Abstract
Stereotactic body radiotherapy (SBRT) is an emerging treatment option for patients with pancreatic cancer, as it can provide a therapeutic benefit with significant advantages for patients’ quality of life over standard conventional chemoradiation (CRT). The objective of this review is to present alternative clinical settings in which SBRT may benefit patients with pancreatic cancer. These include palliation of pain, elderly patients who are not surgical candidates, local therapy in oligometastatic cases and salvaging local failures after surgery or external beam radiation. We will review these individually and provide supporting literature for each.
Keywords: Pancreatic neoplasms, Pancreas cancer, Radiosurgery, Stereotactic, Stereotactic body radiation therapy (SBRT), Radiation, Radiotherapy
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) carries a poor prognosis, with historical median survival rates of 3 months to 24 months, depending on the stage of disease and patient’s performance status. After the results of certain randomized trials showed no improvement in survival with the addition of daily fractionated external beam radiation, clinicians have sought to assess the appropriate situations for its use [1–4].
Historically, radiation therapy (RT) was delivered using 2 beams – 4 beams, given daily for 5 weeks – 6 weeks with concurrent chemotherapy. These 3D treatment techniques, in addition to the large fields required to treat the pancreas and surrounding nodal stations, limited the ability to avoid bowel resulting in high toxicity rates. The development of methods to spare normal tissue, including beam modulation (dynamic shaping), arc therapy, breath hold, immobilization, and placement of fiducials and spacers, allow for delivery of highly focused radiation to the tumor while limiting the dose to adjacent organs at risk using stereotactic radiation. This has resulted in lower rates of skin, gastrointestinal and even hematologic toxicity [5].
In initial studies, stereotactic body radiation therapy (SBRT) showed equivalent control rates to those of chemoradiation (CRT) [6]. From there, clinicians sought to study whether dose escalation could improve local control. The safety of 1, 3, 5 and 15 fraction regimens were examined, using techniques such as a simultaneous integrated boost (SIB) to just the tumor with a lower dose given to a margin surrounding the gross disease [7–11]. This further increased the rate and durability of local control.
Much of the data published on the use of SBRT in pancreatic cancer pertains to delaying local progression in locally advanced (LAPC) cases and improving R0 resection rates and local control when used in the neoadjuvant setting [12,13]. The objective of this review is to present other potential clinical settings in which SBRT may benefit patients with pancreatic cancer. These include palliation of pain, local therapy for elderly patients who are not surgical candidates, local therapy to the primary and/or metastases in oligometastatic cases, and salvaging local failures after surgery or external beam radiation. We will review these individually and provide supporting literature for each.
Palliation of Pain
PDAC is an aggressive disease, with 80% of patients presenting with LAPC and/or metastatic disease (MPC) at diagnosis [14]. Local tumor burden accounts for significant morbidity and mortality as a result of invasion of surrounding structures [15]. This causes increased sensitivity due to pancreatic tissue innervation by sympathetic and parasympathetic systems, leading to the sensation of pain [16]. Approximately 30% – 40% of patients report pain as the dominant symptom at diagnosis, which climbs to 90% shortly before death [14]. Hence pain relief is a major goal of palliative treatment in patients with PDAC [17,18].
While they do not provide a local control benefit, pain management with narcotics or interventional techniques such as neurolytic celiac plexus blockage and intraspinal drug delivery may offer temporary symptomatic relief [18]. RT has also been shown to provide pain palliation [19–23].
Ebrahimi et al. reported on 61 PDAC patients who underwent palliative RT for pain relief. On Numeric Rating Scale (NRS) 0–10, median baseline pain score was 8, with mean pain duration before consultation of 4.5 months. 85% of patients used strong opioids with mean oral morphine equivalent (OME) daily dose of 147 mg on presentation. 13%, 39% and 46% of patients were treated with one, two or three weekly fractions of 8.0 Gy, respectively. Only 30% of patients were treated with highly conformal techniques such as intensity modulated radiation therapy (IMRT) or volumetric modulated arc therapy (VMAT), while the rest received anterior-posterior-posterior-anterior (AP/PA) or 3D conformal techniques. After treatment, 66% of patients had reduction of pain and 7% had complete pain relief (NRS of 0). First pain relief began one week after completion of RT, with median duration of 2.5 months. Transient flare of pain occurred in 33% of patients. While 51% and 21% of patients experienced nausea and vomiting grade ≤2, respectively, there were no grade 3 toxicities. Patients who responded to treatment had significantly improved median overall survival of 4.4 months (95% Confidence Interval [CI]: 2.0 – 6.8) versus 1.7 months (95% CI: 1.1 – 2.3) in non-responders (p <0.001) [14].
