Abstract
The aim of this study is to evaluate the prevalence, pathogenesis and management of mucormycosis in post covid 19 patients in our tertiary care covid dedicated hospital. A prospective cross sectional study was done in 70 patients who were admitted in the covid department of BJ Medical College, Civil hospital Ahmedabad and presented with mucormycosis during admission or after discharge over a period of 10 months from March 2020 to December 2020. Middle aged to elderly population were found to be most commonly affected with mucormycosis. It was found that majority of the affected population was uncontrolled diabeteic and had a delayed presentation to hospital due to ongoing covid pandemic crisis. Covid infection had major effect on the hormonal balance of the body as evident from the uncontrolled blood glucose levels of affected patients. In patients with mucormycosis, early detection, surgical debridement, suitable antifungal therapy, and control of risk factors like diabetes mellitus are the main parameters of successful management of this lethal infection. Early diagnosis and treatment of mucormycosis can be life saving as it is a rapidly progressing disease and have been proven fatal.
Keywords: Diabetes mellitus, Immunocompromised, Mucormycosis, COVID 19, Modified denker, Open maxillectomy, Fungal, Amphoterecin B, Debridement, Palatal erosion
Introduction
In December 2019, clusters of pneumonia cases of unknown etiology emerged in Wuhan, China. Deep sequencing analysis from lower respiratory tract samples indicated a novel coronavirus as the causative agent, which was named Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2), and the disease it caused was called COVID-19 [1, 2]. Although SARS-CoV-2 has shown phylogenetic and clinical similarities with SARS-CoV, the novel coronavirus appears to have a higher transmissibility and lower case fatality rates [3]. On 30 January 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a Public Health Emergency of International Concern, and on March 11, the epidemic was upgraded to pandemic [4].
Rhinocerebral mucormycosis is an acute and often lethal opportunistic fungal infection typically affecting diabetic (50% of the cases) or immunocompromised patients caused by fungi of the class zygomycetes [5–7]. Recent series have described a mortality of approximately 40% in diabetics with rhinocerebral mucormycosis. The infection has high incidence in diabetic patients due to the greater availability of glucose to the pathogen, lower response of T-cells, reduced serum inhibitory activity against the Rhizopus in lower pH, and increased expression of some host receptors that mediate the invasion of human epithelial cells through microorganism. [8]
Diabetes and uncontrolled glycaemia were reported as significant predictors of severity and deaths in patients infected with different viruses, including the 2009 pandemic influenza A (H1N1) [9], SARS-CoV [10] and MERS-CoV [11]. Older patients with chronic diseases, including diabetes, were at higher risk for severe COVID-19 and mortality. Scarce data exist regarding glucose metabolism and development of acute complications of diabetes (e.g., ketoacidosis) in patients with COVID-19. Infection of SARS-CoV-2 in those with diabetes possibly triggers higher stress conditions, with greater release of hyperglycemic hormones, e.g., glucocorticoids and catecholamines, leading to increased blood glucose levels and abnormal glucose variability [12].
An Indian study has reported diabetes as the main risk factor in 70% of the patients [13] Most common sites for mucormycosis are sinus (39%), lungs (24%), skin (19%), brain (9%), GIT (7%), disseminated disease (6%) and other sites (6%) [14].Clinically it may manifest with necrosis of paranasal sinuses or palate and tongue which may progress towards orbit before reaching intracranial structure [15]. Unresolved rhinosinus mucormycosis leads to thrombosis of cavernous sinus and cranial invasion. Poor prognostic indicators are delay in treatment of more than 6 days, evidence of intracranial invasion, bilateral involvement, palate invasion and associated haematological malignancies [16].
Materials and Methods
A prospective cross sectional study was carried out in 70 patients who were admitted in the covid department of BJ Medical College, Civil hospital Ahmedabad and presented to ENT with mucormycosis during admission or after discharge over a period of 10 months from March 2020 to December 2020.
Evaluation at presentation included a detailed history, otorhinolaryngological, ophthalmic and neurological examinations to assess the extent of the disease (Fig. 1). Initial investigations included complete blood counts, blood urea, serum creatinine, serum glucose, urine for ketone bodies and blood gas analysis. Diagnosis was made on histopathological examination and KOH preparation of biopsy specimens obtained from the nasal cavity and/or paranasal sinuses and the palate.
Fig. 1.
