Abstract
Proactive efforts towards the development of new vaccines and antivirals, and the elimination of bottlenecks in vaccine development, will be essential to containing and eradicating future pandemics.
Increased hygienization, facial protection and social-distancing rules, and the reduction of large gatherings and industrial and commercial activity, have helped to ‘flatten the case curve’ of the coronavirus disease 2019 (COVID-19) pandemic. However, these non-pharmacological measures alone are insufficient in the long term. Successful containment and eradication of pandemic viruses is only possible with prophylactic vaccines. Antiviral drugs (mostly small molecules and neutralizing monoclonal antibodies) can only reduce the morbidity and mortality associated with infection.
There is therefore a pressing need for global-preparedness programs that potentiate our ability to rapidly test existing and newly designed antiviral drugs, and for developing safe, effective, easy-to-produce and reasonably priced vaccines in a timely manner. The unprecedented speed of research and development focused on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2020 may serve as a template for mitigating the potentially devastating social and economic consequences of viral pandemics.
The rise of new pathogenic outbreaks in the future is not a matter of ‘if’, but of ‘when’. It is thus imperative that the a priori development of drugs and prophylactic vaccines against viruses, bacteria and other pathogens with pandemic potential is given due consideration. Programs for global pandemic preparedness are based on experiences from the multiple viral epidemics of the past two decades, including those caused by severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1), H1N1 influenza, chikungunya, middle east respiratory syndrome (MERS), Ebola and Zika.
The high infectivity of pandemic respiratory viruses contrasts with the traditionally slow research and development protocols for new antivirals and vaccines, particularly those based on novel technologies or drug classes. The need for careful safety evaluation and for the expansion of the production capacity of antivirals and vaccines in the setting of a worldwide pandemic are additional time-consuming challenges. Current timeframes for drug development, production and distribution are thus not feasible for tackling active pandemic outbreaks. A case in point is when an influenza virus vaccine was finally developed and mass-produced within 6 months to combat the pandemic brought by the H1N1 influenza virus (or ‘swine flu’) a decade ago, the relatively short development timeframe achieved still proved to be too long to influence the outcome of that outbreak1. We are experiencing a similar problem today with SARS-CoV-2, for which there is little hope for a mass-produced prophylactic vaccine for human use before 2021.
Developing novel antiviral agents, including antiviral drugs and vaccines, is financially costly, leaving some to argue that the development of drugs against emerging viruses and other pathogens with pandemic potential is infeasible, especially as viruses can mutate over time and render treatments less effective or even completely ineffective. However, whereas a proactive approach requires significant upfront financial investment, it is the most appropriate action to be prepared for future pandemics. Preparedness involves studying the biology of potentially pandemic pathogens (to understand the mechanism of host cell tropism and to identify small-molecule and vaccine targets, for example) and the pre-emptive development of new drugs or ‘prototype’ vaccines against a given pathogen or group of pathogens in inter-pandemic periods. Then, in a pandemic scenario, such prototype vaccines (particularly those with genetic vaccine formats) can be quickly modified to obtain effective agents. Moreover, because many human pathogens do not replicate well in animals, it can take a long time to generate appropriate animal models to test the protective efficacy of new vaccines, which highlights the importance of laying the groundwork for drug future research and development prior to a pandemic. We can certainly learn from past studies on SARS-CoV-1 and MERS as a stepping stone for developing effective vaccines or other drugs for SARS-CoV-2 in a reduced timeframe. In particular, structure-based antigen design is likely to be critical for quickly designing potent vaccines against coronaviruses and against other difficult pathogens (such as the human immunodeficiency virus (HIV), the respiratory syncytial virus (RSV) and the influenza virus)2. Moderna Therapeutics, in collaboration with the Vaccine Research Center at the National Institutes of Health was able to develop and produce a SARS-CoV-2 mRNA vaccine candidate for human trials in just 42 days, an achievement that might not have been possible without earlier antigen-design studies on MERS3. Additionally, prior work on developing SARS-CoV-1-specific monoclonal antibodies may become useful as passive immunotherapy against SARS-CoV-2, as both viruses use the same cellular receptor for entry4. Clearly, we would probably be in a better position in the battle against SARS-CoV-2 if licensed vaccines against SARS-CoV-1 were available, but no such vaccines have been licensed for human use in the 15 years since the original SARS outbreak5. This was a missed opportunity towards the development of a potentially cross-protective, ready-to-use vaccine regimen against the novel coronavirus.
