Abstract
As clinical advances with chimeric antigen receptor (CAR) T cells are increasingly described and the potential for extending their therapeutic benefit grows, optimizing the implementation of this therapeutic modality is imperative. The recognition and management of cytokine-release syndrome (CRS) marked a milestone in this field; however, beyond the understanding gained in treating CRS, a host of additional toxicities and/or potential late effects of CAR T cell therapy warrant further investigation. A multicentre initiative involving experts in paediatric cell therapy, supportive care and/or study of late effects from cancer and haematopoietic stem-cell transplantation was convened to facilitate the comprehensive study of extended CAR T cell-mediated toxicities and establish a framework for new systematic investigations of CAR T cell-related adverse events. Together, this group identified six key focus areas: extended monitoring of neurotoxicity and neurocognitive function, psychosocial considerations, infection and immune reconstitution, other end-organ toxicities, evaluation of subsequent neoplasms, and strategies to optimize remission durability. Herein, we present the current understanding, gaps in knowledge and future directions of research addressing these CAR T cell-related outcomes. This systematic framework to study extended toxicities and optimization strategies will facilitate the translation of acquired experience and knowledge for optimal application of CAR T cell therapies.
TOC blurb
A host of additional toxicities and/or potential late effects of chimeric antigen receptor (CAR) T cell therapy beyond cytokine-release syndrome (CRS) warrant further investigation. Herein, experts in paediatric cell therapy, supportive care and/or study of late effects from cancer and haematopoietic stem-cell transplantation present six key focus research areas related to CAR T cell-related outcomes beyond CRS.
Introduction
The clinical application of chimeric antigen receptor (CAR) T cell therapy has been one of the most important advances in paediatric cancer therapy. CD19-targeted CAR T cells were infused for the first time in children with B cell acute lymphoblastic leukaemia (B-ALL) in 20121,2. In 2017, the CD19-targeted CAR T cell product tisagenlecleucel was approved by the FDA for children and young adults (aged <25 years) with relapsed and/or refractory (R/R) B-ALL3. Comparable successes have been achieved in adults with R/R B cell lymphomas, for whom multiple CD19-targeted CAR T cell products are currently approved (tisagenlecleucel,4 axicabtagene ciloleucel,5 and bexucabtagene autoleucel6). Now, the field continues to evolve; aspects under evaluation include new antigens as potential CAR T cell targets in a growing range of malignancies, combinatorial targeting strategies and optimization strategies in every capacity.
These successes, however, would not have been feasible without overcoming the potentially life-threatening adverse events (AEs) seen in the acute phase (that is, within the first month) after CAR T cell infusion. Managing cytokine-release syndrome (CRS), the most notable acute toxicity of CAR T –cell therapy, was the first major complication that needed to be solved for a safe and effective delivery of such treatment7,8. Standardized grading algorithms for CRS have now been developed and are used across CAR T cell trials9. First-line therapies to mitigate CRS are well-established10, and second-line agents and/or treatment of refractory CRS are under active clinical investigation11,12. Current studies are focused on further limiting toxicities, optimizing the timing of cytokine-targeted interventions13 and developing constructs14 or methodologies15 that enable fine-tuning of CAR T cell expansion and associated inflammatory responses16.
Beyond efficacy and CRS, a host of considerations must be addressed as this field moves forward. These aspects are of particular relevance to children and young adults, who can experience long-term benefits but also potentially long-term adverse effects, including defects in neurocognitive development and chronic health conditions, that can have life-long implications. With a primary goal of comprehensively identifying and studying non-CRS toxicities and/or late effects of CAR T cells, we established a collaborative network to focus on key domains associated with CAR T cell therapy and subsequent toxicities that warrant further investigation. This initiative led to the establishment of the multicentre CAR T cell Beyond the Storm consortium, involving experts in paediatric cell therapy (including children and young adults), supportive care, and/or study of late effects from cancer and haematopoietic stem cell transplantion, to primarily focus on six key domains: extended monitoring of neurotoxicity and neurocognitive function, psychosocial considerations, infection and immune reconstitution, other end-organ toxicities, evaluation of subsequent neoplasms, and strategies to optimize remission durability (FIG. 1). In this Review, we provide a comprehensive overview and practice recommendations based on expert opinion from experience acquired with anti-CD19 and anti-CD22 CAR T cells in children and young adults. With the ultimate goal of developing evidenced-based guidelines in the future, we identify limitations in our knowledge and address future directions that will be particularly important as CAR T cells are used in new indications and to target novel antigens (TABLE 1).
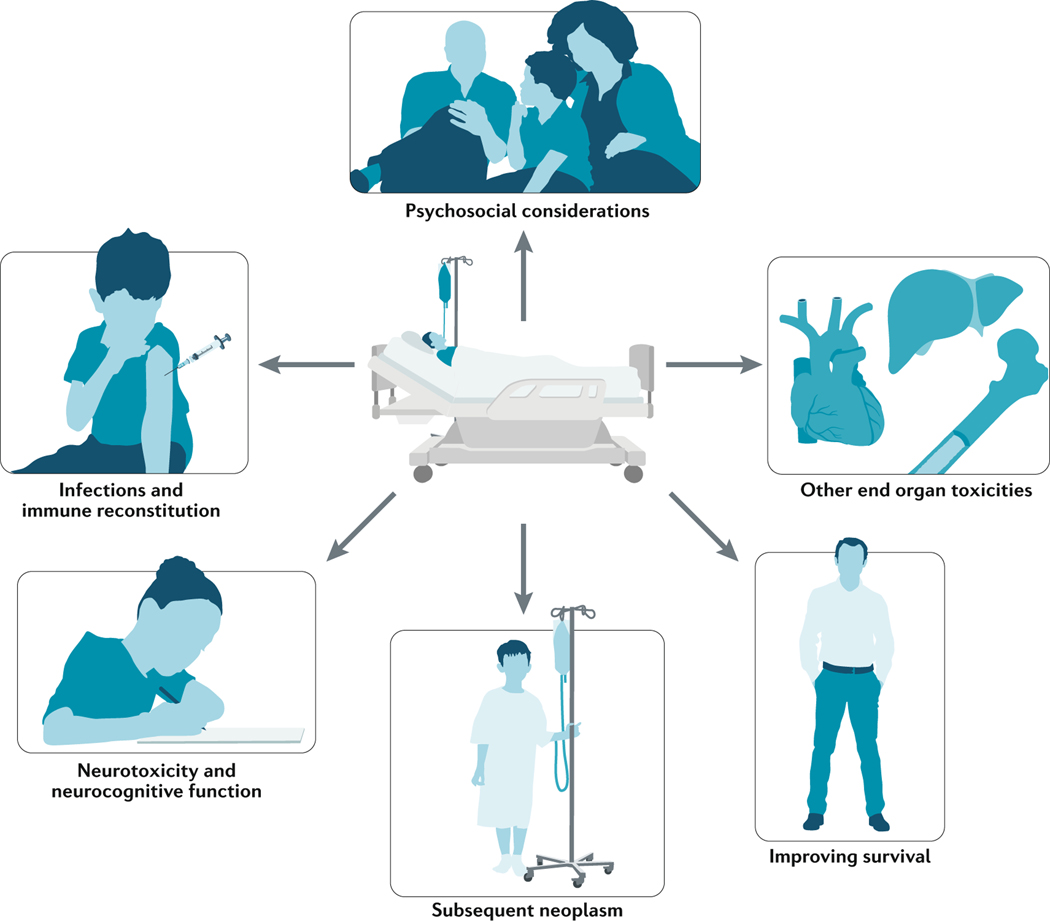
Fig. 1 |. Comprehensive overview of CAR T cell-related toxicities and subacute effects.
Pictorial depiction of six key areas of research focused on toxicities and subacute effects and monitoring for patients after CAR T cell therapy beyond cytokine-release syndrome (CRS).
Table 1 |.
Comprehensive overview of CAR T-cell related toxicities and subacute effects
| Domain | Current knowledge | Limitations and gaps in knowledge | Active areas of research and future directions |
|---|---|---|---|
| Neurotoxicity and neurocognitive function | ICANS is common (25– 44% of treated patients) and independent from but related to CRS. The serum levels of several cytokines are elevated in ICANS. Predictive models based on symptoms and cytokine levels have been described but they are not prospectively validated. Prospective short-term neurocognitive studies after CAR T cell infusion in children are feasible. Neuroimaging abnormalities during acute ICANS (<28 days or during CRS) are variable; specific findings include symmetric white matter and thalamic T2 FLAIR hyperintensities on MRI. |
No consensus recommendations exist regarding ICANS treatment or imaging guidelines. Whether ICANS or its treatment affects long-term neurocognitive outcomes is unknown. Heterogeneous patient populations in terms of treatment exposure make evaluation of late neurocognitive outcomes challenging. Centres delivering CAR T cells tend to be far from patients’ home institutions, creating a barrier in long-term neurocognitive evaluation. |
Long-term neurocognitive outcomes after CAR T cell therapy. Prospective validation of the clinical utility of neuroimaging. Biomarker development for acute and chronic neurological injury. Examination of correlation between acute ICANS and its treatment, imaging findings, biomarkers and long-term neurocognitive outcomes to guide evidence-based interventions. |
| Psychosocial considerations | PROs are crucial in understanding the physical, emotional and psychological effect of CAR T cell treatment on patients. Adults who have a response to CAR T cell treatment have sustained improvement in HRQOL measures. Collection of PROs from CAR T cell-treated children is feasible and has revealed clinically meaningful QOL improvement over time. |
Centres delivering CAR T cells tend to be far from patients’ home institutions, creating a barrier to longitudinal data collection. A standard approach using prespecified and validated instruments to measure PROs across relevant domains in paediatric patients is lacking. |
Development and implementation of robust patient-centred outcomes research to design evidence-based models for CAR T cell therapy. Development of consensus measures to capture specific domains at baseline and over time that could be performed onsite or remotely. |
| Infections and immune reconstitution | Acute infection after infusion occurs in 22– 42% of patients; bacterial infections are most common during the first month. Risk factors associated with infection include an B-ALL diagnosis, ≥4 prior lines of therapy, higher CAR T cell doses (>2 × 107 CAR Tcells/kg) and higher grade CRS (grades 3–4). Most centres in the USA implement antimicrobial prophylaxis, although the type and duration are centre-specific. Prolonged BCA can persist for months or years, and IVIG treatment might result in fewer infections. |
Utility and duration of prophylactic IVIG therapy is not well defined. Vaccine strategies might be more cost-effective and durable, but immunogenicity and safety in the context of CAR T cell therapy remain unknown. Antimicrobial prophylaxis practice has not been optimized. |
Developmentment of algorithms for antimicrobial prophylaxis based on patient riskstratification. Prospective studies to evaluate risk–benefit profile of prophylactic IVIG and response to vaccines after infusion. |
| Other end-organ toxicities | CARHLH, bone marrow, cardiovascular, pulmonary, ocular and renal toxicities can all occur after CAR T cell infusion, with varying clinical presentations. CarHLH is increasingly becoming recognized as distinct from CRS and is a hyperinflammatory entity. Cytopenias, including grade 3–4 thrombocytopenia or neutropenia, occur in >40% of patients treated and can persist in some patients (~15%) beyond 3 months after infusion. Prolonged severe cytopenias have multiple causes. |
Identifying carHLH has been challenging owing to variability in symptom manifestations based on the construct and target antigen, leading to difficulty in diagnosis and treatment recommendations. Whether acute toxicities seen in the cardiovascular, pulmonary, ocular and renal systems result in sub-acute or late dysfunction is unknown. | Identification of biomarkers and/or predictive models to determine the risk of developing carHLH, to enable early mitigation and/or prevention. Collaborative and comprehensive data collection regarding end-organ toxicities to profile novel adverse events and provide a systematic approach to monitoring patients for long-term toxicity |
| Subsequent neoplasms | No events of leukaemogenesis related to replication-competent retrovirus or lentivirus have been reported in patients treated with CAR T cells. Immunophenotypic evolution of underlying primary malignancy (lineage switching) after CAR T cell infusion has been reported in patients with B cell malignancies. Secondary neoplasms following immunotherapy are a cause of concern but not well-described. |
Elucidating the risk of neoplasms subsequent to CAR T cell therapy alone is difficult. | Establish protocols for close monitoring for second malignancies in all patients who receive CAR T cell therapy, with particular emphasis on long-term monitoring. |
| Disease optimization | BCA and loss thereof can help to predict risk of CD19+ disease relapse. Several CAR T cell persistence optimization strategies are currently being investigated, with promising early results. Antigen escape remains a substantial concern; trials of dual-targeted CAR T cells are underway and additional targets are being explored. Consolidative HSCT has shown benefit. CAR T cell treatment of patients with extramedullary disease can lead to objective responses, although the time to best response might be prolonged. |
Variability in the definition of loss of BCA exists. Which patients might need consolidative HSCT immediately post CAR T cell-induced remission remains unknown. Limited data are available on how effectively CAR T cells traffic to sites of extramedullary disease, the durability of response or the influence of extramedullary disease on CAR T cell expansion, persistence and toxicities. |
Establish a consensus on definitions involving BCA. Identify risk factors in patients for whom transplantation might be beneficial. Systematically identify the incidence of extramedullary disease in ALL, and optimize disease targeted strategies with CAR T cells to promote delivery and persistence of cells at extramedullary sites while limiting toxicities within these organ systems. |
ALL, acute lymphoblastic leukaemia; BCA, B cell aplasia; CAR, chimeric antigen receptor; carHLH, CAR T-related haemophagocytic lymphohistiocytosis; CRS, cytokine-release syndrome; CNS, central nervous system; HRQOL, health-related quality of life; HSCT, haematopoietic stem cell transplant; ICANS, immune effector cell-associated neurotoxicity syndrome; IVIG, intravenous immunoglobulin; PROs, patient-reported outcomes; T2 FLAIR, T2-weighted fluid-attenuated inversion recovery.