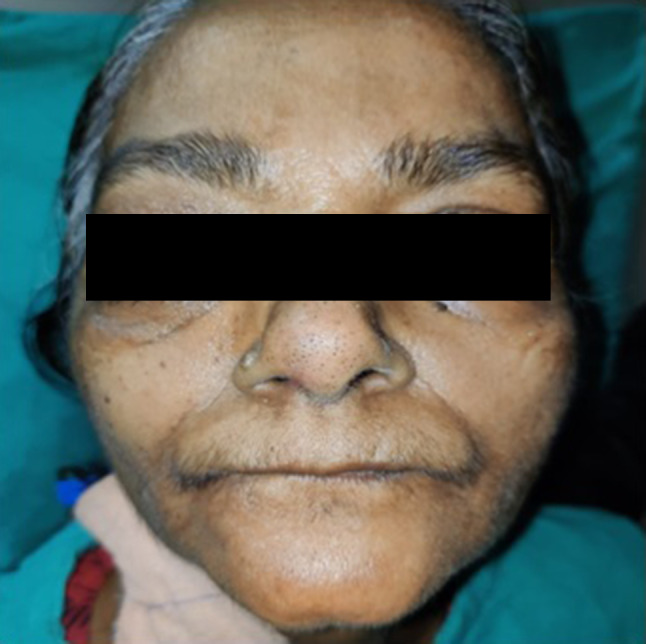
Showing pre op picture of patient with left eye ptosis and complete opthalmoplegia Patient consent- written informed consent for patient information and image to be published was provided by the patient
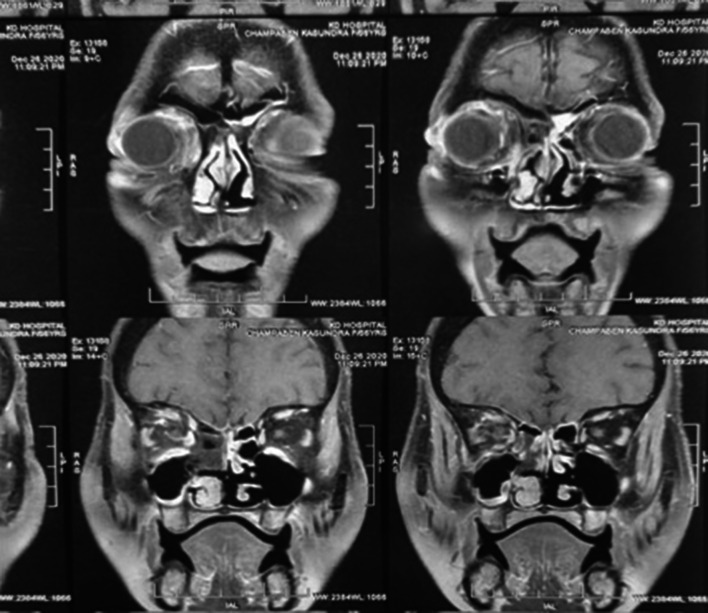
CT scan of paranasal sinuses and orbit, MRI paranasal sinuses with orbit with brain, and nasal endoscopy were done in all 70 patients selected for our study (Fig. 2).
Fig. 2.
Showing preoperative MRI gadolinium enhanced image of above patient
All the patients were started on injection human regular insulin according to 6 hourly RBS scale to control the underlying diabetes.
Treatment with systemic Amphotericin B was started as soon as a diagnosis of mucor was established in all patients, along with treatment to stabilize the underlying metabolic derangement and patient was planned for surgery for debridement of necrosed areas caused by mucor. Approach of surgery was decided depending upon the extent of the disease.
Liposomal amphoterecin B was given in all patients in dose of 3-5 mg/kg body weight diluted in 250 ml of 5% dextrose given slowly over 4 h Table 1–6.
Table 1.
Showing age wise distribution of patients
| Age group(Years) | Number of patients | Percentage (%) |
|---|---|---|
| 10–20 | 0 | 0 |
| 21–30 | 1 | 1.5 |
| 31–40 | 7 | 10 |
| 41–50 | 17 | 24.2 |
| 51–60 | 21 | 30 |
| 61–70 | 20 | 28.5 |
| 71–80 | 4 | 5.8 |
Table 6.
