Abstract
Introduction
Few prospective studies analyze, with sufficient duration, the impact of an antimicrobial stewardship program (AMSP) carried out entirely in a hospital.
Methods
Descriptive study evaluating the consumption of antimicrobials expressed in defined daily doses (DDD) per 100 hospital occupied bed-days (OBDs) stratified in medical, surgical and intensive care unit (ICU) and the incidence of densities (ID) per 1,000 hospital OBDs of the prevalent multidrug-resistant organisms (MDRO) in a tertiary hospital, over a period of 5 years before and after the implementation of an AMSP. Analysis of direct costs and those associated with hospital stay and mortality.
Results
A total of 32,802 patients with antibiotic treatment were included in the intervention period (2013–2017). Non-imposed advice was exercised in 14.9%. The degree of adherence to recommendation was 87.9%, direct treatment and de-escalation being the most frequently admitted interventions (P<0.001). Overall hospital consumption of antibacterials in DDD/100s decreased by 5.7% (77.04 vs. 71.33) between 2008 and 2017. In ICU, the average DDD/100s showed a reduction from 155 to 113 (mean difference -18, P=0.005). There was a decrease in the DI/1000 OBDs of MDROs in the post-intervention period (RR 0.78; CI 95% [0.73, 0.84], P<0.001). The average annual cost of antibacterials declined from €1,435,048 to €955,805 (mean difference -€469,243; P=0.001).
Conclusion
Long-term maintenance of a hospital AMSP was associated with a reduction in antibiotic consumption, especially in ICU, as well as a beneficial ecological impact and economic savings.
Keywords: Antimicrobial stewardship, Use antimicrobials, Multidrug-resistant organisms, Antibiotics cost
Introduction
Antibiotic resistance is a serious health problem with rapid spread worldwide. In 2019, the presence of multidrug-resistant organism (MDRO) outbreaks is considered one of the top 10 global risk factors according to Word Economic Forum [1]. By 2050, antimicrobial resistance is expected to be the leading cause of death attributable to infection [2].
Recently, several nations at the United Nations Assembly asked for the implementation of corrective measures [3]. Intervention strategies include prescribers, patients, pharmaceutical industry and general health providers [4]. One of the most effective interventions is the implementation of an antimicrobial stewardship program (AMSP), especially in hospitals [5,6]. AMSPs show a positive impact on the reduction of stays, shortening treatment duration, and minimizing the incidence of resistant bacteria infection [7,8]. However, there are only a few studies that, prospectively and with long-term maintenance, evaluate their effect beyond an action on critical patients [9], groups of specific antimicrobials [10] or the presence of remarkable microorganisms [11].
The main objective of our study is to assess the impact of an AMSP on a General Hospital over 5 years. The hypothesis formulated is how the establishment of an institutional AMSP could contribute to a reduction in the consumption of antibiotics, the presence of MDROs and their cost.
Methods
Study design
This is a prospective intervention study with historic cohort (before and after). The prospective study period was from January 2013 to December 2017 (5 years) compared to an equivalent pre-intervention period where AMSP actions had not been established.
Study setting
The study was carried out in a 400-bed General University Hospital belonging to the public health network of Catalonia (CatSalut), Spain, with a reference population of 450,000 inhabitants. It is a tertiary hospital that has several medical and surgical specialties, except transplant program, as well as an Intensive Care Unit (ICU) (32 beds). Since 1999, the hospital has had a Hospital-acquired Infection Control Unit (HICU) recognized in the institution that has been actively monitoring intra-hospital infections by MDRO and outbreak control. Annually, the center participates in the surveillance and prevention of Hospital-acquired Infections of Catalan hospitals (VINCat) [12] and is attached to the Zero Infection Projects sponsored by the Spanish Ministerio de Sanidad y Asuntos Sociales [13,14].
Intervention
In 2012, the hospital's infections and antibiotic policy board entrusted HICU (two doctors and two nurses trained in infectious diseases), along with a hospital pharmacist and a clinical microbiologist, the implementation of an institutional AMSP without any cost. The design was created considering the consensus AMSP document published by the Spanish Society of Infectious Diseases and Clinical Microbiology [15]. For its implementation, it received administrative support and was approved by the institution's clinical research ethics committee.
AMSP program design
The program included the following actions: 1. Biennial development and updating of diagnostic protocols and antibiotic treatment of the most prevalent infections; 2. Training of professionals; 3. Daily review of all positive microbiological results (blood cultures and any other samples), except weekends and holidays; 4. Daily written non-imposed advice for professionals on computerized SAP “Systems, Applications, Products in Data Processing” medical history, advice on site or by telephone. The actions could take place in relation to any positive microbiological result and/or systemic antibiotic prescription made for admitted patients. The consulting emphasized the suitability of empirical therapy, targeted treatments, dose adjustments, drug monitoring, de-escalating, early enteral conversions, shortening duration, toxicity or interaction; 5. On-site enhancement of advice in specific units (ICU and Hematology); 6. Perform annual consumption monitoring reports, density of incidence of MDROs and local microbiological sensitivity.
