Abstract
Mesenchymal stem/stromal cells (MSCs) facilitate repair in experimental diabetic kidney disease (DKD). However, the hyperglycemic and uremic milieu may diminish regenerative capacity of patient-derived therapy. We hypothesized that DKD reduces human MSC paracrine function. Adipose-derived MSC from 38 participants with DKD and 16 control subjects were assessed for cell surface markers, trilineage differentiation, RNA sequencing (RNA-seq), in vitro function (coculture or conditioned medium experiments with T cells and human kidney cells [HK-2]), secretome profile, and cellular senescence abundance. The direction of association between MSC function and patient characteristics were also tested. RNA-seq analysis identified 353 differentially expressed genes and downregulation of several immunomodulatory genes/pathways in DKD-MSC versus Control-MSC. DKD-MSC phenotype, differentiation, and tube formation capacity were preserved, but migration was reduced. DKD-MSC with and without interferon-γ priming inhibited T-cell proliferation greater than Control-MSC. DKD-MSC medium contained higher levels of anti-inflammatory cytokines (indoleamine 2,3-deoxygenase 1 and prostaglandin-E2) and prorepair factors (hepatocyte growth factor and stromal cell–derived factor 1) but lower IL-6 versus control-MSC medium. DKD-MSC medium protected high glucose plus transforming growth factor-β–exposed HK-2 cells by reducing apoptotic, fibrotic, and inflammatory marker expression. Few DKD-MSC functions were affected by patient characteristics, including age, sex, BMI, hemoglobin A1c, kidney function, and urine albumin excretion. However, senescence-associated β-galactosidase activity was lower in DKD-MSC from participants on metformin therapy. Therefore, while DKD altered the transcriptome and migratory function of culture-expanded MSCs, DKD-MSC functionality, trophic factor secretion, and immunomodulatory activities contributing to repair remained intact. These observations support testing of patient-derived MSC therapy and may inform preconditioning regimens in DKD clinical trials.
Introduction
By 2045, global estimates predict that nearly 693 million adults will carry a diabetes mellitus (DM) diagnosis (1). Presently, 37% of U.S. adults with DM have concomitant chronic kidney disease (CKD) or diabetic kidney disease (DKD), which represents the most common cause of end-stage kidney disease (ESKD) in the U.S. (2,3). Given the escalating prevalence of obesity and DM, the incidence rate of ESKD is expected to rise exponentially. Therefore, urgent efforts to find novel therapies capable of delaying DKD progression are warranted.
Recent advances in cell-based therapies using mesenchymal stem/stromal cells (MSCs) offer hope for DKD and other kidney diseases (4–7), as MSCs possess antifibrotic, antioxidant, proangiogenic, antiapoptotic, and immunomodulatory activities (8–11). MSCs impart beneficial effects to the injured kidney by releasing soluble trophic factors and extracellular vesicles and participate in cell-cell interactions in the setting of organ injury (10,12–15). Collectively, MSCs reduce glomerulosclerosis, microalbuminuria, interstitial fibrosis, inflammation, and oxidative stress, thereby improving kidney function in animal DKD models (16,17).
Given a favorable safety profile in DM and preclinical studies, early-stage clinical trials are under way in humans with DKD (ClinicalTrials.gov identifiers: NCT02585622 [U.K.], NCT04869761, NCT03840343, NCT02008851, NCT03270956, and NCT02836574 [U.S.], NCT04125329 [Japan], and NCT04216849 [China]) (18,19). Overall, autologous transplantation offers advantages over allogeneic MSCs because it minimizes risk of allosensitization and immune rejection, which may negatively affect future kidney transplant recipients and result in reduced therapeutic effects of MSCs (20–27). However, the endogenous hyperglycemic and uremic environments of DKD might promote senescent MSC accumulation and diminish regenerative capacity (20–24,28–30). Indeed, DM impairs stem cell mobilization and therapeutic effect in experimental DKD (29,31), alters the MSC secretome, and reduces neoangiogenesis in DM ischemic limb disease (29,32,33). We and others also identified altered paracrine MSC function in non-DM conditions, suggesting that disease hinders endogenous repair activities (21,34,35). The functionality of culture-expanded MSCs in human DKD remains to be established. In primarily participants with non-DM CKD, MSCs exhibited preserved cytokine and growth factor gene expression and therapeutic effect (34,36,37), but subsequent studies in human ESKD and in vitro uremic toxin exposure revealed functional impairment (34,35,38).
The objective of this study was to test the hypothesis that DKD alters the transcriptome and induces dysfunction of prorepair responses of human MSCs. Therefore, we compared the MSC transcriptome, functionality, secretome profile, and senescence of adipose tissue–derived MSCs from participants with DKD and control subjects.
Research Design and Methods
Study Participants
Adipose tissue was collected from participants with DKD in an outpatient surgical suite. Control subjects were age-matched individuals without diabetes or CKD who were undergoing laparoscopic nephrectomy for kidney donation. DKD was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 and/or abnormal albuminuria with preserved kidney function (eGFR ≥60 mL/min/1.73 m2) in the setting of DM without another concomitant kidney disease beyond hypertension. Other inclusion criteria were age ≥18 years, type 1 or type 2 DM treated pharmacologically, and ability to give informed consent. To minimize bias, those with dialysis dependency, kidney transplant, immunosuppressive therapy, hemoglobin A1c (HbA1c) >11% (97 mmol/mol), or malignancy were excluded. Participants were accrued January 2016–May 2019 in Rochester, Minnesota. The Mayo Clinic institutional review board approved these studies.
MSC Harvesting and Phenotyping
MSCs were isolated from abdominal subcutaneous fat (0.5–2 g), cultured, and characterized as previously described (Supplementary Materials) (13,21,22,39,40).
MSC RNA Sequencing
Library preparation for the RNA samples (1 μg total RNA) was performed using Illumina TruSeq RNA Library Prep Kit v2. At the Mayo Clinic Sequencing Core, comprehensive analysis of raw RNA sequencing (RNA-seq) paired-end reads was performed using MAP-RSeq version 3.1.3 (41), an in-house bioinformatics pipeline. MAP-RSeq uses STAR, a splice-aware aligner, for aligning reads to the reference human genome (build hg38). Aligned reads were for gene and exon expression quantification performed using the Subread package to obtain raw and normalized fragments per kilobase of transcript per million mapped reads. Raw gene counts were supplied to ComBat-Seq (42) to adjust for batch effects that were due to stranded and unstranded protocols used for samples during RNA library preparation. R bioinformatics package edgeR was used for differential gene expression analysis between participants with DKD and control subjects. Genes with an adjusted P < 0.05 and |log2fold change| >1 were considered differentially expressed (DE). Genes analyzed through Ingenuity Pathway Analysis to compare perturbed pathways with –log(Benjamini-Hochberg values) >1.3 were consider statistically significant at P < 0.05. Genes of interest were then visualized with volcano plots using the R package ggplot2.
MSC Function and Secretome Studies
MSC migration was tested using a QCM Chemotaxis Cell Migration Assay (ECM-508; Millipore, Burlington, MA), and an MTS assay (CellTiter 96 Non-Radioactive Cell Proliferation Assay; Promega, Madison, WI) was used to test for MSC proliferation activity. When MSC (passages 3–5) reached 70–80% confluency, cells were serum starved for 36 h. Following incubation in serum-free Advanced MEM, conditioned media were centrifuged to remove cellular debris, and cytokine levels were measured in supernatants using ELISA. Commercial kits for bone morphogenetic protein 7 (BMP-7) (DBP700; R&D Systems), indoleamine 2,3 deoxygenase 1 (IDO) (LS-F4790 [Lifespan Biosciences], ab245710 [Abcam]), IL-6 (D6050; R&D Systems), stromal cell–derived factor 1 (SDF-1 or C-X-C motif chemokine ligand 12 [CXCL12]) (DY350; R&D Systems), and prostaglandin-E2 (PGE2) (ab133055; Abcam) were used following manufacturers’ protocols. Vascular endothelial growth factor A (VEGF-A), epidermal growth factor (EGF), and hepatocyte growth factor (HGF) (HAGP1MAG; Millipore) were measured by Luminex assay. For functional interpretation, cytokines were grouped on the basis of their putative roles in kidney repair activity (9,14,43) as follows: proangiogenic (VEGF-A), antifibrotic (HGF, BMP-7, and SDF-1), antiapoptotic (EGF, HGF, VEGF, and SDF-1), and immunomodulatory (IDO, IL-6, and PGE2).
In Vitro T-Cell Proliferation
MSCs were grouped as Control-MSC, DKD-MSC, or IFN-γ–primed DKD-MSC for T-cell proliferation studies. IFN-γ–primed DKD-MSCs were treated with IFN-γ (10 ng/µL) for 48 h. MSC-conditioned medium (MSCcm) cytokines were measured with Luminex multiplex assay (PPX-19-MXZTEZR; Invitrogen). Alloreactive T-cell proliferation assays were performed as previously described (44). Briefly, six loci HLA-mismatched (discordant HLA-A, HLA-B, and HLA-DR haplotypes from MSC donors) healthy human peripheral blood mononuclear cells (PBMC) were used. The stimulator PBMCs were irradiated (11 Gy) and plated in 96-well plates (1 × 105 cells/well) with responder PBMCs at a 1:2 ratio with or without the 1 MSC per 10 responder PBMCs. After 6 days, 1 μCi3[H] was added for 18 h. Cells were harvested, and incorporation was assessed using a β-scintillation counter. Negative controls included stimulator and recipient cells alone, and positive controls included Concanavalin A stimulation of recipient cells. Three technical replicates were performed for each experiment, and experiments were repeated at least once using the same samples. Results are expressed as a percentage of the concurrent median counter/min of control PBMC.
