Abstract
Adequate pullet nutrition is essential to reach BW and suitable body composition for reproduction. An experiment was conducted to determine the effects of 4 dietary amino acid (AA) levels on BW, flock uniformity, body conformation, organ, leg, and feathering development of broiler breeder pullets during the rearing phase from 5 to 24 wk. A total of 1,360 Cobb-500 slow-feathering (SF) pullets were randomly placed in 16-floor pens with 85 females per pen. Diets with corn, soybean meal, and wheat-midds were formulated without protein restriction maintaining minimum ratios between essential AA and Lys on a digestible (dig) ideal basis. Treatments consisted of 4 dietary AA levels with 80% (low-AA), 90% (moderate-AA), 100% (standard-AA), and 110% (high-AA) of the Cobb-Vantress recommendations guided by dig Lys using balanced protein. Up to 4 wk, all pullets were fed a common starter crumble diet. Grower and developer mash diets were fed to pullets from 5 to 15 wk and from 16 to 24 wk, respectively. Pullets fed standard-AA and high-AA diets were heavier (P < 0.001) than those fed low-AA diets at 10, 15, and 20 wk of age. High-AA diets resulted in better (P = 0.040) flock uniformity at 10 wk. Pullets fed a high-AA diet had the highest (P = 0.041) relative breast weight at 20 wk of age and the lowest (P = 0.044) deposits of abdominal fat at 15 wk of age. Fleshing increased (P < 0.05) as AA content rise in the diet, while the relative shank length (P < 0.001) and the number of wing juvenile feathers (P = 0.004) decreased. Pullets fed the lowest dietary AA level had the longest (P = 0.007) small intestine relative to BW at 10 wk of age, but a smaller (P = 0.001) liver than those fed moderate and standard-AA diets at 20 wk of age. Dietary AA levels have important effects on pullet BW, fleshing, and organ development during rearing with potential reproductive performance impacts.
Key words: pullet, body weight, flock uniformity, body composition, ideal protein
INTRODUCTION
Dietary amino acids (AA) and crude protein (CP) have been demonstrated to affect BW, body composition, organ development, and feathering in broiler breeder pullets (Hudson et al., 2000; Ekmay, 2011; van Emous et al., 2013). Breeder pullets are fed to achieve target BW, and meeting growth objectives during the rearing phase has been related to improvements in reproductive parameters such as fertility, hatchability, and embryo mortality (Leeson and Summers, 2000; Renema et al., 2001; Fisher and Gous, 2009). Feed allocation and feeding programs to obtain a target BW could trigger an excess of AA and CP intake that can lead to a greater breast muscle (Ekmay et al., 2013; van Emous et al., 2013), reducing abdominal fat deposition (Renema et al., 2001; de Beer and Coon, 2007). It has been reported that BW and breast weight also increased in broiler breeder pullets fed higher CP levels or AA-supplemented diets (Hudson et al., 2000; de Beer and Coon, 2006; van Emous et al., 2013). In contrast, low-CP diets resulted in more abdominal fat deposition (van Emous et al., 2013; Lesuisse et al., 2017). van Emous et al. (2013) described a decrease of breast muscle in Ross 308 pullets fed diets with low levels of CP (starter 14.59, grower 12.27, and pre-breeder 12.79%). In another report, the same authors (van Emous et al., 2015a) observed an increase of 97% in abdominal fat at 22 wk (0.66% of BW), as a result of feeding pullet diets with 13.81, 11.32, and 12.62% CP compared to 16.65, 14.15, and 15.11% CP. Even when abdominal fat is a desirable trait before light stimulation, it has been proven that the hen uses her breast protein during the transition from pullet to sexual maturity (Vignale et al., 2016) and peak production (Caldas et al., 2018). Therefore, an adequate amount of breast or fleshing is desirable to achieve during the rearing period to reach egg production standards. A balance of both breast development and fat reserves has been proposed before light stimulation and onset of production (de Beer and Coon, 2007; Decuypere et al., 2010; Chang et al., 2016; Vignale et al., 2016).
Previous studies have reported that early nutrition has an important impact on appropriate skeletal size. Leeson and Summers (1984) and Hudson et al. (2000) have identified that starter diets up to 4 or 6 wk of age with 22, and 20% CP increased shank length 2.5 and 2.1 mm, respectively, reducing the bone density of broiler breeder pullets. However, other researchers have not detected dietary CP modification effects on skeletal measurements of broiler breeder pullets at 8, 12, and 16 wk of age (Lilburn et al., 1989). Therefore, the impact of dietary CP or AA levels for breeders on leg growth varies with age and is still controversial. Likewise, less CP or AA levels have shown adverse effects on the feather growth, decreasing the feather cover at 6 and 11 wk in pullets fed diets with low CP (14.59 and 12.27% CP) during starter and grower phases than those fed with high (19.2 and 14.13% CP) and medium CP levels of 17.96, and 12.96% (van Emous et al., 2014). Feathering is highly coordinated with the physiological conditions (Leeson and Summers, 2000) and might be used as an approach to evaluate pullets' development and sexual maturation.
Studies during rearing commonly have been focused on extreme protein levels, and the effects of AA have been evaluated in the laying phase (Lopez and Lesson, 1995; Enting et al., 2007; Mejia et al., 2012, 2013; Ekmay et al., 2013; Butler, 2019). Very few studies have evaluated levels of AA during rearing close to the ones recommended by genetic lines. An adequate BW, body composition in terms of fleshing and abdominal fat reserve is essential before light stimulation; otherwise, breeders may exhibit poor egg peak production and persistency during the reproductive phase (Lien and Yuan, 1994; Lewis, 2006). Based on these statements, the objective of this study was to determine the effects of four dietary AA levels on BW, and the development of breast muscle, organs, legs, and feathering on Cobb 500 slow-feathering (SF) broiler breeder pullets during the rearing phase from 5 wk to onset of egg production. We hypothesized that AA levels would affect BW, flock uniformity, body composition, organ and leg development, and feathering in the rearing period.
MATERIALS AND METHODS
The experiment was conducted with the approval of animal use by the Animal Care and Use Committee and in compliance with the Guidelines for Care and Use of Laboratory Animals at North Carolina State University.
Treatments
All females were fed one common starter diet on crumbles from placement to 28 d of age. Corn-soybean meal diets were formulated without CP restriction maintaining minimum ratios between essential AA and Lys on a digestible (dig) ideal basis (Table 1), according to the nutritional recommendations for Cobb 500 SF pullets (Cobb-Vantress, 2018). Birds were assigned one of 4 dietary treatments from 5 wk to the onset of egg production that occurred at 24 wk of age. Treatments consisted of four dietary AA levels with 80% (low-AA), 90% (moderate-AA), 100% (standard-AA), and 110% (high-AA). Diets were guided by an ideal protein profile based on dig Lys level. Pullets were fed grower diets from 5 to 15 wk of age with 0.48, 0.54, 0.60, and 0.66% of dig Lys, and developer diet from 16 up to 24 wk of age with 0.51, 0.57, 0.63, and 0.69% dig Lys. All diets were offered in a coarse mash with approximately 1,000 and 1,300 μm geometric mean particle size and contained 2,700 kcal/kg AMEn and 2,800 kcal/kg AMEn for the grower and developer phases, respectively. Corn, soybean meal, and wheat midds were analyzed before feed formulation of experimental diets by near-infrared spectroscopy (NIRS, DS2500, FOSS, Denmark). Diets were analyzed according to the Association of Official Analytical Chemists International, 2006 using standard methods for dry matter (930.15), ether extract (920.39), ash (942.05), acid detergent fiber (973.18), neutral detergent fiber (2002-04), crude protein (N x 6.25) by combustion (990.03), and AA through HPLC (Evonik Nutrition & Care GmbH, Hanau, Hessen, Germany). The composition for CP and AA were closed to formulated values.
Table 1.