Neurotoxicity and neurocognition
Immune effector cell-associated neurotoxicity (ICANS) is a common AE associated with CAR T cell therapy9,17,18, with a frequency of 25–44% in published trials of anti-CD19 or anti-CD22 CAR T cells in children with haematological malignancies2,3,19–21. ICANS is typically preceded by CRS and usually presents within the first 7–10 days after CAR T cell infusion as a syndrome involving confusion, language disturbance and/or depressed consciousness. More severe presentations, such as seizures or coma, have been described in 10–20% of patients, and an estimated 1% of patients receiving some CAR T cell constructs have died from rapidly progressive cerebral oedema5,17,22–24. A high tumour burden, severe CRS, neurological comorbidities, high CAR T cell doses and high peak levels of CAR T cell expansion have all been implicated as risk factors for severe ICANS22,25,26, whereas no correlation has been found between the presence of active or previously-treated central nervous system (CNS) disease in patients with haematological malignancies and increased ICANS severity26,27.
No consensus or high-quality evidence exists to guide the treatment of ICANS, especially in children with B-ALL28. First-line therapy is typically focused on supportive care and/or short-term use of corticosteroids, with other agents (for example, anakinra) under study11. Overt ICANS symptoms, such as confusion and language disturbances, typically resolve by 28 days after CAR T cell infusion. Chronic neurological sequelae (such as epilepsy), tend to be rare in paediatric patients29, although subtle neurocognitive dysfunction might not be recognized without prospective monitoring. No prospective studies have addressed whether acute neurotoxicity affects long-term neurocognitive outcomes. To address the key question of whether CAR T cell therapy can have long-term neurocognitive sequelae, we advocate for the importance of longitudinal neurocognitive evaluations, neuroimaging, and the development of biomarkers for neurotoxicity risk and quantification of neurological injury using the tools outlined below.
Neurocognitive effects of CAR T cells
Acute neurocognitive effects observed during ICANS include confusion and difficulties with memory, attention and/or language2,3,18,19,26,29,30. To systematically monitor for acute neurotoxicity in early phase trials of CAR T cells, investigators at the National Cancer Institute (NCI) developed a caregiver-reported neurological symptom checklist that was administered at baseline (n = 22) and at days 14 (n = 21) and 28 (n = 19) after–CAR T cell infusion. Patients also underwent neurocognitive testing that assessed attention, cognitive flexibility, working memory and processing speed before and after infusion. This evaluation was deemed feasible in acute treatment settings, captured relevant neurological changes in patients and showed overall stable cognitive functioning in children and young adults with and without neurotoxicity30.
Currently, no longitudinal studies of the long-term neurocognitive outcomes of children treated with CAR T cells using validated performance tests or measures of patient-reported outcomes (PROs) have been published. In adults, one cross-sectional study used the Patient-Reported Outcomes Measurement Information System (PROMIS) forms to assess psychosocial functioning and a newly developed four-item questionnaire to evaluate neurocognitive function at one time point between 1–5 years (median 3 years) after CAR T cell infusion31. Among 40 patients, 15 (38%) reported one or more difficulties in the cognitive functions assessed (concentration, word finding, memory and problem solving)31.
To address the lack of long-term neurocognitive research in children treated with CAR T cell therapy, we convened a multidisciplinary group of psychologists, neuropsychologists, neurologists and oncologists to develop a systematic neuropsychological testing paradigm. The proposed core cognitive domains to assess across trials of CAR T cell therapies include attention, processing speed, working memory, cognitive flexibility and verbal fluency. These domains constitute cognitive processes that are typically vulnerable to impairment in survivors of childhood ALL treated with cranial radiation and/or chemotherapy32,33 and also might be affected by CAR T cell-related neurotoxicity18. Furthermore, impairments in these core cognitive processes might lead to secondary deficits in intellectual, academic and social functioning34. Key considerations when planning neurocognitive assessments for CAR T cell trials are: 1) baseline testing prior to infusion to assess the core cognitive domains, which must be concise given the acutely ill status of the patients, time constraints and the multiple demands on their family; 2) assessments of the sequelae of ICANS should be simple, brief and easy to administer at the bedside; and 3) follow-up testing for late effects should evaluate a wider range of domains deemed at risk of secondary impairments (such as intellectual, academic, socioemotional and adaptive functioning), consist of tests with multiple versions available to minimize the impact of repeated testing (that is, practice effects), and include a remote testing option available to reduce hospital-based assessments and promote compliance (Supplementary Table 1).
Additional complexities in characterizing the neurological sequelae of CAR T cell therapy arise from prior exposure to other potentially neurotoxic therapies, including radiation, intrathecal chemotherapy and haematopoietic stem cell transplantation (HSCT). Indeed, neurocognitive AEs of these therapies can be delayed, sometimes years after completion of treatment32,33. Thus, collecting a detailed medical history in addition to developmental and demographic information in order to examine the effect of a range of factors on neurocognitive outcomes is crucial. Assessing patients systematically over time will help in understanding the effects of these treatments on neurocognitive functioning and provide opportunities for interventions to mitigate long-term toxicity.
Imaging in ICANS
The role of neuroimaging in the long-term monitoring of ICANS outcomes remains unclear. Although brain MRI is performed during acute neurotoxicity in 40–80% of paediatric and adult patients22,25,26,29, no prospective studies have been published that determine the utility of baseline neuroimaging, imaging during acute AEs or follow-up brain MRI. Recommendations are based on expert opinion35 and specific guidelines for prospective neuroimaging are lacking.
Abnormal brain MRI findings are observed in 20–40% of patients during acute ICANS22,25. These specific findings include T2-weighted fluid-attenuated inversion recovery (T2 FLAIR) hyperintensities in the bilateral thalami and pons, and white matter changes, often involving the extreme and external capsule, as well as less-specific findings of vasogenic oedema, leptomeningeal enhancement or restricted diffusion in the splenium of the corpus callosum. When follow-up MRI is available, changes typically resolve by day 28 after CAR T cell infusion22,29. Interpretation of acute imaging findings is often hampered by the lack of baseline imaging, which frequently reveals abnormalities in children who have received numerous courses of chemotherapy32. Moreover, whether any correlation exists between acute neuroimaging findings and ultimate clinical outcomes remains unknown. Current approaches for baseline neuroimaging in children receiving CAR T cell therapy are variable, ranging from screening all patients to limiting imaging to a subset of patients with pre-existing neurological risk factors, such as a history of neurotoxicity, seizures and/or CNS disease. Baseline MRI might become more feasible with the availability of rapid MRI protocols, which even young children (<8 years) might tolerate without sedation. Whether imaging findings can be used to predict clinical outcome and guide management, and for how long, subtle chronic neuroimaging findings persist, remains to be determined.
Biomarkers of ICANS
No validated serum biomarkers are currently available to assess the risk, severity and long-term outcomes of ICANS. In clinical trials involving children and adults, elevated serum levels of cytokines such as IL-6, IL-10, IL-15 and IFNγ have been consistently associated with ICANS17,18. In many patients, however, concurrent CRS confounds the interpretation of biomarker assessments, and findings are discrepant across studies. For instance, changes in angiopoietin–TIE2 signalling that are indicative of endothelial injury were shown to be associated with neurotoxicity in adults but not in children22,29. Gofshteyn et al.26 identified IL-2, soluble IL-4R, HGF and IL-15 as markers for which high serum concentration were exclusively associated with neurotoxicity and not CRS, which differs from other reports16,36. Acute rises of the levels of two markers of glial injury (S100-B and glial fibrillary acidic protein) in cerebrospinal fluid (CSF) have been reported in a paediatric cohort receiving CD19-targeted CAR T cells, but the persistence of such abnormalities, or whether they correlate with neurocognitive outcomes, remain to be determined29. Several studies have proposed predictive models based on clinical parameters and cytokines16,22,25,26, but these predictive models were developed in small cohorts and require validation in prospective studies with larger cohorts. Additional biomarkers of acute and chronic neurological injury, such as neurofilaments, might also prove useful for predicting and measuring neurotoxicity37.
Practice recommendations
Obtain a thorough medial history, including prior treatment-related neurotoxicity or neurological comorbidities; perform baseline neuroimaging in high-risk patients or in those with history of neurotoxicity; and consider baseline imaging in patients without known neurological risk factors.
Establish standardized neurocognitive assessments before and after infusion that include core cognitive domains to facilitate comparison of acute and late effects across trials.
Future directions
Determining the clinical features and biomarkers that enable the identification of patients at highest risk of developing ICANS, the late effects of ICANS on neurocognitive functioning and whether treatment of ICANS mitigates the potential long-term toxicities are crucial needs. These data will guide future interventions for improved clinical care of patients experiencing neurotoxicity and will help to prevent long-term toxicity. Although the current evidence is insufficient for making universal clinical management recommendations for ICANS, we propose the following research agenda to move toward evidence-based clinical practice: 1) conduct prospective multicentre neurocognitive studies that assess a core set of domains using similar neuropsychological tests and PROs or caregiver-reported outcome measures (Supplementary Table 1); 2) implement prospective studies to determine the utility of baseline and follow-up neuroimaging in paediatric patients receiving CAR T cell therapy; 3) investigate biomarker development on correlative samples (including CSF and serum); and 4) conduct future research examining correlation between neurocognitive performance, neuroimaging and biomarkers to explore the aetiology of CAR T cell-related late effects.
