Abstract
This article describes the advances in catheter ablation for AF that have allowed the creation of more durable and efficient lesions. It describes advances in high-power, short-duration radiofrequency ablation, radiofrequency balloon devices, ultra-low cryoablation and irreversible electroporation. It also considers the way these devices may change the way catheter ablation is performed for AF.
Keywords: AF, ablation, novel technologies
AF is one of the most widespread and sustained cardiac arrhythmias and it affects more than 30 million people worldwide.[1] While prevalence in developed nations tends to be small (1–4%), it is steadily increasing and it is well known that AF is associated with an increased risk of all-cause mortality, heart failure, thromboembolism and dementia.[2–5]
Catheter ablation is an alternative treatment option that is more effective than antiarrhythmic medications. Pulmonary vein isolation (PVI), which involves electrically isolating the pulmonary veins (PV) from the left atrium, remains the cornerstone of AF ablation.[6] However, despite numerous advances in mapping and ablation technologies over the past few years, its efficacy for maintaining sinus rhythm, especially in persistent and long-standing cases is still <70%.[7] One of the main limitations of the current technologies is their ability to achieve chronic durable lesions. PV reconnection rates still range from 15–50% depending on the energy and the catheter used for the procedure.[8–10] Other concerns include the efficiency of ablation procedures as point-by-point radiofrequency (RF) encirclement of the PVs is very time-consuming. Similarly, safety is an ongoing concern with phrenic nerve and oesophageal injury occurring in up to 5–10% and 0.1–0.5% of patients, respectively.[11,12]
In this review, we describe the new and developing ablative technologies which may improve the efficacy, safety and efficiency of ablation for persistent AF. These techniques include high-power, short-duration (HPSD) RF delivery, single-shot RF balloons, advances in cryoablation, and electroporation.
High-power, Short-duration Radiofrequency Ablation
Lesion formation using contemporary RF ablation catheters has two simultaneous phases: resistive heating and conductive heating. Resistive heating is caused by the direct contact between the tip of the catheter and the tissue, creating superficial lesions. Conductive heating is time dependent and it extends deeper in the tissue creating transmural lesions (Figure 1).[13] Standard power and duration RF ablation with open saline irrigation results in a small region of resistive heating and deeper conductive heating which can produce transmural lesions in thin atrial tissue, but can limit the surface area of lesions and potentially cause collateral injury to the oesophagus, phrenic nerve and other adjacent structures.[14] HPSD ablation uses power of 50 W or higher for a shorter period of time, typically 5–15 seconds. This causes a larger zone of resistive heating, while conductive heating is limited resulting in shallower and broader lesions. This modality may increase the efficiency of ablation while potentially reducing the opportunity for collateral damage. Furthermore, shorter duration deliveries may improve catheter stability and reduce oedema formation and smaller lesions.[15,16]
Figure 1: High-power, Short-duration Radiofrequency Compared with Standard Radiofrequency Ablation.
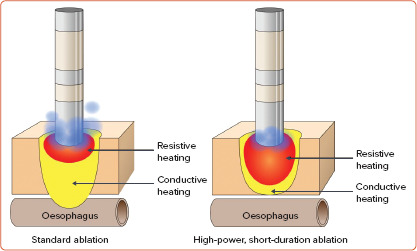
Longer applications using conventional parameters (20.30 W for 20.40 seconds) result in more conductive heating that reaches deep structures (left). The presumed advantage of higher powers with shorter durations (≥50 W for 5.12 seconds) is the larger endocardial lesion as a result of the high energy applied from the tip of the catheter to the myocardium (resistive heating) but less conduction of heat to deeper structures (right). Higher irrigation used in conventional ablation also translates into deeper lesions (left). Conversely, lower irrigation only creates superficial and non-transmural lesions (right).
The new trend of HPSD ablation using conventional RF ablation catheters has been proved to be efficient by reducing the total RF delivery time and total procedure time with a similar or even better safety profile compared with conventional RF ablation.[17] With the exception of thicker areas such as the mitral or tricuspid annulus, HPSD seems to create durable lesions and therefore, improve PVI durability.[18] However, oesophageal injuries are still seen post procedure when RF ablation is guided by time or ablation index.[19,20] Kaneshiro et al. reported that oesophageal thermal injury was seen in 37% of the patients using HPSD, although these lesions were limited to the shallow layer of the oesophageal wall.[21] Other groups have suggested that using higher power (60 W) for a shorter period could be associated with a lower risk of complications.[22]
The concept of HPSD using contemporary available catheters has two main limitations. The first problem is the use of high flow irrigation (15–30 cc/min) in combination with high power. Cold saline irrigation cools the tissue surface during ablation creating lower quality lesions by reducing the area of resistive heating which is important in the thin atrial tissue. Irrigation also enhances the depth of the lesion, increasing the risk of collateral damage in the atrium.
