Abstract
Recent studies defined a potentially important role of the microbiome in modulating pancreatic ductal adenocarcinoma (PDAC) and responses to therapies. We hypothesized that antibiotic usage may predict outcomes in patients with PDAC.
We retrospectively analyzed clinical data of patients with resectable or metastatic PDAC seen at MD Anderson Cancer from 2003 to 2017. Demographic, chemotherapy regimen and antibiotic use, duration, type, and reason for indication were recorded.
A total of 580 patients with PDAC were studied, 342 resected and 238 metastatic patients, selected retrospectively from our database. Antibiotic use, for longer than 48 hrs, was detected in 209 resected patients (61%) and 195 metastatic ones (62%). On resectable patients, we did not find differences in overall survival (OS) or progression‐free survival (PFS), based on antibiotic intake. However, in the metastatic cohort, antibiotic consumption was associated with a significantly longer OS (13.3 months vs. 9.0 months, HR 0.48, 95% CI 0.34–0.7, p = 0.0001) and PFS (4.4 months vs. 2 months, HR 0.48, 95% CI 0.34–0.68, p = <0.0001). In multivariate analysis, the impact of ATB remained significant for PFS (HR 0.59, p = 0.005) and borderline statistically significant for OS (HR 0.69, p = 0.06). When we analyzed by chemotherapy regimen, we found that patients who received gemcitabine‐based chemotherapy as first‐line therapy (n = 118) had significantly prolonged OS (HR 0.4, p 0.0013) and PFS (HR 0.55, p 0.02) if they received antibiotics, while those receiving 5FU‐based chemotherapy (n = 98) had only prolonged PFS (HR 0.54, p = 0.03).
Antibiotics‐associated modulation of the microbiome is associated with better outcomes in patients with metastatic PDAC.
Keywords: antibiotics, autophagy, chemotherapeutic agents, immunity, microbiota, pancreatic adenocarcinoma
Short abstract
We have analyzed the effect of antibiotics’ intake on two cohorts of patients with pancreatic adenocarcinoma, resectable, and metastatic. We have found that on the metastatic cohort, antibiotics use was significantly associated with better outcomes, particularly, on patients that received gemcitabine based‐chemotherapy as the first line.
1. INTRODUCTION
It is estimated that in 2020, there will be 57,600 new cases of pancreatic adenocarcinoma (PDAC), and approximately 47,050 deaths. The 5‐year survival rate of PDAC is 9.3% making it is the third most common cause of cancer‐related death in the United States. 1 The dismal prognosis has been attributed to the lack of early detection, aggressive biology, and the absence of effective therapies. Recent studies have shown that PDAC‐associated gut and tumor microbiome can play a significant role in disease progression and responses to therapy in preclinical models. 2 , 3 Bacterial diminution via antibiotic administration in PDAC orthotopic mouse models altered the tumor microenvironment, resulting in higher T cell activation and ultimately improved immunosurveillance and increased sensitivity to immunotherapy. 2 , 4
The presence of specific microbes within tumors is linked to resistance to gemcitabine as a consequence of the metabolism of this chemotherapeutic agent. 5 One of those microbes, gammaproteobacteria is highly prevalent in human PDAC. Recent studies from our group have further identified a specific microbial signature in tumors from PDAC long‐term survivors. 6 We found that higher tumor alpha‐diversity correlated with better outcomes. 6 One could expect that changes in the microbiome by the administration of antibiotics could result in differential outcomes.
Antibiotics can alter the gut microbiota diversity and composition, leading to modified responses to chemotherapeutic agents and immunotherapy regimens. 5 , 7 , 8 , 9 There have been several recent studies examining the effect of antibiotic use in patients with solid tumors on immune checkpoint inhibitor treatment. Through retrospective studies as well as meta‐analyses, some studies revealed that antibiotics intake during the period of immunotherapy initiation was associated with worse overall and progression‐free survival in several tumor types including renal cell carcinoma, non‐small cell lung cancer, melanoma, sarcoma, hepatocellular carcinoma, urothelial carcinoma, and GI stromal tumors. 8 , 10 , 11 , 12 , 13 , 14 In metastatic colorectal cancer patients, antibiotic use prior to starting 5FU‐based chemotherapy was associated with worse OS and PFS. 15 In contrast, antibiotic use in metastatic colorectal cancer patients treated with bevacizumab is associated with decreased mortality. 16
Antibiotics have been linked to lower efficacy of immunotherapy in several solid tumors, but none of these studies specifically examined PDAC. 7 , 8 , 9 While most studies suggest that lower alpha‐diversity of the gut microbiome is linked to worse responses to immunotherapy, preclinical studies in PDAC have suggested a potential positive role of antibiotics in disease progression and therapy responses. 2 , 3 , 5 Systematic assessment of the potential value of antibiotic administration in patients with pancreatic cancer remains to be performed. In this retrospective study, we evaluated the effect of antibiotics on outcomes in patients with resected and metastatic pancreatic cancer.