Psychosocial considerations
Patients with B-ALL deemed eligible for CAR T cell treatment are a unique population: they typically have treatment-resistant disease, a highly uncertain prognosis, a high incidence of acute toxicities and often receive treatment far from their home institution38. Many of these patients and their families have been prepared for the likelihood of death from their disease39, and CAR T cell therapy is presented as their only hope for a durable remission. This complex situation demands an interdisciplinary40, thoughtful approach to patient-centred care. A comprehensive care team model optimizes health outcomes, minimizes distress and improves quality of life38.
Psychosocial assessment and PROs
Assessing how patients feel and function is a crucial aspect of understanding the effect of anticancer treatment and ensuring appropriately tailored care41. This attention to the ‘whole person’ is particularly important for patients receiving CAR T cells, whose previous medical experience has already been complex and whose disease course after treatment can be complicated by serious acute toxicities as well as by the longer-term issues discussed herein. Information about the symptoms, physical functioning and AEs should be obtained directly from patients receiving CAR T cells using PROs42,43, because understanding a child’s experience during therapy enables appropriate anticipatory guidance, and improves patient-centred decision-making, supportive care and psychosocial support. Systematic data collection from paediatric patients and their families is essential, both in routine care and clinical trials.41,44–47
In trials of CAR T cells in adults, studies of PROs have demonstrated sustained improvements in health-related quality-of-life (HRQOL) in responders48 and suggest superior HRQOL outcomes over those of patients undergoing HSCT49. Similarly, an international CAR T cell trial in paediatric patients showed that longitudinal collection of PROs in children is feasible and most patients have clinically meaningful HRQOL improvements over time50.
As described in adults51,52, the identification and harmonization of patient-reported domains and instruments is necessary for a standard approach to treating children with CAR T cells. These instruments must also be utilized in patients receiving other therapies for R/R leukaemia, including HSCT, to enable meaningful comparisons between treatment modalities that might reveal benefits of one treatment over another. Measures should include baseline social determinants of health, upfront and longitudinal assessments of psychosocial needs, and early and ongoing symptom assessments. Physical function, disease-related and treatment-related symptoms, emotional, social and cognitive function (Supplementary Table 1), as well as financial toxicity and global HRQOL, are important domains to capture. Mixed methods and longitudinal qualitative research approaches will ensure that the domains captured are the most meaningful for patients and their families. Specific to paediatrics, proxy reporters are necessary to capture experiences of patients too young (<8 years) or too ill to self-report53.
Practice recommendations
Establish comprehensive care models that begin with the decision to proceed with CAR T cell therapy, in order that expectations are understood, psychosocial risk factors are identified and educational needs are addressed.
Involve the referring team throughout the CAR T cell therapy process to provide comfort and essential continuity for the patient and their family and to enable evaluation of different care models following CAR T cells, including pursuit of HSCT in remission.
Future directions
Cooperative engagement of patients, their families and interdisciplinary clinicians51 should be leveraged to implement robust patient-centred outcomes research in order to design evidence-based models of care for CAR T cell therapy. A longitudinal focus on the patient’s psychiatric and psychosocial needs, as well as their long-term symptoms, will enable delivery of continued high-quality, tailored care and improve the outcomes of this vulnerable population. As novel CAR T cells are developed, evaluating the outcomes, including psychosocial and subjective symptom experiences, associated with emerging approaches to targeting novel antigen targets in B cells will be imperative, given the unknown elements of toxicity and response.
Infections and immune reconstitution
Incidence and type of infection
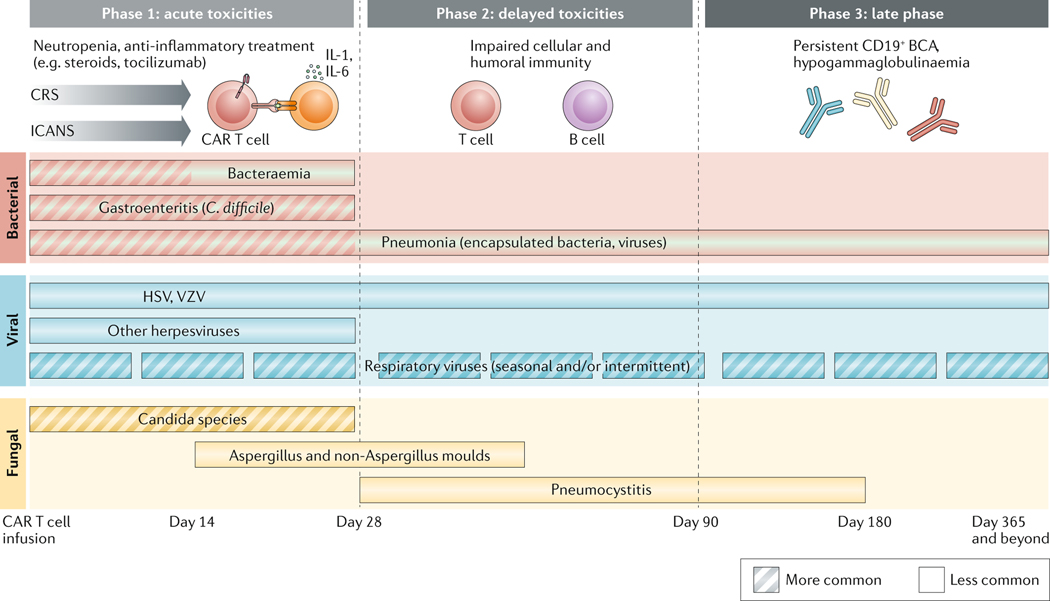
CAR T cell recipients are immunocompromised and at a high risk of infection owing to their underlying malignancy, prior cytotoxic treatments, potentially prior HSCT and lymphodepletion before CAR T cell infusion. Studies in patients with R/R B cell malignancies after infusion demonstrated that infections can occur both early (<30 days after infusion) and late (>30 days after infusion), although the majority (22–42%) occurred within the first month3,54–56. In this early period, the most common infections were bacterial (16–30%, including infections with multi-drug resistant organisms), usually bacteremias, followed by viral (8–19%) and fungal (1–8%) infections (FIG. 2). After 1 month, this pattern changed, with viral respiratory infections being the most frequent (11–28%), followed by bacterial (6–16%) and fungal infections (1–3%).
Fig. 2 |. Risk and timing of infection post CAR T-cell therapy.
Overview of the phases and timeline of common opportunistic infections in the acute and subacute period post CAR T cell therapy. Adapted with permission from Hill J.A. et al. 2020.
Risk factors associated with infection
Several factors have been associated with the risk of infection after CAR T cell treatment, including the diagnosis of ALL, ≥4 prior lines of antitumour therapy, high CAR T cell doses (such as 2 × 107 CAR T cells/kg), and higher grade (grade 3–4) CRS55,56. During CAR T cell therapy, antimicrobial prophylaxis varies; in one survey involving 30 institutions, the frequencies of administration of prophylaxis for infection with Pneumocystis jirovecii, viruses, bacteria and fungi were 100%, 100%, 90% and 87%, respectively, with varying practices on discontinuation after infusion57. Severe cytopenias are also a complication arising from CAR T cell therapy58 and can contribute to the increased risk of infection54,59.
BCA and immune reconstitution
‘On-target, off-tumour’ activity of CAR T cells targeting B cell markers (such as CD19), in addition to baseline immune dysfunction and the effects of lymphodepleting chemotherapy, can result in depletion of non-transformed CD19+ B cells. As a result, many CAR T cell recipients with ongoing remissions have prolonged B cell aplasia (BCA) persisting for months to years58–60. Nevertheless, polyclonal CD19+ B cells recover in up to 50% of paediatric and adult patients in remission 6–12 months after CAR T cell infusion, although recovery might indicate a greater risk of CD19+ disease relapse23,55,61,62. Importantly, restoration of immunoglobulin production upon B cell recovery has not been well described. Persistence of CAR T cells with anti-CD19 and 4–1BB co-stimulatory domains (CD19/4–1BB) can result in profound hypogammaglobulinaemia or agammaglobulinaemia2, generally indicating a need for immunoglobulin replacement therapy. In adults, pathogen-specific antibodies can remain present in the blood owing to the persistence of CD19− plasma cells63,64, although the plasma cell mass is smaller in children, and serum IgM and IgA have been found progressively decline or become undetectable in children after receipt of tisagenlecleucel65. No data supporting the safety of withholding IgG replacement in paediatric patients are available and thus, replacement remains standard practice despite associated costs and logistical challenges. Immunoglobulin replacement strategies differ depending on institutional preferences and the duration of BCA, but often involve treatment with monthly intravenous infusions targeting trough serum levels of IgG >400–500 mg/dl or, on the basis of a single-centre study, with subcutaneous IgG preparations to keep serum IgG levels >1,000 mg/dl66.
Considering T cell recovery, one study reported that absolute CD4+ T cell counts remained low (median of 155 cells/μl, range 33–269 cells/μl) 1 year after treatment with axicabtagene ciloleucel for R/R large B cell lymphomas58. Given the lack of B cells to mount a response, the efficacy of vaccination as an infection prevention strategy in patients lacking seroprotective antibody titres is unknown, but this approach is under active clinical investigation67.
Practice recommendations
Patients receiving CAR T cell therapy should receive prophylaxis against bacterial and fungal infections until neutropenia resolves.
Prophylaxis against Pneumocystis jirovecii-caused pneumonia should be instituted and continued for at least 3 months, and potentially longer in patients with delayed CD4+ T cell count recovery.
Viral prophylaxis for the prevention of Varicella zoster virus reactivation should be administered; further studies are required to determine duration of use.
Future directions
Aspects that remain to be elucidated include the infection profiles with CAR T cell constructs beyond CD19-targeted strategies68, the risks associated with CAR T cell treatment and lymphodepletion, and the optimal antimicrobial prophylaxis approaches in the period after cell infusion. Herein, we provide an overview of best practices for infection prevention, as benchmarks to guide future studies (TABLE 2). Standardization through a risk-stratification-based algorithm remains a goal. Dedicated studies are needed to determine the risk–benefit profile of prophylactic IgG and vaccination strategies in recipients of CAR T cells. Lastly, improved knowledge of inherent infectious disease complications will also facilitate management of emerging infections in CAR T cell recipients.
Table 2 |.