Showing distribution of patients according to HbA1c level
| Hb1Ac (%) | Number of patients | Percentage of patients (%) |
|---|---|---|
| 6–6.5 | 6 | 8.5 |
| 6.5–7 | 17 | 24.2 |
| 7–7.5 | 14 | 20 |
| >7.5 | 33 | 47.2 |
Three distinct treatment groups were identified based on the nature of surgery that the patients underwent. Patients in treatment group 1 underwent sino-nasal debridement only. Treatment group 2 consisted of sino-nasal debridement with orbital exenteration and palatal resection. Treatment group 3 received only medical treatment with intravenous amphotericin B as all these patients refused surgery (Table 7). Adverse effects were overcome by pre medications, avoiding concomitant nephrotoxic drug, and by administering liposomal amphotericin. Figs. 3 and 4.
Table 7.
Showing distribution of patients in different treatment groups
| Treatment | No. of patients | Percentage (%) |
|---|---|---|
| Sino nasal debridement only | 41 | 58 |
| Sino nasal debridement with orbital exenteration/ palatal resection | 23 | 33 |
| Injection amphoterecin B only | 6 | 8 |
Fig. 3.
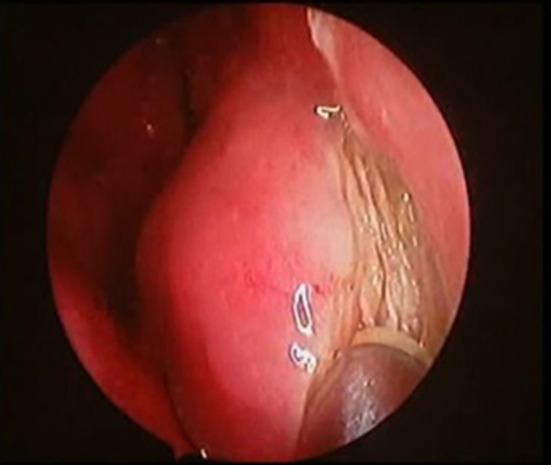
Showing endoscopic picture of modified denker approach keeping incison on pyriform aperture
Fig. 4.
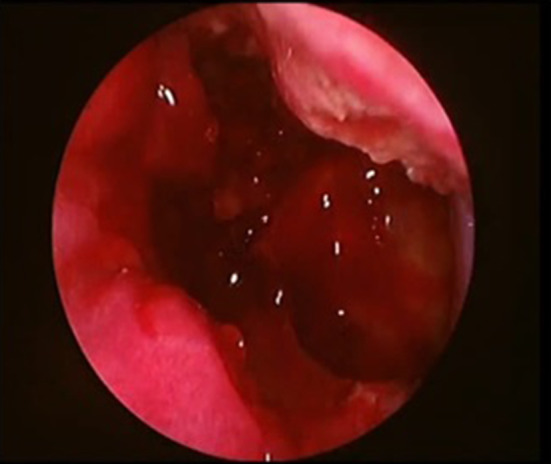
Showing endoscopic picture of exposed posterolateral wall of maxilla and other opened sinuses
In most of our patients in whom only sino nasal debridement was required, the surgical approach used was ‘endoscopic modified denker approach’. This approach gives a very detailed visualisation of the lateral extension of the disease including postero lateral wall of maxilla, pterygopalatine fossa, infratemporal fossa, parapharyngeal space and thus allowing the complete debridement of the affected areas.
Those patients who presented with the non salvageable eye disease, underwent orbital exenteration along with the sino nasal debridement.
Patients who had palatal erosion and required palatal resection, surgical approach of choice was open maxillectomy via weber ferguson incision followed by closure of palatal defect using temporalis musce flap (Figs. 5, 6, 7).
Fig. 5.
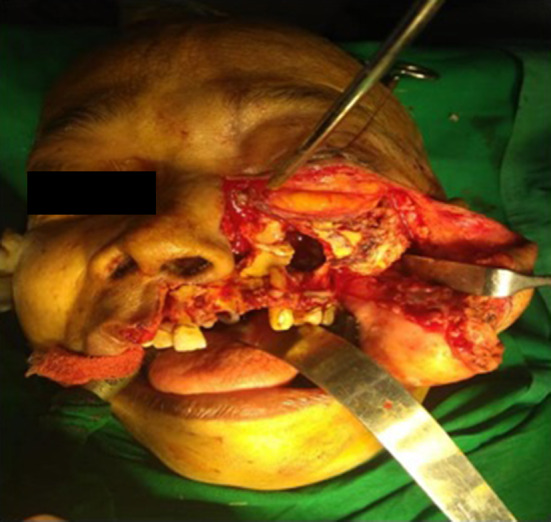
Showing open approach of maxillectomy by weber ferguson incision
Fig. 6.
