Abstract
Thromboembolism is the most serious complication of AF, and oral anticoagulation is the mainstay therapy. Current guidelines place all AF types together in terms of anticoagulation with the major determinants being associated comorbidities translated into risk marker. Among patients in large clinical trials, those with non-paroxysmal AF appear to be at higher risk of stroke than those with paroxysmal AF. Higher complexity of the AF pattern is also associated with higher risk of mortality. Moreover, continuous monitoring of AF through cardiac implantable devices provided us with the concept of ‘AF burden’. Usually, the larger the AF burden, the higher the risk of stroke; however, the relationship is not well characterised with respect to the threshold value above which the risk increases. The picture is more complex than it appears: AF and underlying disorders must act synergically respecting the magnitude of its own characteristics, which are the amount of time a patient stays in AF and the severity of associated comorbidities.
Keywords: AF, AF type, stroke, thromboembolism, mortality, atrial high-rate episode, subclinical AF
AF is the most common sustained arrhythmia encountered in clinical practice; the current estimated prevalence of AF in adults is 2–4%,[1] and a steady increase is expected due to extended longevity in the general population and intensifying search for undiagnosed AF.[2] It is estimated that the number of patients with AF will double over the next 40 years.[3]
AF is associated with substantial morbidity and mortality, thus posing significant burden to patients, the healthcare system, and the healthcare economy. One of the most serious complications of AF is thromboembolism, in particular stroke, which exists regardless of the presence or absence of symptoms.[4,5] The risk of thromboembolism correlates with increases in CHADS2 and CHA2DS2-VASc scores.[6–8]
The temporal pattern expressed as the type of AF, has shown conflicting results in consideration of its impact on the risk of thromboembolism and major outcome. Therefore, the current risk scoring systems do not include the pattern of AF and current practice guidelines make identical recommendations for anticoagulation in patients at moderate or high risk, regardless of the type of AF.[9–11]
The aim of the present paper is to critically review data from literature with the purpose of understanding the relationship between the temporal pattern of AF on the risk of thromboembolic events or mortality.
Classification of Clinical AF
AF is a progressive disorder, and the transition from intermittent to continuous form of arrhythmia may occur in up to 25% of patients at variable follow-up, mainly depending upon age, underlying heart disease, and concurrent treatments.[12]
Based on clinical presentation, available anamnestic data on AF duration, and spontaneous termination, different types of AF have been described, regardless of the presence/absence of symptoms (Table 1).[9]
Table 1: Patterns of AF and Relative Abbreviations.
| AF Pattern | Definition | Comment |
|---|---|---|
| First diagnosed AF | AF that has not been diagnosed before, irrespective of the duration of the arrhythmia or the presence and severity of AF-related symptoms | The form diagnosed at the first clinical presentation of AF, irrespective of severity of symptoms, or the arrhythmia duration |
| Paroxysmal AF | Self-terminating, in most cases within 48 h. Some AF paroxysms may continue for up to 7 days. AF episodes that are cardioverted within 7 days should be considered paroxysmal | The classification extends the duration of the single AF episode up to 7 days, but the probability of spontaneous conversion to sinus rhythm is low after 48 h |
| Persistent AF | AF that lasts longer than 7 days, including episodes that are terminated by cardioversion, either with drugs or by direct current cardioversion, after 7 days or more | The form of AF that persists beyond 7 days or requires active termination for sinus rhythm restoration |
| Long-standing persistent AF | Continuous AF lasting for ≥1 year when it is decided to adopt a rhythm control strategy | A form of AF lasting for ≥12 months, when the adoption of a rhythm control strategy is required |
| Permanent AF | AF that is accepted by the patient (and physician). Hence, rhythm control interventions are, by definition, not pursued in patients with permanent AF. Should a rhythm control strategy be adopted, the arrhythmia would be re-classified as ‘long-standing persistent AF’ | A form of AF for which cardioversion is not attempted since the arrhythmia is accepted by the patient and physician. Permanent AF represents a therapeutic attitude rather than an inherent pathophysiological attribute of AF, and the term should not be used in the context of a rhythm control strategy with antiarrhythmic drug therapy or AF ablation |
Source: Kirchhof et al. 2016.[9] Used with permission from Oxford University Press.
In the general population, permanent (PRM) AF is reported as the most frequent form of diagnosed AF.[12] In the REALIZE-AF Registry, a contemporary, large-scale, international survey of patients with AF who had one or more episodes in the past year, 2,606 of 9,816 patients (26.5%) had paroxysmal (PRX), 2,341 (23.8%) had persistent (PRS) and 4,869 (49.6%) had PRM AF.[13]
The figure provided under the present classification is unfortunately incomplete in view of AF episodes that are often asymptomatic and because it depends on AF detection by ECG recording, which also depends on the variable intensity of ECG monitoring.
Device-detected Atrial Arrhythmias
Unlike ambulatory ECG monitoring tools, cardiac implantable electronic devices (CIEDs) capable of detecting atrial signals provide full-time continuous monitoring of individual atrial arrhythmias that can be stored by the device through auto-triggered alerts. A full array of diagnostic information is available, including date and time of onset, duration, and atrial arrhythmia cycle length, as well as day-level AF. Because many ICDs implanted for the prevention of sudden death are single-ventricular-chamber devices, newer technologies have emerged to also allow for AF detection based on irregularity of R-R cycle length detected with conventional ventricular leads.[14] Implantable loop recorders, which also mainly rely on R-R intervals for arrhythmia detection, have lower sensitivity and specificity for AF identification than CIEDs with an atrial lead.[15]
The definitions of device-detected atrial arrhythmias are shown in Table 2.[16]
Table 2: Definition Related to Device-detected Atrial Arrhythmia and Relative Abbreviations.
| Type of Arrhythmia | Definition |
|---|---|
| Atrial high-rate event | Atrial high-rate episodes are defined as atrial tachyarrhythmia episodes with rate >190 BPM detected by cardiac implantable electronic devices |
| Subclinical AF | Atrial high-rate episodes (>6 min and <24 h) with lack of correlated symptoms in patients with cardiac implantable electronic devices, detected with continuous ECG monitoring (intracardiac) and without prior diagnosis (ECG or Holter monitoring) of AF |
| Silent or asymptomatic AF | Documented AF in the absence of any symptoms or prior diagnosis often presenting with a complication related to AF (e.g. stroke, heart failure, etc.) |
Source: Gorenek et al. 2017.[16] Used with permission from Oxford University Press.
Atrial high-rate episodes (AHRE) correspond to all atrial tachycardias (AT) above a predefined atrial rate threshold.[17] Several technical issues are involved in the process of detecting and recording AHRE, including atrial sensitivity, and the programming of atrial rate and episode duration cutoffs, with some variability according to the device manufacturer, and the ability of storing AHRE electrograms.
Caution is needed in interpreting device-detected AHREs and considering them as a surrogate for AF. False detection can occur because of myopotentials or other sources of electrical interference, far-field R wave over-sensing by the atrial lead or sustained runs of premature atrial complexes. Therefore, validation of the arrhythmia detected through device diagnostics is indicated by reviewing electrograms stored in the device’s memory to rule out artefacts and confirm the diagnosis of AT/AF.
