Abstract
BACKGROUND
The cancer stem cell (CSC) hypothesis of tumor genesis suggests that unlike most cancer cells within tumor CSC resist chemotherapy and can regenerate various cell types in tumor thereby causing relapse. Hence drugs that selectively target CSC may offer great promise for cancer therapy especially when combined with chemotherapy. Current treatment options for colorectal cancer (CRC) and other gastrointestinal (GI) tumors rely on combination of surgical resection, cytotoxic and targeted drugs. Recent findings showed that metformin, an ant diabetic drug was associated with a significantly lower risk of CRC (0.63 [0.47 – 0.84]; P = 0.002) in patients with type 2 diabetes. We therefore hypothesize that administration of metformin will reduce CSC.
METHODS
Patients with CRC and other GI cancers undergoing resection were enrolled. Metformin was administered at 500 mg orally twice daily for up to 14 days and terminated 24 hours, prior to planned surgery. Both tumor and normal tissue was procured. Adverse events (AEs) were graded according to NCI CTCAE Version 3.0. Primary objective was to establish the safety of administering metformin prior to resection. Secondary objective was to evaluate the effects of metformin on the expression of CSC markers by measuring relative mRNA levels of CD133, OCT4 and NANOG by RT-PCR and immunohistochemistry.
RESULTS
A total of 10 patients (4 Male; 6 Female) received metformin. Grade 3 AEs included anemia, hypoalbuminemia, alanine aminotransferase elevation, abdominal pain and nausea but none of these were related to metformin. No hypoglycemia and lactic acidosis were observed. No unexpected post-operative complications were witnessed. Comparison of markers of CCSC results showed that expression of CD133, OCT4 and NANOG expression were decreased following metformin.
CONCLUSIONS
Our pilot study showed feasibility of metformin before surgery in GI cancers and indicated impact on CSC. This preliminary data warrants further investigation in a larger randomized placebo-control study to assess these markers and their correlation with survival.
Keywords: Metformin, Chemotherapy, mTOR, AMP-activated protein kinase (AMPK), Biguanide, Anti-diabetic, Diabetes, Cancer, Colorectal cancer, Cancer stem cells
INTRODUCTION
Colorectal cancer (CRC) continues to cause major mortality and morbidity burdens on our society. Significant progress has been made for patients with metastatic disease and localized disease by improving surgical and chemotherapeutic approaches over the past decade [1,2]. Patients with localized disease undergo potentially curative resection, yet despite no visible disease at the time of resection, many of these patients still have recurrent disease at some point in the future [3,4]. The implementation of adjuvant and/or neoadjuvant chemotherapy has clearly reduced the incidence of recurrence in such patients [3–5]. This suggests that such chemotherapy is likely to achieve elimination of tumor cells in a subset of patients.
One theory that attempts to explain the ability of cancer to evade therapeutic intervention describes cancer stem cells [6]. Cancer stem cells (CSCs) populate the minority of any given tumor mass; however it is from these cells that the tumor is able to regenerate. CSCs tend to be slowly replicating and lack certain surface antigens, and thus can be more resistant to many chemotherapeutic insults compared to the more abundant, mature, rapidly dividing cells in the bulk of the tumor [7,8]. Thus, while initial chemotherapy may be able to reduce tumor volume, the tumor mass can be repopulated by these CSCs. Efforts toward the goal of elimination should be directed toward enhancing elimination of CSCs.
Agents that modulate AMPK have been reported to enhance CSC skilling and delay/prevent tumor xenografts re-growth when combined with a chemotherapeutic agent [9]. Metformin, which is known to act through AMPK, has been shown to have ant proliferative activity against colorectal cancer cell lines, and this effect is most prominent in the p53−/− setting [9–11]. From a cancer prevention perspective, metformin suppresses polyp growth in Apc Min/+ Mice, and population studies have shown diabetics taking metformin have lower incidence of cancer [12–14].
An important link between AMPK/glucose metabolism and colorectal cancer is the observation that Peutz-Jeghers syndrome involves a mutation in the LKB1 tumor suppressor gene, and the finding that LKB1 acts through AMPK for signaling [15–17]. Peutz-Jeghers syndrome is a hereditary polyposis syndrome associated with a high risk for gastrointestinal cancers. We therefore hypothesize that administration of metformin will reduce CCSC in human CRC. The implication of this could be enhanced elimination of CCSC and ultimately increased portion of patients who remain disease-free following chemotherapy treatment in the adjuvant or neoadjuvant setting.