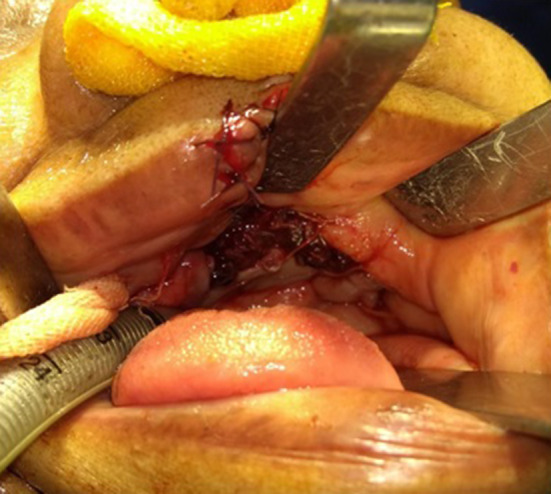
Showing closure of palatal defect by temporalis muscle flap
Fig. 7.
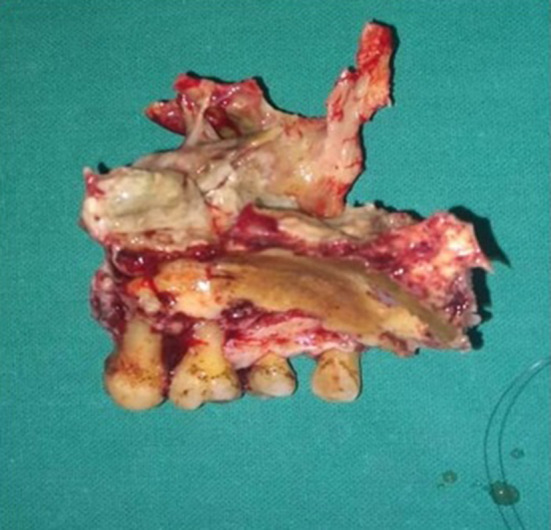
Showing image of resected specimen of maxilla
Surgical Technique of Endoscopic Modified Denker
Step 1: Mucosal Cuts
Under visualization with a 4-mm 0-degree rod-lens endoscope, 2% lignocaine with 1:200,000 epinephrine is first injected into the anticipated incision sites along the nasal floor, lateral nasal wall, and anterior to the head of the inferior turbinate. A coblator is used to incise the mucosa inferiorly at the junction of the nasal floor and lateral nasal wall, carrying the incision through the periosteum. A second mucosal incision is then made superiorly along the lateral nasal wall and carried anteroinferiorly to lie just in front of the anterior head of the inferior turbinate overlying the edge of the pyriform aperture (Fig. 3).
Step 2: Soft Tissue Dissection Over the Maxilla
A subperiosteal dissection is performed with a suction freer elevator to expose the anterior maxilla, the infraorbital foramen, and its neurovascular bundle as well as the lateral nasal wall.
Step 3: Bony Cuts to the Maxilla
A high-speed drill or osteotome is utilized to create a bony window into the anterior maxilla, taking care to stay inferior to the infraorbital nerve. Osteotomes are used to connect the window to the inferior bony cut of the medial maxillectomy, thereby allowing access to the anterior portion of the maxillary sinus. Specifically, a superior cut is made at the level of the roof of the maxillary sinus, an inferior cut at the junction of the nasal floor and medial maxillary wall, and a posterior cut along the posterior wall of the maxillary sinus (Fig. 4). The disease can then be cleared along with the removal of necrosed bone. At the end of the procedure, the nasolacrimal duct is identified, preserved, and sharply cut at an oblique angle to prevent stenosis. Complete exposure of the posterior aspect of the maxillary sinus is also achieved, facilitating use of a 4-handed technique to remove the disease involving the pterygopalatine or infratemporal fossae if needed.
Results
Seventy patients presented to us between march 2020 to December 2020, out of 70 patients, 47 were males (67%), and 23 were females (33%). Distribution of patients among genders is shown in Fig. 8. The age group ranged between 20 and 75 years. Age distribution of patients is shown in Table 1. Most common cause of immunosuppression was found out to be diabetes mellitus. All 70 patients in our study were diabetic (100%). Out of which 23 patients (33%) had diabetes under control while 47 patients (67%) had uncontrolled diabetes (Table 5). 33 patients (47.2%) had their HbA1c level > 7.5 those who had uncontrolled diabetes (Table 6). Other associated causes of immunosuppression were 5 post renal transplant patients (7%) who were on long term immunosuppressive therapy, 2 cases of acute myeloid leukemia (3%) who were on long term chemotherapy (Table 2). Symptoms presentation is shown in Table 3. Thirty seven patients out of 70 (53%) presented with symptom of periorbital oedema and chemosis of eye, 13 patients (19%) showed drooping of ipsilateral eyelid, while 27 patients (38%) presented with complete loss of vision on ipsilateral side and only 3 patients (4%) had complaint of diplopia. Facial swelling was seen in 32 patients (46%). Symptoms of acute sinusitis such as nasal blockage was seen in 5 patients (7%). Palatal involvement was found out in 11 patients (15%). All the patients taken in our study were tested positive for covid by RT-PCR, and all the patients underwent HRCT thorax preoperatively. CORADS grading distribution of patients in HRCT thorax is shown in Table 4.