AF can be missed if episodes of AF are very brief or slow. Therefore, diagnostic accuracy becomes reliable when episodes ≥5–6 minutes in duration are considered, because, with this cutoff, the appropriateness in AF detection is 95%, minimising the risk of over-sensing.[18] In a sub-analysis of the ASSERT study, 17.3% of AHREs at >190 BPM that lasted ≥6 minutes were found to be false-positive for AF.[19]
Patients with CIEDs are at particularly high risk for AF, likely related to the high prevalence of underlying cardiac pathology, such as sinus node dysfunction (SND) and cardiomyopathies, which can predispose to AF.[20]
The diagnostic capabilities of CIEDs can detect AF episodes of sustained duration (>48 hours) much more frequently than conventional follow-up with ECG, and those episodes may be completely asymptomatic and unpredictable.[21] Moreover, patients may experience both symptomatic and asymptomatic episodes of AF, of variable duration, and the symptoms attributed to the arrhythmia have, in fact, a relatively low positive predictive value for AF.[22]
Subclinical AT/AF episodes are common in patients implanted with CIEDs: the reported incidence varies from 25% to 50% with the design of the study (retrospective/prospective), the underlying heart disease (SND, atrioventricular block, or heart failure [HF]), the presence/absence of history of clinically overt atrial arrhythmias, the definition of AHRE duration, type of device detecting the atrial arrhythmias, and the observation period.[23–28]
In the ASSERT study, subclinical ATs with at least 6-minute duration were detected in approximately 25% of patients, during a follow-up of 2.5 years, and about 16% of those who had subclinical ATs developed a clinical AF.[28]
The capability of continuous monitoring of AF through CIEDs has led to the concept of ‘AF burden’, which is defined as the overall time spent in AF that an individual has in each day (daily AF burden) in a specific follow-up period, adopting it to describe the dynamic pattern of AF, not only in term of presence, but also in terms of duration of AF episodes.[20,29–31]
The measurement of total AF burden includes asymptomatic as well as symptomatic episodes. This is important since the ratio of asymptomatic/symptomatic episodes is about 12:1 in patients with symptomatic PRX AF, and the assessment of symptomatic burden alone would greatly underestimate the total burden.[32] The advantage of using burden over other endpoints is that it is not subject to investigator bias. The sampling error introduced by relying on patient symptoms or episodic monitoring is eliminated. Unfortunately, literature on AF burden is sparse simply because continuous monitoring would be required to capture this information.
Risk of Stroke in Different AF Pattern and Type
The relationship between the AF pattern and the risk of stroke, regardless the CHADS2 and CHA2DS2-VASc scores, is currently a matter of huge discussion. The analysis of the relationship between AF pattern and outcomes is complicated by the evidence that the patient profile of PRX AF is different from the other types, because they are generally younger, with a lower prevalence of structural heart disease, and other comorbidities (HF, chronic kidney disease, chronic obstructive pulmonary disease, peripheral vascular disease), as well as lower estimated thromboembolic and bleeding risks.[33]
The above-considered factors, as well as the proportion of patients appropriately treated with antithrombotic therapy, may act as relevant confounders, thus making the assessment of the causal relationship problematic.
Randomised Clinical Trials on Oral Antithrombotic Agents
Antiplatelet therapy plays no more role in preventing stroke in AF; however, a brief re-examination of studies involving antiplatelet therapy can shed some light on the relationship between AF pattern and stroke in patients who are not assuming oral anticoagulants (OAC).[9] An analysis from the ACTIVE-A and AVERROES databases suggests that the pattern of AF is related to stroke risk in patients who were unsuitable for vitamin K antagonists (VKA). In a population of 6,563 aspirin-treated patients, the yearly ischaemic stroke rates were 2.1%, 3.0%, and 4.2% for PRX, PRS, and PRM AF, respectively, with an adjusted HR of 1.83 (p<0.001) PRM versus PRX AF and 1.44 (p=0.02) PRS versus PRX AF.[34] Multivariable analysis identified age ≥75 years, sex, history of stroke or transient ischaemic attack (TIA), and AF pattern as independent predictors of stroke, with a PRM AF pattern being the second strongest predictor after prior stroke/TIA.
Conversely, other randomised controlled trials (RCTs) have not confirmed the finding. The SPAF trial demonstrated similar annualised rate of ischaemic stroke in aspirin-treated patients with intermittent (3.2%) or sustained (3.3%) form of AF, and, similarly, the ACTIVE-W trial, demonstrated a comparable risk of ischaemic stroke or systemic embolism (SE) in patients treated with aspirin plus clopidogrel with PRX or sustained form of AF (HR 0.94; p=0.755).[35,36] These studies might be comparatively underpowered in a population not treated with OAC, that might be potentially less representative of contemporary practice outcome.
Large RCTs on OACs have offered the opportunity to revisit the role of AF pattern in predicting thromboembolic events in the direct oral anticoagulant era.
Some post-hoc analyses of RCTs on direct oral anticoagulants reported that the risk of stroke/SE is lower in patients with PRX AF compared with a non-PRX (mainly PRS) AF.
Post-hoc analyses of ROCKET-AF, ARISTOTLE and ENGAGE-AF have shown significantly lower stroke rates for patients with PRX AF at enrolment than for those with PRS AF, even after adjustment for baseline characteristics (ARISTOTLE: HR 0.70; 95% CI [0.51–0.93]; p=0.0159; ROCKET-AF: HR 0.79; 95% CI [0.63–1.0]; p=0.0481; and ENGAGE-AF: HR 0.79; 95% CI [0.66–0.96]; p=0.0151).[37–39]
In the RE-LY study, the stroke rates were lower in PRX versus PRS AF (1.32% versus 1.55%), but no formal adjusted comparisons were made, and patients with PRX AF tended to have lower CHADS2 scores.[40]
Older data on patients in the SPORTIF III and V trials (randomised to either VKA or ximelagatran) demonstrated an annual stroke/SE rate of 1.73% for PRS AF and 0.93% for PRX AF (HR 1.87; 95% CI [1.04–3.36]; p=0.037).[41] However, in patients with two or more risk factors for stroke, the PRX AF pattern was not associated with significantly lower stroke risk, suggesting that at the higher level of the risk spectrum, clinical risk factors play a much important role than AF pattern.
All those data have been collected in a large meta-analysis focusing on the efficacy and safety of OACs in 70,447 AF patients (78.7% non-PRX). Compared to PRS or PRM AF, the incidence of stroke/SE was lower in PRX AF patients (HR 0.79; 95% CI [0.71–0.88]; p<0.00001). Interestingly, annualised major bleeding rates were similar across AF types (HR 1.06; 95% CI [0.96–1.17]; p=0.22). The absence of an association with bleeding events supports the hypothesis that the association of non-PRX with thromboembolism might be a specific effect attributable to AF pattern.[42]
However, these studies suffer a major limitation of having included post-hoc analyses of trials done for other purposes than to assess the pure role of AF type in predicting major outcomes.