It is important to discuss here that the approaches to eliminate CSCs is challenging for several reasons. Since the cells often represent only a small fraction of the tumor volume, many bioassays may overlook the CSC population amidst more prominent signals within a tumor. Recently the ability to better define and identify CSCs has developed. Functionally, a CSC must be able to self-renew and be able to recapitulate a tumor [18–20].
A number of cell-surface markers have been discovered that are associated with normal stem cells, some of which appear also to be associated with CSCs. CD133 is a cell-surface marker that identifies circulating endothelial progenitors [21]. It turns out that CD133 is associated with a number of tumors, including CRC, and predicts for poor outcome [22,23]. Demonstration that a CD133+ population enriches for CCSC has also been described [24,25]. Hence, CD133+ staining can be used as major determinant of effect of any agent such as metformin on CSC.
Other pharmacodynamic markers associated with CCSC include OCT4 and NANOG. Both OCT4 and NANOG are transcription factors that play significant roles in maintaining pluripotency and self-renewal of embryonic stem cells as well as in adult stem cells [26,27]. OCT4 is a nuclear protein belonging to a family of transcription factors containing the POU DNA-binding domain [28]. NANOG is a homeobox transcription factor that contains DNA-binding domains involved in the regulation of key eukaryotic developmental processes [29].
We therefore hypothesize that administration of metformin will reduce CSC and performed a pilot study in patients with CRC and other GI cancers undergoing resection.
PATIENTS AND METHODS
The study was approved by Institutional review board at the Tufts Medical Center, Boston, MA and was conducted in compliance with the International Council for Harmonization Good Clinical Practice guidelines and with the general ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all patients before study entry. This study was registered on ClinicalTrials.gov under NCT01440127 and enrolled patients from 2010 through 2013.
Metformin was administered at 500mg orally twice daily for up to 14 days and terminated 24 hours. prior to planned surgery. Both tumor and normal tissue was procured. Adverse events (AEs) were collected and graded according to NCI CTCAE Version 3.0 [30]. Primary objective was to establish the safety of administering metformin prior to resection. Secondary objective was to evaluate the effects of metformin on the expression of CSC markers by measuring relative mRNA levels of CD133, OCT4 and NANOG by RT-PCR and immunohistochemistry.
Patient selection
Patients were enrolled if they met the following criteria: histologically documented colorectal, appendiceal cancer or peritoneal carcinomatosis from gastrointestinal source, who were intended to undergo disease resection or biopsy at least 14 days from the treatment start date (allowing for a minimum of 12 days of treatment plus 24 hours break), medically fit for resection of their primary tumor or for biopsy, age 18 years - 79 years, adequate renal function (serum creatinine levels <1.5 mg/dL [males], <1.4 mg/dL [females] or estimated creatinine clearance ≥60 ml/min), adequate hepatic parameters, including aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels ≤2.5 × Upper limit of normal (ULN), total bilirubin ≤1.5 × ULN, and alkaline phosphatase levels ≤2.5 × ULN, and ability to understand and willingness to sign a written informed consent document.
Patients with the following situations were excluded: intent to administer neoadjuvant chemotherapy or radiation therapy during study treatment period prior to the surgery or biopsy, intent to perform surgery or biopsy within 14 days of study treatment start; any situation where participation in this trial would alter, or cause significant risk of altering the ability or timing of a subject to undergo resection of their tumor, current use of metformin (within the past month), blood glucose using point of care test <70 mg/dl, renal disease or renal dysfunction not meeting inclusion criteria, significant medical conditions such as cardiovascular collapse (shock), acute myocardial infarction, septicemia, acute or chronic metabolic acidosis, history of, or states associated with, lactic acidosis such as shock or pulmonary insufficiency, alcoholism (acute or chronic), conditions associated with hypoxemia and pancreatitis, severe dehydration, clinical or laboratory evidence of hepatic disease, congestive heart failure requiring pharmacologic treatment, or unstable or acute congestive heart failure, known hypersensitivity to metformin hydrochloride, pregnant or lactating women (pregnancy test must be negative), and psychiatric illness or social situation that would limit compliance with study requirements and/or obscure results.