Wang et al. retrospectively reviewed 63 patients with LAPC and MPC receiving IMRT with a median dose of 46.0 Gy (range 26.8 – 54.0) to the pancreas. 27 patients received concurrent CRT most commonly with gemcitabine or capecitabine. Median survival for LAPC and MPC patients was 15.7 months and 8.0 months, respectively (p = 029). Pain was graded via the visual analogue scale (VAS). Symptomatic improvements were observed after RT in all 44 patients who had presented with abdominal and/or back pain. RT provided greater amelioration in patients with severe pain (VAS 7 – 10) than those with only moderate pain at consult (VAS 4 – 6), with decreased scores of 5.0 ± 1.5 versus 3.4 ± 0.8, respectively (p <.001). Only 13.9% and 14.8% of patients experienced grade ≥3 hematologic toxicities in the RT and CRT cohorts, and no non-hematologic grade ≥3 toxicities were reported [24].
Buwenge et al. published a systematic review of fourteen studies reporting pain control after SBRT in 479 LAPC patients. Median prescription dose was 27.8 Gy (range: 16.5 – 45.0) and the median number of fractions was 3.5 (range: 1 – 6). 84.9% of patients experienced at least a partial relief of pain (95% CI: 75.8% – 91.5%). Acute and late grade ≥3 toxicity ranged between 3.3% – 18.0% and 6.0% – 8.2%, respectively [16].
Sharrett et al. retrospectively reviewed 60 patients with PDAC (10 with medically inoperable and 12 with MPC). All were treated with five fractions of IMRT with a SIB dose painting technique, with mean doses to Planning Target Volume 1 (PTV1) and PTV2 of 39.0 Gy and 37.0 Gy, respectively. 50% received chemotherapy. Pain was reduced at 6 weeks (p <0.001) and 3 months (p <.001), with significant reductions in narcotic usage. Average pain score was 8.3 before SBRT and 0.97 at 3 months post-RT. One grade ≥3 acute toxicity and 5 grade ≥3 late toxicities occurred [25].
Thus, pain relief can be achieved by RT via conventional fractionation or SBRT, with data suggesting SBRT to be superior to standard CRT with respect to toxicity and efficacy [26,27].
Local Therapy for Elderly and Medically Inoperable Patients
PDAC is common in the elderly, with more than two thirds of patients over 65-years old at diagnosis and a median age of 70 [28]. In addition to affecting performance status and survival outcomes, the increased incidence of comorbid conditions in older patients often precludes the potential for curative-intent multimodality treatment [29,30]. Thus, elderly patients who undergo upfront surgery are at an increased risk of early mortality [30].
One third of patients with localized disease are not candidates for surgical therapy due to pre-existing comorbidities, poor performance status and/or advanced age, and are commonly offered only palliative single-agent chemotherapy or supportive care [31]. Despite the absence of metastatic disease, median survival rates for these patients range from a mere 5 months in LAPC to 12–15 months in resectable or borderline resectable disease [32,33]. RT may represent a viable treatment option for this cohort.
Ciabatti et al. [34] performed a systematic review of eleven studies with 1830 elderly patients treated with RT utilizing PRISMA methodology. Median doses ranged from 33.0–50.4 Gy, with conventional and hypo fractionation being used in five and four studies respectively (two did not report dose and fractionation schemes). Reported median survival rates ranged from 6.4 months to 69.0 months (median survival of all patients in this study was 11 months). Median acute grade ≥3 and late grade ≥2 toxicity rates were 0.5% and 0.0%, respectively. They concluded that irrespective of the treatment setting, RT seems to be tolerable and safe for older patients, particularly in terms of late toxicity [34].