2. METHODS
2.1. Patients and antibiotic use
We studied 580 patients with evaluable chemotherapy data belonging to the following two groups: (1) Patients with localized/borderline disease that underwent resection (n = 342) and (2) Patients with metastatic disease (n = 238). Patients with resectable or metastatic disease diagnosed and/or treated at MD Anderson Cancer Center between 2003–2015 and 2009–2017, respectively, were included in the analysis.
We reviewed patients’ medical records and collected data on patient demographics, antibiotic use, duration, class and indication, disease and treatment characteristics, and survival data including progression, death, and last follow‐up dates. Antibiotic use was assessed from diagnosis to death or the date of the last follow‐up for metastatic cohort, and diagnosis to surgical resection for the resected cohort. All patients were followed up until death or data lock (February 2019 for both cohorts). Antibiotic use was defined as any use of antibiotics for longer than 48 hrs, which excludes the usual one‐time antibiotic given before or during procedure or surgery. Information regarding the class of the antibiotics and indication of use were also provided. This study was approved by the institutional review board.
2.2. Statistical analysis
Patients characteristics were described according to antibiotics intake and compared using Fisher or chi‐squared test for categorical data and Wilcoxon rank‐sum test for continuous data. Frequencies and percentages were reported for categorical variables. Summary statistics such as median, minimum, and maximum were provided for continuous data (such as age). Overall survival (OS) was defined as the time from diagnosis to death from any cause. Patients were censored to the date of the last follow‐up. Progression‐free survival (PFS) was defined as the time from surgery to progression or death, whichever occurred first, in the resected patients; and as the time from first‐line chemotherapy to progression or death, whichever occurred first, in the metastatic group.
Univariate and multivariable Cox proportional hazard models were used to determine the effects of potential risk factors and antibiotic status variables on OS and PFS. Clinically and statistically important variables were included in the multivariable models. Hazard ratios and 95% confidence intervals were provided. Kaplan–Meier curves were estimated for the survival distributions by antibiotic status. The Log‐rank test was used to test the difference in survival distributions between treatment groups. All tests were two‐sided. P‐values less than 0.05 are considered statistically significant. All analyses were conducted using SAS 9.4 (SAS, Cary, NC) and S‐Plus 8.0 (TIBCO Software Inc., Palo Alto, CA) software.
3. RESULTS
3.1. Demographical and clinical characteristics
A total of 580 patients were studied: 342 patients with resected PDAC and 238 patients with metastatic PDAC. All patients were seen between 2003 and 2017 at a single institution: University of Texas MD Anderson Cancer Center.
3.1.1. Resected Cohort
The median age was 64 years (range of 34–85 years), including 147 (43%) females and 195 (57%) males. A total of 284 (83%) patients in the cohort were White followed by 33 (10%) Hispanic, 10 (3%) Black, 8 (2%) Asian, and 7 (2%) from other racial backgrounds. Most patients had PDAC Stage IIB (n = 185, 54%), followed by IIA (n = 111, 32%), Stage 0/I (n = 37,11%), and 9 (3%) patients were found to be stage III/IV during surgical exploration (Table 1).
TABLE 1.
Baseline characteristics of both resectable and metastatic pancreatic ductal adenocarcinoma cohorts
| Resectable Cohort | ||||
|---|---|---|---|---|
| Total (n = 342) | Antibiotics (n = 209) | No antibiotics (n = 133) | p Value | |
| Age at diagnosis‐median (range) | 64 (34–85) | 65 (38 – 85) | 62 (34 – 83) | 0.0085 |
| Gender, n (%) | ||||
| Female | 147 (43%) | 88 (42%) | 59 (44%) | 0.6812 |
| Male | 195 (57%) | 121 (58%) | 74 (56%) | |
| Race, n (%) | ||||
| White | 284 (83%) | 177 (85%) | 107 (80%) | 0.7992 |
| Black | 10 (3%) | 6 (3%) | 4 (3%) | |
| Hispanic | 33 (10%) | 17 (8%) | 16 (13%) | |
| Asian | 8 (2%) | 5 (2%) | 3(2%) | |
| Others | 7 (2%) | 4 (2%) | 3(2%) | |
| Stage, n (%) | ||||
| 0/I | 37 (11%) | 17 (8%) | 20 (15%) | 0.2519 |