Developing antivirals for SARS-CoV-2
Three approaches form the cornerstones of how to confront an outbreak or a new pandemic: community and behaviour-based actions (isolation, quarantining, and social distancing, in particular), small-molecule drugs and monoclonal-antibody therapies (for treating the sick and reducing the morbidity and mortality of infected patients), and prophylactic vaccines (for reducing transmission and eventually eradicating the virus from the population). Although community-based actions can be established quickly and be effective in slowing the spread of pathogens, they lose effectiveness in the medium- to long-term, owing to their impact on local and global economies; in fact, they can lead to serious and long-lasting financial and social consequences6. Pharmaceutical interventions are thus critical for combating pandemics.
Small-molecule drugs can improve the handling of pandemic outbreaks by reducing morbidity and mortality, as exemplified by the currently used antivirals against the hepatitis B virus, the hepatitis C virus, HIV and the herpes simplex virus7. These drugs save millions of lives every year. Neutralizing antibodies and small-molecule drugs against SARS-CoV-2 are currently being explored. One possible shortcut would be to find an already-licensed drug that would also be effective against SARS-CoV-2, and which could be mass-produced; indeed, a large number of drugs have entered clinical trials to assess their potential for repurposing (a regularly updated list of candidates https://covid-19tracker.milkeninstitute.org/ is available).
Chloroquine and hydroxychloroquine, which are licensed antimalarial drugs that are also used to treat patients with rheumatic diseases, have received considerable media attention. But data from multiple clinical trials suggest that these drugs do not provide clinical benefit for COVID-19 patients, and that they can cause adverse effects in these patients8. Antivirals developed for other types of RNA viruses — in particular, favipiravir (against the influenza virus) and lopinavir and ritonavir (used in patients with HIV) — have also been studied for their potential activity against SARS-CoV-2. In fact, favipiravir showed some efficacy in patients with mild COVID-19, whereas lopinavir and ritonavir provided no benefit9,10 Remdesivir, an adenosine nucleoside analogue drug developed by Gilead Sciences for treating Ebola virus infections, also entered clinical trials. As remdesivir showed efficacy against SARS-CoV-1, MERS, SARS-CoV-2 and other coronaviruses in in vitro studies11,12, it represents a promising drug candidate for the treatment of SARS-CoV-2 infection in humans. Indeed, a recent publication provided valuable data about its efficacy in a double-blind, randomized, placebo-controlled trial with 1062 participants13. Beigel and colleagues found that remdesivir treatment shortened the time of recovery of SARS-CoV-2-infected individuals compared to the placebo-injected participants. Based on the positive clinical results, remdesivir has been approved for human use (https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19). Taken together, broad-spectrum antivirals (in particular, nucleoside analogues that target conserved catalytic sites of essential viral enzymes) are amongst the most promising drug candidates against SARS-CoV-2.
A number of new drugs not yet approved by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA) are also under study. These include neutralizing monoclonal antibodies. Notably, Regeneron Pharmaceuticals, using their transgenic mouse model that produces human antibodies14, have generated neutralizing monoclonal antibodies against SARS-CoV-2 for treatment or as a prophylactic. Clinical evaluation of the safety and efficacy of sarilumab, a human monoclonal antibody against the interleukin-6 receptor, is underway (NCT04315298). A new publication reported on the clinical efficacy of LY-CoV555 (developed by Eli Lilly), a neutralizing monoclonal antibody directed against the spike glycoprotein of SARS-CoV-215. Study participants, recently diagnosed with SARS-CoV-2 infection, received a single dose of 700, 2800 or 7000 mg of LY-CoV555 or placebo intravenously and the change of viral load from baseline at day 11 was measured. Interestingly, only the 2800 mg dose provided clinical benefit for LY-CoV555-treated patients compared to the placebo-injected group. Plasma therapy — the transfer of serum from convalescent patients to acutely ill subjects as a passive immunization strategy — is FDA-approved, yet its efficacy against SARS-CoV-2 infection is still under investigation16. Because it proved to be effective in reducing mortality in humans infected with pandemic H1N1 influenza virus17, it may be applicable against SARS-CoV-2 as well. Of note, no strong evidence has been provided about the effectivity of plasma therapy for COVID-19 patients to date18, and further adequate and well-controlled randomized trials will need to investigate the clinical efficacy of this approach.
