Abstract
Purpose
Colorectal cancer (CRC) diagnosis is associated with high mortality in the United States and thus warrants the study of novel treatment approaches. Vascular changes are well observed in cancers and evidence indicates that antihypertensive (AH) medications may interfere with both tumor vasculature and in recruiting immune cells to the tumor microenvironment based on preclinical models. Extant literature also shows that AH medications are correlated with improved survival in some forms of cancer. Thus, this study sought to explore the impact of AH therapies on CRC outcomes.
Patients and Methods
This study was a non‐interventional, retrospective analysis of patients aged 65 years and older with CRC diagnosed from January 1, 2007 to December 31st, 2012 in the Surveillance, Epidemiology, and End‐Results (SEER)‐Medicare database. The association between AH drug utilization on AJCC stage I–III CRC mortality rates in patients who underwent treatment for cancer was examined using Cox proportional hazards models.
Results
The study cohort consisted of 13,982 patients diagnosed with CRC. Adjusted Cox proportional hazards regression showed that among these patients, the use of AH drug was associated with decreased cancer‐specific mortality (HR: 0.79, 95% CI: 0.75–0.83). Specifically, ACE inhibitors (hazard ratio [HR]: 0.84, 95% CI: 0.80–0.87), beta‐blockers (HR: 0.87, 95% CI: 0.84–0.91), and thiazide diuretics (HR: 0.83, 95% CI: 0.80–0.87) were found to be associated with decreased mortality. An association was also found between adherence to AH therapy and decreased cancer‐specific mortality (HR: 0.94, 95% CI: 0.90–0.98).
Conclusion
Further research needs to be performed, but AH medications may present a promising, low‐cost pathway to supporting CRC treatment for stage I–III cancers.
Keywords: antihypertensive agent, colorectal neoplasms, SEER program
The use of antihypertensive agents following colorectal cancer diagnosis is associated with lower mortality (both all‐cause and cancer‐specific) in elderly Medicare patients. Among the studied classes of antihypertensives, ACE inhibitors and beta‐blockers seem to be associated with protective associations.

1. INTRODUCTION
Colorectal cancer (CRC) is the third most diagnosed cancer and the second and third leading cause of cancer deaths among men and women, respectively, in the United States. Its incidence continues to rise in developing nations. 1 As with the majority of cancer types, surgery is the primary treatment approach, and in the case of metastasized cancers, it is preceded or followed by cytotoxic approaches such as neoadjuvant and adjuvant therapies, respectively. 2
Solid tumor growth is associated with angiogenesis and there is a wide evidence base speaking to the relationship between cancer patient prognosis and the angiogenic potential of tumors. 3 , 4 However, tumor vasculature is often irregular in form due to compression by the mechanical stress induced by the proliferation of surrounding cells. Further, they have high microvascular hydrostatic pressure and transcapillary fluid movement. This, combined with poor drainage via lymphatic vessels, interstitial fibrosis, and contraction of the interstitial matrix, leads to the collection of liquid in the interstitium of solid tumors and high interstitial fluid pressure (IFP). 5 Increases in IFP, in turn, contribute to low blood flow through the area due to high viscous resistance which has been shown to cause poor delivery of therapeutic agents to tumors and worsened outcomes. 6 , 7 , 8 , 9 , 10 These drugs are further inhibited in their diffusion by the aforementioned increase in matrix density in solid tumors, which serves to disrupt the flow of oxygen through the microenvironment and ultimately, hypoxia. Hypoxia and uneven tumor vascularization have also been shown to be a factor contributing to the failure of cancer therapies by promoting metastases, complicating surgery, and limiting the efficacy of a variety of known cancer therapies. 11 , 12 , 13 , 14 Therefore, strategies that normalize tumor vasculature function and hypoxia to normalize the underlying tumor microenvironment may be effective for the optimization of different modalities of cancer patient management. 15 , 16 , 17 , 18 , 19 , 20
Hypertension is one of the most significant comorbidities encountered in patients with cancer, as evidenced by a study from Fraeman et al which found new‐onset hypertension in roughly one‐third of cancer patients. 21 Issues may be exacerbated by chemotherapy, given that hypertension is a known risk factor for chemotherapy‐induced cardiotoxicity and has a correspondingly large influence on cancer management approaches. 22 Of note, vascular tone, structure, and function are also altered in systemic hypertension. 23 Common approaches to hypertension management include the application of calcium‐channel blockers, (ARB), thiazide diuretics (TD), angiotensin‐converting enzyme inhibitors (ACEI), and adrenergic β2‐receptor blockers (BB). Preference for treatment regimens is determined largely by clinical status, ethnicity, and age. Increasing evidence indicates that drugs used in the control of hypertension may also interfere with tumor vasculature function and the recruitment of immune cells to the tumor microenvironment based on preclinical models. This is effectively the reverse of the previously stated relationship between anti‐cancer drugs and hypertension. Further, elevated VEGF in patients with hypertension is correlated with cardiovascular disease risk. Given that hypertension treatment reduces VEGF levels, it may offer a novel avenue for cancer treatment with reduced risks. 24 Unfortunately, there does not seem evidence of studies that have examined the potential associations between antihypertensive (AH) medication use on outcomes in CRC patients.
To examine whether AH medications in cancer confer protective benefits, we evaluated the impact of AH regimens used by patients with stage I–III CRC in the combined SEER‐Medicare database. We also analyzed if the use of specific classes of AH drugs would impact the efficacy of further cancer treatment and CRC‐specific mortality.
2. METHODS
The SEER‐Medicare data are a combination of the SEER program of cancer registries in the United States which collects clinical, demographic, and cause of death information for those with cancer. As a subset of the SEER Database, it specifically collects Medicare claims for covered health services for Medicare patients until their death. The Medicare insurance program generally accepts those who are over the age of 65, younger individuals with disabilities, or those with End‐Stage Renal Disease (kidney failure requiring dialysis or transplant). The SEER‐Medicare database analytic variables include patient demographics at diagnosis (e.g., age and gender), tumor characteristics, Medicare enrollment information, International Classification of Diseases, Ninth Revision, Clinical Modification diagnoses and procedure codes, Healthcare Common Procedure Coding System and Current Procedural Terminology codes, and prescription claims data. 25
We received Institutional Review Board approval for this analysis prior to analytic procedures. The initial study cohort included patients diagnosed with CRC from January 1, 2007, to December 31, 2011. This was defined as SEER codes (identifiers for diagnosis and/or procedures in the database) C18.0–C18.9 (malignant neoplasm of colon), C20.9 (rectum), and C19.9 (rectosigmoid junction). Inclusion criteria were first CRC diagnosis, age 65 years or older, if the diagnosis was pathologically confirmed, and whether the diagnosis was reported from autopsy or death report. Patients were included only if they were continuously enrolled in fee‐for‐service Medicare Parts A and B 12 months before diagnosis, and continuously enrolled in fee‐for‐service Medicare Parts A and B as well as Medicare Part D prescription drug benefit program at least 12 months post‐diagnosis. Patients with AH drugs prior to diagnosis, patients with AJCC stage 0 and stage IV tumors were also excluded. Patients with AH prior to diagnosis were excluded as outcomes for these patients would not be available and would not be relevant to the analysis of AH drugs on tumor progression. AJCC Stage 0 tumors were excluded as it refers to tumors that have not yet been staged and stage IV, already metastasized tumors, were excluded as this paper examined disease progression. Figure 1 shows how the study sample was derived.