The annualised rate of stroke or SE in major RCTs on antithrombotic therapy in different temporal pattern of AF is depicted in Figure 1.
Figure 1: Rate per Patient/Year of Stroke or Systemic Embolism in Major Randomised Clinical Trials on Antithrombotic Therapy in Different Temporal Pattern of AF.
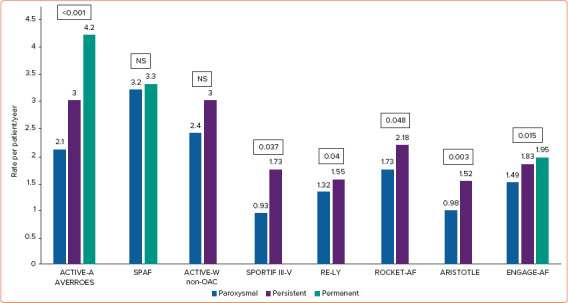
SPAF trial, comparison between intermittent (paroxysmal and persistent) and permanent atrial fibrillation. ACTIVE-W trial and ARISTOTLE trials, comparison between paroxysmal and non-paroxysmal (persistent and permanent) AF. ACTIVE-W, presentation of results only related to the clopidogrel plus aspirin arm (non-anticoagulant arm). RE-LY, presentation of results only related to the lower dose (110 mg twice daily) arm. Source: Vanassche et al. 2015,[34] Hart et al. 2000,[35] Hohnloser et al. 2007,[36] Steinberg et al. 2015,[37] Al-Khatib et al. 2013,[38] Link et al. 2017,[39] Flaker et al. 2012[40] and Lip et al. 2008.[41]
Real-life Data from AF Registries and Population-based Studies
Data from AF registries and population-based studies are also heterogeneous, reflecting the complexity in risk adjustment between AF pattern and therapy, most of all, rate of OACs, which are confounders in evaluating the relationship between AF pattern and the risk of thromboembolism. It is well known that patient characteristics differ significantly by AF type. PRM AF carries a trend toward higher-risk features compared to non-PRM AF, as well as PRS AF compared to PRX AF, which makes any rigorous adjustment difficult for all population-based studies.[13]
Some observational registries reported that patients with PRX AF have a risk of stroke/SEEs comparable to patients with non-PRX AF. In the prospective Euro Heart Survey that enrolled 5,333 AF patients, followed for 1 year, PRX AF had comparable risk for thromboembolic events as PRS and PRM AF (PRX 22/1170 – 1.9%; PRS 11/886 – 1.2%; PRM 19/1126 – 1.6%).[43] Also, in the Loire Valley AF Project, the rates of stroke differed significantly by pattern of AF; however, clinical factors, not AF pattern, independently increased the risk of stroke in multivariate analyses.[44] On the basis of this finding, the authors concluded that the choice for antithrombotic therapy should be based on clinical risk factors, not on AF pattern.
The Chinese AF Registry, involving a total of 8,529 AF patients, concluded that AF type was not an independent predictor of thromboembolism. In non-anticoagulated patients, the PRS AF group demonstrated a higher risk of stroke/SE, compared to the PRX AF group, while no significant difference was found in anticoagulated subjects. On multivariate analysis in non-anticoagulated patients, age ≥75 years (p=0.046) and prior stroke/TIA (p=0.018) but not AF type were significantly associated with the risk of stroke/SE.[45]
The same result has provided by the analysis of 29,181 patients enrolled in the observational GARFIELD-AF, where a multivariable Cox regression was used to assess the risks of stroke/SE across patterns of AF, and whether this changed with anticoagulation on outcomes.[46] Median CHA2DS2-VASc score was similar across AF patterns. During a 2-year follow-up, after adjustment, non-PRX AF patterns were associated with significantly higher rates of stroke/SE, than PRX AF in non-anticoagulated patients only. No difference remained in anticoagulated patients in non-PRX compared with PRX AF patterns.[46] The latter two studies demonstrated that AF pattern is no longer prognostic for thromboembolic events when patients are treated with anticoagulants.
Conversely, the observational Fushimi AF Registry found that PRX AF was associated with a significantly lower risk of stroke/SE than non-PRX forms even after adjusting for a series of potential confounders, including oral anticoagulation.[47] The results were also reinforced by the evidence that the risk of stroke was lower in patients maintaining a PRX AF pattern than those with PRX AF at the baseline who progressed to a sustained AF during the 2-year follow-up.[47] Moreover, a study in patients with previous stroke demonstrated a nearly two-fold increased risk of stroke with PRS AF compared to PRX AF, even after adjustment for age, sex, previous anticoagulation, and severity of the index event.[48]
In this context of uncertainty, it may be interesting to consider the results of systematic reviews and meta-analysis of all the studies that have compared PRX and non-PRX AFs regarding the occurrence of thromboembolic events, although the heterogeneity of study design, type of treatment, and evaluation of outcomes in the various studies suggest caution in the interpretation.
The incidence of thromboembolism and bleeding were analysed in a systematic review of indexed publications of RCTs, cohort studies, and case series reporting collected clinical outcomes stratified by AF type. Data from nearly 100,000 patients indicated that non-PRX AF is associated with a highly significant increase in thromboembolism with multivariable adjusted HR 1.38 (95% CI [1.19–1.61]); p<0.001, compared with PRX AF, while again rates of bleeding were similar, with adjusted HR 1.025 (95% CI [0.89–1.17]); p=0.715.[49]
This meta-analysis suggests that patients with PRX AF have a lower risk of stroke than those with non-PRX AF, but it remains unclear if AF pattern is an independent predictor of stroke or rather a reflection of a different patient profile in terms of risk factors and comorbidities.
Device-detected AF Duration and Risk of Stroke
The increased ability of CIEDs to detect silent AF through continuous monitoring for long periods of time has highlighted the potential opportunity to examine the AF burden, or threshold of AF burden, that is associated with a significant risk of stroke/SE to appropriately consider the benefit of anticoagulant prophylaxis in patients at risk, as evaluated through clinical score schemes.
Studies have generally shown that higher AF burden is associated with a higher risk of stroke; however, thresholds have not been reproducibly identified. Confounding the observation is that patients with higher AF burden also tend to have a higher prevalence of other conditions that increase risk of stroke.
Several studies in different populations with CIEDs have analysed the association of different AF burden thresholds with stroke/SE (Table 3).[26,28,31,50–57] In these studies, with data collected from more than 35,000 patients, the participants were categorised according to the maximum duration of detected AHREs or by the maximum detected daily AF burden. The cutoff points of AF duration were generally arbitrarily pre-specified rather than empirically derived.