Developing vaccines for SARS-CoV-2
Given that no coronavirus vaccine for human use currently exists, the ongoing SARS-CoV-2 pandemic is an extraordinary challenge for rapid vaccine development. Even according to the most optimistic predictions, an effective, safe and mass-produced vaccine will not come before 2021 — clearly too late to have an impact on the current winter outbreaks of SARS-CoV-2 infections. Nevertheless, a vaccine will still be incredibly valuable, as it is the only intervention that can prevent widespread infections effectively.
Although SARS-CoV-2 is a novel human virus, we can take advantage of existing knowledge stemming from research into vaccines for the related SARS-CoV-1 and MERS. Potential SARS-CoV-2 vaccine targets have been identified (Fig. 1a), and the scientific community has experience with the design of coronavirus antigens3. Importantly, the genetic sequence of the virus has been publicly available since January 2020, and animal models that have been developed for the evaluation of SARS-CoV-1 vaccines may also be useful for assessing SARS-CoV-2 vaccine candidates19. Many pharmaceutical companies and academic research institutions have since launched SARS-CoV-2 vaccine programs using both conventional and genetic or viral vector-based platforms20 (a regularly updated list of drug developers is available https://covid-19tracker.milkeninstitute.org/).
Fig. 1 |. Prophylactic and therapeutic strategies against SARS-CoV-2.
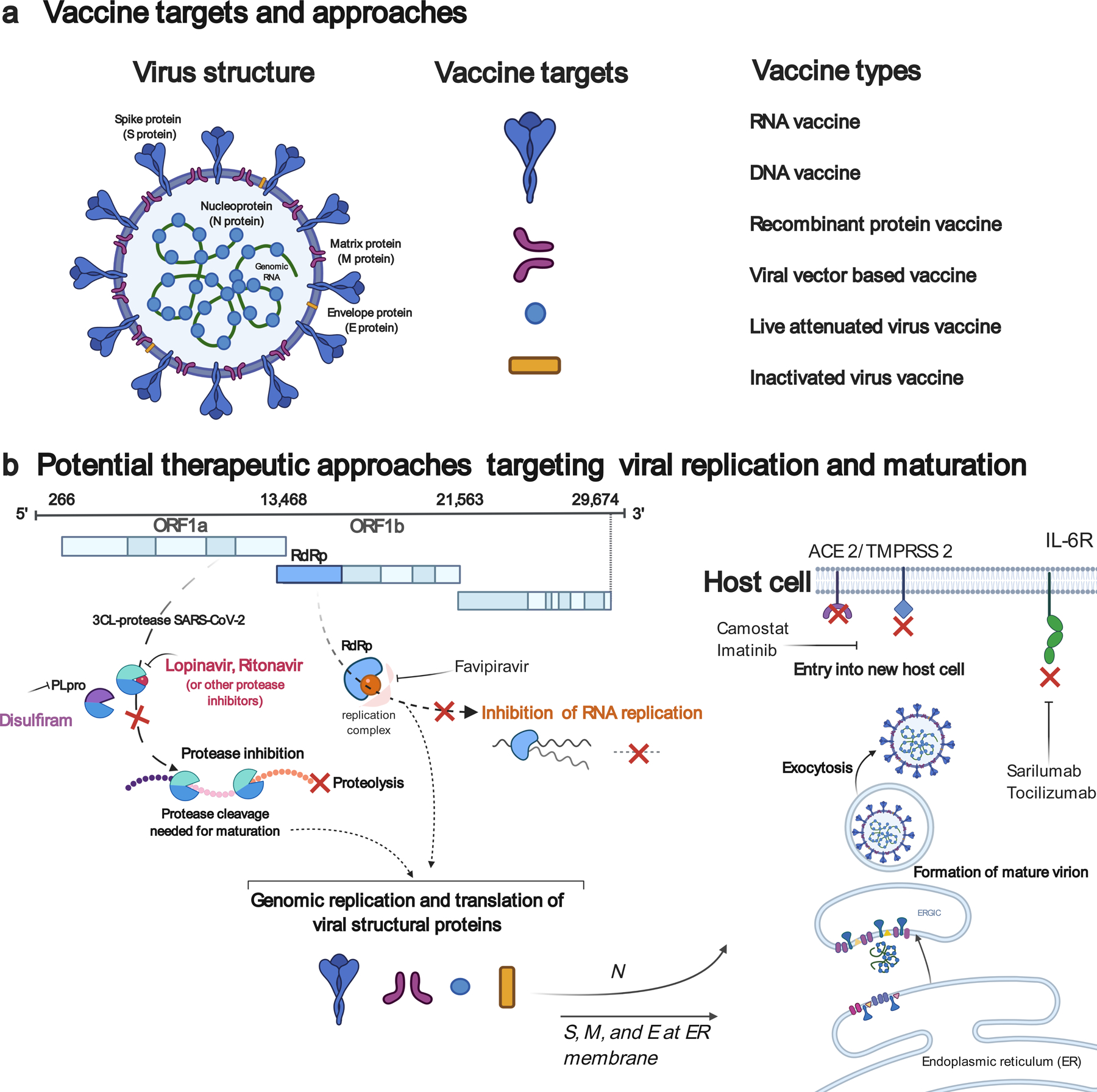
a, Vaccine targets and vaccine platforms against SARS-CoV-2. b, Examples of viral targets and of potential antiviral drugs against SARS-CoV-2. ORF, open reading frame; RdRp, RNA-dependent RNA polymerase; PLpro, papain-like protease; ACE 2, angiotensin-converting enzyme 2; TMPRSS 2, transmembrane protease, serine 2; ERGIC, endoplasmic reticulum-Golgi intermediate compartment; IL-6R, interleukin-6 receptor.
SARS-CoV-2 vaccines can be divided into three general groups: nucleic-acid-based, protein-based (including the use of inactivated viruses and virus-like particles), and live-vector-based. Table 1 provides a summary of the leading SARS-CoV-2 clinical vaccine candidates supported by Operation Warp Speed in the United States.
Table 1 |.
Leading SARS-CoV-2 vaccine candidates supported by Operation Warp Speed in the United States.
