocele, and those with neurologic diseases, such as cerebral palsy.19
Multiple risk factors for developing NRLA have been described in medical personnel and patients, including a history of atopy16 and exposure to latex-derived products during surgery, either through skin contact or by inhalation.17, 18, 19, 20 Table 1 lists the risk groups for latex sensitization and allergy.
Table 1.
Identified and potential Risk groups patients for NRL sensitization and allergy
| Occupations and other situations with frequent contact with latex | Medical conditions with an increased predisposition for latex sensitization |
|---|---|
|
|
Clinical manifestations
Clinical manifestations induced by type I hypersensitivity reactions to NRL vary greatly depending on the route of exposure (cutaneous, percutaneous, mucosal, or parenteral), the amount and features of the allergens, the level of sensitization, and individual factors.19 They may get worse at each following exposure.
Skin symptoms
Immunological contact urticaria (ICU) occurs in previously sensitized individuals and is a type I hypersensitivity reaction mediated by latex-specific immunoglobulin E. ICU is the most frequent manifestation of latex allergy and may be the only one or may precede respiratory and/or systemic manifestations. Latex is the most common cause of occupational contact urticaria.
Protein contact dermatitis (PCD) is a slightly controversial entity.21 Its mechanism is not well known. It probably represents a combination of immediate-type hypersensitivity (type I) and delayed-type hypersensitivity (type IV).16 Clinically, PCD manifests as chronic eczema with episodes of recurrent acute attacks of itchy, sometimes vesicular, eczema on the contact site.16,21 ICU and PCD are rarely diagnosed outside occupational dermatology.
Allergic contact dermatitis (type IV hypersensitivity) due to additives used in latex rubber processing (eg, 1,3-diphenylguanidine) is also observed.22
Respiratory symptoms
NRL was recognized as a major cause of IgE-mediated occupational asthma in the early 1990s, especially in the healthcare setting.19 Because of successful prevention strategies aimed at reducing exposure to NRL protein allergens in gloves, the incidence of NRL-induced allergy and occupational asthma has markedly decreased, although this condition may still remain a cause of concern in workers with frequent NRL exposure, especially in developing countries.
Respiratory symptoms, such as rhinitis, conjunctivitis, cough, and asthma, are caused by contact with latex particles adsorbed by cornstarch used as a lubricating powder to facilitate the donning of latex gloves.23 The high concentrations of airborne latex particles found in the past in some hospital settings (eg, operating or cystoscopy rooms) confirm the importance of sensitization by air.
The latex particles, due to their aerodynamic characteristics, tend to completely pollute the environment in which the gloves are used, causing the onset of allergic symptoms even when staying in the polluted rooms, regardless of the direct use of gloves.
Although asthmatic symptoms may be the first manifestation of latex allergy, it more frequently appears in workers with a history of initial contact skin reactions and/or rhino-conjunctivitis and represents the progression of sensitization following persistence of allergen exposure.
Eosinophilic bronchitis due to latex is an infrequent occupational respiratory manifestation.24
Systemic reactions
Anaphylactic reactions25 generally occur during medical-surgical procedures, such as surgery, gynaecological interventions, or dental examination.19 They are attributable to parenteral release of allergens during surgery by surgical gloves or NRL instruments, particularly catheters or tubes.
Cardiovascular collapse is the most common form of presentation in anesthetized patients, although skin rashes and bronchospasm are also frequent. Reactions to latex normally occur during the maintenance phase of anaesthesia.24
Hevea latex allergens and cross reactivity with foods
Latex comes from the sap of the rubber tree, Hevea brasiliensis; however, it is also found in nearly 10% of plants denoted as “lactiferous” or latex-secreting plants. Therefore, latex derived from Hevea brasiliensis may not be the only source of allergenic latex proteins. Hundreds of allergens have been identified from NRL, of which 15 (Hev b1 to Hev b15) were officially listed by the World Health Organization (WHO)/International Union of Immunological Societies (IUIS) Allergen Nomenclature Sub-Committee (Table 2). The natural proteins in rubber are associated with both asymptomatic sensitization and IgE-mediated hypersensitivity.
Table 2.
Characterized Latex Allergens and clinical relevance
| allergen | Identification | kDa | Cross reactivity |
|---|---|---|---|
| Hev b 1 | Rubber elongation factor (REF) | 14.6 | Papain |
| Hev b 2 | 1,3 glucanase | 41.3 | Other glucanases |
| Hev b 3 | REF homologue | 23 | |
| Hev b 4 | Microhelix protein | 50–57 | |
| Hev b 5 | Acid protein | 16 | Kiwi acid protein |
| Hev b 6.01 | Prohevein | 20 | Chitinases (banana, avocado) |
| Hev b 6.02 | Hevein | 4.7 | Chitinases (banana, avocado, chestnut) |
| Hev b 6.03 | Hevein C | 14 | |
| Hev b 7 | Patatin homologue | 42.9 | Storage protein in Solanaceae |
| Hev b 8 | Profilin | 14 | Panallergen |
| Hev b 9 | Enolase | 51 | |
| Hev b 10 | Superoxide-dismutase | 26 | |
| Hev b 11 | Class I chitinase | 33 | Panallergen |
| Hev b 12 | LTP | 9.3 | Panallergen |
| Hev b 13 | Esterase | 42 | |
| Hev b 14 | Hevamine (chitinase) | 30 | |
| Hev b 15 | Serine protease inhibitor | 60–90 | PR-6 |
Different studies have reported that a high percentage of NRLA patients are also sensitized to foods, especially fruits. The first cases described were latex and banana associated allergy in 199126 and latex, avocado, and banana in 1992.27 In the same year, latex-fruit-associated allergy was also described.28 The next year, in 1993 it was latex-chestnut hypersensitivity.29,30 In 1994, Blanco et al31 proposed the term latex-fruit syndrome, based on the clinical observation of an unexpectedly high rate of fruit hypersensitivity in a group of 25 latex-allergic patients. Overall, 50% of them showed hypersensitivity to one or more fruits and half of the reported episodes were anaphylaxis.