| Grower (4–15 wk) |
Developer (15–21 wk) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ingredient, % | Starter (1–4 wk) | Low -AA | Moderate-AA | Standard -AA | High -AA | Low -AA | Moderate -AA | Standard -AA | High -AA |
| Corn | 56.01 | 54.87 | 54.11 | 53.70 | 53.20 | 58.10 | 57.29 | 58.98 | 56.15 |
| Wheat midds | 15.00 | 32.50 | 31.06 | 30.49 | 29.96 | 26.09 | 24.59 | 22.00 | 23.09 |
| Soybean meal, 46% | 24.36 | 8.11 | 10.33 | 11.23 | 12.12 | 9.85 | 12.17 | 13.48 | 14.60 |
| Poultry fat | 1.00 | 1.10 | 1.10 | 1.10 | 1.10 | 1.85 | 1.85 | 1.32 | 1.85 |
| Limestone fine | 1.26 | 1.53 | 1.51 | 1.51 | 1.50 | 2.10 | 2.09 | 2.07 | 2.08 |
| Dicalcium phosphate | 1.03 | 0.73 | 0.74 | 0.74 | 0.74 | 0.84 | 0.85 | 0.88 | 0.85 |
| Salt, NaCl | 0.30 | 0.28 | 0.28 | 0.27 | 0.25 | 0.25 | 0.25 | 0.24 | 0.23 |
| Sodium bicarbonate | 0.29 | 0.22 | 0.21 | 0.23 | 0.26 | 0.25 | 0.25 | 0.26 | 0.27 |
| Mineral-vitamin premix3 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| Choline chloride, 60% | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| DL-Met, 99% | 0.14 | 0.09 | 0.09 | 0.09 | 0.14 | 0.10 | 0.11 | 0.13 | 0.17 |
| L-Lys HCl, 78.8% | 0.02 | 0.001 | 0.001 | 0.05 | 0.10 | - | 0.01 | 0.05 | 0.09 |
| L-Thr, 98% | 0.06 | 0.03 | 0.01 | 0.04 | 0.08 | 0.02 | 0.01 | 0.05 | 0.07 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated composition | |||||||||
| ME, kcal/kg | 2,800.00 | 2,700.00 | 2,700.00 | 2,700.00 | 2,700.00 | 2,800.00 | 2,800.00 | 2,800.00 | 2,800.00 |
| CP, % | 18.50 | 12.87 | 13.63 | 14.00 | 14.40 | 12.93 | 13.73 | 14.15 | 14.72 |
| NPP, % | 0.34 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 |
| Calcium, % | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 1.15 | 1.15 | 1.15 | 1.15 |
| Total P, % | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.62 | 0.62 | 0.62 | 0.62 |
| Sodium, % | 0.21 | 0.19 | 0.19 | 0.19 | 0.19 | 0.18 | 0.18 | 0.18 | 0.18 |
| Potassium, % | 0.82 | 0.63 | 0.66 | 0.67 | 0.69 | 0.63 | 0.66 | 0.67 | 0.69 |
| Chloride, % | 0.25 | 0.24 | 0.24 | 0.24 | 0.24 | 0.22 | 0.22 | 0.22 | 0.22 |
| Total Lys, % | 1.02 | 0.57 | 0.63 | 0.69 | 0.75 | 0.59 | 0.66 | 0.72 | 0.78 |
| Total TSAA, % | 0.77 | 0.55 | 0.58 | 0.60 | 0.64 | 0.57 | 0.59 | 0.62 | 0.67 |
| Total Thr, % | 0.74 | 0.48 | 0.50 | 0.53 | 0.58 | 0.48 | 0.50 | 0.55 | 0.60 |
| Total Val, % | 0.92 | 0.63 | 0.67 | 0.67 | 0.67 | 0.64 | 0.68 | 0.69 | 0.72 |
| Total Arg, % | 1.21 | 0.83 | 0.89 | 0.86 | 0.86 | 0.84 | 0.90 | 0.93 | 0.97 |
| dig Lys, % | 0.90 | 0.49 | 0.54 | 0.60 | 0.66 | 0.51 | 0.57 | 0.63 | 0.69 |
| dig TSAA, % | 0.69 | 0.48 | 0.51 | 0.52 | 0.56 | 0.50 | 0.52 | 0.56 | 0.60 |
| dig Thr, % | 0.64 | 0.40 | 0.41 | 0.45 | 0.50 | 0.40 | 0.42 | 0.47 | 0.51 |
| dig Val, % | 0.84 | 0.57 | 0.62 | 0.63 | 0.65 | 0.60 | 0.62 | 0.64 | 0.68 |
| dig Arg, % | 1.15 | 0.79 | 0.86 | 0.87 | 0.91 | 0.86 | 0.87 | 0.89 | 0.97 |
| Analyzed nutrient4 | |||||||||
| CP, % | 18.46 | 12.80 | 13.57 | 13.75 | 14.60 | 13.24 | 13.36 | 14.13 | 15.03 |
| Calcium. % | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 1.15 | 1.15 | 1.15 | 1.15 |
| Total P, % | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.62 | 0.62 | 0.62 | 0.62 |
| Total Lys, % | 0.97 | 0.54 | 0.62 | 0.65 | 0.72 | 0.61 | 0.65 | 0.72 | 0.81 |
| Total Met, % | 0.44 | 0.27 | 0.31 | 0.30 | 0.33 | 0.31 | 0.32 | 0.37 | 0.40 |
| Total Cys, % | 0.33 | 0.24 | 0.25 | 0.25 | 0.26 | 0.24 | 0.24 | 0.25 | 0.26 |
| Total TSAA, % | 0.77 | 0.52 | 0.56 | 0.55 | 0.59 | 0.55 | 0.56 | 0.62 | 0.66 |
| Total Thr, % | 0.71 | 0.48 | 0.50 | 0.53 | 0.57 | 0.49 | 0.50 | 0.55 | 0.62 |
| Total Val, % | 0.84 | 0.57 | 0.62 | 0.63 | 0.65 | 0.60 | 0.62 | 0.64 | 0.68 |
| Total Arg, % | 1.15 | 0.79 | 0.86 | 0.87 | 0.91 | 0.86 | 0.87 | 0.89 | 0.97 |
Low-AA, moderate-AA, standard-AA, and high-AA diets corresponding to 80, 90, 100, and 110% of the Cobb-Vantress (2018) recommendations, guided by dig Lys using balanced protein.
All diets contained Quantum Blue 5G, 80 g/ton to supply 1,000 FYT (AB Vista) delivering 0.11% of available P, and 0.10% of calcium.
The trace mineral and vitamin premix contained per kilogram of diet: Selenium premix, 500 mg; manganese (Mn SO4), 60 g; zinc (ZnSO4), 60 g; iron (FeSO4), 40 g; copper (CuSO4), 5 g; iodine (Ca[IO3]2),1.25 g; vitamin A, 13,227,513 IU; vitamin D3, 3,968,253 IU; vitamin E, 66,137 IU; vitamin B12, 39.6 mg; riboflavin, 13,227 mg; niacin, 110,229 mg; d-pantothenic acid, 22,045 mg; menadione, 3,968 mg; folic acid, 2,204 mg; vitamin B6, 7,936 mg; thiamine, 3,968 mg; biotin, 253.5 mg.
Values represent mean of 3 samples.
Husbandry of Broiler Breeder Pullets
At placement, a total of 1,360 Cobb-500 SF pullets and 288 Cobb MV male chicks were obtained from a commercial hatchery (North Carolina Cobb Hatchery, Wadesboro, NC). Birds were individually identified with neck tags and randomly placed in an environmentally controlled dark house with solid-sided walls and light-traps. This house is divided into 32 floor-pens, 16 pens of 4.65 × 3.38 m where 85 females were placed with a density of 5.41 pullets/m2 and 16 cockerel pens (3.99 × 1.17 m) of 18 males/pen with a density of 3.85 cockerels/m2. Each pullet floor pen had fresh wood shavings and was equipped with 4 tube feeders and 2 automatic bell drinkers, and 1 tube feeder was added at 13 wk.
Feeding Program
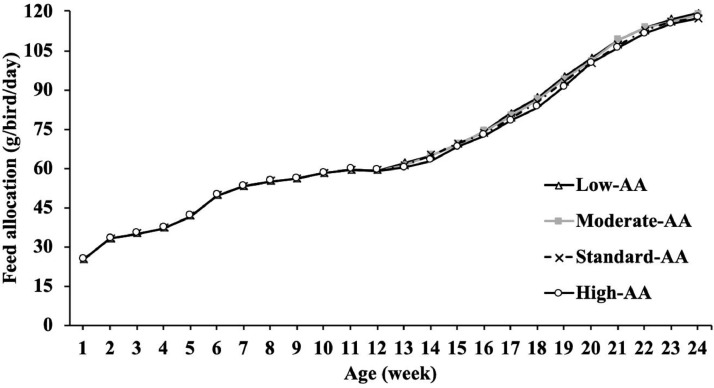
The feeding program from 1 to 2 wk was ad libitum, and after 2 wk, it was restricted. A skip-a-day (6/1) feeding schedule (fed Monday to Saturday) was established from 4 to 5 wk. From 6 wk until the end of the experiment, a skip-2-d (5/2) program was applied (fed on Monday, Tuesday, Thursday, Friday, and Saturday). From 9 wk onward, feed allocation for pullets varied slightly (±3 g/bird/day) among treatments to maintain BW close (± 2%) to the target BW curves recommended by Cobb-Vantress (2018). The feeder space fluctuated between 6.6 and 8.8 cm/pullet because this experiment started with 85 pullets, but several pullets were sampled for body part composition, culled or died, and feeders were added as they grew up. Each feeder pan had a circumference of 132 cm. The amounts of feed offered each week per pullet are presented in Figure 1. Since treatments were for female pullets only and males were not under evaluation, the male data is not presented. Males were fed the standard grower and developer treatment (treatment 100% Cobb-Vantress specifications, 2018).
Figure 1.
Feed allocation (g/bird/day) from 1 to 24 wk of age of Cobb 500 SF broiler breeder pullets fed four different dietary amino acid levels during rearing low-AA, moderate-AA, standard-AA, high-AA corresponding to 80, 90, 100 and 110% of the Cobb-Vantress (2018) recommendations, guided by dig Lys using balanced protein. n = 4 replicate pens per treatment of 85 pullets each.
Lighting Program
The light program started with 24-h lighting for the first d, followed by a daily reduction of 1 h until reach a program of 8 h of light:16 h of darkness (8L:16D) from 17 d to 21 wk. The light duration was decreased daily from 24 to 8 h during the first 17 d. Light intensity was gradually reduced from 45 to 12 lux during the first 11 d. Lighting of 4 to 5 lux was used after 12 d, and almost total darkness was maintained for the remaining 16 h per day until 21 wk.
Vaccination Program
Vaccination In Ovo against Marek’s-Gumboro was made at 18 d of incubation with a full commercial dose of the Vaxxitek HVT/IBD plus HVT+Rispens MREF 0515 (Merial, Inc., Gainesville, GA) and injected with Victrio (Bayer Corporation, Whippany, NJ). At hatch, breeder pullets and cockerels were injected via subcutaneous with antibiotic (Ceftiofur) and spray with Coccivac-D2 (Merck & Co., Inc., Kenilworth, NJ). Henceforth, commercial vaccines produced by Zoetis (Zoetis Inc., Durham, NC) were used. At 2 wk of age, the birds were protected against New Castle-Bronchitis with Newcastle B1 + Bronchitis Conn Mass in the eye. At 6, 11, and 18 wk, Newcastle LaSota + Bronchitis Mass II strain was applied in the water. Finally, at 15 wk, a live virus vaccine of Avian Encephalomyelitis-Fowl Pox was applied by the wing-web puncture method, and a Newcastle + Bronchitis + Salmonella inactivated vaccine was injected intramuscularly.