| IIA | 111 (32%) | 69 (33%) | 42 (32%) | |
| IIB | 185 (54%) | 117 (56%) | 68 (51%) | |
| III/IV | 9 (3%) | 6 (3%) | 3 (2%) | |
| Type of Chemotherapy, n (%) | ||||
| 5FU Based | 44 (13%) | 34 (16%) | 10 (8%) | 0.0533 |
| Gemcitabine | 206 (60%) | 118 (56%) | 88 (66%) | |
| Gemcitabine and 5FU | 7 (2%) | 3 (2%) | 4 (3%) | |
| No chemotherapy | 85 (25%) | 54 (26%) | 31 (23%) | |
| Metastatic Cohort | ||||
| Total (n = 238) | Antibiotics (n = 195) | No antibiotics (n = 43) | p Value | |
| Age at Diagnosis‐Median (range) | 64 (25–84) | 62 (25–84) | 65 (46–83) | 0.31 |
| Gender, n (%) | ||||
| Female | 117 (49%) | 98 (50%) | 19 (44%) | 0.47 |
| Male | 121 (51%) | 97 (50%) | 24 (56%) | |
| Race, n (%) | ||||
| White | 176 (74%) | 140 (72%) | 36 (85%) | 0.73 |
| Black | 22 (9%) | 19 (10%) | 3 (7%) | |
| Hispanic | 10 (4%) | 9 (5%) | 1 (2%) | |
| Asian | 11 (5%) | 10 (5%) | 1 (2%) | |
| Others | 8 (3%) | 7 (3%) | 1 (2%) | |
| Not known | 11 (5%) | 10 (5%) | 1 (2%) | |
| Site of the Disease, n (%) | ||||
| Head | 111 (47%) | 98 (50%) | 13 (30%) | 0.058 |
| Body & Neck | 76 (32%) | 58 (30%) | 18 (42%) | |
| Tail | 51 (21%) | 39 (20%) | 12 (28%) | |
| Type of Chemotherapy, n (%) | ||||
| 5FU Based | 98 (41%) | 78 (40%) | 20 (47%) | 0.68 |
| Gemcitabine | 118 (50%) | 97 (50%) | 21 (49%) | |
| Other | 22 (9%) | 20 (10%) | 2 (4%) | |
3.1.2. Metastatic Cohort
Median age was also 64 years (range of 25–84 years) for the patients in this group, including 117 (49%) females and 121 (51%) males. A total of 176 (74%) patients in the cohort were White, 22 (9%) Black, 11 (5%) Asian, 10 (4%) Hispanic, and 8 (3%) from other races. The most common location for the primary tumor was the head of pancreas in 111(47%) patients, followed by body and neck in 76 (32%) patients, with 51 (21%) patients having the primary tumor in the tail (Table 1).
3.2. Antibiotics intake description
3.2.1. Resected Cohort
The frequency of antibiotic use from diagnosis to surgical resection was 61% (n = 209). Single dose of antibiotics administered prior to operation or ancillary procedures was not considered for this analysis. While 133 patients (39%) did not receive any antibiotics, 209 patients (61%) received antibiotics prior to surgery. Within the group received antibiotics (209 patients, 61% of total resected cohort), 62 patients (18%) received less than 7 days, 147 patients (43%) received 7 days or more duration of antibiotics. A total of 206 (60%) patients received gemcitabine‐based chemotherapy (mostly gemcitabine/Nab‐paclitaxel), while 44 (13%) received 5‐fluorouracil‐based chemotherapy, and 7 (2%) both chemotherapies. Eighty‐five patients (25%) did not receive chemotherapy prior to surgical exploration and resection (Table 1). The following antibiotics were received by the resected PDAC patients: quinolones (n = 168), beta‐lactams (n = 80), nitroimidazoles (n = 48), glycopeptides (n = 32), tetracyclines (n = 18), macrolides (n = 14), and sulfa drugs (n = 4) (Table S1).
3.2.2. Metastatic Cohort
The frequency of antibiotic use from diagnosis to death or the date of the last follow‐up was 82% (n = 195). Forty‐three patients (18%) did not receive any antibiotics. Among 195 patients (82%) that received antibiotics, 35 patients (15%) received antibiotics less than 7 days, and 160 patients (67%) received antibiotics 7 days and more. A total of 118 (50%) patients received gemcitabine‐based chemotherapy, while 98 (41%) received 5‐Fluorouracil‐based chemotherapy, and 22 (9%) received other chemotherapeutic regimens in the first‐line setting (Table 1). The antibiotics received by metastatic PDAC patients were as follows: quinolones (n = 141), beta‐lactams (n = 135), glycopeptides (n = 59), nitroimidazoles (n = 51), macrolides (n = 31), tetracyclines (n = 27), and sulfa drugs (n = 21). (Tabls S1).
3.3. Influence of antibiotics use in clinical outcomes
3.3.1. Resected Cohort
The median PFS of patients who took antibiotics prior to surgery was 12.3 months versus 10.2 months in those who did not (HR 0.95, 95% CI 0.74–1.22, p = 0.68; Table 2, Figure 1A), while OS was 32.7 months versus 32.8 months (HR 0.99, 95% CI 0.76–1.28, p = 0.93; Table 2, Figure 1B) on univariate analysis. Therefore, antibiotics intake prior to surgery did not affect outcomes in those patients who underwent surgical resection.
TABLE 2.