Almost 40% of patients with latex allergy are known to have the latex–fruit syndrome caused by cross-reactivity with food allergens (mainly banana, avocado, chestnut, and kiwifruit), in which Hev b 2, Hev b 6.02, Hev b 7, Hev b 8, and Hev b 12 allergens were reported to be responsible.32
Latex-fruit syndrome has been described in 21%–58% of individuals with NRLA, where class 1 chitinases (Hev b 6) play a major role;33,34 class 1 chitinases have a defensive function, and Hev b 6 shows high sequence homology with chitinases present in fruits such as banana, avocado, and chestnut. Other NRL allergens (eg, profilin, glucanases, and ns-LTPs) may be involved in the latex-fruit syndrome. Furthermore, cassava (Manihot esculenta) and curry spice have been reported to cross-react with latex foods, and these reactions are thought to be due to sensitization to Hev b 5 (protein with an unknown function) and Hev b 8 (profilin), respectively. Cross-reactive reactions to potato and tomato were reported, which were found to be associated with sensitization to Hev b 7, a patatin-like protein. In addition, cross-reactivity to bell pepper has been described, which is thought to be due to cross-reactivity between Hev b 2, a beta-1,3-glucanase, and the bell pepper l-ascorbate peroxidase.33
Diagnosis: usefulness and limitations of available tests
The initial step in diagnosing latex allergy is obtaining a complete clinical history.15 The history should record the presence or absence of other allergies, atopy, previous operations or medical procedures involving latex products, and if the patient belongs to an identified and potential risk group (Table 1).16,34
The interview should be directed to the search for localized symptoms, such as erythema, itching, and urticaria, after wearing latex gloves as well as systemic symptoms, including coughing, sneezing, and wheezing, and any history of anaphylaxis.15 Finally, it should be noted if the patient is allergic to any fruit, especially those with cross reactions, such as, banana, kiwi, figs, papaya, avocado, and chestnuts.35
The diagnosis is made based on skin tests and the determination of specific IgE (sIgE) using the different methods available (Table 3).16,32 A positive result in any of these investigations may be considered indicative of latex sensitization.2 When skin test materials are standardized in terms of their allergen content and stability, they are a safe diagnostic procedure. When available, skin testing is the first line diagnostic method34 with a sensitivity of 93% and a specificity of 100%.16 Some countries such as the United States do not have standardized reagents available for skin testing. Use of unstandardized reagents have resulted in false negative results as well as adverse allergic events.3 In this case, the medical history and serologic assays must be relied on.36
Table 3.
Testing procedures for NRLA diagnosis
| Testing procedures | ||
|---|---|---|
| Type of test | Test | Description |
| Skin Tests | Prick testsa | Method of choice to confirm or rule out latex allergy. Intradermal tests are not recommended. |
| Patch tests | Used in suspected delayed-type hypersensitivity reactions, most of which are attributable to additives. | |
| Laboratory Tests | Latex-specific IgE | Two serologic methods, currently in use worldwide, as the ImmunoCAP and the IMMULITE autoanalyzer, both have a diagnostic sensitivity of 80% and a specificity of >95%. |
| Challenge Tests (With suggestive medical history but negative skin and laboratory tests) | Glove use test | Put a latex glove on one finger, from 15 min to 2 h. If the result is negative, the full glove is placed on one hand and a vinyl, or nitrile glove, on the other hand. The test is considered positive when it causes itching, erythema, vesicles or respiratory symptoms. |
| Rubbing test | The rubbing test gives false positives and is not standardized. Thus, its diagnostic yield is very low and it is not used. | |
| Specific bronchial provocation test | Are classified into those, the ones that use an aqueous latex extract (with a nebulizer or in a chamber with aerosolized glove extract) and those consisting in handling or shaking gloves to generate a dust aerosol. The lung functions and the occurrence of bronchial symptoms are then evaluated. | |
| Conjunctival provocation and nasal challenge | They have been used in some studies, however they have little significance. | |
Standardized allergens are recommended
Serologic tests for the diagnosis of IgE-mediated NRLA have been developed based on the quantification of IgE to the natural crude allergenic extract. However, the diagnosis of NRLA via quantification of latex-sIgE may pose significant difficulties.32 Serologic tests have a sensitivity of 70%–80%; therefore, 20%–30% of latex-allergic patients may show a negative result.34 Cut-off values depend on the method being used, which is not the same in all countries, and differ according to the population investigated to establish those values.
Challenge tests are considered to be the gold standard for the diagnosis of latex allergy. The provocation use test may be applied when there is a positive clinical history and the latex-sIgE antibody serologic and skin test results are incongruent.15 Despite the efficacy of provocation tests, they are considered to have a high risk of causing anaphylaxis and they are reserved for inconclusive cases due to this inherent risk.15,34
The consequences of false-negative results are obvious, as they entail a risk of life-threatening anaphylaxis upon subsequent exposure. However, overdiagnosis due to false-positive results may also have dramatic consequences.32 Identification of clinically irrelevant latex sensitization should avoid unnecessary and generally costly measures to avoid latex. Therefore, it is important to perform confirmatory tests to make accurate diagnoses that provide reliable results.37,38
Basophil activation test (BAT)
Flow cytometry is also used by some groups in Europe to determine the activation of basophils after stimulation with recombinant latex allergens. This might be able to distinguish clinically relevant allergy from asymptomatic sensitization39, 40, 41
Component-resolved diagnosis (CRD)
Over the past decade, increased availability of allergenic molecules for diagnosis, known as precision allergy molecular diagnostic applications (PAMD@), has improved the management of allergic diseases. This diagnostic strategy has also been termed component-resolved diagnostics (CRD), molecular-based allergy diagnostics (MBAD), or molecular allergy diagnostics (MAD).42,43
In addition to the above-mentioned limitations, detection of serum-specific IgE to the crude latex extract has another problem represented by the suboptimal content of some allergens.44 Therefore, already in 2002, the ImmunoCAP test (Thermo Fisher Scientific, Phadia, Uppsala, Sweden) for latex was improved by spiking the extract with Hev b 5, considerably increasing sensitivity.45
Furthermore, the sensitization profile to different allergens is clinically relevant. Currently, tests for sIgE to the 15 described individual latex allergens and to cross-reactive carbohydrate determinants (CCD) are commercially available, either in singleplex or multiplex assays. The ImmunoCAP ISAC microarray (Thermo Fisher Scientific, Phadia, Uppsala, Sweden) currently contains rHev b 1, rHev b 3, rHev b 5, rHev b 6, rHev b 8, and CCD. Some small studies suggest a better performance of ImmunoCAP compared to the ISAC microarray in latex allergy diagnosis, although concordance between methods is good.46,47 Other manufacturers such as Immulite (Siemens Healthcare Diagnostics Inc., Erlangen, Germany), Alex MADX (MacroArray Diagnostics GmbH Vienna, Austria), and Euroline (EUROIMMUN AG, Lübeck, Germany) have marketed latex-specific IgE diagnostic assays.