Study Design
Adult patients with CRC for whom biopsy, or resection of disease was planned, but who did not require neoadjuvant chemo or radiotherapy were enrolled onto this study. This trial will be divided into two stages (Figure 1). The first/pilot stage assessed safety of administering metformin to subject prior to surgery. We plan to perform the second stage as a randomized study treating subjects with or without metformin prior to tissue removal (primary tumor).
Figure 1:
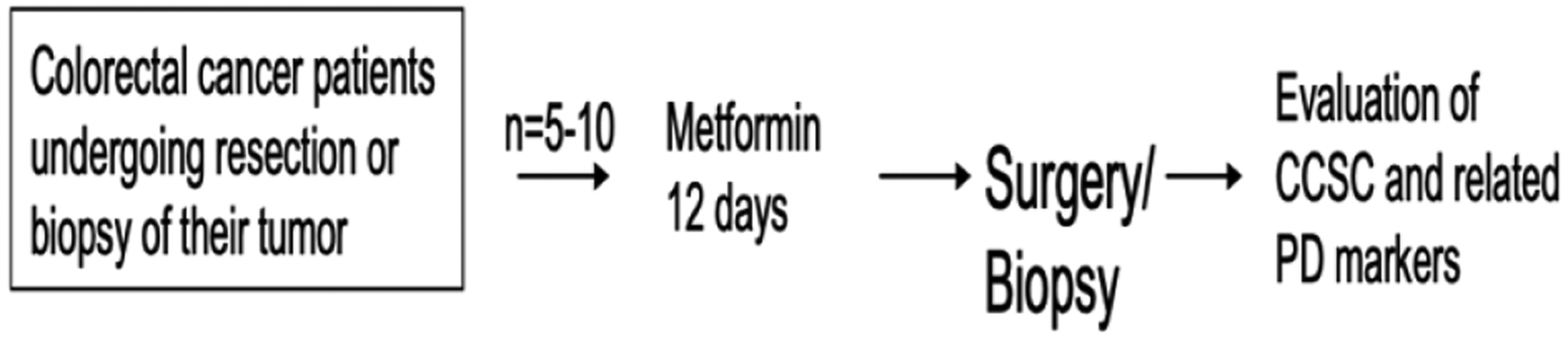
Study schema.
The main objective of this trial was to assess safety of metformin treatment prior to tumor resection or biopsy and to collect CRC or other GI cancers specimens at the time of removal for the purpose of optimizing a reliable platform for evaluating CCSC and related pharmacodynamic markers. Secondary aims included the measure CCSC and CCSC pharmacodynamic markers in subject tumors.
The pilot study panned to enroll up to 5 patients - 10 patients who received treatment with metformin with the goal of establishing safety of this treatment in a population of patients planning to undergo tumor resection or biopsy. The brief duration of treatment with metformin and break is short enough not to interfere with standard of care for this population; however, subjects who are intended to have their surgery or biopsy in less 14 days of treatment start will be excluded.
Importantly, based on prior work in a different system, an exposure to metformin of 2 or more days is likely to be sufficient to induce an effect on CSC [31]. This timeframe was evaluated in Stage 1 of this protocol. Metformin was discontinued 2 days prior to the surgical resection as recommended in the product label. This time period is likely sufficiently short as to not cause significant reversal of any effect on CCSC.
The metformin dose for this study of 500 mg twice daily has been safely administered to non-diabetic patients in other settings. We also performed the largest study in cancer patients and confirmed safety of administrating metformin at this dose in combination with various chemotherapeutic regimens [32]. This dose is also expected to yield exposures in the 1–2 ug/ml range at steady-state based on information in the product label [33]. It is not currently known whether this exposure is sufficient to affect CCSC; however this question was addressed in this study.
The resection and timing of the resection of the subject’s tumor was not being altered from the planned standard of care when a subject participated on this study. If a subject’s planned surgery was less than 14 days from the time of treatment start, then the subject was not be enrolled, and the surgery was not be delayed on account of this protocol.