Data Collection
Growth Performance
Group BW of all birds in a pen was collected at placement, 4, 10, 15, 20, and 21 wk of age to determine the average BW per pen. Individual BW of the whole pen was recorded to determine flock uniformity, guarantee similar BW before starting the experimental period (4 wk), and monitor BW curves between dietary AA levels. All BW were collected in dry, in the morning before feeding and access to water, and after 1 d without feed. Pullet mortality was observed twice a day, and the weight of dead birds was recorded.
Body Conformation and Organ Development
At 11, 15, 17, 20, and 21 wk of age, the fleshing score was measured in all birds by a specialist to estimate individual fleshing changes during the experimental period. Fleshing were scored on a 5-point scale, where score 1 represented an undersized breast muscle and 5 oversized breast muscle. At 10, 15, and 20 wk of age, 3 pullets per pen (12 per treatment, 48 total) were selected, representing the BW of the replicate pen mean and euthanatized by cervical dislocation and weighed. The weight of pectoral muscles with the keel bone (Pectoralis major and P. minor), abdominal fat, and liver were recorded (g), and their relative weight was calculated as a percentage of live empty BW (24-h feed withdrawal). The small intestine and ceca length were measured in cm and presented as a relative of the live weight.
Leg Development
The left (L) and right (R) shanks of the three euthanatized pullets per pen were weighed on an analytical scale (± 0.001 g). The length was measured (mm) with electronic calipers (ProMax Fred V. Fowler Co. Inc., Newton, MA) from the hock to the footpad at 10, 15, and 20 wk of age. The relative asymmetry of shanks was calculated as (|R –L|/[(R + L)]/2) × 100 (Møller et al., 1999), and the bone weight relative to the BW was presented. Likewise, at 10, 11, 15, 17, 20, and 21 wk of age, the shank length of all pullets per pen was measured (mm) using an L-shape metal ruler designed by Cobb-Vantress (2018). Briefly, one assistant held each pullet from the wings with the legs up. Later, the expert placed the metal ruler with the 90° hook on the footpad, adjusting the device to be flat on the shank. According to the ruler, shank length was expressed as the distance from the footpad until the end of the hock joint. Length relative to BW (mm / 100 g) was calculated for both euthanatized and alive pullets.
Feathering Development
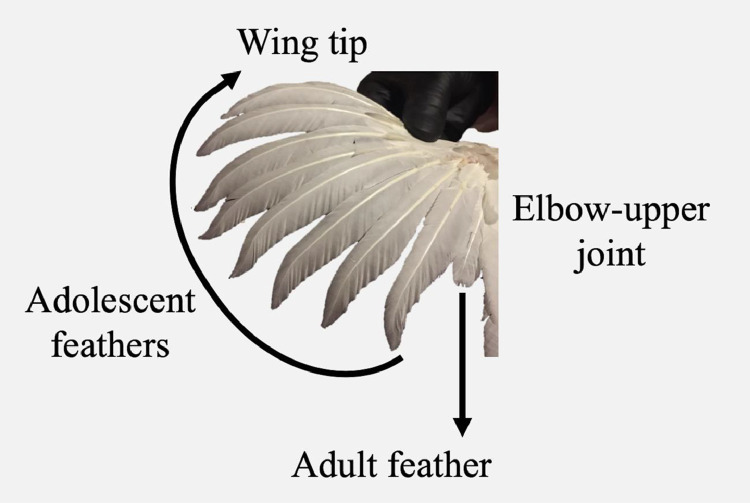
The development of adolescent feathers to an adult phase was observed and recorded at 11, 17, and 21 wk of age. According to the structure and number of primary feathers in the wing, at 7 wk of age, broiler breeders accounted for ten primary adolescent feathers that differed later in shape from the adult stage. Adolescent feathers were described as elongated and pointed structures that molted in a proximal-distal sense from the elbow-upper junction until the wingtip (Figure 2). In contrast, adult feathers were shorter, with a rounded shape in the tip.
Figure 2.
Feathering evaluation, according to feather structure following a proximal-distal sense of change from elbow-upper joint until wing tip.
Statistical Analysis
Data were analyzed in a completely randomized design with four dietary treatments using JMP PRO 14 (SAS Institute, Cary, NC). Each treatment had four replicate pens of 85 pullets each. One-way ANOVA was used to determine the effects of dietary treatments, and Tukey's HSD test was used for mean comparison. Regression analyses were conducted, and significant relationships between BW and response variables were evaluated through pairwise correlations. Percentage data for mortality were transformed to arcsine square-root percentage for analysis. Statistical significance was established at P < 0.05.
RESULTS
Growth Performance
As expected, no differences in BW (P > 0.05) were detected before starting (1 and 4 wk of age) the experimental period (Table 2). The two highest AA levels (standard-AA and high-AA diets) resulted in heavier pullets (P < 0.001) at 10 and 20 wk of age, as compared to pullets fed low-AA and moderate-AA diets. Linear effects (P < 0.001) demonstrated that BW increased as AA dietary levels rise at 10 (BW, g = 963.274 + 2.291 × AA%; R2 = 0.86), and 20 wk of age (BW, g = 2,070.176 + 3.310 × AA%; R2 = 0.78). At 15 wk, the lightest pullets were observed when low-AA diets were fed compared to other treatments. Likewise, BW increased (P < 0.001) linearly (BW, g = 1,432.726 + 2.786 × AA%; R2 = 0.90) with dietary AA concentration. Before photostimulation, pullets fed 110% AA was 104 g on average heavier (P < 0.001) than those fed low-AA and moderate-AA (2,661 vs. 2,557g); however, both weights were ± 2% of the standard BW, which is considered an acceptable variability by the genetic line. Standard-AA diets presented intermediate responses on BW between treatments at 21 wk. On BW uniformity, pullets fed high-AA diets were more uniform at 10 wk (P = 0.040) than breeders fed low-AA diets. No effects (P > 0.05) due to treatments were observed on BW uniformity in the other weeks evaluated. Mortality was low (mean of 0.5%) during the experimental period and was not affected by dietary AA levels.
Table 2.
Effect of dietary AA levels on BW and BW uniformity of Cobb 500 SF breeder pullet females1.
| Item | Age (weeks)2 |
|||||
|---|---|---|---|---|---|---|
| 1 | 4 (start) | 10 | 15 | 20 | 21 | |
| BW | ————————— (g) ————————— | |||||
| Low-AA | 176 | 486 | 1,151b | 1,651c | 2,347b | 2,542b |
| Moderate-AA | 178 | 480 | 1,162b | 1,693ab | 2,346b | 2,572b |
| Standard-AA | 176 | 485 | 1,197a | 1,715ab | 2,416a | 2,607ab |
| High-AA | 180 | 487 | 1,216a | 1,738a | 2,437a | 2,661a |
| SEM | 4 | 3 | 5 | 6 | 19 | 20 |
| P-value | 0.851 | 0.300 | <0.001 | <0.001 | <0.001 | <0.001 |
| BW uniformity | ————————— (%) ————————— | |||||
| Low-AA | 87.71 | 88.88a | 89.16 | 90.94 | 91.96 | |
| Moderate-AA | 87.68 | 89.88ab | 89.76 | 90.07 | 91.67 | |
| Standard-AA | 86.64 | 90.22ab | 89.90 | 89.79 | 92.19 | |
| High-AA | 87.82 | 90.49b | 90.78 | 89.90 | 92.01 | |
| SEM | 0.64 | 0.36 | 0.43 | 0.54 | 0.31 | |
| P-value | 0.551 | 0.040 | 0.113 | 0.491 | 0.707 | |
a-b Means in columns followed by different superscript letters are statistically different by Tukey's test (P < 0.05).
Low-AA, moderate-AA, standard-AA, and high-AA diets corresponding to 80, 90, 100, and 110% of the Cobb-Vantress (2018) recommendations, guided by dig Lys using balanced protein.
Values represent the mean of 4 replicate pens with 85 pullets each.
Results of feed allocation and nutrient intake for each phase are presented in Table 3. Dietary AA concentration affected (P < 0.001) cumulative feed allocation and nutrient intake in grower and developer phases. In these periods and before photostimulation, fewer feed amounts were offered (P < 0.001) to pullets fed high-AA diets than other treatments. Feed allocation (P < 0.001) decreased linearly as dietary AA increased at 15 wk (Feed allocation, g = 4,472.681 – 0.966 × AA%; R2 = 0.67), 21 wk (Feed allocation, g = 8,637.632 – 5.016 × AA%; R2 = 0.97) and 24 wk of age (Feed allocation, g = 6,717.632 – 5.360 × AA%; R2 = 0.98). Similar results (P < 0.001) were observed on AMEn, calcium (Ca), and phosphorus (P) intake in grower, developer, and prior lighting. Lower intake of AMEn, Ca, and P was observed as the AA concentration increased (P < 0.001) in the grower and developer diets. Despite the reduction in feed allocation to reach a target BW, consumption of CP, and all dig AA differed significantly (P < 0.001) among treatments. These nutrients increased linearly (P < 0.001) with the dietary AA level.
Table 3.