HK-2 Culture, DKD Injury, and Coincubation in MSCcm Studies
An immortalized human proximal tubule epithelial cell line (HK-2) (CRL-2190; ATCC) (45) was cultured in keratinocyte serum-free medium (Invitrogen) plus 5 ng/mL EGF and 0.05 mg/mL bovine pituitary extract at 37°C/5% CO2 until 80% confluent. HK-2 cells were seeded (3.5 × 105 cells/well) in the bottom chamber of a six-well culture plate (Corning CLS3412; Sigma-Aldrich) or 0.5 × 105 cells/well in chamber slides (Nunc Lab-Tek II Chamber Slide System, 154526; Thermo Fisher Scientific) until 70% confluent (46). On the basis of our preliminary studies (47), HK-2 cells were incubated with high glucose (HG) (d-glucose 20 mmol/L + 5 mmol/L HK-2 medium, 5504; R&D Systems) plus recombinant human transforming growth factor-β1 (TGF-β1) protein (5 ng/mL, 240-B; R&D Systems) at 37°C/5% CO2 for 12–15 h. Afterward, HG + TGF-β1–treated HK-2 medium was replaced with MSCcm or control medium.
MSCcm incubation was conducted in DKD-MSC and control-MSC, with positive (HG + TGF-β1 medium) and negative (untreated HK-2s in complete medium) HK-2 controls. MSCcm was generated from 1–2 × 106 cells/subject, with normalization for MSC density per sample (1 mL conditioned medium for every 0.1 × 106 MSCs). After a 24-h incubation period (5 mL/well in six-well plate and 1 mL/well in chamber slides), HK-2 cells were collected, and lysates were used for gene expression analyses. Expression of mRNA transcripts of genes relevant to epithelial-mesenchymal transition (EMT), including epithelial (E-cadherin) and mesenchymal (type I collagen) markers, profibrotic (activin-A) (47,48), proinflammatory factor (MCP-1 [CCL2]), and senescence-associated secretory phenotype (SASP) markers (matrix metalloproteinase-3 [MMP-3] and plasminogen activator inhibitor 1 [PAI-1], i.e., Serpine1) was quantified by relative quantitative RT-PCR. Apoptosis was evaluated by TUNEL assay and activated Caspase-3 staining (49). Briefly, HK-2 cells were assayed using the manufacturer’s directions and visualized by green fluorescence for fragmented DNA (incorporation of fluorescein-12-dUTP). Percentages of TUNEL-positive cells were quantified in five fields of view using a Zeiss inverted microscope. Caspase-3 was assessed by staining with anti-rabbit Caspase-3 (9661; Cell Signaling Technology, Boston, MA). Fluorescence intensity was calculated using ImageJ software.
MSC Tube Formation
Human umbilical vein endothelial cells (HUVECs) (Cell Applications, San Diego, CA) were cultured with DiI-labeled MSCs (D282; Life Technologies) in a BioCoat Angiogenesis System (BD Biosciences, Bedford, MA). HUVECs and MSCs (2 × 104) were plated in each well and incubated at 37°C for 16 h with endothelial cell growth medium (Lonza, Walkersville, MD). Formed tubes were imaged using a fluorescence microscope (Zeiss Axio Observer Z1), counted randomly in six to eight 10× fields per sample in a blinded fashion, and measured using ZEN 2012 Blue Edition image analysis software (Zeiss, Oberkochen, Germany).
MSC Senescence
MSC senescence was overall evaluated by senescence-associated β-galactosidase (SA-β-gal), cdkn2a (p16), and cdkn1a (p21) gene expression as well as levels of SASP markers (e.g., PAI-1, MMP-3) in MSCcm (vide supra). SA-β-gal activity was measured using a Cellular Senescence Activity Assay Kit (Enzo Life Sciences, Farmingdale, NY) as well as a staining kit (Cat #9860; Cell Signaling Technology). Cells were visualized by the EVOS FL Cell Imaging System (200× total magnification; Thermo Fisher Scientific). In one nonoverlapping fields of view per sample, senescence was indicated by SA-β-Gal positivity (blue). Concentrations of tumor necrosis factor-α (TNF-α) (DTA00C; R&D Systems) were measured in MSCcm by ELISA. Gene expression of senescence markers (p16 and p21) was tested with RT-PCR.
RT-PCR
For RT-PCR, HK-2 cells (0.5–1.0 × 106) were homogenized in 350 μL of ice-cold lysis buffer supplied by mirVana PARIS total RNA isolation kit (Cat# AM1556; Life Technologies). Total RNA was then isolated, and its concentrations measured by a NanoDrop spectrophotometer. First-strand cDNA was produced from 800 ng of total RNA using SuperScript VILO cDNA Synthesis Kit (#11754-050; Life Technologies). Relative quantitative PCR was performed using Taqman assays containing 4 μL of cDNA products. The following probes were purchased from Thermo Fisher Scientific: E-cadherin (Hs01023895), type I collagen (Hs00164004), activin-A (Hs00426835), MCP-1 (Hs00234140), p16 (Hs00923894), p21 (Hs00355782), PAI-1 (Hs00167155), MMP-3 (Hs00968305), and GAPDH (Hs02786624) as a reference control. Negative controls with no cDNA were cycled in parallel. PCR analysis was done on an Applied Biosystems ViiA 7 Real-Time PCR System using the following conditions: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min. Fold change of gene expression was calculated using the 2−ΔΔCT method.
Clinical Laboratory Studies
At the time of adipose tissue sampling, blood and urine samples were clinically tested for kidney function, proteinuria, and diabetes control, including eGFR calculation by Chronic Kidney Disease-Epidemiology equation (50), urine albumin-to-creatinine ratio (UACR), and HbA1c.
Statistical Analysis
Descriptive characteristics were summarized as mean and SD and counts and percentages, where applicable. Comparisons among groups were performed with the χ2 test for categorical variables and Kruskal-Wallis test or uneven means test for continuous variables. Tukey test was used for multiple group comparisons. Spearman correlation coefficient (rs) tested the direction of the association between MSC and kidney parameters (eGFR and UACR) and participant characteristics (exploratory analysis). Kruskal-Wallis test was used to compare the effect of sex and metformin (antiaging effect) (51) on MSC parameters (exploratory analysis). A sensitivity analysis was conducted repeating all comparisons in control subjects. Missing data were not included. Statistical significance was considered for P < 0.05. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and GraphPad Prism 8 software.
Data and Resource Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
Adipose-derived MSCs were harvested from 38 participants with DKD and 16 control subjects without DM or CKD (Supplementary Table 1). Age, sex, and race of participants with DKD did not statistically differ from control subjects (P > 0.1). By design, mean eGFR was lower in participants with DKD (44.7 [SD 19.7] vs. 77.6 [12.5 mL/min/1.73 m2]; P < 0.0001).
MSC Characterization and Differentiation Potential
Similar to Control-MSC, DKD-MSC showed typical fibroblast-like morphological features (Fig. 1A) and plastic adherence. Their multilineage potential was further confirmed by differentiation into adipocytes, chondrocytes, and osteocytes (Fig. 1B). All MSCs expressed surface marker positivity to CD73, CD90, and CD105 and negativity to CD34 and CD45 by flow cytometric analysis (Fig. 1C), as subsequently confirmed by expression levels of selected genes (Fig. 1D).
Figure 1.
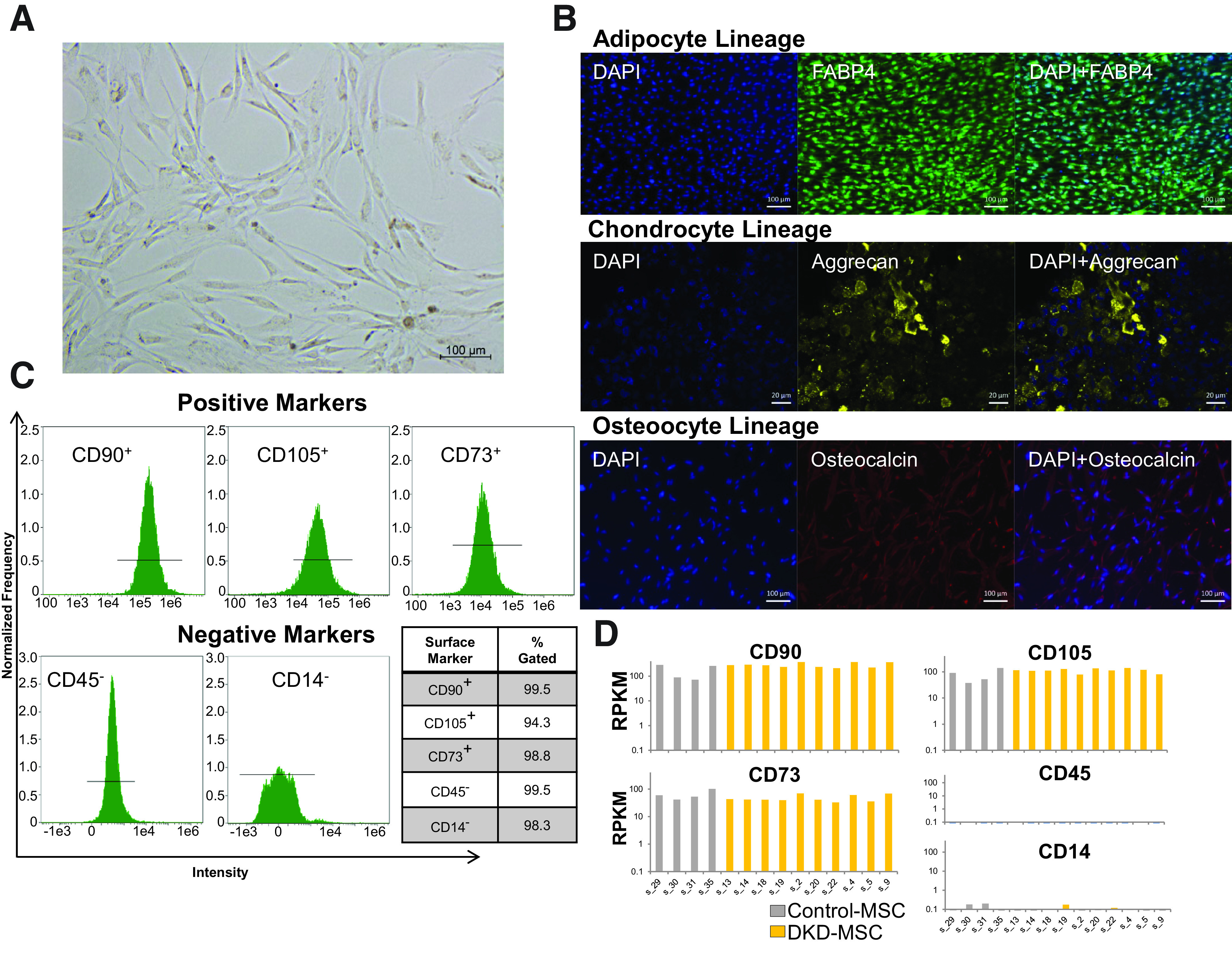
MSC phenotype and characterization. A: Adipose tissue-derived MSC harvested from participants with DKD show typical morphological appearance under the microscope as spindle-shaped, fibroblast-like cells in culture. B: Trilineage differentiation into adipocyte (FABP4, positive staining represents adipocytes), chondrocyte (aggrecan, positive staining represents chondrocytes), and osteocyte (osteocalcin, positive staining represents osteocytes) lineages is achieved in DKD-MSC. C: Cell surface markers characteristic of MSC positivity to CD90, CD105, and CD73 and negativity to CD45 and CD14 are expressed in DKD-MSC through flow cytometric analysis. D: In concordance, gene expression of MSC surface markers is similarly expressed both in Control-MSC and DKD-MSC. RPKM, reads per kilobase million reads.