Assessment of Safety
The safety of administering metformin prior to surgery or biopsy, ending 24 hours prior to the procedure was assessed. Adverse events (AEs) were graded according to NCI CTCAE Version 3.0 [30]. Metformin was discontinued if the patient experienced any grade ≥3 AE that was considered related to metformin, or any AE that could compromise patient’s ability to proceed with surgical intervention. A descriptive analysis of safety parameters covering the period prior to surgery, the surgery itself, and the recovery from surgery was prepared. An institutional Data and Safety Monitoring Board (DSMB) was utilized, and was responsible for periodic safety review. The committee reviewed safety at least quarterly.
Tumor procurement and Procedures for CCSC
Resection and initial surgical pathologic evaluation of the tumor occurred using standard procedures. Once the pathologist was satisfied that adequate tissue and evaluation for surgical staging have been obtained, a portion of the primary tumor was processed for evaluation of CCSC and related PD markers. A sample at least 5 mm3 was desired. Microdissection technique was performed to collect only cancer cells, excluding stromal cells as much as possible. Then micro dissected samples were digested with proteinase K in lysis buffer containing Tris-HCl, EDTA and sodium dodecyl sulfate as previously reported with minor modifications [34–38]. RNA was purified by phenol and chloroform extraction. cDNA was synthesized with random hexamer primers and Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Then real-time quantitative RT-PCR analysis was performed using an Applied Biosystems 7500 real-time PCR system according to the manufacturer’s instructions (Applied Biosystems, Inc., Foster City, CA, USA). Primers and probes for PROM (CD133), POU5F1 (OCT4), and NANOG designed with primer3 software (Biology Workbench Version 3.2, San Diego Supercomputer Center, University of California, San Diego, CA, USA). Primer sequences were as follows: PROM-specific: sense, GCT TTGCA ATCTCCCTGTTG and antisense, TTGATCCGG GTTCTTACCTG; POU5F1-specific: sense, CTGGAGAAGG AGA AGCTGGA and antisense, CA A ATTGCTCGAGTTCT TTCTG; NANOG-specific: sense, GAGATGCCTCACACG GAGAC and antisense, CTTTGGGACTGGTGGA AGA A. PCR was performed in a final volume of 25 μl with a SYBR-Green PCR master mix using 1 μl cDNA and 400 nM of each primer for the respective genes. Cycling conditions were 50°C for 2 min and 95°C for 10 min followed by 40 cycles at 95°C for 15 sec and 60°C for 1 minute. Relative mRNA levels were determined by the standard curve method as previously described. Real-time PCR assays were performed in duplicate for each sample and the mean values were used for calculations of the mRNA levels.
Statistical Analysis
The pilot study panned to enroll up to 5 patients - 10 patients who received metformin before undergoing tumor resection or biopsy with the aim to of establish safety in this setting. For each study subject, we collected demographic data, medical history data, and information on the course of their cancer and effect on CSC.
All statistical analyses were performed using JMP version 5 (SAS Institute Inc. Cary, NC, USA). Relative mRNA levels of each gene were expressed as median values (inter-quartile range). Box and whisker plots were used to summarize the distribution of mRNA levels, in which the horizontal line in the box represents the 50th quartile (median), and the upper and lower lines of the box represent 75th quartiles and 25th quartiles, respectively. The whiskers indicate the range of the measurements. P <0.05 was considered to be statistically significant.
RESULTS
Demographics
A total of 10 patients were consented (4 males; 6 females) and received metformin as summarized in Table 1. The most common site was appendix followed by rectum. Nine patients completed the full course of metformin while one first ended up in urgent surgery and took it only for 6 days. Median duration of metformin treatment was 12 days (Range: 6–12).
Table 1:
Summary of demographic characteristics of patients enrolled in the study.