| Vaccine | Technology platform | Developers | Development status and clinical-trial number |
|---|---|---|---|
| mRNA-127324 | mRNA | Moderna Therapeutics & NIAID | Phase 3 NCT04470427 |
| BNT162b225 | mRNA | BioNTech RNA Pharmaceuticals & Pfizer | Phase 3 NCT04368728 |
| AZD122226 | Adenovirus vaccine | University of Oxford & AstraZeneca | Phase 3 NCT04516746 |
| Ad26.COV2-S | Adenovirus vaccine | Johnson & Johnson | Phase 3 NCT04505722 |
| - | VSV and measles-based | Merck Sharp & Dohme Corp. | Phase 1/2 NCT04498247 |
Among the nucleic-acid formulations, mRNA-based vaccines are a promising strategy and include multiple vaccine candidates against COVID-19. Several recent publications provided preclinical data about SARS-CoV-2 mRNA vaccine candidates21–23. One such strategy employs a nucleoside-modified mRNA-lipid nanoparticle (mRNA-LNP) vaccine encoding the full-length SARS-CoV-2 spike glycoprotein (with a mutated furin cleavage site) or the receptor binding domain of the spike protein as a monomer23. In mice, both vaccines induced robust type-1 CD4+ and CD8+ T-cell responses in the spleen and lungs after administration of a single dose of 30 µg. Moreover, the vaccines elicited strong long-lived plasma-cell and memory B-cell responses that were associated with the production of antibodies with potent anti-SARS-CoV-2 neutralization activity. Two preclinical reports of Moderna’s mRNA-1273 vaccine, which contained nucleoside-modified mRNA-LNPs encoding the spike protein of SARS-CoV-2 stabilized in the pre-fusion state21,22, also elicited robust T-helper-1-dominant (TH1-dominant) CD4+ and CD8+ T-cell responses after two immunizations with only 1 µg of mRNA-LNPs in mice21. Importantly, the vaccine induced strong neutralizing-antibody responses and protection from viral replication in the lungs of SARS-CoV-2-challenged mice. When tested in rhesus macaques, two immunizations with 10 µg or 100 µg of mRNA-1273 induced TH1-biased CD4+ T-cell responses and neutralizing-antibody responses in a dose-dependent manner22. Interestingly, no CD8+ T-cell responses could be measured in vaccinated non-human primates, but the mRNA-1273 vaccine could induce a high-level of protection (particularly in the high dose group) from viral replication in the upper and lower respiratory tracks.
