Summary
Carbapenemase-producing Enterobacteriaceae (CPE) are a significant challenge to healthcare and infection prevention and control teams. In the UK, OXA-48-like carbapenemases are frequently reported. We describe an outbreak of OXA-48-like producing Enterobacteriaceae and the control measures that proved effective in containing further spread.
Aim
To describe epidemiologic and laboratory features of outbreak and highlight key control interventions.
Findings
Following the introduction of an increased sensitivity CPE screening protocol, OXA-48-like CPE were identified in screening and clinical samples from 96 patients across five hospital wards between November 2017 and July 2018. Klebsiella pneumoniae and Enterobacter cloacae were the most frequently isolated organisms, although a range of OXA-48-like positive organisms were identified. The outbreak was contained utilising certain key interventions, including the modification of laboratory screening processes, engagement of hospital senior management, clear and frequent communication and a strong ‘ward presence’ by the infection prevention and control team (IPCT).
Conclusion
Our report describes how a change in laboratory CPE screening process unmasked a CPE outbreak. The range of bacterial species harbouring the OXA-48-like mechanism suggested plasmid-mediated transfer of resistance. The timely implementation of interventions using a clinical, ‘ward-based’ approach to infection prevention and control highlights the importance of behavioural change in infection control interventions and enabled the termination of a large outbreak without recourse to environmental sampling, major remedial construction work or extensive molecular strain or plasmid typing.
Introduction
The rising incidence of carbapenemase producing Enterobacteriaceae (CPE) presents a significant challenge to public health and infection prevention and control teams (IPCT) as CPE infections are associated with adverse outcomes [1]. In the United Kingdom (UK), Public Health England (PHE) have published a Toolkit providing guidance on the detection, management, and control of CPE [2] and published guidance on the standards for microbiological investigations for carbapenemases (SMI B 60)3. However, there is recognised uncertainty in the UK regarding several aspects of CPE screening and the management of CPE colonised patients, with consequent variability in practice related to screening methodology and protocols [4]. OXA-48-like carbapenemases belong to the Ambler class D beta-lactamases. OXA-48 has been detected in a variety of Enterobacteriaceae and may be transmitted between species via plasmids [5]. The OXA-48-like family were the most frequently reported carbapenemase in the UK in 2017, accounting for 48.5% of confirmed CPE [6], however OXA-48-like producers may be missed by routine antimicrobial susceptibility testing methods using clinical breakpoints [7], due in part to their relative susceptibility to carbapenems and cephalosporins compared with other CPE common in the UK (e.g. New Delhi metallo-beta-lactamases and Klebsiella pneumoniae carbapenemases) [11].
Here we report an outbreak of OXA-48-like-producing Enterobacteriaceae in a district general hospital in the UK. The outbreak took place from November 2017 to July 2018. A total of 96 cases were identified across five hospital wards. OXA-48 was detected in 10 different Enterobacteriaceae species. Limited species specific molecular typing identified three probable clusters of six to nine cases each. We aim to highlight that control of the outbreak required modification of laboratory detection methods and a bundle of infection prevention and control interventions implemented across the hospital, with involvement of staff “from board to ward”. These methods proved effective in controlling the outbreak without environmental sampling, major environmental modification or extensive molecular typing or sequencing of isolates.
Methods
Healthcare setting and affected patient population
The outbreak took place at Barnet Hospital (BH), a district general hospital, with additional cases on a rehabilitation ward at Chase Farm Hospital (CFH), which are both part of the Royal Free London NHS Foundation Trust group of hospitals in London, UK. The Trust group also includes the Royal Free Hospital (RFH), an acute and tertiary referral specialist centre.
The population affected included hospital inpatients at BH on the critical care unit (CCU) and surgical wards, and at CFH on the rehabilitation ward. In December 2017, Barnet Hospital microbiology and IPCT teams were alerted to a patient who had been identified as CPE colonised with an OXA-48-like-producing Klebsiella pneumoniae on a rectal swab screening culture following transfer to RFH from BH CCU. The patient had previously had three negative CPE screens on BH CCU. At the onset of the outbreak, admission and weekly screening was in place at BH on the CCU and haematology ward. The microbiology laboratory that processed BH and CFH samples used a different screening method than the separate laboratory serving RFH. Subsequently, the BH and CFH laboratory used a different CPE screening method and further cases were detected resulting in the declaration of an outbreak in January 2018. The outbreak was concluded in July 2018.