Table 3: Summary of Studies Regarding AF Detected by Cardiac Implantable Electronic Devices and Thromboembolic Risk.
| Author | Trial | Patients (n) | Follow-up Duration | Atrial Rate Cutoff | AF Burden Threshold | TE Event, HR (p-value) | TE Event Rate (Below Versus Above AF Burden Threshold) |
|---|---|---|---|---|---|---|---|
| Glotzer et al. 2003[26] | Ancillary MOST | 312 | 27 months (median) | >220 BPM | 5 min | 6.7 (p=0.020) | 3.2% overall (1.3% versus 5%) |
| Capucci et al. 2005[50] | Italian AT500 Registry | 725 | 22 months (median) | >174 BPM | 24 h | 3.1 (p=0.044) | 1.2% annual rate |
| Bottoet al. 2009[31] | Italian AT500 Registry | 568 | 1 year (mean) | >174 BPM | CHADS2+ AF burden | N/A | 2.5% overall (0.8% versus 5%) |
| Glotzeret al. 2009[51] | TRENDS | 2,486 | 1.4 years (mean) | >175 BPM | 5.5 h | 2.2 (p=0.060) | 1.2% overall (1.1% versus 2.4%) |
| Shanmugam et al. 2012[52] | Home Monitor CRT | 560 | 370 days (median) | >180 BPM | 3.8 h | 9.4 (p=0.006) | 2.0% overall |
| Healey et al. 2012[28] | ASSERT | 2,580 | 2.5 years (mean) | >190 BPM | 6 min | 2.5 (p=0.007) | (0.69% versus 1.69%) |
| Boriani et al. 2014[53] | SOS | 10,016 | 2 years (median) | >175 BPM | 1 h | 2.11 (p=0.008) | 0.39% per year overall |
| Turakhia et al. 2015[54] | Veterans HCS | 9,850 | 1–30 and 91–120 days before stroke | >175 BPM | 5.5 h | 4.2 VKA adjusted | N/A |
| Witt et al. 2015[55] | Danish National Registry | 394 | 4.6 years (median) | Nominal setting | 6 min | 2.30 (p=0.028) | 1.80% per year |
| Swiryn et al. 2016[56] | RATE Registry | 5,379 | 22.9 months | Nominal setting | AT onset/offset on different EGM recordings | 1.51 (p<0.05) | N/A |
| Van Gelder et al. 2017[57] | ASSERT Substudy | 2,455 | 2.5 years (mean) | >190 BPM | 24 h | 3.24 (p=0.003) | 3.1 per year |
AT = atrial tachycardia; EGM = electrogram; N/A = not available; TE = thromboembolic.
In an ancillary analysis of the MOST trial of 316 patients with SND and dual-chamber pacemakers, an AHRE (atrial rate >220 BPM) cutoff of 5 minutes was chosen to avoid false-positive results from overs-ensing.[26] Presence of 5-minute AHREs was associated with increased risk of death or nonfatal stroke (HR 2.79; 95% CI [1.51–5.15]; p=0.0011) and of clinical AF (HR 5.93; 95% CI [2.88–12.2]; p=0.0001). This study was limited by its small size, retrospective design, and the fact that only AHREs that lasted >5 minutes were considered; thus, the prognostic significance of much longer episodes was not evaluated.
The TRENDS study was a prospective, observational study of 2,846 patients with pacemakers or defibrillators and risk factors for stroke.[51]. The median value of AT/AF burden of 5.5 hours on any single day in a 30-day window was chosen as the cutoff between low/high-risk threshold. Compared with no AT/AF, the stroke risk was doubled in those with high AT/AF burden (≥5.5 hours) but not in those with low AT/AF burden (<5.5 hours), suggesting that stroke risk is a quantitative function of AT/AF burden.[51]
In the ASSERT study, subclinical episodes of AT (atrial rates ≥190 BPM lasting >6 minutes) were associated with an increased risk of ischaemic stroke/SE (HR 2.49) during a 2.5-year follow-up.[28] The cutoff of 6 minutes was pre-specified. However, albeit important, data from ASSERT do not identify a specific threshold of AF duration or AF burden that may justify, from a risk–benefit perspective, the starting of prophylaxis with OACs.[58] A further sub-analysis of the ASSERT study has given the answer, demonstrating that subclinical AT only increased the risk of stroke/SE for episodes >24 hours (adjusted HR 3.24; 95% CI [1.51–6.95]; p=0.003) and that risk of stroke in patients with subclinical AT between 6 minutes to 6 hours (adjusted HR 0.75; 95% CI [0.29–1.96]; p=0.562) and between 6 hours to 24 hours (adjusted HR 1.32; 95% CI [0.40–4.37]; p=0.646) was not significantly different from that of patients without subclinical AT.[57]
The SOS-AF project has collected the largest dataset of patients previously implanted with CIEDs, a pooled analysis of individual patient data from three prospective studies, with an overall population of 10,016 patients with median age of 70 years, without permanent AF.[53] During a median follow-up of 24 months, 43% of patients experienced at least 1 day with ≥5 minutes of AF burden; and in a Cox regression analysis adjusted for CHADS2 score and use of OACs at baseline, the AF burden was an independent predictor of stroke, with a 1-hour threshold of AF burden associated with the highest HR for ischaemic stroke of 2.11 (95% CI [1.22–3.64]; p=0.008) in a dichotomised analysis that compared various potential threshold cutoffs for AF burden.[53]
In a case-crossover analysis involving 9,850 patients with CIEDs remotely monitored in the Veterans Administration Health Care System, it was found that AF burden of ≥5.5 hours in a given day (based on the previous TRENDS study) raised the short-term risk of stroke almost five-fold.[51,54] Moreover, they found that majority of strokes occurred temporally dissociated from AF. Therefore, although a transient increase in risk based on AF onset was identified, the overall attributable risk was low.
It is noteworthy that a device-detected AF burden of >5 minutes has been recently found to be significantly associated with silent ischaemic brain lesions (IBL) at CT scan. The study prospectively analysed 109 patients, mean aged 74±9 years with a mean CHA2DS2VASc scores of 3.9 ± 1.6. Seventy-five patients (69%) had no history of AF or stroke/TIA. CT scan showed silent IBLs in 28 (25.7%) patients. Multivariable analysis demonstrated that AHRE was an independent predictor for silent IBL in the overall population (HR 3.05; 95% CI [1.06–8.81]; p<0.05) but also in patients without prior history of AF or stroke/TIA (HR 9.76; 95% CI [1.76–54.07]; p<0.05).[59] This finding may be of some value for interpreting the risk of cognitive impairment in AF patients, since there is compelling evidence to support an association of greater cognitive decline and risk of dementia and AF independently of a history of stroke.[60]
Mechanistic models have been proposed to explain the association of AF and dementia. Alterations of brain perfusion from embolic events, bleeding, and rhythm-related hypoperfusion underlie many of these models. Those observations have valuable relevance because potential therapeutic opportunities to reduce dementia risk, including early and effective use of OACs and strategies to improve brain perfusion through rhythm and rate control approaches.[61,62] However, prospective trials are needed to evaluate these therapeutic opportunities.
Type and Burden of AF and Mortality
AF is associated with an increased risk of mortality.[63–65] Importantly, higher AF burden is associated with higher risk of mortality. However, the role of AF type has shown conflicting results in term of its impact on the risk of death.