Strategies to prevent infections after CD19-targeted CAR T cell therapy
| Types of immune dysfunction | Causes | Infectious complications | Mitigation strategies | Active research topics |
|---|---|---|---|---|
| Neutropenia | Underlying disease Lymphodepleting chemotherapy Prolonged cytopenia after CAR T cell treatment | Bacterial infections Fungal infections | Prophylaxis for bacterial and fungal infections until neutropenia resolves Consideration for filgrastim | Define best practice for prophylaxis in this patient population Aetiology of prolonged cytopenia Correlation between CRS and infections |
| Lymphopenia | Prior chemotherapy Underlying disease Lymphodepleting chemotherapy | Viral reactivation (for example, of VZV) Viral and fungal infection (for example, resulting in PJP) | Viral prophylaxis Prophylaxis against PJP | Determination of duration of prophylaxis Correlation between BCA and CD4+ T cell counts |
| Hypogammaglobulinaemia | BCA | Sinopulmonary infections | Immunoglobulin replacement with IVIG or subcutaneous immunoglobulin | Define extent of humoral immunity preservation in patients with ongoing BCA Define optimal immunoglobulin replacement strategy, including duration of administration Determine efficacy and utility of vaccination in the setting of ongoing BCA |
BCA, B cell aplasia; CRS, cytokine release syndrome; IVIG: intravenous immunoglobulin; PJP, Pneumocystis jirovecii-caused pneumonia; VZV, Varicella zoster virus
Other end-organ toxicities
HLH-like toxicities
Haemophagocytic lymphohistiocytosis (HLH) or macrophage activation syndrome-like toxicities, including hyperferritinaemia, cytopenias, hypofibrinogenaemia, hepatic dysfunction and other end-organ involvement, have been described as distinct toxicities occurring in a subset of patients who receive CAR T cells69,70. The overlap between CAR T cell-associated HLH (carHLH) and severe CRS associated with currently approved or investigational CD19-targeted CAR T cell products is substantial, with similar cytokine profiles and clinical manifestations16,70, but might vary with alternative CD19-targeted CAR T cell constructs or CD22-targeted CAR T cells. Additionally, carHLH can occur beyond the onset and resolution of CRS12,71. Treatment paradigms also vary in accordance with the timing of carHLH onset; carHLH might be resolved with standard management of CRS28,70 or can necessitate alternative approaches, particularly when clinical manifestations are delayed and more suggestive of secondary HLH such that common CRS-directed therapies (such as tocilizumab) are not indicated12,70,71. Given the variability in onset and manifestations71 and lack of well-defined diagnostic criteria69, and with symptoms ranging from asymptomatic to life-threatening, a better understanding of the pathophysiology of carHLH is needed to more optimally identify, treat and potentially prevent this toxicity.
Bone marrow aplasia
Initial studies of CD19-targeted CAR T cells have demonstrated that more than 40–50% of patients will have persistent grade 3–4 thrombocytopenia and/or neutropenia beyond day 28 after cell infusion, and at least 15% of patients will continue to have severe cytopenias beyond 3 months after infusion3,61. Furthermore, the pattern of cytopenias occurring after CAR T cell infusion can vary and can present in bimodal fashion, with both early and delayed (that is, after CRS) cytopenias72,73.
Previous leukaemic bone marrow infiltration, infection, cumulative effects of prior chemotherapy and/or radiotherapy and, occasionally, previous HSCT, probably contribute to bone marrow dysfunction and subsequent persistent cytopenias in patients treated with CAR T cells.60,73,74 Standard lymphodepleting regimens, which do not generally cause prolonged cytopenias, might exert additional myelosuppression in patients with pre-existing bone-marrow dysfunction.60,73,74 Moreover, cytokine profiling in such patients revealed elevations of the serum levels of IFNγ, IL-6 and IL-8 in patterns similar to those seen in acquired bone marrow failure states (such as acquired aplastic anaemia and hypocellular myelodysplastic syndrome)16,75. In particular, high levels of IFNγ might suppress haematopoiesis via multiple mechanisms, including a role in carHLH, as well as by inducing stem-cell exhaustion through stress haematopoiesis and inducing damage to bone-marrow stem-cell niches76. Finally, immune dysregulatory conditions, such as antibody-mediated autoimmune cytopenias and thrombotic microangiopathy, can occur after CAR T cell infusion leading to cytopenias extrinsic to bone-marrow dysfunction. Further preclinical and clinical studies defining relative contributions of these factors in precipitating cytopenias will be crucial in developing cause-specific targeted therapies to prevent complications from cytopenias after CAR T cell infusion.
Characterizing patterns of cytopenias and related complications during CAR T cell therapy, and defining the use and efficacy of both pharmacological agents and allogeneic stem cell boosts in preventing cytopenia complications are future goals. The state of haematopoiesis after CAR T cell treatment has many similarities with other bone marrow failure states, although limited published data is available on the use of growth factor support after CAR T cell treatment. Anecdotal experiences indicate that GM-CSF might exacerbate HLH77, and theoretical concerns that thrombopoietin mimetics might promote clonal hematopoiesis remain78. Nonetheless, both agents have anecdotally been used in patients after resolution of CRS and/or day 28 and warrant further study, particularly with emerging preclinical data suggesting a potential role in managing cytopenias79. G-CSF is used routinely in many adults receiving CAR T cells in order to mitigate cytopenia73. Cytokine-mediated cytopenias could potentially be mitigated with cytokine-targeted therapies, but given the immunosuppressive nature of these agents, whether these treatments could compromise long-term CAR T cell efficacy and/or further contribute to the risk of infection and immunosuppression is unknown. In patients who have previously received allogeneic HSCT, the bone marrow reserve can be particularly poor; limited experience suggests a role for CD34-selected allogeneic stem cell boosts80,81 in restoring blood counts for prolonged CAR T cell-associated cytopenias, although systematic studies are needed to define indications for and the optimal timing of this intervention.
Other end-organ toxicities
Cardiac toxicities
Cardiovascular dysfunction is an important, yet incompletely defined, component of CRS and can manifest as tachycardia, haemodynamic instability and depressed cardiac ejection fraction.82 Risk factors include a high disease burden, pre-existing cardiac dysfunction, high-grade CRS (grade ≥3), and delays in the administration of tocilizumab for CRS83–86. In a single-centre study83, 7% (n = 7) of paediatric patients had new cardiac dysfunction at discharge, which was reversible in six of them within 6 months. Guidelines have been developed in 2019 for the evaluation of cardiac risk factors and their management in adults84. However, long-term outcomes data are scarce, and consensus on a systematic approach to continued clinical surveillance and testing in children is needed.
Pulmonary toxicities
Patients with pulmonary manifestations of their malignancy (such as malignant pleural effusions or pleural-based disease) are potentially at risk of developing severe respiratory toxicity associated with CAR T cell-related inflammation87, in addition to complications associated with CRS. Critical care medicine has had an essential role in the optimal management of patients during the acute phases of CRS, especially regarding fluid resuscitation in those with capillary leak syndrome; however, limited data on long-term pulmonary complications or sequelae and their management are available88.
Ophthalmological toxicities
Insufficient information is available on the effects of CRS and CAR T cells on ophthalmological function89. Ophthalmologic manifestations of acute leukaemia and/of complications from leukaemia treatment can involve all parts of the eye90, and can be affected during CAR T cell therapy. Progression of leukaemic ocular lesions during treatment can threaten vision, and distinguishing between CAR T cell-associated inflammation (or ‘pseudoprogression’) and primary disease progression can be difficult. A case report has highlighted the potential for and treatment of retinal detachment as a consequence of CAR T cell therapy in a patient with established ocular leukaemic involvement91. Transient ocular manifestations, such as conjunctivitis, photophobia and blurred vision as a prodrome to CRS, have also been reported in studies of CD22-targeted CAR T cells12. Only 28 CAR T cell therapy-associated ocular AEs amongst 1,421 patients (1.9%), the majority comprising vision impairments, have been reported to the FDA, but these AEs might be underreported92. Collaborative efforts to compile specific data on ophthalmological AEs will be a necessary component of future efforts to more comprehensively profile these CAR T cell-related toxicities.
Renal toxicities
CAR T cell-derived nephrotoxicity can vary in presentation and can be either an indirect consequence of CRS on kidney function or a direct effect of renal-targeted off-tumour toxicity in patients with renal involvement by leukaemia. Manifestations can include electrolyte disturbances related to CRS or tumour cell lysis, pre-renal acute kidney injury or acute tubular injury resulting from CAR T cell-related hypotension, inflammatory cytokine-mediated injury or endothelial activation and/or injury affecting renal function, all of which can range from mild to severe93,94. CAR T cell-related atypical haemolytic uremic syndrome occurred in 3 of 58 participants in a study of CD22-targeted CAR T cells12. Limited data are available on the direct effects of CAR T cells in patients with malignant extramedullary lymphomatous involvement of the kidneys; however, knowledge of such effects could also inform future CAR T cell therapies for primary renal cancers. Long-term nephrotoxicities are seen infrequently95, but are potentially understudied and underreported.
Practice recommendations
Close monitoring for end-organ toxicities related to CRS and/or toxicities extending beyond the period of CRS is needed to inform the potential for longer-term toxicities and/or late effects that might require additional intervention or optimization strategies (TABLE 3).
Table 3 |.
Recommendations for monitoring of non-neurological end-organ toxicities after CAR T cell therapy
| Organ system | Toxicity | Proposed surveillance studies |
|---|---|---|
| Bone marrow | Haemophagocytic lymphohistiocytosis Cytopenias, including grade 3–4 neutropenia and thrombocytopenia | Baseline CBC/D and bone marrow prior to lymphodepletion Frequent laboratory assessments including CBC/D, hepatic panel, inflammatory markers (such as C-reactive protein and ferritin), bone marrow aspirate or biopsy to evaluate cellularity and assess for haemophagocytosis, dysplasia or relapse |
| Cardiac | Sinus tachycardia Hypotension Shock requiring inotropic support Depressed systolic ejection fraction Cardiac arrhythmias Cardiac failure or arrest | Baseline electrocardiogram, transthoracic echocardiogram, biomarkersa Cardiology consultb Consider cardiac monitoring, repeat transthoracic echocardiogram and biomarker assessmentsa in patients with ≥grade 2 CRS Follow-up electrocardiogram, transthoracic echocardiogram, and biomarkersa 1 month after infusion, with serial evaluations in patients who developed cardiac dysfunction or in those with persistent symptoms |
| Pulmonary | Hypoxia Cough Pulmonary oedema Acute respiratory failure | Baseline pulse oximetry and consideration of pulmonary imaging prior to lymphodepletion in patients with neutropenia or history of fungal disease During CRS, consider continuous pulse oximetry and/or pulmonary imaging in patients with respiratory symptoms Routine follow-up assessments, with repeat imaging in clinically indicated situation only |
| Ocular | Conjunctivitis Photophobia Vision changes and/or impairment Papilloedema Retinal detachment | Baseline clinical eye exam Ophthalmology consult as clinically indicated In patients with a known history of ocular involvement from malignancy perform consultation prior to CAR T cell infusion |
| Renal | Acute kidney injury Electrolyte disturbances Atypical haemolytic uremic syndrome Renal failure | Baseline urinalysis and laboratory assessments including electrolyte panel, creatinine and albumin Daily laboratory assessments during CRS, including electrolyte panel, creatinine, albumin and urinalysis Nephrology consultation as clinically indicated |
CBC/D, complete blood count with differential; CRS, cytokine-release syndrome.
Troponin, and B-type natriuretic peptide or N-terminal pro B-type natriuretic neptide.
Consider cardiology consult if patient has a history of heart failure, cardiomyopathy and/or arrhythmias, and/or has received prior mediastinal radiotherapy.
Future directions
In order to optimize further therapy, an enhanced understanding of extended toxicities and the effects of CRS on other end-organs is needed to elucidate the underlying pathophysiology and clinical sequelae. A study in adults with diffuse large B cell lymphomas receiving CAR T cells (n = 60) published in 2020 described a high burden of end-organ toxicities and their effect on outcomes96. Retrospective multicentre studies compiling the collective data from single-institutional trials are needed in order to determine the toxicity profiles of CAR T cell therapies, and several efforts are underway including those undertaken by this group.
