Abstract
This paper reviews the current status of the knowledge on body surface potential mapping (BSPM) and ECG imaging (ECGI) methods for patient selection, left ventricular (LV) lead positioning, and optimisation of CRT programming, to indicate the major trends and future perspectives for the application of these methods in CRT patients. A systematic literature review using PubMed, Scopus, and Web of Science was conducted to evaluate the available clinical evidence regarding the usage of BSPM and ECGI methods in CRT patients. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement was used as a basis for this review. BSPM and ECGI methods applied in CRT patients were assessed, and quantitative parameters of ventricular depolarisation delivered from BSPM and ECGI were extracted and summarised. BSPM and ECGI methods can be used in CRT in several ways, namely in predicting CRT outcome, in individualised optimisation of CRT device programming, and the guiding of LV electrode placement, however, further prospective or randomised trials are necessary to verify the utility of BSPM for routine clinical practice.
Keywords: Body surface potential mapping, ECG imaging, heart failure, CRT
Heart failure (HF) is a common condition with significant morbidity and mortality. One of the therapeutic options for advanced management of patients with HF with reduced or preserved left ventricular ejection fraction (HFrEF or HfpEF, respectively) is CRT. In a selected population of candidates, it improves symptoms, quality of life, and prognosis. However, not all patients respond favourably to CRT, which indicates that there are unsolved issues in accurate patient selection and the proper delivery of CRT.[1] Several characteristics predict improvement in morbidity and mortality, and the extent of reverse remodelling is one of the most important mechanisms of action of CRT. Of the clinical parameters, QRS duration is always used as an outcome parameter in all available trials, but consensus has not been reached regarding the optimal ECG-based criteria for patient selection for a CRT device.
The strategy for CRT device optimisation also remains challenging. The available methods include echocardiography, ECG QRS-based assessment, invasive haemodynamic measurements, and/or non-invasive cardiac mapping.[2] Among others, ECG imaging (ECGI) may be a comprehensive tool for measuring ventricular electrical dyssynchrony.[3–5] However, the results are fragmentary and have not been summarised. This paper reviews the current knowledge on non-invasive ECG mapping methods for patient selection, left ventricular (LV) lead positioning, and optimisation of CRT programming, to determine the major trends and future perspectives for the application of these methods in CRT patients.
Given that the body surface potential mapping (BSPM) and ECGI methods are used mostly in the research field rather than in clinical settings, no standardised terminology concerning the specification of approaches exists. For this review, the methods related to the analysis and interpretation of body surface multi-lead ECG are referred to as BSPM. Techniques involved in the reconstruction of myocardial electrical potentials using body surface ECGs are termed ECGI.
Methods
A systematic literature review was carried out to evaluate the available clinical evidence regarding non-invasive cardiac mapping methods for patient selection, lead placement, and optimisation of CRT programming. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement was used as a basis for this review.[6]
In this review, we included randomised controlled trials and observational studies, such as cohort studies or case–control studies. Conference abstracts, letters and case reports were excluded. To cover all available evidence, we did not use any publication date restrictions.
The search was conducted using MEDLINE (through PubMed), Web of Science and Scopus databases. No limits were applied to language and foreign papers were translated. The reference lists of all included publications were checked to identify additional relevant studies. We also examined any relevant retraction statements and errata for the included studies.
The search terms included ‘CRT’, ‘BSPM’, ‘ECGI’, ‘electrocardiographic mapping (ECM)’, ‘congestive HF’, ‘HFrEF’, and various combinations of these terms. The full search strategies are given in the Supplementary Material.
The selected review publications were used to identify other publications not covered by our search. From the original studies included in the qualitative analysis, we extracted the following characteristics for each included study: participants (number of participants, target population); BSPM type (ECGI or simple BSPM); and BSPM parameters (definition and findings).
Duplicate articles were identified and excluded. Title and abstract screening for eligibility was performed by two reviewers independently. The full text of citations judged as potentially eligible was retrieved and screened in a blinded manner. The disagreement between the reviewers was then resolved by discussion or, if required, with the help of a third independent reviewer.
Results
Search Results
The literature search was performed on 21 January 2020. In total, 420 publications were identified in the main sources (PubMed, Scopus, and Web of Science). After the title and abstract screening, 156 records were excluded. The full text of 74 records was assessed for eligibility, and 23 original studies met the inclusion criteria. The procedure for selecting publications is shown in the PRISMA diagram (Figure 1).
Figure 1: PRISMA Flow Diagram.