The second limitation is the concept of time- or ablation index-guided ablation when using this approach. High-flow irrigation does not allow precise temperature feedback from the tip of the catheter. The quality of the lesion and the possible collateral damage is directly related to the temperature achieved at the level of the tissue, not so much to the power delivered. There are two novel catheters – the diamond-tipped catheter and the micro catheter – used to perform HPSD that introduce the concept of temperature-controlled ablation from the tip of the catheter to limit power delivery, reduce open irrigation to allow for better assessment of the tip temperature and reduce the total duration of RF delivery.
Diamond-tipped Catheter Technology
A novel diamond-tipped catheter ablation system merges development in catheter structure, temperature sensing technology, high-resolution ECG and diamond cooling. The catheter is 7.5 Fr with a 4.1 mm tip. A network of chemical vapour deposit industrial diamonds is designed to act as heat shunting material at the catheter tip. The structure allows for high thermal diffusivity as thermal energy undergoes quick conduction through the shunting network. This heat and cool transfer is 200–400 times faster using a chemical vapour deposit diamond network compared to conventional platinum-iridium structures.[23]
Irreversible tissue damage occurs when tissue temperature exceeds 50°C. Overshooting this temperature point is a common safety challenge in catheter ablation. The DiamondTemp catheter (Medtronic) protects against temperature-derived tissue damage as the system starts by running in temperature control mode where the temperature is sampled every 20 ms by each sensor. The system continuously monitors and records the highest temperature while the power is automatically adjusted. The catheter includes six thermocouples for temperature sensing, which are thermally isolated from the RF electrode. Since these structures are located on the catheter tip, they provide accurate tissue-tip temperature measurements. The low irrigation rate of 8 cc/min allows for better real-time temperature measurement. The maximum power delivery is 50 W and lesions in the atrium are typically applied for 5–10 seconds. The conventional width separating electrodes is 8 mm but the small distal tip electrodes are separated by a 1.1 mm distance. Short spacing between high-resolution electrodes (HREs) at the catheter tip allows for highly localised signals to be recorded at the ablation centre.[23]
Many studies support the improved safety profile and effectiveness of the DiamondTemp catheter. Clinically, the TRAC-AF pilot study (NCT02821351) demonstrated that the catheter could achieve 100% acute procedural success in all subjects, but in a shorter period and with less RF delivery compared with historical controls.[24] The FASTR-AF trial (NCT036266499) also showed procedural safety and effectiveness with the DiamondTemp catheter. Freedom from AF at 12 months was 74.3% while total RF time was only 19.8 ± 8.6 minutes. No steam pops occurred and there was no evidence of char formation on the tip of the catheter.[25] The DIAMOND-AF study (NCT03334630), to be published soon, showed that there was no difference in success between the diamond-tipped catheter and traditional contact force sensing (CFS) catheters in 1-year follow-up for patients with paroxysmal AF. The success rate at 1 year for the diamond tip group was 71% compared with 72% for CFS. The study also showed that procedural time and RF delivery and saline volume were all reduced. The DIAMONDAF II study (NCT03643224) will compare results between the diamond-tip catheter system and traditional RF in patients with persistent AF.
QDOT Micro Catheter
The QDOT Micro Catheter (Biosense Webster) is another emerging system also designed to achieve better monitoring of tissue temperature. It has six thermally isolated thermocouples embedded in the tip and side of the catheter rather than proximally. The tip is made of traditional platinumiridium composite. There are two delivery modes: very high power for very short durations (QMODE+) and more standard delivery mode (QMODE). In QMODE+, the catheter delivers 90 W for 4 seconds, while in QMODE, powers of 40 to 50 W are typically used for 5–15 seconds. The irrigation rate varies between 5–15 ml depending on the tip temperature and power output. This optimised system ensures the tip remains within the allowed temperature to avoid overheating and tissue damage to contiguous areas.[13]
Preclinical data demonstrated that broader, shallower lesions could be obtained. Compared to standard ablation, the use of HPSD resulted in 100% contiguous and transmural lines, whereas standard ablation showed gaps in 25% and non-transmural lesions in 29%. Ablation at 90 W was identified as having no steam pops or char formation. Clinical data from the QDOT FAST study showed shorter procedure and fluoroscopy times of 105.2 ± 24.7 minutes and 6.6 ± 8.2 minutes respectively.[26] Acute success was achieved in 94.2% of the patients at 3 months post ablation and no major side effects including death, stroke, oesophageal fistula or PV stenosis were reported.
Overall, optimising HPSD techniques is a continuous work in progress as the optimal power to be used has yet to be determined. Although HPSD boasts shorter procedure times and less fluid load than for patients undergoing conventional RF, procedural complications may be difficult to assess in real time when lesions are delivered between 3–4 seconds. For instance, oesophageal temperature rises are latent after the first RF delivery and procedural complications may be difficult to mitigate if the RF application is too fast. As time goes on, an optimal RF power output with optimal duration will hopefully be determined for different tissue types.