Univariate analysis for OS and PFS in resectable and metastatic cohorts
| Resectable Cohort (n = 342) | |||||
|---|---|---|---|---|---|
| Overall survival (OS) | Progressive‐free survival (PFS) | ||||
| Prognostic factors | n (%) | OS HR (95% CI) | p value | PFS HR (95% CI) | p value |
| Gender | |||||
| Female | 147 (43) | 0.82 (0.64–1.06) | 0.13 | 0.83 (0.65–1.07) | 0.15 |
| Male | 195 (57) | ||||
| Age | |||||
| </=65 | 193 (56) | 0.94(0.73–1.21) | 0.64 | 1.04 (0.82–1.33) | 0.73 |
| >65 | 149 (44) | ||||
| Antibiotics received | 209 (61) | 0.99 (0.76–1.28) | 0.93 | 0.95 (0.74–1.22) | 0.68 |
| Intra‐abdominal infections | 133 (39) | 1.13 (0.88–1.47) | 0.34 | 1.06 (0.83–1.35) | 0.65 |
| Urinary Infections | 30 (9) | 1.04 (0.67–1.61) | 0.88 | 1.03 (0.67–1.56) | 0.91 |
| Respiratory Infections | 26 (8) | 0.83 (0.53–1.32) | 0.43 | 0.75 (0.48–1.17) | 0.21 |
| Skin/Soft Tissue Infections | 30 (9) | 0.78 (0.51–1.20) | 0.26 | 0.87 (0.58–1.31) | 0.51 |
| Blood Infections | 8 (2) | 1.17 (0.48–2.83) | 0.73 | 1.15 (0.48–2.79) | 0.75 |
| Other Infections | 62 (18) | 0.91 (0.66–1.26) | 0.59 | 0.86 (0.64–1.17) | 0.34 |
| Metastatic Cohort (n = 238) | |||||
| Overall Survival (OS) | Progressive‐Free Survival (PFS) | ||||
| Prognostic factors | n (%) | OS HR (95% CI) | P value | PFS HR (95% CI) | P value |
| Gender | |||||
| Female | 117(49) | 0.73(0.56–0.97) | 0.0327 | 0.97(0.75–1.26) | 0.82 |
| Male | 121(51) | ||||
| Age | |||||
| </=65 | 131(55) | 1.09(0.83–1.44) | 0.52 | 1.09(0.84–1.41) | 0.49 |
| >65 | 107(45) | ||||
| Antibiotics Received | 195(82) | 0.49(0.34– 0.7) | 0.0001 | 0.48(0.34–0.68) | <0.0001 |
| Intra‐abdominal Infections | 102(43) | 0.79(0.6 – 1) | 0.1 | 0.86(0.66–1.12) | 0.28 |
| Urinary Infections | 55(23) | 0.83(0.61–1.15) | 0.27 | 0.83(0.61–1.13) | 0.25 |
| Respiratory Infections | 81(34) | 0.58(0.43–0.78) | 0.0003 | 0.64(0.48–0.84) | 0.0017 |
| Skin/Soft Tissue Infections | 39(16) | 0.55(0.37–0.81) | 0.0028 | 0.60(0.42–0.85) | 0.0047 |
| Blood Infections | 37(16) | 1.13(0.78–1.64) | 0.5 | 0.89(0.62–1.27) | 0.52 |
| Other Infections | 92(39) | 0.75(0.56–1.00) | 0.052 | 0.8(0.61–1.05) | 0.11 |
FIGURE 1.

Kaplan–Meier Survival Curves of resectable and metastatic PDAC cohorts according to antibiotic use. PFS (A) and OS (B) in resectable cohort. PFS (C) and OS (D) in metastatic cohort
3.3.2. Metastatic Cohort
The use of ATB was associated with significantly longer PFS (4.4 months vs. 2 months, HR 0.48, 95% CI 0.34–0.68, p = <0.0001; Table 3, Figure 1C) and median OS (13.3 months vs. 9.0 months, HR 0.48, 95% CI 0.34– 0.7, p = 0.0001; Table 3, Figure 1D) in metastatic patients.
TABLE 3.
Multivariate analysis for OS and PFS in the metastatic cohort
| Prognostic factors | OS HR (95% CI) | p value | PFS HR (95% CI) | p value |
|---|---|---|---|---|
| Antibiotics received | 0.69(0.47–1.02) | 0.06 | 0.59(0.41–0.85) | 0.005 |
| Age | 0.94 (0.98–1) | 0.41 | 0.99(0.97– 1) | 0.18 |
| Gender | 0.75(0.57–1) | 0.05 | 0.96(0.74–1.24) | 0.76 |
| Respiratory infections | 0.63(0.45–0.86) | 0.0045 | 0.74(0.5–1) | 0.052 |
| Skin Infections | 0.56(0.38–0.84) | 0.0057 | 0.65(0.45–0.93) | 0.02 |
Given these results, we carried out multivariate analysis to assess the robustness of this finding accounting for other significant variables on the univariate analysis (gender, age, respiratory infections, and skin/soft tissue infections). Antibiotics use remained independently associated with significantly prolonged PFS (HR 0.59, p = 0.005), while for OS antibiotics usage was associated with borderline statistically significant OS (HR 0.69, p = 0.06). On univariate analysis respiratory and skin infections were associated with an improved outcome. Furthermore, on multivariate analysis respiratory and skin infections independently remained associated with an improved OS and PFS (Table 3).