Laboratory methods
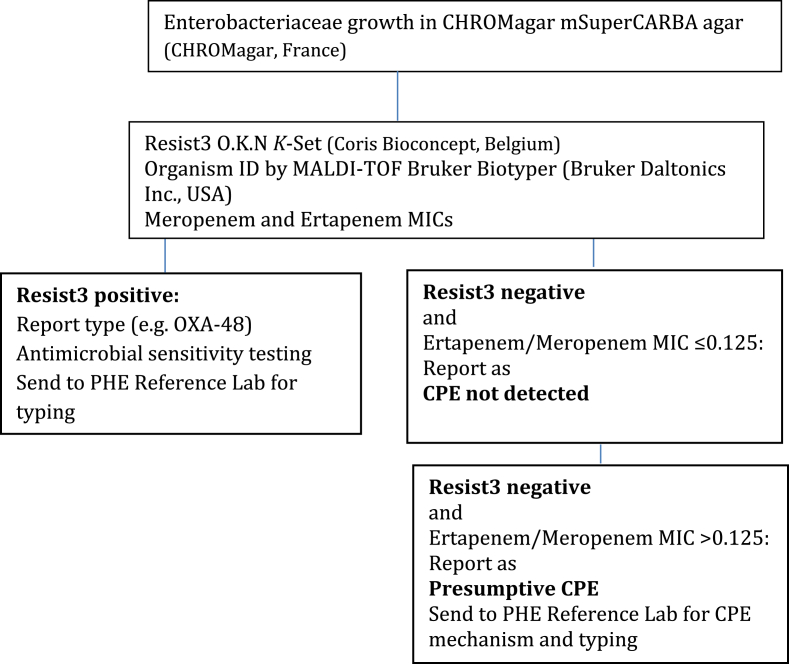
Following the detection of the index case, laboratory methods at BH and RFH were reviewed. The BH screening method comprised inoculation of selective agar (CHROMagar mSuperCARBA agar, CHROMagar, France) followed by antimicrobial susceptibility testing (AST) using the clinical susceptibility breakpoint (the minimum inhibitory concentration (MIC) in mg/L) for meropenem (2 mg/L) according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (version 7.1)7. It was identified that RFH utilised an increased sensitivity screening method for the detection of the OXA-48-like resistance mechanism. This method, which utilised phenotypic rules - resistance to temocillin and coamoxiclav and piperacillin-tazobactam, or third-generation cephalosporins prompted reflex testing for carbapenemase presence by meropenem and ertapenem E-test (bioMérieux UK)- and a lower, meropenem and ertapenem screening MIC cut off (>0.125 mg/L) as parameters triggering CPE testing by means of a lateral flow assay, the Resist3 O.K.N. K-Set test (Coris Bioconcept, Belgium) [15]. This screening algorithm was subsequently adopted by the Barnet Microbiology laboratory with some modifications. (Refer Table I).
Table I.
Updated CPE screening method at Barnet Hospital (February 2018)
The UK SMI B 60 and EUCAST recommend the use of meropenem AST for CPE screening with a cut-off MIC of >0.12mg/L2,7. However, OXA-48-like producers may be susceptible to cephalosporins and carbapenems at the clinical breakpoints, and may be missed by both classical and automated antimicrobial susceptibility testing systems [9,10]. Consequently, high level temocillin resistance (MICs≥128mg/L) has been proposed as a screening test for OXA-48 production [8].
Analytical methods
Cases were defined as patients from whom OXA-48-like-producing CPE was isolated during an admission to BH or CFH, or from whom these organisms were identified within twelve months of admission to BH or CFH, in the absence of other risk factors such as travel to and/or healthcare in a country outside the UK or exposure to another known OXA-48 outbreak.
Case details were collected prospectively during the outbreak. In addition the IPC database was reviewed for additional cases back to January 2017. Case details were collected from the laboratory information system, electronic health record and patient notes. Data was recorded and analysed on an Excel spreadsheet.
