Abstract
In the burn treatment landscape, a variety of skin substitutes, human tissue-sourced products, and other products are being developed based on tissue engineering (ie, the combination of scaffolds, cells, and biologically active molecules into functional tissue with the goal of restoring, maintaining, or improving damaged tissue or whole organs) to provide dermal replacement, prevent infection, or prevent or mitigate scarring. Skin substitutes can have a variety of compositions (cellular vs acellular), origins (human, animal, or synthetically derived), and complexities (dermal or epidermal only vs composite). The regulation of tissue-engineered products in the United States occurs by one of several pathways established by the U.S. Food and Drug Administration, including a Biologics License Application (BLA), a 510(k) (Class I and Class II devices), Premarket Approval (Class III devices), or a human cells, tissues, and cellular and tissue-based products designation. Key differentiators among these regulatory classifications include the amount and type of data required to support a filing. For example, a BLA requires a clinical trial(s) and evaluation of safety and efficacy by the Center for Biologics Evaluation and Research. Applicable approved biological products must also comply with submission of advertising and promotional materials per regulations. This review provides a description of, and associated requirements for, the various regulatory pathways for the approval or clearance of tissue-engineered products. Some of the regulatory challenges for commercialization of such products for the treatment of burns will be explored.
Tissue engineering, as defined by the National Institutes of Health, refers to the field of science in which cells, scaffolds, and biologically active molecules are combined to generate functional tissues, with the aim of restoring or improving damaged tissue or whole organs.1 Tissue engineering continues to advance the field of regenerative medicine and provides a set of biomedical tools for use across a wide range of biomedical applications.2
In the burn treatment landscape, skin substitutes and other tissue-engineered (TEng) products are in development to provide dermal and/or epithelial replacement, prevent or reduce infection, or prevent or mitigate scarring.3 Skin substitutes can vary in composition (cellular and/or tissue products vs acellular), origin (human, animal, or synthetic), processing (minimal vs extensive), and complexity (dermal or epidermal only vs composite).3,4 Furthermore, based on their origin, skin substitutes can be categorized into xenografts (eg, other species' [bovine, equine, ovine, porcine, and piscine] dermis, purified collagen, and intestinal or bladder submucosa), synthetic grafts (eg, polymeric or composed of a porous matrix containing collagen and extracellular matrix components combined with a polymer), allogeneic grafts (eg, human newborn foreskin fibroblasts and/or keratinocytes), and autologous grafts (eg, human keratinocytes, fibroblasts, adipose tissue, and dermis).3
The development of skin substitutes has advanced and is likely to continue to evolve as further advances in this field are made. The wide range of skin substitutes available for the treatment of acute thermal skin injuries (ie, burns) are regulated through one of several available U.S. Food and Drug Administration (FDA) pathways (Table 1).5 The appropriate pathway for product commercialization depends on the skin substitute’s composition, origin, and intended use.5
Table 1.
Select skin substitutes for the treatment of burns and their respective FDA regulatory pathway
| Commercial Product | Company | Brief Description | Type of Approval/ Clearance/Regulation (Year)* | Predicate |
|---|---|---|---|---|
| Integra Dermal Regeneration Template; Omnigraft Dermal Regeneration Matrix | Integra LifeSciences | Porous matrix of bovine collagen and chondtroitin- 6-sulfate with semipermeable polysiloxane (silicone) layer | PMA (1996) | N/A |
| OrCel | Forticell Bioscience | Bilayered cellular matrix in which normal human allogeneic epidermal keratinocytes and dermal fibroblasts are cultured into a type I bovine collagen sponge | PMA† (2001) | N/A |
| ReCell | Avita Medical | Autologous cell harvesting system (device) that enables production of a suspension of Spray-On Skin cells using a small sample of the patient’s own skin. The suspension contains the cells necessary to regenerate the outer layer of skin | PMA (2018) | N/A |
| TransCyte (originally Dermagraft-TC) | Organogenesis | Human fibroblast-derived temporary interactive wound and burn dressing consisting of polymer membrane and donated bioactive neonatal human fibroblast cells cultured under aseptic conditions in vitro on a nylon mesh | PMA (1997) | N/A |
| Cadaver skin | Tissue banks | Cryopreserved cadaver skin allografts/split-thickness graft containing the epidermis and part of the dermis | HCT/P 361 | N/A |
| AlloDerm | LifeCell | Acellular human cadaver skin allograft | HCT/P 361 | N/A |
| EpiBurn‡ | MiMedx Group | Placental membrane allograft | HCT/P 361 | N/A |
| GammaGraft | Promethean LifeSciences | Acellular-irradiated human-cadaver skin allograft | HCT/P 361 | N/A |
| SkinTE | PolarityTE | Autologous, homologous product to repair, reconstruct, replace, or supplement a patient’s damaged or missing skin tissue | HCT/P 361§ | N/A |
| Biobrane | Smith & Nephew | Acellular biosynthetic matrix composed of a silicone membrane bonded to a nylon mesh to which peptides from a porcine dermal collagen source have been bonded | 510(k) FRO (1990) | Predicate not found in CDRH public domain |
| Cytal/MatriStem Wound Matrix | ACell | Acellular matrices derived from porcine urinary bladder | 510(k) KGN (2010) | MatriStem wound sheet |
| Cytal/MatriStem Wound Matrix-meshed sheet | 510(k) KGN (2015) | ACell UBM lyophilized wound dressing | ||
| EZ Derm | Mölnlycke | Acellular biosynthetic matrix from porcine collagen cross-linked with aldehyde | 510(k) KGN (1994) | Predicate not found in CDRH public domain |
| Hyalomatrix | Anika Therapeutics | Acellular synthetic matrix hyaluronic-acid 3D fibrous matrix + thin silicone layer | 510(k) FRO (2007) | Hyalomatrix KC (Laserskin) Wound Dressing |
| Integra BMWD; Integra Meshed Bilayer Wound Matrix | Integra LifeSciences | Porous matrix of X-linked bovine tendon collagen & GAG with semi-permeable polysiloxane (silicone layer) | 510(k) KGN (BMWD, 2002); 510(k) FRO (Meshed Bilayer Wound Matrix, 2008) | Oasis SIS Wound Dressing II, Fortaderm Wound Dressing, VitaChoice Wound Dressing, Biobrane II Temporary Wound Dressing, Integra BMWD |