FIGURE 1.
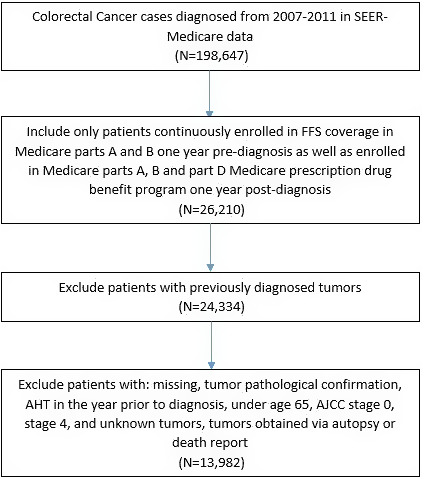
Derivation of the study population
The following variables were extracted for analysis from the SEER cancer registry database: age at diagnosis, year at diagnosis, sex, race (non‐Hispanic White, non‐Hispanic Black, and others), marital status, and clinical tumor characteristics including stage and tumor grade. Surgery and chemotherapy were identified from claims. Adherence was assessed by the proportion of days covered (PDC) measured. This measure has been validated in several studies. 26 A conventional cutoff of 0.80 for PDC was used to categorize adherence (PDC > 0.8) and non‐adherence (PDC ≤ 0.8), truncated to the range of 0 to 1. Adherence was then calculated for the patients who were continuously enrolled in the Part D prescription drug benefit program for at least 1‐year post‐initiation of AH treatment (AHT). AHT drugs included ACE inhibitors, angiotensin receptor blockers, beta‐blockers, and TD. AHT drugs were identified using NDC codes. Only new initiation of AHT was included and counted to calculate adherence. The follow‐up started 1 year after the initiation of AHT medication, and only subjects who survived up until the end of the follow‐up were included. The comorbidities were assessed using the Klabunde et al modification of the Charlson comorbidity index available from SAS macros 27 , based on physician and outpatient claims separated by at least 30 days to identify unique health conditions. Claims were searched during a 1‐year time window pre‐diagnosis date in physician, outpatient, and hospital claims, as described elsewhere. 28 Monotherapy was defined as patients taking only one class of AHT. Cancer type was defined as patients having either colon cancer or rectal cancer. Radiation was defined as a patient receiving radiation therapy.
The primary outcome of statistical analysis was CRC‐specific (CRCS) mortality, as determined using the underlying cause of death found in SEER files. The association between AHT application and cancer‐specific mortality was modeled using Cox proportional hazards regression models to produce hazard ratios (HRs) and the associated 95% confidence intervals (CIs). Kaplan–Meier Survival Analysis was also conducted and survival was tested using the log‐rank test. The follow‐up started 1 year after diagnosis. Adherence to AHT was calculated using the PDC measure. A p value of 0.05 was considered statistically significant. The analysis was performed using SAS software (version 9.4 SAS Institute Inc., Cary, NC, US).
3. RESULTS
The patient population diagnosed with CRC during 2007–2011 included in this study (n = 13,982) was stratified based on AHT use during follow‐up. The demographic and clinical characteristics of this cohort are summarized in Table 1.
TABLE 1.
Baseline characteristics of patients diagnosed with colorectal cancer during 2007–2012 based on antihypertensive use during follow‐up (N=13,982)
| Antihypertensive Use a | Non‐AH users (n = 2553) | ACEI users b (n = 5803) | ARB users b (n = 1171) | BB users b (n = 8025) | TD users b (n = 6927) | Total (n = 13,982) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Year of diagnosis | ||||||||||||
| 2007 | 464 | 18.17 | 1380 | 23.78 | 219 | 18.70 | 1826 | 22.75 | 1625 | 23.46 | 3003 | 21.48 |
| 2008 | 522 | 20.45 | 1269 | 21.87 | 226 | 19.30 | 1711 | 21.32 | 1533 | 22.13 | 2955 | 21.13 |
| 2009 | 485 | 19.00 | 1150 | 19.82 | 236 | 20.15 | 1576 | 19.64 | 1383 | 19.97 | 2727 | 19.50 |
| 2010 | 491 | 19.23 | 1030 | 17.75 | 246 | 21.01 | 1464 | 18.24 | 1246 | 17.99 | 2605 | 18.63 |
| 2011 | 591 | 23.15 | 974 | 16.78 | 244 | 20.84 | 1448 | 18.04 | 1140 | 16.46 | 2692 | 19.25 |
| Age at diagnosis (years) | ||||||||||||
| 65–69 | 603 | 23.62 | 1116 | 19.23 | 212 | 18.10 | 1322 | 16.47 | 1089 | 15.72 | 2568 | 18.37 |
| 70–74 | 636 | 24.91 | 1390 | 23.95 | 276 | 23.57 | 1777 | 22.14 | 1419 | 20.49 | 3209 | 22.95 |
| 75–79 | 528 | 20.68 | 1245 | 21.45 | 272 | 23.23 | 1736 | 21.63 | 1483 | 21.41 | 2992 | 21.40 |
| 80–84 | 457 | 17.90 | 1066 | 18.37 | 245 | 20.92 | 1622 | 20.21 | 1473 | 21.26 | 2728 | 19.51 |
| 85+ | 329 | 12.89 | 986 | 16.99 | 166 | 14.18 | 1568 | 19.54 | 1463 | 21.12 | 2485 | 17.77 |
| Sex | ||||||||||||
| Female | 1492 | 58.44 | 3289 | 56.68 | 760 | 64.90 | 4727 | 58.90 | 4289 | 61.92 | 8283 | 59.24 |
| Male | 1061 | 41.56 | 2514 | 43.32 | 411 | 35.10 | 3298 | 41.10 | 2638 | 38.08 | 5699 | 40.76 |
| Marital status | ||||||||||||
| Single | 1261 | 49.39 | 3303 | 56.92 | 612 | 52.26 | 4583 | 57.11 | 4123 | 59.52 | 7763 | 55.52 |