MSC Transcriptome Alterations in DKD
RNA-seq of mRNAs expressed by MSCs was performed to compare profiles in DKD-MSC (n = 29) and Control-MSC (n = 9) (Supplementary Table 2). A total of 15,086 genes had average counts per million reads mapped >1 in at least one group of comparisons, and 353 (n = 224 upregulated and n = 129 downregulated) DE genes were identified with adjusted P < 0.05 and |log2fold change| >1. Functional annotation clustering analysis of genes upregulated in DKD-MSC revealed involvement in fibrosis, cell adhesion, and cellular (i.e., ILK, Notch) signaling. In contrast, genes downregulated in DKD were primarily implicated in inflammatory categories, including granulocyte adhesion and diapedesis, agranulocyte adhesion and diapedesis, and IL-17A signaling (Supplementary Fig. 1). We then focused on DE genes involved in inflammatory, immunomodulatory, apoptosis, and fibrosis pathways. Volcano plots (Fig. 2A and B) demonstrate that GSTM1 (52), profibrotic, immunomodulatory, adhesion (VCAM1, i.e., CD106), and profibrotic (MYH2, ACTA2, COL11A1) genes were upregulated, although several genes with overlapping inflammatory, immunomodulatory, fibrosis, and apoptosis activities (CXCL8, CXCL3, MMP3, IL33, IL1RL1, COL17A1) were downregulated in DKD-MSC versus Control-MSC. Given the paracrine action of MSC, gene expression of selected proteins involved in renal repair activity was compared (Fig. 2C). In DE analysis, no significant differences were observed between groups. Overall, transcriptomic analysis suggested that culture-expanded DKD-MSCs may be less responsive to a proinflammatory environment but more likely to contribute to matrix production than those derived from similarly aged individuals without DM or CKD. To further explore the therapeutic implications of these gene expression alterations, functional experiments were next performed in MSCs, MSCs cocultured with T cells with and without INF-γ MSC priming, and HK-2 cells exposed to HG + TGF-β1 and incubated in MSCcm.
Figure 2.
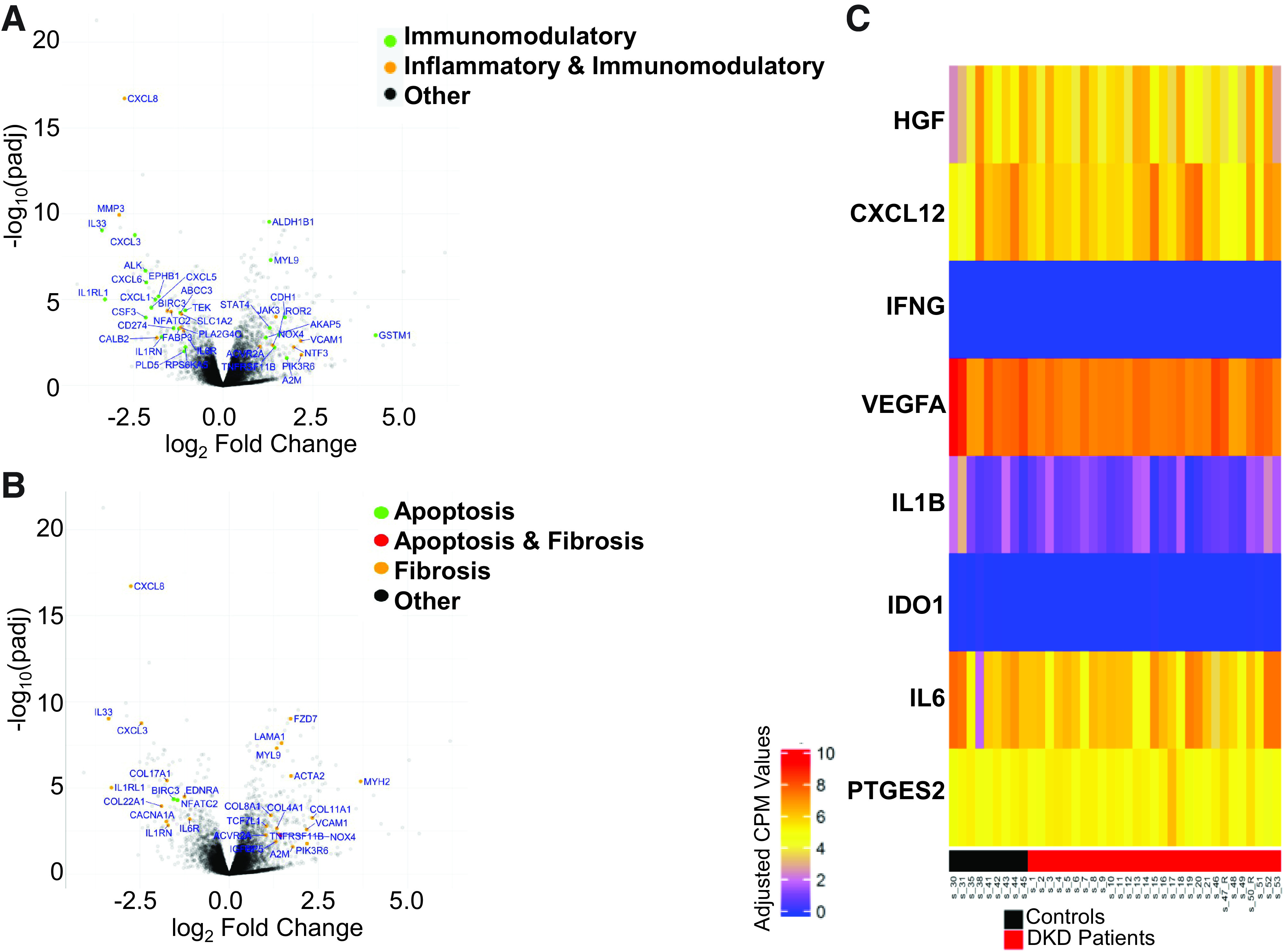
Top Differentially expressed genes involved in fibrosis, apoptosis, inflammation, and immune modulation. Next-generation sequencing (RNA-seq) and differential expression analysis were performed in MSCs harvested from participants with DKD (n = 29) and control subjects (n = 9). A and B: Volcano plots demonstrate the distribution of differentially expressed genes, with specific genes highlighted for their involvement in inflammation and immunomodulation or fibrosis and apoptosis pathways. C: Heatmap is shown of genes for growth factor and cytokines involved in kidney repair activity of MSC (adjusted P ≥ 0.1 for all). CPM, counts per million reads; IFNG, IFN-γ; PTGES2, prostaglandin-endoperoxide synthase 2; padj, adjusted P value.
MSC Immunomodulatory Activity
MSC Secretome
MSC paracrine function was assessed by measuring soluble factors released into culture supernatants (Supplementary Table 3). Basal secretion of immunomodulatory factors IDO and PGE2 was higher (0.30 [0.18] vs. 0.05 [0.05] ng/mL [P = 0.0002] and 1.57 [1.10] vs. 0.27 [0.09] ng/mL [P = 0.001], respectively) in DKD-MSC, whereas secretion of IL-6, a proinflammatory cytokine with antiapoptotic activities (53,54) was lower compared with Control-MSC (Fig. 3A).
Figure 3.