Cumulative feed allocation and nutrient intake of Cobb 500 SF broiler breeder pullet females during the grower, and developer phases including period prior photostimulation1.
| Item | Low2-AA | Moderate2-AA | Standard-AA2 | High-AA2 | SEM | CV | P-value | Linear regression equation | R2 | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Grower (5–15 wk) | ||||||||||
| Feed allocation, g | 4,392a | 4,385b | 4,388ab | 4,359c | 1.43 | 0.07 | <0.001 | Feed allocation = 4,472.681 - 0.965 × AA% | 0.67 | <0.001 |
| AMEn, kcal | 11,859a | 11,840b | 11,846ab | 11,769c | 3.87 | 0.07 | <0.001 | AMEn = 12,076.227 - 2.607 × AA% | 0.67 | <0.001 |
| CP, g | 565.26d | 597.68c | 614.26b | 627.70a | 0.20 | 0.07 | <0.001 | CP = 407.517 + 2.039 × AA% | 0.95 | <0.001 |
| dig Lys, g | 21.52d | 23.68c | 26.32b | 28.77a | 0.01 | 0.07 | < 0.001 | dig Lys = 1.9017525 + 0.2439167 × AA | 0.99 | <0.001 |
| dig TSAA, g | 21.08d | 22.36c | 22.81b | 24.41a | 0.01 | 0.07 | <0.001 | dig TSAA = 12.752 + 0.104 × AA% | 0.96 | <0.001 |
| dig Thr, g | 17.57d | 17.98c | 19.74b | 21.79a | 0.01 | 0.07 | <0.001 | dig Thr = 5.547 + 0.144 × AA% | 0.93 | <0.001 |
| dig Val, g | 25.03d | 27.19c | 27.64b | 28.33a | 0.01 | 0.07 | <0.001 | dig Val = 17.216 + 0.103 × AA% | 0.88 | <0.001 |
| Calcium, g | 39.53a | 39.46b | 39.49ab | 39.23c | 0.01 | 0.07 | <0.001 | Ca = 40.254 - 0.009 × AA% | 0.68 | <0.001 |
| Phosphorus, g | 28.55a | 28.50b | 28.52ab | 28.33c | 0.01 | 0.07 | <0.001 | P = 29.072 - 0.006 × AA% | 0.68 | <0.001 |
| Prior to photostimulation (5–21 wk) | ||||||||||
| Feed allocation, g | 8,227a | 8,194b | 8,150c | 8,074d | 2.65 | 0.06 | <0.001 | Feed allocation = 8,637.632 - 5.016 × AA% | 0.96 | <0.001 |
| AMEn, kcal | 22,595a | 22,505b | 22,380c | 22,172d | 7.33 | 0.07 | <0.001 | AMEn = 23,738.12 - 13.948 × AA% | 0.96 | <0.001 |
| CP, g | 1,061.08d | 1,120.64c | 1,146.60b | 1,174.57a | 0.37 | 0.07 | <0.001 | CP = 777.580 + 3.665 × AA% | 0.95 | <0.001 |
| dig Lys, g | 41.08d | 45.39c | 50.02b | 54.40a | 0.02 | 0.07 | <0.001 | dig Lys = 5.338 + 0.446 × AA% | 0.99 | <0.001 |
| dig TSAA, g | 40.25d | 42.17c | 43.88b | 46.70a | 0.01 | 0.07 | <0.001 | dig TSAA = 23.251 + 0.210 × AA% | 0.98 | <0.001 |
| dig Thr, g | 32.91d | 33.98c | 37.43b | 40.74a | 0.01 | 0.07 | <0.001 | dig Thr = 10.653 + 0.270 × AA% | 0.96 | <0.001 |
| dig Val, g | 48.04d | 50.80c | 51.72b | 53.60a | 0.02 | 0.07 | <0.001 | dig Val = 34.338 + 0.176 × AA % | 0.96 | <0.001 |
| Calcium, g | 83.63a | 83.27b | 82.75c | 81.96d | 0.03 | 0.07 | <0.001 | Ca = 88.151 - 0.055 × AA% | 0.96 | <0.001 |
| Phosphorus, g | 52.32a | 52.11b | 51.84c | 51.37d | 0.02 | 0.06 | <0.001 | P = 54.895 - 0.031 × AA% | 0.95 | <0.001 |
| Developer (15–24 wk) | ||||||||||
| Feed allocation, g | 6,281a | 6,243b | 6,189c | 6,121d | 2.08 | 0.07 | <0.001 | Feed allocation = 6,717.632 - 5.360 × AA% | 0.98 | <0.001 |
| AMEn, kcal | 17,588a | 17,480b | 17,328c | 17,138d | 5.82 | 0.07 | <0.001 | AMEn = 18,809.355 - 15.008 × AA% | 0.98 | <0.001 |
| CP, g | 812.18d | 857.14c | 875.70b | 900.97a | 0.29 | 0.07 | <0.001 | CP = 590.810 + 2.849 × AA% | 0.96 | <0.001 |
| dig Lys, g | 32.03d | 35.58c | 38.99b | 42.23a | 0.01 | 0.07 | <0.001 | dig Lys = 4.9112325 + 0.340 × AA% | 0.99 | <0.001 |
| dig TSAA, g | 31.41d | 32.46c | 34.66b | 36.72a | 0.01 | 0.07 | <0.001 | dig TSAA = 16.573 + 0.181 × AA% | 0.98 | <0.001 |
| dig Thr, g | 25.12d | 26.22c | 29.09b | 31.21a | 0.01 | 0.07 | <0.001 | dig Thr = 7.831 + 0.211 × AA% | 0.97 | <0.001 |
| dig Val, g | 37.69d | 38.70c | 39.61b | 41.62a | 0.01 | 0.07 | <0.001 | dig Val = 27.340 + 0.127 × AA% | 0.96 | <0.001 |
| Calcium, g | 72.23a | 71.79b | 71.17c | 70.39d | 0.02 | 0.07 | <0.001 | Ca = 77.252 - 0.062 × AA% | 0.96 | <0.001 |
| Phosphorus, g | 38.94a | 38.70b | 38.37c | 37.95d | 0.01 | 0.07 | <0.001 | P = 41.649 - 0.033 × AA% | 0.96 | <0.001 |
a-dMeans in columns followed by different superscript letters are statistically different by Tukey's test (P < 0.05).
All pullets were fed 909 grams of the same starter diet from 1 to 4 wk for a total intake of 2,545 Kcal of AMEn, 8.18 g of Ca, 5.91 g of P, 168.18 g of CP, 8.18 g of dig Lys, 6.27 g of dig TSAA, 5.82 g dig Thr, and 7.64 g of dig Val.
Low-AA, moderate-AA, standard-AA, and high-AA diets corresponding to 80, 90, 100, and 110% of the Cobb-Vantress (2018) recommendations, guided by dig Lys using balanced protein.
Body Composition and Organ Development
Pullets fed diets with the highest AA level resulted in greater (P = 0.041) relative breast weight at 20 wk of age than pullets fed low-AA diets (Table 4). In contrast, abdominal fat depositions were larger (P = 0.044) with 80% AA diets at 15 wk, as compared to 110% AA diets. Relative liver weight was also affected (P < 0.001) by treatments. At 20 wk of age, moderate-AA and standard-AA diets resulted in pullets with livers, on average, 0.35% heavier (P < 0.001) than those fed low-AA diets. A shorter (P = 0.007) small intestine relative to BW was observed in pullets fed 100% AA and 110% AA compared to those fed 80% AA diets at 10 wk of age. No significant effects (P > 0.05) of AA levels were detected on relative ceca length or in the other wk in which the organ development was evaluated.
Table 4.
Effect of dietary AA levels on body composition of Cobb 500 SF breeder pullet females at 10, 15, and 20 wk1.
| Item | Age (weeks)2 |
||
|---|---|---|---|
| 10 | 15 | 20 | |
| Relative breast weight | ————————— (%) ————————— | ||
| Low-AA | 18.41 | 21.47 | 22.31b |
| Moderate-AA | 18.37 | 21.14 | 22.81ab |
| Standard-AA | 18.46 | 21.41 | 23.64ab |
| High-AA | 19.45 | 21.60 | 24.30a |
| SEM | 0.33 | 2.05 | 0.51 |
| P-value | 0.076 | 0.761 | 0.041 |
| Relative abdominal fat | ————————— (%)————————— | ||
| Low-AA | 0.41 | 0.35a | 1.31 |
| Moderate-AA | 0.42 | 0.15ab | 1.01 |
| Standard-AA | 0.34 | 0.22ab | 0.86 |
| High-AA | 0.26 | 0.11b | 0.74 |
| SEM | 0.06 | 0.06 | 0.18 |
| P-value | 0.218 | 0.044 | 0.257 |
| Relative liver weight | ————————— (%) ————————— | ||
| Low-AA | 2.05 | 1.61 | 1.39b |
| Moderate-AA | 2.18 | 1.66 | 1.75a |
| Standard-AA | 2.06 | 1.52 | 1.73a |
| High-AA | 2.03 | 1.63 | 1.64ab |
| SEM | 0.07 | 0.06 | 0.07 |
| P-value | 0.491 | 0.336 | 0.001 |
| Relative length of small intestine | ———————- (cm/ 100 g) ———————– | ||
| Low-AA | 16.30a | 11.80 | 6.99 |
| Moderate-AA | 15.70ab | 11.20 | 7.16 |
| Standard-AA | 14.90b | 11.40 | 7.11 |
| High-AA | 14.70b | 10.90 | 6.72 |
| SEM | 0.40 | 0.30 | 0.28 |
| P-value | 0.007 | 0.105 | 0.453 |
| Relative length ceca | ———————- (cm/ 100 g) ———————– | ||
| Low-AA | 2.00 | 1.19 | 3.33 |
| Moderate-AA | 1.92 | 1.22 | 3.95 |
| Standard-AA | 1.67 | 1.20 | 4.11 |
| High-AA | 1.75 | 1.19 | 3.27 |
| SEM | 0.10 | 0.02 | 0.02 |
| P-value | 0.115 | 0.823 | 0.156 |
a-bMeans in columns followed by different superscript letters are statistically different by Tukey's test (P < 0.05).
Low-AA, moderate-AA, standard-AA, and high-AA diets corresponding to 80, 90, 100, and 110% of the Cobb-Vantress (2018) recommendations, guided by dig Lys using balanced protein.
Values represent the mean of 4 replicate pens per treatment with 3 pullets each.