Two recent publications reported the safety and efficacy of mRNA-based SARS-CoV-2 vaccine candidates tested in a small number of people24,25. In a study of Moderna’s mRNA-1273 vaccine24, 45 (mainly white) volunteers 18–55 years of age received two intramuscular immunizations, 4 weeks apart, of 25 µg, 100 µg or 250 µg of mRNA-LNPs. The results are promising, with the medium magnitude of neutralizing-antibody responses measured in vaccines in the upper quartile of values in convalescent serum samples. Antibody titres correlated with the vaccine dose. In general, the vaccine was well-tolerated, although over half the participants receiving the 100 µg and 250 µg doses reported fever and other adverse events (fatigue, chills, headache, or pain at the injection site) after administration of the second dose. Vaccine evaluation in a phase-3 clinical trial will be necessary to confirm these results and to provide more detailed comparative data on vaccine safety and efficacy in different age and ethnic groups. The nucleoside-modified mRNA-LNP vaccine developed by Pfizer/BioNTech25, which encodes a trimeric form of the receptor binding domain of the SARS-CoV-2 spike protein, has also been tested in human volunteers. 45 individuals (mainly white) 19–54 of age received two intramuscular immunizations of 10 µg or 30 µg of mRNA-LNPs with a 3-week interval, or a single dose of 100 µg of mRNA-LNPs. The safety and immunogenicity results were similar to those of the Moderna vaccine, with dose-dependent high neutralizing-antibody titres and mild or moderate adverse events. The booster immunizations elicited significantly stronger neutralizing antibody responses. Of note, using lower vaccine doses (10–30 µg) to achieve protection from viral infection can be a critical advantage when millions or even billions of doses of a vaccine need to be rapidly manufactured. Similar to the Moderna vaccine, a larger study will shed light on the potential differences in safety and efficacy between different ethnicities and age groups.
Preclinical and clinical data are also already available for other vaccine platforms. One of the SARS-CoV-2 vaccine candidates in most advanced development is based on a replication-deficient simian adenovirus vector, ChAdOx1, which contains the full-length spike protein with a tissue-plasminogen-activator leader sequence26. A single intramuscular immunization of 5 × 1010 viral particles in healthy adults induced neutralizing-antibody responses in 100% of the participants; a subsequent immunization further increased the neutralizing-antibody titres to levels comparable to those measured in convalescent plasma samples. The vaccine was well-tolerated, as only mild and moderate adverse events were observed after vaccine administration. Another trial of human volunteers receiving a single intramuscular immunization of a non-replicative adenovirus type-5 (Ad5) vaccine containing the SARS-CoV-2 spike protein27 showed that immunization induced cellular and humoral immune responses in most immunized individuals, but that at least one adverse reaction was reported from more than 75% of the vaccines within the first 7 days post-immunization. Three quarters of the highest dose group had a 4-fold increase in neutralizing antibodies, but no comparison with titres from convalescent patients was performed. Of note, most humans have pre-existing neutralizing antibodies against several adenovirus serotypes, including Ad5, which can negatively affect the performance of such vaccines28.
Inovio’s DNA vaccine (INO-4800), which targets the spike protein of SARS-CoV-2, was also shown to be immunogenic in mice and guinea pigs, although the protective efficacy of the vaccine has not yet been evaluated29. A series of SARS-CoV-2 spike-protein-based DNA vaccines (both soluble and transmembrane) tested in rhesus macaques30 induced neutralizing-antibody responses and variable levels of protection from SARS-CoV-2 infection following two intramuscular immunizations (on week 0 and week 3) with 5 mg of the vaccine. Others have evaluated an alum-adjuvanted purified inactivated SARS-CoV-2 vaccine (PiCoVacc) in mice, rats and rhesus macaques31, reporting that the vaccine is immunogenic in mice and rats after two immunizations, and that it elicited protective immune responses from SARS-CoV-2 infection after three immunizations (on days 0, 7 and 14) with 3-µg or 6-µg doses in non-human primates.