3.4. Influence of antibiotics by chemotherapy type
We next examined if antibiotics differentially affected survival based on the type of chemotherapy that patients received as first‐line within the metastatic cohort.
To this end, we further analyzed outcomes in patients receiving Gemcitabine‐based chemotherapy (n = 118) and 5‐FU based chemotherapy (n = 98). After applying the multivariate modeling in the gemcitabine‐based subgroup, patients who received antibiotics had a significantly improved PFS (HR 0.55, P 0.02) and OS (HR 0.4, P 0.0013); (Table 4, Figure 2A, Figure 2B). For the FOLFIRINOX group, following multivariate analysis, patients who had received ATB also had a significantly prolonged PFS (HR 0.54, p = 0.003), but did not have a significantly different OS (HR 1.17, p = 0.59) (Table 4, Figure 2C, D).
TABLE 4.
Subgroup analysis in the metastatic PDAC cohort based on chemotherapy type
| Gemcitabine based (n = 118) | ||||
|---|---|---|---|---|
| Prognostic factors | OS HR (95% CI) | p value | PFS HR (95% CI) | p value |
| Antibiotics received | 0.4(0.23–0.7) | 0.0013 | 0.55(0.33–0.94) | 0.02 |
| Age | 0.98(0.96–1) | 0.14 | 0.99(0.97–1.01) | 0.48 |
| Gender | 0.92(0.61–1.39) | 0.69 | 0.91(0.62–1.33) | 0.64 |
| Respiratory infections | 0.67(0.42–1.07) | 0.09 | 0.76(0.49–1.16) | 0.2 |
| Skin infections | 0.8(0.45–1.41) | 0.44 | 0.74(0.44–1.25) | 0.27 |
| 5FU‐based (n = 98) | ||||
| Prognostic factors | OS HR (95% CI) | p value | PFS HR (95% CI) | p value |
| Antibiotics received | 1.17(0.64–2.14) | 0.59 | 0.54(0.31–0.94) | 0.03 |
| Age | 0.98(0.96–1) | 0.39 | 0.97(0.95–0.99) | 0.03 |
| Gender | 0.62(0.39–0.98) | 0.04 | 0.87(0.57–1.32) | 0.52 |
| Respiratory infections | 0.55(0.32–0.93) | 0.02 | 0.69(0.42–1.19) | 0.13 |
| Skin infections | 0.42(0.22–0.82) | 0.01 | 0.44(0.24–0.81) | 0.008 |
FIGURE 2.
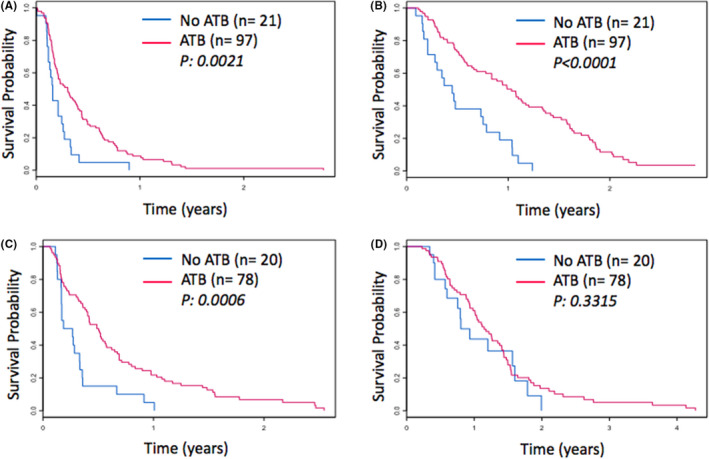
Kaplan–Meier Survival Curves of subgroup analysis per chemotherapy type in metastatic PDAC cohort according to antibiotic use. PFS (A) and OS (B) for Gemcitabine‐ based chemotherapy group. PFS (C) and OS (D) for 5FU‐based chemotherapy group
4. DISCUSSION
The present study reports the impact of antibiotic usage on overall survival and progression‐free survival in PDAC patients, contrasting results in early resected patients and those with metastatic disease. To the best of our knowledge, this is the first study that systemically examined the effect of ATB usage in a large population of pancreatic cancer patients with resectable and metastatic disease. Use of antibiotics in patients with metastatic PDAC was associated with better outcomes: increased PFS and OS and only PFS after multivariate analysis. Furthermore, subgroup analysis revealed that antibiotic usage in patients who received gemcitabine independently prolonged OS and PFS. In the 5FU‐based chemotherapy group, patients who had received ATB had a significantly prolonged PFS but no differences in OS.