Sutera et al. [35] reported on 145 PDAC patients with a median age of 79 years. All were treated with SBRT to median dose of 36.0 Gy. While only 33.8% of patients underwent surgical resection, local control for all patients was 72.0% and 63.0% at 12 months and 24 months, suggesting SBRT provided durable control for patients who could not undergo surgery (control rates were not reported for this subset alone). Median survival was 14.0 months with an overall survival rate at 24 months of 27.0%. There was no statistically significant difference between patients who underwent surgery and those who did not on univariate and multivariate analysis of survival. Acute and chronic grade ≥2 toxicity occurred in 4.1% and 2.0%, respectively [35].
Zhu et al. [36] presented a propensity score-matched analysis of a prospectively collected database of 100 patients with early stage but medically inoperable pancreatic cancer who were treated with SBRT plus chemotherapy. They reported median overall survival and progression-free survival of 23.1 months and 18.0 months, respectively [36].
The data presented above showing relatively high control and survival rates, combined with the limited options for this patient population, led the International Society of Geriatric Oncology (SIOG) task force to recommend SBRT for pancreatic cancer when comorbidities preclude surgery [37]. SBRT may be preferred in this setting, as data has suggested longer survival with lower rates of toxicity than those provided by conventional fractionation [38,39].
SBRT was also recommended more recently for patients with LAPC who have a decline in ECOG performance status in the American Society of Clinical Oncology (ASCO) clinical practice guidelines [40].
Local Therapy in Oligometastatic Cases
Despite promising results from trials showing survival improvements in the treatment of oligometastatic disease, questions remain for malignancies with poor prognoses which were underrepresented in these trials [41]. Historically the role of localized therapy for MPC has been limited due to its poor prognosis, with median survival rates of less than six months [42]. So how do we approach a pancreatic cancer patient with a limited number of metastases? Is there a benefit to an aggressive approach to care in certain circumstances, or is palliative care the only option?
Several retrospective multicenter analyses have suggested that synchronous resection of liver metastases in MPC may provide a survival benefit without compromising safety and quality of life in a highly selected group of patients [42–45]. Investigators at Mayo Clinic reviewed twenty patients with MPC who underwent locoregional therapies performed by interventional radiologists. Median survival was 25.0 months from initial diagnosis of PDAC, with median survival from the time of MPC diagnosis and from the time of locoregional therapy of 16.3 and 9.7 months, respectively. Difference in median survival rates between responders and those with stable or progressive disease approached significance (9.0 vs. 6.0 months, p = 0.08), possibly limited by a low number patients in each subset [46].
Treatment of limited hepatic metastases with SBRT has shown promising results with high rates of local control in the setting of other primary malignancies such as colorectal cancer [47–49]. The data pertaining to treating hepatic metastases from PDAC with SBRT is limited but provides promising results.
Oladeru et al. [50] reported on the outcomes of 41 patients with MPC whose hepatic metastases were treated with SBRT to a median dose of 50.0 Gy (range: 8.0 – 60.0) in 5–6 fractions. At 12 months, rates of local control and overall survival were 75.8% and 36.3% respectively. Pre-RT CA19-9 was associated with local control on univariate analysis (HR 2.28, p = 0.03), while pre-RT CEA (HR 1.01, p = 0.03), pre-RT CA19-9 (HR 1.67, p = 0.01) and response to chemotherapy (HR 6.42, p <0.01) were associated with survival. Response to chemotherapy remained significant on multivariate analysis for overall survival [50].
There may also be clinical settings in which SBRT to the primary pancreatic lesion may be considered in patients with a limited number of metastases, such as a patient with oligometastatic disease that has been stable for more than six months or has responded well to systemic therapy [51].
Lischalk et al. [18] treated the primary pancreatic lesion in twenty patients with MPC using SBRT to doses of 25.0 – 30.0 Gy in five fractions. Twelve-month rates of local control and overall survival were 43.0% and 53.0% respectively. They found that patients with PTV’s smaller than 147.3 cc (the median PTV in their cohort) had high rates of 12month local control of 78.0% and had longer median survival (16.7 vs. 9.8 months, p = 0.09) and time to progression (17.8 vs. 3.0 months, p = 0.02) than patients with larger PTV’s. They also found a trend towards improvement in local control (17.8 vs. 4.3 months, p = 0.17) and median survival (16.7 vs. 9.7 months, p = 0.09) in patients who received concurrent versus sequential chemotherapy [18].
