Abstract
Fibrotic interstitial lung abnormalities in survivors of severe COVID-19 depicted on 6-month CT scans were persistent on 1-year CT scans and were negatively correlated with the lung diffusion capacity.
Introduction
Given the large scale of the COVID-19 pandemic worldwide, lung sequelae after COVID-19 are a major concern to all populations. Our previous study (1) showed that about one-third of survivors with severe COVID-19 had lung “fibrotic-like” changes (fibrotic interstitial lung abnormalities [ILAs] according to Fleischner Society Glossary [2]) at 6-month follow-up. However, whether these fibrotic ILAs changes are permanent, progressive, or reversible remained unclear and little is known about the 1-year sequela of COVID-19. The purpose of this study was to assess the chest CT changes of fibrotic ILAs at 1-year follow-up in survivors of COVID-19.
Materials and Methods
This prospective study was approved by the ethics commission of Wuhan Jin Yin-tan Hospital and Wuhan Union Hospital. All participants remained anonymous, and written informed content was acquired by June 1st, 2020.
A total of 71 participants (41 men, 30 women; mean age ± standard deviation, 57 years ± 10) with lung sequelae (40 participants with fibrotic ILAs [previously described as fibrotic-like changes]; 31 participants without fibrotic ILAs, including ground-glass opacification, consolidation, or reticular abnormalities) at 6-month follow-up in our previous study (1) were invited to this 1-year follow-up study. Nine individuals were excluded due to refusal to participate in the study. Finally, 62 participants (34 men, 28 women; mean age, 57 years ± 10; 35 participants with fibrotic ILAs, 27 participants without fibrotic ILAs) were prospectively enrolled in this study. The flowchart of inclusion is shown in Figure E1 (online).
The World Health Organization’s interim guidance diagnostic criteria for adults with severe COVID-19 pneumonia were used (3). Within 1 week of the follow-up CT scans, 57 participants (32 men, 25 women; mean age, 57 years ± 10) underwent standard pulmonary function testing (PFT) at 6 months, whereas 53 participants (31 men, 21 women; mean age, 57 years ± 10) underwent PFT at 1 year. The severity of the diffusing lung capacity drop was assessed by using a standardized grading system (normal, >75%–140%; mild, 60%–75%; moderate, 40%–59%; and severe, <40%) (4).
All 62 participants underwent 1-year follow-up CT examinations using the same scanners (SOMATOM Definition AS+ or SOMATOM Perspective, Siemens Healthineers) as the 6-month CT scans. CT images obtained at 6 months and 1 year were reviewed side by side in random order by three senior cardiothoracic radiologists (H.S.S., Y.Q.F., and X.Z., with 31, 13 and 8 years of experience in thoracic radiology, respectively). After independent evaluation, discussion and consensus resolved any disagreement. For each patient, CT features according to the Fleischner Society Glossary were assessed and recorded (Appendix E1 [online]).
A semiquantitative CT score (Appendix E1 [online])) was used to quantify the extent of pulmonary abnormalities (all lesions, ground-glass opacification, consolidation, ILAs, and fibrotic ILAs abnormalities and traction bronchiectasis).
Results
The final study group consisted of 62 participants (34 men, 28 women; mean age, 57 years ± 10; range, 34–84 years), of whom 35 of 62 (56%) participants (group 1) showed fibrotic ILAs and the remaining 27 of 62 (44%) participants (group 2) showed no fibrotic ILAs on 6-month follow-up CT scans. Six-month and 1-year follow-up CT scans were obtained at 182 days (interquartile range, 169–196 days) and 363 days (interquartile range, 355– 372 days) after symptoms onset, respectively.
In group 1, all participants (35 of 35, 100%) demonstrated persistent fibrotic ILAs on 1-year follow-up CT scans. Specifically, 27 of 35 (77%) participants had stable lung fibrotic ILAs (Fig A, B), whereas the extent of fibrotic ILAs was slightly reduced in eight of 35 (23%) cases (Fig C). Seventeen of 27 (63%) participants in group 2 showed complete resolution at 1-year CT (Fig F), whereas the remaining 10 of 27 (37%) participants showed either partial resorption of the abnormalities (six of 27, 22%) (Fig D), or static radiologic changes (four of 27, 15%) (Fig E).
