Abstract
Objective
To inform the update of the European Association for the Study of Diabetes clinical practice guidelines for nutrition therapy.
Design
Systematic review and meta-analysis of randomised controlled trials.
Data sources
Medline, Embase, and the Cochrane Library searched up to 13 May 2021.
Eligibility criteria for selecting studies
Randomised controlled trials of three or more weeks investigating the effect of diets with low glycaemic index (GI)/glycaemic load (GL) in diabetes.
Outcome and measures
The primary outcome was glycated haemoglobin (HbA1c). Secondary outcomes included other markers of glycaemic control (fasting glucose, fasting insulin); blood lipids (low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), non-HDL-C, apo B, triglycerides); adiposity (body weight, BMI (body mass index), waist circumference), blood pressure (systolic blood pressure (SBP) and diastolic blood pressure (DBP)), and inflammation (C reactive protein (CRP)).
Data extraction and synthesis
Two independent reviewers extracted data and assessed risk of bias. Data were pooled by random effects models. GRADE (grading of recommendations assessment, development, and evaluation) was used to assess the certainty of evidence.
Results
29 trial comparisons were identified in 1617 participants with type 1 and 2 diabetes who were predominantly middle aged, overweight, or obese with moderately controlled type 2 diabetes treated by hyperglycaemia drugs or insulin. Low GI/GL dietary patterns reduced HbA1c in comparison with higher GI/GL control diets (mean difference −0.31% (95% confidence interval −0.42 to −0.19%), P<0.001; substantial heterogeneity, I2=75%, P<0.001). Reductions occurred also in fasting glucose, LDL-C, non-HDL-C, apo B, triglycerides, body weight, BMI, systolic blood pressure (dose-response), and CRP (P<0.05), but not blood insulin, HDL-C, waist circumference, or diastolic blood pressure. A positive dose-response gradient was seen for the difference in GL and HbA1c and for absolute dietary GI and SBP (P<0.05). The certainty of evidence was high for the reduction in HbA1c and moderate for most secondary outcomes, with downgrades due mainly to imprecision.
Conclusions
This synthesis suggests that low GI/GL dietary patterns result in small important improvements in established targets of glycaemic control, blood lipids, adiposity, blood pressure, and inflammation beyond concurrent treatment with hyperglycaemia drugs or insulin, predominantly in adults with moderately controlled type 1 and type 2 diabetes. The available evidence provides a good indication of the likely benefit in this population.
Study registration
ClinicalTrials.gov NCT04045938.
Introduction
The glycaemic index (GI) ranks a carbohydrate containing food according to the amount by which it raises blood glucose levels after it is consumed in comparison with reference food (pure glucose or white bread), for which a GI of ≤55 is low, 56-69 is medium, and ≥70 is high, based on a glucose scale.1 The glycaemic load (GL) of a food is the GI multiplied by the available carbohydrate (g) in the serving divided by 100.2
Clinical practice guidelines recommend dietary and lifestyle changes as the basis of treatment to prevent and manage diabetes and cardiovascular disease.3 4 5 6 Many dietary patterns are recommended that reduce cardiovascular risk for those with diabetes. Approaches that target postprandial glycaemic excursions through changes to carbohydrate quality and quantity of the diet might have particular advantages.
Systematic reviews and meta-analyses have shown that low GI/GL dietary patterns, which incorporate elements of carbohydrate quality and quantity, result in lower postprandial glycaemic excursions and improve longer term glycaemic control and cardiometabolic risk factors in randomised controlled trials in people at risk for, and with, diabetes,7 8 9 10 11 12 and are associated with a reduced incidence of diabetes and cardiovascular disease in prospective cohort studies inclusive of people with diabetes.12 13 14 15 16 These benefits are recognised by major international clinical practice guidelines in Canada, USA, Australia, UK, and Europe,1 17 18 19 20 with low GI/GL dietary patterns recommended for those with diabetes. Despite this recognition, the European Association for the Study of Diabetes (EASD) last updated their clinical practice guidelines in 200418 and the last comprehensive systematic review and meta-analysis in diabetes was published in 2010,7 8 with numerous randomised controlled trials published after the census for these syntheses.21 22 23 24 25 26 27 28 29 30 31 To inform the update of EASD clinical practice guidelines for nutrition treatment, the Diabetes and Nutrition Study Group (DNSG) of EASD commissioned a systematic review and meta-analysis of randomised controlled trials to summarise the effect of low GI/GL dietary patterns on glycaemic control and other established cardiometabolic risk factors in people with type 1 and type 2 diabetes and assess the certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
Methods
The supplemental methods present our methodology in detail. We followed the Cochrane Handbook for Systematic Reviews of Interventions (version 6.1)32 for the conduct and the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines33 (supplemental table S1). The protocol was registered at ClinicalTrials.gov (NCT04045938).
Search strategy and selection criteria
Supplemental tables S2 and S3 shows the search strategy.33 Validated filters from the McMaster University Health Information Research Unit were applied to limit the database search to controlled studies only.34 We searched Medline, Embase, and the Cochrane Central Register of Controlled Trials through 13 May 2021. These searches were supplemented with manual searches of the reference lists from included trials.
We included randomised controlled trials with a follow-up of three or more weeks investigating the effect of low GI or low GL diets on measures of glycaemic control, blood lipids, adiposity, blood pressure, or inflammation in those with type 1 or type 2 diabetes. We excluded trials that were multimodal with cointerventions (that is, trials which were designed in such a way that the effect of GI or GL could not be isolated), had non-energy matched controls, were in pregnant or breastfeeding women, or did not report viable endpoint data. No restrictions were placed on language.
Data extraction
Two investigators (LC and DL, AA, or AC) independently reviewed and extracted relevant data from each included report using a standardised form including sample size, participant characteristics, study setting, design, feeding control, intervention, control, GI and GL dose (glucose scale) during intervention and control, dietary macronutrients, energy balance, follow-up, funding source, and outcome data. When GL was not reported but GI and carbohydrate (g/d) were, we calculated GL from these values as GI×carbohydrate (g/d)/100. If carbohydrate was reported as percentage of energy, we calculated grams per day using total kilojoules when available, otherwise we assumed an 8368 kJ diet. Authors were contacted for missing data. In the absence of outcome data and inability to obtain the original data from authors, values were extracted from figures using Plot Digitizer,35 where available. Discrepancies were resolved through consensus.
Risk of bias assessment
Included trials were independently assessed by two investigators (LC and DL, AA, or AC) for risk of bias using the Cochrane Risk of Bias Tool.32 Assessment was made across five domains of bias (sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting). Risk of bias was assessed as either low (proper methods taken to reduce bias), high (inadequate methods creating bias), or unclear (insufficient information provided) for each of the five domains of bias (supplemental table S4). Reviewer discrepancies were resolved by consensus or arbitration by the senior author (JLS).
Outcomes
The prespecified primary outcome was difference in glycated haemoglobin (HbA1c). Secondary outcomes included difference in other markers of glycaemic control (fasting glucose, fasting insulin); blood lipids (low density lipoprotein cholesterol (LDL-C), non-high density lipoprotein cholesterol (non-HDL-C), apo B, HDL-C, triglycerides); adiposity (body weight, BMI (body mass index), waist circumference), blood pressure (systolic blood pressure (SBP) and diastolic blood pressure (DBP)), and inflammation (C reactive protein (CRP)). Change in hyperglycaemia drugs or insulin, adverse events, and intervention acceptability were added as a post hoc secondary outcomes that were assessed narratively.
Data analyses
All analyses were conducted using STATA software, version 16.1 (StataCorp, College Station, TX). Separate pooled analyses of study trial comparisons were conducted for each outcome using the generic inverse variance method with DerSimonian and Laird random effects meta-analyses.36 Mean differences between the intervention and control arms and their respective variance terms were extracted and used as the basis for analysis for each trial comparison. If mean differences were not provided, they were derived from available data using published formulas.32 When median data were reported, they were converted to mean data with corresponding variances using established methods.37 38 When no variance data were available, the standard deviation was taken from a trial similar in size, participants, and nature of intervention. Mean differences and standard errors were computed using change in values from baseline in preference to over end differences. For crossover trials and for within arm changes in parallel trials, we used a correlation coefficient of 0.5 in pairwise analysis to calculate standard errors.39 40 41 To mitigate a unit of analysis error, when arms of trials with multiple interventions or control arms were used more than once, the corresponding sample size was divided accordingly.32 Non-HDL-C values that were not reported were derived by subtracting HDL-C from total cholesterol values with standard errors derived from HDL-C and total cholesterol variance data using the inverse variance law.42 For trials in which the change in BMI was not reported, but body weight was reported, then if baseline BMI was available, these data were used to calculate the height, which could then be used to calculate the end BMI and change in BMI. The change in BMI variance was imputed using published formula32 and a correlation coefficient of 0.5.39 40 41
Data were expressed as mean differences with 95% confidence intervals. Heterogeneity was assessed using the Cochran Q statistic and quantified using the I2 statistic. Significance for heterogeneity was set at P<0.10, with an I2 >50% considered to be evidence of substantial heterogeneity.32 Sources of heterogeneity were explored using sensitivity and subgroup analyses.
Sensitivity analyses were performed in which each individual trial comparison was removed from the meta-analysis and the effect size recalculated to determine whether a single trial comparison exerted an undue influence. A trial comparison whose removal explained the heterogeneity, changed the significance of the effect, or altered the effect size by one or more minimally important difference (supplemental table S5) was considered an influential comparison. Sensitivity analyses were also performed using correlation coefficients of 0.25 and 0.75 to determine whether the overall results were robust to the use of different correlation coefficients. Where 10 or more trial comparisons were available, a priori subgroup analyses were conducted using random effects meta-regression where heterogeneity of effect estimates (effect modification) was explored using prespecified subgroups (diabetes type, study design, follow-up duration, comparator diet, baseline outcome level, diabetes duration, and domains of risk of bias).43 44
Additional post hoc subgroup analyses were conducted by age, energy balance, feeding control, test GI/GL (absolute value of GI or GL achieved in trial in the low GI/GL diets), difference in GI/GL (test control), and funding source. Further post hoc categorical subgroup analyses were conducted by presence of a washout period for crossover trials and continuous subgroup analyses by test fibre (absolute value achieved in trial for dietary fibre in the low GI/GL diets) and difference in fibre (test control).