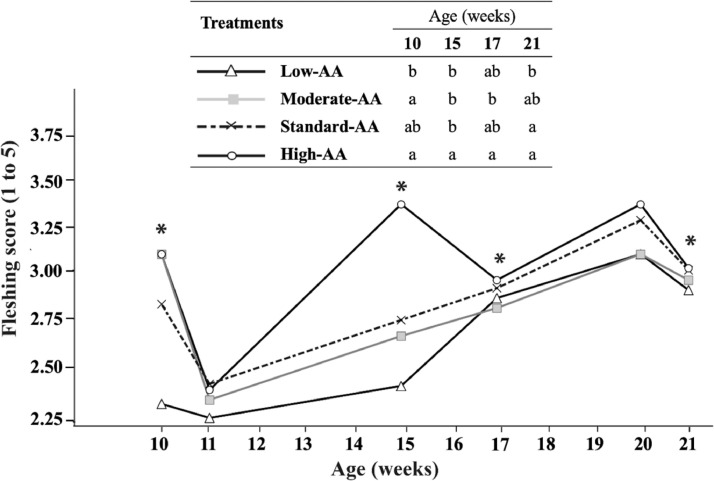
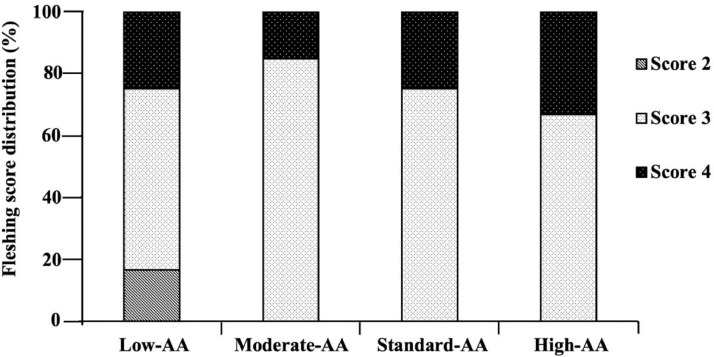
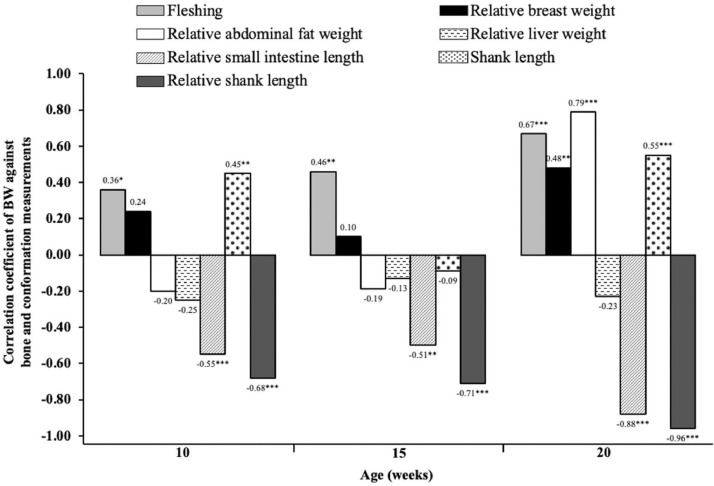
High-AA levels increased (P < 0.01) fleshing score along with the rearing phase (Figure 3). Standard-AA and high-AA diets resulted in fleshing scores closer to recommend values of the breeder line with scores 3 and 4 in around 64 and 35% of the flock at 20 wk of age, respectively (Figure 4). Conversely, the 2 lower AA diets (80% AA and 90% AA) resulted in a lower fleshing, as score 4 was observed in less than 22% of the flock. The linear increase of BW due to treatments resulted in positive correlations (P < 0.05) with the fleshing score at 10, 15, and 20 wk of age and with relative breast weight at 20 wk (Figure 5). As pullets aged, more significant correlations were observed between BW and fleshing scores.
Figure 3.
Effect of dietary AA levels1 on fleshing score of Cobb 500 SF breeder females at 10, 11, 15, 17, 20, and 21 wk of age. *Significant difference between dietary AA levels (P < 0.05). a-b Dietary treatments not sharing a lowercase letter in columns differ significantly by Tukey's test at P < 0.05 level. n = 4 replicate pens per treatment of 3 pullets each. 1 Low-AA, moderate-AA, standard-AA, and high-AA diets corresponding to 80, 90, 100, and 110% of the Cobb-Vantress (2018) recommendations, guided by dig Lys using balanced protein.
Figure 4.
Effect of dietary AA levels1 on fleshing score distribution in the flock of Cobb 500 SF breeder females at 20 wk of age. n = 4 replicate pens per treatment of 3 pullets each.1 Low-AA, moderate-AA, standard-AA, and high-AA diets corresponding to 80, 90, 100, and 110% of the Cobb-Vantress (2018) recommendations, guided by dig Lys using balanced protein.
Figure 5.
Correlation coefficient of BW against conformation and bone measurements of Cobb 500 SF breeder females fed AA levels at 10, 15, and 20 wk of age. *P < 0.05; **P < 0.01; ***P < 0.001.
Similarly, heavier pullets were correlated (P < 0.01) to those with the shortest small intestine relative to BW at 10, 15, and 20 wk of age, and the highest relative fat deposition at 20 wk of age (Figure 5). A negative correlation (P < 0.05) between breast weight and relative abdominal fat weight (r = −0.37) was detected. Meanwhile, breast weight was increased by dietary AA concentration, lower fat deposits due to treatments were observed at 10 wk.
Leg Development
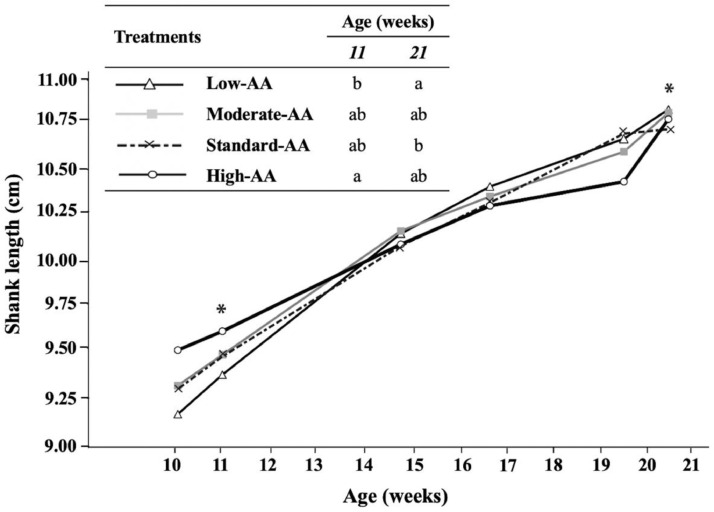
Shank length was evaluated in all pullets alive (n = 85 to 68 per pen) at 11, 17, and 21 wk (Figure 6) and in samples (n = 12 per treatment) taken at 10, 15, and 20 wk (Table 5). Pullets fed high-AA diets had the greatest (P = 0.024) relative asymmetry of shank length at 10 wk of age (Table 5), and the longest (P < 0.001) shank at 11 wk of age (Figure 6). Relative shank length decreased (P < 0.001) as dietary AA increased at 10 (Rel. shank length, mm/100 g = 10.065 – 0.024 × AA%; R2 = 0.99), 15 (Rel. shank length, mm/100 g = 8.782 – 0.016 × AA%; R2 = 0.80), 17 (Rel. shank length, mm/100 g = 6.407 – 0.012 × AA%; R2 = 0.88), 20 (Rel. shank length, mm/100 g = 5.709 – 0.010 × AA%; R2 = 0.91), and 21 wk of age (Rel. shank length, mm/100 g = 4.872 – 0.007 × AA%; R2 = 0.77). No significant (P > 0.05) effects of AA levels were found on relative shank length at 11 wk. Positive correlations (P < 0.01) between shank length and BW were observed. The increase of AA in the diets that produced heavier pullets, and longer shanks resulted in positive correlations between BW and shank length at 10 (r = 0.45; P < 0.01) and 20 wk (r = 0.55; P < 0.001) of age. Likewise, the linear decrease observed in relative shank length resulted in negative correlations (P < 0.001) with BW due to dietary AA concentration at 10 (r = −0.68), 15 (r = −0.71), and 20 wk (r = −0.96) of age.
Figure 6.
Effect of dietary AA levels1 on shank length of Cobb 500 SF breeder females at 10, 11, 15, 17, 20, and 21 wk of age. Shank length was measured from the hock to the footpad of live birds. *Significant difference between dietary AA levels (P < 0.05). n = 4 replicate pens per treatment of 3 pullets each. a-b Dietary treatments not sharing a lowercase letter in columns differ significantly by Tukey's test at P < 0.05 level. 1 Low-AA, moderate-AA, standard-AA, and high-AA diets corresponding to 80, 90, 100, and 110% of the Cobb-Vantress (2018) recommendations, guided by dig Lys using balanced protein.
Table 5.
Effect of dietary AA levels on the relative asymmetry of shank length and relative shank length of Cobb 500 SF breeder females at 10, 15, and 20 wk1,2.
| Item | Age (weeks)3 |
||
|---|---|---|---|
| 10 | 15 | 20 | |
| Relative asymmetry shank length | ———————— (%) ———————— | ||
| Low-AA | 1.28b | 1.28 | 1.32 |
| Moderate-AA | 1.51ab | 1.83 | 1.74 |
| Standard-AA | 1.63ab | 1.83 | 1.48 |
| High-AA | 1.99a | 2.89 | 1.80 |
| SEM | 0.40 | 0.02 | 0.25 |
| P-value | 0.024 | 0.069 | 0.784 |
| Relative shank length | ——————– (mm/ 100 g) —————— | ||
| Low-AA | 8.30a | 7.80a | 4.90 |
| Moderate-AA | 8.00a | 7.10b | 4.80 |
| Standard-AA | 7.80ab | 7.10b | 4.70 |
| High-AA | 7.40b | 7.20b | 4.50 |
| SEM | 0.10 | 0.10 | 0.21 |
| P-value | <0.001 | <0.001 | 0.734 |
a-bMeans in columns followed by different superscript letters are statistically different by Tukey's test (P < 0.05).