Subsequent neoplasm
The risk of second malignancies is of particular concern in patients with R/R disease, for whom cumulative therapies can increase the risk of mutagenesis. Experience thus far suggests that no second malignancies have been caused by CAR T cell therapy, but the issue remains of regulatory concern given the theoretical risks of insertional mutagenesis when using lentiviral and retroviral vectors for CAR T cell production97. Of note, no malignancies associated with replication-competent retroviruses or lentiviruses have been reported to date98,99. In addition to the US FDA guidance regarding monitoring for secondary malignancies100 in this high-risk population, a systematic approach is needed for the evaluation of subsequent neoplasms following treatment with CAR T cells, with categorization according to one of two primary presentations: 1) immunophenotypic evolution of the underlying primary disease, and 2) distinct subsequent neoplasm.
Lineage switching from B-ALL to a myeloid malignancy is a mechanism of immune escape following CAR T cell therapy. Lineage switching as a mechanism for post-CAR T cell relapse is now well appreciated, in particular in patients with KMT2A rearrangement, who can initially present with either acute myeloid leukaemia (AML) or ALL, and in whom treatment directed at one immunophenotype can lead to relapse with the alternative leukaemic subtype101. Several case reports have described lineage switching after treatment with CD19-targeted CAR T cells or blinatumomab (a CD3/CD19-bispecific T cell engager) in patients with B cell malignancies with and without KMT2A rearrangement102–107.
Beyond lineage switching, development of myelodysplastic syndrome (MDS) or AML as a de novo treatment-related malignancy or subsequent neoplasm is a well-established risk associated with chemotherapy and/or radiotherapy108,109. The occurrence of subsequent neoplasms following novel immunotherapies, however, is less well-described. Determining whether prolonged cytopenias after CAR T cell treatment are a result of MDS from prior therapy or of ongoing CAR T cell activity can be difficult59,110. Insufficient information is available on the risks of developing solid tumours or carcinomas after treatment with CAR T cells, which can be further complicated by the inherent risks of HSCT. A case report has described a young adult who developed cholangiocarcinoma following CAR T cell therapy and subsequent HSCT111. Given the potentially long latency of development of secondary malignancies, long-term monitoring will be necessary.
Practice recommendations
In addition to following the FDA guidance for monitoring of all patients receiving CAR T cells for the occurrence of secondary malignancies, a thorough evaluation to distinguish lineage switching of the pre-existing malignancy from development of a secondary (new) neoplasm should be undertaken in the event that a second malignancy is detected.
Future directions
Given the lack of knowledge on long-term risks of subsequent malignancies following CAR T cell therapy, close prospective monitoring for subsequent neoplasms will be important. Owing to the risks associated with the evolution of the primary malignancy, the acquisition of new cytogenetic abnormalities or the increased life-expectancy after anticancer treatment112,113, a systematic framework for the evaluation of subsequent neoplasms will be needed. Given the importance of long-term surveillance, engaging with large cooperative groups (for example, the European Society for Blood and Marrow Transplantation, or the Center for International Blood and Marrow Transplant Research) to share CAR T cell-related data can facilitate such efforts. Indeed, the NCI-funded Cellular Immunotherapy Data Resource was developed specifically to serve the biomedical community by capturing data on recipients of CAR T cells and other cellular immunotherapies, as well as to meet the FDA post-marketing requirement of a 15-year follow-up to assess long-term effects of these therapies. Coordinating efforts within these types of infrastructures will facilitate the development of a robust outcomes database available for future research.
Optimizing remission durability
CD19-targeted CAR T cells have elicited high response rates in children with R/R B-ALL, some of whom seem to be cured with various CD19-targeted approaches alone. Nevertheless, disease relapses occur in 40–50% of patients20,114,115, leaving room for improvement of long-term disease free survival outcomes (FIG. 3).
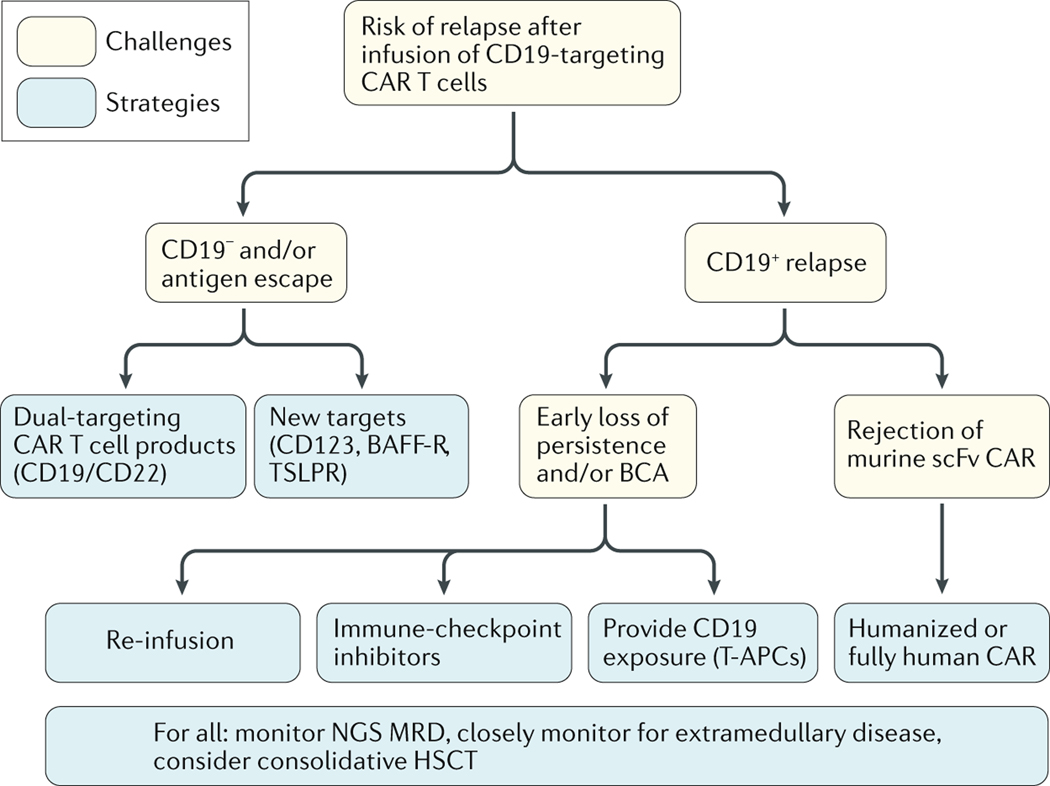
Fig. 3 |. Strategies to prevent relapse after CAR T cell therapy in children.
Proposed algorithm for monitoring of relapse after CAR T cell infusion and optimization strategies for subsequent treatment based on the phenotype of relapsed and/or refractory B cell acute lymphoblastic paediatric leukaemia.
Monitoring for loss of BCA
BCA is a pharmacodynamic marker of ongoing CAR T cell activity; thus, loss of BCA might enable to predict relapse with CD19+ ALL associated with early loss of functional CAR T cells20. Monthly monitoring for loss of BCA is useful in predicting a patient’s risk of CD19+ disease relapse, although the definition for loss of BCA remains variable. The Children’s Hospital of Philadelphia team developed a definition of an increase of 3% or 50 CD19+ cells per ml of peripheral blood, or of 1% CD19+ haematogones in bone marrow. The appearance of CD19+ haematogones in the marrow can precede B cell recovery by several months and, thus, follow-up assessment at 3 months might be warranted. The Seattle Children’s group defines BCA as a 1% increase in CD19+ cells in bone marrow or peripheral blood between two consecutive time points. While loss of BCA prior to 6 months after infusion might increase the risk of CD19+ disease relapse, no clear time thresholds have been defined, and late CD19+ relapses after prolonged BCA have been described116. Furthermore, while ongoing functional persistence of CAR T cells can protect against CD19+ disease relapse, the risk of CD19– relapse remains.
Antigen-negative disease relapse
Efforts to prevent CD19− disease relapse117–120 have included the use CAR T cells dually targeting CD19 and CD22121–123. While these products have been well-tolerated and induce similar rates of remission as single-antigen targeting approaches, longer follow-up is needed to determine whether this strategy effectively prevents antigen escape. Additional targets on B cells (such as CD123, BAFF-R and TSLPR) are being explored for the treatment of B-ALL, with potential roles both in single-agent and/or combinatorial strategies124–126. A comprehensive discussion regarding antigen-negative relapse is outside the scope of this manuscript.
Role for consolidative HSCT
Given the unpredictable risk of disease relapse following treatment with CD19-targeted CAR T cells, the role of consolidative HSCT remains undefined. In two trials of CD19/4–1BB CAR T cells in adults, consolidative HSCT was identified as a factor conferring a leukaemia-free survival or event-free survival (EFS) benefit127,128, although this effect was not shown in a trial of CD19-targeted CAR T cells with a CD28 co-stimulatory domain involving adult patients129. Two studies showed superior outcomes in children and young adults who underwent consolidative HSCT following therapy with CD19-targeted CAR T cells, particularly among those who had not undergone a prior HSCT130 and in those with very early loss of BCA (<2 months after CAR T cell infusion)116. A randomized controlled study would be the optimal method to determine whether consolidative HSCT is superior for long-term cure relative to CAR T cells alone, although the ability to identify those patients at high-risk of relapse who could potentially benefit from HSCT remains a valid goal.
Next-generation sequencing (NGS) assessment of minimal residual disease (MRD) can help to distinguish those patients who are likely to maintain a durable remission without further therapy. In a trial involving adult patients, those with NGS-assessed MRD negativity 3 weeks after CAR T cell infusion had a longer median EFS duration than those with MRD positivity (8.4 months versus 3.6 months; P = 0.036)131. Among children and young adults involved in the ELIANA trial, those with NGS-assessed MRD negativity 1 month after cell infusion had superior progression-free survival than those with MRD positivity (80% versus 30%), the majority of whom had not undergone consolidative HSCT131. An updated analysis of this cohort revealed that NGS-assessed MRD negativity at 3 months was predictive of long-term durable remission (M.A.P., unpublished work). Therefore, early assessment of MRD status with NGS specifically might enable the prediction of which patients do not require consolidative HSCT; however, the optimal timing of this assessment requires validation.
Of note, the described investigations and optimization strategies are specific for CD19-targeted CAR T cells. Different considerations might apply to alternative CAR T cell constructs associated with higher relapse rates12 or to the treatment of diseases other than B cell malignancies.
Novel strategies to enhance persistence
Preferential use of CD19/4–1BB CARs might extend the persistence of functional CAR T cells. A Low antigen burdens might be associated with shorter durations of CAR T cell persistence, as was shown by one study demonstrating better long-term persistence if there was a higher presence of CD19+ cells at baseline20, although additional studies are needed. CAR cells. In an ongoing pilot clinical trial132, patient-derived CD19+ T cell-antigen presenting cells (T-APCs) are being used to provide periodic CD19 antigen stimulation to CD19-targeting CAR T cells in vivo with the aim of improving their persistence. Preliminary results have demonstrated secondary expansions of CAR T cells in patients following CD19+ T-APC infusion without recrudescence of CRS133.
Immunogenicity against murine-derived scFv domains can also limit the persistence of CAR T cells134. Accordingly, humanized and fully-human anti-CD19 scFv-containing CARs have been developed to reduce the risk of immunological rejection. A phase I trial of a humanized CD19-targeted CAR T cell product135 in 30 children and young adults revealed MRD-negative complete response rates of 45% and 100% in individuals who had received prior CD19-targeted CAR T cells and those who had not, respectively136. Several ongoing clinical trials are investigating fully-human CD19-targeted CAR products, which might further mitigate rejection responses, although whether these products will prolong durable responses remains to be determined.