We assessed significant difference within each subgroup category or, where possible, as a continuous variable. Residual I2 was estimated to measure the remaining heterogeneity after accounting for any effect modification. We also conducted dose-response analyses to assess linear dose-response gradients and non-linear dose-response thresholds for dietary GI and GL (by both the absolute value of GI/GL achieved in trial in the low GI/GL diets and difference in GI/GL, test control) if there were six or more trial comparisons.45 Linear dose-response analyses were assessed by random effects meta-regression. Non-linear dose-response associations were assessed with restricted cubic splines with three knots at Harrell’s recommended centiles (15%, 50%, 85%).46 Departure from linearity was assessed using the Wald test and its significance conferred non-linear model as the best fit. When 10 or more trial comparisons were available, publication bias was investigated by inspection of contour enhanced funnel plots47 and formal testing using the Egger and Begg tests (at P<0.05).48 49 If publication bias was suspected, we attempted to adjust for funnel plot asymmetry by imputing the missing study data using the Duval and Tweedie trim-and-fill method and assessed for small study effects.50
GRADE assessment
We used the GRADE approach to assess the overall certainty of the evidence and produce profiles in which evidence was graded as high, moderate, low, or very low certainty.51 52 53 Two investigators (LC and DL, AA, AC, or JLS) independently performed GRADE assessments for each outcome. Randomised controlled trials receive an initial grade of high by default and were downgraded based on the following prespecified criteria: risk of bias (assessed by the Cochrane Risk of Bias Tool), inconsistency (substantial unexplained interstudy heterogeneity, I2 >50%, and P<0.10), indirectness (presence of factors that limit the generalisability of the results), imprecision (the 95% confidence interval for effect estimates overlap the minimally important differences for benefit or harm), and publication bias (significant evidence of a small study effect), or upgraded.
Patient and public involvement
No patients were involved in the design or conduct of the study, development of patient relevant outcomes, interpretation of the results, or writing or editing of the manuscript as there was no funding for this as part of the guidelines development.
Results
Flow of the literature
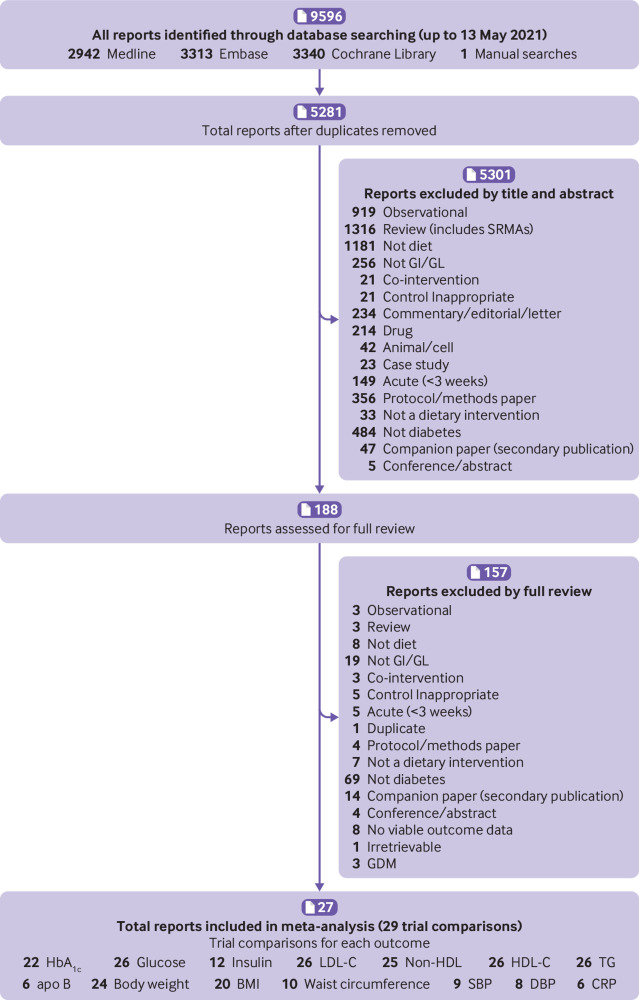
Figure 1 shows the literature search and selection process. Of 9596 reports identified, 9408 were excluded based on titles and abstracts. Of 188 reports reviewed in full, 161 were excluded based on eligibility criteria. A total of 27 reports containing data for 29 trial comparisons involving 1617 participants with diabetes were included in the final analyses.21 22 23 24 26 27 28 30 31 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71
Fig 1.
Literature search and selection strategy. Apo B=apolipoprotein B; CRP=C reactive protein; DBP=diastolic blood pressure; GDM=gestational diabetes; GI=glycaemic index; GL=glycaemic load; HbA1c=glycated haemoglobin; HDL-C=high density lipoprotein cholesterol; LDL-C=low density lipoprotein cholesterol; non-HDL-C=non-high density lipoprotein cholesterol; SBP=systolic blood pressure; SRMA=systematic review and meta-analysis; TG=triglycerides
Trial characteristics
Table 1 and supplemental table S6 show the characteristics of the 29 trial comparisons for each outcome. All trial comparisons were conducted in outpatient settings, with most in Canada (21%) and Australia (17%), and also in France (10%), the United States (7%), Israel (7%), Mexico (7%), and the rest across European and Asian countries. Trials had a median follow-up duration of 12 weeks (range 3-52), an approximately equal distribution of men and women (median percentage women 47%, range 0-100%), and 45% had a crossover design (6 (46%) of 13 trial comparisons had no washout period between interventions). Most trials included adult participants (93%) with type 2 diabetes (90%). Most participants were middle aged (median age 56 years, range 11-67), overweight or obese (median BMI 31, range 19-36), with moderate glycaemic control (median baseline HbA1c 7.7%, range 6.2-13.8%) treated by hyperglycaemia drugs (69%) or insulin (14%) or a mix of both (7%), with a few included participants treated exclusively with diet alone (10%). Mean duration of diabetes varied from 4.9 to 9.5 years for those with type 2 diabetes (n=16 trial comparisons), 10.3 to 14.6 years for adults with type 1 diabetes (n=2), 3 to 3.7 years for children with type 1 diabetes (n=2), 11.5 years for those with mixed type 1 and type 2 diabetes (n=1), otherwise it was unspecified (n=8).
Table 1.
Summary of characteristics of included trial comparisons assessing the effect of low GI/GL dietary patterns on cardiometabolic outcomes*
| Cardiometabolic risk factor | Total No of trial comparisons | Total No† | Sample size‡ | Diabetes type (No of trials) | Age (years)‡ | Diabetes duration (years)‡ | F/U (weeks)‡ | Trial design (No of trials) | Baseline value‡ § | Intervention GI‡ ¶ and GL‡ ¶ |
Control GI‡ ¶ and GL‡ ¶ |
Feeding control (No of trials) | Energy balance** (No of trials) | Funding†† (No of trials) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c (%) | 22 | 1502 | 58.5 (7-210) |
18 T2DM 3 T1DM 1 Mixed |
56 (11-67) |
8 (3-12) |
12 (3.4-52) |
8 C, 14 P | 7.7 (6.2-13.8) |
49 (38-57) 92 (53-176) |
64 (56-75) 137 (89-175) |
15 DA 1 Met 6 Supp |
20 Neutral: 2 Negative |
13 A 3 I 4 AI 2 NR |
| Fasting blood glucose (mmol/L) | 26 | 1369 | 20 (6-210) |
22 T2DM 3 T1DM 1 Mixed |
57 (12-67) |
7 (3-15) |
9 (3-52) |
13 C, 13 P | 9.6 (6.5-13.1) |
49 (38-58) 100 (33-176) |
63 (51-86) 140 (39-175) |
15 DA 2 Met 9 Supp |
23 Neutral: 3 Negative |
14 A 3 I 6 AI 3 NR |
| Fasting insulin (pmol/L) | 12 | 733 | 71 (10-130) |
12 T2DM | 57 (53-67) |
6 (5-9) |
18 (3-52) |
5 C, 7 P | 88.2 (61.0-210) |
43 (39-57) 104 (78-133) |
63 (59-71) 135 (110-155) |
5 DA 1 Met 6 Supp |
11 Neutral: 1 Negative |
6 A 1 I 4 AI 1 NR |
| LDL-C (mmol/L) | 26 | 1373 | 31 (6-210) |
22 T2DM 3 T1DM 1 Mixed |
56 (12-67) |
8 (3-15) |
12 (3-52) |
12 C, 14 P | 3.1 (2.2-4.6) |
49 (38-58) 100 (33-176) |
63 (51-86) 135 (39-175) |
16 DA 2 Met 8 Supp |
23 Neutral: 3 Negative |
15 A 2 I 7 AI 2 NR |
| Non-HDL-C (mmol/L) | 25 | 1353 | 40 (6-210) |
21 T2DM 3 T1DM 1 Mixed |
55 (12-67) |
8 (3-15) |
12 (3-52) |
11 C, 14 P | 3.8 (2.7-5.7) |
49 (38-58) 100 (33-176) |
63 (51-86) 137 (39-175) |
15 DA 2 Met 8 Supp |
22 Neutral: 3 Negative |
14 A 2 I 7 AI 2 NR |
| HDL-C (mmol/L) | 26 | 1373 | 31 (6-210) |
22 T2DM 3 T1DM 1 Mixed |
56 (12-67) |
8 (3-15) |
12 (3-52) |
12 C, 14 P | 1.1 (0.7-1.5) |
49 (38-58) 100 (33-176) |
63 (51-86) 135 (39-175) |
16 DA 2 Met 8 Supp |
23 Neutral: 3 Negative |
15 A 2 I 7 AI 2 NR |
| Triglycerides (mmol/L) | 26 | 1373 | 36 (6-210) |
22 T2DM 3 T1DM 1 Mixed |
56 (12-67) |
8 (3-15) |
12 (3-52) |
12 C, 14 P | 1.8 (0.7-5.0) |
49 (38-58) 100 (33-176) |
63 (51-86) 135 (39-175) |
16 DA 2 Met 8 Supp |
23 Neutral: 3 Negative |
15 A 2 I 7 AI 2 NR |
| Apo B (g/L) | 6 | 241 | 19 (8-103) |
4 T2DM 1 T1DM 1 Mixed |
54 (44-67) |
10 (9-15) |
5 (3-52) |
4 C, 2 P | 2.0 (1.0-2.1) |
42 (38-55) 102 (78-133) |
63 (59-71) 144 (135-155) |
3 DA 1 Met 2 Supp |
6 Neutral | 2 A 4 AI |
| Body weight (kg) | 24 | 1335 | 43 (6-210) |
21 T2DM 2 T1DM 1 Mixed |
56 (28-63) |
8 (5-15) |
12 (3-52) |
11 C, 13 P | 86.0 (66.1-106.9) |
49 (38-58) 100 (53-133) |
63 (51-86) 135 (89-170) |
15 DA 1 Met 8 Supp |
21 Neutral: 3 Negative |
12 A 3 I 8 A 1 NR |
| BMI | 20 | 1166 | 43 (8-210) |
20 T2DM | 55 (49-63) |
7 (5-9) |
12 (3-52) |
8 C, 12 P | 30.7 (25-36.3) |
49 (39-57) 91 (31-121) |
63 (51-72) 121 (39-164) |
13 DA 7 Supp |
18 Neutral 2 Negative |
11 A 3 I 4 AI 2 NR |
| Waist circumference (cm) | 10 | 863 | 90 (20-141) |
10 T2DM | 54 (42-62) |
8 (5-10) |
32 (4-52) |
10 P | 105.1 (91.4-113) |
54 (43-57) 87 (33-133) |
63 (57-72) 105 (39-135) |
6 DA 4 Supp |
9 Neutral 1 Negative |
7 A 1 I 1 AI 1 NR |
| SBP (mm Hg) | 9 | 919 | 100 (40-210) |
9 T2DM | 59 (53-61) |
9 (8-10) |
24 (8-52) |
9 P | 129.4 (120.0-135.1) |
51 (43-57) 91 (53-133) |
63 (57-75) 120 (89-164) |
5 DA 4 Supp |
7 Neutral 2 Negative |
5 A 1 I 2 AI 1 NR |
| DBP (mm Hg) | 8 | 816 | 90 (40-210) |
8 T2DM | 58 (53-61) |
9 (8-10) |
18 (8-52) |
8 P | 75.3 (71.0-80.6) |
50 (43-57) 90 (53-108) |
62 (57.1-75) 118 (89-164) |
5 DA 3 Supp |
6 Neutral 2 Negative |
5 A 1 I 1 AI 1 NR |
| CRP‡‡ (mg/L) | 6 | 622 | 92 (20-210) |
6 T2DM | 55 (42-61) |
7 (5-9) |
24 (4-52) |
6 P | 3.81 (0.33- 8.04) | 54 (43-57) 92 (33-133) |
64 (59-72) 119 (39-135) |
3 DA 3 Supp |
5 Neutral 1 Negative |
2 A 2 AI 2 NR |
A=agency; AI=agency-industry; apo B=apolipoprotein B; C=crossover; CRP=C reactive protein; DA=dietary advice; DBP=diastolic blood pressure; DM=diabetes; F/U=follow-up; GI=glycaemic index; GL=glycaemic load; HbA1c=glycated haemoglobin; HDL-C=high density lipoprotein cholesterol; I=industry; LDL-C=low density lipoprotein cholesterol; Met=metabolic; non-HDL-C=non-high density lipoprotein cholesterol; NR=not reported; P=parallel; SBP=systolic blood pressure; Supp=supplement; T1DM=type 1 diabetes; T2DM=type 2 diabetes.