Fig. 8.
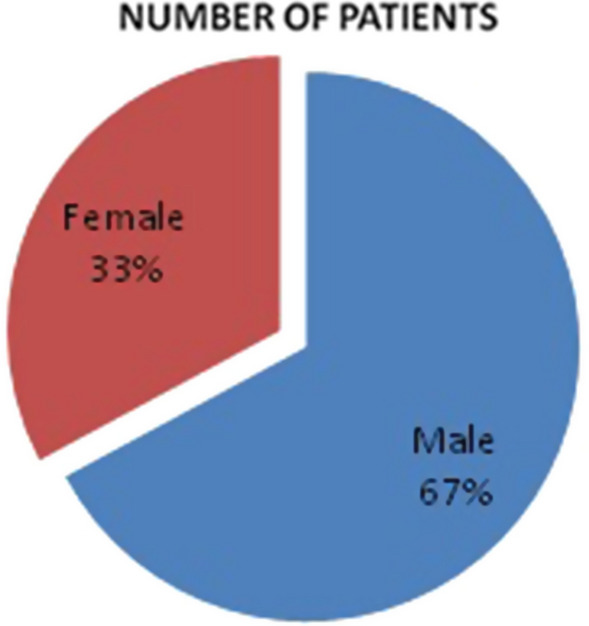
Showing sex distribution of patients
Table 5.
Showing distribution of patients according to RBS values
| Random blood sugar levels (mg/dl) | Number of patients | Percentage of patients (%) |
|---|---|---|
| 150–200 | 11 | 15.7 |
| 201–250 | 12 | 17.1 |
| 251–300 | 9 | 12.8 |
| 301–350 | 19 | 27.1 |
| 351–400 | 10 | 14.2 |
| >400 | 9 | 12.8 |
Table 2.
Showing distribution of immunosuppressive factors among patients
| Immunosuppressive factors | Number of patients | Percentage of patients (%) |
|---|---|---|
| Diabetes | ||
| Under control | 23 | 33 |
| Uncontrolled | 47 | 67 |
| Post renal transplant | 5 | 7 |
| Acute myeloid leukemia | 2 | 3 |
Table 3.
Showing distribution of presenting symptoms among patients
| Symptoms | Number of patients | Percentage of patients (%) |
|---|---|---|
| Periorbital swelling | 37 | 53 |
| Facial swelling | 32 | 46 |
| Loss of vision | 27 | 38 |
| Palatal ulcer | 11 | 16 |
| Drooping of eyelid | 13 | 19 |
| Nasal blockage | 5 | 7 |
| Diplopia | 3 | 4 |
Table 4.
Showing distribution of patients according to CORADS grading in HRCT thorax
| CORADS score | Number of patients | Percentage (%) |
|---|---|---|
| CORADS 1 | 0 | 0 |
| CORADS 2 | 13 | 19 |
| CORADS 3 | 22 | 31 |
| CORADS 4 | 16 | 23 |
| CORADS 5 | 16 | 23 |
| CORADS 6 | 3 | 4 |
Optimal medical therapy relies on rapid correction of underlying systemic abnormalities, such as acidemia and hyperglycemia, along with prompt antifungal initiation and aggressive surgical intervention. Lipid-based amphotericin B, which destroys the cell wall of the fungus, is the first-line medical treatment for mucormycosis and should be initiated as soon as the diagnosis is suspected. High doses are required, and nephrotoxicity may result; however, liposomal formulations may deliver high doses while protecting renal function.
Posaconazole, a triazole that inhibits growth of the fungus, has been proposed as a promising adjunctive or alternative treatment for mucormycosis [6, 9]
Four out of 70 patients died in our study, out of which two were operated while the other two died who refused for surgery and had extensive spread of mucormycosis. Mortality rate in our study was found to be 5.7%. The cause of mortality in above patients was late presentation due to COVID-19 lockdown and associated severe systemic illness.