Low-AA, moderate-AA, standard-AA, and high-AA diets were corresponding to 80, 90, 100, and 110% of the Cobb-Vantress (2018) recommendations, guided by dig Lys using balanced protein.
Shank length measured from the hock to the footpad of sacrificed birds.
Values represent the mean of 4 replicate pens per treatment with 3 pullets each.
Feathering Development
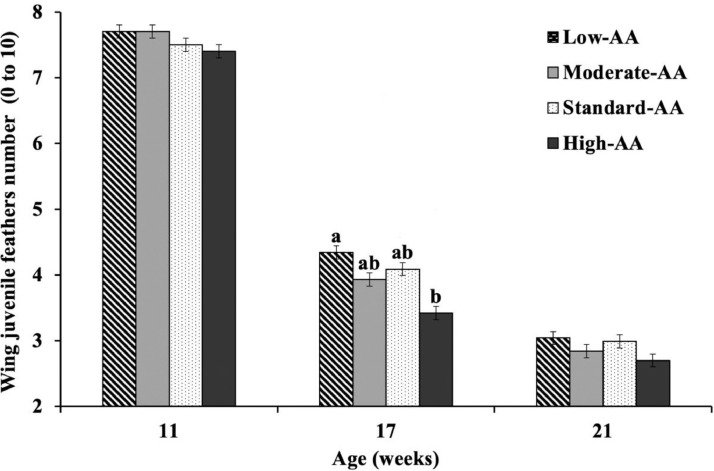
At 17 wk of age, dietary AA content affected the number of adolescent feathers (Figure 7). Pullets fed 80% AA had the highest number of immature wing feathers compared to pullets fed 110% AA diets (4.34 vs. 3.42). Moderate-AA and standard-AA diets resulted in intermediate responses for this week. The number of adolescent feathers were reduced (P = 0.001) as dietary AA levels increased at 17 wk of age (Num. of feathers = 5.935 – 0.021 × AA%; R2 = 0.44). No significant effects (P > 0.05) of treatments were observed at 11 or 21 wk, but pullets showed 7.57 and 2.89 juvenile feathers on average, respectively.
Figure 7.
Effect of dietary AA levels1 on wing juvenile feathers number of Cobb 500 SF breeder females at 11, 17, and 21 wk of age. Results are presented as means ± SEM. n = 2 replicate pens per treatment of 85 pullets each. a-b Means not sharing a lowercase letter differ significantly by Tukey's test at P < 0.05 level. 1 Low-AA, moderate-AA, standard-AA, and high-AA diets corresponding to 80, 90, 100, and 110% of the Cobb-Vantress (2018) recommendations, guided by dig Lys using balanced protein.
DISCUSSION
Growth Performance
At the beginning of the experiment, pullet BW and BW uniformity were similar between pens that received later the four dietary AA levels. The two higher AA levels (100 and 110% AA) resulted in 3% heavier pullets than those fed the two lowest levels (80 and 90% AA diets) in most of the periods evaluated. However, differences in BW due to AA levels were slightly reduced as pullets approached to photostimulation due to extra feed offered to reach a target BW at the onset of egg production. A study by van Emous et al. (2013) reported that CP influenced BW. At 5 wk, pullets fed diets with 19.21% CP were 4.2% heavier than birds fed with 14.59% CP, 0.60% of dig Lys, and 0.48% of Met+Cys. In contrast, the lower CP on starter (14.59%) and grower diets (12.27%) resulted in pullets with 2% more BW compared to those fed high CP levels around 19.21 and 14.13% at 10 wk, respectively. Conversely, another report from the same authors (van Emous et al., 2015a), indicated that no effects on BW were observed when using either low levels of CP during rearing (13.81 starter, 11.32 grower, 12.62% pre-breeder) or high CP content (16.65 starter, 14.15 grower, 15.11% pre-breeder) on Ross 308 pullets. In those studies (van Emous et al., 2013, 2015a), feed amounts also varied to achieve a target BW, which is an important and valid reason since breeders would be fed in this way under commercial conditions.
In the present study, at 10 wk, high-AA diets resulted in pullet flocks of 1.61% more uniform in BW (CV) than those fed low-AA diets (9.51 vs. 11.12%). Similarly, Hudson et al. (2000) described an improvement in BW uniformity of 22.2% on average up to 6 wk (73.7 vs. 51.5%) with diets containing 16 and 20% CP vs. 12%, without effects 14 and 22 wk of age. van Emous et al. (2013) reported that starter, grower, and pre-breeder diets low in CP (14.56, 12.27, and 12.79% CP) did not affect CV% at 5, 10, and 15 wk of age, but resulted in 3.3% lower CV% at 20 wk compared to high (19.21, 14.13, and 14.88% CP) and medium levels of CP (17.96, 12.96, and 13.96%). In the present study, dietary AA effects on BW uniformity were not detected after 10 wk of age, probably due to the feed allowance adjustments to achieve a predetermined target BW.
Less feed allocation in grower (4,392, 4,385, 4,388, and 4,360 g) and developer phase (6,281, 6,243, 6,189, and 6,121 g) was necessary to reach the target BW when AA increased in the diets. The cumulative intake of AMEn, Ca, and P was reduced as feed offered decreased, even with similar dietary content across all treatments. On the contrary, despite a linear decrease of feed allocation with the increase of dietary AA levels, pullets still obtained more cumulative CP and AA intake at 24 wk. van Emous et al. (2013) reported a CP intake of 1,510 and 1,427 g on Ross 308 pullets from 2 to 22 wk when using starter, grower, and pre-breeder diets with high (19.21, 14.13, and 14.18% CP) and low CP (14.59, 12.27 and 12.79%), respectively. In the present study, pullets fed high-AA diets resulted in a cumulative CP intake of 1,425 g, while pullets from low-AA treatment ingested 1,299 g CP between 2 and 22 wk of age. The CP intake for 110% AA and 80% AA reported herein were 85 and 128 g less, respectively, compared to the high and low CP diets reported by van Emous et al. (2013). Moreover, making the same comparison with dig Lys intake, the differences between the two studies were 9.63 g more (66.4 vs. 56.77 g) and only 0.75 g less (51.75 vs. 52.50 g) for the high and low-AA in the present study, compared to high and low CP diets in van Emous et al. (2013). These variations could be associated with the fact that nutrient requirements are different for genetic lines (Aviagen, 2007; Cobb-Vantress, 2018), and dietary treatments in the experiment presented herein were established from 5 wk, while nutrient content modifications reported by van Emous et al. (2013) were performed from 15 d of age. However, the nutrient intake of the standard-AA treatment in the present study was very close to those suggested by Cobb-Vantress guidelines (2020). At 21 wk, the recommendations for cumulative CP, dig Lys, and Met+Cys consumption per pullet are 1,341 g, 57.13 g, and 47.96 g, respectively.
Body Composition and Organ Development
Dietary 110% AA resulted in greater relative breast weight than breeders fed 80% AA at 20 wk (24.30 vs. 22.31%). Similarly, van Emous et al. (2013) reported heavier breasts at 10 (0.9%) and 20 wk of age (1.5%) in pullets fed high CP diets with 14.13 and 14.88% inclusion, as compared to those fed with low CP content of 12.27 and 12.79%. In contrast, de los Mozos et al. (2017) found that Ross 308 pullets fed with rearing diets with CP levels of 14.5 (0.64% dig Lys) and 12.8% (0.47% dig Lys) had a breast meat yield 8% lower at 6 (9.92 vs. 10.9% BW), 13 (13.6 vs. 15.2% BW), and 19 wk of age (18.6 vs. 19.6% BW), in comparison to pullets fed control diets with 17.0 (0.75% dig Lys), and 15.0% of CP (0.55% dig Lys). Some studies have described the influence of dietary CP content on breast weight since lean body tissue is considered as a protein reserve which could influence the size, number, and persistency of egg production during the laying phase (Fisher and Gous, 2009; Ekmay, 2011; Vignale et al., 2016; Caldas et al., 2018). At 10, 15, and 21 wk of age, the fleshing score was enhanced by higher AA diets that promoted breast muscle growth. Although AA consumption increased in higher AA diets, the distribution of fleshing conformation in the flock remained close to recommended values of the breeder line (Cobb-Vantress, 2018) in standard-AA and high-AA diets with observed fleshing scores at 3 and 4 in around 64 and 35% respectively of the flock at 20 wk of age. Conversely, the two lower AA diets resulted in an insufficient fleshing distribution, as score 4 was found in lower than 22% of the flock when it is recommended to have 35% (Cobb-Vantress, 2016). Therefore, the correct dietary AA content associated with a monitored feeding program is essential to build birds with characteristics suitable for their production phase.