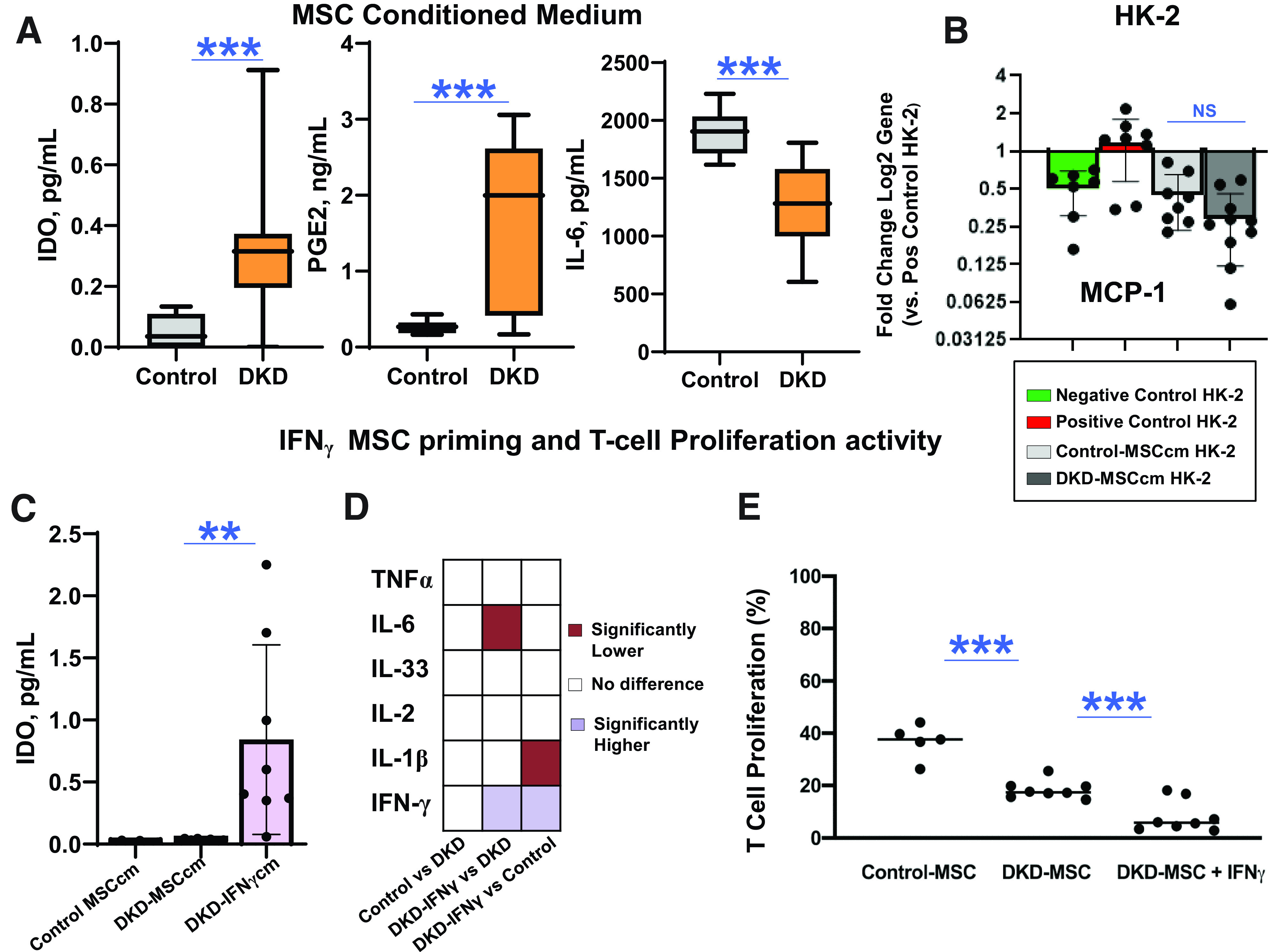
MSC immunomodulatory activity. A: Secretion of immunomodulatory factors IDO (DKD-MSC, n = 38; Control-MSC, n = 10) and PGE2 (DKD-MSC, n = 21; Control-MSC, n = 10) into culture supernatant was higher (P = 0.0002 and P = 0.001, respectively) in DKD-MSC. However, secretion of IL-6, a proinflammatory cytokine with antiapoptotic activities, was lower in DKD-MSC vs. Control-MSC (DKD-MSC, n = 38; Control-MSC, n = 10; P < 0.0001). B: Effect of 24-h MSCcm incubation on HG + TGF-β1–exposed HK-2 cells; gene expression showed DKD-MSCcm decreased MCP-1 gene expression but not greater than Control-MSCcm (negative control, n = 7; positive control, n = 8; Control-MSCcm, n = 8; DKD-MSCcm, n = 8; P = 0.7). C: IFN-γ priming of DKD-MSC resulted in a higher release of IDO into DKD-IFN-γcm (Control-MSCcm, n = 6; DKD-MSCcm, n = 8; DKD-IFN-γcm, n = 8; P = 0.006). D: Heatmap demonstrates comparisons between immunomodulatory and inflammatory cytokine levels in the unstimulated DKD-MSCcm, Control-MSCcm, and DKD-IFN-γcm. IFN-γ DKD-MSC priming resulted in lower levels of IL-6 (P = 0.008 by paired t test) and TNF-α (P = 0.06 by paired t test) compared with unstimulated DKD-MSCcm and lower IL-1β levels compared with Control-MSCcm (P = 0.002). E: DKD-MSC primed with IFN-γ inhibited alloreactive T-cell proliferation greater than Control-MSC (P = 0.002) and unstimulated MSC (Control MSCcm, n = 6; DKD-MSCcm, n = 8; DKD-IFN-γcm, n = 8; P = 0.007). **P ≤ 0.01, ***P ≤0.005. pos, positive.
Paracrine Effect on HG + TGF-β1–Exposed HK-2 cells
HK-2 gene expression of MCP-1, a macrophage chemoattractant in DKD, decreased significantly after exposure to DKD-MSCcm but was not different from Control-MSCcm (Fig. 3B).
Inhibition of Alloreactive T-cell Activation and Proliferation With and Without IFN-γ Priming of MSC
IFN-γ priming of DKD-MSC increased (nearly 10-fold) IDO release into conditioned medium compared with unstimulated Control-MSC and DKD-MSC (Fig. 3C). In unstimulated MSC, no differences were found in immunomodulatory and inflammatory cytokine levels in DKD-MSCcm and Control-MSCcm (Fig. 3D). However, IFN-γ DKD-MSC priming resulted in lower levels of IL-6 compared with unstimulated DKD-MSCcm and lower IL-1β levels compared with Control-MSCcm. To further assess their immunomodulatory capacity, the alloreactive T-cell inhibitory effects of DKD-MSC and Control-MSC were compared in vitro in PBMC cocultures. Control-MSC inhibited T-cell proliferation significantly (36.8 [6.6%] of controls). However, DKD-MSC inhibited alloreactive T-cell proliferation more effectively (18.4 [3.4%] of controls; P = 0.002), with even more profound effects following IFN-γ priming of DKD-MSC (8.1 [5.9%] of controls; P = 0.007) (Fig. 3E).
MSC Antifibrotic Activity
MSC Secretome
Interestingly, secretion of HGF, a renal trophic, antiapoptotic, and antifibrotic factor (9,43,55), was almost 10-fold higher (4,454.3 [2,947.78] vs. 485.71 [297.45] pg/mL; P < 0.0001) in DKD-MSC compared with Control-MSC (Fig. 4A). Similarly, release of SDF-1, a stem/progenitor homing and homeostasis factor (11,56), was higher in DKD-MSC (105.0 [112.33] vs. 6.72 [10.21] pg/mL; P = 0.03). Other secreted trophic factors, such as EGF and BMP-7, were undetectable in MSCcm.
Figure 4.
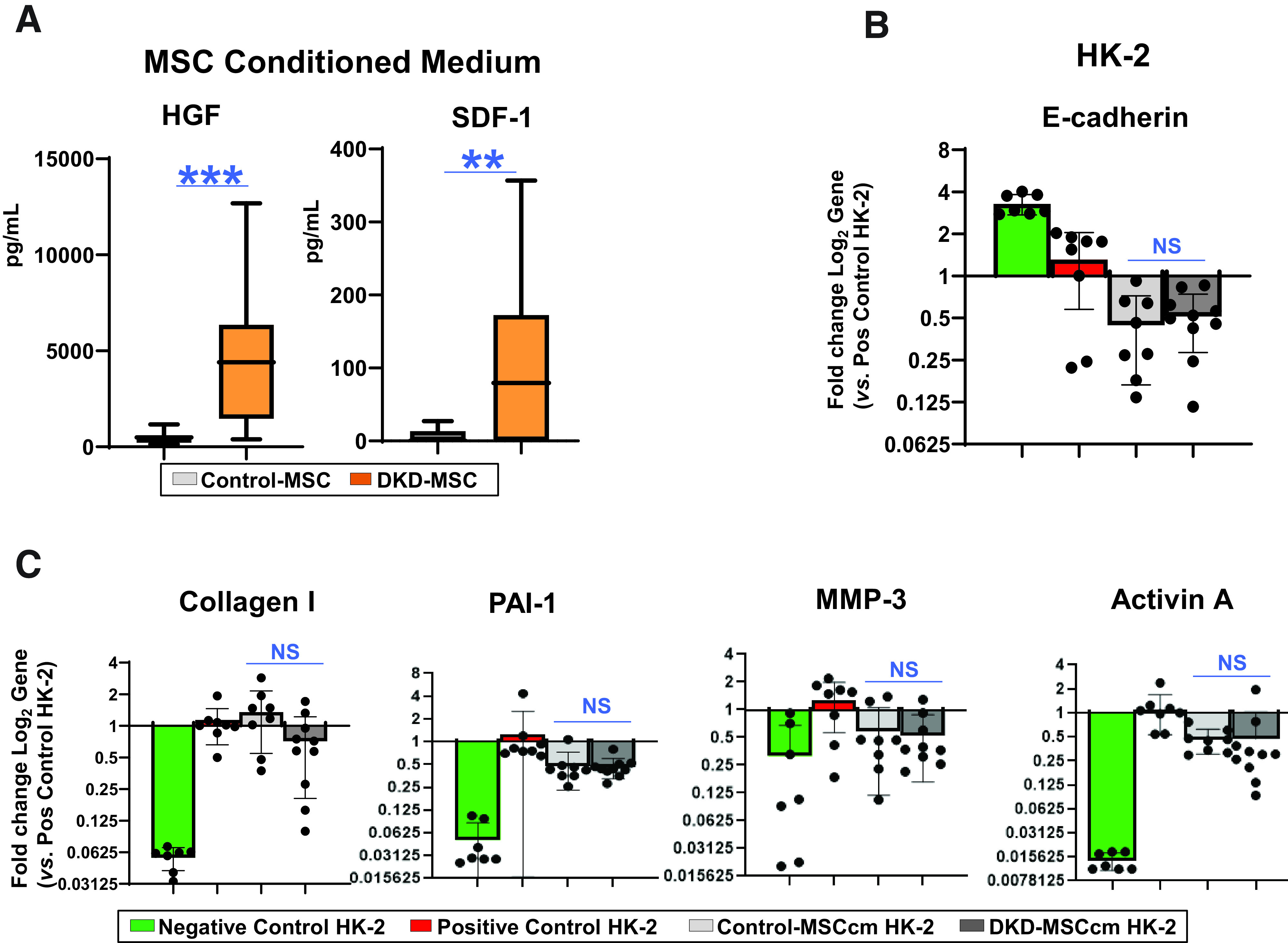
MSC antifibrotic activity. A: Secretion of HGF was substantially higher in DKD-MSC (n = 38) vs. Control-MSC (n = 10; P < 0.0001). Similarly, the trophic factor SDF-1 was higher in DKD-MSC (n = 20; P = 0.002 [Control-MSC, n = 10]). B: Effect of 24-h MSCcm incubation on HG + TGF-β–exposed HK-2 cells. Gene expression of E-cadherin, an EMT marker, was not restored in DKD-injured kidney cells cocultured with MSCcm from participants with DKD or control subjects. C: Among profibrotic markers, type I collagen was not reduced, although PAI-1 (P = 0.05), MMP-3 (P = 0.02), and activin A (P = 0.02) were decreased by DKD-MSC vs. positive HK-2 control. No differences were observed between DKD-MSCcm– and Control-MSCcm–treated groups for type I collagen, PAI-1, MMP-3, or activin A. Negative control, n = 7; positive control, n = 8; Control-MSCcm, n = 8; DKD-MSCcm, n = 8. **P ≤ 0.01, ***P ≤ 0.005. pos, positive.