All numbers with the exception of baseline values were rounded to the nearest whole number to improve readability.
All sample sizes reflect participants included in the data analysed.
Data are medians and ranges where the range represents the range of the mean (age or diabetes duration or follow-up) in the included trial comparisons.
Note: not all trials reported baseline values. Baseline values were not reported for: HbA1c (n=1 trial comparison), fasting glucose (n=2), insulin (n=3), LDL-C (n=7), non-HDL-C (n=7), HDL-C (n=5), triglycerides (n=3), apo B (n=2), body weight (n=2), BMI (n=0), waist circumference (n=0), SBP (n=0), DBP (n=0), CRP (n=0).
Note: not all trials reported a GI/GL value for the intervention. For those trials that did report GI/GL values, most reported intakes achieved in trial intakes based on food records. GI units are on the glucose scale.
Negative energy balance refers to a deficit in normal energy intake or intake below energy requirements. Neutral energy balance refers to the maintenance of usual energy intake or meeting energy requirements.
Agency funding is that from government, university, or not-for-profit sources. Industry funding is that from trade organisations that obtain revenue from the sale of products.
Five of six trial comparisons explicitly report that high sensitivity CRP was measured.
The median GI values prescribed or achieved in trial in the intervention or control diets were 49 (range 38-58) and 63 (51-86), respectively; this value was reported for 24 of 29 trial comparisons and approximated for two trial comparisons. The median difference in GI (test – control) between the intervention and control diets was a reduction of 12 (range −32 to −1). The median (range) GL prescribed or achieved in trial values in the intervention or control diets were 102 (33-176) and 138 (39-175), respectively; GL values were reported for about one third of trial comparisons (n=12) and calculated for nearly half (n=13). The median difference in GL (test – control) between the intervention and control diets was a reduction of 29 (−77 to 5). Most trial comparisons investigated the effect of a low GI diet (90%), but only three trials explicitly defined their interventions as low GL (10%). Macronutrient composition of intervention and control diets varied across trials. Across intervention arms, the median (range) intake values, reported as percentages of energy were: carbohydrate 49% (range 38-60%), protein 20% (13-23%), fat 32% (18-42%), saturated fat 8.2% (5.1-13.2%), and fibre 30.7 g/d (12.2-53.0). Across control arms, percentages of energy were: carbohydrate 48% (36-64%), protein 19% (15-23%), fat 32% (17-43%), saturated fat 8.6% (6.1-14.2%), and fibre 26.3 g/d (11-35.4). Most trials had neutral energy balance (90%), provided dietary advice (59%; 34% supplemented; 7% metabolic), and were funded by agency alone (55%) or agency-industry (24%; 10% industry; 10% not reported).
Risk of bias
Supplemental figures S1 and S2 show the Cochrane Risk of Bias assessments for the included trials. Most trials were judged as having a low or unclear risk of bias across domains and none were rated as high.
Primary outcome
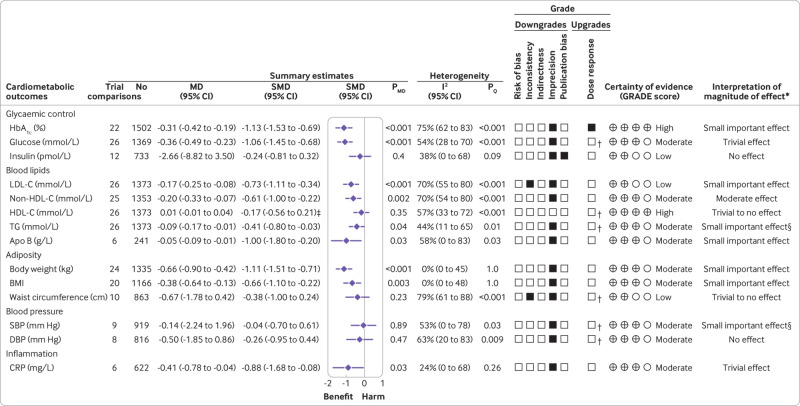
Figure 2 and supplemental figure S3 show the effect of low GI/GL dietary patterns on the primary outcome HbA1c. In 22 trial comparisons involving 1502 participants (18 in those with type 2 diabetes (n=1319), three in those with type 1 diabetes (n=165), and one in those with mixed type 1 and 2 diabetes (n=18)), low GI/GL diets led to a small important reduction in HbA1c compared with control diets (mean difference −0.31% (95% confidence interval −0.42% to −0.19%), P<0.001; substantial heterogeneity, I2=75%, P<0.001).
Fig 2.
Summary plot of the effect of low glycaemic index/glycaemic load dietary patterns on glycaemic control and cardiometabolic outcomes in randomised controlled trials in diabetes. Data are expressed as weighted mean differences with 95% confidence intervals using the generic inverse variance method modelled by random effects meta-analyses. To allow the pooled effect estimates for each end point to be displayed on the same axis, mean differences were transformed to standardised mean differences (SMDs). Pseudo-95% confidence intervals for each transformed SMD were derived directly from the original mean difference and 95% confidence intervals. Between study heterogeneity was assessed by the Cochran Q statistic, where P<0.10 is considered statistically significant, and quantified by the I2 statistic, where I2 ≥50% is considered evidence of substantial heterogeneity.51 The grading of recommendations, assessment, development, and evaluation (GRADE) of randomised controlled trials are rated as “high” certainty of evidence and can be downgraded by five domains and upgraded. The filled black squares indicate downgrade or upgrade for each outcome. *For interpretation of the magnitude, we used the minimally important differences for each outcome and dose-response analyses to assess the importance of magnitude of our point estimate using the effect size categories according to new GRADE guidance (see supplemental methods and supplemental tables S5 and S10). †Not upgraded for dose-response (see supplemental table S10 for details). ‡Owing to the difference in directionality of high density lipoprotein cholesterol compared with the other outcomes with regards to signal for benefit or harm, the sign for the SMD was changed. §The interpretation of the magnitude of the effect was based on the positive linear dose-response gradient (see supplemental table S10 for details). To convert blood glucose to mg/dL, multiply by 18.02; LDL-C, non-HDL-C, and HDL-C to mg/dL, multiply by 38.67; to convert triglycerides to mg/dL, multiply by 88.57; to convert CRP to nmol/L, divide by 9.52. Apo B=apolipoprotein B; CI=confidence interval; CRP=C reactive protein; DBP=diastolic blood pressure; HbA1c=glycated haemoglobin; HDL-C=high density lipoprotein cholesterol; LDL-C=low density lipoprotein cholesterol; MD=mean difference; non-HDL-C=non-high density lipoprotein cholesterol; PMD=P value of the mean difference; PQ=P value of the heterogeneity; SBP=systolic blood pressure; SMD=standardised mean difference; TG=triglycerides
Secondary outcomes
Figure 2 and supplemental figures S4-16 show the effect of low GI/GL dietary patterns on cardiometabolic outcomes. Low GI/GL diets showed moderate reductions in non-HDL-C (mean difference −0.20 mmol/L (95% confidence interval −0.33 to −0.07), P=0.002; substantial heterogeneity, I2=70%, P<0.001); small important reductions in LDL-C (−0.17 mmol/L (−0.25 to −0.08), P<0.001; substantial heterogeneity, I2=70%, P<0.001), apo B (−0.05 g/L (−0.09 to −0.01), P=0.03; substantial heterogeneity, I2=58%, P=0.03), triglycerides (−0.09 mmol/L (−0.17 to −0.01), P=0.04; no substantial heterogeneity, I2=44%, P=0.01), body weight (−0.66 kg (−0.90 to −0.42), P<0.001; no heterogeneity, I2=0%, P=1.0), BMI (−0.38 (−0.64 to −0.13), P=0.003; no heterogeneity, I2=0%, P=1.0), and trivial reductions in fasting blood glucose (−0.36 mmol/L (−0.42 to −0.19), P<0.001; substantial heterogeneity; I2=54%, P<0.001) and CRP (−0.41 mg/L (−0.78 to −0.04), P=0.03; no substantial heterogeneity, I2=24%, P=0.26). Other secondary outcomes demonstrated non-significant improvements.