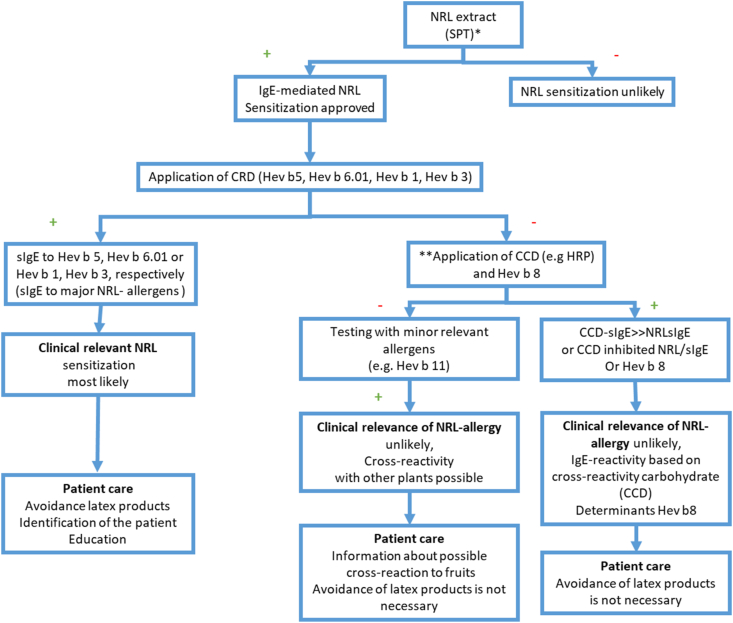
Once positivity in skin prick test or serum-sIgE to latex is confirmed, a reaction to certain components, such as Heb v 5, Hev b 6, Hev b 1, or Hev b 3, points to relevant sensitization. On the other hand, isolated sensitization to CCD or profilin (Hev b 8) found in patients without clinical symptoms, suggest a cross-reactive reaction with negligible clinical impact.48, 49, 50 CCD-inhibition assays may aid in proving such false positives. These findings have implications for the necessity to avoid latex, for example, in medical or surgical procedures.51,52 (Fig. 1)
Fig. 1.
Diagnostic serologic algorithm in suspected latex allergy. Modified52 reused with permission. ∗ Use of non-standardized NRL reagents may result in false positive test or even adverse allergic reactions. ∗ ∗Application of CCD tools [like horseradish peroxidase (HRP)] and/or inhibition studies with ‘cross-reactive carbohydrate determinants’ (CCD) to clarify protein epitopes (with clinical relevance) or the glyco-epitopes (with low clinical relevance)
Hev b 5 and Hev b 6.01 are the major allergens sensitizing health care workers, while spina bifida patients recognize mainly Hev b 1, Hev b 3, Hev b 5 and Hev b 6.01.44 Latex-fruit cross-reactivity has been associated with multiple allergens, such as Hev b 2 (β-1,3-Glucanase), Hev b 6.02 (Prohevein), Hev b 7 (Patatin-like protein), Hev b 8 (profilin), and Hev b 12 (Non-specific Lipid Transfer Protein type 1), and relevance of such positivity should be assessed case by case.52
Management of latex allergy
Individual strategies
Management of IgE-mediated latex allergy involves primary prevention of sensitization through reduction of natural rubber exposure (which will be discussed under the workplace strategy) and strategies for the latex-allergic patient that involve avoidance and education.
Avoidance
Subjects diagnosed with latex allergy should strive to avoid latex allergens contacting skin, mucosa, and respiratory epithelium. In addition, as latex-sensitized subjects may experience life-threatening anaphylaxis during unintentional contact with latex allergens, self-administered auto-injector epinephrine should be prescribed for these individuals. In resource-poor settings or when the auto-injector is unavailable, prescription of adrenaline ampoules and continuous education for the patients on how to administer them is recommended. Even if a sensitized individual has never experienced an event, the risk of developing anaphylaxis is substantial.
Patients generally do not realize that latex allergens may become airborne and inhaled. The fact that some rubber products may release proteins and/or have a lubricant powder that carries latex proteins into the air or dust possibly resulting in an allergic reaction is important information for the patient.
Education
Numerous articles and websites note that there are >40,000 products that contain NRL. It is likely that many more products contain NRL today. Thus, writing down all the potential products for a patient is not feasible. Rather, providing guidance on what might be a high-risk product makes more sense. Most medical devices are now required to be labeled with a warning if they contain NRL; however, unfortunately, this is not a uniform rule in all countries, and most consumer products are no mandated to contain such labeling.
It appears that products made by a dipping process with low-heat vulcanization to crosslink the polyisoprene contain substantial intact allergenic proteins in the finished product. The most recognizable products are gloves, condoms, balloons, rubber bladder catheters, tourniquets, adhesives, koosh balls, and some rubber toys. It has been estimated that around 10–12% of NRL products are made by this method. The majority of common rubber products are heat vulcanized at very high temperatures for prolonged lengths of time. In these products, most allergenic proteins are denatured and cleaved into small peptides. This prevents the crosslinking of IgE and renders these latex products virtually incapable of causing a clinical reaction. For the patient, the most obvious product is a rubber car tire. Teaching the patient about these differences is often helpful to prevent allergic reactions and to reduce the anxiety about developing anaphylaxis during daily living activities.
There are numerous educational resources on the importance to avoid latex allergens. Some of the most compelling work comes from colleagues in Germany in workplace settings. An obvious reduction in sensitization occurred after measures of latex avoidance were taken consisting of stopping the use of powdered latex gloves.53 Most interesting were the longitudinal data collected from latex-allergic healthcare workers who had a positive skin and serology test. Personal and workplace avoidance of latex allergens resulted in approximately 25% of the sensitized subjects to lose evidence of sensitization to NRL after a year or more.54 These promising results show that inadvertent contact with NRL does not always cause an allergic reaction if individuals strictly adhere to avoidance measures. For the individual who fits this category of reduced sensitization, it is not clear whether reintroduction of latex contact will induce an expansion of IgE-specific antibodies resulting in clinical reactions in the future.
There are 2 situations regarding latex-allergic patients in a healthcare setting that should be especially mentioned. The first involves patients with spina bifida: as this population appears to be at extreme risk of sensitization, no latex products should ever be used in contact with these individuals, if feasible, from the time of birth.
The second involves a practice that evolved in the early days after latex allergy was discovered. Patients who have latex allergy may need surgery or other specialized procedures in operating/procedure rooms. It was recommended that in elective situations, these subjects be cared for as the first case of the day. Latex allergens bound to cornstarch powder, although able to become airborne and inhaled, is a physically heavy particle that settles down from the air in several hours. Thus, it appeared safe to have these patients receive their care after an overnight time of no activity in the procedure suites. This is still a very sound practice when powdered latex gloves are utilized in an institution. Nevertheless, it is unlikely that this is necessary when a source of airborne latex is no longer used. Many institutions will continue the “first case of the day” tradition for latex allergic subjects in order to minimize risk and achieve consistency, but this is not always possible in all hospitals settings. In fact, some guidelines recommend that if powdered latex gloves are used, the operating room should be clean and free of powdered latex gloves for at least 3 h,55 which in many cases constitutes a complication.