Among the patients affected with mucormycosis, majority falls in the middle aged -elderly age group. As evident from our study,approximately 50% patients presented with opthalmic symptoms, showing the effect of covid pandemic had in delaying the presentation to hospitals and seeking treatment on time. SARS-CoV-2 infection triggers the release of hyperglycemic hormones like glucocorticoids leading to uncontrolled blood glucose levels in diabetic patients, as observed from our study ie. among covid positive patients 67% had uncontrolled DM. Among the 70 patients we had selected for our study all were tested covid positive previously, tested by RTPCR of nasopharyngeal and throat swabs. HRCT Thorax was done in all covid positive patients and changes suggestive of covid infection was there for all positive patients in our study.
Post operatively all the patients were started again on inj. Liposomal amphoterecin B in the dose of 3–5 mg/kg body weight for minimum of 14 days after which nasal endoscopy was done to assess the clinical improvement and any crusting or suspicious tissue if present was sent for KOH and biopsy. Radiological assessment was also done by post operative gadolinium enhanced MRI. Those showing improvement clinically and radiologically with proven repeat KOH and biopsy negative were planned for step down therapy and were switched to syrup posaconazole in the dose of 400 mg twice a day overlapping with amphoterecin B for at least 2 days. Those patients who don’t respond to or cannot tolerate amphoterecin B were started on salvageable therapy initially only with syrup posaonazole in the same dosage as above. Patients in whom repeat KOH and biopsy came positive even after 14 days of injection amphoterecin B post operatively were planned for repeat debridement along with the control of systemic predisposing factor such as diabetes. Those who underwent orbital exenteration and palatal resection were given were given obturator before being discharge from hospital.
Discussion
Mucormycosis is found predominantly in patients with poorly controlled diabetes mellitus and diabetic ketoacidosis [17, 18]. Impaired neutrophil and phagocyte response and increased available serum iron are the two underlying conditions in the majority of mucormycosis patients [18]. Earlier studies had proven that diabetic ketoacidosis impairs chemotaxic and phagocytic activity of neutrophils and increased available serum iron respectively [17]. Angioinvasion by the fungi has been studied to a greater extent and is considered central to its ability to cause tissue necrosis and dissemination [18, 19]. Facial pain and unilateral facial swelling are also important parts of the clinical picture of the patient with variable grade fever being present [20]. The clinical picture may further progress to include unilateral opthalmoplegia representing involvement of the orbital contents either by infection or vascular compromise [21]. Progression of the infection to central nervous system is heralded by development of confusion and disorientation [18]. Central nervous system damage may also result from cavernous sinus thrombosis and internal carotid artery encasement leading to cerebral infarction and hematogenous dissemination of the disease to other organ sites [22]. Early diagnosis of rhinocerebral mucormycosis and prompt and effective treatment are very important for good outcome of the patient [17, 18]
Histopathological examination of surgical specimens can confirm the clinical diagnosis with the appearance of right-branching aseptate hyphae, which are considered typical of mucor species, along with evidence of angioinvasion and tissue necrosis [23]. Fungal cultures provide further confirmation [24]. CT scans can be used to evaluate the progression of disease although correlation with the clinical findings may not always be accurate [20]
Reversal of underlying predisposing conditions is of paramount importance. Euglycemia should be restored rapidly and any immunosuppressive conditions reversed if possible [25]. The surgical approach should be based on the clinical state of the patient with timely interventions for appropriate debridement of infected areas [26]. Spellberg et al. specify the resolution of immunosuppression, radiographical signs and clinical symptomatology as the objectives of treatment [25].
In our attempt to find out a correlation between mucormycosis and covid 19 we found out that the infection of SARS-CoV-2 in those with diabetes possibly triggers higher stress conditions, with greater release of hyperglycemic hormones, e.g., glucocorticoids and catecholamines, leading to increased blood glucose levels and abnormal glucose variability [12] Glucocorticoids have been widely used in syndromes closely related to Covid-19, including SARS, Middle East respiratory syndrome (MERS), severe influenza, and community-acquired pneumonia [27].
Hyperglycemia and insulin resistance promote increased synthesis of glycosylation end products (AGEs) and pro-inflammatory cytokines, oxidative stress, in addition to stimulating the production of adhesion molecules that mediate tissue inflammation [28, 29]. This inflammatory process may compose the underlying mechanism that leads to a higher propensity to infections, with worse outcomes thereof in patients with diabetes [28]. The prognosis depends on the extent of infectin, underlying disease and the eastablishment of an early treatment.