| Married | 1292 | 50.61 | 2500 | 43.08 | 559 | 47.74 | 3442 | 42.89 | 2804 | 40.48 | 6219 | 44.48 |
| Race/ethnicity | ||||||||||||
| Non‐Hispanic white | 2097 | 82.14 | 4673 | 80.53 | 852 | 72.76 | 6467 | 80.59 | 5642 | 81.45 | 11229 | 80.31 |
| Non‐Hispanic black | 164 | 6.42 | 567 | 9.77 | 86 | 7.34 | 739 | 9.21 | 683 | 9.86 | 1195 | 8.55 |
| Others | 292 | 11.44 | 563 | 9.70 | 233 | 19.90 | 819 | 10.21 | 602 | 8.69 | 1558 | 11.14 |
| Chemotherapy | 1768 | 69.25 | 4148 | 71.48 | 834 | 71.22 | 5843 | 72.81 | 5092 | 73.51 | 3993 | 28.56 |
| Surgery | 2438 | 95.50 | 5640 | 97.19 | 1141 | 97.44 | 7782 | 96.97 | 6694 | 96.64 | 13497 | 96.53 |
| Stage at diagnosis | ||||||||||||
| I | 874 | 34.23 | 1910 | 32.91 | 405 | 34.59 | 2654 | 33.07 | 2288 | 33.03 | 4640 | 33.19 |
| II | 875 | 34.27 | 2153 | 37.10 | 414 | 35.35 | 2939 | 36.62 | 2549 | 36.80 | 5054 | 36.15 |
| III | 804 | 31.49 | 1740 | 29.98 | 352 | 30.06 | 2432 | 30.31 | 2090 | 30.17 | 4288 | 30.67 |
| Grade | ||||||||||||
| Well‐differentiated | 225 | 8.81 | 506 | 8.7 | 95 | 8.1 | 701 | 8.74 | 623 | 8.99 | 1246 | 8.91 |
| Moderately differentiated | 1701 | 66.63 | 4052 | 69.83 | 823 | 70.28 | 5533 | 68.95 | 4730 | 68.28 | 9544 | 68.26 |
| Poorly differentiated | 401 | 15.71 | 838 | 14.44 | 179 | 15.29 | 1226 | 15.28 | 1062 | 15.33 | 2147 | 15.36 |
| Undifferentiated | 65 | 2.55 | 116 | 2.00 | 17 | 1.45 | 162 | 2.02 | 150 | 2.17 | 297 | 2.12 |
| Unknown | 161 | 6.31 | 291 | 5.01 | 57 | 4.87 | 403 | 5.02 | 362 | 5.23 | 748 | 5.35 |
| Metformin use | ||||||||||||
| Yes | 239 | 9.36 | 1462 | 25.19 | 273 | 23.31 | 1695 | 21.12 | 1498 | 21.63 | 2618 | 18.72 |
| No | 2314 | 90.64 | 4341 | 74.81 | 898 | 76.69 | 6330 | 78.88 | 5429 | 78.37 | 11364 | 81.28 |
| Diabetes | ||||||||||||
| Yes | 393 | 15.39 | 2268 | 39.08 | 391 | 33.39 | 2857 | 35.60 | 2586 | 37.33 | 4297 | 30.73 |
| No | 2160 | 84.61 | 3535 | 60.92 | 780 | 66.61 | 5168 | 64.40 | 4341 | 62.67 | 9685 | 69.27 |
| CCI | ||||||||||||
| 0 | 1604 | 62.83 | 2025 | 34.90 | 474 | 40.48 | 2833 | 35.30 | 2239 | 32.32 | 5882 | 42.07 |
| 1 | 579 | 22.68 | 1676 | 28.88 | 348 | 29.72 | 2186 | 27.24 | 1955 | 28.22 | 3822 | 27.34 |
| 2+ | 370 | 14.49 | 2102 | 36.22 | 349 | 29.80 | 3006 | 37.46 | 2733 | 39.45 | 4278 | 30.60 |
| Monotherapy | – | – | 945 | 16.28 | 365 | 31.17 | 1710 | 21.31 | 1238 | 17.87 | 4258 | 30.45 |
| Hypertension | ||||||||||||
| Yes | 975 | 38.19 | 4621 | 79.63 | 962 | 82.15 | 6337 | 78.97 | 5492 | 79.28 | 9741 | 69.67 |
| No | 1578 | 61.81 | 1182 | 20.37 | 209 | 17.85 | 1688 | 21.03 | 1435 | 20.72 | 4241 | 30.33 |
| Cancer type | ||||||||||||
| Colon | 1934 | 75.75 | 4498 | 77.51 | 956 | 81.64 | 6347 | 79.09 | 5482 | 79.14 | 10899 | 77.95 |
| Rectal | 619 | 24.25 | 1305 | 22.49 | 215 | 18.36 | 1678 | 20.91 | 1445 | 20.86 | 3083 | 22.05 |
| Radiation | ||||||||||||
| Yes | 304 | 11.1 | 596 | 10.7 | 89 | 7.60 | 754 | 9.40 | 645 | 9.31 | 1407 | 10.06 |
| 220 | 86.6 | 516 | 88.1 | 1076 | 91.89 | 7184 | 89.52 | 6203 | 89.55 | 12418 | 88.81 | |
| Unknown | 29 | 1.14 | 71 | 1.22 | 6 | 0.51 | 87 | 1.08 | 79 | 1.14 | 157 | 1.12 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BB, beta‐blockers; TD, thiazide diuretics.
Columns of antihypertensive medication use are not mutually exclusive. Those using combination antihypertensive medications are included in multiple columns.
Antihypertensive use during the year following diagnosis.
3.1. Correlates of colorectal cancer‐specific mortality
A range of factors was found to be associated with higher cancer‐specific mortality in elderly Medicare patients (Table 2). Male sex was found to be significantly associated with increased CRCS mortality (HR: 1.07, 95% CI: 1.03–1.13). Patients with a marital status of single had greater mortality for patients as well (HR: 1.08, 95% CI: 1.03–1.13). With respect to procedures, patients who did not receive chemotherapy possessed a higher mortality rate than those who did (HR: 1.07, 95% CI: 1.01–1.13). Further, not receiving surgery was found to be associated with higher mortality (HR: 1.39, 95% CI: 1.23–1.58). Tumor stage and tumor grade were not found to be significantly associated with patient mortality. In addition, as determined by the Charlson Comorbidity index, patients with a higher comorbidity index (1 or 2+ comorbidities relative to no comorbidities) were associated with a higher risk of mortality (HR for 1 comorbidity: 1.08, 95% CI: 1.02–1.14 and HR for 2+ comorbidities: 1.21, 95% CI: 1.14–1.28). 29 Cancer type and receipt of radiation therapy were not significantly associated with patient mortality.
TABLE 2.