Antibiotics are known to reduce microbial diversity and have been associated with worse outcomes in the setting of immunotherapy for other tumor types. 8 , 10 , 11 , 12 , 13 , 14 Based on preclinical pancreatic cancer studies 2 , 3 and outcomes from this retrospective clinical study, antibiotics may be playing a potentially beneficial role in PDAC. Gammaproteobacteria (GP) in tumors can affect gemcitabine activity, converting gemcitabine (20,20‐difluorodeoxycytidine) into its inactive form (20,20 ‐difluorodeoxyuridine), implying that the tumor microbiome in PDAC may be responsible for the tumor resistance to gemcitabine. 5 The use of antibiotics may result in decreased proportions of those drug inactivating‐bacteria which would result in higher chemotherapy activation and improved outcomes. 5 Better responses to gemcitabine (27.6 vs. 15.1%), and clinically better OS (13.83 vs. 7.53 months), and PFS (4.9 vs. 2.5 months) were observed in patients that received antibiotics with several solid tumors that were examined including PDAC. 17 Besides altering the microbiome, there are multiple other mechanisms antibiotics could prolong the survival in PDAC patients at a cellular level. Antibiotics alone or in combination with other chemotherapeutic agents could act as anti‐neoplastic agents, by cytotoxic mechanisms such as loss of STAT 3 activation 18 or inducing direct morphologic changes such as a decrease of microvilli‐like protrusions on cell surface membrane and the swelling of mitochondria. 19 Antibiotics could also be involved in reducing the expression of proteins such as CD 47, which would promote macrophage and tumor cell interaction, ultimately leading to tumor cell death. 19
Immune response modulation by infections, especially chronic infections, and tumors has similarities 20 causing an immune‐suppressive microenvironment. Evasion of the infection with antibiotics might undo the immunosuppressive microenvironment that would otherwise promote tumor growth.
Use of antibiotics in resectable patients was not associated with better outcome, which may be explained by the fact that surgery‐related outcomes may play a more important role with other prognostic factors in resected patients (surgical margins, pathological response, lymph node invasion, nerve or vascular invasion). 21 , 22 A biologic understanding of which patients require antibiotics and those who do not in the metastatic setting is not immediately forthcoming. It is known that patients with a low neutrophil/lymphocyte ratio predict for better outcome in patients on Nab‐paclitaxel regimens. 23 , 24 , 25 Furthermore, our studies with neoadjuvant administration of gemcitabine‐containing regimens along with autophagy inhibition with hydroxychloroquine administration revealed improved outcomes. 26 , 27 , 28 It would be interesting to retrospectively examine completed randomized studies to determine whether antibiotic usage defined better outcomes. 29 , 30 An increased neutrophil to lymphocyte ratio may predict which patients might best respond to a course of antibiotics prior to chemotherapy, suggesting the possibility of necrosis and/or occult infection. 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50
Our study has several limitations. First, the study was a single‐center study of retrospective nature. Second, the analysis did not take into consideration additional factors which could alter the microbiome composition such as diet, country of origin, other medications or supplements. We conducted a multivariate and subgroup analysis to avoid confounding factors within our data set that replicated similar results after adjustment with age, gender, initial treatment type and the reason for antibiotic use. It would be worth mentioning here, there has been increasing evidence that probiotics could possibly prolong survival in PDAC by altering the microbiome. 51 However, due to the retrospective nature of our study and the fact that probiotics are often not documented in the medication list during both outpatient visits and inpatient admissions, we were not able to capture this data for our study. In the resected PDAC cohort, we collected the antibiotic exposure data prior to surgical extirpation, not after. It is unclear if post‐surgical antibiotic use affects the survival of the patients, and there is no available correlative data in the literature examining this measure.
Finally, recent studies have described differences in the microbiome of individuals of different races and ethnicity. 52 Therefore, a clear study limitation is the enrichment of patients of the white race in both resected and metastatic PDAC groups. While the data strongly support the benefit of antibiotics consumption in PDAC patients, there could be alternative explanations for this association. Antibiotics may improve prognosis by limiting local and systemic infections, completely independent of their effect on the tumor and its microenvironment. Also, there may be survivor bias since patients living longer may also have more opportunities to receive any type of therapy, besides antibiotics.
In conclusion, antibiotic use among patients with metastatic PDAC is associated with an increased progression‐free and overall survival, even more marked on patients who received concomitant gemcitabine‐based chemotherapy. These findings need to be validated within a larger group of patients within the context of a prospective randomized clinical trial that is being planned now (MTL).
CONFLICT OF INERTEST
No conflict of interests reported by authors.
AUTHORS’ CONTRIBUTIONS
CM, MH, and FM designed, extracted data, performed analysis, and wrote the manuscript. FM supervised the study. WD performed statistical analysis. JR, LP, SB, and JM had worked on data tabulation and extraction. MO, GV, RW, MJ, DF, MK, MK, SP, CWT, and FM contributed with patients’ data, study revision, analysis, and writing revision. ML contributed by the critical analysis of manuscript and contribution in resubmission write up.
Supporting information
Table S1
Chirayu Mohindroo, Merve Hasanov, and Jane Rogers contributed equally to this work.
Funding information
Dr. McAllister received support from the Cancer Prevention Research Institute of Texas, V Foundation (Translational Award), Sabin Family Foundation, and AGA Foundation.