In the meta-analysis by Zhang et al., solely based on RCTs in patients with moderate-to-high risk of stroke receiving anticoagulation, PRX AF showed significantly improved efficacy and a similar safety profile compared to PRS or PRM AF patients. Overall, the results included a reduction of all-cause mortality in PRX compared to non-PRX AF patients (HR 0.72; 95% CI [0.66–0.79]; p<0.00001).[42]
The meta-analysis by Ganesan et al. has compared outcomes by type of AF, representing the largest aggregated AF patient dataset. Overall unadjusted all-cause mortality was higher in patients with non-PRX AF than in those with PRX AF (HR 1.46; 95% CI [1.26–1.70]; p<0.001); multivariable adjustment only partially attenuated this association (HR 1.22; 95% CI [1.09–1.37]; p<0.001).[49] The mechanisms by which non-PRX patients experienced increased mortality include worsened HF or more severe stroke events, or perhaps a higher burden of underlying non-cardiovascular diseases.[65,66]
To further appreciate the complex picture of AF patients presenting with PRX on non-PRX AF, it is also interesting to consider an analysis of the predictors of outcome taking into account all-cause mortality instead of stroke. In the EORP-AF General Pilot Registry, patients with non-PRX AF had a worse outcome for all-cause mortality at 1 year than those with PRX AF; however, in the multivariable Cox model, non-PRX AF was not an independent predictor of death during follow-up being the adverse outcome maybe related to the worse clinical risk profile for age, underlying cardiac disease, comorbidities, and risk factors.[33]
The data are even less clear on how AHREs relate to mortality, with a suggestion that these low-burden events carry lower mortality risk, in part because studies have been smaller with less precision.
In a study of 224 patients, 17% had AHREs of ≥5-minute duration within 6 months after pacemaker implantation; over a mean follow-up of 6.6 ± 2.0 years, the rate of all-cause mortality was 29%. In multivariate analysis adjusted for age, sex, and cardiovascular diseases, presence of AHREs was associated with a significant increase in cardiovascular mortality (HR 2.80; 95% CI [1.24–6.31]; p=0.030) and stroke mortality (HR 9.65; 95% CI [1.56–59.9]; p=0.015), with a trend toward increased all-cause mortality (HR 1.79; 95% CI [0.98–3.26]; p=0.079). The subgroup of patients with AHREs of ≥5-minute but <24-hour duration also had a significantly increased cardiovascular mortality (HR 3.24; 95% CI [1.37–7.66]; p=0.007).[67]
By contrast, in one study of 394 patients implanted with cardiac resynchronisation therapy devices, and included in the Danish National Registry, although the 20% of patients with AHREs (compared with those without) had an increased risk of clinical AF (HR 2.35; 95% CI [1.47–3.74]; p<0.001) and thromboembolic events (HR 2.30; 95% CI [1.09–4.83]; p=0.028), the risk of mortality was not increased (HR 0.97; 95% CI [0.64–1.45]; p=0.87).[55] Adjusting the analysis for pre-selected baseline risk factors (age at implantation, estimated glomerular filtration rate, left ventricular ejection fraction, QRS width, presence of coronary artery disease, and functional class) had no impact on this result (HR 1.08; 95% CI [0.71–1.65]; p=0.70).
The story is much more complex than simply considering AF type/burden and the presence/absence of specific comorbidities (such as those used in risk marker scores). Moreover, comorbidities should also be considered in a qualitative-quantitative way rather than just as binomial (present/absent) factors.[68,69]
Complex Relationship Between AF Type or Burden and Stroke: Magnitude Synergism and the Concept of Atrial Myopathy
According to the studies previously discussed it is possible to conclude that (even not consistently) more sustained patterns of AF are associated with higher risk of major events (including stoke and death, but not bleeding).[42,49] These studies have included device-detected AF studies, which have the advantage of truly assessing total AF burden. The greater the AF burden, the higher the association with thromboembolism. However, further considerations are mandatory: non-device-assessed AF cannot provide assessment of AF burden, and, consequently, AF type/pattern does not equate with AF burden. For example, frequent PRX-AF may result in more time in AF (greater AF burden) than occasional episodes of cardioverted PRS-AF. The latter could explain the discordant results among trials that simply looked at AF type versus outcome events.
Moreover, AF burden, beyond AF pattern, is not considered in the risk scoring systems, while some studies have demonstrated that this should be. Botto et al. assessed the interaction between AF and CHADS2 factors with respect to risk for stroke. Three groups: no AF, AF >5 minutes <24 hours, AF >24 hours. The rate of TE events increased linearly with the presence and duration of AF, so too as the CHADS2 score increased. Patients with a CHADS2 score of 0 were at low risk, even if they had long-lasting AF, as were patients with a score of 1 if AFB was >5 minutes but <24 hours, and patients with a score of 2 if they had no AF. By contrast, patients with a CHADS2 score ≥3 demonstrated high risk, even without AF being recorded, as did patients with a score of 2 if they had AF >5 min.[31] Thus, the mere presence/absence of AF is not enough of a consideration, especially in those with very-low or very-high risk score. That is: we cannot evaluate outcome events in AF without considering the state of the atria, that immediately refers to the concept of ‘atrial cardiomyopathy’ (ACMP).
A consensus document and detailed reviews have discussed aspects of the definition, histopathology, atrial-specific physiology, atrial pathology, impact on arrhythmia occurrence, imaging, mapping, and ablation of the ACMP.[70–72]
ACMP may be the cause and/or the consequence of AF, can vary with the number and severity of associated comorbidities as well as the amount of AF present over time (AF burden better than AF pattern with that regard) in a synergistic combination, and may finally results in thrombus formation. In patients with AF and stroke-risk comorbidities, the atria are not normal. Rather, in the atria there are endothelial, metabolic, anatomic, histopathologic, and contractile alterations. Those data suggest that the absolute rate of stroke should be expected higher with a greater AF as well as a greater degree on combined contributors.
Therefore, magnitude synergism of contributing factors should be considered and our current risk scoring systems fail by missing this point. Certainly, the CHA2DS2-VASc score relates well to the number of contributory comorbidities, but only age is considered in any semiquantitative way. Yet, if one considers pathophysiologically how disease can contribute to thrombus formation in the left atrium, the process cannot simply be ‘all or nothing’. It is a clear limitation of the score systems.
Therefore, the greater the atrial pathology created by the synergism of AF and underlying disease, the greater the risk. Here is the concept of ‘magnitude synergism’ that should be applied to understand the complex relationship between AF and outcomes.[68,73] It is not enough to just note the presence of AF and its longest duration; rather, a quantitative description of the setting in which it occurs is also a necessity (quantitative and qualitative comorbidity).[74]
The KP-RHYTHM study clearly demonstrated this concept: AF burden, not just the presence of AF, is important in quantitating the risk for stroke since the highest tertile of AF burden was associated with a more than three-fold higher adjusted rate of thromboembolism compared with the combined lower 2 tertile.[75]
Conclusion
AF is associated with substantial mortality and morbidity, of which, the most serious is thromboembolism. The risk stratification for stroke is a crucial step in the clinical management of AF patients and is currently based on the evaluation of a series of clinical factors included in the CHADS2 and CHA2DS2-VASc scores.