Although many advances in HPSD seem promising for the field of electrophysiology, it is crucial that the proper technologies are employed. Temperature control is one of the keystones for HPSD ablation. Without temperature control, power delivery cannot be actively titrated by the generator to optimise the lesion and avoid complications like steam pops, perforation or char formation. For example, Kottmaier et al. presented findings where they applied 70 W of energy for 7 seconds at the anterior left atrium and 5 seconds at posterior left atrium using a standard, highirrigation catheter without any temperature control. Despite some early promising results, the findings should be interpreted with caution. For example, the group found three participants with pericardial effusion with HSPD, as opposed to two in the standard ablation group. This rate of effusion is much higher than reported in other traditional technologies.[27] Without temperature control to limit power delivery, HPSD lesions can result in steam pops and micro-perforations.
Single-shot Radiofrequency Balloons
Point-by-point ablations are time-consuming and require high technical experience on the part of physicians. However, balloon-based ablation allows for quick, easy isolation of the PVs in a single shot. The two most prominent developments in RF balloon ablation are the HELIOSTAR RF balloon (Biosense Webster) and the LUMINIZE RF balloon (Boston Scientific).
HELIOSTAR RF Balloon
The HELIOSTAR is a 13.5 Fr RF balloon with a 3 Fr circular mapping catheter, a 13.5 Fr steerable sheath and a dedicated multichannel generator. The balloon is 28 mm in diameter with 10 irrigated, flexible, gold-plated electrodes on the distal end of the catheter. Integrated thermistors monitor tissue temperature throughout the procedure. The multi-electrode RF balloon is manipulated over a guidewire using a deflectable sheath. The RF balloon is sited at the antra of each of the PVs, inflated, and irrigated at a rate of 35 ml/min. Ablation is delivered in the unipolar mode at 15 W and is temperature-controlled to a target of 60°C for 60 seconds. Electrodes of the RF balloon that are adjacent to the posterior wall can be identified and RF can be stopped at these electrodes after 20 seconds of ablation. The ablation electrodes are gold-plated and are bonded to the surface of the balloon. The electrodes are teardrop in shape and measure 14.4 mm in length with a width of 1.1 mm distally to 4.4 mm proximally. This technology uses a magnet-based visualisation system (CARTO3, Biosense Webster) to map and navigate the balloon around the veins and left atrium.[28]
The RADIANCE trial evaluated the 1-year outcome and safety of the HELIOSTAR balloon. The results demonstrated acute PV isolation in 100% of the 39 patients enrolled. The mean duration of ablation lesion delivery was 5.9 (4.5–7.6) minutes, which is short compared to cryoablation. Isolation was achieved after just one delivery in 79.6% of PVs. Acute PV reconnection was seen in 4.6% (7/150) of PVs. Freedom from documented atrial arrhythmia at 12 months was 86.4% (32/37) or 75.7% from antiarrhythmic medications. The larger, ongoing investigational device exemption (IDE) trial STELLAR (NCT03683030) will give further results.
The clearest advantage of RF balloons for PV isolation lies in their efficiency. Compared to the cryoballoon, where applications are 2–3 minutes each and an additional ‘bonus’ lesion is often required, RF balloons can achieve isolation within one minute in the majority of veins with a single application. However, the long-term safety of such powerful devices remains to be seen. In RADIANCE, for example, asymptomatic cerebral lesions were seen in 30% of patients, which is on the higher end of accepted ablation technologies. Gastroscopy revealed asymptomatic oesophageal erythema in 5 of 39 patients (13%) which is also higher than reported for other ablation technologies.[29]
LUMINIZE RF Balloon
The LUMINIZE RF balloon is 28 mm in diameter and is compliant with 12 equatorial and six forward-facing irrigated electrodes. Microelectrodes are also printed along splines of the catheter which can map ECGs. The catheter has built-in cameras with LED lighting for visualisation and no additional diagnostic catheters are needed. An over-the-wire technique and steerable sheath is used to navigate the balloon in the left atrium. The catheter delivers RF energy in either a bipolar or unipolar fashion. Specifically, the equatorial electrodes deliver bipolar RF at 6–10 W for 60 seconds with an irrigation rate of 30 ml/min. The forward-facing electrodes can deliver bipolar or unipolar energy and can be used for non-PV ablation. LUMINIZE uses electrode impedance readings to determine contact. The built-in camera allows for real-time visualisation of the electrodes on the tissue to confirm contact and energy delivery.[30]
The two-phase AF-FICIENT 1 study examined acute and procedural success and safety for LUMINIZE in a cohort of 100 patients with paroxysmal AF. The first phase of the study investigated the original design of the device, while phase 2 investigated changes which enhanced manoeuvrability with added dedicated pacing and sensing electrodes. Overall, the study demonstrated a high rate of acute PV isolation (99.4% in phase 2) with no device-related serious adverse events. The median time the balloon spent in the left atrium went from 91 minutes in phase 1 to 29 minutes in phase 2, which brought the total procedure time down to a median of 71 minutes.[31] Although the preliminary results with LUMINIZE RF balloon seemed promising, the future of this device is now unclear.