Contributor Information
Merve Hasanov, Email: mhasanov@mdanderson.org.
Florencia McAllister, Email: fmcallister@mdanderson.org.
REFERENCES
- 1. Institute NC . SEER cancer stat facts: pancreatic cancer. In. National Cancer Institute. 2019.
- 2. Pushalkar S, Hundeyin M, Daley D, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8:403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sethi V, Kurtom S, Tarique M, et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology. 2018;155(1):33–37.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science. 2018;359:97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geller LT, Barzily‐Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178(795–806):e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lalani AA, Xie W, Braun DA, et al. Effect of antibiotic use on outcomes with systemic therapies in metastatic renal cell carcinoma. Eur. Urol Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pinato DJ, Howlett S, Ottaviani D, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elkrief A, Derosa L, Kroemer G, et al. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor? Ann Oncol. 2019;30:1572‐1579. [DOI] [PubMed] [Google Scholar]
- 10. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non‐small‐cell lung cancer. Ann Oncol. 2018;29:1437‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang X‐Z, Gao P, Song Y‐X, et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology. 2019;8:e1665973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinato DJ, Gramenitskaya D, Altmann DM, et al. Antibiotic therapy and outcome from immune‐checkpoint inhibitors. J Immunother Cancer. 2019;7:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sen S, Carmagnani Pestana R, Hess K, et al. Impact of antibiotic use on survival in patients with advanced cancers treated on immune checkpoint inhibitor phase I clinical trials. Ann Oncol. 2018;29:2396‐2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson BE, Routy B, Nagrial A, Chin VT. The effect of antibiotics on clinical outcomes in immune‐checkpoint blockade: a systematic review and meta‐analysis of observational studies. Cancer Immunol Immunother. 2020;69:343‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdel‐Rahman O, Ghosh S, Walker J. Outcomes of metastatic colorectal cancer patients in relationship to prior and concurrent antibiotics use; individual patient data analysis of three clinical trials. Clin Transl Oncol. 2020. [DOI] [PubMed] [Google Scholar]
- 16. Lu L, Zhuang T, Shao E, et al. Association of antibiotic exposure with the mortality in metastatic colorectal cancer patients treated with bevacizumab‐containing chemotherapy: a hospital‐based retrospective cohort study. PLoS One. 2019;14:e0221964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Imai H, Saijo K, Komine K, et al. Antibiotic therapy augments the efficacy of gemcitabine‐containing regimens for advanced cancer: a retrospective study. Cancer Manag Res. 2019;11:7953‐7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quinn BA, Dash R, Sarkar S, et al. Pancreatic cancer combination therapy using a BH3 mimetic and a synthetic tetracycline. Can Res. 2015;75:2305‐2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang R‐Q, Geng J, Sheng W‐J, et al. The ionophore antibiotic gramicidin A inhibits pancreatic cancer stem cells associated with CD47 down‐regulation. Cancer Cell Int. 2019;19:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldszmid RS, Dzutsev A, Trinchieri G. Host immune response to infection and cancer: unexpected commonalities. Cell Host Microbe. 2014;15:295‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen JWC, Bhandari M, Astill DS, et al. Predicting patient survival after pancreaticoduodenectomy for malignancy: histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB (Oxford). 2010;12:101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. John BJ, Naik P, Ironside A, et al. Redefining the R1 resection for pancreatic ductal adenocarcinoma: tumour lymph nodal burden and lymph node ratio are the only prognostic factors associated with survival. HPB (Oxford). 2013;15:674‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldstein D, El‐Maraghi RH, Hammel P, et al. nab‐Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long‐term survival from a phase III trial. J Natl Cancer Inst. 2015;107. [DOI] [PubMed] [Google Scholar]
- 25. Hidalgo M, Plaza C, Musteanu M, et al. SPARC expression did not predict efficacy of nab‐paclitaxel plus gemcitabine or gemcitabine alone for metastatic pancreatic cancer in an exploratory analysis of the phase III MPACT trial. Clin Cancer Res. 2015;21:4811‐4818. [DOI] [PubMed] [Google Scholar]
- 26. Boone BA, Murthy P, Miller‐Ocuin J, et al. Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps. BMC Cancer. 2018;18:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borhani AA, Dewan R, Furlan A, et al. Assessment of response to neoadjuvant therapy using CT texture analysis in patients with resectable and borderline resectable pancreatic ductal adenocarcinoma. AJR Am J Roentgenol. 2020;214:362‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeh HJ, Bahary N, Boone BA, et al. A randomized phase II preoperative study of autophagy inhibition with high‐dose hydroxychloroquine and gemcitabine/nab‐paclitaxel in pancreatic cancer patients. Clin Cancer Res. 2020;26:3126‐3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430‐1438. [DOI] [PubMed] [Google Scholar]