Gkika et al. [52] reported the results of eighteen patients with local recurrences (n = 9) or oligometastatic disease (n = 11) in whom the primary pancreatic lesion was treated via SBRT to a median dose of 37.5 Gy. Median survival was 13.2 months, with overall survival rates at 6 and 12 months of 87.0% and 58.0%, respectively. Freedom from local progression at 6 months and 12 months was 93.0% and 67.0%. One patient experienced grade ≥3 acute toxicity and 1 patient experienced a grade ≥3 late toxicity [52].
Herman et al. [53] reported on the safety with SBRT as liver directed therapy after Whipple resection or a biliary bypass. They urged caution when treating metastatic lesions with surgery, ablation, or radio embolization due to the potentially higher risk of liver abscess formation attributable to obligate colonization of the bile ducts from the biliary-enteric anastomoses [53]. This risk may be lower with the use of SBRT as this technique is noninvasive, and necrosis occurs more slowly when compared with ablation or trans arterial chemoembolization. However, further investigation is necessary [54].
Salvage for Patients who Fail Surgery or Radiation
Despite multimodal treatment, local disease progression and recurrence are common. The majority of patients develop disease recurrence within two years of surgical resection [55]. Management of recurrent or progressive PDAC proves challenging.
Moletta et al. [55] presented a systematic review of fourteen studies with 301 patients who experienced recurrence of PDAC after resection. The recurrence was local or regional in 230 patients and distant in 71. Median survival was 70.0 months after resection of the primary tumor, and 26.0 months after surgery for recurrent disease. The disease-free interval after the resection of recurrences was 14.2 months [55].
Groot et al. [56] published a systematic review of treatment options for this patient population. Eighteen studies were included, reporting on 313 patients who experienced isolated local recurrence after surgery and were salvaged with surgical re-resection, CRT or SBRT. Morbidity was lower with SBRT (3%) than surgery (29%) and CRT (54%), while median survival was higher with re-resection (32 months) than with CRT (19 months) and SBRT (16 months). Given the retrospective nature of this study, the possibility exists that baseline differences in patients who were surgical candidates versus those who were not could also contribute to survival differences [56].
SBRT has also been shown to be effective for salvaging patients who experience isolated local recurrence or progression following previous conventional CRT. Wild et al. reported on the safety and feasibility of re-irradiation in this setting [57]. Eighteen patients were treated with salvage SBRT to a median dose of 25.0 Gy (Range: 20.0–27.0 Gy) in 5 fractions after failing previous CRT +/− surgery. Six and twelve months progression free survival rates were 78.0% and 62.0%, respectively. Median survival was 8.8 months from completion of SBRT. Patients who had failed previous treatment within nine months had worse median survival than those who had a disease-free interval after initial treatment of more than nine months (3.4 vs. 11.3 months, p = 0.02).
Dagoglu et al. [58] reported on thirty patients who failed initial RT, including 20 patients treated with conventional fractionation and 10 treated with SBRT. All subsequently received salvage SBRT re-irradiation, with a median prescription dose of 25.0 Gy (range: 24.0 – 36.0) in 5 fractions with chemotherapy. Median survival was 14.0 months, and rates of 12 months and 24 months local control were both 78.0%. Grade 3 acute toxicity occurred in 10.0% of patients, and 7.0% developed grade 3 long-term bowel obstructions [58].
Sutera et al. [59] reviewed 38 patients with locally progressive or recurrent pancreatic cancer after previous conventionally fractionated RT who were treated with salvage SBRT re-irradiation to a median dose of 24.5 Gy in 1–3 fractions. The median survival from diagnosis was 26.6 months with a 24 months overall survival rate of 53.0%. Median survival from re-irradiation SBRT was 9.7 months. The 24 months rate of freedom from local and regional progression were 58.0% and 82.0%, respectively. Rates of late grade ≥2 and grade ≥3 toxicity were 18.4% and 10.5%, respectively [59].
SUMMARY
The treatment of pancreatic cancer continues to evolve. As the prognosis of this patient population improves with advancing systemic therapy regimens, additional clinical settings in which SBRT may provide benefit emerge. These include palliation of pain, providing durable local control in patients who are ineligible for surgery, treating primary and metastatic lesions in oligometastatic cases, and salvaging local failures. Several studies are currently evaluating combinations of SBRT with targeted agents such as PARP inhibitors to enhance the local tumor response. RT may also enhance the systemic immune response when combined with checkpoint inhibitors and/or vaccines. Additional studies are needed to evaluate how imaging and molecular and genomic predictors can be used to identify which patients with pancreatic cancer are most likely to benefit from SBRT (Figure 1).