Risk of mortality (cancer‐specific survival) among the users of antihypertensive medication (PDC ≥ 80% vs. PDC < 80%). (N = 13,982)
| Hazard Ratio (95% CI) | ||
|---|---|---|
| PDC | >=80% | 0.94 (0.90 to 0.98) |
| <80% | Reference | |
| Age | 1.01 (1.00 to 1.01) | |
| Sex | Male | 1.07 (1.03 to 1.12) |
| Female | Reference | |
| Marital status | Single/Other | 1.08 (1.03 to 1.13) |
| Married | Reference | |
| Race | Black | 0.97 (0.91 to 1.05) |
| Race | Other | 1.04 (0.98 to 1.11) |
| White | Reference | |
| Chemotherapy | No | 1.07 (1.01 to 1.13) |
| Yes | Reference | |
| Surgery | No | 1.39 (1.23 to 1.58) |
| Yes | Reference | |
| Stage | Stage II | 0.98 (0.93 to 1.03) |
| Stage III | 1.00 (0.95 to 1.06) | |
| Stage I | Reference | |
| Tumor grade | Moderately differentiated | 1.04 (0.97 to 1.11) |
| Poorly differentiated | 1.05 (0.96 to 1.14) | |
| Undifferentiated | 1.30 (1.11 to 1.51) | |
| Well differentiated | Reference | |
| Metformin | Yes | 1.04 (0.98 to 1.10) |
| No | Reference | |
| Diabetes | Yes | 1.02 (0.96 to 1.08) |
| No | Reference | |
| CCI | 1 | 1.08 (1.02 to 1.14) |
| CCI | 2+ | 1.21 (1.14 to 1.28) |
| 0 | Reference | |
| Monotherapy | Yes | 1.15 (1.10 to 1.20) |
| No | Reference | |
| Hypertension | Yes | 1.13 (1.07 to 1.18) |
| No | Reference | |
| Cancer Type | Rectal | 0.99 (0.94–1.06) |
| Colon | Reference | |
| Radiation | Yes | 1.01 (0.92–1.10) |
| Unknown | 1.18 (0.98–1.43) | |
| No | Reference |
3.2. Relationship between adherence and mortality
We also examined the relationship between adherence to AH medication, represented by the PDC measure (Table 2). Lower adherence was set at PDC lower than 80% and higher adherence was set as equal to or greater than 80%. A Kaplan–Meier analysis was also used to determine CRC‐specific in the groups based on adherence. There was a clear association found between increased adherence to AH medications and reduced CRCS mortality in patients starting these medications after CRC diagnosis relative to those who did not after adjusting for cancer stage and treatment (HR: 0.94, 95% CI: 0.90–0.98) (Figure 2). At follow‐up, patients in the <80% adherence group had a CRCS survival of 74% compared with 83.6% in the group with ≥80% adherence group.
FIGURE 2.
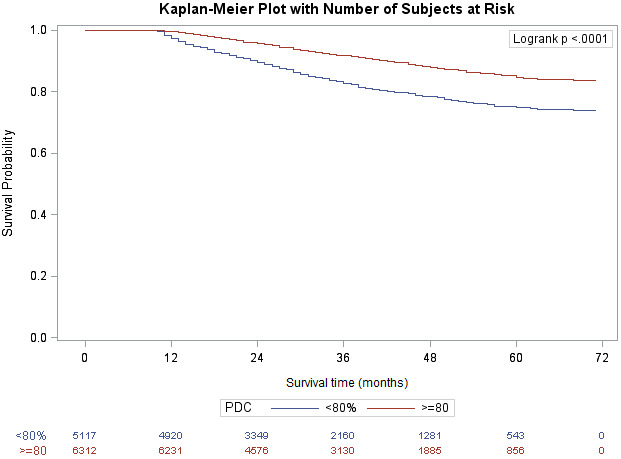
Kaplan–Meier survival curve over 6 years, by the proportion of days covered (PDC) (N = 11,429)
3.3. Correlates of cancer‐specific mortality among users of antihypertensive, ACEIs, ARBs, BBs, and TDs
We subsequently focused on exploring CRCS mortality differences between patients using each of the studied AH therapeutic drugs: Overall, ACEIs, ARBs, BBs, and TDs (Table 3). Overall, AH users conferred positive associations on patient mortality. Among the different classes of drugs, it was found that ACEIs (HR: 0.84, 95% CI: 0.80–0.87), BBs (HR: 0.87, 95% CI: 0.84–0.91), and TDs (HR: 0.83, 95% CI: 0.80–0.87) also conferred positive associations on patient mortality. No association between drug class and patient mortality was found in the case of ARBs. Broadly, the use of AH medications showed a significant correlation with decreased mortality (Table 3).
TABLE 3.
Cox proportional hazards model evaluating cancer‐specific survival for all patients diagnosed with colorectal cancer among antihypertensive and non‐hypertensive medication users (N = 13,982)
| Hazard† Ratio (95%CI)* | |
|---|---|
| Antihypertensive users | 0.79 (0.75 to 0.83) |
| ACEI users | 0.84 (0.80 to 0.87) |
| ARB users | 0.96 (0.89 to 1.03) |
| BB users | 0.87 (0.84 to 0.91) |
| TD users | 0.83 (0.80 to 0.87) |
Adjusted for age, sex, marital status, race and/or ethnicity, chemotherapy, surgery, stage, grade, metformin, diabetes, Charlson comorbidity index, monotherapy, hypertension, cancer type, radiation.
ACEI, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blocker; BB, beta‐blockers; CI, confidence interval; TD, thiazide diuretics.