| Patient # | Age | Gentler | Diagnosis | Stage |
|---|---|---|---|---|
| I | 63 | F | Signet ring adenocarcinoma of appendix | III |
| 2 | 53 | F | Mucinous adenocarcinoma of appendix | IVA |
| 3 | 39 | F | Adenocarcinoma of appendix | II |
| 4 | 51 | F | Mucinous adenocarcinoma of appendix | III |
| 5 | 58 | F | Rectal adenocarcinoma | IV |
| 6 | 75 | M | Rectal adenocarcinoma | IIIA |
| 7 | 74 | M | Rectal adenocarcinoma | IIIB |
| 8 | 61 | M | Rectal adenocarcinoma | III |
| 9 | 61 | M | Mucinous adenocarcinoma of appendix | IV |
| 10 | 41 | F | Pseudomyxoma peritonei | UK |
Safety
Overall, metformin was tolerated well. Grade ≥3 AEs included anemia (1; related to underlying cancer), hypoalbuminemia (1; unrelated to metformin), alanine aminotransferase elevation (1: possibly related to liver metastasis), abdominal pain (1; probable but possibly related to underlying cancer) and nausea (1; thought to be not related to metformin). Neither hypoglycemia nor lactic acidosis was observed. No unexpected post-operative complications were witnessed.
CCSC markers
Relative mRNA levels of CD133 (PROM), OCT4 (POU5F1) and NANOG were measured using real-time reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemistry. Comparison of post-metformin levels of CCSTs markers to pre-metformin showed that expression of CD133, OCT4 and NANOG were all decreased following metformin (Figure 2 – Figure 4).
Figure 2:
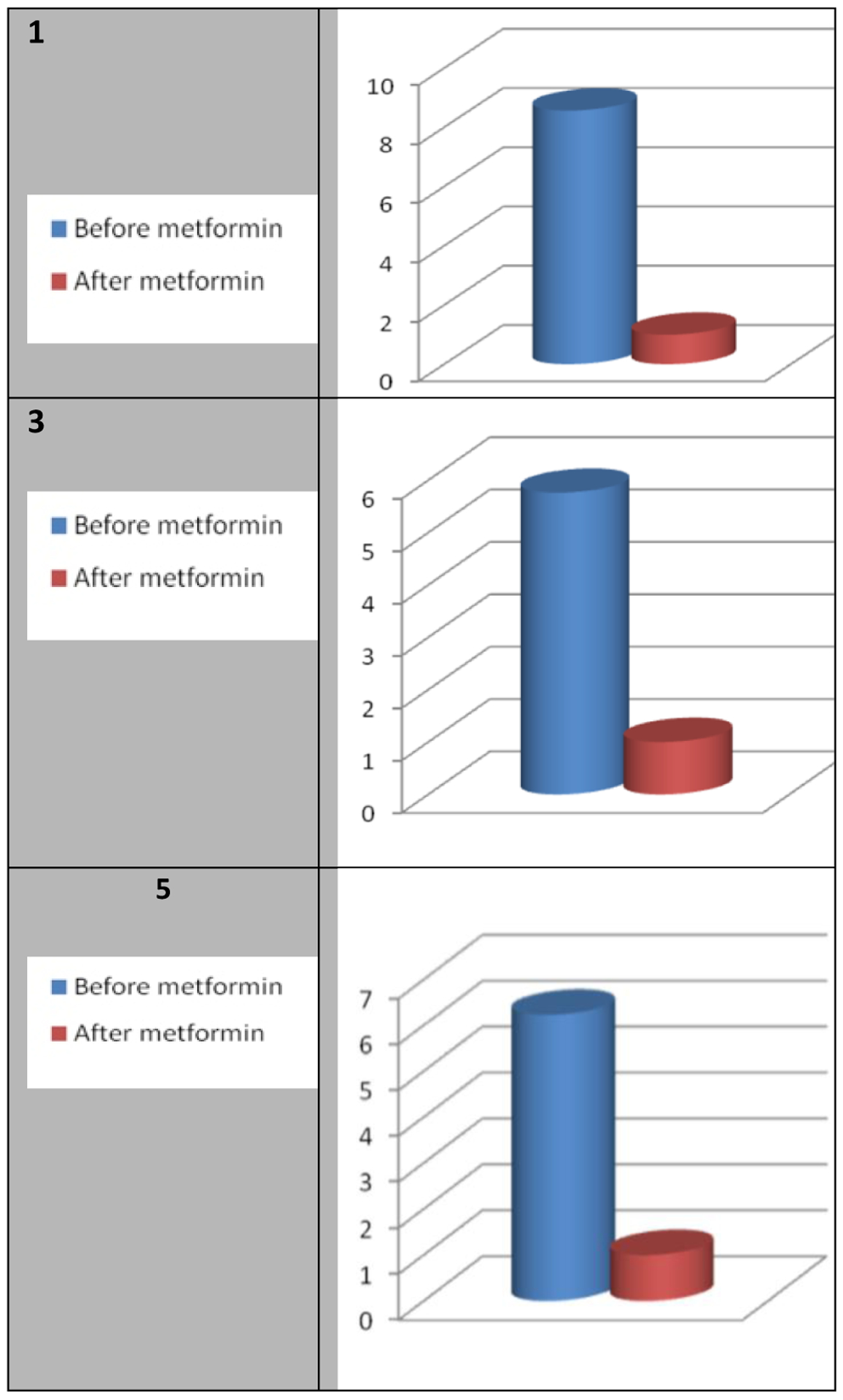
Effect of metformin on the relative mRNA levels of CD133 (PROM): Fold decrease.