Current guidelines place all AF types together in term of anticoagulation with the major determinants being associated comorbidities translated into risk marker scores: Patients with a substantial clinical risk (CHA2DS2-VASc scores ≥2) should receive OACs regardless of their AF pattern; therefore, PRX AF should not be an element to deny any anticoagulation in patients at risk.[76] At the higher level of the risk spectrum, clinical risk factors play a much important role than AF pattern.[41]
Conversely, in deciding whether or not to offer anticoagulation to patients at lower risk (CHA2DS2-VASc scores =1), for whom the risk/benefit ratio of OACs is less clear, it might be useful to consider the type of AF (PRX versus non-PRX) since in studies among contemporary patients, the strongest evidence suggests that patients with PRX AF are at lower risk of stroke than those with non-PRX AF.[34,37–39] However, it must be emphasised that these studies suffer a major limitation of having included post-hoc analyses of trials done for other purposes than to assess the pure role of AF type in predicting major outcomes.
The capability of continuous monitoring of AF through CIEDs has led to the concept of ‘AF burden’ defined as the overall time spent in AF that an individual has in each day in a specific follow-up period. The measurement of total AF burden includes asymptomatic as well as symptomatic episodes. Although increasing AF burden is generally associated with an increasing risk of stroke, the relationship is not well characterised with respect to the definition of threshold value above which the risk increases or the duration of any transient risk. Therefore, some caution is needed in interpreting AF burden–related ischaemic stroke risk derived from pivotal studies. In general, the higher the clinical risk as expressed by the CHA2DS2-VASc score, the lower the threshold of AF burden that should be considered for eventually initiating OACs. However, the latter is still matter of debate, since no intervention trial is in support of this reasonable choice, and newer specifically designed trials are ongoing.[77,78]
Nevertheless, AF alone cannot be the sole factor that can explain the increased risk of thromboembolism. The total burden of AF and its effect on introducing fibrotic and mechanical abnormalities together with the magnitude of atrial pathophysiology consequent to any atrial-affecting disorder must interact synergistically to magnify the thromboembolic risk.[49,73] Moreover, the synergism cannot be simply dichotomous (while the risk scores, unfortunately, are), it must have magnitude depending on the severity of the associated comorbidities and the total amount of AF.[69,73,74]
Even today, with the availability of sophisticated and advanced diagnostic tools, the primary approach to a patient with documented AF remains primarily clinical, based on the evaluation of underlying heart disease and associated comorbidities, the correction of precipitating risk factors, and the stratification for stroke risk. With that regard, current stroke risk scores are practical, but limited in their capacity to predict stroke risk accurately in individual patients. Stroke prediction might be improved by the addition of emerging risk factors, many of which are expressions of atrial fibrosis. The use of novel parameters, including biomarkers and imaging data, regardless of AF pattern or burden, might improve stroke risk prediction and inform optimal treatment for patients with AF.[79–83]
Clinical Perspective
Current guidelines place all AF types together in terms of anticoagulation, with the major determinants being associated co-morbidities translated into risk marker scores.
Patients with non-paroxysmal AF are at higher risk of stroke and death than those with paroxysmal AF.
Continuous monitoring of AF through cardiac implantable devices has led to the concept of ‘AF burden’. Although increasing AF burden is generally associated with an increasing risk of stroke, the relationship is not well characterised with respect to the threshold value above which the risk increases.
Underlying disorders alone cannot be the sole factors, nor can AF alone; those two factors must be considered within a more complex synergism.
The synergistic risk must also have magnitude, depending on the amount of time a patient is in AF and the number and the severity of associated co-morbidities.
The new knowledge will trigger further investigation into the pathological interplay between AF type or burden and underlying disorders, allowing us to better determine optimal risk assessment and therapy.
References
- 1.Benjamin EJ, Muntner P, Alonso A et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Staerk L, Sherer JA, Ko D et al. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120:1501–17. doi: 10.1161/CIRCRESAHA.117.309732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krijthe BP, Kunst A, Benjamin EJ et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–51. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–88. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 5.McManus DD, Rienstra M, Benjamin EJ. An update on the prognosis of patients with atrial fibrillation. Circulation. 2012;126:e143–6. doi: 10.1161/CIRCULATIONAHA.112.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gage BF, Waterman AD, Shannon W et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 7.Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648–61. doi: 10.1016/S0140-6736(11)61514-6. [DOI] [PubMed] [Google Scholar]
- 8.Lane DA, Lip GY. Use of the CHA2DS2-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126:860–5. doi: 10.1161/CIRCULATIONAHA.111.060061. [DOI] [PubMed] [Google Scholar]
- 9.Kirchhof P, Benussi S, Kotecha D et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 10.Hindricks G, Potpara T, Dagres N et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2002;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 11.January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation. Circulation. 2019;140:e125–51. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 12.Zoni-Berisso M, Lercari F, Carazza TS. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–20. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang CE MD, Naditch-Brûlé L, Murin J et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice. Insight From the Real-Life Global Survey Evaluating Patients with Atrial Fibrillation International Registry. Circ Arrhythm Electrophysiol. 2012;5:632–9. doi: 10.1161/CIRCEP.112.970749. [DOI] [PubMed] [Google Scholar]
- 14.Deshmukh A, Brown ML, Higgins E et al. Performance of atrial fibrillation detection in a new single-chamber ICD. Pacing Clin Electrophysiol. 2016;39:1031–7. doi: 10.1111/pace.12918. [DOI] [PubMed] [Google Scholar]
- 15.Podd SJ, Sugihara C, Furniss SS et al. Are implantable cardiac monitors the “gold standard” for atrial fibrillation detection? A prospective randomized trial comparing atrial fibrillation monitoring using implantable cardiac monitors and DDDRP permanent pacemakers in post atrial fibrillation ablation patients. Europace. 2016;18:1000–5. doi: 10.1093/europace/euv367. [DOI] [PubMed] [Google Scholar]
- 16.Gorenek B, Bax J, Boriani G et al. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management—an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardıacay Electrofisiologıa (SOLEACE). Europace. 2017;19:1556–78. doi: 10.1093/europace/eux163. [DOI] [PubMed] [Google Scholar]