4. DISCUSSION
This study examined the potential influence of AH medication initiation following CRC diagnosis based on the hypothesis that addressing hypertension, a common comorbidity of cancers, could offer protection for CRC patients. Our study evaluated the impact of these AHs from the Medicare SEER database during a contemporary time frame and found that ACEIs and TD provide the most significant benefit to the patient's survival and outcomes for those with stage I–III cancers. Our findings of the protective association between survival and the AH treatment with ACEIs are consistent with a previous study that described a significantly increased rate of pathologic complete response after neoadjuvant treatment among patients with rectal cancer. 30 Moreover, our results show an association between increased adherence to AH medications and reduced CRCS mortality in patients starting these medications after stage I, II or III CRC diagnosis relative to those who did not. Although further analysis is necessary, this increment of survival may be associated with a higher dose exposure, as a long‐term/high‐dose exposure to ACE‐Is/ARBs was associated with a decreased incidence of CRC mortality. 31
Several AH drugs have been recently studied in the context of cancer treatment. The angiotensin‐receptor blocker losartan has been shown to be potentially antiangiogenic in the maximally tolerated dose. 32 Losartan may also be used in the normalization of tumor vascularization, which was indicated previously as a potential avenue for improving the distribution of oxygen and drugs, such as 5‐fluorouracil, a chemotherapeutic agent used in the management of CRCs. 33 , 34 Recent clinical trials seeking to apply total neoadjuvant approaches to advanced pancreatic cancer applied Losartan, a drug used to treat hypertension, in tandem with FOLFIRINOX (fluorouracil, leucovorin, oxaliplatin, and irinotecan) found evidence of downstaging of locally advanced pancreatic ductal adenocarcinoma and an R0 resection rate of 61%. 35 Further, a phase II clinical trial is recruiting pancreatic cancer patients to assess the impact of combining chemoradiotherapy and losartan with nivolumab (immunotherapy) on survival and the proportion of patients with R0 resection. 36 Another study is recruiting pancreatic cancer patients for a phase I clinical trial to compare the safety and efficacy of losartan plus hypo‐fractionated radiation therapy following chemotherapy. 37 In addition, a double‐blinded, placebo‐controlled, randomized, phase III trial found that, despite the possible interference of losartan on VEGF‐mediated angiogenesis, its use did not show any impact on steroid requirements during radiotherapy to reduce peritumoral edema in newly diagnosed glioblastoma patients. 38
Losartan has also been shown as an effective means of targeting the angiotensin signaling axis in order to reduce extracellular matrix content, thereby increasing chemotherapeutic efficacy in ovarian cancer. 39 Aligned with this, an open‐label study is recruiting patients with glioblastoma and metastatic brain tumors from non‐small cell lung cancer to assess the dose–response relationship of losartan on imaging‐based measures of tissue perfusion and mechanical forces. 40 Other trials are evaluating the use of losartan as neoadjuvant treatment. 41 , 42 Therefore, neoadjuvant therapy with losartan or others ARBs, which is relatively inexpensive and considered safe, is a promising treatment. However, the treatment’s efficacy must be further elucidated by the way of randomized clinical trials. 43 Bevacizumab, an anti‐VEGF monoclonal antibody, has also been shown to prolong survival in ovarian and cervical cancer with chemotherapy. 44 In addition, FOLFOXIRI plus bevacizumab has been demonstrated to improve the overall survival and progression‐free survival of CRC patients. Because of that, the treatment pathway presents a very effective first‐line regimen, regardless of ethnicity, to improve the outcome of patients with metastatic CRC. 45 A common side effect of treatment with bevacizumab is arterial hypertension, which is easily managed by standard AH therapy. Although limited by the small sample size, a meta‐analysis reported that the occurrence of bevacizumab‐induced hypertension in patients with metastatic CRC was highly associated with improvements in progression‐free survival and overall survival. 46 Additionally, a retrospective study of 315 patients with CRC found that patients with bevacizumab‐induced hypertension had a median overall survival of 42.6 months, while normotensive patients had 20.6 months (p = 0.00071). 47 Thus, this bevacizumab‐induced hypertension might work as a prognostic predictor, once it probably has the potential to estimate the anti‐VEGF efficacy and activity of the treatment. But we should not lose sight of the possible influence that the AH drugs, used to manage this elevation of blood pressure, can have on cancer outcomes. Therefore, this correlation needs to be assessed by further research.
In this study, we found a statistically significant protective association of the use of β‐blockers on CRC‐specific mortality. Propranolol, a β2‐adrenergic receptor blocker, has been shown to interfere with NK cell homing to tumors. 48 It has also been shown to reduce the production of angiogenic factors, such as VEGF, by macrophages and cancer cells while also downregulating the rapidly accelerated fibrosarcoma (RAF)–mitogen‐activated protein kinase (MAPK) pathway, inhibiting angiogenesis. 49 Propranolol has been used to treat infantile hemangiomas (IH) with relative success. 50 , 51 Although the precise mechanisms of action of propranolol on IHs remain unclear, its angiogenic inhibition properties are one of the hypotheses for its action. According to this, propranolol and possibly other beta‐blockers may have a role in normalizing tumor vasculature and, consequently, improving the delivery of drugs to the tumor environment. Barron et al. 52 have shown that the use of a beta‐blocker with beta‐2 receptor activity before breast cancer diagnosis can reduce breast cancer progression and mortality. R. Udumyan et al. 53 have found that b‐adrenergic receptor blockers, particularly non‐selective types, are associated with lower liver cancer mortality in patients with primary hepatocellular carcinoma.
Haldar et al. and Shaashua et al. have evaluated the safety and short‐term efficacy of perioperative propranolol and etodolac (COX2 inhibitor) treatment on colorectal and breast cancers, respectively. 54 , 55 Drugs were well tolerated and the treatment significantly decreased tumor‐infiltrating CD14+ monocytes, which are associated with tumor progression and metastatic disease, and reduced markers of epithelial‐to‐mesenchymal transition (EMT), reducing the pro‐metastatic capacity of the malignant tissue. Based on this finding, a randomized phase II clinical trial is recruiting CRC patients that are going to undergo curative surgery, to assess the consequences of the treatment with propranolol and etodolac on disease‐free survival and on pro‐ and anti‐metastatic processes, through biomarkers in blood and in extracted tumor tissue. 56 Similarly, a double‐blind placebo‐controlled two‐arm phase II clinical trial that combines a beta‐blocker with a COX2 inhibitor is recruiting primary pancreatic cancer patients to assess the efficacy and safety of this therapy. 57 Some other clinical trials, which will help us to understand the impact of the use and effectiveness of propranolol on cancer patient management, are still ongoing. These trials include the treatment of metastatic soft tissue sarcoma, melanoma, refractory solid tumors in children and teenagers, gastric cancer, prostate cancer, and breast cancer. 58 , 59 , 60 , 61 , 62 , 63 , 64
Beyond its use on neoadjuvant treatment, several clinical trials seek to assess whether AH treatment may have an action on the prevention of anthracycline‐ and trastuzumab‐induced cardiotoxicity. Although evidence is not clear, it seems that the addition of a beta‐blocker early in the treatment of cancer patients who are undergoing anthracycline or trastuzumab treatment can have beneficial associations in preserving left ventricular ejection fraction and preventing chemotherapy‐induced cardiotoxicity. 65 A recent clinical trial, however, found that neither lisinopril nor carvedilol led to a difference in LVEF reduction in patients with HER2 breast cancer receiving trastuzumab. The same study found that both lisinopril (ACEi) and carvedilol (BB) prevented cardiotoxicity in patients with HER2‐positive breast cancer treated with anthracyclines. 66 In contrast with this, despite a significant reduction in troponin levels and diastolic dysfunction, a prospective, randomized, double‐blind, placebo‐controlled study did not find any difference in LVEF between carvedilol‐ and placebo‐treated patients. 67 Treatment of CRC patients with TD also showed a protective association with cancer‐specific mortality. A similar finding was described in another retrospective SEER‐Medicare cohort study, in which thiazide diuretics reduced ovarian cancer‐specific mortality (HR of 0.82, 95% CI 0.68–0.99%). 68
Although data are data that AH drugs can interfere with tumor vasculature and microenvironment, it is hard to determine whether our results are due to the impact of the AH medication or the hypertension control. Some studies have already associated hypertension with increased cancer risks, 69 , 70 which might suggest that hypertension control could be an approach for cancer prevention. In patients with mild hypertension and low‐moderate cardiovascular risk, treatment often began with lifestyle changes. 71 There are little literature data that analyzed these interventions with cancer outcomes. Some of these strategies, like the DASH diet and physical activity, were related to decreased incidence of CRC and reduced cancer mortality, though the correlation with hypertension control was not assessed. 72 , 73 , 74 Therefore, it is possible that AH drugs can help achieve better outcomes on cancer patients by both mechanisms, interfering with tumor microenvironment and vasculature and also controlling blood pressure, but more research is needed to assess these correlations and potential shared mechanisms.