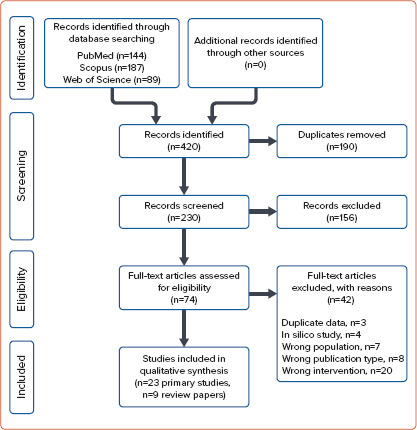
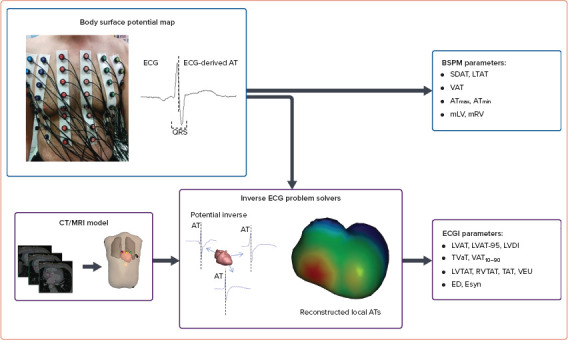
Table 1 summarises the 23 selected original studies in the population, intervention, control and outcomes (PICO) format.[3,5,7–27] They include both BSPM (n=8) and ECGI methods (n=15). The median number of body surface leads used for ECGI was higher than for BSPM techniques (252 [IQR 192–252] versus 64 [IQR 53–87], respectively, p<0.0001). Non-invasive cardiac mapping approaches were applied in patients with CRT in several scenarios: the prediction of CRT response and patient selection; the selection of optimal LV pacing site; and the optimisation of CRT programming. Quantitative parameters of ventricular depolarisation are listed in Table 2. BSPM characteristics of depolarisation were mostly based on the detection of activation time, defined as the minimum of the first derivative of potential with respect to time during the QRS complex in unipolar body surface ECG leads. Parameters of ventricular activation obtained from reconstructed epicardial electrical potentials using ECGI techniques included local characteristics of ventricular depolarisation such as LV activation time, the interventricular difference in activation time, and intraventricular activation time distribution (Figure 2).
Table 1: Summary of Included Original Studies.
| Objective | Population | n | Intervention, No. of Leads |
|---|---|---|---|
| CRT optimisation | |||
| Bank et al. 2018[8] | 69 ± 11 years; 68% men; EF 26 ± 7%; QRSd 159 ± 23 ms; MI 50%; LBBB 62% | 94 | BSPM, 53 |
| Berger et al. 2005[9] | 61 ± 8 years; 76% men; EF 21 ± 5%; QRSd 150 ± 24 ms; MI 44%; LBBB 100% | 25 | BSPM, 64 |
| Lumens et al. 2013[15] | 66 ± 12 years; 71% men; EF 27 ± 3%; QRSd 164 ± 22 ms; MI 33% | 24 | ECGI, 252 |
| Pastore et al. 2006[17] | 61 ± 9 years; 71% men; EF 28 ± 8%; QRSd 180 ± 19 ms; MI 11%; LBBB 100% | 28 | BSPM, 87 |
| Pereira et al. 2018[18] | 68 ± 13 years; 79% men; MI 63%; LBBB 74% | 19 | ECGI, 252 |
| Pereira et al. 2019[19] | 69 ± 12 years; 81% men; EF 27 ± 10%; QRSd 162 ± 21 ms; MI 62%; LBBB 71% | 21 | ECGI, 252 |
| Samesima et al. 2013[24] | 61 ± 10 years; 60% men; EF 28 ± 9%; QRSd 182 ± 24 ms; MI 20% | 55 | BSPM, 87 |
| Sieniewicz et al. 2019[26] | 61 ± 13 years; 80% men; EF 28 ± 9%; QRSd 168 ± 8 ms; MI 80%; LBBB 80% | 5 | ECGI, 252 |
| LV lead positioning | |||
| Arnold et al. 2018[7] | 67 ± 10 years; 53% men; EF 26 ± 7%; QRSd 158 ± 21 ms (BiV pacing); MI 38%; LBBB 100% | 23 | ECGI, 252 |
| Ghosh et al. 2011[11] | 51 ± 18 years; 44% men; EF 22 ± 5%; QRSd 138 ± 27 ms; MI 0%; | 25 | ECGI, NA |
| Johnson et al. 2017[13] | 65 ± 14 years; 75% men; EF 27 ± 7%; QRSd 165 ± 26 ms; MI 40%; LBBB 58% | 60 | BSPM, 53 |
| Nguyen et al. 2019[16] | 71 ± 8 years; 81% men; EF 24 ± 8%; QRSd 142 ± 21 ms; MI 69%; LBBB 38% | 16 | ECGI, 184 |
| Rudy 2006[22] | 72 ± 11 years; 75% men | 8 | ECGI, 220 - 250 |
| Varma 2014[27] | 54 ± 15 years; 33% men; EF 18 ± 3%; QRSd 146 ± 7 ms; MI 33%; LBBB 66% | 3 | ECGI, >200 |
| Patient selection | |||
| Dawoud et al. 2016[10] | 63 ± 10 years; 75% men; EF 20 ± 5%; QRSd 149 ± 9 ms; MI 37%; LBBB 100% | 8 | ECGI, 120 |
| Gage et al. 2017[3] | 70 ± 11 years; 67% men; EF 27 ± 7%; QRSd 152 ± 26 ms; MI 48%; LBBB 55% | 66 | BSPM, 53 |