Institutional and workplace avoidance
Starting after 2010, purchasing practices at healthcare institutions in high-income countries reduced the use of latex gloves. Most examination gloves (the major source of contact with powdered latex gloves in health care) was reduced to 30% or less by 2012 and is now negligible.56 Labeling of medical devices and personal protective equipment as to the content of latex became mandatory in many countries. A comprehensive occupational health program to assist individuals who develop latex allergy became the standard.51 Nevertheless, recent data from Wu et al show that latex allergy has not been reduced in many countries because of the lack of resources to substitute latex products for non-latex products.57 Although the initial cost of conversion is high, long-term healthcare costs may be lowered with reductions in healthcare workers’ compensation claims for latex-related disability.58 Many institutions formed purchasing consortiums to lower the cost of products bought in bulk quantity, which also contributed to cost reduction.
Because of the nature of their work, hospitals and healthcare institutions (eg, clinics or surgical centers) may introduce contact of a patient with an NRL product, requirements to ensure a “latex-safe” environment are essential. It is likely not possible to make a medical facility completely latex free, given the numerous devices and pharmaceutical containers (eg, multi-dose vials) that contain latex. The first task of these facilities is to eliminate sources of airborne latex proteins. This obviously includes powdered latex gloves (although not sold or distributed in some countries due to government regulations) and other products that are inadvertently brought into a facility (eg, balloons as a “get well” gift) by well-meaning personnel. Every year during the holiday seasons, lactifer plants are often delivered to patients in the hospital. One of the most common is the poinsettia plant. Poinsettia is one of >200 lactiferous plants that secrete latex when injured. That latex contains similar allergenic proteins that could theoretically induce an allergic reaction in a latex-allergic patient. It is extremely unlikely that this latex source emits latex proteins that can be inhaled. It would be prudent to ban these and NRL balloons from entering healthcare institutions.
It is critical that a latex allergy committee be active in the healthcare centers and formed by the Chief Executive of the hospital in order to demonstrate the importance of this committee. The responsibility of this committee is to assure that high-risk products (eg, powdered latex gloves) are not permitted to be bought, brought into the institution, or used when an acceptable alternative is available. In addition, this committee would write policies and procedures that staff are mandated to follow.
Because of the importance of such a committee, high-level management and front-line leaders should be active members:
-
•
Chief medical officer
-
•
Chief surgical officer
-
•
Chief nursing officer
-
•
Chief operating officer
-
•
Director of purchasing and central supply
-
•
Director of pharmacy
-
•
Medical/surgical specialists including anesthesiology
-
•
neurosurgery
-
•
cardiology
-
•
critical care
-
•
general surgery
-
•
urology
-
•
emergency medicine
-
•
radiology
-
•
allergy/immunology
-
•
Operating room nursing director
-
•
ICU nursing and general floor nursing directors
-
•
Director of endoscopy and/or vascular intervention
-
•
Director of employee health
A subunit of this group should be responsible for identifying all latex containing medical, surgical, endoscopy, and vascular catheters that contain latex in the institution and central supply. This list should be available in all areas of patient care or online and should also contain “substitute” devices that are latex safe when a latex-allergic patient is being cared for. When a substitute product is desired, the chief of the specialty using that device should be consulted about an acceptable alternative.
Even in a highly regulated healthcare system environment, inadvertent latex exposure still occurs. Recently, a publication citing the work from the Pennsylvania-Patient Safety Reporting System (PA-PSRS) reported that there were 616 inadvertent exposures to latex in the state hospitals over a 3-year period. There were 72 near misses and 544 exposures documented. These exposures resulted in temporary patient harm in 7 subjects.59
Every hospital and healthcare center should have an action protocol that is activated at the moment of patient admission. Each patient should wear an identification bracelet or collar of medical alert.
Table 4 summarizes different working scenarios and institutional measures for NRLA patients and their impact at institutional and personal levels.60,61
Table 4.
| Possible actions for latex avoidance in the workplace | Description | Positive effect | Pitfalls | Personal impact |
|---|---|---|---|---|
| Qualifies for disability.Termination of employment | The patient qualifies for disability due to an occupational disease | Definitively avoids contact with the allergen in the workplace | Loss of human resources and increased social costs | Affected individuals feel alone and abandoned. They have to reconsider their professional life. The economic impact may lead to depression |
| Relocation of the patient | The patient is relocated to places without direct contact with latex, such as administrative areas | Avoids contact with latex in the workplace | This may lead to a loss of employment status and human resources. May lead to contact with latex when moving to other areas in the institution | Feelings of loss as the patient feels obliged to change his/her job |
| Creation of latex-free areas | The area where the patient works is adapted. Options are: 1) The use of latex-free gloves both for the affected workers and their colleagues 2) The use of latex-free gloves for the affected workers and non-powdered, low-protein gloves for the remaining colleagues |
No loss of human resources | The patient may be at risk in other areas of the institution and potential new cases are not avoided. This type of avoidance requires changes in the institutional policies | The patient can continue working which compensates for the fact that the appearance of the disease was work related |
| Creation of a completely latex-free environment | Turn the institution into a latex-free environment | No loss of human resources, new sensitizations are avoided. Beneficial for the workers and the quality of the work environment |
This requires a delicate balance between the management of the budget and the human resources. This long-term measure implies a financial risk and can likely not be achieved in many institutions worldwide. | The feeling of loss is dramatically reduced. |
School environment
Little has been written about latex allergy in the school environment. One consumer advocate source for latex allergy is the Asthma and Allergy Foundation of America (AAFA). However, since several children with special needs (eg, spina bifida) attend school and will require medical interventions at times, many of the same precautions are necessary at the school as in the healthcare environment. The nursing office and front desk should carry non-latex examination gloves and sterile non-latex gloves when possible. This is not only safe for the student but also for the school personnel who may have to use these gloves and other products.
The school should be made aware of the student with latex allergy and what class they are in. An anaphylaxis plan and auto-injector epinephrine should be readily available. Bringing NRL and powdered balloons into the school should be avoided. Fortunately, common school items (eg, erasers) although made of NRL often contain a very small or undetectable amount of latex allergen. Working with the guardian of the child at risk and their physician to avoid NRL products that could present a risk of an allergic reaction is key in the school.