| MariGen | Kerecis | Piscine dermis | 510(k) KGN (2013) | Mesynthes Endoform dermal template, ACeII MatriStem, Integra wound matrix, LTM wound dressing, PriMatrix, HemCon chitoflex surgical dressing, Oasis |
| MatriStem MicroMatrix particles | ACell | Acellular matrices from porcine urinary bladder | 510(k) KGN (2016) | ACell powder wound dressing |
| Novosorb BTM | PolyNovo | Polyurethane porous foam bonded with a polyurethane adhesive layer to a fenestrated transparent sealing membrane | 510(k) FRO (2017) | Suprathel wound and burn dressing Integra BMWD |
| Oasis wound matrix & Oasis burn matrix | Smith & Nephew | Acellular matrices from porcine small intestine submucosa | 510(k) KGN (2006) | SIS Wound Dressing II |
| PriMatrix | Integra LifeSciences | Acellular matrix from fetal bovine dermis | 510(k) KGN (2008) | Oasis |
| PriMatrix Dermal Repair Scaffold | 510(k) KGN (2016) | PriMatrix Dermal Repair Scaffold | ||
| Suprathel | Polymedix Innovations; BioMed Sciences | Acelluar synthetic matrix composed of copolymer of polylactide, trimethylene carbonate, and s-caprolactone | 510(k) FRO (2009) | BioCore Medical Collatek sheet, Biomet Merck Topkin Foil, Inion OTPS Biodegradable Pin, MacroPore Surgi-Wrap MAST Bioresorbable Sheet, Nymed Group Hydrolyzed Collagen Gel with Silver, Biomet Mesofol, BioDerm BTF Thin-Film Wound Dressing, and Integra Life Sciences HeliDerm |
| Epicel | Vericel | Cultured epidermal autograft–keratinocytes from autologous skin cultured in murine fibroblast | HDE† (2007) | N/A |
BMWD, bilayer matrix wound dressing; BTM, biodegradable temporizing matrix; CDRH, Center for Devices and Radiological Health; FDA, Food and Drug Administration; FRO, FDA product code for unclassified medical devices (dressing, wound, and drug) that are not implanted; GAG, glycosaminoglycan; HCT/P, human cells, tissues, and cellular and tissue-based product; HDE, humanitarian device exemption; KGN, FDA product code for unclassified medical devices (dressing, wound, and collagen) that are implanted; N/A, not applicable; PMA, premarket approval; UBM, urinary bladder matrix. Some of the products listed here are also available for chronic wounds, and there are additional skin substitutes not listed that are available for chronic wounds. For additional information on these products and/or their market-access regulatory pathways, please see fda.gov. Please note that all trademarks are the property of their respective owners.
*Information is based on the initial FDA approval/clearance/regulation.
†Initially regulated as a device but shifted to being regulated through CBER as cellular therapeutic because it contains live cells.6
‡EpiFix (same product description; available in sheet, particulate, and wrap configurations) was marketed in 2006.
§In April 2020, PolarityTE announced that the company plans to file a BLA for SkinTE.7
This review will explore the current FDA regulatory classifications and associated requirements (eg, clinical data, predicate [or previously approved/cleared] medical device) that define the approval, clearance, or regulation of products for the treatment of acute thermal skin injuries. In addition, context will be provided for healthcare practitioners to facilitate a better understanding of the types of data generated to support product commercialization (ie, the level of regulatory scrutiny and clinical evidence required for approval or clearance). The current regulatory challenges, with respect to skin substitutes for the treatment of burns, will also be discussed.
REGULATORY PATHWAYS FOR SKIN SUBSTITUTES FOR THE TREATMENT OF ACUTE THERMAL SKIN INJURIES
The FDA regulates skin substitutes under one of several categories, depending on the product’s origin, composition, and intended use: human cells, tissues, and cellular and tissue-based products (HCT/Ps) for human-derived products that are minimally manipulated and intended for homologous use; Premarket Approval (PMA) or a humanitarian use device (HUD) for human- and human/animal-derived products; and the 510(k) pathway for animal-derived and synthetic products.5 In addition, when human tissues and cells are used to produce cellular-derived material for a specific claim of action (eg, wound healing) or the product undergoes more than minimal manipulation during production, the FDA recommends the use of the Biologics License Application (BLA) pathway.5,8
Each of these pathways varies in submission and clinical requirements, as well as the review and decision timelines. The timing for an FDA decision can vary based on the regulatory pathway and is established by User Fee Goals. For medical devices, the Medical Device User Fee Amendments (MDUFA) for Fiscal Years (FYs) 2018 through 2022 (MDUFA IV) establishes PMA total time to decision goals ranging from 310 to 290 calendar days for FYs 2018 through 2022 (eg, for Original PMA submissions received in FYs 2018 through 2020, the average Total Time to Decision goal for FDA and industry is 310 calendar days).9 For 510(k) submissions, the total time to decision goal ranges from 124 to 108 calendar days (eg, for 510(k) submissions received beginning in FY 2020, the average Total Time to Decision goal for FDA and industry is 116 calendar days).9 The Prescription Drug User Fee Act (PDUFA) for FYs 2018 through 2022 (PDUFA VI) sets a goal to review and act on original new molecular entity (standard review) NDA/BLAs within 10 months of the 60-day filing date and 6 months of the 60-day filing date for priority new molecular entity (priority review).10
Key features of each of these regulatory classifications and governing agencies are highlighted in Table 2, with products being differentiated by the basic and clinical requirements, as well as the level of data required to support a filing. For example, the BLA pathway requires extensive nonclinical and controlled clinical data to support the safety and efficacy of the product in the intended indication, whereas the 510(k) pathway may not require clinical data if the device to be marketed is considered substantially equivalent (ie, as safe and effective) to a previously cleared (predicate) device and is not being marketed for a new indication (ie, intended use).11–15 Each pathway and its requirements are discussed in more detail below. While not addressed in detail herein, combination products are composed of any combination of a drug and a device; a biological product and a device; a drug and a biological product; or a drug, device, and a biological product.16 In particular, “single entity” combination products are comprised of two or more regulated components that are physically, chemically, or otherwise combined or mixed and produced as a single entity.16 Such products are assigned to an FDA center that will have primary jurisdiction for its premarket review and regulation.