No restrictive measures were made to prescriptions. Adherence to the recommendations was evaluated at 24–48h from intervention. The information was collected prospectively to quantify the degree of acceptance.
Measurement of consumption, microbiological and economic impact
The primary outcome was the change in global antimicrobial hospital consumption, stratified by medical (MS), surgical (SS) and ICU services, before and after AMSP implementation. That is from 2008 to 2012 (first pre-intervention period) and between 2013 and 2017 (second post-intervention period). The secondary outcome was the trend in the evolution of common MDROs (Methicillin-resistant Staphylococcus aureus –MRSA-, Acinetobacter spp., extended-spectrum B-lactamase producing Klebsiella pneumoniae -ESBL- or carbapenemase-producing, Pseudomonas spp.). The third outcome was the reduction in expenses attributable to the results of the AMSP program.
Evaluation methods
To evaluate the consumption of antimicrobials, the Anatomical Therapeutic Chemical Classification and Defined Daily Dose System (ATC/DDD) instituted by the World Health Organization (WHO) (http://www.whoc.no/atc_ddd_index/) was used and expressed as the number of DDD/100 hospital occupied bed-days (OBDs). DDDs correspond to the assumed average maintenance dose per day for a drug used for its main indication in adults.
Pharmacy Department evaluated the consumption data obtained from the specific software of electronic prescription and economic management (SILICON) integrated in SAP, together with antimicrobial cost (expressed in euros -€-) according to the standard fee for the center in the period studied. In this calculation, the consumption of non-computerized units (emergencies, pediatrics) and those that did not generate stays were not considered.
The average stay, discharge, mortality and readmissions-related data were provided by the hospital's technical registry.
The evolutionary impact of resistance was assessed by calculating the density of incidence (DI) per 1,000 hospital OBDs. For the definition of MDRO, the international standard criteria proposed in consensus by Magiorakos et al. [16] were used. The identification of new colonization or infection was carried out through the surveillance system of the HICU, on results offered by the Microbiology department that determined antibiotic resistance according to the International Laboratory Standards (ISL) [17].
Statistical analysis
Continuous quantitative variables were expressed as mean ± standard deviation (SD) and categorical variables as frequencies and percentages (%). The comparison of variables between pre-intervention (2008–2012) and post-intervention (2013–2017) periods was performed with the chi-square or exact Fisher test for categorical variables and t-Student or U-Mann-Whitney for quantitative variables. Rates were analyzed applying Poisson logistic regression in the assessment of differences between periods. In IDs, the risk ratio was expressed as (RR) with 95% confidence interval (CI). SPSS software (version 22) was used for statistical analysis. Statistical significance was defined by P <0.05.
Results
Impact on antibiotic consumption
A total of 67,362 patients with antibiotic treatment were included; 34,560 in the period before intervention (2008–2012) and 32,802 in the intervention period (2013–2017), with 212,872 and 194,330 hospital stays, respectively. During the intervention period, 5,825 cases of advice were exercised in 4,920 patients (14.9%), highlighting general surgery and internal medicine among the services advised (43.3%). Of these, 57.2% were men with an average age of 76 years (range 20–97 years).