| Jia et al. 2006[12] | 72 ± 11 years; 75% men; EF 19 ± 7%; QRSd 155 ± 22 ms; MI 75%; LBBB 75%; | 8 | ECGI, NA |
| Kittnar et al. 2018[14] | 62 ± 6 years; 57% men; EF 25 ± 8%; QRSd 170 ± 12 ms; LBBB 52%; | 21 | BSPM, NA |
| Ploux et al. 2013[20] | 65 ± 9 years; 85% men; EF 27 ± 4%; QRSd 152 ± 22 ms; MI 42%; LBBB 55% | 33 | ECGI, 252 |
| Ploux et al. 2015[21] | 65 years (median); 80% men; EF 28%(median); QRSd 146 ms (median); MI 46%; LBBB 43% | 61 | ECGI, 252 |
| Samesima et al. 2007[23] | 60 ± 11 years; 62% men; EF 31 ± 8%; QRSd 185 ± 35 ms; MI 18%; LBBB 100% | 56 | BSPM, 87 |
| Shannon et al. 2008[25] | 64 ± 12 years; 71% men; MI 56% | 34 | ECGI, 80 |
| Strik et al. 2018[5] | 67 ± 10 years; 78% men; EF 29 ± 5%; MI 48%; LBBB 51% | 79 | ECGI, 252 |
Data given as mean ± SD if not otherwise specified. BSPM = body surface potential mapping; ECGI = ECG imaging; EF = ejection fraction; LBBB = left bundle branch block; NA = not available; QRSd = QRS duration.
Table 2: Parameters of Ventricular Activation Derived from Body Surface Potential Mapping or ECG Imaging.
| Reference | Parameters |
|---|---|
| BSPM | |
| Bank et al. 2018[8] | SDAT |
| Gage et al. 2017[3] | SDAT, LTAT |
| Johnson et al. 2017[13] | SDAT, LTAT |
| Kittnar et al. 2018[14] | ATmax, ATmin |
| Pastore et al. 2006[17] | mAS, mLV, mRV |
| Samesima et al. 2007[23] | Global VAT, regional VAT, RV–LV VAT difference |
| Samesima et al. 2013[24] | Global VAT, regional VAT, RV–LV VAT difference |
| ECGI | |
| Arnold et al. 2018[7] | LVAT, LVAT-95, LVDI |
| Ghosh et al. 2011[11] | ED |
| Jia et al. 2006[12] | Esyn |
| Lumens et al.2013[15] | ATLV, ATTOT |
| Pereira et al. 2018[18] | TVaT, VaT10–90 |
| Pereira et al. 2019[19] | TVaT, VaT10–90 |
| Ploux et al. 2013[20] | LVTAT, RVTAT, VEU |
| Ploux et al. 2015[21] | LVTAT, TAT, VEU |
| Rudy 2006[22] | Esyn |
| Sieniewicz et al. 2019[26] | VVsync, VVTAT, LVTAT, LVdisp |
| Strik et al. 2018[5] | ADV |
| Varma 2014[27] | LVTAT |
ADV = activation delay vector; ATLV = left ventricular activation time; ATmax = maximum activation time; ATmin = minimum activation time; ATTOT = total ventricular activation time; BSPM = body surface potential mapping; ECGI = ECG imaging; ED = electrical dyssynchrony; Esyn = electrical synchrony index; LTAT = average left thorax activation time; LVAT = left ventricular activation time; LVAT-95 = left ventricular activation time spanning 95% of activations; LVDI = left ventricular dyssynchrony index; LVdisp = global left ventricular dispersion of activation; LVTAT = left ventricular total activation time; mAS = anterior septal area mean activation time; mLV = left ventricle mean activation time; mRV = right ventricle mean activation time; RVTAT = right ventricular total activation time; SDAT = standard deviation of activation times; TAT = total activation time; TVaT = total ventricular activation time; VAT = ventricular activation time; VaT10–90 = ventricular activation time10–90 (delay between the 10th and 90th percentiles of ventricular activation time); VEU = ventricular electrical uncoupling; VVsync = global right/left ventricular electrical synchrony.
Figure 2: Schematic Presentation of BSPM and ECGI Approaches to Determine Electrical Synchrony in Heart Ventricles.