Table 2.
United States Food and Drug Administration regulatory pathways for skin substitutes
| Pathway | Clinical/Basic Requirements | Review of Data |
|---|---|---|
| Biologics License Application (BLA)26 | • Cultured tissue and/or allogeneic or autologous cells • Requires FDA review of an IND, which includes clinical trial efficacy and safety data in the intended indication • Similar pathway to a drug approval, except full characterization of the active component is not required • More rigorous than HCT/P, HDE, and 510(k) |
CBER, APLB* |
| 510(k) submission (Class I or II device)15,22,27 | • Animal- or plant-derived products/synthetic products† • Must have a terminal sterilization step and demonstrate viral inactivation‡ • Requires premarket notification that results in FDA clearance • Must demonstrate that the device to be marketed is substantially equivalent to a preexisting legally marketed device (predicate) in terms of safety and effectiveness. The predicate must have been legally marketed prior to May 28, 1976, been reclassified to Class I or II from Class III, been approved via the 510(k) pathway, or granted market authorization through the de novo classification pathway • The type of data or information necessary to establish substantial equivalence varies by device and the differences between the new and predicate devices |
CDRH |
| HDE28,29 | • Human- and human/animal-derived products • HUD designation can be granted to medical devices when no comparable device is available to treat or diagnose a condition affecting no more than 8000 individuals in the United States annually • Because the clinical investigation demonstrating the device’s efficacy is not feasible (due to low prevalence), an HDE grants manufacturers an exemption to the usual PMA pathway and allows marketing of the device only for the FDA-labeled HDE indication |
CDRH |
| PMA30 | • Human- and human/animal-derived or synthetic products • Class III high-risk devices require PMA of an IDE application to obtain clinical data to support specific claims for use • PMA is the most stringent type of device-marketing application and often requires clinical data to support safety and efficacy of product use, typically obtained through a randomized trial |
CDRH |
| HCT/P designation8 | • Human-derived tissue and/or cellular products • If the product is minimally manipulated (ie, manufacture cannot involve the combination of the cells or tissues with another article) and intended for homologous use, it is regulated under 21 CFR Part 1271 and section 361 of the PHS Act and requires no FDA clearance or approval • If the product does not meet the criteria of minimal manipulation and homologous use, it is regulated under 21 CFR Part 1271 and Section 351 of the PHS Act • The manufacturer and distributor must register their establishments with the FDA and list their HCT/P |
CDRH, CBER |
21 CFR, Code of Federal Regulations Title 21; APLB, Advertising and Promotional Labeling Branch; CBER, Center for Biologics Evaluation and Research; CDRH, Center for Devices and Radiological Health; FDA, Food and Drug Administration; HCT/P, human cells, tissues, and cellular and tissue-based product; HDE, humanitarian device exemption; HUD, humanitarian use device; IDE, investigational device exemption; IND, investigational new drug; PHS, Public Health Service; PMA, Premarket Approval.
*APLB is responsible for regulating advertising and promotional labeling materials for CBER products to ensure that the information about the risks and benefits of regulated products are communicated in a truthful, accurate, science-based, non-misleading, and balanced manner and is in compliance with pertinent federal laws and regulations.31
†Historically, the 510(k) pathway has been used to regulate animal- and/or plant-derived products. Although possible to develop a human tissue-derived product under 510(k) regulations, it has not been done to date for burn care.
‡For devices that use animal tissue.
TEng Products Regulated as Medical Devices by CDRH
Medical devices may be regulated by the Center for Devices and Radiological Health (CDRH) or the Center for Biologics Evaluation and Research (CBER), both with the mission to ensure that patients and healthcare providers have continued access to safe and effective medical products. Medical products regulated as devices by CBER are discussed later. TEng products may be considered to be medical devices according to the intended use and absence of live cells.17,18 Classification is based on risk, that is, the risk that the device poses to the patient and/or the user is a major factor in the class that it is assigned and the extent of regulatory control required to demonstrate safety and effectiveness.17,18,19 Medical devices are classified into Class I, II, and III with regulatory control increasing from Class I to Class III.19 The device classification defines the regulatory requirements for a general device type. Most Class I devices are exempt from Premarket Notification 510(k); most Class II devices require Premarket Notification 510(k); and most Class III devices require PMA.20 Class I devices are low risk for causing illness or injury and permit the use of general controls, whereas Class II devices present moderate risk and require specialized controls in addition to general controls.21 General controls may include registration and listing, establishment of quality system regulations, and labeling, while specialized controls may include mandatory performance standards and special labeling.21
Some Class I and most Class II devices follow the 510(k) pathway, a premarket submission made to the FDA to demonstrate that a device to be marketed is substantially equivalent to a preexisting, legally marketed device (ie, a predicate; Table 2).12,14,15 To establish “substantial equivalence,” the FDA reviews the scientific methods used to evaluate the technological differences between the device and the predicate to ensure that variances do not affect safety or effectiveness.15 The required data and information change depending on the differences between the device and the predicate. For example, the 510(k) pathway may require supporting preclinical or clinical data, depending on whether an indication has changed, there are significant technological differences, and the appropriateness of existing nonclinical data to the current indication.15,22