Medication use
Supplemental table S7 presents the data available for the reporting of changes in medication or insulin use. Of the 29 trials, 10 reported changes in hyperglycaemia drugs or insulin use, in which two showed a significant reduction in their use for low GI/GL diets compared with control,28 57 four showed a significant reduction within the low GI/GL diet interventions which was not statistically different from control,26 27 55 63 and four showed no change in medication or insulin use within or between the low GI/GL and control diets.31 56 59 60
Adverse events
Adverse events were reported in four trials. One trial by Giacco et al59 reported a statistically significant reduction in hypoglycaemic events (0.73 ±0.7 v 1.5 ±1.2 events per patient per month; P<0.01). Giacco et al59 also reported gastrointestinal side effects, recording that 56% of participants treated with a high fibre/low GI diet had some minor gastrointestinal side effects (flatulence, meteorism, and diarrhoea) in comparison with 40% of the those treated with low fibre/higher GI diets (P>0.05). None of these episodes resulted in participant withdrawals. A trial by Jenkins et al, 200863 showed that more hypoglycaemic symptoms/low blood glucose levels were found in a subset of those who had to reduce their hyperglycaemia drugs on low GI/GL diets compared with control diets (6/106 and 0/104 participants, respectively). The remaining two trials by Jenkins et al, 2014 and Gilbertson et al, 2001 showed no differences in hypoglycaemic events between diets.26 60
Acceptability
Supplemental table S8 presents the data available for the seven trials reporting acceptability. Three trial comparisons26 28 60 reported a preference for the low GI diets and the other four65 67 68 reported that both diets were equally acceptable.
Sensitivity and subgroup analyses
Supplemental figures S17-S30 show influence analyses, in which systematic removal of individual trials altered the results. Removal of single trial comparisons resulted in changes in a gain of significance in the pooled effect estimate for the decrease in waist circumference26; loss of significance for the decrease in triglycerides22 23 26 27 56 57 71 and apoB,62 68 although the direction of the pooled effect estimate still favoured low GI/GL diets; and partial explanation of the evidence of substantial heterogeneity for HbA1c,21 fasting glucose,21 27 68 non-HDL-C,65 HDL-C,22 27 63 apoB,71 waist circumference,26 and SBP and DBP.27
Supplemental table S9 shows sensitivity analyses in which we used different correlation coefficients (0.25 and 0.75) for paired analyses to calculate standard errors. None of the correlation coefficients altered the conclusions for any outcome.
Supplemental figures S31-S62 present the subgroup analyses conducted for all outcomes except apo B, SBP and DBP, and CRP (<10 trial comparisons). For HbA1c, evidence of significant effect modification by baseline HbA1c (P=0.02) was seen in categorical analyses, where significantly greater reductions were found in HbA1c in those trials with greater baseline HbA1c (≥7.7%); in funding (P=0.003), where those not reporting the funding source (two trial comparisons) showed greater reductions than the other categories of funding; and allocation concealment (P=0.04) and blinding (P=0.04), where those studies rated as unclear had greater reductions than those rated as low, although both categories were significant.
For the secondary outcomes fasting insulin, LDL-C, non-HDL-C, HDL-C, triglycerides, and DBP, significant effect modification was observed by at least one of the following: age, baseline outcome level, funding source, allocation concealment, blinding, incomplete outcome reporting, absolute test fibre, and difference in fibre. No effect modification was found by type of diabetes. None of these subgroup effects altered the evidence for heterogeneity for any outcome. No effect modification was found by presence of a washout phase in crossover trials on any outcome except for LDL-C (P=0.03), where seven crossover trials with a washout showed significant reduction, whereas five trials without a washout showed no effect, a trend seen similarly for non-HDL-C (P=0.05). The opposite was found for triglycerides (P=0.002), however, where the reduction was greater in those crossover trials without a washout phase (n=7).
Dose-response analyses
Supplemental figures S63-S70 present linear and non-linear dose-response analyses, and supplemental figures S31-S49 present categorical subgroups analyses for GI and GL. For HbA1c, a significant linear dose-response was seen for difference in GL (P=0.032). For the dose-response analyses of GI, there was a positive linear association with absolute test GI (prescribed or achieved in trial GI in the low GI/GL diets) and SBP, where trials with lower dietary GI saw greater reductions in SBP (which influenced the interpretation of the magnitude of effect, fig 2). Similar non-significant trends were seen for HbA1c, non-HDL-C, apo B, triglycerides, and body weight (P values ranged from 0.122 to 0.301). A non-linear bell shaped association was seen for absolute test GI and fasting glucose (P=0.01) and waist circumference (P=0.002). For difference in GI, no associations were found for any outcome. For the dose-response analyses of GL, for absolute test GL, a non-linear bell shaped association for HDL-C (P=0.04) was seen and a U shaped association for waist circumference (P<0.001). For difference in GL, a non-linear U shaped association was seen for triglycerides (P=0.03) and waist circumference (P<0.001) and a negative linear association for DBP (P=0.01). The associations for triglycerides and DBP were driven by outliers. The association for triglycerides became a significant positive linear dose-response gradient with the removal of a single outlier of effect64 (P=0.04) (which influenced the interpretation of the magnitude of effect, fig 2), and the association for DBP became non-significant with the removal of a single extreme outlier of exposure61 (P=0.07).
Publication bias
Supplementary figures S71–S74 show the assessments for publication bias for all outcomes. No evidence for funnel plot asymmetry was found, and Egger’s and Begg’s tests showed no evidence of small study effects (P>0.05) for HbA1c, or for any secondary outcome except fasting glucose and insulin (P<0.05, Egger’s test). The trim and fill method (supplemental fig S72) shows no evidence of small study effects for fasting glucose, but some evidence for fasting insulin, where the imputation of five trials altered the significance (mean difference −6.68 pmol/L (95% confidence interval −11.99 to −1.37), P=0.01). Publication bias was not assessed for apo B, SBP and DBP, and CRP (<10 trial comparisons).
GRADE assessment
Figure 2 and supplemental table S10 present the GRADE assessments of the overall certainty of the evidence for the effect of low GI/GL dietary patterns on cardiometabolic outcomes. The evidence was graded as high for the primary outcome HbA1c owing to a downgrade for imprecision and an upgrade for a dose-response. The evidence for most secondary outcomes was graded as moderate owing to downgrades for imprecision, except HDL-C which was graded as high, and insulin, LDL-C, and waist circumference which were graded as low owing to downgrades for either inconsistency, imprecision, or evidence of publication bias from small study effects.
Discussion
This systematic review and meta-analysis included 27 randomised controlled trials (29 trial comparisons) in 1617 participants with type 1 or 2 diabetes, who were predominantly middle aged, overweight, or obese with moderately controlled type 2 diabetes treated by hyperglycaemia drugs or insulin. We showed that low GI/GL dietary patterns in comparison with higher GI/GL control diets provide small important benefits for glycaemic control and other established cardiometabolic risk factors over a median follow-up of 12 weeks. An improvement was seen in the primary outcome and primary target of glycaemic control HbA1c of 0.31% with a significant positive linear dose-response gradient for difference in GL showing a reduction of −0.04% HbA1c units per 10 unit reduction in GL. Further improvements were seen in several secondary outcomes, including other established targets of glycaemic control (fasting glucose −0.36 mmol/L); blood lipids (LDL-C −0.17 mmol/L; non-HDL-C −0.20 mmol/L; triglycerides −0.09 mmol/L; apo B −0.05g/L); adiposity (body weight −0.66 kg; BMI −0.38); SBP (positive linear dose-response gradient showing a reduction of 0.49 mm Hg per unit reduction of GI) and inflammation (CRP −0.41 mg/L). A limited number of trials also showed that low GI/GL dietary patterns were preferred or equally acceptable to control diets, without any adverse effects.
Findings in the context of the literature
Our findings provide a comprehensive update on previous systematic reviews and meta-analyses. The last comprehensive analysis in diabetes, which was published in 2010,7 8 also showed a reduction in HbA1c (−0.5%). With similar inclusion criteria, but with a census date of March 2009, it included only 12 randomised controlled trial comparisons, of which only seven reported data for HbA1c. Thus, our 29 trial comparisons, including 22 trial comparisons for HbA1c, provide an update to support a role of low GI/GL diets for glycaemic control in diabetes. Other similar analyses published since 2010 were not comprehensive owing to restrictions in search dates or inclusion criteria, thus including only 3-15 trials. Despite their limitations, each showed important improvements in glycaemic control in diabetes.9 10 11 Thus evidence consistently shows that low GI/GL dietary patterns can improve glycaemic control in comparison with higher GI/control diets.
The effects observed for secondary outcomes are generally supported by previous systematic reviews and meta-analyses. One synthesis by Goff et al of 23 randomised trials comparing low with high GI diets over at least four weeks (14 with diabetes) found similar LDL-C reductions, with no effect found on HDL-C or triglycerides.72 Similarly, another synthesis by Zafar et al of 36 randomised trials over one or more weeks in diabetes and impaired glucose tolerance found a reduction in LDL-C with no effect on HDL-C or triglycerides.73 In contrast to our synthesis, which showed a positive linear dose-response gradient for difference in GL and triglycerides, these two meta-analyses did not find a significant effect on triglycerides, although there was a tendency for a reduction; however, those studies focused on low GI interventions only. This difference suggests that the improvement in triglycerides requires the combination of lower GI and lower carbohydrate intake. The meta-analysis by Zafar et al also showed in 42 trials a reduction in body weight and BMI. Another synthesis of 14 long term randomised trials (≥6 months), four trials in type 2 diabetes, of low GI/GL diets reported a non-significant reduction in body weight compared with higher GI/GL diets.74 Our observed anti-inflammatory effect is supported by previous work,75 particularly with longer follow-up.74 The positive linear dose-response gradient observed for absolute prescribed or achieved in trial dietary GI and SBP found in the present synthesis is supported by a systematic review and meta-analysis of 13 trials in healthy participants (not taking drugs for hypertension), commissioned by the Scientific Advisory Committee on Nutrition (SACN), which demonstrated reductions in SBP and DBP for GI and GL.76 Evidence for the effect of low GI/GL diets on insulin is mixed.73 74
The reductions in intermediate cardiometabolic risk factors seen with low GI/GL dietary patterns align with the reductions in clinical events seen in prospective cohort studies. Systematic reviews and meta-analyses of prospective cohort studies have shown reduced incidence of diabetes and cardiovascular disease. A recent random effects dose-response meta-analysis of 24 prospective studies with validated instruments (correlation >0.55) for carbohydrate, showed a 27% and 26% greater incidence of type 2 diabetes per 10 unit increase in GI and per 80 g/d increase in GL, respectively, and relative risks for global dose-response meta-analyses were 1.87 and 1.89 for GI (range 47.6-76.1 GI units) and GL (range 73-257 g/d GL in an 8368 kJ (2000 kcal) diet), respectively.13 The same dose-response meta-analysis was conducted looking at the risk of coronary heart disease and showed relative risks of 2.71 and 5.5 across the global range for GI (47-82 GI units) and GL (55-290 g/d), respectively.14 Another meta-analysis similarly demonstrated significantly higher risk with higher GI/GL diets for both cardiovascular disease16 and the incidence of heart disease.15
The association between GI and GL and cardiovascular disease was most recently explored in a large international cohort of 137 851 participants aged between 35 and 70 years living on five continents, with a median follow-up of 9.5 years.77 The Prospective Urban Rural Epidemiology (PURE) study found that a diet with a high GI was associated with an increased risk of a major cardiovascular event or death, both among participants with pre-existing cardiovascular disease (hazard ratio 1.51, 95% confidence interval 1.25 to 1.82) and among those without such disease (1.21, 1.11 to 1.34) compared with diets with a low GI.77 The results were similar for GL among the participants with cardiovascular disease at baseline, but the association was not significant among those without pre-existing cardiovascular disease.77 This study showed that the associations found in the meta-analyses, which principally include cohorts from Western countries, are also found in non-Western countries with low or middle incomes. The study also examined the outcomes among participants according to the presence or absence of pre-existing cardiovascular disease, allowing the exploration of associations with implications for both primary and secondary prevention strategies.