Figure 4:
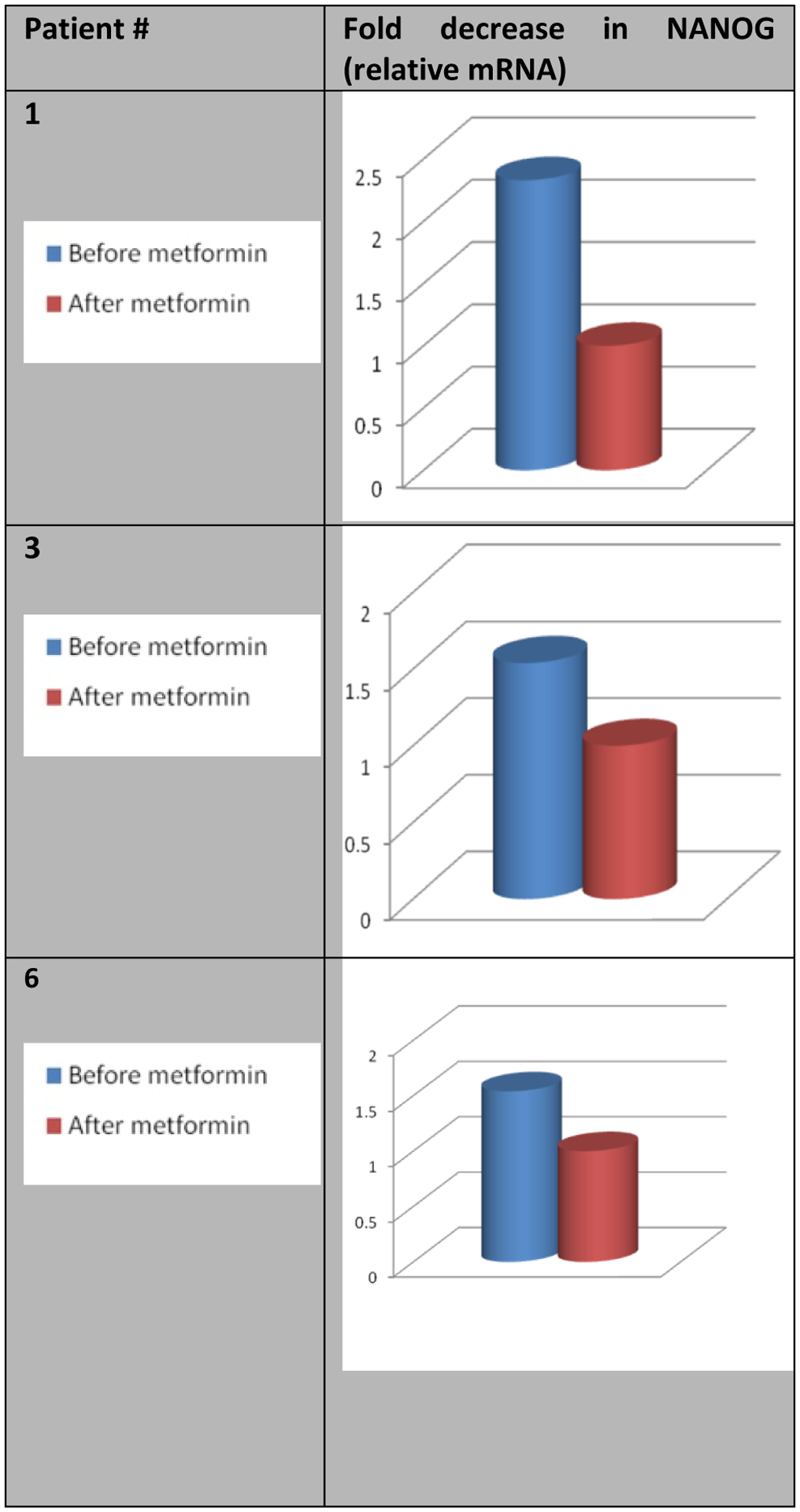
Effect of metformin on the relative mRNA levels of NANOG.