Figure 1:
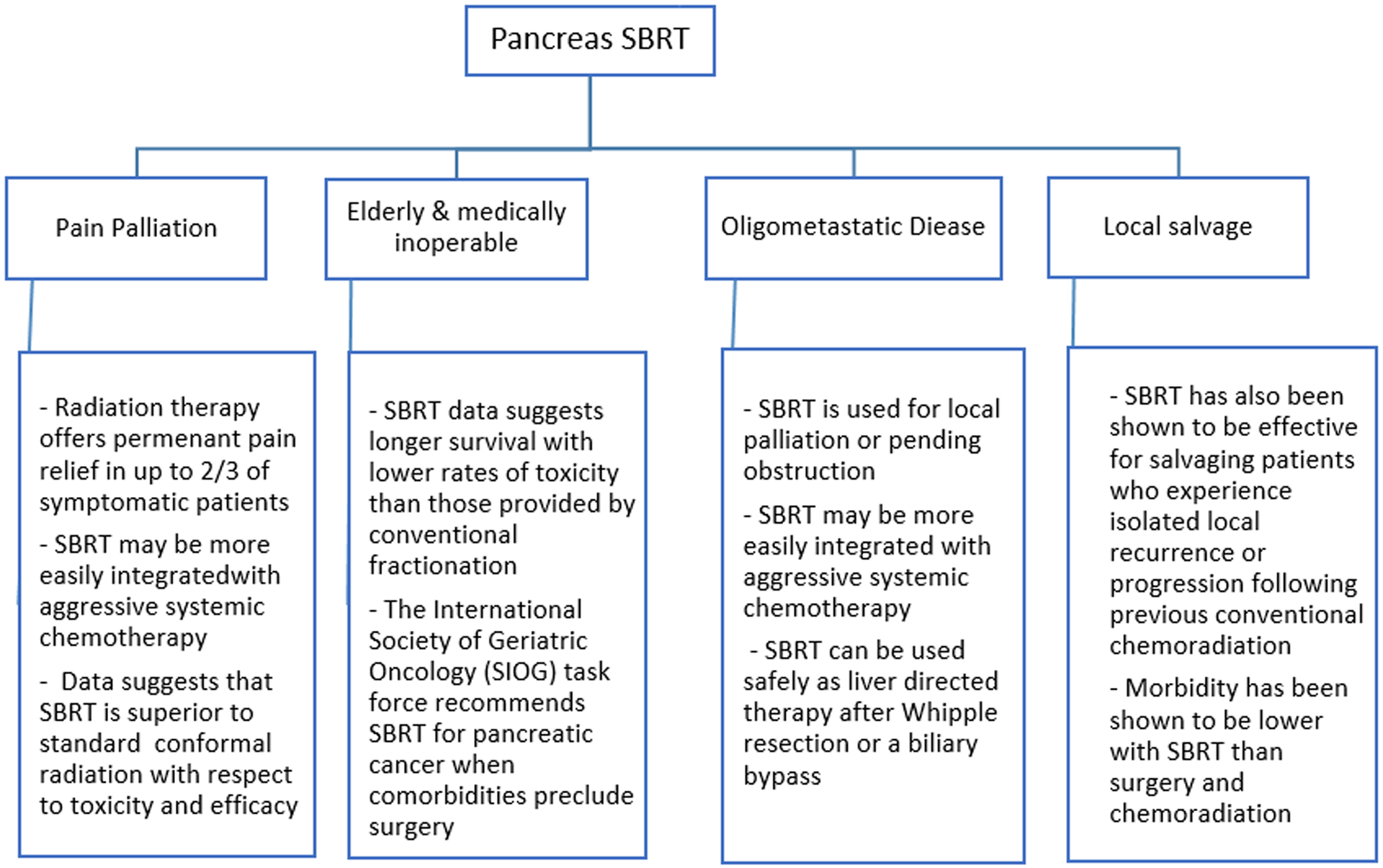
Expanding role of SBRT in pancreas cancer.