Immunotherapy
Since it is still quite difficult to achieve complete avoidance of contact with NRL, allergen-specific immunotherapy (AIT) may be an option, with the chance to reduce the severity of the disease and improve the patient's quality of life.
In 2000 and 2003, 2 double-blind placebo, controlled (DBPC) studies were published on the efficacy of subcutaneous immunotherapy (SCIT) with latex extracts.62,63 In both, significant improvement of symptoms was obtained; however, a remarkable number of side-effects was observed. In 2006, another DBPC trial failed to show a significant improvement of symptoms and medication scores using latex SCIT, probably because of the low level of symptoms at baseline and the low maintenance dose of therapy;64 moreover, the incidence of systemic reactions was very high in the active group.
After the publication of anecdotal cases of latex allergy treated with sublingual immunotherapy (SLIT), subsequent SLIT trials in Europe began to show safety and efficacy of this route of administration. In 2 Italian DBPC trials conducted in 20 children and 35 adults, respectively, using a rapid-induction protocol, cutaneous exposure tolerance significantly improved after 1 year of treatment and very few or no local oral reactions were reported.65,66 In another DBPC trial collecting 28 patients, no significant difference in specific provocation tests was observed after one year of SLIT.67
A case series of 26 latex-allergic patients highlighted the potential safety risk of latex SLIT, as 46% of patients experienced at least one systemic reaction and 88.5% reported at least one local reaction.68 Although these rates were distressing, they could also be at least partially related to trial design as all patients were being openly exposed to the allergen.
In an attempt to find correlations to predict clinical efficacy or safety outcomes, a 12-month open, case-control study of 23 children failed to show consistent significant immunological changes.69 The most recent and largest study (in 76 adults) on latex SLIT was published in 2018.70 In this observational case series, skin reactivity, latex IgE levels, and provocation tests significantly improved, even though IgG4 levels did not change.67
Unfortunately, there are several limitations to SLIT studies. The overall quality of the studies is moderate and the sample size is small in the DBPC trials. Patients with different symptoms are often grouped together in these studies, limiting their power. Moreover, long-term data to confirm sustained efficacy after AIT discontinuation are lacking.71 Nevertheless, although guidelines do not consider allergy to latex as an accepted indication for desensitization, all authors concluded that SLIT may be offered, in addition to symptomatic treatment, to selected patients when avoidance measures are not feasible or effective.
Finally, in a recent systematic review and meta-analysis of randomized controlled trials, including four studies, a statistically significant difference was observed only with the glove provocation test, leading the authors to conclude that more clinical trials are required to better define the clinical usefulness and safety of latex immunotherapy.72
Currently, to our knowledge, no commercial extracts are available for SCIT worldwide, and the only extract available in Europe is made by ALK (Denmark) for SLIT. No trials on latex AIT are currently ongoing.
Biologic drugs
To date there is only 1 study showing that the treatment with the monoclonal anti-IgE antibody omalizumab has clinically relevant ocular and skin antiallergic activity in healthcare workers with occupational latex allergy.73 Further studies are needed to evaluate the cost-effectiveness of this treatment, when exposure at the workplace cannot be avoided.
Conclusions and future perspectives
This review attempts to highlight the international relevance of NRLA. While on the one hand considerable progress has been made regarding this disease, on the other hand there are still great disparities in knowledge, diagnosis, and treatment in different regions of the world. In developed countries, due to the relaxation of preventive measures, anaphylactic accidents still occur since some materials containing latex can go unnoticed. Developing countries do not appear to be exposed to a similar situation, no updated epidemiological data are available and this limited information has an impact on the scope of institutional and governmental policies. This situation, together with economic difficulties, results in a lack of basic measures, such as the provision of non-powdered, low-protein gloves or synthetic gloves.
Moreover, not all patients have access to epinephrine autoinjectors; therefore, they have to carry adrenaline ampoules with them and be trained in its proper use. Many developing countries either do not have either standardized diagnostic methods or more sophisticated tests, such as CDR, to differentiate between true sensitization and cross-reactivity, or these techniques are not accessible to the majority of the population.
It is necessary to conduct epidemiological studies and to assess direct and indirect costs of latex allergy. It is also necessary to maintain the warnings and provide ongoing educational activities on NRLA in training programs, medical internships and residences, nursing education, and for all personnel in contact with the patient.
In addition, the COVID-19 pandemic has increased the use of latex gloves by institutions and the general population. It would be also useful to identify which elements of personal protective equipment other than latex gloves are a source of latex. All of this confers a greater risk of presenting allergic reactions in sensitized individuals in contact with these elements, as well as a potential risk, in the medium term, of an increase in the number of subjects allergic to latex.
AIT, SCIT, or SLIT, continue to play a doubtful role given the scarcity of positive trials, which exposes the need for new and better research in this regard.
Regarding the new biologics, beyond the few studies with omalizumab, exploring those with other therapeutic targets upstream in the T2 inflammatory cascade such as dupilumab or tezepelumab, could have a potential role in the treatment of NRLA in the future.
Finally, more research is needed to find economically and ecologically sustainable alternatives.
Abbreviations
NRLA, Natural Rubber Latex Allergy, IgE, Immunoglobulin E, HIV, Human immunodeficiency Virus, NRL, Natural Rubber Latex, ICU, Immunological Contact Urticaria, PCD, Protein Contact Urticaria, WHO, Word Health Organization, IUIS, International Union of immunological Societies, ns-LTPs, Non specific Lipid Transport Proteins, USA, United States of America, sIgE, specific Immunoglobulin E, BAT, Basophil Activation Test, CDR, Component Resolved Diagnosis, PAMD@, Precision Allergy Molecular Diagnostic Applications, MBAD, Molecular-based Allergy Diagnostic, MAD, Molecular Allergy Diagnosis, CCD, Carbohydrate determinants, ICU, Intensive Care Unit, PA-PSRS, Pennsylvania-Patient Safety Reporting System, AAFA, Asthma and Allergy Foundation of America, AIT, Allergen-specific Immunotherapy, DBPC, double-blind placebo, controlled, SCIT, Subcutaneous Immunotherapy, SLIT, Sublingual Immunotherapy.
Author contributions
All authors have contributed to the writing and revision of the manuscript.
Consent for publication
All authors agreed to the publication of this work in the World Allergy Organization Journal.
Availability of data and materials
The raw data will be made available with the acceptance of the submitted manuscript.
Ethics approval
No ethical consent was required since this study does not involve human or animals.
Funding
The authors have not received any funding to prepare the manuscript.
Declaration of competing interest
The authors declare that they do not have conflict of interests related to the contents of this article.