Patient Selection for CRT
Current European Society of Cardiology guidelines approve CRT as an indication for patients with symptomatic HFrEF and intraventricular conduction abnormality, especially QRS duration >150 ms and left bundle branch block (LBBB) morphology. In contrast, patients with QRS duration <120–130 ms are not indicated for CRT, due to a low success rate and possible worsening of HF.[21,28]
Assessment of electrical dyssynchrony is important for the accurate identification of appropriate CRT candidates. Although QRS duration is used as an indirect measure of dyssynchrony, some studies noted a weak correlation between QRS duration and mechanical dyssynchrony.[29,30] BSPM and ECGI approaches have been applied in clinical studies to develop reliable parameters of ventricular activation for assessment of the CRT effects (Table 2).
BSPM-based Selection Criteria
BSPM-derived activation time (AT) is the duration between the QRS complex onset and the steepest negative slope of the QRS complex.[3] This can then be visualised as body surface isochronal maps of ATs. The isochronal maps of ATs have been obtained using BSPM with 53 ECG leads to assess the changes in electrical dyssynchrony in patients with CRT. Quantitative metrics of dyssynchrony such as the standard deviation of activation times (SDAT) and average left thorax activation time (LTAT) have been suggested. Patients with native SDAT ≥35 ms and QRS duration ≥120 ms had significant reverse LV remodelling (improvement in left ventricular ejection fraction [LVEF] and decrease in end-systolic volume), thus both parameters have been suggested as predictors of CRT response.[3] The longest activation time (ATmax) detected in any of 123 unipolar chest leads, served as a reliable dyssynchrony marker to predict CRT outcome.[14] The right ventricular to left ventricular (RV–LV) activation gradient was identified through measures of QRS durations in 87-lead BSPM. It was suggested that an RV–LV activation gradient <20 ms during biventricular pacing could identify patients with improved functional class after CRT.[23]
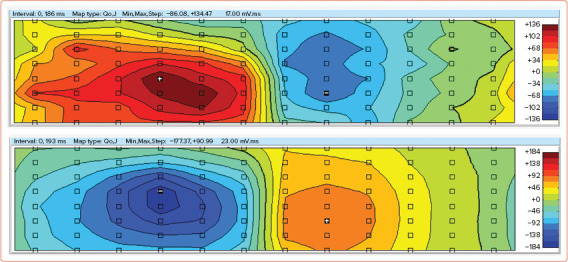
In our pilot study, an analysis of ventricular depolarisation was performed during different pacing configurations in selected patients using the QRS integral maps produced from a 96-lead mapping system (unpublished data). A 46-year-old patient with a history of post-myocarditis cardiomyopathy, LBBB (QRS 172 ms) and progressive LV dysfunction (LVEF 30%) underwent permanent CRT defibrillator (CRT-D) implantation in 2019, leading to reverse remodelling 6 months after CRT-D implantation (improvement of LVEF to 45%). The distribution of positive and negative time integrals correlated with the acute haemodynamic response to the different pacing configurations. Obtained time integral maps of the QRS complex reflected the improvement in LVEF during LV pacing compared with atrial pacing (Figure 3).
Figure 3: QRS Integral Maps of a Patient with Heart failure Under Atrial Pacing (Top) and Left Ventricular Pacing (Bottom).
ECGI-based Selection Criteria
In patients with LBBB and non-specific intraventricular conduction disturbance (NICD), an ECGI-derived index of electrical dyssynchrony, ventricular electric uncoupling (VEU), defined as the difference between the mean epicardial LV and RV activation times, served as a significant predictor of response to CRT. It was concluded that in consecutive CRT candidates with QRS duration ≥120 ms, VEU is a more reliable predictor of clinical CRT response than QRS duration or the presence of LBBB.[20] Supporting data were obtained in the study in which VEU was calculated at baseline and during biventricular pacing to assess the resynchronising effect in relation to the underlying electrical substrate. Responders had higher baseline VEU and more intensive reduction of VEU in response to biventricular pacing than did non-responders.[21]
Given that VEU represents the impairment of ventricular depolarisation only with regard to time, an activation delay vector (ADV) adds an additional parameter: a direction in space. This parameter represents a comprehensive electrical substrate, such that patients may have a similar direction of activation delay but a great difference in its magnitude. This parameter might be used to determine right-to-left activation delay and identify responders.[5]
Electrical synchrony of ventricles was assessed using isochronal activation maps obtained with the ECGI technique. The interventricular synchrony index, Esyn (the difference between activation times in the RV and LV), for estimation of electrical synchrony, however, did not always correlate with clinical improvement.[12] In that case, a non-invasive ECGI approach for reconstruction of epicardial ventricular activation was then applied, in combination with cardiac magnetic resonance used for mechanical imaging of dyssynchrony in the LV. Electromechanical dissociation has been suggested as a marker of reduced response to CRT.[10]
Spatiotemporal myocardial activation maps were constructed using the ECGI method in patients with wide QRS complex before CRT. The different patterns of myocardial activation described suggested an association between electrophysiological pattern and the effect of CRT.[25]
In summary, the most promising predictors of response to CRT appear to be SDAT and LTAT, parameters that can be derived from BSPM, without the need for 3D imaging. These parameters are easy to obtain and analyse.