Paracrine Effects on HG + TGF-β1–Exposed HK-2 Cells
Suggestive of EMT, E-cadherin gene expression decreased and type I collagen increased in HK-2 after injury. Both DKD-MSCcm and Control-MSCcm were ineffective at restoring E-cadherin levels (Fig. 4B). Among profibrotic markers, PAI-1, MMP-3, and activin A were decreased by DKD-MSC versus positive HK-2 controls, but type I collagen was not. There were no differences between DKD-MSCcm– and Control-MSCcm–treated groups for type I collagen, PAI-1, MMP-3, or activin A (Fig. 4C).
MSC Antiapoptotic Activity
Staining of proapoptotic markers caspase-3 and TUNEL was increased in HG + TGF-β1–exposed HK-2 compared with control. TUNEL positivity decreased after exposure to both Control-MSCcm (P < 0.0001) and DKD-MSCcm (P < 0.0001). However, there was no difference between DKD-MSCcm and Control-MSCcm (P = 0.7). For caspase-3 immunoreactivity, only Control-MSCcm reduced expression (P = 0.003), although DKD-MSC trended toward lower expression (P = 0.16). Moreover, there was a trend toward lower Control-MSCcm versus DKD-MSCcm (P = 0.08) caspase-3 expression (Fig. 5A and B).
Figure 5.
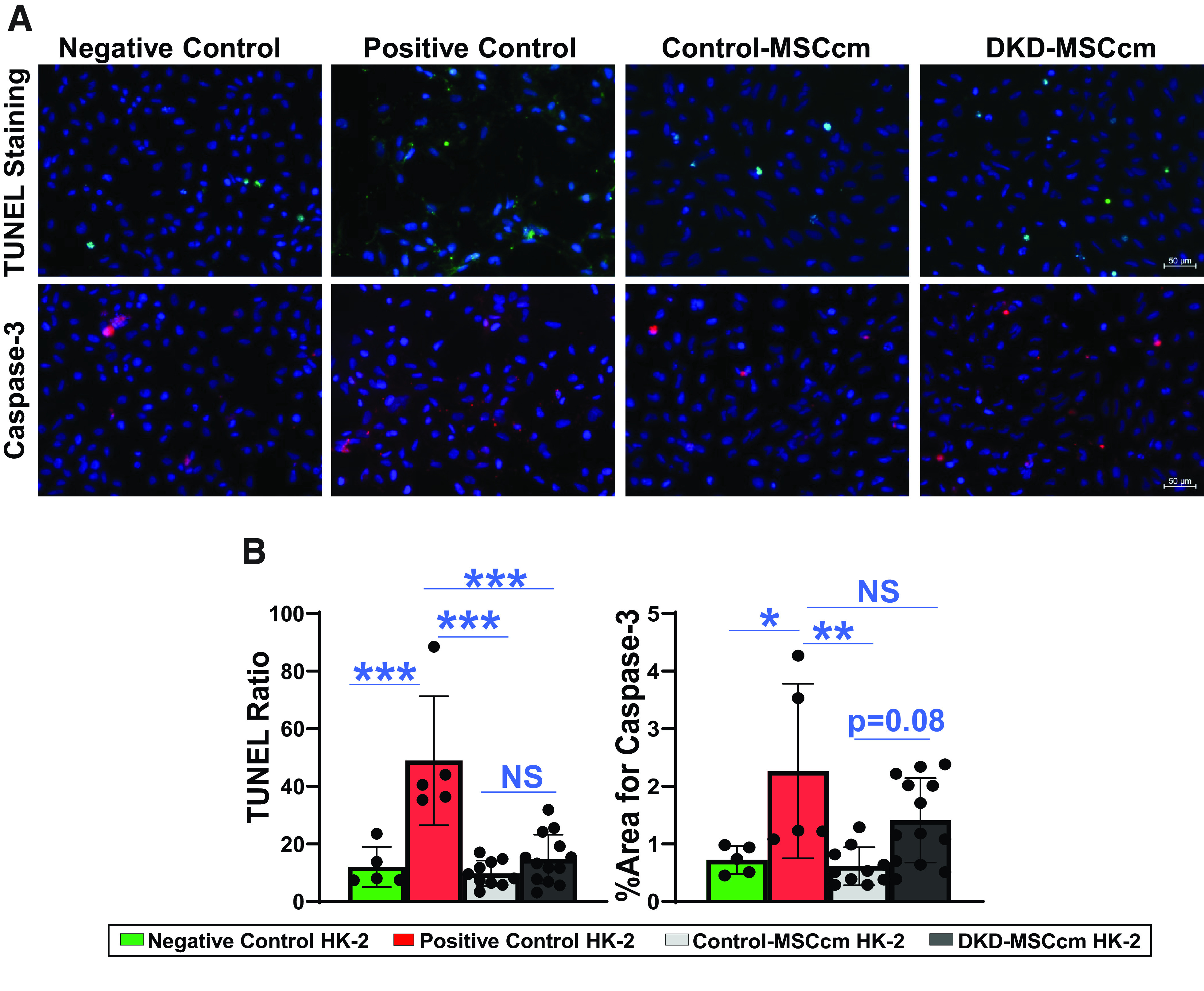
MSC antiapoptotic activity. Apoptosis activity was assessed in HG + TGF-β1–exposed HK-2 cells exposed to MSCcm. A and B: Proapoptotic markers TUNEL and caspase-3 staining was reduced in DKD-injured HK-2 after treatment with DKD-MSCcm (P < 0.0001 and P = 0.2, respectively) and Control-MSCcm (P < 0.0001 and P = 0.002, respectively). Control-MSCcm trended toward greater reduction of caspase-3 immunostaining vs. DKD-MSCcm (P = 0.08) but was not different for TUNEL (P = 0.7). Negative control, n = 5; positive control, n = 5; control-MSCcm, n = 10; DKD-MSCcm, n = 13. *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001.
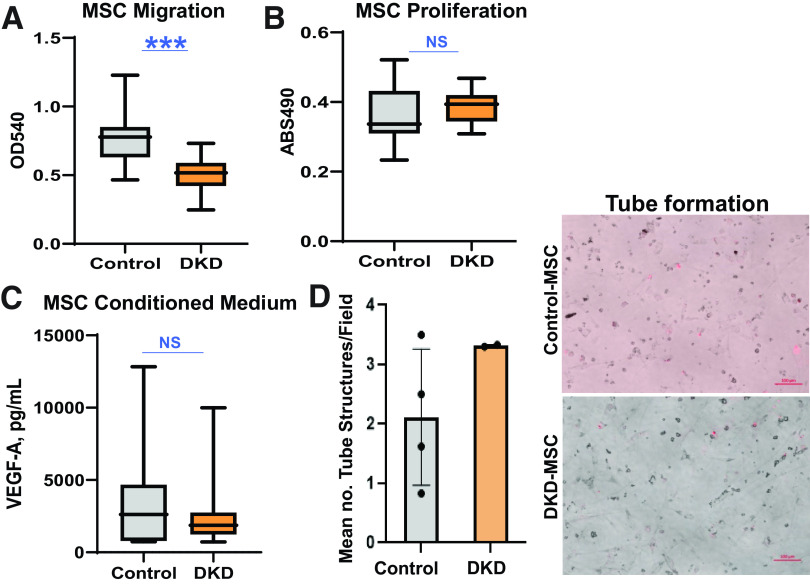
MSC Migration, Proliferation, and Angiogenic Activity
The migratory capacity of DKD-MSC was lower than that of Control-MSC (optical density 540-nm wavelength: 0.51 [0.12] vs. 0.76 [0.19]; P < 0.0001), while their proliferative activity trended higher (absorbance 490-nm wavelength: 0.39 [0.05] vs. 0.36 [0.08]; P = 0.07) (Fig. 6A). There was no difference in secretion of VEGF-A in DKD-MSC vs. Control-MSC (P = 0.6) (Fig. 6C), which also maintained similar capacity to induce tube formation (Fig. 6D).
Figure 6.