Ultra-low Temperature Cryoablation
Unlike RF ablation, cryoablation methods withdraw heat for ablation. The cryoballoon was the first balloon platform for PVI isolation.[2] The cryoballoon uses a single-shot delivery method to ablate in an efficient manner.2 Traditional cryotherapy uses pressurised nitric oxide to absorb heat from surrounding tissues, achieving temperatures as low as -80°C. Ice crystal formation causes cell death and ice crystal expansion during melting causes further cell destruction.[32]
Recently, a system using ultra-low cryoablation was developed using the intelligent continuous lesion ablation system (iCLAS; Adagio Medical). The system uses an 8.5 Fr catheter which is very malleable. Pre-shaped nitinol stylets can then be advanced into the catheter to allow it to take a circular, linear or curved shape suited for specific locations in the left and right atria. Some stylets have a double circular design such that the inner circle records signals within the PV while the outer circle ablates around the PV. Other curves are designed for single shot cavotricuspid isthmus ablation. The device uses highly purified liquid nitrogen to cool the tissue to −190°C. Lesions are administered for 30–60 seconds followed by a repeat application. The profound cooling helps to create more transmural lesions compared to traditional cryoablation.[33] To protect against oesophageal injury, a warmed saline filled balloon is placed in the oesophagus to keep the temperature at 38°C during ablation over the oesophageal regions (Figure 2).
Figure 2: Ultra-low Cryoablation System.
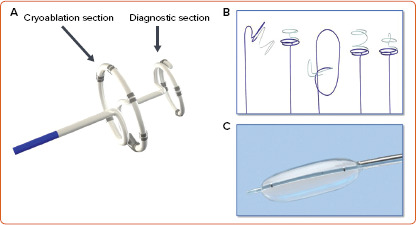
Illustration of the ultra-low cryoablation system (intelligent Continuous Lesion Ablation System – iCLAS, Adagio). A: Cryoablation catheter with a proximal ablation portion and a distal diagnostic portion used to confirm isolation of the pulmonary vein or other structure. B: Different pre-shaped stylets that can be advanced into the ablation catheter, allowing it to take different shapes depending on the specific location or vein size. C: Oesophageal warming balloon with saline at 38°C.
In the CRYO-CURE 2 study (NCT02839304), results from 48 patients were reported (35 for both safety and efficacy and 13 for safety only). Two patients had persisting phrenic nerve palsy, but no other safety events were reported. Five patients with paroxysmal AF and 17 with persistent AF were followed up for 6 months and the success rate was 100%. Success rates dropped to about 78% at 12 months.[34] A larger IDE trial for persistent AF is starting which will provide more safety and efficacy data. The efficiency of the procedure is also good with a mean procedure time of 116 minutes. Ongoing developments include combining cryoablation with novel pulsed field ablation.
Electroporation
Electroporation is a non-thermal ablation technique using electric fields to produce nanoholes in the cell membranes of targeted cardiac tissue cells by exposing them to a high-voltage field. If sufficient voltage is applied, the effects of electroporation are irreversible leading to cellular apoptosis and replacement fibrosis. These changes probably occur over days to weeks but this is not well known. Irreversible electroporation (IRE) is also referred to as pulsed field ablation (PFA).[35] There are multiple ways that IRE can be delivered. Pulses can use alternating current or direct current and delivery may be unipolar (from the catheter tip to a return electrode on the skin of the patient) or bipolar (between adjacent electrodes). It may also be delivered using a monophasic or biphasic waveform. Most systems to date use bipolar, biphasic pulse delivery. The rationale is that bipolar, biphasic delivery can reduce the recruitment of skeletal muscle and the voltage delivered, avoiding twitching or movement of the patient as we might observe during a cardioversion. The disadvantage is that bipolar delivery of higher voltages results in some minor electrode heating. Typically, IRE is delivered in several trains, each consisting of several pulses, in a repetitive series. The width of each pulse is typically measured in nanoseconds or milliseconds, so one delivery of a train of pulses can be delivered in a fraction of a second. This contributes to one of the chief benefits of IRE as an energy source: ultra-rapid and efficient energy delivery.[36,37]
The other advantage of IRE is that cardiac tissue seems to be more susceptible to cell death than other tissues. This may be due to tissue selectivity. It may also be due to the limited regeneration capability of cardiac tissue in comparison to oesophageal tissue, and the insulating effect of multiple tissue layers. For example, nerves are surrounded by a myelin sheath which may make them more resistant to IRE. If IRE is delivered in the left atrium, the outer layer of the myocardium, the pericardial layer and adipose tissue may all insulate the oesophagus. Therefore, IRE’s principal advantage will be enabling ablation in the left atrium while minimising, if not eliminating, the possibility of damage to the phrenic nerve or oesophagus.[38–40]
Since lesions are created by a field of energy and not by selected regions of heat or cold, the lesions seem to be more contiguous than traditional ablation. IRE is also more forgiving when it comes to contact with the tissue since the field can reach the tissue even if the catheter has suboptimal contact force. Some contact, however, is still required. What is unclear, however, is whether PFA will be any more effective than thermal ablation. Current systems are optimised for tissue depths of 3–7 mm which is fine for left atrial tissue but may not be sufficient for ventricular tissue. However, if the same efficacy can be achieved with more safety and efficiency, then IRE will still be a game changer.