Finally, upon B cell recovery following CD19-targeted CAR T cell infusion, immune-checkpoint inhibition (ICI) with pembrolizumab (following either initial cell infusion or reinfusion) re-established functional persistence of CAR T cells in 3 of 6 patients who had early B cell recovery137. CRs occurred in 2 of 4 patients who started pembrolizumab for bulky extramedullary disease unresponsive to CAR T cell monotherapy137. Further studies are needed to determine which patients are more likely to respond to ICI administered to enhance the efficacy of CAR T cells, and when to initiate ICI after CAR T cells.
Extramedullary disease
Published trials of CAR T cells in paediatric patients with R/R B-ALL have largely excluded patients with isolated extramedullary disease relapse2,12,19,20,138. However, several trials have included patients with CNS disease and demonstrated CRs with trafficking of CAR T cells into the CSF19,20. Reports of CAR T cell therapy in patients with non-CNS extramedullary disease (for example, in bone or kidney) are infrequent, although antitumour responses with anti-CD19 or anti-CD22 CAR T cells have been demonstrated in the context of B-ALL12,87,134. Indeed, one of the first paediatric patients treated with CD19-directed CAR T cells experienced resolution of kidney disease and remains in a long-term remission (>8 years)1. Despite the small numbers of patients with non-CNS extramedullary disease treated to date, the available evidence indicates that the kinetics of disease resolution can be delayed and complete radiographic resolution of such disease can take several weeks to months87.
One strategy to fully optimize the efficacy, delivery and potency of CAR T cells in patients with extramedullary disease could involve immune modulation with ICI139. Several groups have demonstrated the safety and efficacy combining PD-1 inhibition with CD19-targeted CAR T cells in children with extramedullary B-ALL140 and in adults with B-cell lymphoma141,142. An additional strategy in this setting involves the use of radiotherapy, either for local disease control as a bridge to CAR T cell therapy or to synergize with and enhance CAR T cell-induced cytotoxicity143–147. Future prospective studies will be needed to identify optimal combinatorial strategies to improve CAR T cell efficacy and durability in patients with extramedullary disease.
Practice recommendations
For eligible patients, HSCT should be encouraged if CAR T cell persistence does not last 6 months (as evidenced by loss of BCA).
Experimental options exist for patients in remission with loss of CAR T cell persistence before 6 months who are not eligible for HSCT, who have already undergone HSCT previously or who decline HSCT. These approaches include re-infusion with another aliquot of the original treatment product or a fully human or humanized CD19-targeted CAR T cell product, and/or re-infusion followed by treatment with an immune-checkpoint inhibitor (such as pembrolizumab). Limited published data are available on these options, which are best pursued in the context of a clinical trial.
Future directions
Extending remission durability, preventing relapse and optimizing response are areas of active investigation. Defining BCA, identifying the role and timing of combinatorial ICI therapy, improving outcomes after second CAR T cell infusions and gaining a better understanding of which patients are most likely to benefit from HSCT will be essential to extending the therapeutic benefits of B cell-targeted CAR T cells.
Conclusions
In response to a need for systematic evaluation of late effects and/or subacute toxicities of CAR T cells, we have sought to address gaps and limitations in the current understanding of challenges in CAR T cell therapy that extend beyond CRS. As more patients receive CD19 CAR T cell products with the goal of long-term durable remission, a systematic approach to evaluating CAR T cell-related toxicities will be essential for monitoring of long-term outcomes. Furthermore, lessons learned from the initial studies B cell antigen-targeted CAR T cells will be useful when testing similar products with novel targets.
Herein, we provide a contextual framework for evaluating toxicities of novel CAR T cell constructs, as well as an overview of strategies currently used to optimize responses and assess other long-term outcomes of CAR T cell therapy, which is of particular relevance in children and young adults who might subsequently become cancer survivors. Future multicentre prospective studies focused on toxicity mitigation and optimization strategies will provide much needed insight that facilitate the formulation of hypothesis-generating questions imperative to further address the biology and pathophysiology underscoring the multitude of extended toxicities and impact on late effects of CAR T cell therapies.
Supplementary Material
Key points.
A host of extended toxicities and/or potential late effects of CAR T cell therapy and cytokine release syndrome (CRS) in children warrant further investigation with a systematic approach and with prospective studies.
Herein, we provide a contextual framework for evaluating toxicities of novel CAR T cell constructs, as well as an overview of strategies currently used to optimize responses and assess other long-term outcomes of CAR T cell therapy.
Longitudinal neurocognitive evaluations, established time points for and interpretation of neuroimaging, and development of biomarkers for assessment of neurotoxicity risk and quantification of neurological injury across CAR T cell trials will be needed to further optimize neurological outcomes.
Comprehensive care models that include the patient, family, referring and primary teams should be embedded into all CAR T cell therapy programmes to provide comfort, address expectations, and understand psychosocial risk factors and educational needs.
CAR T cell recipients are at high risk for infection owing to a host of factors and developing optimal guidelines for infection prevention during both acute CRS and longitudinally is necessary to optimize outcomes. Monitoring immune reconstitution after treatment will help to identify future risks of infection and how vaccination strategies might be effective in this population.
Extending the durability of remission following CAR T cell treatment remains a primary goal, for which the study of late effects remains imperative. The results of ongoing studies of a host of strategies will inform the role for remission consolidation and risk stratification to identify patients at highest risk of relapse.
Acknowledgements
The initial efforts from the CAR T cell Therapy Beyond the Storm Consortium were presented on 14 May 2020 in a conference jointly sponsored by the National Cancer Institute (NCI), and the Pediatric Transplantation and Cell Therapy Consortium (PTCTC). The authors would like to acknowledge all the speakers and the >200 international participants. The audio recording from the conference can be found on the conference website. The authors would like to acknowledge the NCI and the PTCTC for their support, and specifically Dr L. Schultz for sharing her efforts on CAR T cell consortia studies. PTCTC receives support through a Johnny Crisstopher Children’s Charitable Foundation St. Baldrick’s Consortium Grant and the NHLBI/NCI grant (2UG1HL069254). The work of N.N.S. is supported in part by the National Institute of Neurological Disorders and Stroke Child Neurology Career Development Program K12 (1K12NS098482-02) (Gust), and the Intramural Research Program of the NCI and NIH Clinical Center (ZIA BC 011823).
Footnotes
Competing interests
J.G. is a consultant for Johnson & Johnson. T.W.L. has consulted for Bayer, Cellectis and Novartis; and his institution has research funding from Bayer, Novartis and Pfizer. R.A.G. has received honoraria from Novartis. J.A.H. has consulted for Allogene Therapeutics. K.J.C. has received research support from Juno Therapeutics and Novartis; has consulted and participated in advisory boards or educational seminars for Juno Therapeutics, Mesoblast and Novartis. M.C.P. has received research support from BMS, Kite and Novartis, and has been a consultant for Amgen and BMS. S.A.G. receives research support from Kite, Novartis and Servier; is a consultant for CBMG, GSK, Humanigen, Janssen/Johnson & Johnson, Novartis and Roche; is a study steering committee or scientific advisory board member for Adaptimmune, Allogene, Cellectis, Jazz, Juno, Novartis, TCR2 and Vertex/CRISPR; and has a patent (WO 2014011984 A1) that is managed according to the University of Pennsylvania patent policy. M.A.P. has received fees from Novartis, grants and personal fees from Miltenyi, grants from Adaptive, and personal fees from Mesoblast. H.S., A.T., A.B.L., C.S., P.L.W., L.W., V.N., J.K., C.N.D. and N.N.S. declare no competing interests.
Disclaimer
The content of this publication does not necessarily reflect the views and policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Peer review information
Nature Reviews Clinical Oncology thanks J. Gauthier, N. Kapoor, R. Phelan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Related links
CAR T-Cell Therapy: Beyond The Storm conference: https://ncifrederick.cancer.gov/events/conferences/car-t-cell-therapy-beyond-storm
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41571-020-00456-y
References
- 1.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 5.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Administration USFaD. FDA approves brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma; 2020. [Google Scholar]
- 7.Tragedy Rosenbaum L., Perseverance, and Chance - The Story of CAR-T Therapy. N Engl J Med. 2017;377(14):1313–1315. [DOI] [PubMed] [Google Scholar]
- 8.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. [DOI] [PubMed] [Google Scholar]
- 10.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strati P, Ahmed S, Kebriaei P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4(13):3123–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J Clin Oncol. 2020;38(17):1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner RA, Ceppi F, Rivers J, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. 2019;134(24):2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perales MA, Kebriaei P, Kean LS, Sadelain M. Building a Safer and Faster CAR: Seatbelts, Airbags, and CRISPR. Biol Blood Marrow Transplant. 2018;24(1):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber EW, Lynn RC, Sotillo E, Lattin J, Xu P, Mackall CL. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv. 2019;3(5):711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teachey DT, Lacey SF, Shaw PA, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016;6(6):664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DW, Shah NN. Chimeric Antigen Receptor T-Cell Therapies for Cancer E-Book: A Practical Guide: Elsevier Health Sciences; 2019. [Google Scholar]
- 18.Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity Associated with CD19-Targeted CAR-T Cell Therapies. CNS Drugs. 2018;32(12):1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gust J, Hay KA, Hanafi LA, et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017;7(12):1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med. 2017;377(26):2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torre M, Solomon IH, Sutherland CL, et al. Neuropathology of a Case With Fatal CAR T-Cell-Associated Cerebral Edema. J Neuropathol Exp Neurol. 2018;77(10):877–882. [DOI] [PubMed] [Google Scholar]
- 25.Santomasso BD, Park JH, Salloum D, et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018;8(8):958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gofshteyn JS, Shaw PA, Teachey DT, et al. Neurotoxicity after CTL019 in a pediatric and young adult cohort. Ann Neurol. 2018;84(4):537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman H, Leahy AEB, Li Y, et al. CD19-targeted chimeric antigen receptor (CAR) T cells in CNS relapsed acute lymphoblastic leukemia (ALL). Journal of Clinical Oncology. 2020;38(15_suppl):10511–10511. [Google Scholar]