The degree of acceptance of the advice was 87.9%, the most accepted intervention being targeted and de-escalated therapy according to microbiological results at 29.5% (P<0.001). Specifically in MSs, early enteral conversion (P=0.001) and drug monitoring (P=0.003), and in SSs the discontinuation of treatment (P=0.023).
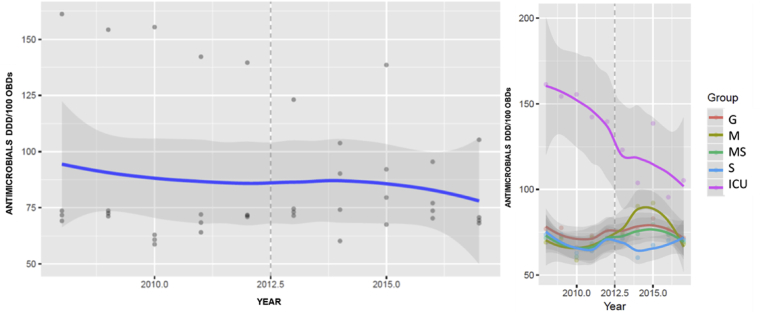
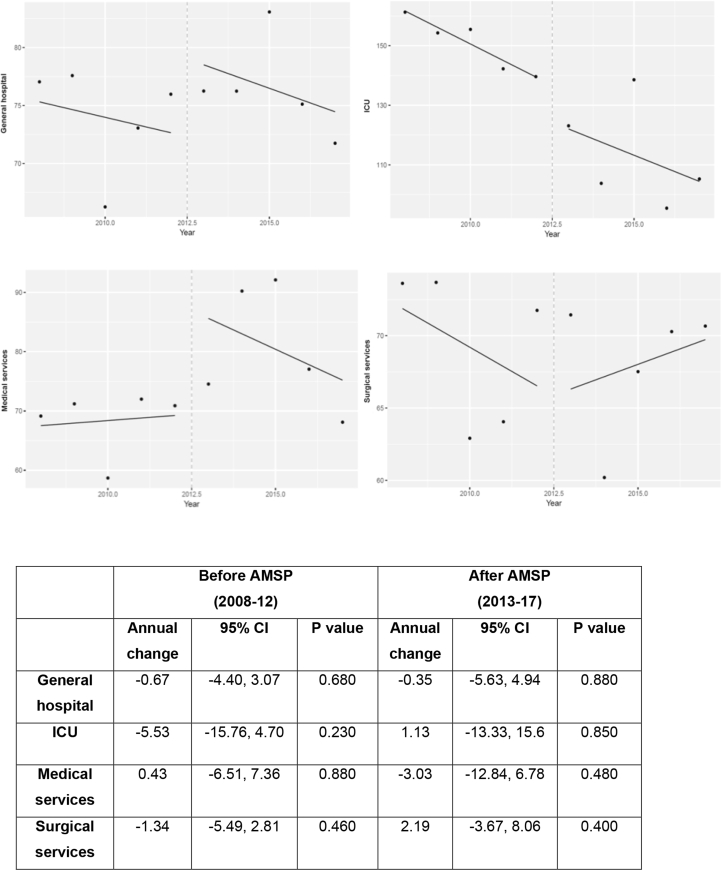
DDD/100 OBDs for each antibiotic divided into groups used in general hospital and by type of service, in each study period, are shown in Table 1. The overall hospital consumption of antibacterials in DDD/100 OBDs decreased by 5.7% (77.04 vs 71.33) between 2008 and 2017 although not significantly (P=0.395) (Figure 1). In ICU, the DDD/100 OBDs showed the most significant decline, from 155 (9.24) in 2008–12 to 113 (17.4) in 2013–17 (mean difference -18, P=0.005). Annual changes in antimicrobial consumption by DDD/100s in pre- and post-intervention period globally and according to type of service are shown in Figure 2.
Table 1.
Defined daily doses (DDD) per 100 hospital occupied bed-days (OBDs) in the consumption of antibacterials, before and after the implementation of AMSP, in the hospital and according to type of service
|
Antimicrobial classes |
Hospital |
P∗ overall | ICU |
P∗ overall | MS |
P∗ overall | SS |
P∗ overall | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non AMSP 2008–12 |
AMSP 2013–17 |
Non AMSP 2008–12 |
AMSP 2013–17 |
Non AMSP 2008–12 |
AMSP 2013–17 |
Non AMSP 2008–12 |
AMSP 2013–17 |
|||||
| Penicillins | 28.0 | 27.2 | 0.368 | 40.4 | 37.2 | 0.419 | 25.2 | 30.9 | 0.020 | 29.1 | 22.4 | 0.011 |
| Cephalosporins | 13.6 | 16.3 | 0.061 | 25.2 | 28.8 | 0.154 | 11.2 | 13.2 | 0.127 | 14.3 | 17.8 | 0.089 |
| 1st generation | 1.67 | 3.39 | 0.013 | 1.08 | 0.65 | 0.234 | 0.16 | 0.41 | 0.523 | 3.36 | 6.40 | 0.052 |
| 2nd generation | 3.12 | 1.10 | 0.152 | 0.47 | 0.08 | 0.032 | 1.09 | 0.75 | 0.243 | 5.30 | 1.45 | 0.032 |
| 3rd generation | 6.34 | 9.76 | 0.001 | 8.54 | 19.0 | <0.001 | 9.04 | 9.46 | 0.842 | 4.99 | 8.30 | 0.128 |
| 4th generation | 2.47 | 2.07 | 0.126 | 15.08 | 9.12 | 0.001 | 2.61 | 2.57 | 0.745 | 0.67 | 0.61 | 0.375 |
| Carbapenems | 4.60 | 3.77 | 0.102 | 21.3 | 9.10 | <0.001 | 3.12 | 3.73 | 0.346 | 3.83 | 3.08 | 0.090 |
| Monobactam | 0.05 | 0.08 | 0.355 | 0.01 | 0.09 | 0.257 | 0.05 | 0.13 | 0.239 | 0.05 | 0.03 | 0.551 |
| Fluoroquinolones | 11.0 | 10.0 | 0.272 | 12.1 | 4.14 | <0.001 | 13.1 | 14.1 | 0.491 | 8.75 | 6.97 | 0.048 |
| Macrolides | 3.78 | 4.05 | 0.776 | 1.87 | 3.26 | 0.147 | 5.96 | 5.98 | 0.990 | 1.92 | 2.28 | 0.451 |
| Aminoglycosides | 3.06 | 2.95 | 0.745 | 6.39 | 6.45 | 0.976 | 2.67 | 2.51 | 0.755 | 3.08 | 2.93 | 0.694 |
| Glycopeptides | 1.96 | 1.54 | 0.546 | 1.98 | 3.13 | 0.126 | 2.70 | 1.15 | 0.034 | 1.26 | 1.72 | 0.576 |
| Glicilcyclines | 0.25 | 0.22 | 0.453 | 3.25 | 1.14 | <0.001 | 0.02 | 0.06 | 0.001 | 0.10 | 0.26 | 0.003 |
| Colistin | 1.69 | 0.37 | <0.001 | 17.88 | 2.67 | <0.001 | 0.52 | 0.28 | 0.022 | 0.22 | 0.14 | 0.450 |
| Oxazolidinones | 0.87 | 0.97 | 0.485 | 7.55 | 2.70 | <0.001 | 0.39 | 0.76 | 0.050 | 0.25 | 0.95 | 0.003 |
| Sulfamides and trimethoprim | 0.89 | 1.55 | 0.001 | 2.76 | 4.61 | 0.028 | 1.47 | 2.28 | 0.007 | 0.37 | 0.89 | 0.009 |
| Lincosamides | 1.10 | 1.07 | 0.821 | 0.71 | 1.32 | 0.140 | 0.48 | 0.92 | 0.010 | 1.78 | 1.34 | 0.098 |
| Metronidazole | 2.03 | 3.25 | 0.014 | 1.05 | 2.06 | 0.069 | 0.78 | 1.28 | 0.126 | 3.34 | 5.37 | 0.625 |
| Tetraciclines | 0.36 | 0.53 | 0.015 | 3.44 | 1.81 | 0.034 | 0.40 | 0.73 | 0.077 | 0.44 | 0.64 | 0.377 |
| Lipoglycopeptides | 0.53 | 1.28 | 0.015 | 4.17 | 4.44 | 0.876 | 0.35 | 1.16 | 0.023 | 0.24 | 0.96 | 0.001 |