Microbubble formation has been noted with IRE, with some forms of delivery being more prone to this than others. While it is assumed that such microbubbles are due to fluid electrolysis and have a very short half-life, it is unclear what risk, if any, they pose. Early cerebral MRI data has suggested that they do not create any acute, asymptomatic cerebral lesions. Further data will be forthcoming.
In this section, we will summarise the IRE systems closest to market release. However, there are multiple companies developing newer IRE systems all the time, so this section is not meant to be comprehensive.
Medtronic Pulsed Field Ablation System
The Medtronic pulse direct system consists of a circular catheter with nine gold electrodes with bipolar and biphasic delivery. Alternating current’s electric fields can be adjusted via different energy profiles. Typically, four applications are made at each catheter position and each application takes a fraction of a second. Pre-clinical data has shown that the oesophagus and phrenic nerve are quite resistant to any damage from this system. PV stenosis is also very unlikely to occur despite delivery deep inside the veins. Animal data at 1 and 3 months also suggest that the lesions created are durable and contiguous and capable of 3–7 mm tissue depth. Human studies have just begun. The PULSED AF pilot study has demonstrated 100% PV isolation with no serious adverse events, including no change in oesophageal temperature or phrenic nerve injury. Patients did require heavy sedation or general anaesthesia for pain management but no skeletal muscle twitching or paralytics were required.[41]
Farapulse Irreversible Electroporation System
The Farapulse system includes a 20-electrode catheter arranged in a flower-like configuration with five ‘petals’. The catheter can also resemble a basket. It delivers DC bipolar and biphasic trains.[36,42] This system was the first used in a human trial. The IMPULSE (NCT03700385) and PEFCAT (NCT03714178) trials were prospective feasibility trials investigating the use of the Farapulse PFA system for the treatment of paroxysmal AF and cavotricuspid isthmus-dependent atrial flutter and all the participants in both groups achieved acute PV isolation. Primary safety endpoints were achieved with no adverse events other than cardiac perforation or tamponade in one patient in the IMPULSE cohort. There were no oesophageal lesions or enhancement, no silent ischaemia in post-procedure MRIs in 13 patients and phrenic nerve assessment showed no paresis or palsy after 3 months. There was also no PV stenosis or narrowing. The system underwent several iterations in pulse delivery from monophasic pulses (which required general anaesthesia and paralytics) to different biphasic deliveries which improved lesion durability from 18% to 100% at 3 months.[42] A more recent study showed that the system can also create posterior wall lesions in addition to PVI with 100% posterior wall durability at 3 months.[43] Pre-clinical data has shown that the delivery of IRE does not cause oesophageal lesion and also seems to avoid any PV or superior vena cava stenosis.[44,45]
New Lattice Catheter – Combination Irreversible Electroporation and Pulsed Field Ablation
The novel Sphere-9 catheter (Affera) has an expandable spheroid-shaped lattice tip with a 10-fold larger effective area compared to the conventional 3.5 mm electrode. It can deliver higher energy with a lower risk of tissue overheating. The entire lattice framework emits RF energy to deliver a uniform current cloud, and its temperature-controlled mode modulates the current output to avoid overheating (Figure 3).
Figure 3: Lattice-tipped Radiofrequency and Pulsed Field Ablation Catheter.
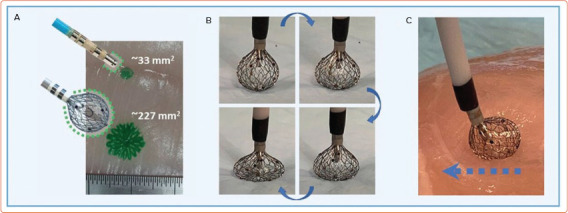
Lattice-tipped, spherical ablation catheter with combined temperature-controlled radiofrequency and pulsed field ablation capabilities (Sphere-9 catheter, Affera). It has an expandable spheroid-shaped lattice tip capable of making large, point-by-point lesions (A and B). The tip is flexible allowing it to conform to tissue (C). Source: Anter et al. 2020.[46] Reproduced with permission from Elsevier.
In the first-in-human study of this device, PVI was achieved in 64 of 65 patients (98.5%) using the lattice alone and mitral block was achieved in 100% of patients.[46] Roof line and cavotricuspid isthmus ablation were achieved in 95.8% and 100% of patients, respectively. The device boasts a high safety profile with no complications after a 3-month follow-up.[46] Despite epicardial cooling, the large surface area of the device enables lesions suitable for varied tissue thickness. Furthermore, the lower current density allows homogenous heating with reduced risks of hot spots and steam pops, or damage due to passive conductive heating. Using high resolution mapping and ablation in one, physicians can define the anatomy of the individual patient as they go, with included voltage and activation mapping. All these integrated advantages of the lattice-tip catheters will advance catheter ablation beyond conventional PVI methods.[47]
The device has demonstrated its capability to deliver PFA.[48] The first-inhuman trial showed 100% acute procedural efficacy with a left atrial ablation time less than 25 minutes and no evidence of oesophageal injury in the patients receiving PFA alone. There were no cases of phrenic nerve injury or PV stenosis. There were 6% of patients with fluid-attenuated inversion recovery (FLAIR) positive lesions on cerebral MRI and 10% with positive diffusion weighted MRI, but the activated clotting time appeared to be sub-therapeutic in all of these cases and patients were not necessarily receiving PFA alone.