- 28.Teachey DT, Bishop MR, Maloney DG, Grupp SA. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘ALL’. Nat Rev Clin Oncol. 2018;15(4):218. [DOI] [PubMed] [Google Scholar]
- 29.Gust J, Finney OC, Li D, et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann Neurol. 2019;86(1):42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shalabi H, Wolters PL, Martin S, et al. Systematic Evaluation of Neurotoxicity in Children and Young Adults Undergoing CD22 Chimeric Antigen Receptor T-Cell Therapy. J Immunother. 2018;41(7):350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruark J, Mullane E, Cleary N, et al. Patient-Reported Neuropsychiatric Outcomes of Long-Term Survivors after Chimeric Antigen Receptor T Cell Therapy. Biol Blood Marrow Transplant. 2020;26(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung YT, Sabin ND, Reddick WE, et al. Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy: a longitudinal analysis. Lancet Haematol. 2016;3(10):e456–e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung YT, Krull KR. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: A systematic review. Neurosci Biobehav Rev. 2015;53:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulhern RK, Butler RW. Neurocognitive sequelae of childhood cancers and their treatment. Pediatr Rehabil. 2004;7(1):1–14; discussion 15–16. [DOI] [PubMed] [Google Scholar]
- 35.Mahadeo KM, Khazal SJ, Abdel-Azim H, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol. 2019;16(1):45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577–589. [DOI] [PubMed] [Google Scholar]
- 38.Steineck A, Wiener L, Mack JW, Shah NN, Summers C, Rosenberg AR. Psychosocial care for children receiving chimeric antigen receptor (CAR) T-cell therapy. Pediatr Blood Cancer. 2020;67(5):e28249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun W, Malvar J, Sposto R, et al. Outcome of children with multiply relapsed B-cell acute lymphoblastic leukemia: a therapeutic advances in childhood leukemia & lymphoma study. Leukemia. 2018;32(11):2316–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callahan C, Barry A, Fooks-Parker S, Smith L, Baniewicz D, Hobbie W. Pediatric Survivorship: Considerations Following CAR T-Cell Therapy. Clin J Oncol Nurs. 2019;23(2):35–41. [DOI] [PubMed] [Google Scholar]
- 41.Leahy AB, Feudtner C, Basch E. Symptom Monitoring in Pediatric Oncology Using Patient-Reported Outcomes: Why, How, and Where Next. Patient. 2018;11(2):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kluetz PG, O’Connor DJ, Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol. 2018;19(5):e267–e274. [DOI] [PubMed] [Google Scholar]
- 43.Kirch R, Reaman G, Feudtner C, et al. Advancing a comprehensive cancer care agenda for children and their families: Institute of Medicine Workshop highlights and next steps. CA Cancer J Clin. 2016;66(5):398–407. [DOI] [PubMed] [Google Scholar]
- 44.Kazak AE, Abrams AN, Banks J, et al. Psychosocial Assessment as a Standard of Care in Pediatric Cancer. Pediatr Blood Cancer. 2015;62 Suppl 5:S426–459. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Singh H, Ayalew K, et al. Use of PRO Measures to Inform Tolerability in Oncology Trials: Implications for Clinical Review, IND Safety Reporting, and Clinical Site Inspections. Clin Cancer Res. 2018;24(8):1780–1784. [DOI] [PubMed] [Google Scholar]
- 46.Schepers SA, Haverman L, Zadeh S, Grootenhuis MA, Wiener L. Healthcare Professionals’ Preferences and Perceived Barriers for Routine Assessment of Patient-Reported Outcomes in Pediatric Oncology Practice: Moving Toward International Processes of Change. Pediatr Blood Cancer. 2016;63(12):2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yen HJ, Eissa HM, Bhatt NS, et al. Patient-reported outcomes in survivors of childhood hematologic malignancies with hematopoietic stem cell transplant. Blood. 2020;135(21):1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maziarz RT, Waller EK, Jaeger U, et al. Patient-reported long-term quality of life after tisagenlecleucel in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(4):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sidana S, Thanarajasingam G, Griffin J, et al. Patient Experience of Chimeric Antigen Receptor (CAR)-T Cell Therapy Vs. Stem Cell Transplant: Longitudinal Patient Reported Adverse Events, Cognition and Quality of Life. Blood. 2019;134(Supplement_1):794–794. [Google Scholar]
- 50.Laetsch TW, Myers GD, Baruchel A, et al. Patient-reported quality of life after tisagenlecleucel infusion in children and young adults with relapsed or refractory B-cell acute lymphoblastic leukaemia: a global, single-arm, phase 2 trial. Lancet Oncol. 2019;20(12):1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chakraborty R, Sidana S, Shah GL, Scordo M, Hamilton BK, Majhail NS. Patient-Reported Outcomes with Chimeric Antigen Receptor T Cell Therapy: Challenges and Opportunities. Biol Blood Marrow Transplant. 2019;25(5):e155–e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Services CfMM. MEDCAC Meeting 8/22/2018 - Chimeric Antigen Receptor (CAR) T-Cell Therapy and Patient Reported Outcomes. Chimeric Antigen Receptor (CAR) T-Cell Therapy and Patient Reported Outcomes; 2018. [Google Scholar]
- 53.Pinheiro LC, McFatrich M, Lucas N, et al. Child and adolescent self-report symptom measurement in pediatric oncology research: a systematic literature review. Qual Life Res. 2018;27(2):291–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vora SB, Waghmare A, Englund JA, Qu P, Gardner RA, Hill JA. Infectious Complications Following CD19 Chimeric Antigen Receptor T-cell Therapy for Children, Adolescents, and Young Adults. Open Forum Infect Dis. 2020;7(5):ofaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park JH, Romero FA, Taur Y, et al. Cytokine Release Syndrome Grade as a Predictive Marker for Infections in Patients With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia Treated With Chimeric Antigen Receptor T Cells. Clin Infect Dis. 2018;67(4):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahmoudjafari Z, Hawks KG, Hsieh AA, Plesca D, Gatwood KS, Culos KA. American Society for Blood and Marrow Transplantation Pharmacy Special Interest Group Survey on Chimeric Antigen Receptor T Cell Therapy Administrative, Logistic, and Toxicity Management Practices in the United States. Biol Blood Marrow Transplant. 2019;25(1):26–33. [DOI] [PubMed] [Google Scholar]
- 58.Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late Events after Treatment with CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol Blood Marrow Transplant. 2020;26(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54(10):1643–1650. [DOI] [PubMed] [Google Scholar]
- 61.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kochenderfer JN, Somerville RPT, Lu T, et al. Long-Duration Complete Remissions of Diffuse Large B Cell Lymphoma after Anti-CD19 Chimeric Antigen Receptor T Cell Therapy. Mol Ther. 2017;25(10):2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhoj VG, Arhontoulis D, Wertheim G, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood. 2016;128(3):360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hill JA, Krantz EM, Hay KA, et al. Durable preservation of antiviral antibodies after CD19-directed chimeric antigen receptor T-cell immunotherapy. Blood Adv. 2019;3(22):3590–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deya-Martinez A, Alonso-Saladrigues A, Garcia AP, et al. Kinetics of humoral deficiency in CART19-treated children and young adults with acute lymphoblastic leukaemia. Bone Marrow Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arnold DE, Maude SL, Callahan CA, DiNofia AM, Grupp SA, Heimall JR. Subcutaneous immunoglobulin replacement following CD19-specific chimeric antigen receptor T-cell therapy for B-cell acute lymphoblastic leukemia in pediatric patients. Pediatr Blood Cancer. 2020;67(3):e28092. [DOI] [PubMed] [Google Scholar]
- 67.US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04410900 (2020). [DOI] [PubMed]
- 68.Hill JA, Seo S. How we prevent infections in patients receiving CD19-targeted chimeric antigen receptor T-cells for B-cell malignancies. Blood. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandler RD, Tattersall RS, Schoemans H, et al. Diagnosis and Management of Secondary HLH/MAS Following HSCT and CAR-T Cell Therapy in Adults; A Review of the Literature and a Survey of Practice Within EBMT Centres on Behalf of the Autoimmune Diseases Working Party (ADWP) and Transplant Complications Working Party (TCWP). Front Immunol. 2020;11:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hashmi H, Bachmeier C, Chavez JC, et al. Haemophagocytic lymphohistiocytosis has variable time to onset following CD19 chimeric antigen receptor T cell therapy. Br J Haematol. 2019;187(2):e35–e38. [DOI] [PubMed] [Google Scholar]
- 72.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 73.Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4(15):3776–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strati P, Varma A, Adkins S, et al. Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large B-cell lymphoma. Haematologica. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng X, Scheinberg P, Wu CO, et al. Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2011;96(4):602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morales-Mantilla DE, King KY. The Role of Interferon-Gamma in Hematopoietic Stem Cell Development, Homeostasis, and Disease. Curr Stem Cell Rep. 2018;4(3):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang S, Degar BA, Zieske A, Shafi NQ, Rose MG. Hemophagocytosis exacerbated by G-CSF/GM-CSF treatment in a patient with myelodysplasia. Am J Hematol. 2004;77(4):391–396. [DOI] [PubMed] [Google Scholar]
- 78.Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med. 2009;60:193–206. [DOI] [PubMed] [Google Scholar]
- 79.Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133(7):697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haen SP, Schumm M, Faul C, Kanz L, Bethge WA, Vogel W. Poor graft function can be durably and safely improved by CD34+-selected stem cell boosts after allogeneic unrelated matched or mismatched hematopoietic cell transplantation. J Cancer Res Clin Oncol. 2015;141(12):2241–2251. [DOI] [PubMed] [Google Scholar]
- 81.Mainardi C, Ebinger M, Enkel S, et al. CD34(+) selected stem cell boosts can improve poor graft function after paediatric allogeneic stem cell transplantation. Br J Haematol. 2018;180(1):90–99. [DOI] [PubMed] [Google Scholar]
- 82.Guha A, Addison D, Jain P, et al. Cardiovascular Events Associated with Chimeric Antigen Receptor T Cell Therapy: Cross-Sectional FDA Adverse Events Reporting System Analysis. Biol Blood Marrow Transplant. 2020. [DOI] [PubMed] [Google Scholar]
- 83.Burstein DS, Maude S, Grupp S, Griffis H, Rossano J, Lin K. Cardiac Profile of Chimeric Antigen Receptor T Cell Therapy in Children: A Single-Institution Experience. Biol Blood Marrow Transplant. 2018;24(8):1590–1595. [DOI] [PubMed] [Google Scholar]
- 84.Ganatra S, Carver JR, Hayek SS, et al. Chimeric Antigen Receptor T-Cell Therapy for Cancer and Heart: JACC Council Perspectives. J Am Coll Cardiol. 2019;74(25):3153–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J Am Coll Cardiol. 2019;74(25):3099–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shalabi H, Sachdev V, Kulshreshtha A, et al. Impact of Cytokine Release Syndrome on Cardiac Function Following CD19 CAR-T Cell Therapy in Children and Young Adults with Hematologic Malignancies. Journal for ImmunoTherapy of Cancer. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yates B, Shalabi H, Civelek AC, Delbrook C, Fry TJ, Shah NN. Efficacy and Kinetics of CAR-T Cell Therapy in Children and Young Adults with Extramedullary Acute Lymphoblastic Leukemia (ALL) and Non-Hodgkin Lymphoma (NHL) [Abstract]. Biology of Blood and Marrow Transplantation. 2018;Vol. 24(3). [Google Scholar]