Certain devices may be wholly exempt from 510(k) requirements if the FDA determines reasonable assurance of safety and effectiveness can be established without the 510(k) pathway.23 Examples would be devices with preamendment status, such as a device that has been classified into Class I or Class II, a device that has been classified into Class III, but for which a regulation under section 515(b) of the Federal Food, Drug, and Cosmetic (FD&C) Act (21 U.S.C. 360e[b]) requiring the submission of an application for PMA has not yet been issued, or a device that has not yet been classified.24 Manufacturers of such devices are required to register their establishment and list the generic category or classification name.25
PMA is the FDA pathway for scientific and regulatory review for Class III high-risk devices. Class III devices are defined as those that support or sustain human life, are of substantial importance in preventing impairment of human health, or present a potential, unreasonable risk of illness or injury.32 These are generally novel products for which insufficient information exists to assure safety and effectiveness solely through general or specialized controls.13,21 The most stringent type of device marketing application for TEng devices or combination products, PMA requires the provision of safety and effectiveness data from both nonclinical (ie, bench and/or animal testing) and clinical studies (ie, placebo-controlled clinical trial). Historically, wound care products containing live cells were regulated under the PMA pathway due to the presence of scaffold materials; however, in 2013, the FDA determined that products containing live cells should be regulated as a biologic through CBER’s Office of Tissues and Advanced Therapies (formerly known as the Office of Cellular, Tissue and Gene Therapies).33 Regardless of the risk classification, a novel device with no predicate is automatically designated Class III and is subject to PMA.34 In cases for which the device is of a type that has not yet been classified and the manufacturer can provide data supporting a lower risk classification, a manufacturer can submit a reclassification request in order to market the de novo product as Class I or Class II.34,35 If the device meets the requirements of section 513(a)(1)(A) or (B) of the FD&C Act, it can be classified as Class I or II and marketed immediately.34
An approved humanitarian device exemption (HDE) authorizes marketing of a HUD for its specified indication for use. This unique marketing approval pathway was created specifically to stimulate the development of, and provide earlier access to, devices intended for use in patients with rare diseases (Table 2).28 In contrast to the PMA pathway, manufacturers of an HDE device need to prove “probable benefit” rather than reasonable assurance of safety and effectiveness.28 According to guidance provided for the HDE pathway, the medical device is intended to benefit patients in the treatment or diagnosis of a disease or condition that affects or is manifested in ≤8000 individuals in the United States per year.29 For example, in the burn treatment landscape, Epicel (cultured epidermal autografts) received HDE status in the United States in 2007 for the treatment of full-thickness burns that are ≥30% total body surface area. Specific guidance documents for medical devices are available from the FDA.36
TEng Products Regulated as BLA or HCT/P by CBER
TEng products may be regulated under BLA or HCT/P pathways. A BLA, which assumes a potential systemic effect of a biologic product or a claim that is for nonhomologous use, is a rigorous pathway that requires extensive nonclinical and controlled clinical data to support the safety and efficacy of the product in the treatment of the specific indication from which the data were obtained.11,26 The BLA pathway is regulated by either CBER or the Center for Drug Evaluation and Research (CDER), depending on the product type, and is analogous to a new drug application to market new drug products.37 Products in the BLA pathway regulated by CDER include monoclonal antibodies for in vivo use, most therapeutic proteins, and immunomodulators.37 Products regulated by CBER include medical devices to safeguard blood, blood components, and cellular products from infectious agents (eg, HIV, hepatitis, and syphilis) and human tissues and cellular products, as well as a wide range of gene therapies, xenotransplantation products, and vaccines.37,38 Similar to CDER and CDRH, CBER is responsible for the continued monitoring of the safety and stability of biological products, and with ensuring that information regarding the benefits and risks of a product is truthful, nonmisleading, and balanced.38,39 CBER-approved biologics regulated under a BLA must comply with advertising and promotional labeling regulations defined in the Code of Federal Regulations.39 Unlike CBER, for medical devices approved or cleared by CDRH, there is no statutory requirement that advertisements and promotional material be submitted to FDA at the time of initial dissemination. Similar to CDER, all branded advertising and promotional labeling materials directed at both healthcare providers and consumers are required to be submitted to CBER at the time of use.39
The HCT/P designation may be assigned to human skin or cultured skin cells prepared on synthetic membranes or combined with collagen. While HCT/Ps that qualify as medical devices are regulated by CDRH, the remainder of HCT/Ps are regulated by CBER and provisions of the Public Health Service (PHS) Act.40 Human cells, tissues, and cellular and tissue-based products that fall within detailed exceptions or meet the 21 CFR Part 1271 criteria of “minimally manipulated” (according to FDA guidance, “manufacture cannot involve the combination of the cells or tissues with another article, and does not alter the tissue's relevant biological characteristics”) and intended for “homologous use” (according to FDA guidance defined as “the repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor”) are regulated by section 361 of the PHS Act.8 The HCT/Ps regulated under section 361 and 21 CFR Part 1271 require infectious disease testing, donor screening, and record-keeping, but do not require a PMA or BLA.8
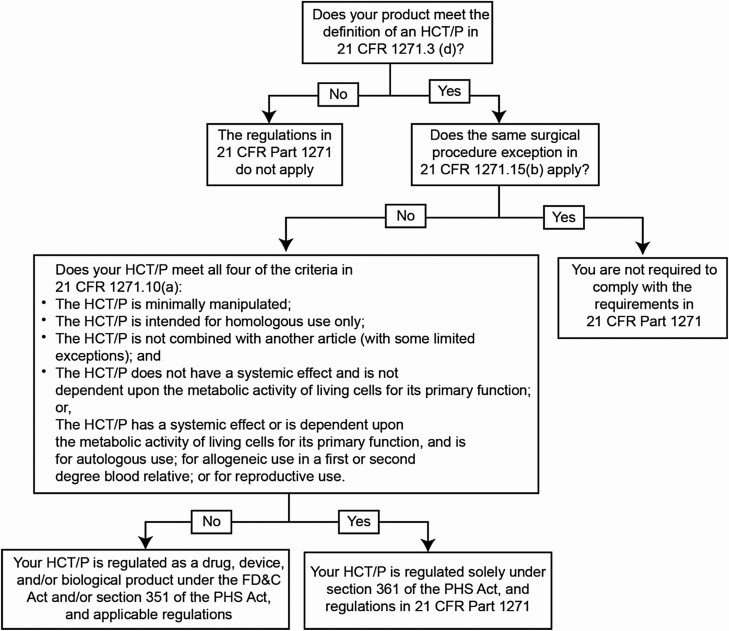
In contrast, if an HCT/P does not meet the criteria outlined in 21 CFR Part 1271 and the manufacturer is not exempt, the HCT/P will be regulated as a drug, device, and/or biologic under section 351 of the PHS Act. In this case, premarket review is required through either the PMA or BLA pathway (Figure 1).8 Because products falling under this category are not minimally manipulated and/or intended for homologous use, substantial safety and efficacy evidence must be provided through bench and animal studies, and clinical trials.