| Antipseudomonal antibioticsa | 19.3 | 16.2 | 0.090 | 10.15 | 5.41 | <0.001 | 3.21 | 3.28 | 0.852 | 2.10 | 1.93 | 0.572 |
| Antimicroorganism resistant gram + antibioticsb | 4.80 | 6.70 | 0.350 | 2.83 | 3.11 | 0.450 | 0.51 | 0.91 | 0.520 | 0.58 | 0.84 | 0.826 |
AMSP: antimicrobial stewardship program; ∗Statistical significance; ICU: intensive care unit; MS: medical services (internal medicine, cardiology, neurology, pneumology, digestology, rheumatology, nephrology, hematology and oncology); SS: surgical services (otorhinolaryngology, ophthalmology, traumatology and orthopedics, general surgery, vascular surgery, neurosurgery, gynecology and obstetrics, urology and maxillofacial surgery).
Piperacillin-tazobactam, ceftazidime, cefepime, ciprofloxacin, meropenem, amikacin, colistin, fosfomycin, aztreonam.
Vancomycin, daptomycin, cotrimoxazole, tigecycline, doxycycline, linezolid, clindamycin.
Figure 1.
Fitted growth curve in the general hospital's consumption of antimicrobials in DDD/100 hospital occupied bed-days (OBDs) and according to type of service. G: general; M: medical; MS: medical-surgery; S: surgery; ICU: intensive care unit; OBD: occupied bed-days.
Figure 2.
Annual changes in the consumption of antimicrobials by DDD/100 hospital occupied bed-days (OBDs) in the general hospital and according to type of service before and after implementation of AMSP.
After the implementation of the AMSP there was an annual downward trend in DDD/100 OBDs inverse to the previous trend in the period 2008–12, this behavior being significantly different in both periods, particularly in the use of carbapenems (P=0.050), monobactams (P=0.043), fluoroquinolones (P=0.015), antipseudomonics (P=0.009), tetracyclines (P=0.024), colistin (P<0.001) and glycopeptides, especially teicoplanin (P=0.001). Many antibiotics also decreased in 2013, such as linezolid -0.55; 95% IC [-0.86,-0.23] (P=0.010), daptomycin (P=0.013) and piperacillin-tazobactam (P=0.030). In contrast, cephalosporins tended to decrease annually in the period 2008–12, and then increased in post-intervention (p-0.020) at the expense of cefazolin (P=0.013), ceftriaxone/cefotaxime (P=0.010) and ceftazidime compared with cefepime (P=0.004). Azithromycin (P=0.029) and cloxacillin also increased (P=0.030) in the second period. Sulfamethoxazole-Trimethoprim showed no significant annual trend in any of the periods, but an average increase in 2013 of 0.72; 95% IC [0.23, 1.20], (P=0.010). All other antibiotics showed insignificant data. Sequential therapy maintained a decrease in the previous period (2008–12), with an annual change of -0.69; 95% IC [-1.26,-0.12], P=0.030 that disappears in the subsequent period when an annual trend was not significant (P=0.78). In ICU, a significant reduction in prescription was observed, when the intervention started in 2013, of -17.69; 95% IC [-25.56,-9.82], (P<0.010) in carbapenems and -4.48, 95% CI [-8.36,-0.59], (p-0.030) in fluoroquinolones but with no subsequent significant annual decrease. Macrolides decreased in post-intervention period. Aminoglycosides experienced a significant increase of +8.07; 95% CI [1.71, 14.42], (P=0.020) and cephalosporins lost the downward trend in the pre-intervention period (P=0.014). In MSs, carbapenems showed a significant increase in the pre-intervention period +0.61; 95% IC [0.25, 0.97], (P=0.010) which is fully reversed in the post-intervention period (P<0.001). Finally in SSs, lincosamides and aminoglycosides reversed the upward trend of the pre-intervention period (P=0.040) following an opposite pattern in the next period (P=0.025) and cephalosporins suffered from a significant increase in 2013 of +6.61; 95% IC [0.78, 12.45], (P=0.030) which was subsequently maintained annually (P=0.008).