The development of conventional-type vaccines (such as those based on recombinant protein subunits, and on inactivated or live-attenuated viruses) has a critical advantage over novel vaccine formats: researchers have a deep pool of relevant experience to draw from (including safety data), and similar vaccine formats for humans are already in use. This prior experience would probably speed up the licensing of SARS-CoV-2 vaccines using conventional vaccine platforms, and mass production would be facilitated given that the infrastructure is readily available. Nevertheless, as with any new medicine, their initial development still requires extensive investigation. But genetic vaccines have already showed high efficacy in preclinical models, and offer the advantages of flexible and very fast antigen design, and of rapid production (once sufficient manufacturing capacity is available)32. These potential advantages were demonstrated by Moderna Therapeutics, which generated a SARS-CoV-2 mRNA vaccine for human testing with unprecedented speed. The concerted effort being poured into SARS-CoV-2 vaccine development, with multiple platforms being explored in varied versions, should ensure that there will be at least one viable vaccine licensed for COVID-19. At this point, the bottlenecks are the time-consuming nature of clinical trials (many months in the best case scenario, particularly if the administration of a single vaccine dose does not induce durable protective immune responses), and mass production and distribution of the vaccines (likely months to years if billions of vaccine doses must be produced and administered worldwide).
Bottlenecks in vaccine development
Vaccine development is a complex endeavour. It involves multiple phases, starting with a design stage and including preclinical studies, phases 1–3 of clinical-trial testing, human-use approval, and post-marketing studies. Each development phase faces its challenges and adds to the overall length of the process. Early preclinical studies alone, which aim to establish the safety and efficacy of the vaccine platform in animal models in the context of the pathogen of interest, can take decades to complete: for example, preclinical studies for HIV-vaccine development have been ongoing for almost 40 years. And that is even before beginning human testing, which (in the United States) involves formal toxicity trials in animals, followed by an Investigational New Drug (IND) submission to the FDA, phase-1 tests in small groups of people who receive the vaccine (to test dosing and safety), phase-2 clinical studies that expand testing to additional patients to assess efficacy and adverse effects, and phase-3 trials in which the vaccine is given to thousands of people in a placebo-controlled double-blind protocol for evaluation of efficacy and safety. The duration of phase-3 testing alone depends in part on the incidence of infection and the characteristics of the disease (acute infection versus chronic infection).
Altogether, the sheer length of this formal process is a major roadblock in the development of a safe and efficacious vaccine early enough to tackle the spread of a pandemic virus. In the context of the COVID-19 pandemic, researchers, governments, pharmaceutical companies and regulatory bodies are coordinating an unprecedented overhaul of the vaccine-development process, by condensing — rather than eliminating —steps in the process, without compromising safety. As we have observed with the COVID-19 pandemic, preclinical testing can be condensed into a much shorter time period when exploring vaccine strategies developed for related pathogens. The speed at which the three phases of clinical testing can be completed depends on many factors, including the disease being targeted. One of the most important factors in phase-3 trials is the incidence of the disease in the target population, which determines the size of the study and the duration of follow-up needed to obtain compelling data. Once approved — or during the approval process itself — the protocols for developing the necessary scale of good manufacturing practice (GMP) manufacturing is a critical concern. Finally, comprehensive distribution of the vaccine to the population, and the education and legislation needed to get the majority of people to take the vaccine, are extremely important considerations. Overall, it is believed that at least 8–12 months will be needed to evaluate novel SARS-CoV-2 vaccine candidates and produce sufficient amounts for people at high risk (in particular, healthcare workers and individuals with chronic diseases). Vaccine production for the rest of the world’s population and comprehensive vaccine distribution and administration will likely take at least an additional 6–8 months.