LV Lead Placement
The degree of cardiac resynchronisation response is influenced by many factors, of which the position of the LV lead on the heart is an important one. Many strategies have been used to optimise LV lead placement. Some of them use QLV interval, that is, the time between Q onset on the ECG and local depolarisation at the LV electrode, as measured during implantation, to determine the site of the latest activation. Other strategies involve echocardiography or MRI to evaluate the proximity of the lead to the site of maximal mechanical dyssynchrony.
BSPM-based LV Lead Placement
More recently, BSPM has been suggested as another alternative for LV lead positioning.[2,13] The pacing site with the greatest decrease in SDAT and LTAT has been shown to have a strong correlation with the acute haemodynamic response measured invasively.[13]
ECGI-based LV Lead Placement
In patients with a quadripolar LV lead, ECGI visualisation of endocardial and epicardial activation was applied to identify the optimal area for pacing, that is, the site with the shortest total activation duration of both ventricles.[27,31] ECGI isochronal maps of epicardial ventricular depolarisation also enabled the guidance of LV lead placement for improved clinical outcome.[22] Another study integrated ECGI with CT angiography and cardiac magnetic resonance to develop the ‘CRT roadmap’, which provides data on scar localisation, epicardial activation sequence, and coronary venous anatomy. This CRT roadmap was suggested to be a reliable tool to guide LV lead placement.[16]
In some cases, LV lead placement is suboptimal due to unfavourable anatomy of the coronary venous system, and the response to CRT may be inferior.[32] Recently, His bundle pacing (HBP) has emerged as an alternative to CRT. With the help of ECGI, it was established that HBP reduced LV activation time and LV dyssynchrony index (LVDI) more than twofold compared with biventricular pacing.[7,11] The His-SYNC (His Bundle Pacing versus Coronary Sinus Pacing for Cardiac Resynchronisation Therapy) pilot trial was an investigator-initiated, prospective, randomised controlled study that demonstrated that there were no significant improvements in ECG or echocardiographic parameters compared with biventricular pacing-CRT.[33]
Optimisation of CRT Programming
LV pacing and biventricular pacing have a similar, positive effect on the haemodynamic function of patients with HF, while RV pacing alone is highly ineffective.[12,17,22,34]
BSPM-based CRT Optimisation
BSPM parameters such as SDAT can be useful for both LV pacing and biventricular pacing programming.[8] CRT programmed at baseline settings can reduce dyssynchrony by up to 20%. This improvement is greater in the LBBB group of patients with wide QRS, and is lacking in the non-LBBB group. However, individualised and optimised settings based on BSPM parameters, such as SDAT, can further improve ventricular activation time by 46%, compared with standard pacing settings.[3] SDAT reduction ≥10% was a significant predictor of improved ejection fraction and LV end-systolic volume response. With CRT optimisation, it is possible to achieve twofold improvement in electrical synchrony, regardless of patient baseline characteristics.[8] Another BSPM approach based on measurement of QRS duration in 87 body surface unipolar leads showed that regional activation time in the RV increased in biventricular pacing, but it was compensated for by an even greater decrease in activation time in the LV, therefore the effect of CRT could be optimised by decreasing the inter-regional RV–LV gradients.[23,24] In addition, BSPM parameters of ventricular repolarisation dispersion such as Tpeak–Tend interval, Tpeak–Tend integral, and T wave amplitude were reduced compared with sinus rhythm under biventricular pacing, whereas RV or LV pacing resulted in increased dispersion of repolarisation.[9]
ECGI-based CRT Optimisation
Using ECGI techniques to reconstruct epicardial isochronal maps, it has been shown that, despite the positive haemodynamic response during LV pacing, only biventricular pacing has resulted in reduced electrical dyssynchrony, represented by decreased total RV and LV activation time.[15] The individual configuration of LV quadripolar leads guided by the parameters total ventricular activation time (TVaT) and the time for the bulk of ventricular activation (VaT10–90), which were obtained from ECGI, significantly increased the resynchronisation effect in both ischaemic and non-ischaemic patients.[18] The aforementioned parameters, TVaT and VaT10–90, were also used to identify the optimal atrioventricular delay (AVD) and interventricular pacing interval (VVD). The minimum TVaT and VaT10–90 values were associated with the most improved ventricular haemodynamics, suggesting that ECG mapping approaches are effective for programming optimisation.[19] The potential of ECGI activation maps for detection of the best configuration of multi-polar pacing was demonstrated in a pilot study with five patients.[26]
Discussion
One of the therapeutic options for advanced management of HFrEF patients during the last 30 years has been CRT. To increase the efficiency of CRT, different approaches have been applied, such as clinical and experimental approaches, and computer simulation. The experimental porcine model of LBBB to induce electrical and mechanical dyssynchrony was suggested for the study of the mechanisms of CRT effect in a treatment of HF.[35] Preclinical studies, including both animal experimental models and patient-specific computational models of the heart, demonstrate a high potential for prediction and optimisation of CRT treatment. Nevertheless, clinical studies are required to validate the efficacy of these models in the target HF population, as well as the applicability of these models to the ECGI methods discussed in this work.[36]
BSPM could be useful for the selection of HFrEF patients with a borderline QRS width on standard ECG. Most of the evidence suggests that SDAT ≥35 ms from BSPM with 53 leads can predict reverse LV remodelling after CRT, as can the greater change of SDAT (ΔSDAT) from baseline to post-implant values. ECGI can further improve patient selection with the use of parameters such as ADV or VEU. BSPM has been advocated as an alternative guide for the positioning of the LV lead during the implant procedure. It is based on the presumption that choosing the pacing site with the greatest reduction in SDAT will correspond to an improvement in haemodynamics as evaluated using invasive measurement of acute haemodynamic response. However, there is no comparison between these BSPM-derived parameters and the simple strategy, such as the selection of the pacing site based on the identification of the late local activation in sinus rhythm. In our opinion, BSPM has a large potential for individualising the optimisation of CRT device programming. This conclusion is based on studies showing that the optimised SDAT parameter is predictive of reverse remodelling, regardless of the baseline characteristics of the CRT candidates.