Impact on microbial resistance
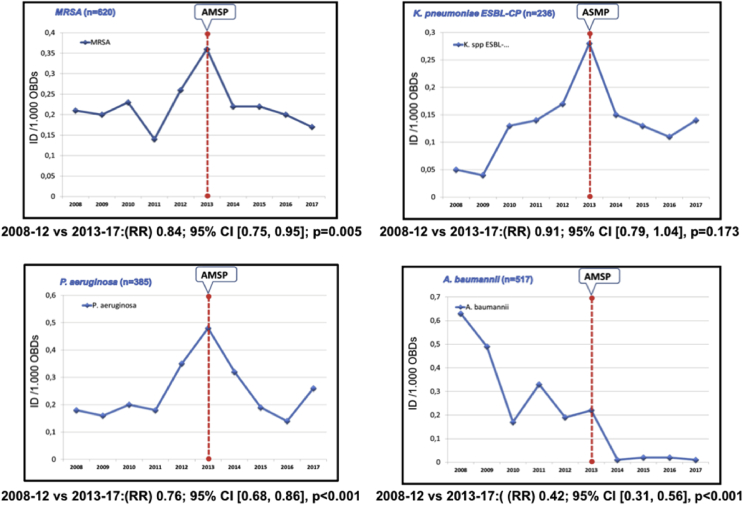
From 2008-2012, the hospital had an average DI/1,000 OBDs of MDROs of 0.98 vs 0.75/1,000 OBDs in 2013–2017 (P=0.030). There is a downward trend that was significant in this post-intervention period (RR 0.78; 95% IC [0.73, 0.84], P<0.001) following a significant increase in 2013 (P<0.001). The evolution in DI/1000 OBDs of MDROs is shown in Figure 3. In ICU, P. aeruginosa and K. pneumoniae in the pre-intervention period experienced an annual increase but only significant in the latter microorganism (P=0.014), with a complete reversion in the post-intervention period with RR 0.71; 95% IC [0.56, 0.89], P=0.004 and RR 1.48; 95% IC [1.03, 1.30], P=0.017, respectively. A. baumanii maintains a downward trend in the period 2008–2012, more pronounced and significant in the second period (RR 0.20; 95% IC [0.08, 0.39], P<0.001). Most of the MDROs studied suffer from a significant rise in 2013.
Figure 3.
Evolution of the incidence density (ID) of MDR organisms most frequently found in hospital by 1,000 occupied bed-days (OBDs). AMSP: antimicrobial stewardship program; MDR: hospital-acquired multidrug-resistant; RR: rate ratio; ESBL: extended-spectrum β-lactamase-producing Enterobacteriaceae; CP: carbapenemase-producing.
Clostridioides difficile maintained an unchanged DI between periods of around 0.05/1,000 OBDs, that increased in the last year of the post-intervention period (2016), following the introduction of new techniques as a diagnostic method. Multi-resistant Enterococcus spp were not found in either of the periods.
Various health indicators were monitored in order to detect other issues that could interfere with MDRO IDs (Table 2). In the post-intervention period, there was a reduction in episodes of central venous catheter-related infections in patients with parenteral nutrition (RR 0.50; 95% CI [0.43, 0.56], P<0.001), colon-rectum surgery rates (RR 0.65; 281 CI 95% [0.57, 0.72], P=0.032) and per organ/space in colon-rectum surgery (RR 0.68; 95% 282 [0.59, 0.71], P=0.048).
Table 2.
Health variables during the study period by year
| Health indicators | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|---|---|---|---|
| No. Patients | 19.676 | 19.654 | 20.120 | 19.677 | 19.066 | 18.278 | 18.488 | 18.522 | 18.675 | 19.192 |
| No. OBDs | 125.416 | 118.260 | 126.247 | 117.862 | 116.965 | 112.769 | 110.159 | 110.070 | 109.110 | 109.968 |
| No. Bacteremia associated with central vascular catheter for parenteral nutrition | 145 | 85 | 59 | 60 | 59 | 62 | 47 | 52 | 64 | 60 |
| Knee prosthesis infection rate | 0 | 0 | 1.8 | 1 | 1.9 | 0.8 | 0 | 1.4 | 0 | 0.6 |
| Hip prosthesis infection rate | 2.6 | 0 | 0 | 0 | 0 | 1.7 | 4.4 | 0 | 1.6 | 0 |
| Colon Surgery (CS) infection rate | 28.7 | 14.4 | 23.9 | 25.2 | 26.7 | 15.8 | 18.1 | 21.7 | 11.9 | 9.4 |
| Organ/space infection rate in CS | 16.1 | 8.5 | 8.3 | 10.7 | 15.8 | 4.2 | 11.4 | 11.5 | 7.9 | 5.5 |
OBDs: occupied bed-days.