Figure 1.
Flowchart to illustrate how to apply the criteria in 21 CFR 1271.15(b) and 1271.10(a) for HCT/Ps. 21 CFR, Code of Federal Regulations Title 21; FD&C, Food, Drug, & Cosmetic Act; HCT/P, human cells, tissues, and cellular and tissue-based product; PHS, Public Health Service.8
Companies are permitted to self-designate tissue products as meeting the specific set of criteria set forth under 21 CFR Part 1271, but the manufacturer must register their establishment and list their HCT/P with CBER annually.41 These regulations explain the types of HCT/Ps that do not require PMA and define the registration, manufacturing, and reporting steps that must be taken to prevent the introduction, transmission, and spread of communicable disease, including the application of current good tissue practice.8 The FDA’s Tissue Reference Group (TRG), comprising representatives from both CBER and CDRH, serves as a resource for answering product-specific questions related to 21 CFR Part 1271 criteria.42 The TRG Rapid Inquiry Program was instituted to help manufacturers of HCT/Ps obtain a rapid, preliminary assessment regarding the regulation of specific HCT/Ps and was extended through October 2020.43 The FDA has stated that it intends to exercise enforcement discretion of provisions for certain HCT/Ps,8 and expects companies to confirm the appropriate regulatory status of their HCT/Ps and apply for a PMA or BLA if appropriate.8 The period during which the FDA intends to exercise enforcement discretion has been extended through May 2021.8 In cases in which a product has a biologic component, CBER may be an active participant in the review pathway completed by CDRH. Additional guidance documents are available from CBER and CDRH and can be searched and narrowed by filters.44
CHALLENGES ASSOCIATED WITH REGULATORY PATHWAYS
The complexity and evolving science of TEng products along with differences when comparing cellular therapies to drugs, biologics (eg, monoclonal antibodies), and medical devices presents a challenge for organization of these types of treatments into the available regulatory guidance and application structure. The intended use of the product and primary mode of action are important factors that define the selection of a regulatory pathway. Robust and predictive preclinical models to support efficient development of TEng products are needed. For novel products with unique manufacturing challenges, such as 3D bioprinting, the lack of regulatory precedent for similar products can pose significant challenges, given the absence of relevant guidance.45
Chemistry, manufacturing, and control requirements represent a significant (and complex) component of the BLA regulatory pathway. Whereas a traditional drug molecule requires a summary of formulation development and manufacturing processes, the manufacturing process for biological products are typically more complicated (due in part to genetic variability in source material) and must contain a thorough description of product development and relevant manufacturing procedures, as well as all steps taken to ensure consistency across batches. Supplementary Table S1 summarizes the key FDA requirements for the commercialization of biologics, devices, and HCT/Ps. Early engagement with the FDA can help determine how a product should be regulated, and which clinical and manufacturing requirements apply. This is especially important for novel products that do not easily fit into a defined regulatory pathway.
The FDA has acknowledged the importance of advancing the understanding of complex novel products, both within the organization and among innovators, with the establishment of the CBER Advanced Technologies Team (CATT), the INitial Targeted Engagement for Regulatory Advice on CBER ProducTs (INTERACT) meeting program, and the “Simplicity” strategy.46–48 CATT focuses on regulatory implications of novel technologies that can have a significant impact on product development, manufacturing process, and control strategies.47 INTERACT meetings enable companies to engage with the FDA early in the development process, in order to obtain advice regarding the path from preclinical to clinical development for biological products, which helps a company fulfill the FDA’s science-based requirements and avoid potentially unnecessary preclinical studies.48 CDRH’s “simplicity” strategic priority recognizes the importance of building on existing policies and processes, while making regulatory processes as streamlined as possible in order to help improve decision-making and ensure consistent implementation and adherence to regulatory policies.46,53
Modernization of 510(k)
In 2018, the FDA made a statement about modernizing the 510(k) program, saying that “new medical devices that come to market under the 510(k) pathway should either account for advances in technology or demonstrate that they meet more modern safety and performance criteria.” 54 In addition, the FDA believed that there should be greater competition to adopt modern features, so that newer medical devices reflect up-to-date technology and standards that can improve patient care and outcomes.54 In 2018, the FDA planned to pursue additional actions that will allow it to retire outdated predicates (>10 years old)54 and CDRH was considering the release of an online list of cleared devices that demonstrate substantial equivalency to predicate devices that are >10 years old.54 As a first step to achieve this modernization, the FDA released updated draft guidance in 2019 for four devices (no TEng products were included) outlining recommendations for premarket performance criteria and testing methodologies.55
REGENERATIVE MEDICINE ADVANCED THERAPY (RMAT) DESIGNATION
The BLA pathway is lengthy, requiring both preclinical data and clinical data demonstrating the safety and efficacy of a product.11 In order to help address this issue, Congress created the RMAT designation in December 2016, via the 21st Century Cures Act, that offered an expedited option for certain eligible biologics products, thereby facilitating reduced time to market for innovative medicines with the potential to address an unmet medical need for serious or life-threatening diseases or conditions.52,56,57 Benefits of an RMAT designation include increased opportunities to meet with the FDA and early meetings to discuss potential surrogate or intermediate study endpoints.52,56
As of October 19, 2020, 55 RMAT designations have been granted by the FDA, with 47 products announced publicly (Table 3).49,50,51 The first bioengineered allogeneic cellularized construct that has received RMAT designation is StrataGraft® (Mallinckrodt Pharmaceuticals, plc., Hampton, NJ), which is in development for the treatment of thermal burns. StrataGraft has been evaluated in a phase III clinical trial for the treatment of patients with deep, partial-thickness thermal burns in comparison to autograft (standard of care). Although StrataGraft is not yet approved by the FDA, Mallinckrodt is seeking CBER approval via the BLA pathway.