MSC migration, proliferation, and angiogenic activity. A: MSC migratory function is reduced in DKD-MSC compared with Control-MSC (Control-MSC, n = 16; DKD-MSC, n = 38; P < 0.0001). B: Mean proliferation trended higher in DKD-MSC vs. Control-MSC (Control-MSC, n = 16; DKD-MSC, n = 38; P = 0.07). C: Secretion of proangiogenic VEGF-A was not different (Control-MSC, n = 10; DKD-MSC, n = 38; P = 0.6). D: DKD-MSC maintained tube formation capacity with no differences between groups for mean number of tube structures per field (P = 0.8). Representative images are shown for tube structures induced by DKD-MSC and Control-MSC incubation with HUVECs. ***P ≤ 0.001. ABS490, absorbance 490-nm wavelength (proliferation); OD540, optical density 540-nm wavelength (migration).
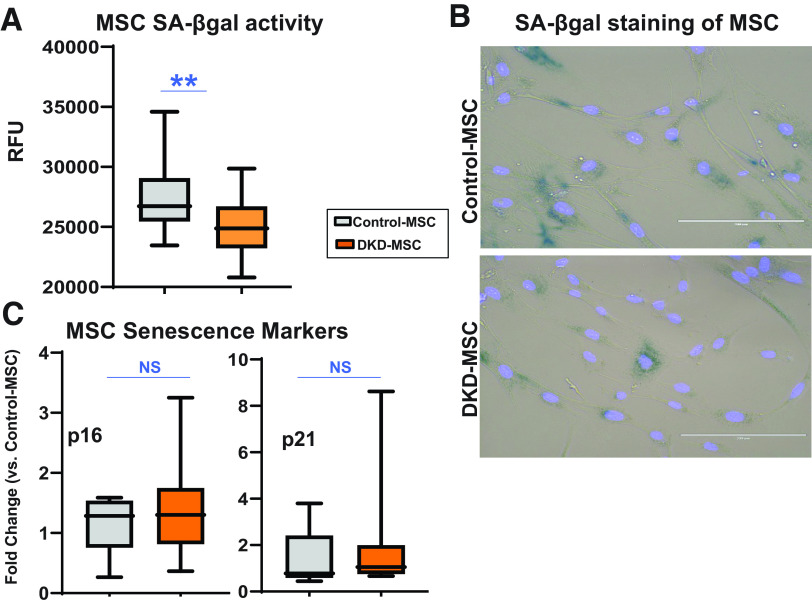
MSC Senescence
Increased senescent cell abundance might contri-bute to endogenous MSC dysfunction in disease states (22,24,48,57,58). Interestingly, SA-β-gal activity levels were lower in DKD-MSC (Fig. 7A and Supplementary Table 4), and SA-β-gal staining of MSCs was similar between groups (Fig. 7B). Similarly, gene expression of senescence markers p16INK4A and p21CIP1 was not different between Control-MSC and DKD-MSC (Fig. 7C). The majority of proinflammatory cytokines (SASP) measured in MSCcm (shown above), excluding IL-6 which was lower in DKD-MSC compared with Control-MSC, were not significantly different between groups. Overall, MSC senescence burden appeared similar between DKD-MSC and matched controls, and most reparative functionality remained intact.
Figure 7.
MSC senescence. A: SA-β-gal activity was lower in DKD-MSC (Control-MSC, n = 14; DKD-MSC, n = 27; P = 0.01). B: Representative images are shown for SA-β-gal staining in Control-MSC and DKD-MSC (scale bar = 200 µm). C: Fold change gene expression of the senescence markers p16INK4A and p21CIP1 was elevated in DKD-MSC compared with control, but the relationship was not significantly different (Control-MSC, n = 5; DKD-MSC, n = 22; P = 0.5 and P = 0.6, respectively). **P ≤ 0.01. RFU, relative fluorescence unit.
Relationship Between MSC and Demographic and Laboratory Findings in Participants With DKD
The association between functional kidney parameters (eGFR, UACR) and MSC properties (function, secretome profile, senescence) was evaluated in participants with DKD. Older age was associated with higher SDF (rs = 0.46; P = 0.04) and PGE2 release (rs = 0.52; P = 0.04) (Table 1) into DKD-MSCcm. PGE2 release decreased with lower kidney function (rs = 0.53; P = 0.01) and with proteinuria (rs = −0.45; P = 0.046), suggesting that PGE2 release by DKD-MSC may be differentially altered by age and kidney dysfunction. Interestingly, senescence burden assessed by SA-β-gal activity was inversely associated with BMI (rs = −0.51; P = 0.007) in this cohort. Male or female sex was not associated with any measurable differences in MSC studies (Supplementary Table 5). Type 2 DM tended to have higher IDO and IL-6 DKD-MSCcm levels and lower SA-β-gal activity than type 1 DM, although this was not significantly different (P = 0.07, P = 06, and P = 0.1, respectively) (Supplementary Table 6). SA-β-gal activity was also lower in DKD-MSC from participants on metformin therapy (P = 0.03 vs. nonuse). Overall, DKD-MSC studies showed little variation by diabetes control, kidney function, or urine protein excretion rate, but age, BMI, and metformin medication exposure may affect their function.
Table 1.
Univariate correlation coefficients between MSC in vitro studies and patient characteristics among participants with DKD
| Age (per year) | BMI (per kg/m2) | HbA1c (per % unit change) | Glucose (per mg/dL) | eGFR (per mL/min/1.73 m2) | UACR (per mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In vitro study | rs | P value | rs | P value | rs | P value | rs | P value | rs | P value | rs | P value |
| MSC function | ||||||||||||
| Migration | −0.175 | 0.3 | −0.034 | 0.8 | 0.118 | 0.5 | 0.034 | 0.8 | 0.075 | 0.7 | 0.33 | 0.06 |
| Proliferation | 0.046 | 0.8 | −0.094 | 0.6 | −0.174 | 0.3 | −0.117 | 0.5 | 0.090 | 0.6 | −0.151 | 0.4 |
| MSC secretome | ||||||||||||
| Proangiogenesis, antiapoptosis, antifibrosis | ||||||||||||
| VEGF-A | −0.104 | 0.5 | −0.084 | 0.6 | −0.221 | 0.2 | −0.159 | 0.3 | −0.167 | 0.3 | −0.101 | 0.6 |
| HGF | 0.076 | 0.6 | −0.012 | 0.9 | 0.137 | 0.4 | 0.192 | 0.3 | −0.015 | 0.9 | −0.206 | 0.3 |
| SDF | 0.458 | 0.04 | −0.151 | 0.5 | 0.114 | 0.6 | 0.227 | 0.3 | 0.00 | 0.9 | −0.144 | 0.6 |
| Immunomodulatory | ||||||||||||
| IDO | 0.187 | 0.3 | 0.262 | 0.1 | 0.075 | 0.7 | −0.035 | 0.8 | 0.262 | 0.1 | −0.062 | 0.7 |
| PGE2 | 0.522 | 0.02 | 0.047 | 0.8 | −0.214 | 0.4 | −0.336 | 0.1 | 0.531 | 0.01 | −0.451 | 0.046 |
| IL-6 | 0.012 | 0.9 | 0.059 | 0.7 | −0.012 | 0.9 | −0.130 | 0.4 | −0.063 | 0.7 | −0.170 | 0.3 |
| MSC senescence | ||||||||||||
| SA-β-gal | −0.254 | 0.2 | −0.507 | 0.007 | 0.134 | 0.5 | 0.317 | 0.1 | −0.174 | 0.4 | 0.053 | 0.8 |
SDF, n = 20; PGE2, n = 21; SA-β-gal activity, n = 27; all other MSC studies, n = 38. Units of measurement: ng/mL for IDO, PGE2, and activin A; pg/mL for VEGF-A, VEGF-C, HGF, and IL-6. Other studies are expressed as optical density 540-nm wavelength (migration), absorbance 490-nm wavelength (proliferation), and relative fluorescence unit (SA-β-gal). Additional studies: EGF (angiogenic), BMP-7 (antifibrosis), and TNF-α (proinflammatory) were undetectable in MSC culture medium by ELISA/Luminex assay. Boldface type indicates significance for P < 0.05.
In exploratory analyses, we also tested the relationships for Control-MSC. No significant correlations were identified related to sex and protein secretion (data not shown). Older age associated with lower IDO levels. VEGF-A and HGF levels decreased with higher BMI (Supplementary Table 7). However, as seen in DKD-MSC, PGE2 release increased with higher kidney function (rs = 0.71; P = 0.02) in Control-MSCcm.
Discussion
This study identified alterations in the transcriptome, in vitro function, and secretome profile of adipose-derived MSCs from participants with DKD compared with control subjects. Our results demonstrate that the hyperglycemic and uremic milieu of DKD is associated with reduced in vitro MSC migration, which may impair the ability to move to areas of injury, yet proliferation rates were higher and senescent cell burden was decreased compared with control. Despite identified transcriptome differences, the paracrine reparative and immunomodulatory capacity of MSCs appeared relatively preserved in DKD.
This study represents the largest evaluation to date of MSCs from humans with DKD or CKD. A limited number of published studies have assessed either MSC functionality in human CKD or the effect of uremic toxins on human MSCs, mostly in participants without DM (34,35,37,38,57,59). Our study uniquely provides insight into transcriptome alterations, comprehensive functional capacity (including migration, proliferation, tube formation, antiapoptosis, antifibrosis, and immunomodulation), and senescence burden of MSCs harvested from individuals with DKD. Some proinflammatory and profibrotic signaling pathways were found to be upregulated in DKD-MSC, suggesting less efficient immunomodulation and prorepair activity in culture-expanded DKD-MSC. Upon further exploration, we found preserved phenotype and trilineage differentiation capacity but diminished migration capability in DKD-MSC. Other reparative indices, like proangiogenic, antifibrotic, and antiapoptotic trophic factors, were either increased or similar to Control-MSC, and interestingly, HGF and SDF-1 secretion was substantially higher. We previously identified higher in vitro HGF release by MSCs in hypertension (21), which correlated with the ability of MSCs to reduce stenotic kidney hypoxia in vivo (60). HGF reduces kidney fibrosis by blocking EMT in tubular epithelial cells (61) and inhibits diabetic kidney expression of MCP-1 and macrophage infiltration (55). In the current study, the T-cell inhibitory capacity of DKD-MSC was superior to Control-MSC, possibly related to HGF, which maintains anti-T-cell proliferative properties and is one of the mediators through which MSCs avoid allorecognition (62–64). Furthermore, secretion of the immunomodulatory mediators IDO and PGE2 was higher in DKD. IFN-γ priming of DKD-MSC led to more robust IDO release and further suppression of T-cell proliferation beyond unstimulated DKD-MSC. Therefore, increased HGF, IDO (8), and PGE2 release by DKD-MSC may account for immunomodulatory and anti-inflammatory effects of DKD-MSC observed in the current study and might reflect enhancement of reparative properties in DKD. Conceivably, these might reflect compensatory magnification of MSC repair capacity to offset the noxious microenvironment in DM. Additional studies are needed to determine whether these are lost in uncontrolled DM or CKD.