As for the crude mortality rate, there was no variation in either of the two periods, 3.34 vs 3.14 (P=0.210). A decrease in the annual ratio between days of stay and hospital discharge was observed in both periods, but with an upward annual pattern change in the discharges in MSs in the period 2013–2017 (P=0.029). The rate of hospital readmission in the first month remained almost unchanged from 6.9% to 7.0%.
Impact on economic cost
The average annual cost of antibacterials between MSs and SSs decreased from €1,152,151 to €835,568 (mean difference -€316,583; P=0.001) and in ICU went from €282,897 to €120,237 (mean difference -€122,660; P<0.001). The 4,330 patients in whom the advice was accepted presented 0.55 inpatient days post intervention compared with cases that ignored recommendations, generated 2,375.2 days less of hospitalization, which is quantified as an economic amount of €1,254,111. The total savings between antibacterials and days of hospitalization were €3,450,326.
Discussion
The rise of AMSPs in hospitals, as a consequence of concern about the negative effects of the inappropriate use of antibiotics and increased resistance, has led to an improvement in the centers that implemented them [4,5,18]. Long-term benefit is practically unknown, most experiences in the research literature not exceeding one year [19].
This study confirms how the implementation of an AMSP in a tertiary hospital and for a prolonged period, carried out by a team of professionals exclusively specialized in infectious diseases, was associated with a reduction in consumption of antimicrobials, decreasing MDROs and favorable economic and cost savings.
The outcomes are the result of the high degree of acceptance (about 90%) of non-imposed advice, unlike the results achieved with restrictive measures as described in the literature [20]. This high percentage response has also been observed in other studies carried out in hospitals of a similar level [21]. The yield is better when they also work on the control and prevention of hospital-acquired infections [10,22]. Although advice interventions made 1–3 days a week may be effective [23], daily intervention has helped to consolidate the impact [18].
Although there are other types of measurement units in antibiotic consumption, this study has used DDD as a numerical assessment of international comparison. Our general consumption of antibacterials percentage (5.7%) is similar to other publications [19,24]. In the specific case of ICU, the reduction in DDD/100 OBDs was less (155 vs 113 (mean difference -18, P=0.005)) than that observed in the literature, perhaps because they have a higher starting point of DDD in the pre-intervention period [9] and as a consequence of active surveillance projects. In contrast, the non-significant increase in antibiotic use in SSs during the intervention period may have been due to an increase on combining two antimicrobials, as an alternative to carbapenem or ureidopenicillin monotherapy. Perhaps these are the reasons why general antibiotic reduction has not been significant.
Overall, we observed that the decrease in consumption occurred especially in the antibiotics that are associated with a greater induction of resistance and the emergence of Clostridioides difficile [14]. This reduction was achieved with an early de-escalation and restriction of these antibiotics both in community infections and in empirical treatments by modifying local protocols. This is quite the opposite of what has been happening in the case of carbapenems, in other hospitals near us [25]. After the implementation of a specific AMSP, Álvarez-Lerma et al. [9] achieved a decrease in various antimicrobials, except in piperacillin-tazobactam and carbapenems. Also noteworthy is the reduction in teicoplanin compared to vancomycin (P<0.001), justifiable by the choice of the latter because of the possibility of monitoring levels and lower cost [26]. The increase in the use of cloxacillin was possibly caused by the substitution of lipo and glycopeptides in the cases of beta-lactamic gram-positive cocci infection.
Some studies point to a reduction in mortality and stays in the groups intervened with AMSPs. Tedeschi et al. [27] show how de-escalation is not linked to higher mortality. In the case of bacteremia, especially Staphylococcus aureus, a rapid targeted treatment even increases the cure rate, reducing relapses and mortality [28]. In our case, the crude mortality remained unchanged, although its relationship with infections could not be investigated. The stays remained stable and the discharges increased slightly, indicating that a smaller amount of antimicrobials has been consumed and that more patients have been treated with the same number of antimicrobials.
In our study we observed a global reduction in the presence of MDROs and specifically in ICU after starting the AMSP, although we do not know the main reason for exponential growth before its beginning. Karanika et al. [29] analyzed the effect of AMSPs in 7 studies in which there was a significant decrease in the presence of MDROs. In a recent meta-analysis by Baur et al. [11] of 32 studies conducted over 60 years, a reduction was shown in the incidence of infection and MDROs colonization.
From an economic point of view, our experience shows that the AMSP has been cost effective, with a potential annual saving of approximately €500,000, similar to other studies [30]. The cost of HICU staff can be financed by the indirect saving derived from the reduction in hospital stays, which in our case is around €250,822 per year.
Our work has several limitations: (1) the AMSP was applied in units with electronic medication dispensers which left out pediatrics and emergencies, (2) the introduction of Zero projects in the ICU and institutional projects in hospitalization rooms that condition the decrease in surgical infection rates in the AMSP's period may have influenced our results.
Finally, we think that the fact that it is a prospective comparative trial with a reproducible methodology makes it possible to generalize our results.
In conclusion, the results of this study show that, after 5 years, the strategy of implementing a global AMSP in a tertiary hospital was associated with significant benefits in reducing antimicrobial consumption, protection of the ecosystem and lower economic cost, without prejudice to the patient.