Even putting aside the lengthiness of formal vaccine-development programs, bottlenecks and delays may occur at any step of the way. Most vaccines do not move out of preclinical testing, owing to a lack of efficacy or to toxicity, and multiple factors — toxicity, adverse events (including unexpected incidents), lack of efficacy, poor magnitude or short durability of the protective response, inability to effectively implement GMP manufacturing, and projected cost — determine whether a vaccine will continue its development and be submitted for approval. Individual adverse effects identified in human volunteers during clinical trials may lead to the trial being placed on hold (allowing the investigation of the adverse effect and assessing if it compromises vaccine safety), as has occurred with several of the SARS-CoV-2 vaccine formulations currently in trials. Vaccine developers can address many bottlenecks, such as altering the adjuvant or other components to reduce toxicity, developing new production methods to reduce cost, and adjusting the dose, the location of delivery, and the timing and number of immunizations, to increase efficacy.
Outlook
The SARS-CoV-2 pandemic is an extraordinary health emergency that has profoundly altered lives around the globe. It requires unprecedented and globally coordinated steps. The virus is likely to stay with us until a prophylactic vaccine is developed, produced and administered worldwide, which is not expected to happen until 2021. Besides social isolation, our current best chance to reduce SARS-CoV-2-related morbidity and mortality is to find and apply a licensed drug (such as a small molecule, Fig. 1b) with at least partial effectiveness against SARS-CoV-2 infection. Owing to tremendous research efforts in this area, the identification of an effective antiviral drug that can be widely distributed is a concrete possibility. But the typical development program and careful evaluation of novel antivirals and vaccines take years, a timeframe that falls short in the context of an ongoing pandemic. To avoid or mitigate the consequences of future pandemics, policy makers (such as governments and the World Health Organization) and the pharmaceutical industry need to recognize that sustained and well-organized global pandemic preparedness is critical. Activities should include monitoring the natural reservoirs of pathogens with pandemic potential, improving communication between different national and international centres of disease control, the development and licensure of novel (preferably broad-spectrum) antivirals and antibacterials, the development of ‘prototype’ vaccines that can quickly be adjusted to a pandemic pathogen and produced and distributed at large scale, and the generation of appropriate animal models for testing the safety and protective efficacy of novel vaccine candidates. These measures require substantial investment from national governments, but these expenses more than make up for the financial (and human) costs of handling a pandemic, as is patent from the heavy effects of lockdowns on global (and personal) economies during the first wave of SARS-CoV-2 outbreaks. Resources should also be poured into communicating frankly and effectively about vaccines, and the science of pandemics and the measures to slow down transmission, with the public. Effective science communication is particularly critical when addressing anti-vaccine (and anti-science) activities in the general population, which can undermine the bench-to-bedside translation of successful research.
Acknowledgements
The authors thank Michael J. Hogan and Keely Walsh for a critical reading of the manuscript, and Mohamad-Gabriel Alameh for helping to create the figure. N.P. is supported by the National Institute of Allergy and Infectious Diseases (AI146101 and AI153064) and the Global Health Innovative Technology Fund (GHIT). D.W. is supported by the National Institute of Allergy and Infectious Diseases (R01-AI124429, BAA2018, AI124429, AI142596, 1U19AI135902, UM1-AI-144371) and BioNTech RNA Pharmaceuticals.
Footnotes
Competing interests
The authors are named on patents that describe the use of nucleoside-modified mRNA as a platform to deliver therapeutic proteins and vaccines. These competing interests have been fully disclosed to the University of Pennsylvania, and an approved plan for managing any potential conflicts arising from the licensing of the patents is in place.