REFERENCES
- 1.Neoptolemos JP, Stocken DD, Friess H, et al. (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. The New England Journal of Medicine 350(12): 1200–1210. [DOI] [PubMed] [Google Scholar]
- 2.Stocken DD, Büchler MW, Dervenis C, et al. (2005) Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. British Journal of Cancer 92(8): 1372–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chauffert B, Mornex F, Bonnetain F, et al. (2008) Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Annals of Oncology 19(9): 1592–1599. [DOI] [PubMed] [Google Scholar]
- 4.Hammel P, Huguet F, van Laethem JL, et al. (2016) Effect of chemoradiotherapy vs. chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without Erlotinib: The LAP07 randomized clinical trial. JAMA 315(17): 1844–1853. [DOI] [PubMed] [Google Scholar]
- 5.Wild AT, Herman JM, Dholakia AS, et al. (2016) Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. International Journal of Radiation Oncology, Biology, Physics 94(3): 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghaly M, Gogineni E, Saif MW (2019) Theevolving field of stereotactic body radiation therapy in pancreatic cancer. Pancreas (Fairfax) 3(1): 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang DT, Schellenberg D, Shen J, et al. (2009) Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 115(3): 665–672. [DOI] [PubMed] [Google Scholar]
- 8.Pollom EL, Alagappan M, von Eyben R, et al. (2014) Single-versus multifraction stereotactic body radiation therapy for pancreatic adenocarcinoma: Outcomes and toxicity. International Journal of Radiation Oncology, Biology, Physics 90(4): 918–925. [DOI] [PubMed] [Google Scholar]
- 9.Mahadevan A, Miksad R, Goldstein M, et al. (2011) Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. International Journal of Radiation Oncology, Biology, Physics 81(4): e615–e622. [DOI] [PubMed] [Google Scholar]
- 10.Koay EJ, Hanania AN, Hall WA, et al. (2020) Dose-escalated radiation therapy for pancreatic cancer: A simultaneous integrated boost approach. Practical Radiation Oncology 10(6): e495–e507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passoni P, Reni M, Cattaneo GM, et al. (2013) Hypofractionated image-guided IMRT in advanced pancreatic cancer with simultaneous integrated boost to infiltrated vessels concomitant with capecitabine: A phase I study. International Journal of Radiation Oncology, Biology, Physics 87(5): 1000–1006. [DOI] [PubMed] [Google Scholar]
- 12.Oar A, Lee M, Le H, et al. (2020) Australasian gastrointestinal trials group (AGITG) and trans-tasman radiation oncology group (TROG) guidelines for pancreatic stereotactic body radiation therapy (SBRT). Practical Radiation Oncology 10(3): e136–e146. [DOI] [PubMed] [Google Scholar]
- 13.Jung J, Yoon SM, Park JH, et al. (2019) Stereotactic body radiation therapy for locally advanced pancreatic cancer. PLoS One 14(4): e0214970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebrahimi G, Rasch CRN, van Tienhoven G (2018) Pain relief after a short course of palliative radiotherapy in pancreatic cancer, the academic medical center (AMC) experience. Acta Oncologica 57(5): 697–700. [DOI] [PubMed] [Google Scholar]
- 15.Freelove R, Walling AD (2006) Pancreatic cancer: Diagnosis and management. American Family Physician 73(3): 485–492. [PubMed] [Google Scholar]
- 16.Buwenge M, Macchia G, Arcelli A, et al. (2018) Stereotactic radiotherapy of pancreatic cancer: A systematic review on pain relief. Journal of Pain Research. Dove Medical Press Ltd. 11: 2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruarus A, Vroomen L, Puijk R, et al. (2018) Locally advanced pancreatic cancer: A review of local ablative therapies. Cancers 10(1): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lischalk JW, Burke A, Chew J, et al. (2018) Five fraction stereotactic body radiation therapy (SBRT) and chemotherapy for the local management of metastatic pancreatic cancer. Journal of Gastrointestinal Cancer 49(2): 116–123. [DOI] [PubMed] [Google Scholar]
- 19.Kunk PR, Bauer TW, Slingluff CL, et al. (2016) From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. Journal for ImmunoTherapy of Cancer 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunkler IH, Audisio R, Belkacemi Y, et al. (2014) Review of current best practice and priorities for research in radiation oncology for elderly patients with cancer: The International society of geriatric oncology (SIOG) task force. Annals of Oncology 25(11): 2134–2146. [DOI] [PubMed] [Google Scholar]