Table 3.
| Product | Company | Therapeutic Area |
|---|---|---|
| ABO-102 | Abeona Therapeutics | Mucopolysaccharidosis type IIIA |
| ADP-A2M4 | AdaptImmune | Synovial sarcoma |
| AmnioFix | MiMedx | Osteoarthritis of the knee |
| AST-OPC1 | Asterias Biotherapeutics | Spinal cord injury |
| AT132 | Audentes Therapeutics | X-linked myotubular myopathy |
| ATIR101 | Kiadis Pharma | Leukemia |
| Avance | AxoGen | Nerve injuries |
| CAP-1002 | Capricor Therapeutics | Duchenne muscular dystrophy |
| CD30 CAR-T | Tessa Therapeutics | Relapsed or refractory CD30-positive Hodgkin lymphoma |
| CLBS14 (CD34+ cell therapy) | Caladrius Biosciences | Refractory angina |
| CEVA101 | Cellvation | Traumatic brain injury |
| CT053 | CARsgen Therapeutics | Relapsed or refractory multiple myeloma |
| CTX001 | CRISPR Therapeutics and Vertex Pharmaceuticals | Severe hemoglobinopathies |
| EB-101 | Abeona Therapeutics | Epidermolysis bullosa |
| ECT-0012 | ExCellThera | Hematologic malignancies |
| FCR-001 | Talaris Therapeutics | Prevention of renal transplant rejection |
| FCX-007 | Fibrocell | Recessive dystrophic epidermolysis bullosa |
| Humacyl | Humacyte | Vascular access for hemodialysis |
| Ilixadencel | Immunicum | Metastatic renal cell carcinoma |
| Ixmyelocel-T | Vericel | Dilated cardiomyopathy |
| JCAR017 | Juno Therapeutics | Large B-cell lymphoma |
| jCell | jCyte | Retinitis pigmentosa |
| KB103 | Krystal Biotech | Recessive dystrophic epidermolysis bullosa |
| Kymriah | Novartis | Relapsed or refractory follicular lymphoma |
| LentiGlobin | bluebird bio | Severe combined immune deficiency |
| Lifileucel | Iovance | Advanced melanoma |
| Liso-cel | Bristol Myers Squibb | Large B-cell lymphoma |
| MB-107 | Mustang Bio/St. Judes | X-linked severe combined immunodeficiency |
| MDR-101 | Medeor Therapeutics | Prevention of renal transplant rejection |
| MGTA-456 | Magenta Therapeutics | Inherited metabolic disorders |
| MPC-150-IM | Mesoblast | Heart failure |
| MultiStem | Athersys | Ischemic stroke Acute respiratory distress syndrome |
| NSR-REP1 | Nightstar Therapeutics | Choroideremia |
| Orca-T | Orca Bio | Blood cancers eligible for hematopoietic stem cell transplant |
| OTL-103 | Orchard Therapeutics | Wiskott-Aldrich syndrome |
| P-BCMA-101 | Poseida Therapeutics | Relapsed/refractory multiple myeloma |
| Romyelocel-L | Cellerant Therapeutics | Prevention of infection in neutropenia |
| RP-L102 | Rocket Pharmaceuticals | Fanconi anemia |
| RVT-802 | Enzyvant | DiGeorge syndrome |
| SB-525 | Sangamo Therapeutics | Severe hemophilia A |
| SB623 | SanBio | Chronic motor deficits secondary to traumatic brain injury |
| StrataGraft® construct | Stratatech (Mallinckrodt Pharmaceuticals) | Thermal burns |
| TTAX02 | Tissue Tech | Spina bifida in utero |
| Viralym-M (ALVR105) | Allovir | BK virus-associated hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation |
| VM202 | Helixmith Co. | Diabetic peripheral neuropathy |
| VY-AADC | Voyager Therapeutics | Parkinson’s disease |
CONCLUSIONS
The path to regulatory approval and data requirements for TEng products can vary widely based on many factors, including the tissue composition, intended use, primary mechanism of action, and treatment indication. Different regulatory pathways, with varying degrees of data and regulatory oversight, can be used to achieve market authorization of TEng products. Understanding the different regulatory pathways and data requirements necessary for authorization along each path is critical to the understanding and selection of TEng products in this rapidly advancing field. Technology and demands for TEng products continue to evolve, presenting unique challenges, and opportunities for their development and commercialization.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Mary Lokuta, PhD, Director of Regulatory Affairs, Mallinckrodt Pharmaceuticals, and Helen Hahn, BSN, MBA, Director of Medical Affairs, Mallinckrodt Pharmaceuticals, for their support and intellectual contributions to the presented poster that served as the basis for this publication. Medical writing and editorial support, conducted in accordance with Good Publication Practice (GPP3) and the International Committee of Medical Journal Editors (ICMJE) guidelines, were provided by Oxford PharmaGenesis Inc., Newtown, PA, funded by Mallinckrodt Pharmaceuticals, plc., Hampton, NJ.
Funding: This work was funded by Mallinckrodt Pharmaceuticals, plc., Hampton, NJ.
Conflict of interest statement: Kimberly Belsky and Janice Smiell are employees and shareholders of Mallinckrodt Pharmaceuticals.
REFERENCES
- 1. National Institute of Biomedical Imaging and Bioengineering. Tissue engineering and regenerative medicine. Accessed 2 June 2020; available from https://www.nibib.nih.gov/science-education/science-topics/tissue-engineering-and-regenerative-medicine.