Authors' contribution
Alfredo Jover-Sáenz: Conceptualization, Methodology, Writing - original draft, Supervision, Writing - review & editing; María Fernanda Ramírez-Hidalgo: Methodology, Validation, Writing - original draft, Writing - review & editing; Montserrat Vallverdú Vidal: Visualization, Writing - review & editing; Merce García González: Resources, Writing - review & editing; Santiago Manuel Cano Marrón: Software, Methodology, Writing - review & editing; Alfredo Escartín Arias: Visualization, Writing - review & editing; Miquel Falguera Sacrest: Visualization, Writing - review & editing; Dolors Castellana-Perelló: Software, Visualization, Writing - review & editing; Fernando Barcenilla-Gaite: Conceptualization, Methodology, Supervision, Writing - review & editing
Conflict of interest
The authors declare that they have no conflicts of interest and did not receive external financial support on conducting the study.
Acknowledgements
Our most sincere thanks to medical professionals and hospital nurses, responsible for our patients, for their support on accepting and implementing the recommendations; pharmacists, microbiologists and technicians for their invaluable contribution to the completion of advice, dosing and drug monitoring and early identification of microorganisms, and to the hospital's technical registry for supplying the administrative information necessary for the study.
Contributor Information
Alfredo Jover-Sáenz, Email: ajover.lleida.ics@gencat.cat.
Fernando Barcenilla-Gaite, Email: fbarcenilla.lleida.ics@gencat.cat.
References
- 1.The Global Risks Report 2019 14th Edition. Word economic fórum. www.weforum.org Available at:
- 2.Antimicrobial resistance (AMR): applying all our health. 2019. Public heath England.https://www.gov.uk/government/publications/antimicrobial-resistance-amr-applying-all-our-health Available at: [Google Scholar]
- 3.World Health Organizatión General Assembly of the United Nations: High-level Meeting programme and documents. 2016. http://www.un.org/pga/71/wpcontent/uploads/sites/40/2016/09/DGACM_GAEAD_ESCAB-AMR-Draft-Political-Declaration-1616108-Spanish.pdf Available at:
- 4.Cox J.A., Vlieghe E., Mendelson M., Wertheim H., Ndegwa L., Villegas M.V. Antibiotic stewardship in low and middle income countries: the same but different? Clin Microbiol Infect. 2017;23:812–818. doi: 10.1016/j.cmi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Bucket W.R., Veillettte J.J., Vento T.J., Stenehjem E. Antimicrobial stewardship in community hospitals. Med Clin N Am. 2018;102:913–928. doi: 10.1016/j.mcna.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Nathwani D., Varghese D., Stephens J., Ansari W., Martin S., Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control. 2019;8:35. doi: 10.1186/s13756-019-0471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilholm H., Holmstrand L., Ahl J., Mansson F., Odenholt I., Tham J. An audit based-infectious disease specialist guided antimicrobial stewardship program profoundly reduced antibiotic use without negatively affecting patient outcome. Open Forum Infect Dis. 2015;2:ofv042. doi: 10.1093/ofid/ofv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marwick C.A., Guthrie B., Davey P.G. Hospital antimicrobial stewardship: the way forward. Lancet Infect Dis. 2017;17:1119–1120. doi: 10.1016/S1473-3099(17)30566-2. [DOI] [PubMed] [Google Scholar]
- 9.Álvarez-Lerma F., Grau S., Echeverría-Esnal D., Martínez-Alonso M., Gracia-Arnillas M.P., Horcajada J.P. A Before-and-after study of the effectiveness of an antimicrobial stewardship program in critical care. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.01825-17. e01825-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-Rodríguez J.F., Bardán-García B., Peña-Rodríguez M.F., Álvarez-Díaz H., Mariño-Callejo A. Meropenem antimicrobial stewardship program: clinical, economic, and antibiotic resistance impact. Eur J Clin Microbiol Infect Dis. 2019;38:161–170. doi: 10.1007/s10096-018-3408-2. [DOI] [PubMed] [Google Scholar]
- 11.Baur D., Gladstone B.P., Burkert F., Carrara E., Foschi F., Döbele S. Effect of antibiotic stewardship on the incidence of infection and colonization with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990–1001. doi: 10.1016/S1473-3099(17)30325-0. [DOI] [PubMed] [Google Scholar]
- 12.VINCat Program Programa de Vigilància de les Infeccions Nosocomials a Catalunya. Generalitat de Catalunya. Departament de Salut. http://catsalut.gencat.cat/ca/proveidors-professionals/vincat/ Available at:
- 13.Spanish Society of Intensive Care Medicine, Units Coronary. Working Group of Infectious Diseases and Sepsis (GTEIS). National Surveillance Study of Nosocomial Infection in the ICU (ENVIN-HELICS). Bacteremia Zero project, Pneumonia Zero project, Resistance Zero project. SEMICYUC, Madrid, Spain. 2018. http://hws.vhebron.net/envin-helics Available at:
- 14.Plan estratégico y de acción para reducir el riesgo de selección y diseminación de la resistencia a los antibióticos (2014). Agencia española de medicamentos y productos sanitarios. Ministerio de Sanidad, Servicios Sociales e Igualdad. Available at: https://www.aemps.gob.es/publicaciones/publica/planestrategico-antibioticos/v2/docs/plan-estrategico-antimicrobianos-AEMPS.pdf.