We identified interesting and unexpected correlations between MSC function/senescence and patient-related factors. First, higher kidney function was associated with increased in vitro PGE2 secretion by DKD-MSC and Control-MSC, although PGE2 levels were higher in DKD-MSCcm than Control-MSCcm. Renal PGE2 mediates several actions in the kidney, including renin release, vascular tone/systemic blood pressure, and tubular transport (65). In diabetes mouse models, inhibition of one of the PGE2 receptors attenuated kidney injury (66). On the contrary, MSC-secreted PGE2 exerts beneficial immunomodulatory effects partly by mediating regulatory T-cell differentiation (12). However, the cause/effect relationship between kidney function and MSC PGE2 secretion in DKD remains to be established. Furthermore, we found that paradoxically, lower BMI correlated with higher SA-β-gal activity (senescence) in DKD-MSC. Possibly, these findings reflect an increase in senescent cell burden among thin individuals or poor nutritional status reflective of increased overall comorbid disease burden and subsequent DNA damage in MSCs of individuals with DKD. Interestingly, MSC senescence burden was also lower among metformin users and trended lower in those with type 2 (vs. type 1) DM, supporting its putative antiaging benefits (51). Furthermore, metformin use may have masked the relationship between aging and obesity with inflammation and cellular senescence markers in MSC. Finally, we did not identify associations between sex and MSC function. Additional studies are needed to better define these relationships. Collectively, a minority of alterations observed (migration) in DKD-MSC may reflect impairments in the endogenous cellular repair programs that might ultimately contribute to the pathophysiology of DM and CKD.
MSCs possess a potential to differentiate into multiple cell lineages, are believed to reside in the kidney as pericytes or periendothelial cells, and support kidney repair through paracrine and cell-cell interaction activities (9,12,43). To exert renal trophic and immunomodulatory effects, MSCs migrate to the site of injury, release soluble factors and extracellular vesicles, interact with other cells, and signal for progenitor/stem cell support. Our observations of reduced migration and enhanced immunomodulatory function of DKD-MSC may be useful for tailoring autologous MSC interventions in DKD, including the route of delivery (to counteract limited migration to injured organ), cell preconditioning (cell survival in harsh microenvironments, homing, or priming), or patient preconditioning. Several investigations are under way to improve MSC function, such as the removal of senescent MSC in humans (ClinicalTrials.gov identifiers: NCT02848131 and NCT03325322), with encouraging interim findings (67). Nonetheless, at present, our understanding of the safety, tolerability, and early efficacy signals of MSC therapy in individuals with DKD (n = 30; mesenchymal precursor cells) or non-DM CKD (n = 7) is limited to only two early-phase clinical trials (18). However, additional trials using allogeneic (Novel Stromal Cell Therapy for Diabetic Kidney Disease [NEPHSTROM]; Ireland, Italy, U.K., China, Japan) and autologous (U.S.) MSCs in participants with DKD are under way (ClinicalTrials.gov identifiers: NCT02585622, NCT04216849, NCT04125329, and NCT03840343). Hopefully, these studies will expedite the clinical translation of MSC-based therapy for DKD.
Our study has limitations. First, the predominantly Caucasian and older study cohort potentially limits generalizability but remains reflective of the U.S. CKD population. Second, control arms of CKD without DM and/or DM without CKD were unavailable, and intragroup variation (RNA-seq) in control subjects was found. Furthermore, adipose-derived MSCs may differ from other autologous cell sources, such as bone marrow. While bone marrow–derived MSCs function is impaired in rats with DKD (29), studies are needed to determine the impact of DKD on human MSC functionality in vivo. Third, noted differences between MSC mRNA and measured protein secretion may be attributable to various mechanisms (i.e., posttranslational modifications, protein degradation rate, differences in secretion pathways) and warrant future mechanistic studies. Finally, loss-of-function (siRNA) studies will be necessary to establish which of the differentially regulated genes are involved in alteration of DKD-MSC function.
In conclusion, compared with MSCs from control subjects, adipose-derived MSCs harvested from participants with DKD possess altered transcriptome, reduced migratory capacity, but preserved or enhanced immunomodulatory, antifibrotic, antiapoptotic paracrine kidney repair activities in vitro. Identification of MSC functional deficiencies or strengths may allow for development of methods to improve MSC function and help to eliminate barriers to successful MSC transplantation. Importantly, this study also suggests that moderate kidney function impairment in individuals with DKD should not deter a patient-derived MSC intervention.
Article Information
Acknowledgments. The authors thank the patients who participated in the study. In addition, they thank Mayo Clinic employees Donna K. Lawson, Jennifer M. Manggaard, Tammie L. Volkman, Erin Wissler-Gerdes, Marcia K. Mahlman, Beverly K. Tietje, and Tamara K. Evans (study coordination); Karena K. DiNicola and Shannon K. Meier (secretarial support); Rebekah Samsonraj, Allyson K. Palmer, John R. Woollard, Shane A. Bobart, and Abdelrhman M. Abumoawad (study design); Ariel J. Caride, Gift Ben-Bernard, Anastasia L. Smith, and Michael J. Hansen (cell studies); Lisa E. Vaughan (statistical support); and Mrunal Dehankar, Pritha Chana, and Asha Nair (Bioinfomatics Core, RNA-seq).
Funding. This project was supported by funding from the Extramural Grant Program of Satellite Healthcare, a not-for-profit care provider (L.J.H.), Regenerative Medicine Minnesota (RMM 091718, L.J.H.), Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery (L.J.H.); National Institutes of Health (NIH) grants DK-109134 (L.J.H.), DK-123492 (L.J.H.), UL1-TR-002377 (L.J.H., Mayo Clinic), and UL1-TR-000135 (L.J.H., Mayo Clinic Center for Clinical and Translational Science); and National Institute of Diabetes and Digestive and Kidney Diseases Diabetic Complications Consortium (RRID:SCR_001415, https://www.diacomp.org) grants DK-076169 and DK-115255. Additional support was provided by NIH grants DK-106427 (A.E.), T32-DK-07013 (S.M.C.), DK-118120 (S.M.H.), AG-013925 (J.L.K.), AG-062413 (J.L.K.), R01-DK-100081 (S.C.T.), and R01-DK-120292, DK-122734, AG-062104 (L.O.L.); the Ted Nash Long Life and Noaber Foundations (J.L.K.); the Connor Group (J.L.K.); and Robert J. and Theresa W. Ryan (J.L.K.). S.M.C. is supported by the Burroughs Wellcome Fund. M.D.G. is supported by the European Commission (Horizon 2020 Collaborative Health Project NEPHSTROM grant 634086), Science Foundation Ireland (CÚRAM Research Centre grant number 13/RC/2073), and the European Regional Development Fund.
Publication content is solely the responsibility of the authors and does not necessarily represent the official views of Satellite Healthcare, NIH, or the European Commission.
Duality of Interest. L.O.L. received grant funding from Novo Nordisk and is an advisor to AstraZeneca. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. L.J.H. researched data, designed the studies, and drafted and revised the manuscript. L.J.H., A.E., S.M.C., T.Ta., X.B., A.S., J.L.K., T.Tc., I.M.S., H.T., K.L.J., X.Z., A.J.v.W., S.C.T., and L.O.L. performed and/or supervised experiments. L.J.H, X.B., and R.A.M. provided statistical support. L.J.H. and L.O.L. obtained funding. A.E., S.M.H., J.L.K., T.Ta., T.Tc., M.D.G., A.D.R., A.J.v.W., S.C.T., and L.O.L. contributed to the results and discussion revision. T.J.M. and T.A.K. collected adipose tissue. All authors reviewed, edited, and approved the manuscript. L.J.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at American Society of Nephrology Kidney Week, New Orleans, LA, 31 October–5 November 2017.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14374106.