- 2. Castells-Sala C, Alemany-Ribes M, Fernández-Muiños T, et al. . Current applications of tissue engineering in biomedicine. J Biochip Tissue Chip. 2013;S2:004. doi: 10.4172/2153-0777.S2-004. [DOI] [Google Scholar]
- 3. Nathoo R, Howe N, Cohen G. Skin substitutes: an overview of the key players in wound management. J Clin Aesthet Dermatol. 2014;7(10):44–8. [PMC free article] [PubMed] [Google Scholar]
- 4. Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7(43):229–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Snyder DL, Sullivan N, Schoelles KM.. Skin substitutes for treating chronic wounds. Technology Assessment Report. Rockville (MD): Agency for Healthcare Research and Quality (US);2012. [PubMed] [Google Scholar]
- 6. Schmidt C. Gintuit cell therapy approval signals shift at US regulator. Nat Biotechnol. 2012;30(6):479. [DOI] [PubMed] [Google Scholar]
- 7. PolarityTE provides update on corporate strategy and regulatory pathway for SkinTE. Business Wire. 2020. Accessed 2 June 2020; available from https://www.businesswire.com/news/home/20200430005967/en/PolarityTE-Update-Corporate-Strategy-Regulatory-Pathway-SkinTE.
- 8. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health. Guidance for Industry and Food and Drug Administration Staff: regulatory considerations for human cells, tissues, and cellular and tissue-based products: minimal manipulation and homologous use.2020. Accessed 31 August 2020; available from https://www.fda.gov/media/109176/download.
- 9. U.S. Food and Drug Administration. MDUFA performance goals and procedures, fiscal years 2018 through 2020. 2016. Accessed 31 August 2020; available from https://www.fda.gov/media/102699/download.
- 10. U.S. Food and Drug Administration. PDUFA reauthorization performance goals and procedures fiscal years 2018 through 2022. Accessed 31 August 2020; available from https://www.fda.gov/media/99140/download.
- 11. The FDA Group, LLC. The biologics license application (BLA) process explained. 2014. Accessed 2 June 2020; available from https://www.thefdagroup.com/blog/2014/07/test-the-biologics-license-application-bla-process/.
- 12. DiPaola D. BIOMEMS: Navigating the medical device FDA approval process. MEMS J; 2012. Accessed 2 June 2020; available from https://www.memsjournal.com/2012/11/biomems-navigating-the-medical-device-fda-approval-process.html. [Google Scholar]
- 13. Teow N, Siegel SJ. FDA regulation of medical devices and medical device reporting. Pharmaceut Reg Affairs. 2013;2:110. [Google Scholar]
- 14. U.S. Food and Drug Administration. 510(k) premarket notification. 2020. Accessed 2 June 2020; available from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm.
- 15. U.S. Food and Drug Administration. Premarket notification 510(k). 2020. Accessed 2 June 2020; available from https://www.fda.gov/medical-devices/premarket-submissions/premarket-notification-510k.
- 16. U.S. Food and Drug Administration. Frequently asked questions about combination products. 2020. Accessed 19 June 2020; available from https://www.fda.gov/combination-products/about-combination-products/frequently-asked-questions-about-combination-products#:~:text=Some%20FDA%20regulated%20products%20are,for%20general%20delivery%20of%20unspecified.
- 17. U.S. Food and Drug Administration. Classify your medical device.2020. Accessed 2 June 2020; available from https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device.
- 18. U.S. Food and Drug Administration. Code of Federal Regulations Title 21. Part 878 General and plastic surgery devices. 2019. Accessed 2 June 2020; available from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=878.
- 19. U.S. Food and Drug Administration. How to study and market your device.2019. Accessed 2 June 2020; available from https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/how-study-and-market-your-device.
- 20. U.S. Food and Drug Administration. Overview of device regulation. 2018. Accessed 2 June 2020; available from https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/overview-device-regulation.
- 21. Sutton WM. Classification overview. U.S. Food and Drug Administration. 2015. Accessed 2 June 2020; available from https://www.fda.gov/media/94057/download.
- 22. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research. Guidance for Industry and Food and Drug Administration Staff: The 510(k) program: evaluating substantial equivalence in premarket notifications [510(k)]. 2014. Accessed 2 June 2020; available from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/510k-program-evaluating-substantial-equivalence-premarket-notifications-510k.
- 23. U.S. Food and Drug Administration. Class I/II exemptions. 2019. Accessed 2 June 2020; available from https://www.fda.gov/medical-devices/classify-your-medical-device/class-i-ii-exemptions.
- 24. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health. Guidance for Industry: Evaluation of devices used with regenerative medicine advanced therapies. 2019. Accessed 2 June 2020; available from https://www.fda.gov/media/120266/download.
- 25. U.S. Food and Drug Administration. Medical device exemptions 510(k) and GMP requirements. 2020. Accessed 19 June 2020; available from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/315.cfm.
- 26. U.S. Food and Drug Administration. Biologics License Applications (BLA) process (CBER). 2018. Accessed 2 June, 2020; available from https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/biologics-license-applications-bla-process-cber.
- 27. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. Guidance for Industry and Food and Drug Administration Staff: medical devices containing materials derived from animal sources (except for in vitro diagnostic devices). 2019. Accessed 19 June 2020; available from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/medical-devices-containing-materials-derived-animal-sources-except-vitro-diagnostic-devices.
- 28. U.S. Food and Drug Administration. Humanitarian device exemption. 2019. Accessed 2 June 2020; available from https://www.fda.gov/medical-devices/premarket-submissions/humanitarian-device-exemption.
- 29. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research. Guidance for Industry and Food and Drug Administration Staff: Humanitarian device exemption (HDE) program. 2019. Accessed 2 June 2020; available from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/humanitarian-device-exemption-hde-program.
- 30. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health (CDRH), Center for Biologics Evaluation and Research. Information sheet guidance for IRBs, clinical investigators, and sponsors: frequently asked questions about medical devices.2006. Accessed 2 June 2020; available from http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM127067.pdf.
- 31. U.S. Food and Drug Administration. About the Advertising and Promotional Labeling Branch (APLB).2018. Accessed 30 June 2020; available from https://www.fda.gov/vaccines-blood-biologics/advertising-labeling-biologics/about-advertising-and-promotional-labeling-branch-aplb
- 32. U.S. Food and Drug Administration. Premarket approval (PMA). 2020. Accessed 2 June 2020; available from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm.