As an alternative to BSPM, a novel approach based on ultra-high-frequency ECG was recently suggested to improve patient selection for CRT treatment. Jurak et al. demonstrated that an ultra-high-frequency 14-lead ECG technique could improve the application of CRT based on new ECG indices of ventricular depolarisation.[37] This technique may prove to be a valuable addition to the discussed BSPM and ECGI methods.
Artificial intelligence techniques have recently been proposed as a promising tool in cardiac electrophysiology to increase the diagnostic accuracy and treatment capabilities of medical technologies such as surface ECG, intracardiac mapping and cardiac implantable electronic devices.[38,39] A machine learning model with nine variables demonstrated improved CRT response prediction compared with guidelines.[40] Kalscheur et al. developed a random forest model that predicted all-cause mortality and HF hospitalisation in patients receiving CRT implantation, based on pre-implant characteristics.[41] Hu et al. successfully applied machine learning techniques with natural language processing to identify a subgroup of patients who were unlikely to benefit from CRT.[42] Machine learning models that relied on pre-implantation clinical, echocardiographic, and ECG characteristics produced understandably better predictions of CRT benefit than those that relied on ECG parameters.[41,42] This integration approach based on analysis of many clinical parameters may provide a new opportunity for personalised management of patients with HF. The combination of ECGI-derived parameters and machine learning models may provide a pathophysiological interpretation of related clinical features and CRT response.
An advantage of ECGI methods relates to the ability to obtain important information on CRT effect through an electrical solution to a mechanical problem. A solid understanding of the electromechanical structure of the heart is required. Future ECGI developments should therefore aim to increase the modelling capabilities used in ECG technology to reduce the number of required ECG leads, preferably to the standard 12-lead ECG configuration. Recent developments in the anatomical localisation of premature ventricular contractions from a 12-lead ECG using ECGI technology show that the potential of the ECGI technology has not been fully explored.[43]
Conclusion
BSPM and ECGI can be used in CRT in several ways. There is a potential for improvement of patient selection for CRT, optimisation of CRT programming and LV lead placement. The most promising parameter (and also the easiest to obtain) is SDAT derived from BSPM. Further prospective or randomised trials are necessary to identify the utility of BSPM for routine clinical practice.
Clinical Perspective
For clinical use, the standard deviation of activation times appears to be the most promising parameter for individualised optimisation of CRT device programming without the need for imaging studies.
Further prospective or randomised trials are necessary to verify the utility of body surface potential mapping for routine clinical practice.
ECG imaging approaches can provide detailed information on the depolarisation process in ventricles with heart failure, which is crucial for understanding the relationship between electromechanical status and CRT effect.