Our synthesis shows that a focus on both carbohydrate quality and quantity through low GI/GL dietary patterns might have similar or broader benefits than a focus on carbohydrate quantity alone. An earlier DNSG commissioned systematic review and meta-analysis of low carbohydrate diets (defined as interventions encouraging diets with carbohydrates as <40% of energy) compared with higher carbohydrate diets (defined as control diets encouraging carbohydrates as >40% of energy) in participants with type 2 diabetes found a trivial effect on HbA1c with no differences in other measures of glycaemic control, blood lipids, blood pressure, or measures of adiposity.78 Other systematic reviews and meta-analyses showed reductions in HbA1c, fasting glucose, and triglycerides over the shorter term (3-6 months) but not the longer term (≥12 months), with no consistent evidence of reductions in body weight or LDL-C using lower carbohydrate diets (defined as <47% of energy).79 These studies also showed higher diabetes remission, with important improvements in weight loss, triglycerides, and insulin sensitivity over the shorter term (≤6-months), which diminished at 12 months in those following low carbohydrate diets (defined as diets with <26% of energy as carbohydrates) but not very low carbohydrate diets (defined as diets with <10% of energy as carbohydrates).80 This synthesis also had an important signal for harmful increases in LDL-C at 12 months.80 On the other hand, our synthesis shows that low GI/GL dietary patterns produce similar improvements in HbA1c and related cardiometabolic risk factors, with the addition of reductions not seen with lower carbohydrate diets in LDL-C, non-HDL-C, apo B, SBP, and CRP, which are established targets for cardiovascular risk reduction.
Acarbose, an oral α-glucosidase inhibitor that effectively converts the diet to a low GI/GL dietary pattern, provides a biological analogy81 to support the ability of low GI/GL dietary patterns to improve clinical outcomes.82 Individual randomised controlled trials83 84 85 and systematic reviews and meta-analyses of randomised controlled trials86 87 have shown that acarbose reduces the incidence of diabetes, hypertension, cardiovascular disease, myocardial infarction, and stroke in people at risk for type 2 diabetes,83 84 85 87 and myocardial infarction and cardiovascular disease in people with type 2 diabetes,86 reductions which have estimates and 95% confidence intervals that contain those seen for the association of low GI dietary patterns with the same clinical outcomes13 14 15 16 and correspond with improvements in glycaemic control similar to those seen in our synthesis. Supplemental Table S11 presents various other mechanisms supporting the effects observed in our analyses.
One of the longstanding criticisms of the GI is the inability to disentangle the effects of a low GI dietary pattern from the individual components that it contains, especially dietary fibre.5 We were able to assess the interaction by fibre in the available trials. No interaction by fibre was found on the primary outcome HbA1c or any of the secondary outcomes related to glycaemic control. Exceptions were other secondary cardiometabolic outcomes, including LDL-C, non-HDL-C, and DBP, where higher fibre intakes on the low GI/GL diets or greater differences in fibre between the low GI/GL and control diets were associated with reductions (P<0.05), possibly related to the intake of viscous fibre. Another exception was for triglycerides, where a higher difference in fibre between the low GI/GL and control diets was associated with a smaller reduction in triglycerides, which might reflect higher carbohydrate intakes. These findings indicate that fibre does not contribute to the beneficial effects on glycaemic control achieved with low GI/GL diets but might relate to improvements in lipids and blood pressure. The underlying criticism that one cannot disentangle the effects of a dietary pattern from its components is applicable to any dietary pattern—for example, Mediterranean, vegetarian, Portfolio, Nordic, Dietary Approaches to Stop Hypertension (DASH), low carbohydrate, and others. The same criticism applies equally to fibre itself, as foods which are rich in fibre also contain vitamin B6, thiamine, folate, vitamin E, magnesium, and other trace minerals and bioactive agents—in particular, phenolic compounds and antioxidants.88 89 90 91
Strengths and limitations
The strengths of the analyses include a rigorous search and selection strategy allowing comprehensive identification of all eligible studies; inclusion of primarily high quality randomised controlled trials providing the highest protection against bias; use of intention to treat data, when available, providing more conservative pooled estimates,92 and using the GRADE approach to assess the overall certainty of evidence.
Our analyses had several limitations. Firstly, the evidence indicated serious inconsistency for the effect of low GI/GL dietary pattens on LDL-C and waist circumference. No inconsistency was present for other outcomes.
Secondly, potential for indirectness was seen in some of the analyses. Few trial comparisons were available in children (two trials) and people with type 1 diabetes (five trials). Removal of the trials in children did not alter the estimates for any outcome and there was no effect modification in subgroup analyses by type of diabetes for any outcome and so we did not downgrade for serious indirectness in either case. Our findings, however, remain most relevant to adults with type 2 diabetes. The relative lack of high GI comparator diets was another potential source of indirectness. The median GI achieved in trial across low GI interventions was 49, whereas it was 63 across the higher GI control diets, with a median difference of more than 10 GI units. Although these GI values suggest that the comparisons were between low GI/GL and medium GI/GL diets, we did not downgrade for serious indirectness, as probably these medium GI control diets would have led only to an underestimation of the true effect of low GI/GL diets, with larger effect sizes expected for their intended substitution with high GI/GL control diets.
Thirdly, the evidence indicated serious imprecision in the pooled estimates across most outcomes. The 95% confidence intervals were wide and could not rule out clinically trivial effects for all outcomes, although they did not contain harm. Instability was seen in the significance of the pooled effect estimates, with the removal of single trial comparisons during sensitivity analyses changing the significance from non-significant to significant for waist circumference and significant to non-significant for triglycerides and apo B. Several cases of imprecision, however, were explained by linear dose-response gradients (HbA1c, triglycerides, and SBP), suggesting that trials with lower prescribed or in trial achieved GI/GL and larger differences in GI/GL (test−control) might produce more precise clinically important reductions. Finally, evidence of small study effects was found for the effect on insulin. Although the overall effect on fasting insulin was not significant, trim-and-fill analyses showed evidence of small study effects, with additional imputed trial comparisons resulting in a significant reduction in insulin. The small number of available trial comparisons (<10) for blood pressure and inflammatory markers meant we were unable to conduct publication bias analyses for these outcomes.
Weighing these strengths and limitations, we graded the certainty in the evidence as high for HbA1c and moderate for most other outcomes, with the exception of high for HDL-C and low for fasting insulin, LDL-C, and waist circumference.
Implications
Diet and lifestyle remain the cornerstone of the management of diabetes. Our synthesis shows that low GI/GL dietary patterns are considered an acceptable and safe dietary strategy that can produce small meaningful reductions in the primary target for glycaemic control in diabetes, HbA1c, fasting glucose, and other established cardiometabolic risk factors. The pooled in trial achieved reduction in HbA1c of −0.31% would meet the threshold of ≥0.3% reduction in HbA1c proposed by the European Medicines Agency as clinically relevant for risk reduction of diabetic complications.93 This effect was observed beyond concurrent hyperglycaemia drugs or insulin, which was reduced in many of the included trial comparisons.26 27 28 55 57 63 Thus low GI/GL dietary patterns might be an especially helpful lifestyle strategy for those with type 2 diabetes as it might assist in the management of glycaemic control as add-on treatment to hyperglycaemia drugs while at the same time reducing the need for these drugs. Given the ultimate target of glycaemic control in those with diabetes is reducing cardiovascular events as the leading cause of death in this population,94 it is likely to be achieved not only by the clinically significant reduction in HbA1c, which previous systematic reviews and meta-analyses of trials have shown,95 96 97 but also by the 0.17 mmol/L reduction (~6%) in LDL-C, which it is predicted would translate to about a 6% risk reduction in major cardiovascular events.98 99 Therefore, there is an important opportunity for those with diabetes to achieve the glycaemic and cardiometabolic advantages of adopting low GI/GL dietary patterns.
Conclusions
In conclusion, our synthesis supports existing recommendations for the use of low GI/GL dietary patterns in the management of diabetes. The available evidence shows that low GI/GL dietary patterns might have advantages for reducing the primary target for glycaemic control, HbA1c, as well as fasting glucose and other established cardiometabolic risk factors beyond concurrent treatment with hyperglycaemia drugs or insulin in predominantly adults with moderately controlled type 1 and type 2 diabetes. Our confidence in the evidence was high for small clinically important reductions in HbA1c and moderate for most cardiometabolic risk factors, suggesting the available evidence provides a good indication of the likely benefit in this population.
The main source of uncertainty, imprecision, should be considered by further large high quality randomised controlled trials, which target lower GI/GL diets with bigger differences between test and control. To confirm whether the improvements in intermediate cardiometabolic risk factors translate to reductions in clinical outcomes, larger randomised trials are needed in those with diabetes of the effect of low GI/GL dietary patterns on outcomes of cardiovascular disease, nephropathy, and retinopathy. We await the results of the Low Glycemic Index Diet for Type 2 Diabetes trial (NCT01063374), a randomised trial of the effect of a low GI dietary pattern on the progression of atherosclerosis by vascular MRI over three years in 169 high risk participants with type 2 diabetes and subclinical atherosclerosis (carotid intima media thickness ≥1.2 mm).100
What is already known on this topic
Previous systematic reviews and meta-analyses have shown that low glycaemic index (GI)/glycaemic load (GL) dietary patterns improve glycaemic control and cardiometabolic risk factors in randomised controlled trials in people at risk for, and with, diabetes and are associated with reduced incidence of diabetes and cardiovascular disease in prospective cohort studies inclusive of people with diabetes
These benefits are recognised by major international clinical practice guidelines in Canada, US, Australia, UK, and Europe, with low GI/GL dietary patterns recommended for those with diabetes
The last comprehensive systematic review and meta-analysis in diabetes was published in 2010, but lacked a GRADE (grading of recommendations assessment, development, and evaluation) assessment for certainty of evidence, and numerous randomised controlled trials have been published after the census for these syntheses
What this study adds
The available evidence suggests that low GI/GL dietary patterns result in small clinically significant reductions in the primary target of glycaemic control HbA1c, and small clinically meaningful improvements in other established cardiometabolic risk factors (blood lipids, body weight, blood pressure, inflammation) in moderately controlled type 1 and type 2 diabetes
As these benefits are seen beyond concurrent treatment with hyperglycaemia drugs or insulin, low GI/GL dietary patterns might be especially helpful as add-on treatment to help individuals with type 1 and type 2 diabetes achieve their targets for glycaemic control and cardiometabolic risk factors
This synthesis includes new data, expands the number of relevant intermediate cardiometabolic outcomes, and assesses the certainty of the evidence using GRADE, providing an update to the last EASD clinical practice guidelines published over 15 years ago and the last systematic review and meta-analysis of low GI/GL dietary patterns in diabetes published over a decade ago
Web extra.