A retrospective cohort study was conducted to review the epidemiology of the outbreak in relation to the control measures instituted. Percentages were used to evaluate the positivity rates of CPE screening.
Results
After the increased sensitivity screening method was introduced, the number of patients detected with CPE positive screens with OXA-48-like resistance mechanism began to increase. CPE colonised patients were identified on the critical care unit weekly screening; contact tracing and re-screening resulted in further patients being identified with CPE colonisation. Species specific molecular typing was performed by the Antimicrobial resistance and healthcare associated infections reference unit (AMRHAI) at the PHE Bacteriology Reference Department, using pulsed-field gel electrophoresis (PFGE) or variable number tandem repeat (VNTR) analysis. Typing results demonstrated three clusters: on the critical care unit and a surgical ward at Barnet Hospital and the rehabilitation unit at Chase Farm Hospital. A CPE outbreak was declared in January 2018.
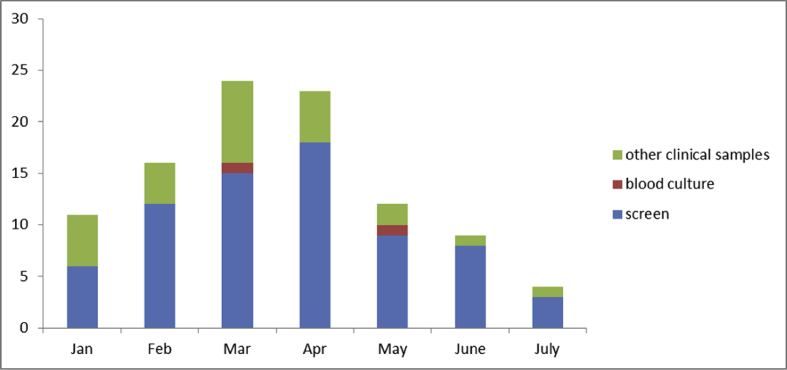
During the period of the outbreak, (November 2017 to July 2018), 96 patients had positive cultures with OXA-48 CPE across five wards. CPE screens were positive in 71 patients and clinical samples returned CPE positive cultures in 25 patients; this included blood culture specimens (n=2), fluid (n=3), urine (n=13), sputum (n=3) and wound swab samples (n=4) (Figure 1). Screening numbers were monitored between April and August 2018: 41 out of 2118 screens returned positive (1.9%).
Figure 1.
CPE positive screens and clinical samples.
Klebsiella pneumoniae and Enterobacter cloacae were the most frequently isolated organisms, although a range of organisms carrying OXA-48-like carbapenemases were identified (Table II).
Table II.
Organisms with OXA-48 resistance mechanism identified during the outbreak (January 2018 to June 2018) (including organisms identified in mixed cultures)
| Organism | Number of isolates |
|---|---|
| Klebsiella pneumoniae | 40 |
| Enterobacter cloacae | 34 |
| Escherichia coli | 19 |
| Citrobacter freundii | 3 |
| Klebsiella variicola | 2 |
| Klebsiella oxytoca | 2 |
| Serratia marcescens | 2 |
| Citrobacter amalonaticus | 1 |
| Klebsiella aerogenes | 1 |
| Leclercia sp | 1 |
Meropenem MIC testing results were available for 72 isolates: MIC values were <0.125 mg/L for seven isolates, 53 isolates had MIC values between 0.125 -0.5 mg/L, five isolates had MIC values between 0.5 and 1 mg/L. Only seven isolates had meropenem MICs >1 mg/L.
Outbreak interventions
At the onset of the outbreak, a range or IPCT interventions were instituted including contact screening (weekly ward screening on affected wards for four weeks following detection of the most recently identified CPE colonised patient), isolation of cases in single rooms with strict contact precautions, enhanced environmental cleaning and increased hand hygiene promotion. These measures were monitored, and reported back to the IPC team during weekly outbreak meetings.