- 15.Rodríguez-Baño J., Paño-Pardo J.R., Alvarez-Rocha L., Asensio A., Calbo E., Cercenado E. Grupo de Estudio de la Infección Hospitalaria-Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica; Sociedad Española de Farmacia Hospitalaria; Sociedad Española de Medicina Preventiva Salud Pública e Higiene. Programs for optimizing the use of antibiotics (PROA) in Spanish hospitals: GEIH-SEIMC, SEFH and SEMPSPH consensus document. Enferm Infecc Microbiol Clin. 2012;22 doi: 10.1016/j.eimc.2011.09.018. e122-e23. (In Spanish). Available at: [DOI] [PubMed] [Google Scholar]
- 16.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 17.Patel J.B. Clinical and laboratory standards institute; 2016. Approved Guideline, M-02 e M-07. Performance standards for antimicrobial susceptibility Testing MS100–26. [PubMed] [Google Scholar]
- 18.Beaulac K., Corcione S., Epstein L., Davidson L.E., Doron S. Antimicrobial stewardship in a long-term acute care hospital using offsite electronic medical record audit. Infect Control Hosp Epidemiol. 2016;37:433–439. doi: 10.1017/ice.2015.319. [DOI] [PubMed] [Google Scholar]
- 19.Ugalde-Espiñeira J., Bilbao-Aguirregomezcorta J., Sanjuan-López A.Z., Floristán-Imízcoz C., Elorduy-Otazua L., Viciola-García M. A program for optimizing the use of antimicrobials (PROA): experience in a regional hospital. Rev Esp Quimioter. 2016;29:183–189. [PubMed] [Google Scholar]
- 20.Mostaghim M., Snelling T., Bajorek B. Factors associated with adherence to antimicrobial stewardship after-hours. Int J Pharm Pract. 2019;27:180–190. doi: 10.1111/ijpp.12486. [DOI] [PubMed] [Google Scholar]
- 21.García-San Miguel L., Cobo J., Martínez J.A., Arnau J.M., Murillas J., Peña C. Third day intervention': an analysis of the factors associated with following the recommendations on the prescribing of antibiotics. Enferm Infecc Microbiol Clin. 2014;32:654–661. doi: 10.1016/j.eimc.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Burnham J.P., Olsen M.A., Stwalley D., Kwon J.H., Babcock H.M., Kollef M.H. Infectious diseases consultation reduces 30-day and 1-year all-cause mortality for multidrug-resistant organism infections. Open Forum Infect Dis. 2018;5:ofy026. doi: 10.1093/ofid/ofy026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagiwara D., Sato K., Miyazaki M., Kamada M., Moriwaki N., Nakano T. The impact of earlier intervention by an antimicrobial stewardship team for specific antimicrobials in a single weekly intervention. Int J Infect Dis. 2018;77:34–39. doi: 10.1016/j.ijid.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins T.C., Knepper B.C., Shihadeh K., Haas M.K., Sabel A.L., Steele A.W. Long-term outcomes of an antimicrobial stewardship program implemented in a hospital with low baseline antibiotic use. Infect Control Hosp Epidemiol. 2015;36:664–672. doi: 10.1017/ice.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grau S., Fondevilla E., Echeverría-Esnal D., Alcorta A., Limon E., Gudiol F., VINCat Program group Widespread increase of empirical carbapenem use in acute care hospitals in Catalonia, Spain. Enferm Infecc Microbiol Clin. 2019;37:36–40. doi: 10.1016/j.eimc.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Abad F., Calbo F., Zapater P., Rodríguez-Vilanova F., García-Pérez L., Sacristán J.A. Comparative pharmacoeconomic study of vancomycin and teicoplanin in intensive care patients. Int J Antimicrob Agents. 2000;15:65–71. doi: 10.1016/s0924-8579(00)00123-0. [DOI] [PubMed] [Google Scholar]
- 27.Tedeschi S., Trapani F., Giannella M., Cristini F., Tumietto F., Bartoletti M. An Antimicrobial Stewardship Program Based on Systematic Infectious Disease Consultation in a Rehabilitation Facility. Infect Control Hosp Epidemiol. 2017;38:76–82. doi: 10.1017/ice.2016.233. [DOI] [PubMed] [Google Scholar]
- 28.Lahey T., Shah R., Gittzus J., Schwartzman J., Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore) 2009;88:263–267. doi: 10.1097/MD.0b013e3181b8fccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karanika S., Paudel S., Grigoras C., Kalbasi A., Mylonakis E. Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob Agents Chemother. 2016;60:4840–4852. doi: 10.1128/AAC.00825-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Standiford H.C., Chan S., Tripoli M., Weekes E., Forrest G.N. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7-year program. Infect Control Hosp Epidemiol. 2012;33:338–345. doi: 10.1086/664909. [DOI] [PubMed] [Google Scholar]