- 21.Vernerey D, Huguet F, Vienot A, et al. (2016) Prognostic nomogram and score to predict overall survival in locally advanced untreated pancreatic cancer (PROLAP). British Journal of Cancer 115(3): 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tempero MA, Malafa MP, Al-Hawary M, et al. (2017) Pancreatic adenocarcinoma, version 2.2017: Clinical practice guidelines in oncology. JNCCN Journal of the National Comprehensive Cancer Network. Harborside Press; 15: 1028–1061. [DOI] [PubMed] [Google Scholar]
- 23.Katz MHG, Pisters PWT, Evans DB, et al. (2008) Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. Journal of the American College of Surgeons 206(5): 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Ren ZG, Ma NY, et al. (2015) Intensity modulated radiotherapy for locally advanced and metastatic pancreatic cancer: A mono-institutional retrospective analysis. Radiation Oncology 10: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharrett JM, Sarihan EI, Magnelli A, et al. (2018) Anatomically restricted dose escalated SBRT to the pancreas effects high local control and rapid and substantial pain relief. International Journal of Radiation Oncology, Biology, Physics 10(3): E77. [Google Scholar]
- 26.Herman JM, Chang DT, Goodman KA, et al. (2015) Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 121(7): 1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin JC, Jen YM, Li MH, et al. (2015) Comparing outcomes of stereotactic body radiotherapy with intensity-modulated radiotherapy for patients with locally advanced unresectable pancreatic cancer. European Journal of Gastroenterology and Hepatology 27(3): 259–264. [DOI] [PubMed] [Google Scholar]
- 28.(2019) Pancreatic cancer-cancer stat facts.
- 29.Higuera O, Ghanem I, Nasimi R, et al. (2016) Management of pancreatic cancer in the elderly. World Journal of Gastroenterology 22(2): 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu CC, Wolfgang CL, Laheru DA, et al. (2012) Early mortality risk score: Identification of poor outcomes following upfront surgery for resectable pancreatic cancer. Journal of Gastrointestinal Surgery 16(4): 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto K, Miyake Y, Kato H, et al. (2011) Effect of low-dose gemcitabine on unresectable pancreatic cancer in elderly patients. Digestion 84(3): 230–235. [DOI] [PubMed] [Google Scholar]
- 32.Koong AC, Le QT, Ho A, et al. (2004) Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. International Journal of Radiation Oncology, Biology, Physics 58(4): 1017–1021. [DOI] [PubMed] [Google Scholar]
- 33.Hodul P, Tansey J, Golts E, et al. (2001) Age is not a contraindication to pancreaticoduodenectomy. The American Journal of Surgery 67(3): 270–275. [DOI] [PubMed] [Google Scholar]
- 34.Ciabatti S, Cammelli S, Frakulli R, et al. (2019) Radiotherapy of pancreatic cancer in older patients: A systematic review. Journal of Geriatric Oncology 10(4): 534–539. [DOI] [PubMed] [Google Scholar]
- 35.Sutera PA, Bernard ME, Wang H, et al. (2018) Prognostic factors for elderly patients treated with stereotactic body radiation therapy for pancreatic adenocarcinoma. Frontiers of Oncology 8: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu X, Li F, Liu W, et al. (2018) Stereotactic body radiation therapy plus induction or adjuvant chemotherapy for early stage but medically inoperable pancreatic cancer: A propensity score-matched analysis of a prospectively collected database. Cancer Management and Research 10: 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunkler IH, Audisio R, Belkacemi Y, et al. (2014) Review of current best practice and priorities for research in radiation oncology for elderly patients with cancer: The International society of geriatric oncology (SIOG) task force. Annals of Oncology 25(11): 2134–2146. [DOI] [PubMed] [Google Scholar]
- 38.Zhong J, Patel K, Switchenko J, et al. (2017) Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer 123(18): 3486–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park JJ, Hajj C, Reyngold M, et al. (2017) Stereotactic body radiation vs. intensity-modulated radiation for unresectable pancreatic cancer. Acta Oncologica 56(12): 1746–1753. [DOI] [PubMed] [Google Scholar]