Supplementary Material
References
- 1.Thomas G, Kim J, Lerman BB. Improving cardiac resynchronisation therapy. Arrhythm Electrophysiol Rev. 2019;8:220–7. doi: 10.15420/aer.2018.62.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pujol-Lopez M, San Antonio R, Mont L et al. Electrocardiographic optimization techniques in resynchronization therapy. Europace. 2019;21:1286–96. doi: 10.1093/europace/euz126. [DOI] [PubMed] [Google Scholar]
- 3.Gage RM, Curtin AE, Burns KV et al. Changes in electrical dyssynchrony by body surface mapping predict left ventricular remodeling in cardiac resynchronization therapy patients. Heart Rhythm. 2017;14:392–9. doi: 10.1016/j.hrthm.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Bear LR, Huntjens PR, Walton RD et al. Cardiac electrical dyssynchrony is accurately detected by noninvasive electrocardiographic imaging. Heart Rhythm. 2018;15:1058–69. doi: 10.1016/j.hrthm.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Strik M, Ploux S, Huntjens PR et al. Response to cardiac resynchronization therapy is determined by intrinsic electrical substrate rather than by its modification. Int J Cardiol. 2018;270:143–8. doi: 10.1016/j.ijcard.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold AD, Shun-Shin MJ, Keene D et al. His resynchronization versus biventricular pacing in patients with heart failure and left bundle branch block. J Am Coll Cardiol. 2018;72:3112–22. doi: 10.1016/j.jacc.2018.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bank AJ, Gage RM, Curtin AE et al. Body surface activation mapping of electrical dyssynchrony in cardiac resynchronization therapy patients: potential for optimization. J Electrocardiol. 2018;51:534–41. doi: 10.1016/j.jelectrocard.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Berger T, Hanser F, Hintringer F et al. Effects of cardiac resynchronization therapy on ventricular repolarization in patients with congestive heart failure. J Cardiovasc Electrophysiol. 2005;16:611–17. doi: 10.1046/j.1540-8167.2005.40496.x. [DOI] [PubMed] [Google Scholar]
- 10.Dawoud F, Spragg DD, Berger RD et al. Non-invasive electromechanical activation imaging as a tool to study left ventricular dyssynchronous patients: implication for CRT therapy. J Electrocardiol. 2016;49:375–82. doi: 10.1016/j.jelectrocard.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh S, Silva JNA, Canham RM et al. Electrophysiologic substrate and intraventricular left ventricular dyssynchrony in nonischemic heart failure patients undergoing cardiac resynchronization therapy. Heart Rhythm. 2011;8:692–9. doi: 10.1016/j.hrthm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia P, Ramanathan C, Ghanem RN et al. Electrocardiographic imaging of cardiac resynchronization therapy in heart failure: observation of variable electrophysiologic responses. Heart Rhythm. 2006;3:296–310. doi: 10.1016/j.hrthm.2005.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson WB, Vatterott PJ, Peterson MA et al. Body surface mapping using an ECG belt to characterize electrical heterogeneity for different left ventricular pacing sites during cardiac resynchronization: relationship with acute hemodynamic improvement. Heart Rhythm. 2017;14:385–91. doi: 10.1016/j.hrthm.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Kittnar O, Riedlbauchova L, Adla T et al. Outcome of resynchronization therapy on superficial and endocardial electrophysiological findings. Physiol Res. 2018;67:601–10. doi: 10.33549/physiolres.934056. [DOI] [PubMed] [Google Scholar]
- 15.Lumens J, Ploux S, Strik M et al. Comparative electromechanical and hemodynamic effects of left ventricular and biventricular pacing in dyssynchronous heart failure: electrical resynchronization versus left-right ventricular interaction. J Am Coll Cardiol. 2013;62:2395–403. doi: 10.1016/j.jacc.2013.08.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen UC, Cluitmans MJM, Strik M et al. Integration of cardiac magnetic resonance imaging, electrocardiographic imaging, and coronary venous computed tomography angiography for guidance of left ventricular lead positioning. Europace. 2019;21:626–35. doi: 10.1093/europace/euy292. [DOI] [PubMed] [Google Scholar]
- 17.Pastore CA, Tobias N, Samesima N et al. Body surface potential mapping investigating the ventricular activation patterns in the cardiac resynchronization of patients with left bundle-branch block and heart failure. J Electrocardiol. 2006;39:93–102. doi: 10.1016/j.jelectrocard.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Pereira H, Jackson TA, Sieniewicz B et al. Non-invasive electrophysiological assessment of the optimal configuration of quadripolar lead vectors on ventricular activation times. J Electrocardiol. 2018;51:714–19. doi: 10.1016/j.jelectrocard.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Pereira H, Jackson TA, Claridge S et al. Comparison of echocardiographic and electrocardiographic mapping for cardiac resynchronisation therapy optimisation. Cardiol Res Pract. 2019;2019:4351693. doi: 10.1155/2019/4351693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ploux S, Lumens J, Whinnett Z et al. Noninvasive electrocardiographic mapping to improve patient selection for cardiac resynchronization therapy: beyond QRS duration and left bundle branch block morphology. J Am Coll Cardiol. 2013;61:2435–43. doi: 10.1016/j.jacc.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 21.Ploux S, Eschalier R, Whinnett ZI et al. Electrical dyssynchrony induced by biventricular pacing: implications for patient selection and therapy improvement. Heart Rhythm. 2015;12:782–91. doi: 10.1016/j.hrthm.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Rudy Y. Noninvasive electrocardiographic imaging of cardiac resynchronization therapy in patients with heart failure. J Electrocardiol. 2006;39:28–30. doi: 10.1016/j.jelectrocard.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samesima N, Douglas R, Tobias N et al. Twenty-millisecond interventricular difference as assessed by body surface potential mapping identifies patients with clinical improvement after implantation of cardiac resynchronization device. Anadolu Kardiyol Derg. 2007;7((Suppl 1)):213–5. [PubMed] [Google Scholar]