Acknowledgement
For the special contribution and support for writing the manuscript: Mario Sanchez Borges and Ignacio J. Ansotegui. We also would like to honor the memory of the late Mario Sanchez Borges, our esteemed colleague.
Footnotes
Full list of author information is available at the end of the article https://doi.org/10.1016/j.waojou.2021.100569
References
- 1.Kawai M., Kondo Y., Nakajima Y. Changes in the characteristics of patients with latex allergy from 1999 to 2014. Fujita medical journal. 2020;6(3):67–72. doi: 10.20407/fmj.2019-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saleh M.M., Forkel S., Schön M.P. Profile shift in latex sensitization over the last 20 years. Int Arch Allergy Immunol. 2019;178(1):83–88. doi: 10.1159/000492191. [DOI] [PubMed] [Google Scholar]
- 3.Kelly K.J., Kurup V., Zacharisen M. Skin and serologic testing in the diagnosis of latex allergy. J Allergy Clin Immunol. 1993;91:1140–1145. doi: 10.1016/0091-6749(93)90316-8. PMID: 8509577. [DOI] [PubMed] [Google Scholar]
- 4.Hourihane J.O., Allard M.N., Wade A.M. Impact of repeated surgical procedures on the incidence and prevalence of latex allergy: a prospective study of 1263 children. J Pediatr. 2002;140:479–482. doi: 10.1067/mpd.2002.123288. PMID: 12006967. [DOI] [PubMed] [Google Scholar]
- 5.Nieto A., Extornell F., Mazon A. Allergy to latex in spina bifida: a multivariate study of associated factors in 100 consecutive patients. J Allergy Clin Immunol. 1996;98:501–507. doi: 10.1016/s0091-6749(96)70082-9. PMID: 8828526. [DOI] [PubMed] [Google Scholar]
- 6.Cremer R., Hoppe A., Korsch E. Natural rubber latex allergy: prevalence and risk factors in patients with spina bifida compared with atopic children and controls. Eur J Pediatr. 1998;157:13–16. doi: 10.1007/s004310050758. PMID: 9461356. [DOI] [PubMed] [Google Scholar]
- 7.Bernardini R., Novembre E., Lombardi E. Risk factors for latex allergy in patients with spina bifida and latex sensitization. Clin Exp Allergy. 1999;29:681–686. doi: 10.1046/j.1365-2222.1999.00541.x. [DOI] [PubMed] [Google Scholar]
- 8.Konz K.R., Chia J.K., Kurup V.P. Comparison of latex hypersensitivity among patients with neurologic defects. J Allergy Clin Immunol. 1995;95:950–954. doi: 10.1016/s0091-6749(95)70094-3. [DOI] [PubMed] [Google Scholar]
- 9.Vogel L.C., Schrader T., Lubicky J.P. Latex allergy in children and adolescents with spinal cord injuries. J Pediatr Orthop. 1995;15:517–520. doi: 10.1097/01241398-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Tarlo S., Sussman G., Holness D. Latex sensitivity in dental students and staff: a cross-sectional study. J Allergy Clin Immunol. 1997;99:396–401. doi: 10.1016/s0091-6749(97)70058-7. 1997. [DOI] [PubMed] [Google Scholar]
- 11.Liss G.M., Sussman G.L., Deal K. Latex allergy: epidemiological study of 1351 hospital workers. Occup Environ Med. 1997;54:335–342. doi: 10.1136/oem.54.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagier F., Vervloet D., Lhermet I. Prevalence of latex allergy in operating room nurses. J Allergy Clin Immunol. 1993;90:319–322. doi: 10.1016/s0091-6749(05)80009-0. [DOI] [PubMed] [Google Scholar]
- 13.Levenbom-Mansour M.H., Oesterle J.R., Ownby D.R., al at. The incidence of latex sensitivity in ambulatory surgical patients: a correlation of historical factors with positive serum IgE levels. Anesth Analg. 1997;85:44–49. doi: 10.1097/00000539-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Orfan N.A., Reed R., Dykewicz M.S. Occupational asthma in a latex doll manufacturing plant. J Allergy Clin Immunol. 1994;94(5):826–830. doi: 10.1016/0091-6749(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 15.Kahn S.L., Podjasek J.O., Dimitropoulos V.A., Brown C.W. Natural rubber latex allergy. Disease-a-Month. 2016;62(1):5–17. doi: 10.1016/j.disamonth.2015.11.002. Internet. [DOI] [PubMed] [Google Scholar]
- 16.Cabañes N., Igea J.M., de la Hoz B. On behalf of the committee of latex allergy of the SEAIC. Latex allergy: position paper. J Investig Allergol Clin Immunol. 2012;22(5):313–330. [PubMed] [Google Scholar]
- 17.Bedolla-Barajas M., Machuca-Rincón M.L. Self-reported prevalence of latex allergy and associated factors in healthcare workers. Rev Alerg Mex. 2017;64(4):430–438. doi: 10.29262/ram.v64i4.289. [DOI] [PubMed] [Google Scholar]
- 18.Parisi C.A.S., Petriz N.A., Busaniche J.N. Prevalence of latex allergy in a population of patients diagnosed with myelomeningocele. Arch Argent Pediatr. 2016;114(1):30–35. doi: 10.5546/aap.2016.eng.30. [DOI] [PubMed] [Google Scholar]
- 19.Kelly K.J., Sussman G. Latex allergy: where are we now and how did we get there? J Allergy Clin Immunol Pract. 2017;5(5):1212–1216. doi: 10.1016/j.jaip.2017.05.029. Internet. [DOI] [PubMed] [Google Scholar]
- 20.Wu M., McIntosh J., Liu J. Current prevalence rate of latex allergy: why it remains a problem? J Occup Health. 2016;58(2):138–144. doi: 10.1539/joh.15-0275-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pesonen M., Koskela K., Aalto-Korte K. Contact urticaria and protein contact dermatitis in the Finnish Register of Occupational Diseases in a period of 12 years. Contact Dermatitis. 2020;83:1–7. doi: 10.1111/cod.13547. [DOI] [PubMed] [Google Scholar]
- 22.Dejonckheere G., Herman A., Baeck M. Allergic contact dermatitis caused by synthetic rubber gloves in healthcare workers: sensitization to 1,3-diphenylguanidine is common. Contact Dermatitis. 2019 Sep;81(3):167–173. doi: 10.1111/cod.13269. [DOI] [PubMed] [Google Scholar]
- 23.Vandenplas O., Froidure A., Meurer U. The role of allergen components for the diagnosis of latex-induced occupational asthma. Allergy. 2016;71:840–849. doi: 10.1111/all.12872. [DOI] [PubMed] [Google Scholar]
- 24.Poley G.E., Slater J.E. Latex allergy. J Allergy Clin Immunol. 2000;105:1054–1062. doi: 10.1067/mai.2000.106925. [DOI] [PubMed] [Google Scholar]
- 25.Turner P.J., Worm M., Ansotegui I.J., WAO Anaphylaxis Committee Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ J. 2019 Oct 31;12(10):100066. doi: 10.1016/j.waojou.2019.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.M'Raihi L., Charpin D., Pons A., Bongrand P., Vervloet D. Cross-reactivity between latex and banana. J Allergy Clin Immunol. 1991 Jan;87(1 Pt 1):129–130. doi: 10.1016/0091-6749(91)90224-c. [DOI] [PubMed] [Google Scholar]
- 27.Lavaud F., Cossart C., Reiter V. Latex allergy in patient with allergy to fruit. Lancet. 1992 Feb 22;339(8791):492–493. doi: 10.1016/0140-6736(92)91100-m. [DOI] [PubMed] [Google Scholar]