There are other devices that are exploring the combination of thermal and PFA energy. The Adagio system described above is looking at combination cryoablation and PFA delivered together to improve tissue contact, minimise any heating or microbubble formation, and potentially create deeper lesions. The novel Kardium Globe catheter (Kardium) offers global multielectrode contact mapping and ablation and combines the benefits of single-tip catheters with the simplicity of balloon catheters. The catheter structure includes 16 flat ribs with 122 gold-plated electrodes, which individually measure tissue contact pressure, temperature, current and intracardiac ECGs, and apply RF accordingly in a temperature-controlled, non-irrigated fashion. In the GLOBAL AF study, 60 patients with symptomatic AF underwent PVI using Globe and 72% were free from AF and atrial tachycardia at 12 months without antiarrhythmic drugs. The Globe catheter seems well suited for PFA and may be used as another combination device (Figure 4).[49]
Figure 4: Spherical, Multipolar Combination Mapping and Ablation Catheter.
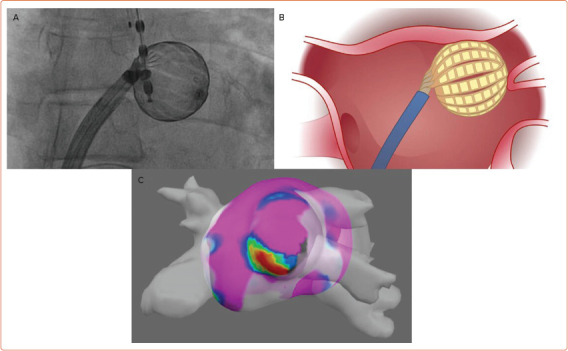
A multipolar, spherical combined mapping and ablation system (Globe, Kardium). A: Fluoroscopic image of the Kardium Globe catheter inside the left atrium. B: Illustration of the catheter outside of the left superior pulmonary vein. C: Map of the left atrium performed with the Globe catheter using an integrated mapping system (Globe Positioning System). The large size of the catheter allows for ‘single-shot’ mapping and energy application.
Despite successes in these trials, certain questions remain for clinical practice. How do we increase the depth of lesions to affect deeper ventricular tissue without creating thermal damage or altering tissue selectivity? Initial pre-clinical studies have shown that ventricular ablation is feasible but probably lacks the depth needed for broad application. Physicians also need to better understand the exact mechanisms of myocardial sensitivity and cell death to guarantee positive safety outcomes.
Conclusion
The optimal ablation strategy is still unknown and it is unlikely that a perfect ablation strategy will ever exist. Comparative clinical trials will dictate which factors physicians should consider when deciding which strategy is most effective, efficient and safe. HPSD, single-shot RF balloons, cryoablation, electroporation and lattice catheters are among few of the many new emerging technologies which could revolutionise the field of electrophysiology.
Clinical Perspective
Higher-power, shorter-duration lesions with temperature control and lower irrigation can produce lesions with more resistive and less conductive heating which can increase lesion width without extending lesion depth.
Single-shot radiofrequency balloon devices can produce rapid pulmonary vein isolation and safety will be determined from larger clinical trials.
Ultra-low cryoablation attempts to address the issue of lesion transmurality and requires oesophageal warming.
Electroporation, either in isolation or combined with other energy sources, may offer much better efficiency and improved safety.
References
- 1.Chugh SS, Havmoeller R, Narayanan K et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e125–51. doi: 10.1161/CIR.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart S, Hart CL, Hole DJ, McMurray JJV. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–64. doi: 10.1016/S0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D’Agostino RB et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.CIR.98.10.946. [DOI] [PubMed] [Google Scholar]
- 5.Marijon E, Le Heuzey JY, Connolly S et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128:2192–201. doi: 10.1161/CIRCULATIONAHA.112.000491. [DOI] [PubMed] [Google Scholar]
- 6.Calkins H, Hindricks G, Cappato R et al. 2017 HRS/EHRA/ ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarnette JA, Brooks AG, Mahajan R et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and meta-analysis. Europace. 2018;20:f366–76. doi: 10.1093/europace/eux297. [DOI] [PubMed] [Google Scholar]
- 8.De Potter T, Van Herendael H, Balasubramaniam R et al. Safety and long-term effectiveness of paroxysmal atrial fibrillation ablation with a contact force-sensing catheter: real-world experience from a prospective, multicentre observational cohort registry. Europace. 2018;20:f410–8. doi: 10.1093/europace/eux290. [DOI] [PubMed] [Google Scholar]
- 9.Aryana A, Singh SM, Mugnai G et al. Pulmonary vein reconnection following catheter ablation of atrial fibrillation using the second-generation cryoballoon versus openirrigated radiofrequency: results of a multicenter analysis. J Interv Card Electrophysiol. 2016;47:341–8. doi: 10.1007/s10840-016-0172-z. [DOI] [PubMed] [Google Scholar]