- 17.Boriani G, Padeletti L. Management of atrial fibrillation in bradyarrhythmias. Nat Rev Cardiol. 2015;12:337–49. doi: 10.1038/nrcardio.2015.30. [DOI] [PubMed] [Google Scholar]
- 18.Purerfellner H, Gillis AM, Holbrook R et al. Accuracy of atrial tachyarrhythmia detection in implantable devices with arrhythmia therapies. Pacing Clin Electrophysiol. 2004;27:983–92. doi: 10.1111/j.1540-8159.2004.00569.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman ES, Israel CW, Nair GM et al. for the ASSERT Steering Committee and Investigators. Positive predictive value of device-detected atrial high-rate episodes at different rates and durations: an analysis from ASSERT. Heart Rhythm. 2012;9:1241–6. doi: 10.1016/j.hrthm.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Gillis AM, Morck M. Atrial fibrillation after DDDR pacemaker implantation. J Cardiovasc Electrophysiol. 2002;13:542–7. doi: 10.1046/j.1540-8167.2002.00542.x. [DOI] [PubMed] [Google Scholar]
- 21.Israel CW, Grönefeld G, Ehrlich JR et al. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol. 2004;43:47–52. doi: 10.1016/j.jacc.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Strickberger SA, Ip J, Saksena S et al. Relationship between atrial tachyarrhythmias and symptoms, Heart Rhythm. 2005;2:125–31. doi: 10.1016/j.hrthm.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 23.Chen-Scarabelli C, Scarabelli TM, Ellenbogen KA et al. Device-detected atrial fibrillation. What to do with asymptomatic patients? J Am Coll Cardiol. 2015;65:281–94. doi: 10.1016/j.jacc.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 24.Cheung JW, Keating RJ, Stein KM et al. Newly detected atrial fibrillation following dual chamber pacemaker implantation. J Cardiovasc Electrophysiol. 2006;17:1323–8. doi: 10.1111/j.1540-8167.2006.00648.x. [DOI] [PubMed] [Google Scholar]
- 25.Healey JS, Martin JL, Duncan A et al. Pacemaker-detected atrial fibrillation in patients with pacemakers: prevalence, predictors, and current use of oral anticoagulation. Can J Cardiol. 2013;29:224–8. doi: 10.1016/j.cjca.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Glotzer TV, Hellkamp AS, Zimmerman J et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614–9. doi: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler PD, Glotzer TV, Daoud EG et al. Detection of previously undiagnosed atrial fibrillation in patients with stroke risk factors and usefulness of continuous monitoring in primary stroke prevention. Am J Cardiol. 2012;110:1309–14. doi: 10.1016/j.amjcard.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 28.Healey JS, Connolly SJ, Gold MR et al. ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–9. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 29.Friedman PA, Dijkman B, Warman EN et al. Atrial therapies reduce atrial arrhythmia burden in defibrillator patients. Circulation. 2001;104:1023–8. doi: 10.1161/hc3401.095039. [DOI] [PubMed] [Google Scholar]
- 30.Israel CW, Hugl B, Unterberg C et al. Pace-termination and pacing for prevention of atrial tachyarrhythmias: Results from a multicenter study with an implantable device for atrial therapy. J Cardiovasc Electrophysiol. 2001;12:1121–8. doi: 10.1046/j.1540-8167.2001.01121.x. [DOI] [PubMed] [Google Scholar]
- 31.Botto GL, Padeletti L, Santini M et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20:241–8. doi: 10.1111/j.1540-8167.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 32.Page RL, Wilkinson WE, Clair WK et al. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89:224–7. doi: 10.1161/01.cir.89.1.224. [DOI] [PubMed] [Google Scholar]
- 33.Boriani C, Laroche I, Diemberger I et al. ‘Real world’ management and outcomes of patients with paroxysmal versus non-paroxysmal atrial fibrillation in Europe: the EURObservational Research Programme–Atrial Fibrillation (EORP-AF) General Pilot Registry. Europace. 2016;18:648–57. doi: 10.1093/europace/euv390. [DOI] [PubMed] [Google Scholar]
- 34.Vanassche T, Lauw MN, Eikelboom JW et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J. 2015;36:281–8. doi: 10.1093/eurheartj/ehu307. [DOI] [PubMed] [Google Scholar]
- 35.Hart RG, Pearce LA, Rothbart RM et al. for the Stroke Prevention in Atrial Fibrillation Investigators. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. J Am Coll Cardiol. 2000;35:183–7. doi: 10.1016/s0735-1097(99)00489-1. [DOI] [PubMed] [Google Scholar]
- 36.Hohnloser SH, Pajitnev D, Pogue J et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol. 2007;50:2156–61. doi: 10.1016/j.jacc.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg BA, Hellkamp AS, Lokhnygina Y et al. Higher risk of death and stroke in patients with persistent vs paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J. 2015;36:288–96. doi: 10.1093/eurheartj/ehu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Khatib SM, Thomas L, Wallentin L et al. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J. 2013;34:2464–71. doi: 10.1093/eurheartj/eht135. [DOI] [PubMed] [Google Scholar]
- 39.Link MS, Giugliano RP, Ruff CT et al. Stroke and mortality risk in patients with various patterns of atrial fibrillation: results from the ENGAGE AF-TIMI 48 Trial (effective anticoagulation with Factor Xa next generation in atrial fibrillation-thrombolysis in myocardial infarction 48). Circ Arrhythm Electrophysiol. 2017;10:1–7. doi: 10.1161/CIRCEP.116.004267. [DOI] [PubMed] [Google Scholar]
- 40.Flaker G, Ezekowitz M, Yusuf S et al. Efficacy and safety of dabigatran compared to warfarin in patients with paroxysmal, persistent, and permanent atrial fibrillation: results from the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) study. J Am Coll Cardiol. 2012;59:854–855. doi: 10.1016/j.jacc.2011.10.896. [DOI] [PubMed] [Google Scholar]
- 41.Lip GY, Frison L, Grind M. Stroke event rates in anticoagulated patients with paroxysmal atrial fibrillation. J Intern Med. 2008;264:50–61. doi: 10.1111/j.1365-2796.2007.01909.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Xiong Y, Yu L et al. Meta-analysis of stroke and bleeding risk in patients with various atrial fibrillation patterns receiving oral anticoagulation. Am J Cardiol. 2019;123:922–8. doi: 10.1016/j.amjcard.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 43.Nieuwlaat R, Prins MH, Le Heuzey JY et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. Eur Heart J. 2008;29:1181–9. doi: 10.1093/eurheartj/ehn139. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee A, Taillandier S, Olesen JB et al. Pattern of atrial fibrillation and risk of outcomes: the Loire Valley Atrial Fibrillation Project. Int J Cardiol. 2013;167:2682–7. doi: 10.1016/j.ijcard.2012.06.118. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Ma CS, Du X et al. Thromboembolic risks associated with paroxysmal and persistent atrial fibrillation in Asian patients: a report from the Chinese atrial fibrillation registry. BMC Cardiovasc Dis. 2019;19:283–11. doi: 10.1186/s12872-019-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atar D, Berge E, Le Heuzey JY et al. The association between pattern of atrial fibrillation, anticoagulation, and cardiovascular events. Europace. 2020;22:195–204. doi: 10.1093/europace/euz292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takabayashi K, Hamatani Y, Yamashita Y et al. Incidence of stroke or systemic embolism in paroxysmal versus sustained atrial fibrillation: the Fushimi Atrial Fibrillation Registry. Stroke. 2015;46:3354–61. doi: 10.1161/STROKEAHA.115.010947. [DOI] [PubMed] [Google Scholar]