Images show changes in CT findings of lung abnormalities for six patients with COVID-19 at baseline (top row) during the acute illness, at 6 months (middle row), and 1 year (bottom row) after hospital discharge. Middle and bottom rows show (A) persisting traction cylindrical bronchiectasis; (B) persisting subpleural bronchiectasis; (C) persistent honeycombing with slightly reduced extension; (D) partial resorption of residual opacifications; (E) static radiologic changes; and (F) complete radiologic resolution. (Figure F [top and middle rows] reprinted, with permission, from reference 1.)
Compared with the 6-month CT scans, decrease in the CT scores of all lesions, ground-glass opacification, reticular pattern and ILAs (all P < .05) were observed on 1-year CT scans. However, CT scores of the fibrotic ILAs and traction bronchiectasis showed no differences between the two CT scans (P = .40, P = .75). Regarding PFT findings, diffusion capacity of the lungs for carbon monoxide (DLCO) abnormality was mostly and consistently mild, with no severe grading. Although the DLCO value slightly improved over time (85% ± 12 vs 89% ± 19), no differences were found between PFT and the severity of DLCO% at 6 months and 1 year (all P > .05) (Table).
Comparison of Clinical Characteristics and CT Findings between Two Follow-ups in Convalescent Patients with Severe COVID-19
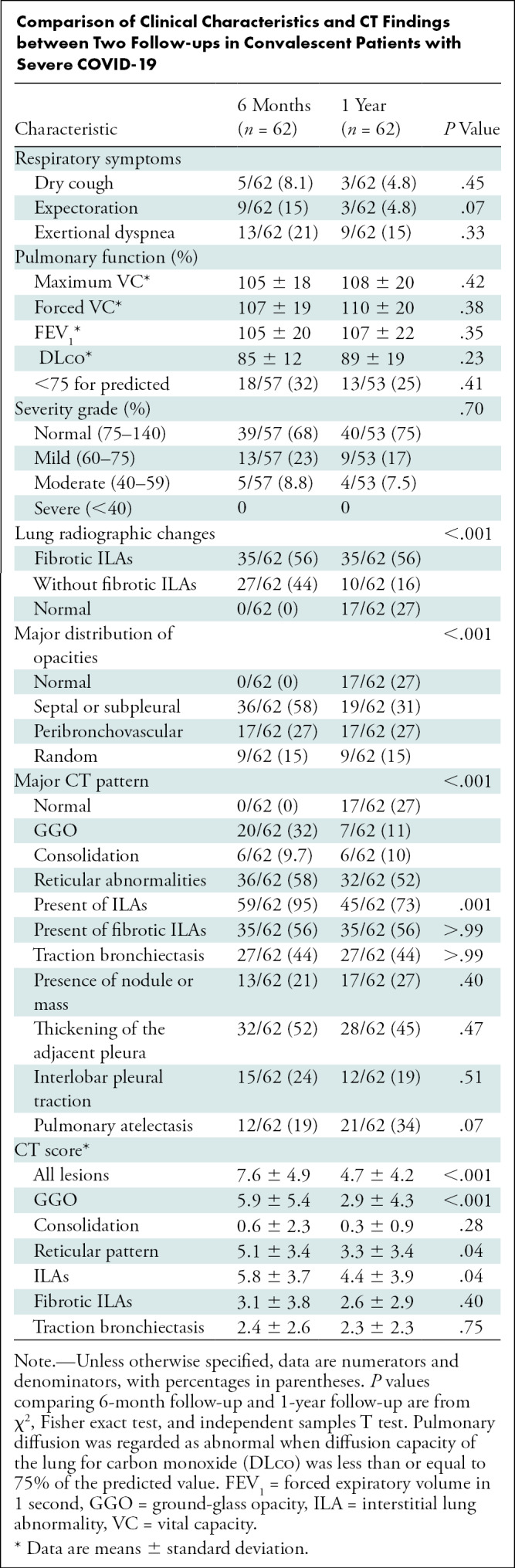
At the 1-year follow up, nine of 62 (15%) participants experienced exertional dyspnea, of whom seven of nine (78%) participants had fibrotic ILAs at CT. There were still 13 of 53 (25%) participants with abnormal pulmonary diffusion on 1-year PFT seen, particularly in those with fibrotic ILAs (11 of 13, 85%) (Table E1 [online]). A negative correlation was found between the score of fibrotic ILAs and DLCO(r = −0.35; P = .01) at 1-year follow-up.