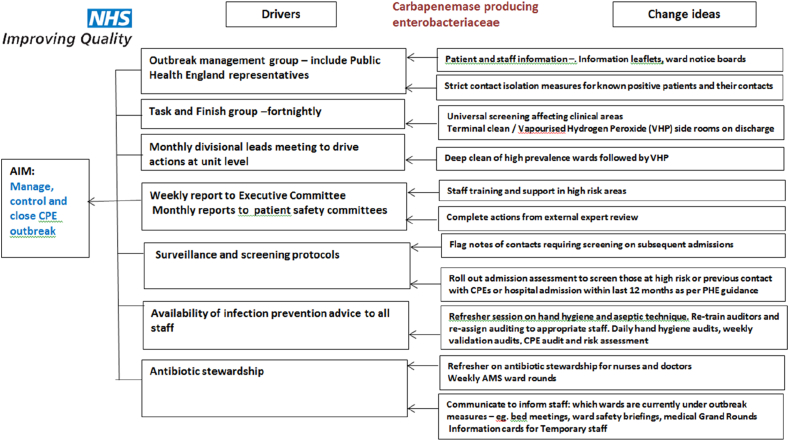
Despite this, as the number of cases continued to rise, it became clear that a fresh approach was required. A combination of interventions using quality improvement (QI) principles were then agreed and instituted, supported by the use of a driver diagram (Table III).
Table III.
CPE outbreak control measures using QI principles
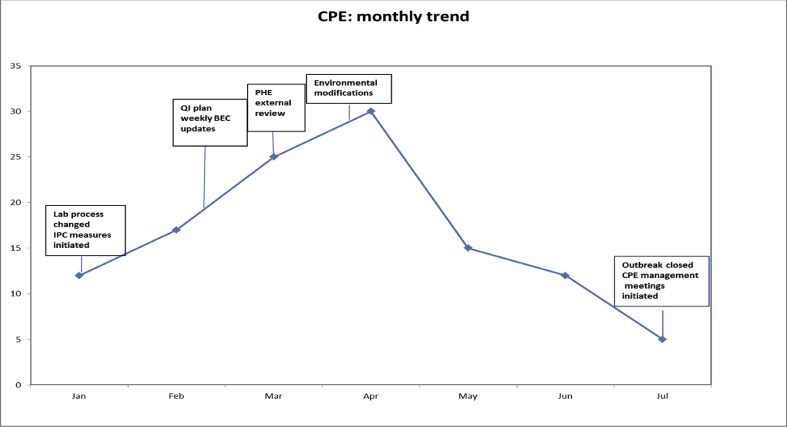
Key interventions that were effective in outbreak control (Figure 2) were:
-
-
Modification of laboratory processes, enabling rapid detection of CPE colonised and infected patients
-
-
Engagement of hospital senior management from an early stage. Hospital executive committee members were updated weekly with outbreak reports and recommendations. Senior level support made it possible to initiate timely interventions, such as terminal cleaning of wards. A monthly report to the Executive Board and Patient Safety Committee was provided in person by the IPCT along with a narrative of the outbreak progress and any issues related to resource provision or staff engagement were raised for action. IPCT attendance at Executive Board meetings continued for the duration of the outbreak.
-
-
Clear and frequent communication by a range of methods. In the early phase of the outbreak, IPC team members recognised that hospital staff needed support to build up their knowledge and confidence in order to engage in discussions and answer questions posed by patients. In response, an extensive teaching exercise was launched. IPCT conducted frequent teaching sessions to a wide range of hospital staff, including paramedical staff and cleaners. Sessions were undertaken out of hours to access staff working different shift patterns. Content included general information regarding the nature and epidemiology of CPE and necessity for strict isolation measures as well as specific guidance related to the management of laundry, waste, crockery and care equipment. Educational resources included information leaflets for staff and patients and pocket cards with prompts to enable quick reference. This was also undertaken in early mornings and late evenings to include staff working on different ‘shift’ patterns. Refresher sessions on basic IPC precautions were undertaken, with the opportunity for face-to-face teaching emphasised over mandatory electronic education packages.
Figure 2.
Timeline of interventions.
CPE updates were provided at Medical Grand Rounds, Divisional Directorate meetings and medical teaching sessions.