- 28.Ceuppens J.L., Van Durme P., Dooms-Goossens A. Latex allergy in patient with allergy to fruit. Lancet. 1992 Feb 22;339(8791):493. PMID: 1346844. [PubMed] [Google Scholar]
- 29.Fernández de Corres L., Moneo I., Muñoz D. Sensitization from chestnuts and bananas in patients with urticaria and anaphylaxis from contact with latex. Ann Allergy. 1993 Jan;70(1):35–39. [PubMed] [Google Scholar]
- 30.Añíbarro B., García-Ara M.C., Pascual C. Associated sensitization to latex and chestnut. Allergy. 1993 Feb;48(2):130–131. doi: 10.1111/j.1398-9995.1993.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 31.Blanco C., Carrillo T., Castillo R., Quiralte J., Cuevas M. Latex allergy: clinical features and cross reactivity with fruits. Ann Allergy. 1994;73:309–314. [PubMed] [Google Scholar]
- 32.Raulf M. Current state of occupational latex allergy. Curr Opin Allergy Clin Immunol. 2020 Apr;20:112–116. doi: 10.1097/ACI.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 33.Fukutomi Y. Occupational food allergy. Curr Opin Allergy Clin Immunol. 2019 Jun;19(3):243–248. doi: 10.1097/ACI.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 34.Gawchik SM. Latex allergy: Diagnosis and management. https://www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/latex-allergy-diagnosis-and-management accessed on January 25, 2021.
- 35.Ebo D.G., Bridts C.H., Rihs H.P. Hevea latex-associated allergies: piecing together the puzzle of the latex IgE reactivity profile. Expert Rev Mol Diagn. 2020;20(4):367–373. doi: 10.1080/14737159.2020.1730817. Internet. [DOI] [PubMed] [Google Scholar]
- 36.Wang M.L., Kelly K.J., Klancnik M., Petsonk E.L. Self-reported hand symptoms-a role in monitoring health care workers for latex sensitization? Ann Allergy Asthma Immunol. 2012;109(5):314–318. doi: 10.1016/j.anai.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton R.G., Peterson E.L., Ownby D.R. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(Suppl 2):S47–S56. doi: 10.1067/mai.2002.125334. [DOI] [PubMed] [Google Scholar]
- 38.Liberatore K. Protecting patients with latex allergies. Am J Nurs. 2019;119(1):60–63. doi: 10.1097/01.NAJ.0000552616.96652.72. [DOI] [PubMed] [Google Scholar]
- 39.Sanz M.L., Gamboa P.M., Garcia-Avila C. Flow-cytometry cellular allergen stimulation test in latex allergy. Int Arch Allergy Immunol. 2003;130:33–39. doi: 10.1159/000068367. [DOI] [PubMed] [Google Scholar]
- 40.Ebo D.G., Lechkar B., Schuerwegh A.J. Validation of a two-color flow cytometric assay detecting in vitro basophil activation for the diagnosis of IgE-mediated natural rubber latex allergy. Allergy. 2002;57(8):706–712. doi: 10.1034/j.1398-9995.2002.23553.x. [DOI] [PubMed] [Google Scholar]
- 41.Trabado A.R., Pereira L.M.F., Romero-Chala S. Evaluation of latex subclinical sensitization by way of the basophil activation test and specific IgE to latex recombinant allergens. Allergol Int. 2013;62(3):385–387. doi: 10.2332/allergolint.12-LE-0502. [DOI] [PubMed] [Google Scholar]
- 42.Ansotegui I.J., Melioli G., Canonica G.W. A WAO-ARIA- GA2LEN Consensus document on molecular-based allergy diagnosis (PAMD@): update 2020. WAO Journal. 2020;13:100091. doi: 10.1016/j.waojou.2019.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raulf M. Allergen component analysis as a tool in the diagnosis of occupational allergy. Curr Opin Allergy Clin Immunol. 2016;16:93–100. doi: 10.1097/ACI.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 44.Huss-Marp J., Raulf M., Jakob T. Spiking with recombinant allergens to improve allergen extracts: benefits and limitations for the use in routine diagnostics. Allergo J. 2015;24:18–25. doi: 10.1007/s40629-015-0072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundberg M., Chen Z., Rihs H.P., Wrangsjö K. Recombinant spiked allergen extract. Allergy Eur J Allergy Clin Immunol. 2001;56:794–795. doi: 10.1034/j.1398-9995.2001.056008794.x. [DOI] [PubMed] [Google Scholar]
- 46.De Sá A.B., Oliveira L.C., Camilo R. Latex sensitization in patients with myelomeningocele: contribution of microarray technique. Eur Ann Allergy Clin Immunol. 2018;50:135–138. doi: 10.23822/EurAnnACI.1764-1489.52. [DOI] [PubMed] [Google Scholar]
- 47.Seyfarth F., Schliemann S., Wiegand C. Diagnostic value of the ISAC® allergy chip in detecting latex sensitizations. Int Arch Occup Environ Health. 2014;87:775–781. doi: 10.1007/s00420-013-0921-6. [DOI] [PubMed] [Google Scholar]
- 48.Raulf-Heimsoth M., Rihs H.P., Rozynek P. Quantitative analysis of immunoglobulin E reactivity profiles in patients allergic or sensitized to natural rubber latex (Hevea brasiliensis) Clin Exp Allergy. 2007;37:1657–1667. doi: 10.1111/j.1365-2222.2007.02833.x. [DOI] [PubMed] [Google Scholar]
- 49.Ebo D.G., Hagendorens M.M., De Knop K.J. Component-resolved diagnosis from latex allergy by microarray. Clin Exp Allergy. 2010;40:348–358. doi: 10.1111/j.1365-2222.2009.03370.x. [DOI] [PubMed] [Google Scholar]
- 50.Schuler S., Ferrari G., Schmid-Grendelmeier P., Harr T. Microarray-based component-resolved diagnosis of latex allergy: isolated IgE-mediated sensitization to latexprofilin Hev b8 may act as confounder. Clin Transl Allergy. 2013;3:1–7. doi: 10.1186/2045-7022-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quercia O., Stefanini G.F., Scardovi A., Asero R. Patients monosensitised to Hev b 8 (Hevea brasiliensis latex profilin) may safely undergo major surgery in a normal (non-latex safe) environment. Eur Ann Allergy Clin Immunol. 2009;41:112–116. [PubMed] [Google Scholar]
- 52.Matricardi P.M., Kleine-Tebbe J., Hoffmann H.J. EAACI molecular allergology user's guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1–250. doi: 10.1111/pai.12563. [DOI] [PubMed] [Google Scholar]
- 53.Allmers H., Schmengler J., Skudlik C. Primary prevention of natural rubber latex allergy in the German health care system through education and intervention. J Allergy Clin Immunol. 2002;110:318–323. doi: 10.1067/mai.2002.126461. [DOI] [PubMed] [Google Scholar]
- 54.Kelly K.J., Wang M.L., Klancnik M., Petsonk E.L. Prevention of IgE sensitization to latex in health care workers after reduction of antigen exposures. J Occup Environ Med. 2011;53(8):934–940. doi: 10.1097/JOM.0b013e31822589dc. [DOI] [PubMed] [Google Scholar]
- 55.Management of Latex-Allergic Individuals ASCIA Guidelines https://www.allergy.org.au/hp/papers/management-of-latex-allergic-patients , accessed on January 25, 2021.