- 10.Kautzner J, Neužil P, Lambert H et al. EFFICAS II: Optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace. 2015;17:1229–35. doi: 10.1093/europace/euv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansour M, Lakkireddy D, Packer D et al. Safety of catheter ablation of atrial fibrillation using fiber optic-based contact force sensing. Heart Rhythm. 2017;14:1631–6. doi: 10.1016/j.hrthm.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Deshmukh A, Patel NJ, Pant S et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation. 2013;128:2104–12. doi: 10.1161/CIRCULATIONAHA.113.003862. [DOI] [PubMed] [Google Scholar]
- 13.Leshem E, Zilberman I, Tschabrunn CM et al. High-power and short-duration ablation for pulmonary vein isolation: biophysical characterization. JACC Clin Electrophysiol. 2018;4:467–79. doi: 10.1016/j.jacep.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Nath S, DiMarco JP, Haines DE. Basic aspects of radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 1994;5:863–76. doi: 10.1111/j.1540-8167.1994.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 15.Shin DG, Ahn J, Han SJ, Lim HE. Efficacy of high-power and short-duration ablation in patients with atrial fibrillation: a prospective randomized controlled trial. Europace. 2020;22:1495–501. doi: 10.1093/europace/euaa144. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Schmidt B, Bordignon S et al. Ablation indexguided 50 W ablation for pulmonary vein isolation in patients with atrial fibrillation: Procedural data, lesion analysis, and initial results from the FAFA AI High Power Study. J Cardiovasc Electrophysiol. 2019;30:2724–31. doi: 10.1111/jce.14219. [DOI] [PubMed] [Google Scholar]
- 17.Winkle RA, Mohanty S, Patrawala RA et al. Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Heart Rhythm. 2019;16:165–9. doi: 10.1016/j.hrthm.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Yavin HD, Leshem E, Shapira-Daniels A et al. Impact of high-power short-duration radiofrequency ablation on longterm lesion durability for atrial fibrillation ablation. JACC Clin Electrophysiol. 2020;6:973–85. doi: 10.1016/j.jacep.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Schmidt B, Seeger A et al. Catheter ablation of atrial fibrillation using ablation index-guided high power (50 W) for pulmonary vein isolation with or without esophageal temperature probe (the AI-HP ESO II). Heart Rhythm. 2020;17:1833–40. doi: 10.1016/j.hrthm.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Chun KRJ, Tohoku S et al. Esophageal endoscopy after catheter ablation of atrial fibrillation using ablationindex guided high-power: Frankfurt AI-HP ESO-I. JACC Clin Electrophysiol. 2020;6:1253–61. doi: 10.1016/j.jacep.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Kaneshiro T, Kamioka M, Hijioka N et al. Characteristics of esophageal injury in ablation of atrial fibrillation using a high-power short-duration setting. Circ Arrhythm Electrophysiol. 2020;13:e008602. doi: 10.1161/CIRCEP.120.008602. [DOI] [PubMed] [Google Scholar]
- 22.Castrejón-Castrejón S, Marínez Cossiani M, Ortega Molina M et al. Feasibility and safety of pulmonary vein isolation by high-power short-duration radiofrequency application: short-term results of the POWER-FAST PILOT study. J Interv Card Electrophysiol. 2020;57:57–65. doi: 10.1007/s10840-019-00645-5. [DOI] [PubMed] [Google Scholar]
- 23.Iwasawa J, Koruth JS, Petru J et al. Temperature-controlled radiofrequency ablation for pulmonary vein isolation in patients with atrial fibrillation. J Am Coll Cardiol. 2017;70:542–53. doi: 10.1016/j.jacc.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Starek Z, Lehar F, Jez J et al. TRAC-AF trial: first-in-man multicenter prospective clinical experience using a novel diamond tip temperature controlled irrigated ablation system: safety results and initial effectiveness performance. Europace. 2018;20:i–61. doi: 10.1093/europace/euy015.168. [DOI] [Google Scholar]
- 25.Neuzil P. First-in-man FASTR-AF Study: novel temperaturecontrolled fast ablation system to rapidly create lesions for the treatment of persistent and paroxysmal atrial fibrillation. Presented at Heart Rhythm Society 2019 Scientific Sessions, San Diego, CA, US, 8–11 May 2019
- 26.Reddy VY, Grimaldi M, De Potter T et al. Pulmonary vein isolation with very high power, short duration, temperaturecontrolled lesions: the QDOT-FAST trial. JACC Clin Electrophysiol. 2019;5:778–86. doi: 10.1016/j.jacep.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Kottmaier M, Popa M, Bourier F et al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Europace. 2020;22:388–93. doi: 10.1093/europace/euz342. [DOI] [PubMed] [Google Scholar]
- 28.Gianni C, Chen Q, Della Rocca D et al. Radiofrequency balloon devices for atrial fibrillation ablation. Card Electrophysiol Clin. 2019;11:487–93. doi: 10.1016/j.ccep.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Reddy VY, Schilling R, Grimaldi M et al. Pulmonary vein isolation with a novel multielectrode radiofrequency balloon catheter that allows directionally tailored energy delivery: short-term outcomes from a multicenter first-in-human study (RADIANCE). Circ Arrhythm Electrophysiol. 2019;12:e007541. doi: 10.1161/CIRCEP.119.007541. [DOI] [PubMed] [Google Scholar]