- 24.Samesima N, Pastore CA, Douglas RA et al. Improved relationship between left and right ventricular electrical activation after cardiac resynchronization therapy in heart failure patients can be quantified by body surface potential mapping. Clinics. 2013;68:986–91. doi: 10.6061/clinics/2013(07)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon J, Navarro CO, McEntee T et al. An early phase of slow myocardial activation may be necessary in order to benefit from cardiac resynchronization therapy. J Electrocardiol. 2008;41:531–5. doi: 10.1016/j.jelectrocard.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Sieniewicz BJ, Jackson T, Claridge S et al. Optimization of CRT programming using non-invasive electrocardiographic imaging to assess the acute electrical effects of multipoint pacing. J Arrhythm. 2019;35:267–75. doi: 10.1002/joa3.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varma N. Variegated left ventricular electrical activation in response to a novel quadripolar electrode: visualization by non-invasive electrocardiographic imaging. J Electrocardiol. 2014;47:66–74. doi: 10.1016/j.jelectrocard.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Sorgente A, Cappato R. A critical reappraisal of the current clinical indications to cardiac resynchronisation therapy. Arrhythm Electrophysiol Rev. 2013;2:91–4. doi: 10.15420/aer.2013.2.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auger D, Bleeker GB, Bertini M et al. Effect of cardiac resynchronization therapy in patients without left intraventricular dyssynchrony. Eur Heart J. 2012;33:913–20. doi: 10.1093/eurheartj/ehr468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bleeker GB, Schalij MJ, Molhoek SG et al. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544–9. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 31.Seger M, Hanser F, Dichtl W et al. Non-invasive imaging of cardiac electrophysiology in a cardiac resynchronization therapy defibrillator patient with a quadripolar left ventricular lead. Europace. 2014;16:743–9. doi: 10.1093/europace/euu045. [DOI] [PubMed] [Google Scholar]
- 32.Lambiase PD, Rinaldi A, Hauck J et al. Non-contact left ventricular endocardial mapping in cardiac resynchronisation therapy. Heart. 2004;90:44–51. doi: 10.1136/heart.90.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Upadhyay GA, Vijayaraman P, Nayak HM et al. His corrective pacing or biventricular pacing for cardiac resynchronization in heart failure. J Am Coll Cardiol. 2019;74:157–9. doi: 10.1016/j.jacc.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Vatasescu R, Berruezo A, Mont L et al. Midterm ‘super-response’ to cardiac resynchronization therapy by biventricular pacing with fusion: insights from electro-anatomical mapping. Europace. 2009;11:1675–82. doi: 10.1093/europace/eup333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rigol M, Solanes N, Fernandez-Armenta J et al. Development of a swine model of left bundle branch block for experimental studies of cardiac resynchronization therapy. J Cardiovasc Trans Res. 2013;6:616–22. doi: 10.1007/s12265-013-9464-1. [DOI] [PubMed] [Google Scholar]
- 36.Lee AWC, Costa CM, Strocchi M et al. Computational modeling for cardiac resynchronization therapy. J Cardiovasc Trans Res. 2018;11:92–108. doi: 10.1007/s12265-017-9779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jurak P, Curila K, Leinveber P et al. Novel ultra-high-frequency electrocardiogram tool for the description of the ventricular depolarization pattern before and during cardiac resynchronization. J Cardiovasc Electrophysiol. 2020;31:300–7. doi: 10.1111/jce.14299. [DOI] [PubMed] [Google Scholar]
- 38.Muthalaly RG, Evans RM. Applications of machine learning in cardiac electrophysiology. Arrhythm Electrophysiol Rev. 2020;9:71–7. doi: 10.15420/aer.2019.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Leur RR, Boonstra MJ, Bagheri A et al. Big data and artificial intelligence: opportunities and threats in electrophysiology. Arrhythm Electrophysiol Rev. 2020;9:146–54. doi: 10.15420/aer.2020.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feeny AK, Rickard J, Patel D et al. Machine learning prediction of response to cardiac resynchronization therapy: improvement versus current guidelines. Circ Arrhythm Electrophysiol. 2019;12:e007316. doi: 10.1161/CIRCEP.119.007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalscheur MM, Kipp RT, Tattersall MC et al. Machine learning algorithm predicts cardiac resynchronization therapy outcomes. Circ Arrhythm Electrophysiol. 2018;11:e005499. doi: 10.1161/circep.117.005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu S-Y, Santus E, Forsyth AW et al. Can machine learning improve patient selection for cardiac resynchronization therapy? PLoS One. 2019;14:e0222397. doi: 10.1371/journal.pone.0222397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra S, van Dam PM, Chrispin J et al. Initial validation of a novel ECGI system for localization of premature ventricular contractions and ventricular tachycardia in structurally normal and abnormal hearts. J Electrocardiol. 2018;51:801–8. doi: 10.1016/j.jelectrocard.2018.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