- 30. Karasic TB, O’Hara MH, Loaiza‐Bonilla A, et al. Effect of gemcitabine and nab‐paclitaxel with or without hydroxychloroquine on patients with advanced pancreatic cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:993‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ong SL, Garcea G, Thomasset SC, et al. Surrogate markers of resectability in patients undergoing exploration of potentially resectable pancreatic adenocarcinoma. J Gastrointest Surg. 2008;12:1068‐1073. [DOI] [PubMed] [Google Scholar]
- 32. Aliustaoglu M, Bilici A, Seker M, et al. The association of pre‐treatment peripheral blood markers with survival in patients with pancreatic cancer. Hepatogastroenterology. 2010;57:640‐645. [PubMed] [Google Scholar]
- 33. An X, Ding P‐R, Li Y‐H, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516‐522. [DOI] [PubMed] [Google Scholar]
- 34. Bhatti I, Peacock O, Lloyd G, et al. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil‐lymphocyte versus platelet‐lymphocyte ratio. Am J Surg. 2010;200:197‐203. [DOI] [PubMed] [Google Scholar]
- 35. Garcea G, Ladwa N, Neal CP, et al. Preoperative neutrophil‐to‐lymphocyte ratio (NLR) is associated with reduced disease‐free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011;35:868‐872. [DOI] [PubMed] [Google Scholar]
- 36. Hamed MO, Roberts KJ, Smith AM, Morris SG. Elevated pre‐operative neutrophil to lymphocyte ratio predicts disease free survival following pancreatic resection for periampullary carcinomas. Pancreatology. 2013;13:534‐538. [DOI] [PubMed] [Google Scholar]
- 37. Ino Y, Yamazaki‐Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schueneman AJ, Sugar EA, Uram J, et al. Low total lymphocyte count is associated with poor survival in patients with resected pancreatic adenocarcinoma receiving a GM‐CSF secreting pancreatic tumor vaccine. Ann Surg Oncol. 2013;20(Suppl 3):S725‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stotz M, Gerger A, Eisner F, et al. Increased neutrophil‐lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Szkandera J, Stotz M, Eisner F, et al. External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PLoS One. 2013;8:e78225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Teo MinYuen, Sharial MSNM, McDonnell F, et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in advanced pancreatic ductal adenocarcinoma: impact of baseline fluctuation and changes during chemotherapy. Tumori. 2013;99:516‐522. [DOI] [PubMed] [Google Scholar]
- 42. Xue P, Kanai M, Mori Y, et al. Neutrophil‐to‐lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med. 2014;3:406‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonnet E, Mastier C, Lardy‐Cléaud A, et al. FOLFIRINOX in patients with peritoneal carcinomatosis from pancreatic adenocarcinoma: a retrospective study. Curr Oncol. 2019;26:e466‐e472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harimoto N, Hoshino K, Muranushi R, et al. Prognostic significance of neutrophil‐lymphocyte ratio in resectable pancreatic neuroendocrine tumors with special reference to tumor‐associated macrophages. Pancreatology. 2019;19:897‐902. [DOI] [PubMed] [Google Scholar]
- 45. Kawada T. Neutrophil‐to‐lymphocyte ratio as an indicator of prognosis in patients with pancreatic cancer. HPB (Oxford). 2019;21:1791. [DOI] [PubMed] [Google Scholar]
- 46. Kim EY, Hong TH. Changes in total lymphocyte count and neutrophil‐to‐lymphocyte ratio after curative pancreatectomy in patients with pancreas adenocarcinoma and their prognostic role. J Surg Oncol. 2019;120:1102‐1111. [DOI] [PubMed] [Google Scholar]
- 47. Kubo H, Murakami T, Matsuyama R, et al. Prognostic impact of the neutrophil‐to‐lymphocyte ratio in borderline resectable pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiotherapy followed by surgical resection. World J Surg. 2019;43:3153‐3160. [DOI] [PubMed] [Google Scholar]
- 48. Matsuda A, Yamada T, Matsumoto S, et al. Pretreatment neutrophil‐to‐lymphocyte ratio predicts survival after TAS‐102 treatment of patients with metastatic colorectal cancer. Anticancer Res. 2019;39:4343‐4350. [DOI] [PubMed] [Google Scholar]
- 49. Nakagawa K, Sho M, Akahori T, et al. Significance of the inflammation‐based prognostic score in recurrent pancreatic cancer. Pancreatology. 2019;19:722‐728. [DOI] [PubMed] [Google Scholar]
- 50. Tanaka R, Kimura K, Eguchi S, et al. Preoperative neutrophil‐to‐lymphocyte ratio predicts tumor‐infiltrating CD8(+) T cells in biliary tract cancer. Anticancer Res. 2020;40:2881‐2887. [DOI] [PubMed] [Google Scholar]
- 51. Kita A, Fujiya M, Konishi H, et al. Probiotic‐derived ferrichrome inhibits the growth of refractory pancreatic cancer cells. Int J Oncol. 2020;57:721‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gupta VK, Paul S, Dutta C. Geography, ethnicity or subsistence‐specific variations in human microbiome composition and diversity. Front Microbiol. 2017;8:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