The average age group affected in our study was around 50–60 years and more commonly affected were males which similar to the study done by balai et al. [30]
Diabetes mellitus is the most commonly reported predisposing factor for rhinocerebral mucormycosis. All the patients in our study were found to be diabetic which was similar to the study done by Nezafati et al. and Kolekar at el. who reported the percentage of patients with underlying diabetes to be 90% and 80% respectively [31, 32].
During this covid 19 pandemic, diabetic patients were reluctant to come to hospitals due to lockdown rules imposed by government,family issues, concommitent covid causes,fear of contracting the disease which resulted in delay in seeking help. Patients who developed symptoms of mucormycosis,did not take adequate treatment on time due to fear of infectivity and also due to the lockdown imposed all over the country, resulting in their late presentation with loss of vision.
Nasal endocopy and debridement was done for those who required the same with proper precautions during COVID 19 pandemic. Following were some of them:
All patients were tested for covid 19 by RTPCR and HRCT thorax was also done in positive patients.
Those who tested positive were shifted to designated covid hospitals for proper control of the infection
Patients who tested positive earlier were taken to surgery after resolution of symptoms and one negative covid report.
Surgery of patients with known COVID19 status or high risk operations were performed in a designated operating room with negative pressures.
Operating surgeons used personal protective equipment (PPE) and Powered air purifying respirators (PAPRs) while operating
Minimal OR team was present during operation, no trainees and observers were allowed in the room both for protection and preservation of PPE.
Conclusion
Since the major victims of the invasive mucormycosis are immunocompromised individuals, they should be under consideration for early intervention. Knowledge of rhinocerebral mucormycosis among these individuals allows an early visit to the hospital or clinic. It assists in early diagnosis and increases survival. Given the high morbidity and mortality of invasive rhino-orbito-cerebral fungal infections, a comprehensive and efficient multidisciplinary approach must be executed. This includes early and aggressive surgical debridement of disease in the paranasal sinuses, orbit, palate as well as initiation of a robust anti-fungal medical regimen with close follow-up. Early diagnosis and early initiaton of treatment increases the chances of survival.
Acknowledgement
Ethical committee- Institutional review board at BJ MEDICAL college approved the study for publication.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declares that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it 2020 [31/03/2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
- 3.Ceccarelli M, Berretta M, Venanzi RE, Nunnari G, Cacopardo B. Differences and similarities between severe acute respiratory syndrome (SARS)-CoronaVirus (CoV) and SARS-CoV-2. Would a rose by another name smell as sweet. Eur Rev Med Pharmacol Sci. 2020;24(5):2781–2783. doi: 10.26355/eurrev_202003_20551. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Rolling updates on coronavirus disease (COVID-19) 2020 [31/03/2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 5.Vijayabala GS, Annigeri RG, Sudarshan R. Mucormycosis in a diabetic ketoacidosis patient. Asian Pac J Trop Biomed. 2013;3(10):830–833. doi: 10.1016/S2221-1691(13)60164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarrami AH, Setareh M, Izadinejad M, Afshar-Moghaddam N, Baradaran-Mahdavi MM, Meidani M. Fatal disseminated mucormycosis in an immunocompotent patient: a case report and literature review. Int J Prev Med. 2013;4(12):1468. [PMC free article] [PubMed] [Google Scholar]
- 7.Herrera DA, Dublin AB, Ormsby EL, Aminpour S, Howell LP. Imaging findings of rhinocerebral mucormycosis. Skull base. 2009;19(2):117. doi: 10.1055/s-0028-1096209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammadi R, Meidani M, Mostafavizadeh K, Iraj B, Hamedani P, Sayedain SM, Mokhtari M. Case series of rhinocerebral mucormycosis occurring in diabetic patients. Caspian J Intern Med. 2015;6(4):243–246. [PMC free article] [PubMed] [Google Scholar]