- 48.Koga M, Yoshimura S, Hasegawa Y et al. for the SAMURAI Study Investigators. Higher risk of ischemic events in secondary prevention for patients with persistent than those with paroxysmal atrial fibrillation. Stroke. 2016;47:2582–8. doi: 10.1161/STROKEAHA.116.013746. [DOI] [PubMed] [Google Scholar]
- 49.Ganesan AN, Chew DP, Hartshorne T et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016;37:1591–1602. doi: 10.1093/eurheartj/ehw007. [DOI] [PubMed] [Google Scholar]
- 50.Capucci A, Santini M, Padeletti L et al. Italian AT500 Registry Investigators. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913–20. doi: 10.1016/j.jacc.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 51.Glotzer TV, Daoud EG, Wyse DG et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–80. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 52.Shanmugam N, Boerdlein A, Proff J et al. Detection of atrial high-rate events by continuous home monitoring: clinical significance in the heart failure-cardiac resynchronization therapy population. Europace. 2012;14:230–7. doi: 10.1093/europace/eur293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boriani G, Glotzer TV, Santini M et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35:508–16. doi: 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turakhia MP, Ziegler PD, Schmitt SK et al. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8:1040–7. doi: 10.1161/CIRCEP.114.003057. [DOI] [PubMed] [Google Scholar]
- 55.Witt CT, Kronborg MB, Nohr EA et al. Early detection of atrial high rate episodes predicts atrial fibrillation and thromboembolic events in patients with cardiac resynchronization therapy. Heart Rhythm. 2015;12:2368–75. doi: 10.1016/j.hrthm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Swiryn S, Orlov MV, Benditt DG et al. Clinical implications of brief device-detected atrial tachyarrhythmias in a cardiac rhythm management device population: results from the Registry of Atrial Tachycardia and Atrial Fibrillation Episodes. Circulation. 2016;134:1130–40. doi: 10.1161/CIRCULATIONAHA.115.020252. [DOI] [PubMed] [Google Scholar]
- 57.Van Gelder IC, Healey JS, Crijns HJGM et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–44. doi: 10.1093/eurheartj/ehx042. [DOI] [PubMed] [Google Scholar]
- 58.Lamas G. How much atrial fibrillation is too much atrial fibrillation? N Engl J Med. 2012;366:178–80. doi: 10.1056/NEJMe1111948. [DOI] [PubMed] [Google Scholar]
- 59.Benezet-Mazuecos J, Rubio JM, Cortés M et al. Silent ischaemic brain lesions related to atrial high rate episodes in patients with cardiac implantable electronic devices. Europace. 2015;17:364–9. doi: 10.1093/europace/euu267. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 60.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158:338–46. doi: 10.7326/0003-4819-158-5-201303050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bunch TJ, Galenko O, Graves KG et al. Atrial fibrillation and dementia: exploring the association, defining risks and improving outcomes. Arrhythm Electrophysiol Rev. 2019;8:8–12. doi: 10.15420/aer.2018.75.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bunch TJ. Atrial fibrillation and dementia. Circulation. 2020;142:618–20. doi: 10.1161/CIRCULATIONAHA.120.045866. [DOI] [PubMed] [Google Scholar]
- 63.Benjamin EJ, Wolf PA, D’Agostino RB et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 64.Chen LY, Sotoodehnia N, Bůžková P et al. Atrial fibrillation and the risk of sudden cardiac death: the Atherosclerosis Risk in Communities Study and Cardiovascular Health Study. JAMA Intern Med. 2013;173:29–35. doi: 10.1001/2013.jamainternmed.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang TJ, Larson MG, Levy D et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 66.Deguchi I, Fukuoka T, Hayashi T et al. Clinical outcomes of persistent and paroxysmal atrial fibrillation in patients with stroke. J Stroke Cerebrovasc Dis. 2014;23:2840–4. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez M, Keating RJ, Markowitz SM et al. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm. 2014;11:2214–21. doi: 10.1016/j.hrthm.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 68.Reiffel JA. If it were only that simple. Eur Heart J. 2016;37:1603–5. doi: 10.1016/j.amjcard.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Chen LY, Chung MK, Allen LA et al. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation. 2018;137:e623–e644. doi: 10.1161/CIR.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goette A, Kalman JM, Aguinaga L et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18:1455–90. doi: 10.1093/europace/euw161. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guichard JB, Nattel S. Atrial cardiomyopathy: a useful notion in cardiac disease management or a passing fad? J Am Coll Cardiol. 2017;70:756–65. doi: 10.1016/j.jacc.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 72.Rivner H, Mitrani RD, Goldberger JJ. Atrial Myopathy Underlying Atrial Fibrillation. Arrhythm Electrophysiol Rev. 2020;9:61–70. doi: 10.15420/aer.2020.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reiffel JA. Optimum risk assessment for stroke in atrial fibrillation: should we hold the status quo or consider magnitude synergism and left atrial appendage anatomy. Arrhythm Electrophysiol Rev. 2017;6:161–6. doi: 10.15420/aer.2017.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reiffel JA. Complexities in the atrial fibrillation-stroke relationship: improving comprehension of temporal discordance, magnitude synergism, and subclinical atrial fibrillation – three sources of consternation for physicians who care for patients with atrial fibrillation. J Atr Fibrillation. 2018;11:2100–3. doi: 10.4022/jafib.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Go AS, Reynolds K, Yang J et al. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: the KP-RHYTHM Study. JAMA Cardiol. 2018;3:601–8. doi: 10.1001/jamacardio.2018.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boriani G, Pettorelli D. Atrial fibrillation burden and atrial fibrillation type: clinical significance and impact on the risk of stroke and decision making for long-term anticoagulation. Vasc Pharmacol. 2016;83:26–35. doi: 10.1016/j.vph.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation (ARTESiA). https://clinicaltrials.gov/ct2/show/NCT01938248 (accessed 15 March 2021) [DOI] [PubMed]
- 78.Non-vitamin K. Antagonist Oral Anticoagulants in patients with atrial high rate episodes (NOAH) https://clinicaltrials.gov/ct2/show/record/NCT02618577 (accessed 15 March 2021)
- 79.Hijazi Z, Lindbäck J, Alexander JH et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J. 2016;37:1582–90. doi: 10.1093/eurheartj/ehw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yaghi S, Moon YP, Mora-McLaughlin C et al. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke. 2015;46:1488–93. doi: 10.1161/STROKEAHA.115.008711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saha SK, Anderson PL, Caracciolo G et al. Global left atrial strain correlates with CHADS2 risk score in patients with atrial fibrillation. J Am Soc Echocardiogr. 2011;24:506–12. doi: 10.1016/j.echo.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 82.Daccarett M, Badger TJ, Akoum N et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:831–8. doi: 10.1016/j.jacc.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calenda BW, Fuster V, Halperin JL, Granger GB. Stroke risk assessment in atrial fibrillation: risk factors and markers of atrial myopathy. Nat Rev Cardiol. 2016;13:549–59. doi: 10.1038/nrcardio.2016.106. [DOI] [PubMed] [Google Scholar]