Abdominal fat deposits with low-AA diets at 15 wk were higher by 0.24% than high-AA diets. Still, an excess of muscle protein deposition in breeder diets high in CP can bring a negative effect, reducing fat deposition (Mohiti-Asli et al., 2012). Over-fleshed pullets during rearing could have more difficulty getting enough abdominal fat before lighting because those birds exhibit a formal maintenance requirement to preserve the larger muscle mass (Robinson et al., 2003). A higher abdominal fat deposition at 20 (0.42% of BW) and 27 wk of age (1.34% of BW) was previously reported in broiler breeders (van Emous et al., 2013; Lesuisse et al., 2017) fed grower and pre-breeder diets with less than 13% CP. Higher abdominal fat (0.53 vs. 0.29% of BW) in heavier compared to lighter pullets was reported almost 20 yr ago. (Renema et al., 2001). But the higher AA diets used in the present study, which resulted in the heaviest pullets, showed the lowest relative abdominal fat deposition. A correlated response (r = −0.37) between breast weight and relative abdominal fat weight was found (P < 0.05) at 10 wk of age. As breast weight increased, a lower abdominal fat deposition relative to BW was observed. Thus, special attention should be paid to keep the fleshing patterns in rearing to avoid massive breast muscle growth that could reduce pelvic and abdominal fat development. These findings could be due to the higher Lys intake in pullets fed higher AA diets. However, the egg production performance is the final indicator of the best breast and fat pad deposits in the current modern broiler breeders (Ekmay, 2011; Chang et al., 2016; Vignale et al., 2016; Caldas et al., 2018). It has been described that carnitine is synthesized from peptide-bound Lys via posttranslational methylation (Bremer, 1983) and plays a role in transporting long-chain fatty acids into mitochondria to be oxidized for energy production (Rehman et al., 2017). Despite this, carnitine is mainly formed by the methylation of Lys residues in proteins (Bremer, 1983). The utilization of free Lys for growth in supplemented diets could increase the availability of peptide-bound Lys for carnitine biosynthesis. Thus, the largest abdominal fat deposition in pullets fed 80% AA diets may be explained by a lower fatty acid β-oxidation due to lower Lys intake, considering a lower basal metabolism since those birds were lighter. A study by van Emous et al. (2013) reported an increase of 0.26 and 0.66% in abdominal fat on pullets fed low CP levels (12.27 and 12.79%), compared to birds fed high CP (14.13 and 14.88%) that only increased 0.04 and 0.24% at 10 and 20 wk, respectively.
It has been described that the weight and length of digestive organs could be related to the physiological functions (Yu et al., 2018) and the effects of AA or CP content (Adedokun and Olojede, 2019; Zaefarian et al., 2019). Low-AA diets resulted in lower relative liver weight than moderate-AA and standard-AA treatments (1.35 vs. 1.74%) at 20 wk. Zuidhof et al. (2015), using grower diets formulated to have 2,200 kcal/kg of ME, 11.4% CP, 0.56% dig Lys and high fiber content with 25% inclusion of oat hulls, reported 0.12% less relative liver weight compared to control diets with 2,865 kcal/kg of ME, 15% CP and 0.74% dig Lys. In poultry, it has been reported that 11% of all protein synthesis is carried out in the liver, and exceeding AA are catabolized in this organ (Denbow, 2000). Therefore, breeders supplemented with higher AA could increase the relative liver weight as a compensatory mechanism to improve the synthesis of tissue protein, hormones, enzymes, and catabolizing AA (Zaefarian et al., 2019). At 10 wk, 80%AA presented the longest small intestine compared to 100 and 110% AA levels as a compensatory effect to absorb the lower AA available. Negative correlations between BW and small intestine were found at 10, 15, and 20 wk of age, indicating that heavier pullets had shorter intestine. Some authors (Taylor and Jones, 2004; de Verdal et al., 2010) have suggested that lower sections of the intestine (Jejunum and ileum) may result in smaller portions due to an adaptation to greater nutrient availability.
Leg Development
Low-AA diets resulted in the shortest shanks at 11 wk but in the longest measurements at 21 wk. Hudson et al. (2000) observed an increase of 2.2 mm on shank length due to diets containing 20% CP up to 6 wk, and after that age, pullets fed diets with 12% CP tended to have 3 mm on average longer shanks. Conversely, de Beer and Coon (2006) reported that higher CP intake (307.5 vs. 286.7 g per pullet) at 6 wk on Cobb 700 increased shank length at 6 (1.7 mm), 16 (2.2 mm), and 21 wk (0.7 mm), but at sexual maturity and onset of egg production these breeders presented shorter shanks relative to BW as compared to the ones with lower CP intake. In the present study, relative shank length decreased as AA levels increased at 10, 15, 17, 20, and 21 wk, indicating that more dietary protein resulted in relative shorter shanks due to an earlier epiphyseal fusion (Reich et al., 2005; 2008). Similarly, Leeson and Summers (1984) described that pullets fed diets up to 21 d of age with 4 different CP content (13, 15, 18, and 20%) resulted in a decrease of the relative shank length as CP increased at 7 (10.4, 10.1, 10.0, and 9.8 mm/100 g) and 11 wk of age (8.4, 8.3, 8.2, and 8.00 mm/100 g). These results indicate that pullets fed higher AA or CP levels grew faster, got heavier, and weight loading inhibits bone elongation and promotes growth plate ossification and vascularization (Reich et al., 2005, 2008).
Feathering Development
No significant differences (P > 0.05) were observed on the number of juvenile feathers at 11 or 21 wk, and effects (P = 0.001) due to treatments were identified only at 17 wk of age. Overall, pullets showed a decrease in the number of feathers as age increased. At 11 and 21 wk of age, the mean of feathers was estimated at 7.57 and 2.89, respectively. It indicates the reduction of 7.11 feathers on average between 7 wk of age and before photostimulation, which could be used as a marker of sexual maturity. At 17 wk, the number of feathers decreased linearly with the increase of dietary AA level while BW increased linearly with AA content during rearing. Ekmay (2011) reported that heavier hens could reach sexual maturity faster with effects on egg production. Therefore, a linear reduction of the juvenile feathers could be associated with a higher CP and AA intake that produced heavier pullets between 15 and 20 wk of age. van Emous et al. (2015b), using a scoring scale, reported a better feathering wing cover score on pullets fed diets with high CP (16.65, 14.15, and 15.11%), as compared to pullets fed diets with 13.81, 11.32, and 12.62% CP (0.93 vs. 1.51) during rearing. On the other hand, insufficient AA levels could trigger a malformation of spoon-like appearance in the primary and secondary feathers and abnormal curling away to the body due to lacking arginine, valine, and tryptophan (van Emous and van Krimpen, 2019). Overall, the observations of the adequate fleshing score, shorter legs, and fewer juvenile feathers were signs of sexual maturity.
CONCLUSIONS
In conclusion, dietary AA levels have important effects on pullet BW, shank length, fleshing, and organ development during rearing with potential impacts on reproductive performance. BW and breast growth were demonstrated to increase linearly with dietary AA levels while reducing the abdominal fat deposition. Hundred and ten percent AA diets resulted in higher fleshing scores during rearing and produced pullets with shorter legs relative to BW and fewer juvenile feathers at 17 wk. The impact of this dietary AA during rearing on reproductive performance and egg quality is presented by Oviedo-Rondón et al. (2021).
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to thank Cobb-Vantress Inc. (Siloam Springs, AR) and Winfridus Bakker for providing financial and technical support for this trial, Zoetis Inc. (Durham, NC, USA) for the vaccines. Evonik Nutrition & Care (Hanau, Hessen, Germany) for their help with analyzing nutrient content in feed ingredients and feed. Furthermore, thanks to all staff and students of the Prestage Department of Poultry Science at North Carolina State University.
DISCLOSURES
The authors have no conflict of interest to report.
REFERENCES
- Adedokun S.A., Olojede O.C. Optimizing gastrointestinal integrity in poultry: the role of nutrients and feed additives. Front. Vet. Sci. 2019;5:1–11. doi: 10.3389/fvets.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists International. Official Methods of Analysis of AOAC International. Horwitz W. and Latimer G., 18th ed., 2006, AOAC INTERNATIONAL; Gaithersburg, MD.