Despite such research, there is a sparse evidence base regarding the use of the AH drug class for cancer treatment. Thus, the results of this study are novel and suggest future research analyzing the application of AHs as a tool to improve cancer‐related mortality. In this study, we examined CRC outcomes, however, it is necessary to also investigate mortality risk in other cancers such as gastric and bladder cancer. Furthermore, we examined the application of only the four major classes of AH agents, leaving room for further study of substances such as metformin for their potential onco‐protective associations. Research collaborations exploring this space are necessary to discover novel treatment approaches for cancers.
4.1. Limitations
There are several limitations to our study. First, this study only examined data spanning 5 years, and was thereby limited by time span as well its retrospective nature and inherent biases. Second, the use of prescription Part D claims for adherence estimation may not represent patient medication consumption data accurately as it does not speak to patient behaviors. Third, a limitation intrinsic to administrative claims database analysis is the existence of potential coding errors though this likely would not occur differentially. In addition, patient data exploring psychosocial characteristics such as anxiety, depression, knowledge, beliefs, and barriers to treatment are not readily accessible. Future studies may explore these unmeasurable variables which contribute to survival. Fourth, this study only examined cancer patients with stage I–III tumors and thus the results are not generalizable to other stages of CRC. Fifth, given that this data only encompasses real‐world utilization data, it is not necessarily concordant with clinical practice guidelines. Further, it does allow for information on which patients may be ineligible for treatments such as chemotherapy. Finally, the findings of this study may not be generalizable to patients enrolled in insurance programs other than Medicare, under 65 years or with stage IV tumors. Accordingly, future studies may seek replication in more diverse study populations and time intervals.
CONFLICT OF INTEREST
The authors declare that no competing interests exist
ACKNOWLEDGMENTS
RB, FC, and RD work supported by the UVA Cancer Center resources. RC work is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2015/22814‐5). LF work is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2020/15317‐3).
Balkrishnan R, Desai RP, Narayan A, Camacho FT, Flausino LE, Chammas R. Associations between initiating antihypertensive regimens on stage I–III colorectal cancer outcomes: A Medicare SEER cohort analysis. Cancer Med. 2021;10:5347–5357. 10.1002/cam4.4088
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the Center for Medicaid and Medicare Services (CMS). Restrictions apply to the availability of these data, which were used under license for this study. Data used in the analyses are available from the authors with the permission of the CMS.
REFERENCES
- 1. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Gastroenterology Review. 2019;14(2):89‐103. 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kekelidze M. Colorectal cancer: current imaging methods and future perspectives for the diagnosis, staging and therapeutic response evaluation. World J Gastroenterol. 2013;19(46):8502. 10.3748/wjg.v19.i46.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vermeulen P, Gasparini G, Fox S, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38(12):1564‐1579. 10.1016/s0959-8049(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 4. Nico B, Benagiano V, Mangieri D, Maruotti N, Vacca A, Ribatti D. Evaluation of microvascular density in tumors: pro and contra. Histol Histopathol. 2008;23(5):601–607. 10.14670/HH-23.601. [DOI] [PubMed] [Google Scholar]
- 5. Jain R. Barriers to drug delivery in solid tumors. Sci Am. 1994;271(1):58‐65. 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 6. Brown J, Wilson W. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4(6):437‐447. 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 7. Heldin C, Rubin K, Pietras K, Östman A. High interstitial fluid pressure — an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806‐813. 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 8. Yu T, Liu K, Wu Y, et al. High interstitial fluid pressure promotes tumor cell proliferation and invasion in oral squamous cell carcinoma. Int J Mol Med. 2013;32(5):1093‐1100. 10.3892/ijmm.2013.1496. [DOI] [PubMed] [Google Scholar]
- 9. Cairns R. Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol Cancer Re. 2006;4(2):61‐70. 10.1158/1541-7786.mcr-06-0002. [DOI] [PubMed] [Google Scholar]
- 10. Lunt S, Fyles A, Hill R, Milosevic M. Interstitial fluid pressure in tumors: therapeutic barrier and biomarker of angiogenesis. Future Oncol. 2008;4(6):793‐802. 10.2217/14796694.4.6.793. [DOI] [PubMed] [Google Scholar]
- 11. Graham K, Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int J Nanomedicine. 2018;13:6049–6058. 10.2147/ijn.s140462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.[12]Jing X, Yang F, Shao C, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18(1):157. 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hammond E. Hypoxia‐inducible factor‐1 and p53: friends, acquaintances, or strangers? Clin Cancer Res. 2006;12(17):5007‐5009. 10.1158/1078-0432.ccr-06-0613. [DOI] [PubMed] [Google Scholar]
- 14. Jeong H, Bok S, Hong B, Choi H, Ahn G. Radiation‐induced immune responses: mechanisms and therapeutic perspectives. Blood Res. 2016;51(3):157. 10.5045/br.2016.51.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jain R. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605‐622. 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jain R. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205‐2218. 10.1200/jco.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noman M, Hasmim M, Lequeux A, et al. Improving cancer immunotherapy by targeting the hypoxic tumor microenvironment: new opportunities and challenges. Cells. 2019;8(9):1083. 10.3390/cells8091083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Patel S, Roszik J, Qin Y. Hypoxia‐driven immunosuppressive metabolites in the tumor microenvironment: new approaches for combinational immunotherapy. Front Immunol. 2018;9:1591. 10.3389/fimmu.2018.01591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goel S, Duda D, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071‐1121. 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cully M. Tumour vessel normalization takes centre stage. Nat Rev Drug Discov. 2017;16(2):87. 10.1038/nrd.2017.4. [DOI] [PubMed] [Google Scholar]
- 21. Fraeman K, Nordstrom B, Luo W, Landis S, Shantakumar S. Incidence of new‐onset hypertension in cancer patients: a retrospective cohort study. Int J Hypertens. 2013;2013:1‐10. 10.1155/2013/379252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gabrić I. Cardiotoxicity due to biological cancer therapy. Cardiologia Croatica. 2017;12(1–2):16‐22. 10.15836/ccar2017.16. [DOI] [Google Scholar]
- 23. Touyz RM, Alves‐Lopes R, Rios FJ, et al. Vascular smooth muscle contraction in hypertension. Cardiovasc Res. 2018;114(4):529‐539. 10.1093/cvr/cvy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferroni P, Della‐Morte D, Palmirotta R, Rundek T, Guadagni F, Roselli M. Angiogenesis and hypertension: the dual role of anti‐hypertensive and anti‐angiogenic therapies. Curr Vasc Pharmacol. 2012;10(4):479–493. 10.2174/157016112800812836. [DOI] [PubMed] [Google Scholar]
- 25. National Cancer Institute . SEER registries: population characteristics. 2011. https://seer.cancer.gov/registries/characteristics.html. Accessed 14 August 2017. Accessed September 2, 2019.