-
-
A strong ‘ward presence’ by IPCT: Of note, the trust has established IPC nurses as ‘Clinical Practice Educators’ since 2007 to enable IPC specialists to engage in shift work and patient care activities to educate by role modelling and by one-to-one facilitation. Where ‘IPC link nurses’ had previously found problems attending IPC training and had insufficient time for dedicated IPC activities within the wards, the recruitment of IPC trained practice educators within the IPC team who spent all their work hours within the clinical ward area teaching and role-modelling best IPC practice has paid dividends across the Trust sites. Clinical areas with CPE colonised patients were visited on a daily basis by IPC nurses, who discussed practical aspects of care with nursing staff and doctors and highlighted any improvements in practise from their ward inspection.
-
-
Deep Cleaning protocols were used to focus stringent cleaning following identification and discharge of colonised patients. Emphasis was around the quality of the cleaning and repeated cleaning of frequently touched surfaces such as door handles, phones and light switches. The cleaning protocol included the use of peracetic acid wipes (Clinell® wipes, Gama Healthcare, UK) and was followed by hydrogen peroxide decontamination (Glosair 400 misting, Ethicon Inc, USA).
-
-
PHE guidance: PHE representatives were closely involved with outbreak management decisions. A PHE expert visit to affected clinical areas was arranged and important environmental interventions (including changing the direction of opening of the intensive care unit sluice room doors to allow ‘hands-free access) were undertaken following recommendations from the report.
-
-
CPE management meetings: Monthly meetings dedicated to driving improvements to end the outbreak were continued for six months after the outbreak was declared over. This enabled continuous vigilance to detect new cases or clusters and was a forum to review any pending long-term actions from the outbreak management plan.
The outbreak was declared over in July 2018.
Discussion
OXA-48 carbapenemase producing strains have been described as ‘the phantom menace’ [11]: they can be difficult to identify, since meropenem MICs can be much lower than the clinical breakpoint for resistance [3]. In recognition of this, the current EUCAST screening cut off for carbapenem resistance is based on the epidemiological cut off value (ECOFF) for meropenem, which is 0.125 μg/ml [7]. Our report describes how adopting an alternative screening process, utilising both the lower cut-off value and an assay independent of meropenem MIC, unmasked a CPE outbreak and serves as a salutary warning for laboratories to regularly review processes in light of rapidly changing global and local epidemiology. Of note, seven isolates had Meropenem MICs lower than the EUCAST ECOFF value and were identified only as a result of our optimised screening process utilising the Resist3 O.K.N K-Set test for any bacterial growth on the screening agar.
OXA-48-like carbapenemase was identified from several bacterial isolates and VNTR typing enabled the detection of three CPE clusters; however, the range of bacterial species harbouring the OXA-48-like resistance determinant suggests transmission via mobile genetic elements. One of the limitations of our study is the lack of extensive molecular typing or genotypic sequencing of isolates or plasmids, which makes it difficult to ascertain exact routes of spread.
Using a quality improvement (QI) framework for interventions proved to be very effective in rapidly controlling this large outbreak. Another aspect that is difficult to quantify and can be overlooked in outbreak management by IPCTs is the importance of behavioural science in Infection Prevention and Control [12,13]. During the outbreak, there was an emphasis on clear communication with clinical teams, managers and paramedical staff. Several teaching sessions with small groups were conducted and regular, frequent ward visits were undertaken. This had a direct effect on organisational level awareness and education related to CPE, from ward staff to trust board and undoubtedly contributed to the overall recognition and management of CPE within the hospital. Rather than concentrating on resource heavy measures that were difficult to implement, a decision was made to focus on what the ideal measures would be and what steps were possible to ‘start’ to implement them and then take them forward. This is a key role of the QI process [14].
In conclusion, we were successful in rapidly terminating a large CPE outbreak by the timely implementation of interventions using a quality improvement framework and a clinical, ‘ward-based’ approach to infection prevention and control, highlighting the importance of behavioural change in infection control interventions.