- 56.Kelly K.J., Kelly B.T. Latex allergy. In: Leung, editor. Pediatric Allergy. 3r Edition. Elsevier Saunders Edinburgh; 2016. pp. 505–513. (Chapter 56) copyright. [Google Scholar]
- 57.Korniewicz D.M., Chookaew N., El-Masri M. Conversion to low protein, powder-free surgical gloves: is it worth the cost? American Association of Occupational Health Nurses. 2005;53(9):388–393. [PubMed] [Google Scholar]
- 58.Liberatore K., Kelly K.J. Latex allergy risks live on. J Allergy Clin Immunol: In Pract. 2018 Nov - Dec;6(6):1877–1878. doi: 10.1016/j.jaip.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Latex allergy. Asthma and Allergy Foundation of America. www.aafa.org/latex-allergy/- accessed on September 16, 2020.
- 60.Latex in Spain: situation overview. Spanish Latex Allergy Association. https://www.seguridaddelpaciente.es/resources/contenidos/english2/Latex_allergy_Spain.pdf accesed on dicember 6, 2020.
- 61.Vandenplas O., Raulf M. Occupational latex allergy: the current state of affairs. Curr Allergy Asthma Rep. 2017 Mar;17(3):14. doi: 10.1007/s11882-017-0682-5. [DOI] [PubMed] [Google Scholar]
- 62.Leynadier F., Herman D., Vervloet D. Specific immunotherapy with a standardized latex extract versus placebo in allergic healthcare workers. J Allergy Clin Immunol. 2000;106:585–590. doi: 10.1067/mai.2000.109173. [DOI] [PubMed] [Google Scholar]
- 63.Sastre J., Fernandez-Nieto M., Rico P. Specific immunotherapy with a standardized latex extract in allergic workers: a double-blind, placebo-controlled study. J Allergy Clin Immunol. 2003;111:985–994. doi: 10.1067/mai.2003.1390. [DOI] [PubMed] [Google Scholar]
- 64.Tabar A.I., Anda M., Bonifazi F. Specific immunotherapy with standardized latex extract versus placebo in latex-allergic patients. Int Arch Allergy Immunol. 2006;141(4):369–376. doi: 10.1159/000095463. [DOI] [PubMed] [Google Scholar]
- 65.Nettis E., Colanardi M.C., Soccio A.L. Double-blind, placebo-controlled study of sublingual immunotherapy in patients with latex-induced urticaria: a 12-month study. Br J Dermatol. 2007 Apr;156(4):674–681. doi: 10.1111/j.1365-2133.2006.07738.x. [DOI] [PubMed] [Google Scholar]
- 66.Bernardini R., Campodonico P., Burastero S. Sublingual immunotherapy with a latex extract in paediatric patients: a double-blind, placebo-controlled study. Curr Med Res Opin. 2006 Aug;22(8):1515–1522. doi: 10.1185/030079906X115711. [DOI] [PubMed] [Google Scholar]
- 67.Gastaminza G., Algorta J., Uriel O. Randomized, double-blind, placebo-controlled clinical trial of sublingual immunotherapy in natural rubber latex allergic patients. Trials. 2011 Aug 9;12:191. doi: 10.1186/1745-6215-12-191. PMID: 21827704; PMCID: PMC3175458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cisteró Bahima A., Sastre J., Enrique E. Tolerance and effects on skin reactivity to latex of sublingual rush immunotherapy with a latex extract. J Investig Allergol Clin Immunol. 2014;14(1):17–25. [PubMed] [Google Scholar]
- 69.Lasa Luaces E.M., Tabar Purroy A.I., García Figueroa B.E. Component-resolved immunologic modifications, efficacy, and tolerance of latex sublingual immunotherapy in children. Ann Allergy Asthma Immunol. 2012 May;108(5):367–372. doi: 10.1016/j.anai.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Nucera E., Mezzacappa S., Buonomo A. Latex immunotherapy: evidence of effectiveness. Postepy Dermatol Alergol. 2018 Apr;35(2):145–150. doi: 10.5114/ada.2018.75235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith D.M., Freeman T.M. Sublingual immunotherapy for other indications: venom large local, latex, atopic dermatitis, and food. Immunol Allergy Clin. 2020 Feb;40(1):41–57. doi: 10.1016/j.iac.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 72.Sridharan K., Sivaramakrishnan G. Sublingual immunotherapy in patients with latex allergy: systematic review and meta-analysis of randomized controlled trials. J Dermatol Treat. 2017 Nov;28(7):600–605. doi: 10.1080/09546634.2017.1303567. [DOI] [PubMed] [Google Scholar]
- 73.Leynadier F., Doudou O., Gaouar H. Effect of omalizumab in healthcare workers with occupational latex allergy. J Allergy Clin Immunol. 2004;113:360–361. doi: 10.1016/j.jaci.2003.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data will be made available with the acceptance of the submitted manuscript.