Extra material supplied by authors
Web appendix: Supplemental material
Contributors: LC and JLS had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. LC and JLS were responsible for the study concept and design. LC, DL, AA, AC, TAK, SBM, and JLS were responsible for the data acquisition and analyses. All authors contributed to interpretation of the findings. LC drafted the manuscript. All the authors contributed to critical revision of the manuscript for important intellectual content. LC and TAK conducted the statistical analyses. JLS supervised the study. JLS is the study guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD) commissioned this systematic review and meta-analysis and provided funding and logistical support for meetings as part of the development of the EASD Clinical Practice Guidelines for Nutrition Therapy. This work was also supported by the Canadian Institutes of Health Research (funding reference number 129920) through the Canada-wide Human Nutrition Trialists' Network (NTN). The Diet, Digestive tract, and Disease (3D) Centre, funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund, provided the infrastructure for the conduct of this work. LC was funded by a Mitacs-Elevate postdoctoral fellowship award. DL was funded by a St Michael’s Hospital Research Training Centre Scholarship (runner-up). AA was funded by a Toronto 3D MSc scholarship award. TAK was funded by a Toronto 3D postdoctoral fellowship award. DJAJ was funded by the government of Canada through the Canada research chair endowment. JS-S is partially supported by the Catalan Institution for Research and Advanced Studies (ICREA) under the ICREA academia programme. JLS was funded by a PSI Graham Farquharson knowledge translation fellowship, Diabetes Canada clinician scientist award, INMD-CNS New Investigator Partnership Prize, and Banting and Best Diabetes Centre Sun Life financial new investigator award. With the exception of the DNSG of the EASD guidelines committee, none of the sponsors had a role in any aspect of this study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, approval of the manuscript, or decision to publish. All authors had full access to all the data in the study and accept responsibility to submit for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from Diabetes Canada and the Diet, Digestive tract, and Disease Centre through the Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund for the submitted work. LC is a Mitacs-Elevate postdoctoral fellow jointly funded by the government of Canada and the Canadian Sugar Institute. She was previously employed as a casual clinical coordinator at INQUIS Clinical Research, Ltd (formerly Glycemic Index Laboratories, Inc), a contract research organisation. TAK has received research support from the Canadian Institutes of Health Research (CIHR), the International Life Science Institute (ILSI), and National Honey Board. He has been an invited speaker at the Calorie Control Council annual meeting for which he has received an honorarium. DJAJ has received research grants from Saskatchewan and Alberta Pulse Growers Associations, the Agricultural Bioproducts Innovation Programme through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd, Unilever Canada and Netherlands, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg's Company, Canada, Quaker Oats, Canada, Procter and Gamble Technical Centre Ltd, Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut and Dried Fruit Council (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute (SNI), the Canola and Flax Councils of Canada, the Calorie Control Council, CIHR, the Canada Foundation for Innovation (CFI) and the Ontario Research Fund (ORF). He has received in-kind supplies for trials as a research support from the Almond board of California, Walnut Council of California, the Peanut Institute, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (PepsiCo), Pristine Gourmet, Bunge Limited, Kellogg Canada, WhiteWave Foods. He has been on the speaker's panel, served on the scientific advisory board and received travel support and honoraria from 2020 China Glycemic Index (GI) International Conference, Atlantic Pain Conference, Academy of Life Long Learning, the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, Epicure, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative (THI), Heali AI Corp, Institute of Food Technologists (IFT), SNI, Herbalife Nutrition Institute (HNI), Saskatchewan and Alberta Pulse Growers Associations, Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamentals for Health (NFH), Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter and Gamble, Abbott Laboratories, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Programme, the Toronto Knowledge Translation Group (St Michael's Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation, and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry, his two daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the foods described here, The Portfolio Diet for Cardiovascular Risk Reduction (Academic Press/Elsevier 2020 ISBN:978-0-12-810510-8) and his sister, Caroline Brydson, received funding through a grant from the St Michael's Hospital Foundation to develop a cookbook for one of his studies. GL reports non-financial conflicts of interest from Independent Nutrition Logic Ltd (UK), International Carbohydrate Quality Consortium (CA), EASD Nutrition Guidelines Committee (EU), and personal fees from Beneo Institute (DE) outside the submitted work. He is director and holds shares in Independent Nutrition Logic Ltd, a consultancy. He and his wife have benefited from research grants, travel funding, consultant fees, and honoraria from the American Association for the Advancement of Science (USA), the All Party Parliamentary Group for Diabetes (London, UK), Almond Board of California (USA), BENEO GmbH (DE), Biotechnology and Biosciences Research Council (UK), British Nutrition Foundation (UK), Calorie Control Council (USA), Cantox (CA), Colloides Naturel International (FR), Coca Cola (UK), Danisco (UK and Singapore), Diabetes Nutrition Study Group (EASD, EU), DiabetesUK (UK), Elsevier Inc. (USA), European Commission (EU), European Polyol Association (Brussels), Eureka (UK), Food and Agricultural Organisation (Rome), Granules India (Ind), General Mills (USA), Health Canada (CA), Institute of Food Research (UK), International Carbohydrate Quality Consortium (CA), Institute of Medicine (Washington, DC), International Life Sciences Institute (EU and USA), Life Sciences Research Office, FASEB (USA), Nutrition Society of Australia, Knights Fitness (UK), Leatherhead Food Research (UK), LitghterLife (UK), Matsutani (JPN), Medical Research Council (UK), MSL Group (UK), Porter Novelli (UK), Sudzuker (DE), Sugar Nutrition/WSRO (UK), Tate and Lyle (UK), The Food Group (USA), WeightWatchers (UK), Wiley-Blackwell (UK), and the World Health Organisation (Geneva). He and his wife Helen Livesey have benefited from services of the Royal Society of Medicine (UK), Sense Nutrition Consultancy Group (UK), Acumentia Bioscience Consultancy Group UK, and memberships of the Nutrition Society of Great Britain, The Association for Nutrition (UK), The American Nutrition Society and the Canadian Nutrition Society (CA). GL is a professional member of Diabetes UK, and a Fellow of the Royal Society of Medicine (UK). TMSW is part owner and employee of INQUIS Clinical Research, Ltd (formerly Glycemic Index Laboratories, Inc), a contract research organisation. DR is director of Vuk Vrhovac University Clinic for Diabetes, Endocrinology and Metabolic Diseases at Merkur University Hospital, Zagreb, Croatia. He is the president of Croatian Society for Diabetes and Metabolic Disorders of Croatian Medical Association. He serves as an executive committee member of Croatian Endocrine Society, Croatian Society for Obesity and Croatian Society for Endocrine Oncology. He was a board member and secretary of International Diabetes Federation (IDF) Europe and currently he is the chair of the IDF Young Leaders in Diabetes Programme. He has served as an executive committee member of the Diabetes and Nutrition Study Group of EASD and currently he serves as an executive committee member of Diabetes and Cardiovascular Disease Study Group of EASD. He has served as principal investigator or co-investigator in clinical trials of AstraZeneca, Eli Lilly, MSD, Novo Nordisk, Sanofi Aventis, Solvay, and Trophos. He has received travel support, speaker fees, and honoraria from advisory board engagements and consulting fees from Abbott, Amgen, AstraZeneca, Bayer, Belupo, Boehringer Ingelheim, Eli Lilly, Lifescan – Johnson and Johnson, International Sweeteners Association, Krka, Medtronic, Mediligo, Mylan, Novartis, Novo Nordisk, MSD, Merck Sharp and Dohme, Pfizer, Pliva, Roche, Salvus, Sandoz, Solvay, Sanofi Aventis, and Takeda. HK works as director of clinical research at the Physicians Committee for Responsible Medicine, a non-profit organisation that provides nutrition education and research. JS-S reports serving on the board of and receiving grant support through his institution from the INC and the Eroski Foundation. He reports serving on the executive committee of the Instituto Danone Spain. He reports receiving research support from the Instituto de Salud Carlos III, Spain; Ministerio de Educación y Ciencia, Spain; Departament de Salut Pública de la Generalitat de Catalunya, Catalonia, Spain; European Commission; California Walnut Commission, Sacramento, CA, USA; Patrimonio Comunal Olivarero, Spain; La Morella Nuts, Spain; and Borges SA, Spain. He reports receiving consulting fees or travel expenses from Danone, California Walnut Commission, Eroski Foundation, Instituto Danone—Spain, Nuts for Life, Australian Nut Industry Council, Nestlé, Abbot Laboratories, and Font Vella Lanjarón. He is on the Clinical Practice Guidelines Expert Committee of EASD, and served on the Scientific Committee of the Spanish Food and Safety Agency, and the Spanish Federation of the Scientific Societies of Food, Nutrition and Dietetics. He is a member of the ICQC and an executive board member of the DNSG of EASD. CWCK has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada (AAFC), Almond Board of California, Barilla, CIHR, Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd, the Peanut Institute, Pulse Canada, and Unilever. He has received in-kind research support from the Almond Board of California, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Nutrartis, Quaker (PepsiCo), the Peanut Institute, Primo, Unico, Unilever, WhiteWave Foods/Danone. He has received travel support and honoraria from the Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organisation, Lantmannen, Loblaw Brands Ltd, Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, the Peanut Institute, Pulse Canada, Sun-Maid, Tate and Lyle, Unilever, and White Wave Foods/Danone. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organisation, McCormick Science Institute, and Oldways Preservation Trust. He is a founding member of the ICQC, executive board member of the DNSG of EASD, is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of EASD and is a director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, CIHR, Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), ASN, INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, National Honey Board (the US. Department of Agriculture (USDA) honey “Checkoff” programme), ILSI, Pulse Canada, Quaker Oats Center of Excellence, The United Soybean Board (the USDA soy “Checkoff” programme), The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), and The Nutrition Trialists fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received in-kind food donations to support a randomised controlled trial from the Almond Board of California, California Walnut Commission, Peanut Institute, Barilla, Unilever/Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods/Danone, and Nutrartis. He has received travel support, speaker fees, and honoraria from Diabetes Canada, Dairy Farmers of Canada, FoodMinds LLC, International Sweeteners Association, Nestlé, Pulse Canada, Canadian Society for Endocrinology and Metabolism (CSEM), GI Foundation, Abbott, General Mills, Biofortis, ASN, Northern Ontario School of Medicine, INC Nutrition Research and Education Foundation, European Food Safety Authority (EFSA), Comité Européen des Fabricants de Sucre (CEFS), Nutrition Communications, International Food Information Council (IFIC), Calorie Control Council, and Physicians Committee for Responsible Medicine. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate and Lyle, Wirtschaftliche Vereinigung Zucker e.V., Danone, and INQUIS Clinical Research. He is a member of the European Fruit Juice Association Scientific Expert Panel and former member of the SNI Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, EASD, Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves or has served as an unpaid scientific adviser for the Food, Nutrition, and Safety Programme (FNSP) and the Technical Committee on Carbohydrates of ILSI North America. He is a member of the ICQC, executive board member of DNSG of EASD, and director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of AB InBev. DL, AA, AC, SBM and AM declare no competing interests. There are no products in development or marketed products to declare.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: There are plans to disseminate the results to relevant patient and clinician communities. This systematic review and meta-analysis will directly inform the update of the EASD clinical practice guidelines for nutrition therapy and any translation efforts that results from this guideline development, including the development of low glycaemic index symbol programmes, education portal, and food guide. The clinical practice guidelines and associated knowledge translation tools will be disseminated to the diabetes community in Europe through EASD channels. Member EASD countries might use these outputs to develop their own clinical practice guidelines and knowledge translation tools. We will also disseminate the results of this systematic review and meta-analysis and these outputs through our contacts at Diabetes Canada, Obesity Canada, and the Canadian Cardiovascular Society.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
No additional data are available.