- 9.Karla S, Natally H, Guerreiro Nicolau FC, de Castro I, de Giassi KS. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect Dis. 2019 doi: 10.1186/s12879-019-4592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JK, Feng Y, Yuan MY, Yuan SY, Fu HJ, Wu BY. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 11.Rani BG, Salem AA, Robert B, Harunor R. Risk factors for severity and mortality in patients with MERS-CoV: analysis of publicly available data from Saudi Arabia. Virol Sin. 2016;31(1):81–84. doi: 10.1007/s12250-015-3679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aihong W, Zhao Weibo Xu, Jianwen ZG. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020;162:108118. doi: 10.1016/j.diabres.2020.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabarti A, Das A, Mandal J, Shivaprakash MR, George VK, Tarai B, Rao P, et al. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med Mycol. 2006;44:335–342. doi: 10.1080/13693780500464930. [DOI] [PubMed] [Google Scholar]
- 14.Roden MM, Te Z, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis:a review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 15.Effat KG, Karam M, El-Kabani A. Potts puffy tumour caused by mucormycosis. J Laryngol Otol. 2005;119:643–5. doi: 10.1258/0022215054516304. [DOI] [PubMed] [Google Scholar]
- 16.Parfery NA. Improved diagonis and prognosis of mucormycosis. A clinicopathologic study of cases. Medicine (Baltimore) 1986;65:113–23. doi: 10.1097/00005792-198603000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Kim J-G, Park HJ, Park JH, Baek J, Kim HJ, Cha I-H, Nam W. Importance of immediate surgical intervention and antifungal treatment for rhinocerebral mucormycosis. J Korean Assoc Oral Maxillofac Surg. 2013;39:246–250. doi: 10.5125/jkaoms.2013.39.5.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosseini S, Borghei P. Rhinocerebral mucormycosis:pathways of spread. Eur Arch Otorhinolaryngol. 2005;262:932–938. doi: 10.1007/s00405-005-0919-0. [DOI] [PubMed] [Google Scholar]
- 20.Talmi Y, Goldschmied-Reouven A, Bakon M, Barshack I, Wolf M, Horowitz Z, Berkowicz M, Keller N, Kronenberg J. Rhino-orbital and rhino-orbitocerebral mucormycosis. Otolaryngol Head Neck Surg. 2002;127:22–31. doi: 10.1067/mhn.2002.126587. [DOI] [PubMed] [Google Scholar]
- 21.Thajeb P, Thajeb T, Dai D. Fatal strokes in patients with rhino-orbito-cerebral mucormycosis and associated vasculopathy. Scand J Infect Dis. 2004;36:643–648. doi: 10.1080/00365540410020794. [DOI] [PubMed] [Google Scholar]
- 22.Sehgal A, Raghavendran M, Kumar D, Srivastava A, Dubey D, Kumar A. Rhinocerebral mucormycosis causing basilar artery aneurysm with concomitant fungal colonic perforation in renal allograft recipient: a case report. Transplantation. 2004;78:949–950. doi: 10.1097/01.TP.0000129798.22312.1E. [DOI] [PubMed] [Google Scholar]
- 23.Lass-Florl C. Zygomycosis: conventional laboratory diagnosis. Clin Microbiol Infect. 2009;15(SUPPL5):60–65. doi: 10.1111/j.1469-0691.2009.02999.x. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg R, Scott L, Vaughn H, Ribes J. Zygomycosis (mucormycosis): emerging clinical importance and new treatments. Curr Opin Infect Dis. 2004;17:517–525. doi: 10.1097/00001432-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Spellberg B, Walsh T, Kontoyiannis D, Edward JJR, Ibrahim A. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009;48:1743–1751. doi: 10.1086/599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed C, Bryant R, Ibrahim A, Edwards JJR, Filler S, Golderg R, Spellberg B. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47:364–371. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Recovery Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID-19—Preliminary report. N Engl J Med. 2020. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32678530 [DOI] [PMC free article] [PubMed]
- 28.Sylvia K. Diabetes and infection: is there a link?—A mini-review. Gerontology. 2013;59(2):99–104. doi: 10.1159/000345107. [DOI] [PubMed] [Google Scholar]
- 29.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Canadian J Cardiol. 2018;34(5):575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balai E, Mummadi S, Jolly K, Darr A, Aldeerawi H. Rhinocerebral Mucormycosis: a Ten-Year Single Centre Case Series. Cureus. 2020 doi: 10.7759/cureus.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nezafati S, Kazemi A, Asgari K, et al. Rhinocerebral mucormycosis, risk factors and the type of oral manifestations in patients referred to a university hospital in Tabriz, Iran 2007–2017. Mycoses. 2018;61:764–769. doi: 10.1111/myc.12802. [DOI] [PubMed] [Google Scholar]
- 32.Kolekar JS. Rhinocerebral mucormycosis: a retrospective study. Indian J Otolaryngol Head Neck Surg. 2015;67:93–96. doi: 10.1007/s12070-014-0804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]