References
- 1.Krammer F & Palese P Advances in the development of influenza virus vaccines. Nat Rev Drug Discov 14, 167–182, doi: 10.1038/nrd4529 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Graham BS, Gilman MSA & McLellan JS Structure-Based Vaccine Antigen Design. Annu Rev Med 70, 91–104, doi: 10.1146/annurev-med-121217-094234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pallesen J et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A 114, E7348–E7357, doi: 10.1073/pnas.1707304114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, doi: 10.1016/j.cell.2020.02.052 (2020). [DOI] [PMC free article] [PubMed]
- 5.Tse LV, Meganck RM, Graham RL & Baric RS The Current and Future State of Vaccines, Antivirals and Gene Therapies Against Emerging Coronaviruses. Front Microbiol 11, 658, doi: 10.3389/fmicb.2020.00658 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan H, Wang S & Yang C Coronavirus: limit short-term economic damage. Nature 578, 515, doi: 10.1038/d41586-020-00522-6 (2020). [DOI] [PubMed] [Google Scholar]
- 7.De Clercq E & Li G Approved Antiviral Drugs over the Past 50 Years. Clin Microbiol Rev 29, 695–747, doi: 10.1128/CMR.00102-15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulware DR et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med, doi: 10.1056/NEJMoa2016638 (2020). [DOI] [PMC free article] [PubMed]
- 9.Cao B et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med, doi: 10.1056/NEJMoa2001282 (2020). [DOI] [PMC free article] [PubMed]
- 10.Dong L, Hu S & Gao J Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther 14, 58–60, doi: 10.5582/ddt.2020.01012 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Gordon CJ et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem, doi: 10.1074/jbc.RA120.013679 (2020). [DOI] [PMC free article] [PubMed]
- 12.Sheahan TP et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Science Translational Medicine 9, 10.1126/scitranslmed.aal3653 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med, doi: 10.1056/NEJMoa2007764 (2020). [DOI] [PMC free article] [PubMed]
- 14.Macdonald LE et al. Kappa-on-Heavy (KoH) bodies are a distinct class of fully-human antibody-like therapeutic agents with antigen-binding properties. Proc Natl Acad Sci U S A 117, 292–299, doi: 10.1073/pnas.1901734117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen P et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med, doi: 10.1056/NEJMoa2029849 (2020). [DOI] [PMC free article] [PubMed]
- 16.Zhang B et al. Treatment With Convalescent Plasma for Critically Ill Patients With SARS-CoV-2 Infection. Chest, doi: 10.1016/j.chest.2020.03.039 (2020). [DOI] [PMC free article] [PubMed]
- 17.Hung IF et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 52, 447–456, doi: 10.1093/cid/ciq106 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA 324, 460–470, doi: 10.1001/jama.2020.10044 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gretebeck LM & Subbarao K Animal models for SARS and MERS coronaviruses. Curr Opin Virol 13, 123–129, doi: 10.1016/j.coviro.2015.06.009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amanat F & Krammer F SARS-CoV-2 Vaccines: Status Report. Immunity, doi: 10.1016/j.immuni.2020.03.007 (2020). [DOI] [PMC free article] [PubMed]
- 21.Corbett KS et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature, doi: 10.1038/s41586-020-2622-0 (2020). [DOI] [PMC free article] [PubMed]
- 22.Corbett KS et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med, doi: 10.1056/NEJMoa2024671 (2020). [DOI] [PMC free article] [PubMed]
- 23.Laczko D et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity, doi: 10.1016/j.immuni.2020.07.019 (2020). [DOI] [PMC free article] [PubMed]
- 24.Jackson LA et al. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med, doi: 10.1056/NEJMoa2022483 (2020). [DOI] [PMC free article] [PubMed]
- 25.Mulligan MJ et al. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature, doi: 10.1038/s41586-020-2639-4 (2020). [DOI] [PubMed]
- 26.Folegatti PM et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396, 467–478, doi: 10.1016/S0140-6736(20)31604-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu FC et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet, doi: 10.1016/S0140-6736(20)31208-3 (2020). [DOI] [PMC free article] [PubMed]
- 28.Fausther-Bovendo H & Kobinger GP Pre-existing immunity against Ad vectors: humoral, cellular, and innate response, what’s important? Hum Vaccin Immunother 10, 2875–2884, doi: 10.4161/hv.29594 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith TRF et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun 11, 2601, doi: 10.1038/s41467-020-16505-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science, doi: 10.1126/science.abc6284 (2020). [DOI] [PMC free article] [PubMed]
- 31.Gao Q et al. Rapid development of an inactivated vaccine candidate for SARS-CoV-2. Science, doi: 10.1126/science.abc1932 (2020). [DOI] [PMC free article] [PubMed]
- 32.Pardi N, Hogan MJ, Porter FW & Weissman D mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov 17, 261–279, doi: 10.1038/nrd.2017.243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