References
- 1.Diabetes Canada Clinical Practice Guidelines Expert C . Sievenpiper JL, Chan CB, Dworatzek PD, Freeze C, Williams SL. Nutrition Therapy. Can J Diabetes 2018;42(Suppl 1):S64-79. [DOI] [PubMed] [Google Scholar]
- 2.Wolever TMS. The glycaemic index: A physiological classification of dietary carbohydrate. CABI, 2006: 1-227 10.1079/9781845930516.0000. [DOI] [Google Scholar]
- 3.Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol 2016;32:1263-82. 10.1016/j.cjca.2016.07.510 [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein AH, Appel LJ, Brands M, et al. American Heart Association Nutrition Committee . Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82-96. 10.1161/CIRCULATIONAHA.106.176158 [DOI] [PubMed] [Google Scholar]
- 5.Evert AB, Boucher JL, Cypress M, et al. American Diabetes Association . Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013;36:3821-42. 10.2337/dc13-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evert AB, Dennison M, Gardner CD, et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019;42:731-54. 10.2337/dci19-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr 2010;104:797-802. 10.1017/S0007114510001534 [DOI] [PubMed] [Google Scholar]
- 8.Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev 2009;(1):CD006296. 10.1002/14651858.CD006296.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojo O, Ojo OO, Adebowale F, Wang XH. The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018;10:E373. 10.3390/nu10030373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Xia W, Zhao Z, Zhang H. Effects comparison between low glycemic index diets and high glycemic index diets on HbA1c and fructosamine for patients with diabetes: A systematic review and meta-analysis. Prim Care Diabetes 2015;9:362-9. 10.1016/j.pcd.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 11.Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr 2013;97:505-16. 10.3945/ajcn.112.042457 [DOI] [PubMed] [Google Scholar]
- 12.Viguiliouk E, Nishi SK. TMS W, Sievenpiper J. Point: glycemic index an important but oft misunderstood marker of carbohydrate quality. Cereal Foods World 2018;63:158-64. [Google Scholar]
- 13.Livesey G, Taylor R, Livesey HF, et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies. Nutrients 2019;11:E1280. 10.3390/nu11061280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livesey G, Livesey H. Coronary Heart Disease and Dietary Carbohydrate, Glycemic Index, and Glycemic Load: Dose-Response Meta-analyses of Prospective Cohort Studies. Mayo Clin Proc Innov Qual Outcomes 2019;3:52-69. 10.1016/j.mayocpiqo.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirrahimi A, de Souza RJ, Chiavaroli L, et al. Associations of glycemic index and load with coronary heart disease events: a systematic review and meta-analysis of prospective cohorts. J Am Heart Assoc 2012;1:e000752. 10.1161/JAHA.112.000752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirrahimi A, Chiavaroli L, Srichaikul K, et al. The role of glycemic index and glycemic load in cardiovascular disease and its risk factors: a review of the recent literature. Curr Atheroscler Rep 2014;16:381. 10.1007/s11883-013-0381-1 [DOI] [PubMed] [Google Scholar]
- 17.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018;61:2461-98. 10.1007/s00125-018-4729-5 [DOI] [PubMed] [Google Scholar]
- 18.Mann JI, De Leeuw I, Hermansen K, et al. Diabetes and Nutrition Study Group (DNSG) of the European Association . Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis 2004;14:373-94. 10.1016/S0939-4753(04)80028-0 [DOI] [PubMed] [Google Scholar]
- 19.Diabetes UK Nutrition working group. Evidence-based nutrition guidelines for the prevention and management of diabetes. 2011. https://www.diabetes.org.uk/professionals/position-statements-reports/food-nutrition-lifestyle/evidence-based-nutrition-guidelines-for-the-prevention-and-management-of-diabetes.
- 20.Australia D. General practice management of type 2 diabetes – 2014-15. The Royal Australian College of General Practitioners and Diabetes Australia, 2014, https://www.diabetesaustralia.com.au/best-practice-guidelines. [Google Scholar]
- 21.Cai X, Wang L, Wang X, Liu S. Effect of high dietary fiber low glycemic index diet on intestinal flora, blood glucose and inflammatory response in T2DM patients. Biomed Res 2017;28:9371-5. [Google Scholar]
- 22.Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes Obes Metab 2010;12:204-9. 10.1111/j.1463-1326.2009.01151.x [DOI] [PubMed] [Google Scholar]
- 23.Fabricatore AN, Wadden TA, Ebbeling CB, et al. Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: a randomized controlled trial. Diabetes Res Clin Pract 2011;92:37-45. 10.1016/j.diabres.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes JMG, Fabrini SP, Alfenas RCG. Low glycemic index diet reduces body fat and attenuates inflammatory and metabolic responses in patients with type 2 diabetes. Arch Endocrinol Metab 2017;61:137-44. 10.1590/2359-3997000000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutschall MD, Miller CK, Mitchell DC, Lawrence FR. A randomized behavioural trial targeting glycaemic index improves dietary, weight and metabolic outcomes in patients with type 2 diabetes. Public Health Nutr 2009;12:1846-54. 10.1017/S1368980008004680 [DOI] [PubMed] [Google Scholar]
- 26.Jenkins DJ, Kendall CW, Vuksan V, et al. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: a randomized controlled trial. Diabetes Care 2014;37:1806-14. 10.2337/dc13-2990 [DOI] [PubMed] [Google Scholar]
- 27.Jenkins DJ, Kendall CW, Augustin LS, et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med 2012;172:1653-60. 10.1001/2013.jamainternmed.70 [DOI] [PubMed] [Google Scholar]
- 28.Ma Y, Olendzki BC, Merriam PA, et al. A randomized clinical trial comparing low-glycemic index versus ADA dietary education among individuals with type 2 diabetes. Nutrition 2008;24:45-56. 10.1016/j.nut.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavithran N, Kumar H, Menon AS, Pillai GK, Sundaram KR, Ojo O. The Effect of a Low GI Diet on Truncal Fat Mass and Glycated Hemoglobin in South Indians with Type 2 Diabetes-A Single Centre Randomized Prospective Study. Nutrients 2020;12:E179. 10.3390/nu12010179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visek J, Lacigova S, Cechurova D, Rusavy Z. Comparison of a low-glycemic index vs standard diabetic diet. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014;158:112-6. 10.5507/bp.2012.103 [DOI] [PubMed] [Google Scholar]
- 31.Yusof BN, Talib RA, Kamaruddin NA, Karim NA, Chinna K, Gilbertson H. A low-GI diet is associated with a short-term improvement of glycaemic control in Asian patients with type 2 diabetes. Diabetes Obes Metab 2009;11:387-96. 10.1111/j.1463-1326.2008.00984.x [DOI] [PubMed] [Google Scholar]
- 32.Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1, 2019 https://training.cochrane.org/handbook [DOI] [PMC free article] [PubMed]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilczynski NL, Morgan D, Haynes RB, Hedges Team . An overview of the design and methods for retrieving high-quality studies for clinical care. BMC Med Inform Decis Mak 2005;5:20. 10.1186/1472-6947-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bray GA, Popkin BM. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: health be damned! Pour on the sugar. Diabetes Care 2014;37:950-6. 10.2337/dc13-2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 37.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785-805. 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 38.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002;31:140-9. 10.1093/ije/31.1.140 [DOI] [PubMed] [Google Scholar]
- 40.Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769-73. 10.1016/0895-4356(92)90054-Q [DOI] [PubMed] [Google Scholar]
- 41.Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IF. Empirical Assessment of Within-Arm Correlation Imputation in Trials of Continuous Outcomes. Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
- 42.Ku H. Notes on the Use of Propagation of Error Formulas, J Research of National Bureau of Standards-C. Engineering and Instrumentation 1966;70C:263-73. [Google Scholar]
- 43.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559-73. 10.1002/sim.1187 [DOI] [PubMed] [Google Scholar]
- 44.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to metaanalysis. Wiley, 2008. [Google Scholar]
- 45.Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol 2011;64:1187-97. 10.1016/j.jclinepi.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 46.Harrell FEJ. Regression Modeling Strategies-with Applications to Linear Models, Logistic Regression, and Survival Analysis: Springer Series in Statistics. Springer, 2001, 10.1007/978-1-4757-3462-1. [DOI] [Google Scholar]
- 47.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008;61:991-6. 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 48.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 50.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 51.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 52.Schünemann H, Brożek J, Guyatt G, Oxman A, eds. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. GRADE Working Group, 2013. https://gdt.gradepro.org/app/handbook/handbook.html.