References
- 1.Pérez-Blanco V., Redondo-Bravo L., Ruíz-Carrascoso G., Paño-Pardo J.R., Gómez-Gil R., Robustillo-Rodela A. Epidemiology and control measures of an OXA-48-producing Enterobacteriaceae hospital-wide oligoclonal outbreak. Epidemiol Infect. 2018 Apr;146(5):656–662. doi: 10.1017/S0950268818000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PHE . 2013. Acute trust toolkit for the early detection, management and control of carbapenemase-producing enterobacteriaceae.https://www.gov.uk/government/news/phe-carbapenemase-producing-enterobacteriaceae-toolkit-published Available at: [Google Scholar]
- 3.UK Standards for Microbiology Investigations B 60: Detection of bacteria with carbapenem hydrolysing β lactamases (carbapenemases). (SMI B 60), Public Health England, issue number 2.1, issue date 20.09.2016, accessed on line on May 3 2019 at: https://www.gov.uk/government/publications/smi-b-60-detection-of-bacteria-with-carbapenem-hydrolysing-lactamases-carbapenemases.
- 4.Coope C.M., Verlander N.Q., Schneider A., Hopkins S., Welfare W., Johnson A.P. An evaluation of a toolkit for the early detection, management, and control of carbapenemase-producing Enterobacteriaceae: a survey of acute hospital trusts in England. J Hosp Infect. 2018;(4):381–389. doi: 10.1016/j.jhin.2018.03.007. Aug 99. [DOI] [PubMed] [Google Scholar]
- 5.Wrenn C., O'Brien D., Keating D., Roche C., Rose L., Ronayne A. Investigation of the first outbreak of OXA-48-producing Klebsiella pneumoniae in Ireland. J Hosp Infect. 2014 May;87(1):41–46. doi: 10.1016/j.jhin.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 6.English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report 2018. Public Health England; October 2018. https://www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report access online May 3 2019 at: [Google Scholar]
- 7.EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance Version 2. 01 July 2017. [Google Scholar]
- 8.Woodford N., Pike R., Meunier D., Loy R., Hill R., Hopkins K.L. In vitro activity of temocillin against multidrug-resistant clinical isolates of Escherichia coli, Klebsiella spp. and Enterobacter spp., and evaluation of high-level temocillin resistance as a diagnostic marker for OXA-48 carbapenemase. J Antimicrob Chemother. 2014 Feb;69(2):564–567. doi: 10.1093/jac/dkt383. [DOI] [PubMed] [Google Scholar]
- 9.Livermore D.M., Andrews J.M., Hawkey P.M., Ho P.L., Keness Y., Doi Y. Are susceptibility tests enough, or should laboratories still seek ESBLs and carbapenemases directly? J Antimicrob Chemother. 2012 Jul;67(7):1569–1577. doi: 10.1093/jac/dks088. [DOI] [PubMed] [Google Scholar]
- 10.Haldorsen B., Giske C.G., Hansen D.A., Helgason K.O., Kahlmeter G., Lohr I.H. Performance of the EUCAST disc diffusion method and two MIC methods in detection of Enterobacteriaceae with reduced susceptibility to meropenem: the NordicAST CPE study. J Antimicrobial Chemo. 2018 Oct 73;(10):2738–2747. doi: 10.1093/jac/dky276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poirel L., Potron A., Nordmann P. OXA48-like carbapenemases: the phantom menace. J Antimicrobial Chemo. 2012 April 67;(7):1597–1606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 12.Storr Julie, Wigglesworth Dr Neil, Kilpatrick Claire. May 2013. Integrating human factors with infection prevention and control. The Health Foundation Thought Paper. [Google Scholar]
- 13.Pittet D. The Lowbury lecture: behaviour in infection control. JHI. 2004;58:1–13. doi: 10.1016/j.jhin.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Institue for Healthcare improvement (IHI) http://www.ihi.org/resources/Pages/Tools/QI-Project-Management.aspx accessed 3.4.2019.
- 15.Vanstone G.L., Woodhead S., Roulston K., Sharma H., Wey E., Smith E.R. Improving the detection of carbapenemase-producing organisms (CPO) in a low-prevalence setting: evaluation of four commercial methods and implementation of an algorithm of testing. J Med Microbiol. 2018;67(2):208–214. doi: 10.1099/jmm.0.000674. [DOI] [PubMed] [Google Scholar]