Discussion
This study shows that fibrotic ILAs were a common and persistent sequela at 1-year follow-up CT after severe COVID-19. Recent autopsy studies found that diffuse alveolar damage is the primary pathologic finding in patients with fatal COVID-19, which is indistinguishable from other causes of diffuse alveolar damage (5). Since the fibrotic phase of diffuse alveolar damage could occur in the process of lung injury due to the failure of removal of alveolar collagen (6), the fibrotic ILAs detected in our study could be part of diffuse alveolar damage, which could also be similar to other forms of postviral fibrotic changes such as those occurring after SARS-CoV infection. Although reversible CT evidence of lung fibrosis was noted in the early convalescence stage of SARS-CoV infection (7), lung fibrotic changes on CT images persisted in survivors of SARS-CoV until 1 year after discharge (8). Furthermore, a long-term follow-up SARS-CoV study (9) found that the proportion of lung fibrosis could remain stable from 1-year to 15-year follow-up. Similarly, our results confirmed that fibrotic ILAs were persisting at 1-year follow-up, which indicate that fibrotic diseases in late stage might be irreversible, although whether the findings represent actual pathologic fibrosis remain to be confirmed with lung biopsy.
There were some limitations to this study, including the small sample size and the lack of confirmatory pathologic testing, as well as the lack of quantitative analyses of lung parenchyma.
We conclude that interstitial lung abnormalities were found to be persistent on 1-year follow-up CT scans in survivors of severe COVID-19, which were correlated with a reduction in the diffusion capacity of carbon monoxide. These findings need to be confirmed by further investigations on a larger population.
Acknowledgments
Acknowledgments
We would like to thank all colleagues for helping us during the current study and all the selfless volunteers who participated in the study. We are also very grateful to many members of the frontline medical staff for their selfless dedication and heroic dedication in the face of this outbreak, despite the potential threat to their own lives and the lives of their families.
Footnotes
X.H. and Y.F. contributed equally to this work.
Supported by the National Natural Science Foundation of China (grant 82071921), National Key Research and Development Project of China (grant 2020YFC0840800), Zhejiang University Special Scientific Research Fund for COVID-19 Prevention and Control, and Fundamental Research Funds for the Central Universities (grant 2020kfyXGYJ019).
Disclosures of Conflicts of Interest: X.H. No relevant relationships. Y.F. No relevant relationships. O.A. No relevant relationships. X.Z. No relevant relationships. X.J. No relevant relationships. Y.Z. No relevant relationships. H.S. No relevant relationships.
References
- 1. Han X , Fan Y , Alwalid O , et al . Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia . Radiology 2021. ; 299 ( 1 ): E177 – E186 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hatabu H , Hunninghake GM , Richeldi L , et al . Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society . Lancet Respir Med 2020. ; 8 ( 7 ): 726 – 737 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance . https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf Published January 12, 2020. Accessed June 28, 2021 .
- 4. Nguyen LP , Harper RW , Louie S . Using and interpreting carbon monoxide diffusing capacity (DLCO) correctly . Consultant 2016. ; 56 ( 5 ): 440 – 445 . https://www.consultant360.com/articles/using-and-interpreting-carbon-monoxide-diffusing-capacity-dlco-correctly . [Google Scholar]
- 5. Konopka KE , Nguyen T , Jentzen JM , et al . Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 Infection is Morphologically Indistinguishable from Other Causes of DAD . Histopathology 2020. ; 77 ( 4 ): 570 – 578 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sweeney RM , McAuley DF . Acute respiratory distress syndrome . Lancet 2016. ; 388 ( 10058 ): 2416 – 2430 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antonio GE , Wong KT , Hui DS , et al . Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience . Radiology 2003. ; 228 ( 3 ): 810 – 815 . [DOI] [PubMed] [Google Scholar]
- 8. Xie L , Liu Y , Fan B , et al . Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge . Respir Res 2005. ; 6 ( 1 ): 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang P , Li J , Liu H , et al . Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study . Bone Res 2020. ; 8 : 8 [Published correction appears in Bone Res 2020;8:34.] . [DOI] [PMC free article] [PubMed] [Google Scholar]



![Images show changes in CT findings of lung abnormalities for six patients with COVID-19 at baseline (top row) during the acute illness, at 6 months (middle row), and 1 year (bottom row) after hospital discharge. Middle and bottom rows show (A) persisting traction cylindrical bronchiectasis; (B) persisting subpleural bronchiectasis; (C) persistent honeycombing with slightly reduced extension; (D) partial resorption of residual opacifications; (E) static radiologic changes; and (F) complete radiologic resolution. (Figure F [top and middle rows] reprinted, with permission, from reference 1.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/f99e/8630528/b8cc8e231f2e/radiol.2021210972.fig1.jpg)