- 53.Santesso N, Glenton C, Dahm P, et al. GRADE Working Group . GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 2020;119:126-35. 10.1016/j.jclinepi.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 54.Brand JC, Colagiuri S, Crossman S, Allen A, Roberts DC, Truswell AS. Low-glycemic index foods improve long-term glycemic control in NIDDM. Diabetes Care 1991;14:95-101. 10.2337/diacare.14.2.95 [DOI] [PubMed] [Google Scholar]
- 55.Collier GR, Giudici S, Kalmusky J, et al. Low glycaemic index starchy foods improve glucose control and lower serum cholesterol in diabetic children. Diabetes Nutr Metab 1988;1:11-9. [Google Scholar]
- 56.Fontvieille AM, Rizkalla SW, Penfornis A, Acosta M, Bornet FR, Slama G. The use of low glycaemic index foods improves metabolic control of diabetic patients over five weeks. Diabet Med 1992;9:444-50. 10.1111/j.1464-5491.1992.tb01815.x [DOI] [PubMed] [Google Scholar]
- 57.Fontvieille AM, Acosta M, Rizkalla SW, et al. A moderate switch from high to low glycaemic-index foods for 3 weeks improves the metabolic control of Type 1 (IDDM) diabetic subjects. Diabetes Nutr Metab 1988;1:139-43. [Google Scholar]
- 58.Frost G, Wilding J, Beecham J. Dietary advice based on the glycaemic index improves dietary profile and metabolic control in type 2 diabetic patients. Diabet Med 1994;11:397-401. 10.1111/j.1464-5491.1994.tb00292.x [DOI] [PubMed] [Google Scholar]
- 59.Giacco R, Parillo M, Rivellese AA, et al. Long-term dietary treatment with increased amounts of fiber-rich low-glycemic index natural foods improves blood glucose control and reduces the number of hypoglycemic events in type 1 diabetic patients. Diabetes Care 2000;23:1461-6. 10.2337/diacare.23.10.1461 [DOI] [PubMed] [Google Scholar]
- 60.Gilbertson HR, Brand-Miller JC, Thorburn AW, Evans S, Chondros P, Werther GA. The effect of flexible low glycemic index dietary advice versus measured carbohydrate exchange diets on glycemic control in children with type 1 diabetes. Diabetes Care 2001;24:1137-43. 10.2337/diacare.24.7.1137 [DOI] [PubMed] [Google Scholar]
- 61.Heilbronn LK, Noakes M, Clifton PM. The effect of high- and low-glycemic index energy restricted diets on plasma lipid and glucose profiles in type 2 diabetic subjects with varying glycemic control. J Am Coll Nutr 2002;21:120-7. 10.1080/07315724.2002.10719204 [DOI] [PubMed] [Google Scholar]
- 62.Järvi AE, Karlström BE, Granfeldt YE, Björck IE, Asp NG, Vessby BO. Improved glycemic control and lipid profile and normalized fibrinolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes Care 1999;22:10-8. 10.2337/diacare.22.1.10 [DOI] [PubMed] [Google Scholar]
- 63.Jenkins DJ, Kendall CW, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA 2008;300:2742-53. 10.1001/jama.2008.808 [DOI] [PubMed] [Google Scholar]
- 64.Jimenez-Cruz A, Bacardi-Gascon M, Turnbull WH, Rosales-Garay P, Severino-Lugo I. A flexible, low-glycemic index mexican-style diet in overweight and obese subjects with type 2 diabetes improves metabolic parameters during a 6-week treatment period. Diabetes Care 2003;26:1967-70. 10.2337/diacare.26.7.1967 [DOI] [PubMed] [Google Scholar]
- 65.Jimenez-Cruz A, Turnbull WH, Bacardi-Gascon M, Rosales-Garay P. A high-fiber, moderate-glycemic-index, Mexican style diet improves dyslipidemia in individuals with type 2 diabetes. Nutr Res 2004;24:19-27 10.1016/j.nutres.2003.09.005. [DOI] [Google Scholar]
- 66.Komindr S, Ingsriswang S, Lerdvuthisopon N, Boontawee A. Effect of long-term intake of Asian food with different glycemic indices on diabetic control and protein conservation in type 2 diabetic patients. J Med Assoc Thai 2001;84:85-97. [PubMed] [Google Scholar]
- 67.Luscombe ND, Noakes M, Clifton PM. Diets high and low in glycemic index versus high monounsaturated fat diets: effects on glucose and lipid metabolism in NIDDM. Eur J Clin Nutr 1999;53:473-8. 10.1038/sj.ejcn.1600779 [DOI] [PubMed] [Google Scholar]
- 68.Rizkalla SW, Taghrid L, Laromiguiere M, et al. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care 2004;27:1866-72. 10.2337/diacare.27.8.1866 [DOI] [PubMed] [Google Scholar]
- 69.Wolever TM, Gibbs AL, Mehling C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr 2008;87:114-25. 10.1093/ajcn/87.1.114 [DOI] [PubMed] [Google Scholar]
- 70.Wolever TM, Jenkins DJ, Vuksan V, Jenkins AL, Wong GS, Josse RG. Beneficial effect of low-glycemic index diet in overweight NIDDM subjects. Diabetes Care 1992;15:562-4. 10.2337/diacare.15.4.562 [DOI] [PubMed] [Google Scholar]
- 71.Pavithran N, Kumar H, Menon AS, Pillai GK, Sundaram KR, Ojo O. South Indian Cuisine with Low Glycemic Index Ingredients Reduces Cardiovascular Risk Factors in Subjects with Type 2 Diabetes. Int J Environ Res Public Health 2020;17:E6232. 10.3390/ijerph17176232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goff LM, Cowland DE, Hooper L, Frost GS. Low glycaemic index diets and blood lipids: a systematic review and meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis 2013;23:1-10. 10.1016/j.numecd.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 73.Zafar MI, Mills KE, Zheng J, et al. Low-glycemic index diets as an intervention for diabetes: a systematic review and meta-analysis. Am J Clin Nutr 2019;110:891-902. 10.1093/ajcn/nqz149 [DOI] [PubMed] [Google Scholar]
- 74.Schwingshackl L, Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis 2013;23:699-706. 10.1016/j.numecd.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 75.Milajerdi A, Saneei P, Larijani B, Esmaillzadeh A. The effect of dietary glycemic index and glycemic load on inflammatory biomarkers: a systematic review and meta-analysis of randomized clinical trials. Am J Clin Nutr 2018;107:593-606. 10.1093/ajcn/nqx042 [DOI] [PubMed] [Google Scholar]
- 76.Evans CE, Greenwood DC, Threapleton DE, Gale CP, Cleghorn CL, Burley VJ. Glycemic index, glycemic load, and blood pressure: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2017;105:1176-90. 10.3945/ajcn.116.143685 [DOI] [PubMed] [Google Scholar]
- 77.Jenkins DJA, Dehghan M, Mente A, et al. PURE Study Investigators . Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality. N Engl J Med 2021;384:1312-22. 10.1056/NEJMoa2007123 [DOI] [PubMed] [Google Scholar]
- 78.Korsmo-Haugen HK, Brurberg KG, Mann J, Aas AM. Carbohydrate quantity in the dietary management of type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab 2019;21:15-27. 10.1111/dom.13499 [DOI] [PubMed] [Google Scholar]
- 79.Scientific Advisory Committee on Nutrition. Lower carbohydrate diets for adults with type 2 diabetes. 2021. https://www.gov.uk/government/publications/sacn-report-lower-carbohydrate-diets-for-type-2-diabetes.
- 80.Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021;372:m4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hill AB. The environment and disease: Association or causation? Proc R Soc Med 1965;58:295-300. 10.1177/003591576505800503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Augustin LS, Kendall CW, Jenkins DJ, et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis 2015;25:795-815. 10.1016/j.numecd.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 83.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trail Research Group . Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072-7. 10.1016/S0140-6736(02)08905-5 [DOI] [PubMed] [Google Scholar]
- 84.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trial Research Group . Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003;290:486-94. 10.1001/jama.290.4.486 [DOI] [PubMed] [Google Scholar]
- 85.Holman RR, Coleman RL, Chan JCN, et al. ACE Study Group . Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5:877-86. 10.1016/S2213-8587(17)30309-1 [DOI] [PubMed] [Google Scholar]
- 86.Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J 2004;25:10-6. 10.1016/S0195-668X(03)00468-8 [DOI] [PubMed] [Google Scholar]
- 87.van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005;28:154-63. 10.2337/diacare.28.1.154 [DOI] [PubMed] [Google Scholar]
- 88.Pellegrini N, Serafini M, Salvatore S, Del Rio D, Bianchi M, Brighenti F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol Nutr Food Res 2006;50:1030-8. 10.1002/mnfr.200600067 [DOI] [PubMed] [Google Scholar]
- 89.Zaupa M, Scazzina F, Dall’Asta M, et al. In vitro bioaccessibility of phenolics and vitamins from durum wheat aleurone fractions. J Agric Food Chem 2014;62:1543-9. 10.1021/jf404522a [DOI] [PubMed] [Google Scholar]
- 90.Lillioja S, Neal AL, Tapsell L, Jacobs DR, Jr. Whole grains, type 2 diabetes, coronary heart disease, and hypertension: links to the aleurone preferred over indigestible fiber. Biofactors 2013;39:242-58. 10.1002/biof.1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev 2010;23:65-134. 10.1017/S0954422410000041 [DOI] [PubMed] [Google Scholar]
- 92.Porta N, Bonet C, Cobo E. Discordance between reported intention-to-treat and per protocol analyses. J Clin Epidemiol 2007;60:663-9. 10.1016/j.jclinepi.2006.09.013 [DOI] [PubMed] [Google Scholar]
- 93.Committee for Medicinal Products for Human Use. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus (Draft Guidance). CPMP/EWP/1080/00 Rev 2. European Medicines Agency, 2018.
- 94.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229-34. 10.1056/NEJM199807233390404 [DOI] [PubMed] [Google Scholar]
- 95.Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 2009;373:1765-72. 10.1016/S0140-6736(09)60697-8 [DOI] [PubMed] [Google Scholar]
- 96.Mannucci E, Monami M, Lamanna C, Gori F, Marchionni N. Prevention of cardiovascular disease through glycemic control in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2009;19:604-12. 10.1016/j.numecd.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 97.Turnbull FM, Abraira C, Anderson RJ, et al. Control Group . Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288-98. 10.1007/s00125-009-1470-0 [DOI] [PubMed] [Google Scholar]
- 98.Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol 2005;46:1855-62. 10.1016/j.jacc.2005.05.085 [DOI] [PubMed] [Google Scholar]
- 99.Manson JE, Tosteson H, Ridker PM, et al. The primary prevention of myocardial infarction. N Engl J Med 1992;326:1406-16. 10.1056/NEJM199205213262107 [DOI] [PubMed] [Google Scholar]
- 100.Chiavaroli L, Mirrahimi A, Ireland C, et al. Low-glycaemic index diet to improve glycaemic control and cardiovascular disease in type 2 diabetes: design and methods for a randomised, controlled, clinical trial. BMJ Open 2016;6:e012220. 10.1136/bmjopen-2016-012220 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplemental material
Data Availability Statement
No additional data are available.