- 88.Gutierrez C, Brown ART, Herr MM, et al. The chimeric antigen receptor-intensive care unit (CAR-ICU) initiative: Surveying intensive care unit practices in the management of CAR T-cell associated toxicities. J Crit Care. 2020;58:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mumtaz AA, Li A. Ocular Toxicity and Mangement of Chimeric Antigen Receptor T-Cell Therapy. Investigative Ophthalmology & Visual Science. 2020;61(7):5178–5178. [Google Scholar]
- 90.Sharma T, Grewal J, Gupta S, Murray PI. Ophthalmic manifestations of acute leukaemias: the ophthalmologist’s role. Eye (Lond). 2004;18(7):663–672. [DOI] [PubMed] [Google Scholar]
- 91.Denton CC, Gange WS, Abdel-Azim H, et al. Bilateral retinal detachment after chimeric antigen receptor T-cell therapy. Blood Adv. 2020;4(10):2158–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fischer A, Li A. CAR T-Cell Therapy Associated Ocular Adverse Events Reported to the Food and Drug Administration. Investigative Ophthalmology & Visual Science. 2020;61(7):3636–3636. [Google Scholar]
- 93.Jhaveri KD, Rosner MH. Chimeric Antigen Receptor T Cell Therapy and the Kidney: What the Nephrologist Needs to Know. Clin J Am Soc Nephrol. 2018;13(5):796–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupta S, Seethapathy H, Strohbehn IA, et al. Acute Kidney Injury and Electrolyte Abnormalities After Chimeric Antigen Receptor T-Cell (CAR-T) Therapy for Diffuse Large B-Cell Lymphoma. Am J Kidney Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gutgarts V, Jain T, Zheng J, et al. Acute Kidney Injury after CAR-T Cell Therapy: Low Incidence and Rapid Recovery. Biol Blood Marrow Transplant. 2020;26(6):1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wudhikarn K, Pennisi M, Garcia-Recio M, et al. DLBCL patients treated with CD19 CAR T cells experience a high burden of organ toxicities but low nonrelapse mortality. Blood Adv. 2020;4(13):3024–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shah NN, Qin H, Yates B, et al. Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy. Blood Adv. 2019;3(15):2317–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cornetta K, Duffy L, Turtle CJ, et al. Absence of Replication-Competent Lentivirus in the Clinic: Analysis of Infused T Cell Products. Mol Ther. 2018;26(1):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cornetta K, Duffy L, Feldman SA, et al. Screening Clinical Cell Products for Replication Competent Retrovirus: The National Gene Vector Biorepository Experience. Mol Ther Methods Clin Dev. 2018;10:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.FDA-2018-D-2173) CfBEaRDN. Long Term Follow-up After Administration of Human Gene Therapy Products; 2020. [Google Scholar]
- 101.Britten O, Ragusa D, Tosi S, Kamel YM. MLL-Rearranged Acute Leukemia with t(4;11)(q21;q23)-Current Treatment Options. Is There a Role for CAR-T Cell Therapy? Cells. 2019;8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127(20):2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wolfl M, Rasche M, Eyrich M, Schmid R, Reinhardt D, Schlegel PG. Spontaneous reversion of a lineage switch following an initial blinatumomab-induced ALL-to-AML switch in MLL-rearranged infant ALL. Blood Adv. 2018;2(12):1382–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rayes A, McMasters RL, O’Brien MM. Lineage Switch in MLL-Rearranged Infant Leukemia Following CD19-Directed Therapy. Pediatr Blood Cancer. 2016;63(6):1113–1115. [DOI] [PubMed] [Google Scholar]
- 105.Haddox CL, Mangaonkar AA, Chen D, et al. Blinatumomab-induced lineage switch of B-ALL with t(4:11)(q21;q23) KMT2A/AFF1 into an aggressive AML: pre- and post-switch phenotypic, cytogenetic and molecular analysis. Blood Cancer J. 2017;7(9):e607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oberley MJ, Gaynon PS, Bhojwani D, et al. Myeloid lineage switch following chimeric antigen receptor T-cell therapy in a patient with TCF3-ZNF384 fusion-positive B-lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65(9):e27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zoghbi A, Zur Stadt U, Winkler B, Muller I, Escherich G. Lineage switch under blinatumomab treatment of relapsed common acute lymphoblastic leukemia without MLL rearrangement. Pediatr Blood Cancer. 2017;64(11). [DOI] [PubMed] [Google Scholar]
- 108.Schmiegelow K, Levinsen MF, Attarbaschi A, et al. Second malignant neoplasms after treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31(19):2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aguilera DG, Vaklavas C, Tsimberidou AM, Wen S, Medeiros LJ, Corey SJ. Pediatric therapy-related myelodysplastic syndrome/acute myeloid leukemia: the MD Anderson Cancer Center experience. J Pediatr Hematol Oncol. 2009;31(11):803–811. [DOI] [PubMed] [Google Scholar]
- 110.Shouse GP, Xue T, Herrera A, et al. MDS AS A CAUSE FOR PROLONGED HEMATOLOGIC TOXICITY AFTER TREATMENT WITH CD19 TARGETED CAR-T CELL THERAPY IN PATIENTS WITH RELAPSED REFRACTORY LYMPHOMA. Hematological Oncology. 2019;37(S2):507–508. [Google Scholar]
- 111.Good ML, Malekzadeh P, Kriley IR, et al. Intrahepatic cholangiocarcinoma as a rare secondary malignancy after allogeneic hematopoietic stem cell transplantation for childhood acute lymphoblastic leukemia: A case report. Pediatr Transplant. 2020;24(2):e13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tichelli A, Beohou E, Labopin M, et al. Evaluation of Second Solid Cancers After Hematopoietic Stem Cell Transplantation in European Patients. JAMA Oncol. 2019;5(2):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Inamoto Y, Shah NN, Savani BN, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50(8):1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125(26):4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee DW, Stetler-Stevenson M, Yuan CM, et al. Long-Term Outcomes Following CD19 CAR T Cell Therapy for B-ALL Are Superior in Patients Receiving a Fludarabine/Cyclophosphamide Preparative Regimen and Post-CAR Hematopoietic Stem Cell Transplantation. Blood. 2016;128(22). [Google Scholar]
- 116.Summers C, Annesley C, Bleakley M, Dahlberg A, Jensen MC, Gardner R. Long Term Follow-up after SCRI-CAR19v1 Reveals Late Recurrences As Well As a Survival Advantage to Consolidation with HCT after CAR T Cell Induced Remission. Blood. 2018;132. [Google Scholar]
- 117.Asnani M, Hayer KE, Naqvi AS, et al. Retention of CD19 intron 2 contributes to CART-19 resistance in leukemias with subclonal frameshift mutations in CD19. Leukemia. 2020;34(4):1202–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bagashev A, Sotillo E, Tang CH, et al. CD19 Alterations Emerging after CD19-Directed Immunotherapy Cause Retention of the Misfolded Protein in the Endoplasmic Reticulum. Mol Cell Biol. 2018;38(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sotillo E, Barrett DM, Black KL, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015;5(12):1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24(10):1504–1506. [DOI] [PubMed] [Google Scholar]
- 121.Schultz LM, Davis KL, Baggott C, et al. 1 Study of CD19/CD22 Bispecific Chimeric Antigen Receptor (CAR) Therapy in Children and Young Adults with B Cell Acute Lymphoblastic Leukemia (ALL). Blood. 2018;132. [Google Scholar]
- 122.Gardner R, Annesley C, Finney O, et al. Early Clinical Experience of CD19 x CD22 Dual Specific CAR T Cells for Enhanced Anti-Leukemic Targeting of Acute Lymphoblastic Leukemia. Blood. 2018;132. [Google Scholar]
- 123.Amrolia PJ, Wynn R, Hough R, et al. Simultaneous Targeting of CD19 and CD22: Phase I Study of AUTO3, a Bicistronic Chimeric Antigen Receptor (CAR) T-Cell Therapy, in Pediatric Patients with Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia (r/r B-ALL): Amelia Study. Blood. 2018;132. [Google Scholar]
- 124.Ruella M, Barrett DM, Kenderian SS, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126(10):3814–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qin H, Dong Z, Wang X, et al. CAR T cells targeting BAFF-R can overcome CD19 antigen loss in B cell malignancies. Sci Transl Med. 2019;11(511). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qin H, Cho M, Haso W, et al. Eradication of B-ALL using chimeric antigen receptor-expressing T cells targeting the TSLPR oncoprotein. Blood. 2015;126(5):629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frey NV, Shaw PA, Hexner EO, et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia. J Clin Oncol. 2020;38(5):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hay KA, Gauthier J, Hirayama AV, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Park JH, Riviere I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee DW III, Stetler-Stevenson M, Yuan CM, et al. Long-Term Outcomes Following CD19 CAR T Cell Therapy for B-ALL Are Superior in Patients Receiving a Fludarabine/Cyclophosphamide Preparative Regimen and Post-CAR Hematopoietic Stem Cell Transplantation. Blood. 2016;128(22):218–218. [Google Scholar]
- 131.Pulsipher MA, Han X, Quigley M, et al. Potential utility of minimal residual disease (MRD) to identify relapse in pediatric and young adult (AYA) B-cell acute lymphoblastic leukemia (B-ALL) patients treated with tisagenlecleucel. Cancer Research. 2019;79(13). [Google Scholar]
- 132.US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03186118 (2020). [DOI] [PubMed] [Google Scholar]
- 133.Annesley C, Gardner R, Wilson A, et al. Novel CD19t T-Antigen Presenting Cells Expand CD19 CAR T Cells In Vivo. Blood. 2019;134. [Google Scholar]
- 134.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT2374333 (2019). [DOI] [PubMed]
- 136.Maude SL, Hucks GE, Callahan C, et al. Durable Remissions with Humanized CD19-Targeted Chimeric Antigen Receptor (CAR)-Modified T Cells in CAR-Naive and CAR-Exposed Children and Young Adults with Relapsed/Refractory Acute Lymphoblastic Leukemia. Blood. 2017;130. [Google Scholar]
- 137.Li AM, Hucks GE, Dinofia AM, et al. Checkpoint Inhibitors Augment CD19-Directed Chimeric Antigen Receptor (CAR) T Cell Therapy in Relapsed B-Cell Acute Lymphoblastic Leukemia. Blood. 2018;132. [Google Scholar]
- 138.Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134(26):2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yoon DH, Osborn MJ, Tolar J, Kim CJ. Incorporation of Immune Checkpoint Blockade into Chimeric Antigen Receptor T Cells (CAR-Ts): Combination or Built-In CAR-T. Int J Mol Sci. 2018;19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li AM, Hucks GE, Dinofia AM, et al. Checkpoint Inhibitors Augment CD19-Directed Chimeric Antigen Receptor (CAR) T Cell Therapy in Relapsed B-Cell Acute Lymphoblastic Leukemia. Blood. 2018;132(Supplement 1):556–556. [Google Scholar]
- 141.Chong EA, Melenhorst JJ, Lacey SF, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129(8):1039–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hill BT, Roberts ZJ, Rossi JM, Smith MR. Marked Re-Expansion of Chimeric Antigen Receptor (CAR) T Cells and Tumor Regression Following Nivolumab Treatment in a Patient Treated with Axicabtagene Ciloleucel (axi-cel; KTE-C19) for Refractory Diffuse Large B Cell Lymphoma (DLBCL). Blood. 2017;130(Supplement 1):2825–2825. [Google Scholar]
- 143.Qu C, Ping N, Kang L, et al. Radiation Priming Chimeric Antigen Receptor T-Cell Therapy in Relapsed/Refractory Diffuse Large B-Cell Lymphoma With High Tumor Burden. J Immunother. 2020;43(1):32–37. [DOI] [PubMed] [Google Scholar]
- 144.DeSelm C, Palomba ML, Yahalom J, et al. Low-Dose Radiation Conditioning Enables CAR T Cells to Mitigate Antigen Escape. Mol Ther. 2018;26(11):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wright CM, LaRiviere MJ, Baron JA, et al. Bridging Radiation Therapy Before Commercial Chimeric Antigen Receptor T-Cell Therapy for Relapsed or Refractory Aggressive B-Cell Lymphoma. Int J Radiat Oncol Biol Phys. 2020. [DOI] [PubMed] [Google Scholar]
- 146.Sim AJ, Jain MD, Figura NB, et al. Radiation Therapy as a Bridging Strategy for CAR T Cell Therapy With Axicabtagene Ciloleucel in Diffuse Large B-Cell Lymphoma. Int J Radiat Oncol Biol Phys. 2019;105(5):1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Imber BS, Sadelain M, DeSelm C, et al. Early experience using salvage radiotherapy for relapsed/refractory non-Hodgkin lymphomas after CD19 chimeric antigen receptor (CAR) T cell therapy. Br J Haematol. 2020;190(1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.