- 30.Dhillon GS, Honarbakhsh S, Di Monaco A et al. Use of a multi-electrode radiofrequency balloon catheter to achieve pulmonary vein isolation in patients with paroxysmal atrial fibrillation: 12-month outcomes of the RADIANCE study. J Cardiovasc Electrophysiol. 2020;31:1259–69. doi: 10.1111/jce.14476. [DOI] [PubMed] [Google Scholar]
- 31.Al-Ahmad A, Aidietis A, Daly M Assessment of the safety and performance of a novel RF balloon catheter system to isolate pulmonary veins: results of the multicenter AF-FICIENT 1 Trial. Presented at European Heart Rhythm Association Congress, Lisbon, Portugal, 17 March 2019
- 32.Khairy P, Dubuc M. Transcatheter cryoablation part i: preclinical experience. Pacing Clin Electrophysiol. 2007;31:112–20. doi: 10.1111/j.1540-8159.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- 33.De Potter T, Boersma L, Babkina A Novel linear cryoablation catheter to treat atrial fibrillation. Presented at Heart Rhythm Society Scientific Sessions, Boston, MA, US, 9–12 May 2018
- 34.De Potter T. Investigation of the Adagio cryoablation system in patients with atrial fibrillation (CryoCure2). Presented at 24th Annual International AF Symposium, Boston, MA, US, 24–26 January 2019
- 35.Wittkampf FHM, van Es R, Neven K. Electroporation and its relevance for cardiac catheter ablation. JACC Clin Electrophysiol. 2018;4:977–86. doi: 10.1016/j.jacep.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Reddy VY, Koruth J, Jais P et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol. 2018;4:987–95. doi: 10.1016/j.jacep.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Wittkampf FH, van Driel VJ, van Wessel H et al. Feasibility of electroporation for the creation of pulmonary vein ostial lesions. J Cardiovasc Electrophysiol. 2011;22:302–9. doi: 10.1111/j.1540-8167.2010.01863.x. [DOI] [PubMed] [Google Scholar]
- 38.Wojtaszczyk A, Caluori G, Pešl M et al. Irreversible electroporation ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:643–51. doi: 10.1111/jce.13454. [DOI] [PubMed] [Google Scholar]
- 39.Sugrue A, Maor E, Ivorra A et al. Irreversible electroporation for the treatment of cardiac arrhythmias. Expert Rev Cardiovasc Ther. 2018;16:349–60. doi: 10.1080/14779072.2018.1459185. [DOI] [PubMed] [Google Scholar]
- 40.Maor E, Sugrue A, Witt C et al. Pulsed electric fields for cardiac ablation and beyond: a state-of-the-art review. Heart Rhythm. 2019;16:1112–20. doi: 10.1016/j.hrthm.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Verma A, Boersma LV, Hummel JD PULSED AF: first human experience and acute procedural outcomes using a novel pulsed field ablation system. Presented at Heart Rhythm Society, 8 May 2020. [DOI] [PMC free article] [PubMed]
- 42.Reddy VY, Neuzil P, Koruth JS et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74:315–26. doi: 10.1016/j.jacc.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Reddy VY, Anic A, Koruth J et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol. 2020;76:1068–80. doi: 10.1016/j.jacc.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Koruth JS, Kuroki K, Kawamura I et al. Pulsed field ablation versus radiofrequency ablation: esophageal injury in a novel porcine model. Circ Arrhythmia Electrophysiol. 2020;13:e008303. doi: 10.1161/CIRCEP.119.008303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koruth J, Kuroki K, Iwasawa J et al. Preclinical evaluation of pulsed field ablation: electrophysiological and histological assessment of thoracic vein isolation. Circ Arrhythmia Electrophysiol. 2019;12:1–9. doi: 10.1161/CIRCEP.119.007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anter E, Neužil P, Rackauskas G et al. A lattice-tip temperature-controlled radiofrequency ablation catheter for wide thermal lesions: first-in-human experience with atrial fibrillation. JACC Clin Electrophysiol. 2020;6:507–19. doi: 10.1016/j.jacep.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Reddy VY, Neužil P, Peichl P et al. A lattice-tip temperaturecontrolled radiofrequency ablation catheter: durability of pulmonary vein isolation and linear lesion block. JACC Clin Electrophysiol. 2020;6:623–35. doi: 10.1016/j.jacep.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Reddy VY, Anter E, Rackauskas G Point-by-point pulsed field ablation (+/- radiofrequency ablation) to treat atrial fibrillation: a first in human trial. Presented at Heart Rhythm Society, 8 May 2020
- 49.Kottkamp H, Hindricks G, Pönisch C et al. Global multielectrode contact-mapping plus ablation with a single catheter in patients with atrial fibrillation: GLOBAL AF study. J Cardiovasc Electrophysiol. 2019;30:2248–55. doi: 10.1111/jce.14172. [DOI] [PubMed] [Google Scholar]