- 40.Balaban EP, Mangu PB, Khorana AA, et al. (2016) Locally advanced, unresectable pancreatic cancer: American society of clinical oncology clinical practice guideline. Journal of Clinical Oncology 13(4): 265–269. [DOI] [PubMed] [Google Scholar]
- 41.Palma DA, Olson R, Harrow S, et al. (2019) Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): Arandomised, phase 2, open-label trial. Lancet 393(10185): 2051–2058. [DOI] [PubMed] [Google Scholar]
- 42.Voss N, Izbicki JR, Nentwich MF (2019) Oligometastases in pancreatic cancer (Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis). Annals of Gastroenterological Surgery 3(4): 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hackert T, Niesen W, Hinz U, et al. (2017) Radical surgery of oligometastatic pancreatic cancer. European Journal of Surgical Oncology 43(2): 358–363. [DOI] [PubMed] [Google Scholar]
- 44.Tachezy M, Gebauer F, Janot M, et al. (2016) Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery 160(1): 136–144. [DOI] [PubMed] [Google Scholar]
- 45.Shrikhande SV., Kleeff J, Reiser C, et al. (2007) Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Annals of Surgical Oncology 14(1): 118–127. [DOI] [PubMed] [Google Scholar]
- 46.Bailey RE, Surapaneni PK, Core J, et al. (2019) Safety and efficacy of locoregional therapy for metastatic pancreatic ductal adenocarcinoma to the liver: A single-center experience. Journal of Gastrointestinal Oncology 10(4): 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodman BD, Mannina EM, Althouse SK, et al. (2016) Long-term safety and efficacy of stereotactic body radiation therapy for hepatic oligometastases. Practical Radiation Oncology 6(2): 86–95. [DOI] [PubMed] [Google Scholar]
- 48.Kress MAS, Collins BT, Collins SP, et al. (2012) Stereotactic body radiation therapy for liver metastases from colorectal cancer: Analysis of safety, feasibility, and early outcomes. Frontiers in Oncology 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.N K, V der Sluis WB (2013) Stereotactic ablative radiotherapy to treat colorectal liver metastases: Ready for prime-time? Journal of Liver 2: 5. [Google Scholar]
- 50.Oladeru OT, Vaios EJ, Eyler C, et al. (2019) Outcomes following liver SBRT for metastatic pancreatic cancer. Journal of Clinical Oncology 37(4_suppl): 418–418. [Google Scholar]
- 51.Herman JM, Hoffman JP, Thayer SP, et al. (2015) Management of the primary tumor and limited metastases in patients with metastatic pancreatic cancer. Journal of the National Comprehensive Cancer Network 13(5): e29. [DOI] [PubMed] [Google Scholar]
- 52.Gkika E, Adebahr S, Kirste S, et al. (2017) Stereotactic body radiotherapy (SBRT) in recurrent or oligometastatic pancreatic cancer: Atoxicity review of simultaneous integrated protection (SIP) versus conventional SBRT. Strahlentherapie und Onkologie 193(6): 433–443. [DOI] [PubMed] [Google Scholar]
- 53.Groot VP, van Santvoort HC, Rombouts SJE, et al. (2017) Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery, re-resection, chemoradiotherapy and SBRT. HPB (Oxford) 19(2): 83–92. [DOI] [PubMed] [Google Scholar]
- 54.De Jong MC, Farnell MB, Sclabas G, et al. (2010) Liver-directed therapy for hepatic metastases in patients undergoing pancreaticoduodenectomy: a dual-center analysis. Annals of Surgery 252(1): 142–148. [DOI] [PubMed] [Google Scholar]
- 55.Herman HM, et al. (2015) Management of the primary tumor and limited metastases in patients with metastatic pancreatic cancer. Journal of the National Comprehensive Cancer Network 13(5): e29–36. [DOI] [PubMed] [Google Scholar]
- 56.Moletta L, Serafini S, Valmasoni M, et al. (2019) Surgery for recurrent pancreatic cancer: Is it effective? Cancers 11(7): 991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wild AT, Hiniker SM, Chang DT, et al. (2013) Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: Experience from two institutions. Journal of Gastrointestinal Oncology 4(4): 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dagoglu RN, Callery M, Moser J, et al. (2015) Stereotactic body radiotherapy (SBRT) reirradiation for recurrent pancreas cancer. Journal of Clinical Oncology 7(3): 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sutera P, Bernard ME, Wang H, et al. (2018) Stereotactic body radiation therapy for locally progressive and recurrent pancreatic cancer after prior radiation. Frontiers in Oncology 8: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]