- 26. Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15(7):457. [PMC free article] [PubMed] [Google Scholar]
- 27. NCI . SEER‐medicare: calculation of comorbidity weights. https://healthcaredelivery.cancer.gov/seermedicare/considerations/calculation.html. Published 2017. Accessed October 9, 2019.
- 28. Klabunde C, Potasky A, Legler J. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258‐1267. [DOI] [PubMed] [Google Scholar]
- 29. Roffman C, Buchanan J, Allison G. Charlson comorbidities index. J Physiother. 2016;62(3):171. 10.1016/j.jphys.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 30. Morris Z, Saha S, Magnuson W, et al. Increased tumor response to neoadjuvant therapy among rectal cancer patients taking angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers. Cancer. 2016;122(16):2487‐2495. 10.1002/cncr.30079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Makar G, Holmes J, Yang Y. Angiotensin‐converting enzyme inhibitor therapy and colorectal cancer risk. J Natl Cancer Inst. 2014;106(2):djt374. 10.1093/jnci/djt374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Otake A, Mattar A, Freitas H, et al. Inhibition of angiotensin II receptor 1 limits tumor‐associated angiogenesis and attenuates growth of murine melanoma. Cancer Chemother Pharmacol. 2009;66(1):79‐87. 10.1007/s00280-009-1136-0. [DOI] [PubMed] [Google Scholar]
- 33. Diop‐Frimpong B, Chauhan V, Krane S, Boucher Y, Jain R. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA. 2011;108(7):2909‐2914. 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chauhan V, Martin J, Liu H, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4(1):2516. 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murphy J, Wo J, Ryan D, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma. JAMA Oncol. 2018;4(7):963. 10.1001/jamaoncol.2018.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. ClinicalTrials.gov . Losartan and nivolumab in combination with FOLFIRINOX and SBRT in localized pancreatic cancer (NCT03563248). https://clinicaltrials.gov/ct2/show/NCT03563248. Accessed 5 Jul 2020.
- 37. ClinicalTrials.gov . SHAPER: a phase 1 study of losartan and hypofractionated radiation therapy after induction chemotherapy for borderline resectable or locally advanced pancreatic cancer (NCT04106856). https://clinicaltrials.gov/ct2/show/NCT04106856. Accessed 5 Jul 2020. Accessed 5 Jul 2020.
- 38. Ursu R, Thomas L, Psimaras D, et al. Angiotensin II receptor blockers, steroids and radiotherapy in glioblastoma—a randomised multicentre trial (ASTER trial). An ANOCEF study . Eur J Cancer. 2019;109:129‐136. 10.1016/j.ejca.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 39. Zhao Y, Cao J, Melamed A, et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc Natl Acad Sci USA. 2019;116(6):2210‐2219. 10.1073/pnas.1818357116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. ClinicalTrials.gov . Imaging perfusion restrictions from extracellular solid stress ‐ an open‐label losartan study (NCT03951142). https://ClinicalTrials.gov/ct2/show/NCT03951142. Accessed 5 Jul 2020.
- 41. ClinicalTrials.gov . A phase I/Ib study of losartan in combination with sunitinib in the treatment of pediatric and adult patients with relapsed or refractory osteosarcoma (NCT03900793). https://clinicaltrials.gov/ct2/show/NCT03900793. Accessed 5 Jul 2020.
- 42. ClinicalTrials.gov . Tissue pharmacokinetics of intraoperative gemcitabine in resectable adenocarcinoma of the pancreas (NCT01276613). https://clinicaltrials.gov/ct2/show/NCT01276613. Accessed 5 Jul 2020.
- 43. Hauge A, Rofstad E. Antifibrotic therapy to normalize the tumor microenvironment. J Transl Med. 2020;18(1): 10.1186/s12967-020-02376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Plummer C, Michael A, Shaikh G, et al. Expert recommendations on the management of hypertension in patients with ovarian and cervical cancer receiving bevacizumab in the UK. Br J Cancer. 2019;121(2):109‐116. 10.1038/s41416-019-0481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oki E, Kato T, Bando H, et al. A multicenter clinical phase II study of FOLFOXIRI plus bevacizumab as first‐line therapy in patients with metastatic colorectal cancer: QUATTRO study. Clin Colorectal Cancer. 2018;17(2):147‐155. 10.1016/j.clcc.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 46. Cai J, Ma H, Huang F, et al. Correlation of bevacizumab‐induced hypertension and outcomes of metastatic colorectal cancer patients treated with bevacizumab: a systematic review and meta‐analysis. World J Surg Oncol. 2013;11:306. 10.1186/1477-7819-11-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakaya A, Kurata T, Yokoi T, et al. Retrospective analysis of bevacizumab‐induced hypertension and clinical outcome in patients with colorectal cancer and lung cancer. Cancer Med. 2016;5(7):1381‐1387. 10.1002/cam4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pedersen L, Idorn M, Olofsson G, et al. Voluntary Running Suppresses Tumor Growth through Epinephrine‐ and IL‐6‐Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016;23(3):554‐562. 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 49. Engineer D, Burney B, Hayes T, Garcia J. Exposure to ACEI/ARB and β‐blockers is associated with improved survival and decreased tumor progression and hospitalizations in patients with advanced colon cancer. Transl Oncol. 2013;6(5):539‐545. 10.1593/tlo.13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krowchuk D, Frieden I, Mancini A, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2018;143(1):e20183475. 10.1542/peds.2018-3475. [DOI] [PubMed] [Google Scholar]
- 51. Frieden I, Haggstrom A, Drolet B, et al. Infantile Hemangiomas: Current Knowledge, Future Directions. Proceedings of a Research Workshop on Infantile Hemangiomas. April 7‐9, 2005 Bethesda, Maryland. Pediatr Dermatol. 2005;22(5):383‐406. 10.1111/j.1525-1470.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- 52. Barron T, Connolly R, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population‐ based study. J Clin Oncol. 2011;29(19):2635‐2644. 10.1200/jco.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 53. Udumyan R, Montgomery S, Duberg A, et al. Beta‐adrenergic receptor blockers and liver cancer mortality in a national cohort of hepatocellular carcinoma patients. Scand J Gastroenterol. 2020;55(5):597‐605. 10.1080/00365521.2020.1762919. [DOI] [PubMed] [Google Scholar]
- 54. Haldar R, Ricon I, Cole S, Zmora O, Ben‐Eliyahu S. Perioperative beta‐adrenergic blockade and COX2 inhibition in colorectal cancer patients improves pro‐metastatic indices in the excised tumor: EMT, tumor infiltrating lymphocytes (TILs), and gene regulatory pathways. Brain Behav Immun. 2017;66:e9. 10.1016/j.bbi.2017.07.046. [DOI] [Google Scholar]
- 55. Shaashua L, Shabat‐Simon M, Haldar R, et al. Perioperative COX‐2 and β‐adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase‐II randomized trial. Clin Cancer Res. 2017;23(16):4651‐4661. 10.1158/1078-0432.ccr-17-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. ClinicalTrials.gov . Perioperative use of a β‐adrenergic blocker, propranolol, and a COX2 inhibitor, etodolac, in patients undergoing resection with curative intent for primary colon and rectal cancer: effect on tumor recurrence and survival (NCT03919461). https://clinicaltrials.gov/ct2/show/NCT03919461. Accessed 5 Jul 2020.