- 33. Gaffney A. CDRH cedes regulatory authority for certain wound care products to CBER. Regulatory Affairs Professionals Society. 2013. Accessed 2 June 2020; available from https://www.raps.org/regulatory-focus%E2%84%A2/news-articles/2013/8/cdrh-cedes-regulatory-authority-for-certain-wound-care-products-to-cber.
- 34. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research. Guidance for Industry and Food and Drug Administration Staff: De novo classification process (evaluation of automatic class III designation).2017. Accessed 2 June 2020; available from https://www.fda.gov/media/72674/download. [DOI] [PubMed]
- 35. U.S. Food and Drug Administration. De novo classification request. 2019. Accessed 2 June 2020; available from https://www.fda.gov/medical-devices/premarket-submissions/de-novo-classification-request.
- 36. U.S. Food and Drug Administration. Guidance documents (medical devices and radiation-emitting products). 2020. Accessed 15 September 2020; available from https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/guidance-documents-medical-devices-and-radiation-emitting-products.
- 37. U.S. Food and Drug Administration. Frequently asked questions about therapeutic biological products. 2015. Accessed 2 June 2020; available from https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/frequently-asked-questions-about-therapeutic-biological-products.
- 38. U.S. Food and Drug Administration. Center for Biologics Evaluation and Research (CBER) responsibilities questions and answers.2018. Accessed 2 June 2020; available from https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/center-biologics-evaluation-and-research-cber-responsibilities-questions-and-answers.
- 39. U.S. Food and Drug Administration. Submitting biologics advertising & promotional labeling. 2018. Accessed 2 June 2020; available from https://www.fda.gov/vaccines-blood-biologics/advertising-labeling-biologics/submitting-biologics-advertising-promotional-labeling.
- 40. U.S. Food and Drug Administration. FDA regulation of human cells, tissues, and cellular and tissue-based products (HCT/Ps) product list. 2018. Accessed 2 June 2020; available from https://www.fda.gov/vaccines-blood-biologics/tissue-tissue-products/fda-regulation-human-cells-tissues-and-cellular-and-tissue-based-products-hctps-product-list.
- 41. U.S. Food and Drug Administration. Code of Federal Regulations Title 21. Part 1271 Human cells, tissues, and cellular and tissue-based products. 2019. Accessed 2 June 2020; available from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1271&showFR=1.
- 42. U.S. Food and Drug Administration. Tissue reference group. 2018. Accessed 2 June 2020; available from https://www.fda.gov/vaccines-blood-biologics/tissue-tissue-products/tissue-reference-group.
- 43. U.S. Food and Drug Administration. TRG Rapid Inquiry Program (TRIP). 2020. Accessed 2 June 2020; available from https://www.fda.gov/vaccines-blood-biologics/trg-rapid-inquiry-program-trip?utm_campaign=What%27sNew2020-03-26&utm_medium=email&utm_source=Eloqua.
- 44. U.S. Food and Drug Administration. Search for FDA guidance documents. 2020. Accessed 15 September 2020; available from https://www.fda.gov/regulatory-information/search-fda-guidance-documents#guidancesearch.
- 45. Correia Carreira S, Begum R, Perriman AW. 3D Bioprinting: the emergence of programmable biodesign. Adv Healthc Mater. 2020;9(15):e1900554. [DOI] [PubMed] [Google Scholar]
- 46. U.S. Department of Health and Human Services, Food and Drug Administration. FDA Report to Congress: least burdensome training audit. 2018. Accessed 2 June 2020; available from https://www.fda.gov/media/113823/download.
- 47. U.S. Food and Drug Administration. CBER Advanced Technologies Team (CATT). 2019. Accessed 2 June 2020; available from https://www.fda.gov/vaccines-blood-biologics/industry-biologics/cber-advanced-technologies-team-catt.
- 48. U.S. Food and Drug Administration. FDA In Brief: FDA announces program to enhance early communications with biological product developers. 2018. Accessed 2 June 2020; available from https://www.fda.gov/news-events/fda-brief/fda-brief-fda-announces-program-enhance-early-communications-biological-product-developers.
- 49. Hildreth C. What is an RMAT? List of RMAT designations. BioInformant; 2020. Accessed 3 November 2020; available from https://bioinformant.com/rmat/.
- 50. RMAT list. The Niche. 2020. Accessed 2 June 2020; available from https://ipscell.com/rmat-list/.
- 51. U.S. Food and Drug Administration. Cumulative CBER regenerative medicine advanced therapy (RMAT) designation requests received by fiscal year. 2020. Accessed 2 June 2020; available from https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/cumulative-cber-regenerative-medicine-advanced-therapy-rmat-designation-requests-received-fiscal.
- 52. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for Industry: expedited programs for regenerative medicine therapies for serious conditions. 2019. Accessed 2 June 2020; available from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expedited-programs-regenerative-medicine-therapies-serious-conditions.
- 53. U.S. Food and Drug Administration. 2018–2020 strategic priorities. Center for Devices and Radiological Health. 2018. Accessed 2 June 2020; available from https://www.fda.gov/files/about%20fda/published/2018-2020-Strategic-Priorities.pdf.
- 54. U.S. Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D. and Jeff Shuren, M.D., Director of the Center for Devices and Radiological Health, on transformative new steps to modernize FDA’s 510(k) program to advance the review of the safety and effectiveness of medical devices.2018. Accessed 2 June 2020; available from https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-jeff-shuren-md-director-center-devices-and.
- 55. U.S. Food and Drug Administration. FDA continues to take steps to fulfill its commitment to strengthen and modernize the 510(k) medical device program. 2019. Accessed 2 June 2020; available from https://www.fda.gov/news-events/press-announcements/fda-continues-take-steps-fulfill-its-commitment-strengthen-and-modernize-510k-medical-device-program.
- 56. Dennett R, Messmer K. FDA regenerative medicine policy framework and advanced therapy designation. Regulatory focus. May 2018. Regulatory affairs professionals society. 2018. [Google Scholar]
- 57. U.S. Food and Drug Administration. 21st Century Cures Act. 2020. Accessed 30 June 2020; available from https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.