References
- 1. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–281 [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2017. Accessed 31 March 2021. Available from https://www.cdc.gov/diabetes/data/statistics/statistics-report.html
- 3. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019;73(3 Suppl. 1):A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ezquer F, Giraud-Billoud M, Carpio D, Cabezas F, Conget P, Ezquer M. Proregenerative microenvironment triggered by donor mesenchymal stem cells preserves renal function and structure in mice with severe diabetes mellitus. BioMed Res Int 2015;2015:164703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A 2006;103:17438–17443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Papazova DA, Oosterhuis NR, Gremmels H, van Koppen A, Joles JA, Verhaar MC. Cell-based therapies for experimental chronic kidney disease: a systematic review and meta-analysis. Dis Model Mech 2015;8:281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paulini J, Higuti E, Bastos RM, Gomes SA, Rangel EB. Mesenchymal stem cells as therapeutic candidates for halting the progression of diabetic nephropathy. Stem Cells Int 2016;2016:9521629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bulur P, Dietz A. Secretion of indoleamine 2-3 deoxygenase by adipose derived mesenchymal stromal cells as a biomarker for immune suppressive capacity 2018. Accessed 5 August 2018. Available from https://www.celltherapyjournal.org/article/S1465-3249(18)30122-1/abstract
- 9. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076–1084 [DOI] [PubMed] [Google Scholar]
- 10. Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004;109:1292–1298 [DOI] [PubMed] [Google Scholar]
- 11. Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 2007;292:F1626–F1635 [DOI] [PubMed] [Google Scholar]
- 12. Bai M, Zhang L, Fu B, et al. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int 2018;93:814–825 [DOI] [PubMed] [Google Scholar]
- 13. Eirin A, Zhu XY, Puranik AS, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int 2017;92:114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reinders ME, Fibbe WE, Rabelink TJ. Multipotent mesenchymal stromal cell therapy in renal disease and kidney transplantation. Nephrol Dial Transplant 2010;25:17–24 [DOI] [PubMed] [Google Scholar]
- 15. Samsonraj RM, Rai B, Sathiyanathan P, et al. Establishing criteria for human mesenchymal stem cell potency. Stem Cells 2015;33:1878–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. An X, Liao G, Chen Y, et al. Intervention for early diabetic nephropathy by mesenchymal stem cells in a preclinical nonhuman primate model. Stem Cell Res Ther 2019;10:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee KW, Kim TM, Kim KS, et al. Renal ischemia-reperfusion injury in a diabetic monkey model and therapeutic testing of human bone marrow-derived mesenchymal stem cells. J Diabetes Res 2018;2018:5182606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Packham DK, Fraser IR, Kerr PG, Segal KR. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo-controlled, dose escalation study. EBioMedicine 2016;12:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skyler JS, Fonseca VA, Segal KR; MSB-DM003 Investigators . Allogeneic mesenchymal precursor cells in type 2 diabetes: a randomized, placebo-controlled, dose-escalation safety and tolerability pilot study. Diabetes Care 2015;38:1742–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minamino T, Orimo M, Shimizu I, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 2009;15:1082–1087 [DOI] [PubMed] [Google Scholar]
- 21. Saad A, Zhu XY, Herrmann S, et al. Adipose-derived mesenchymal stem cells from patients with atherosclerotic renovascular disease have increased DNA damage and reduced angiogenesis that can be modified by hypoxia. Stem Cell Res Ther 2016;7:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu XY, Ma S, Eirin A, et al. Functional plasticity of adipose-derived stromal cells during development of obesity. Stem Cells Transl Med 2016;5:893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM. Cellular senescence in renal ageing and disease. Nat Rev Nephrol 2017;13:77–89 [DOI] [PubMed] [Google Scholar]
- 24. Klinkhammer BM, Kramann R, Mallau M, et al. Mesenchymal stem cells from rats with chronic kidney disease exhibit premature senescence and loss of regenerative potential. PLoS One 2014;9:e92115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006;108:2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood 2005;106:4057–4065 [DOI] [PubMed] [Google Scholar]
- 27. Liew A, Baustian C, Thomas D, et al. Allogeneic mesenchymal stromal cells (MSCs) are of comparable efficacy to syngeneic MSCs for therapeutic revascularization in C57BKSdb/db mice despite the induction of alloantibody. Cell Transplant 2018;27:1210–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hickson LJ, Eirin A, Lerman LO. Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int 2016;89:767–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagaishi K, Mizue Y, Chikenji T, et al. Umbilical cord extracts improve diabetic abnormalities in bone marrow-derived mesenchymal stem cells and increase their therapeutic effects on diabetic nephropathy. Sci Rep 2017;7:8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noh H, Yu MR, Kim HJ, et al. Uremia induces functional incompetence of bone marrow-derived stromal cells. Nephrol Dial Transplant 2012;27:218–225 [DOI] [PubMed] [Google Scholar]
- 31. Fadini GP, Albiero M, Vigili de Kreutzenberg S, et al. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care 2013;36:943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ribot J, Caliaperoumal G, Paquet J, Boisson-Vidal C, Petite H, Anagnostou F. Type 2 diabetes alters mesenchymal stem cell secretome composition and angiogenic properties. J Cell Mol Med 2017;21:349–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van de Vyver M. Intrinsic mesenchymal stem cell dysfunction in diabetes mellitus: implications for autologous cell therapy. Stem Cells Dev 2017;26:1042–1053 [DOI] [PubMed] [Google Scholar]
- 34. Idziak M, Pedzisz P, Burdzinska A, Gala K, Paczek L. Uremic toxins impair human bone marrow-derived mesenchymal stem cells functionality in vitro. Exp Toxicol Pathol 2014;66:187–194 [DOI] [PubMed] [Google Scholar]
- 35. Khanh VC, Ohneda K, Kato T, et al. Uremic toxins affect the imbalance of redox state and overexpression of prolyl hydroxylase 2 in human adipose tissue-derived mesenchymal stem cells involved in wound healing. Stem Cells Dev 2017;26:948–963 [DOI] [PubMed] [Google Scholar]
- 36. Reinders ME, Roemeling-van Rhijn M, Khairoun M, et al. Bone marrow-derived mesenchymal stromal cells from patients with end-stage renal disease are suitable for autologous therapy. Cytotherapy 2013;15:663–672 [DOI] [PubMed] [Google Scholar]
- 37. Roemeling-van Rhijn M, Reinders ME, de Klein A, et al. Mesenchymal stem cells derived from adipose tissue are not affected by renal disease. Kidney Int 2012;82:748–758 [DOI] [PubMed] [Google Scholar]
- 38. Yamanaka S, Yokote S, Yamada A, et al. Adipose tissue-derived mesenchymal stem cells in long-term dialysis patients display downregulation of PCAF expression and poor angiogenesis activation. PLoS One 2014;9:e102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eirin A, Zhu XY, Krier JD, et al. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 2012;30:1030–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saad A, Dietz AB, Herrmann SMS, et al. Autologous mesenchymal stem cells increase cortical perfusion in renovascular disease. J Am Soc Nephrol 2017;28:2777–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kalari KR, Nair AA, Bhavsar JD, et al. MAP-RSeq: Mayo analysis pipeline for RNA sequencing. BMC Bioinformatics 2014;15:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y, Parmigiani G, Johnson WE. ComBat-seq: batch effect adjustment for RNA-seq count data. NAR Genom Bioinform 2020;2:lqaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meirelles LdaS, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009;20:419–427 [DOI] [PubMed] [Google Scholar]
- 44. Taner T, Gustafson MP, Hansen MJ, et al. Donor-specific hypo-responsiveness occurs in simultaneous liver-kidney transplant recipients after the first year. Kidney Int 2018;93:1465–1474 [DOI] [PubMed] [Google Scholar]
- 45. Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 1994;45:48–57 [DOI] [PubMed] [Google Scholar]
- 46. Leuning DG, Engelse MA, Lievers E, et al. The human kidney capsule contains a functionally distinct mesenchymal stromal cell population. PLoS One 2017;12:e0187118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bian X, Griffin TP, Zhu X, et al. Senescence marker activin A is increased in human diabetic kidney disease: association with kidney function and potential implications for therapy. BMJ Open Diabetes Res Care 2019;7:e000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 2015;4:e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lavi R, Zhu XY, Chade AR, Lin J, Lerman A, Lerman LO. Simvastatin decreases endothelial progenitor cell apoptosis in the kidney of hypertensive hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol 2010;30:976–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stevens PE; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–830 [DOI] [PubMed] [Google Scholar]
- 51. Fang J, Yang J, Wu X, et al. Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell 2018;17:e12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yoshimatsu G, Sakata N, Tsuchiya H, et al. The co-transplantation of bone marrow derived mesenchymal stem cells reduced inflammation in intramuscular islet transplantation. PLoS One 2015;10:e0117561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gu Y, He M, Zhou X, et al. Endogenous IL-6 of mesenchymal stem cell improves behavioral outcome of hypoxic-ischemic brain damage neonatal rats by suppressing apoptosis in astrocyte. Sci Rep 2016;6:18587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood 1995;86:1243–1254 [PubMed] [Google Scholar]
- 55. Lv SS, Liu G, Wang JP, et al. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltration. Int Immunopharmacol 2013;17:275–282 [DOI] [PubMed] [Google Scholar]
- 56. Zhang M, Mal N, Kiedrowski M, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J 2007;21:3197–3207 [DOI] [PubMed] [Google Scholar]
- 57. Lee JH, Yun CW, Hur J, Lee SH. Fucoidan rescues p-Cresol-induced cellular senescence in mesenchymal stem cells via FAK-Akt-TWIST axis. Mar Drugs 2018;16:E121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Palmer AK, Xu M, Zhu Y, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 2019;18:e12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lanza D, Perna AF, Oliva A, et al. Impact of the uremic milieu on the osteogenic potential of mesenchymal stem cells. PLoS One 2015;10:e0116468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abumoawad A, Saad A, Ferguson CM, et al. In a phase 1a escalating clinical trial, autologous mesenchymal stem cell infusion for renovascular disease increases blood flow and the glomerular filtration rate while reducing inflammatory biomarkers and blood pressure. Kidney Int 2020;97:793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang J, Dai C, Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol 2005;16:68–78 [DOI] [PubMed] [Google Scholar]
- 62. Jang YH, You DH, Nam MJ. Protective effects of HGF gene-expressing human mesenchymal stem cells in acetaminophen-treated hepatocytes. Growth Factors 2015;33:319–325 [DOI] [PubMed] [Google Scholar]
- 63. Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Taniguchi F, Harada T, Deura I, Iwabe T, Tsukihara S, Terakawa N. Hepatocyte growth factor promotes cell proliferation and inhibits progesterone secretion via PKA and MAPK pathways in a human granulosa cell line. Mol Reprod Dev 2004;68:335–344 [DOI] [PubMed] [Google Scholar]
- 65. Nasrallah R, Hassouneh R, Hébert RL. Chronic kidney disease: targeting prostaglandin E2 receptors. Am J Physiol Renal Physiol 2014;307:F243–F250 [DOI] [PubMed] [Google Scholar]
- 66. Thieme K, Majumder S, Brijmohan AS, et al. EP4 inhibition attenuates the development of diabetic and non-diabetic experimental kidney disease. Sci Rep 2017;7:3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 2019;47:446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]