- Aviagen . Aviagen, Ltd.; Huntsville, AL: 2007. Parent Stock Nutrition Specifications: Ross 308. [Google Scholar]
- Bremer J. Carnitine metabolism and functions. Physiol. Rev. 1983;63:1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Butler L. PhD Diss. Univ. Arkansas; Fayetteville: 2019. Broiler Breeder Amino Acid Levels and Progeny Nutritional Status. [Google Scholar]
- Caldas J.V., Hilton K., Boonsinchai N., England J.A., Mauromoustakos A., Coon C.N. Dynamics of nutrient utilization, heat production, and body composition in broiler breeder hens during egg production. Poult. Sci. 2018;97:2845–2853. doi: 10.3382/ps/pey133. [DOI] [PubMed] [Google Scholar]
- Chang A., Halley J., Silva M. Can feeding the broiler breeder improve chick quality and offspring performance? Anim. Prod. Sci. 2016;56:1254–1262. [Google Scholar]
- Cobb-Vantress . Cobb-Vantress; Siloam Springs, AR: 2016. Cobb500 Breeder Management Guide. [Google Scholar]
- Cobb-Vantress . Cobb-Vantress; Siloam Springs, AR: 2018. Cobb500 Slow Feather Breeder Management Supplement. [Google Scholar]
- Cobb-Vantress . Cobb-Vantress; Siloam Springs, AR: 2020. Cobb500 Slow Feather Breeder Management Supplement. [Google Scholar]
- de Beer M., Coon C.N. The effect of increased protein intake during the starter and prebreeder periods on reproductive performance of ultra high yield broiler breeders hens. Int. J. Poult. Sci. 2006;5:812–821. [Google Scholar]
- de Beer M., Coon C.N. The effect of different feed restriction programs on reproductive performance, efficiency, frame size, and uniformity in broiler breeder hens. Poult. Sci. 2007;86:1927–1939. doi: 10.1093/ps/86.9.1927. [DOI] [PubMed] [Google Scholar]
- Decuypere E., Bruggeman V., Everaert N., Li Y., Boonen R., de Tavernier J., Janssens S., Buys N. The broiler breeder paradox: ethical, genetic and physiological perspectives, and suggestions for solutions. Br. Poult. Sci. 2010;51:569–579. doi: 10.1080/00071668.2010.519121. [DOI] [PubMed] [Google Scholar]
- de los Mozos J., García-Ruiz A.I., den Hartog L.A., Villamide M.J. Growth curve and diet density affect eating motivation, behavior, and body composition of broiler breeders during rearing. Poult. Sci. 2017;96:2708–2717. doi: 10.3382/ps/pex045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denbow D.M. Gastrointestinal anatomy and physiology. In: Whittow G.C., editor. Sturkie's Avian Physiology. Academic Press; New York, NY: 2000. pp. 299–325. [Google Scholar]
- de Verdal H., Mignon-Grasteau S., Jeulin C., le Bihan-Duval E., Leconte M., Mallet S., Martin C., Narcy A. Digestive tract measurements and histological adaptation in broiler lines divergently selected for digestive efficiency. Poult. Sci. 2010;89:1955–1961. doi: 10.3382/ps.2010-813. [DOI] [PubMed] [Google Scholar]
- Ekmay R.D. Univ. Arkansas; Fayetteville: 2011. Protein Utilization and Requirements in Broiler Breeders. PhD Diss. [Google Scholar]
- Ekmay R.D., de Beer M., Mei S.J., Manangi M., Coon C.N. Amino acid requirements of broiler breeders at peak production for egg mass, body weight, and fertility. Poult. Sci. 2013;92:992–1006. doi: 10.3382/ps.2012-02554. [DOI] [PubMed] [Google Scholar]
- Enting H., Kruip T.A.M., Verstegen M.W.A, Van Der Aar P.J. The effect of low-density diets on broiler breeder performance during the laying period and on embryonic development of their offspring. Poult. Sci. 2007;86:850–856. doi: 10.1093/ps/86.5.850. [DOI] [PubMed] [Google Scholar]
- Fisher C., Gous R.M. Biology of breeding poultry. In: Hocking P., editor. Protein and Amino Acid Responses. CAB International; 2009. pp. 331–361. Edinburgh, UK. [Google Scholar]
- Hudson B.P., Lien R.J., Hess J.B. Effects of early protein intake on development and subsequent egg production of broiler breeder hens. J. Appl. Poult. Res. 2000;9:324–333. [Google Scholar]
- Leeson S., Summers J.D. Influence of nutritional modification on skeletal size of leghorn and broiler breeder pullets. Poult. Sci. 1984;63:1222–1228. doi: 10.3382/ps.0631222. [DOI] [PubMed] [Google Scholar]
- Leeson S., Summers J.D. Pages 137–138 in Nutrition and Feeding. Nottingham University Press; Nottingham, England: 2000. Broiler breeder production. [Google Scholar]
- Lesuisse J., Li C., Schallier S., Leblois J., Everaert N., Buyse J. Feeding broiler breeders a reduced balanced protein diet during the rearing and laying period impairs reproductive performance but enhances broiler offspring performance. Poult. Sci. 2017;96:3949–3959. doi: 10.3382/ps/pex211. [DOI] [PubMed] [Google Scholar]
- Lewis P.D. A review of lighting for broiler breeders. Br. Poult. Sci. 2006;47:393–404. doi: 10.1080/00071660600829092. [DOI] [PubMed] [Google Scholar]
- Lien R.J., Yuan T. Effect of delayed light stimulation on egg production by broiler breeder pullets of low body weight. J. Appl. Poult. Res. 1994;3:40–48. [Google Scholar]
- Lilburn M.S., Ngiam-Rilling K., Myers-Miller D.J. Growth and development of broiler breeders: 2. Independent effects of dietary formulation versus body weight on skeletal and muscle growth. Poult. Sci. 1989;68:1274–1281. doi: 10.3382/ps.0681274. [DOI] [PubMed] [Google Scholar]
- Lopez G., Leeson S. Response of broiler breeders to low-protein diets. 1. Adult breeder performance. Poult. Sci. 1995;74:685–695. doi: 10.3382/ps.0740685. [DOI] [PubMed] [Google Scholar]
- Mejia L., McDaniel C.D., Corzo A. Dietary influence of digestible lysine concentration on Cobb 500 hen broiler breeder reproductive performance. Poult. Sci. 2012;91:426–431. doi: 10.3382/ps.2011-01710. [DOI] [PubMed] [Google Scholar]
- Mejia L., McDaniel C.D., Kidd M.T., Lopez K., Corzo A. Evaluation of carryover effects of dietary lysine intake by Cobb 500 broiler breeder hens. Poult. Sci. 2013;92:709–718. doi: 10.3382/ps.2012-02517. [DOI] [PubMed] [Google Scholar]
- Mohiti-Asli M., Shivazad M., Zaghari M., Aminzadeh S., Rezaian M., Mateos G.G. Dietary fibers and crude protein content alleviate hepatic fat deposition and obesity in broiler breeder hens. Poult. Sci. 2012;91:3107–3114. doi: 10.3382/ps.2011-02040. [DOI] [PubMed] [Google Scholar]
- Møller A.P., Sanotra G.S., Vestergaard K.S. Developmental instability and light regime in chickens (Gallus gallus) Appl. Anim. Behav. Sci. 1999;62:57–71. [Google Scholar]
- Oviedo-Rondón, E. O., Y. A. Matta, A. Ortiz, M. C. Alfaro-Wisaquillo, H. A. Cordova-Noboa, M. Chico, J. S. Hoyos, G. A. Quintana-Ospina, J. V. Caldas, D. Buitrago, J. D. Martinez, J. J. Yanquen. 2021. Effects of amino acid levels during rearing on Cobb 500 slow-feathering broiler breeders: 2. Reproductive performance. Poult. Sci., 100, In press. [DOI] [PMC free article] [PubMed]
- Rehman Z., Naz S., Khan R.U., Tahir M. An update on potential applications of L-carnitine in poultry. World. Poult. Sci. J. 2017;73:823–830. [Google Scholar]
- Reich A., Jaffe N., Tong A., Lavelin I., Genina O., Pines M., Sklan D., Nussinovitch A., Monsonego-Ornan E. Weight loading young chicks inhibits bone elongation and promotes growth plate ossification and vascularization. J. Appl. Physiol. 2005;98:2381–2389. doi: 10.1152/japplphysiol.01073.2004. [DOI] [PubMed] [Google Scholar]
- Reich A., Sharir A., Zelzer E., Hacker L., Monsonego-Ornana E., Shahar R. The effect of weight loading and subsequent release from loading on the postnatal skeleton. Bone. 2008;43:766–774. doi: 10.1016/j.bone.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Renema R.A., Robinson F.E., Goerzen P.R. Effects of altering growth curve and age at photostimulation in female broiler breeders. 1. Reproductive development. Can. J. Anim. Sci. 2001;81:467–476. [Google Scholar]
- Robinson F.E., Fasenko G.M. Female Reproduction: Control of Ovarian Function. Spotted Cow Press; Alberta, Canada: 2003. Optimizing chick production in broiler breeders; pp. 3–10. [Google Scholar]
- Taylor R.D., Jones G.P.D. The incorporation of whole grain into pelleted broiler chicken diets. II. Gastrointestinal and digesta characteristics. Br. Poult. Sci. 2004;45:237–246. doi: 10.1080/00071660410001715849. [DOI] [PubMed] [Google Scholar]
- van Emous R.A., Kwakkel R., van Krimpen M., Hendriks W. Effects of growth patterns and dietary crude protein levels during rearing on body composition and performance in broiler breeder females during the rearing and laying period. Poult. Sci. 2013;92:2091–2100. doi: 10.3382/ps.2012-02987. [DOI] [PubMed] [Google Scholar]
- van Emous R.A., Kwakkel R., van Krimpen M., Hendriks W. Effects of growth pattern and dietary protein level during rearing on feed intake, eating time, eating rate, behavior, plasma corticosterone concentration, and feather cover in broiler breeder females during the rearing and laying period. Appl. Anim. Behav. Sci. 2014;150:44–54. [Google Scholar]
- van Emous R.A., Kwakkel R., van Krimpen M., Hendriks W. Effects of dietary protein levels during rearing and dietary energy levels during lay on body composition and reproduction in broiler breeder females. Poult. Sci. 2015;94:1030–1042. doi: 10.3382/ps/pev079. [DOI] [PubMed] [Google Scholar]
- van Emous R.A., Kwakkel R., van Krimpen M., Hendriks W. Effects of different dietary protein levels during rearing and different dietary energy levels during lay on behaviour and feather cover in broiler breeder females. Appl. Anim. Behav. Sci. 2015;168:45–55. [Google Scholar]
- van Emous R.A., van Krimpen M.M. Pages 133-150 in Protein Feathers and Skin. CAB International; Preston, UK: 2019. Effects of nutritional interventions on feathering of poultry. [Google Scholar]
- Vignale K., Caldas J.V., England J.A., Boonsinchai N., Sodsee P., Putsakum M., Pollock E.D., Dridi S., Coon C.N. The effect of four different feeding regimens from rearing period to sexual maturity on breast muscle protein turnover in broiler breeder parent stock. Poult. Sci. 2016;96:1219–1227. doi: 10.3382/ps/pew369. [DOI] [PubMed] [Google Scholar]
- Yu J., Yang H., Wang Z., Dai H., Xu L., Ling C. Effects of arginine on the growth performance, hormones, digestive organ development and intestinal morphology in the early growth stage of layer chickens. Ital. J. Anim. Sci. 2018;17:1077–1082. [Google Scholar]
- Zaefarian F., Abdollahi M.R., Cowieson A., Ravindran V. Avian liver: the forgotten organ. Animals. 2019;9:1–24. doi: 10.3390/ani9020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidhof M.J, Holm D.E., Renema R.A., Jalal M.A., Robinson F.E. Effects of broiler breeder management on pullet body weight and carcass uniformity. Poult. Sci. 2015;94:1389–1397. doi: 10.3382/ps/pev064. [DOI] [PubMed] [Google Scholar]