- 57. ClinicalTrials.gov . Perioperative use of a beta‐adrenergic blocker and a COX‐2 inhibitor in patients undergoing surgery with primary pancreatic cancer: intervention aiming to reduce pro‐metastatic processes (NCT03838029). https://clinicaltrials.gov/ct2/show/record/NCT03838029. Accessed 5 Jul 2020.
- 58. ClinicalTrials.gov . The use of propranolol hydrochloride combined with anthracyclin based chemotherapy in the treatment of metastatic soft tissue sarcoma (NCT03108300). https://clinicaltrials.gov/ct2/show/NCT03108300. Accessed 5 Jul 2020.
- 59. ClinicalTrials.gov . Melablock: a multicentre randomized, double‐‐‐blinded and placebo‐‐‐controlled clinical trial on the efficacy and safety of once daily propranolol 80 mg retard for the prevention of cutaneous malignant melanoma recurrence (NCT02962947). https://clinicaltrials.gov/ct2/show/NCT02962947. Accessed 5 Jul 2020.
- 60. ClinicalTrials.gov . A phase Ib/II study of propranolol with fixed‐dose pembrolizumab in patients with unresectable stage III and stage IV melanoma (NCT03384836). https://clinicaltrials.gov/ct2/show/NCT03384836. Accessed 5 Jul 2020.
- 61. ClinicalTrials.gov . Study of a propranolol (HEMANGIOL®) and oral metronomic vinorelbine (NAVELBINE®) combination for children and teenagers with refractory/relapsing solid tumors (NCT02897986). https://clinicaltrials.gov/ct2/show/NCT02897986. Accessed 5 Jul 2020.
- 62. ClinicalTrials.gov . Efficacy and safety of propranolol combined with neoadjuvant chemotherapy in stage III‐IV gastric cancer: an open‐lable, single‐arm study (NCT04005365). https://clinicaltrials.gov/ct2/show/NCT04005365. Accessed 5 Jul 2020
- 63. ClinicalTrials.gov . Evaluating the effect of ADRB2 blockers on PKA/BAD/CREB signaling in patients undergoing prostatectomy (NCT03152786). https://clinicaltrials.gov/ct2/show/NCT03152786. Accessed 5 Jul 2020.
- 64. ClinicalTrials.gov . A study of the beta‐blocker propranolol alone and with chemotherapy in patients receiving neoadjuvant treatment for newly diagnosed breast cancer (NCT01847001). https://clinicaltrials.gov/ct2/show/NCT01847001. Accessed 5 Jul 2020.
- 65. Blanter J, Frishman W. The preventive role of angiotensin converting enzyme inhibitors/angiotensin‐II receptor blockers and β‐adrenergic blockers in anthracycline‐ and trastuzumab‐induced cardiotoxicity. Cardiol Rev. 2019;27(5):256‐259. 10.1097/crd.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 66. Guglin M, Krischer J, Tamura R, et al. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol. 2019;73(22):2859‐2868. 10.1016/j.jacc.2019.03.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ayub‐Ferreira S, Avila M, Brandão S, et al. Carvedilol for prevention of chemotherapy‐induced cardiotoxicity: final results of the prospective, randomized, double‐blind, placebo controlled CECCY trial. J Am Coll Cardiol. 2020;75(11):658. 10.1016/s0735-1097(20)31285-7. [DOI] [Google Scholar]
- 68. Harding BN, Delaney JA, Urban RR, Weiss NS. Post‐diagnosis use of antihypertensive medications and the risk of death from ovarian cancer. Gynecol Oncol. 2019;154(2):426‐431. 10.1016/j.gyno.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 69. Christakoudi S, Kakourou A, Markozannes G, et al. Blood pressure and risk of cancer in the European prospective investigation into cancer and nutrition. Int J Cancer. 2020;146(10):2680‐2693. 10.1002/ijc.32576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Seretis A, Cividini S, Markozannes G, et al. Association between blood pressure and risk of cancer development: a systematic review and meta‐analysis of observational studies. Sci Rep. 2019;9(1):8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334‐1357. 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 72. Ali Mohsenpour M, Fallah‐Moshkani R, Ghiasvand R, et al. Adherence to dietary approaches to stop hypertension (DASH)‐style diet and the risk of cancer: a systematic review and meta‐analysis of cohort studies. J Am Coll Nutr. 2019;38(6):513‐525. 10.1080/07315724.2018.1554460. [DOI] [PubMed] [Google Scholar]
- 73. Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The association of dietary approaches to stop hypertension (DASH) Diet with the risk of colorectal cancer: a meta‐analysis of observational studies. Nutr Cancer. 2020;72(5):778‐790. 10.1080/01635581.2019.1651880. [DOI] [PubMed] [Google Scholar]
- 74. McTiernan A, Friedenreich CM, Katzmarzyk PT, et al. Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc. 2019;51(6):1252‐1261. 10.1249/MSS.0000000000001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the Center for Medicaid and Medicare Services (CMS). Restrictions apply to the availability of these data, which were used under license for this study. Data used in the analyses are available from the authors with the permission of the CMS.