DISCUSSION
To the best of our knowledge, no report has investigated the expression of the three stem cell markers considered in this study in the context of CRC and GI malignancies following metformin. Our pilot study showed feasibility of metformin before surgery and indicated impact on CRC stem cells by reducing the expression of CD133, OCT4 and NANOG [21–28]. This preliminary data warrants further investigation in a larger, randomized placebo control study, which should include assessing these markers and correlating them with survival end points. We intend to perform a randomized study treating subjects with or without metformin prior to tissue removal (primary tumor). Assessment of CCSC and related pharmacodynamic markers will also be performed.
Implementation of neoadjuvant therapy, including a therapy targeting CRC and GI cancers stem cells in a patient undergoing curative intervention holds promise for enhancing elimination of the tumor [31]. A highly relevant setting for study of the impact of such therapy on CCSC is in the actual tumor itself [41–43]. The standard of care for patients with stages I-III CRC is initial resection of their tumor, followed in more advanced cases by adjuvant chemotherapy.
Since neoadjuvant therapy is typically not given to this population and since there is typically a window of several weeks from the time that the decision to do surgery is made and the actual surgery, there is an opportunity to evaluate the impact of short exposures of novel agents on the tumor. A similar opportunity exists to study metastatic disease considering that a portion of patients with oligo-metastasis are candidates for resection of their metastatic disease or alternatively, patients with metastatic CRC and other GI malignancies require biopsy of their disease.
It is known fact now that CSC are capable of self-renewal and pluripotency [6,7,12,28,44]. In addition to their contribution to normal tissue development, regeneration and disease, they also seem to play a role in resistance to chemotherapy possibly by the fact that the tumor mass can be repopulated by these CSCs. Therefore, efforts have been advanced to eliminate CSCs. Researchers have been working hard to develop techniques of isolation techniques for cell surface markers for CSCs as well as functional assays. CD133 is probably one of the most recognized cell surface markers of CCSCs. CD133 immunoreactivity has been found to be a prognostic marker for sporadic. Similarly, CSC markers, including OCT4 and NANOG have also been found to be expressed in many cancers, including CRC. OCT4, the gene encoding the POU domain, class 5, transcription factor 1 also called POU5F1 plays a role both in embryonic stem cells (ES) by maintaining the pluripotency and self-renewal of ES cells as well as CSC regulating the proliferation and differentiation, especially epithelial-mesenchymal transition (EMT) [9,22,28]. Some investigators also assess OCT4 in CRC for risk of liver metastases. Our study further supported the role of these CSC markers and above all the impact of metformin on them.
In short, the implementation of adjuvant and/or neoadjuvant chemotherapy for CRC and other GI cancers has clearly given the evidence in reducing the recurrence as well as improved survival the incidence of recurrence. This suggests that such chemotherapy is likely to achieve elimination of tumor cells in a subset of patients. The purpose of this protocol is to explore approaches to enhance this elimination by focusing on the subset of CCSC. While cytotoxic chemotherapy is known to reduce tumor volume, the tumor mass can be repopulated by CCSC. Efforts toward the goal of elimination should be directed toward enhancing elimination of CSCs.
Our pilot study showed that metformin can be administered safely assesses safety prior to tumor resection or biopsy of primary CRC. In addition, we demonstrated that administration of metformin pre-operatively to CRC patients resulted in decreasing the markers of CCSC; including CD133, OCT4 and NANOG evidencing the hypothesis that sufficient to induce an effect on cancer stem cells. The first part I of study was feasibility and hence limited by the relatively small number of patients (n = 10), but we plan to enroll patients to the second part of the study to further confirm our results.
Figure 3:
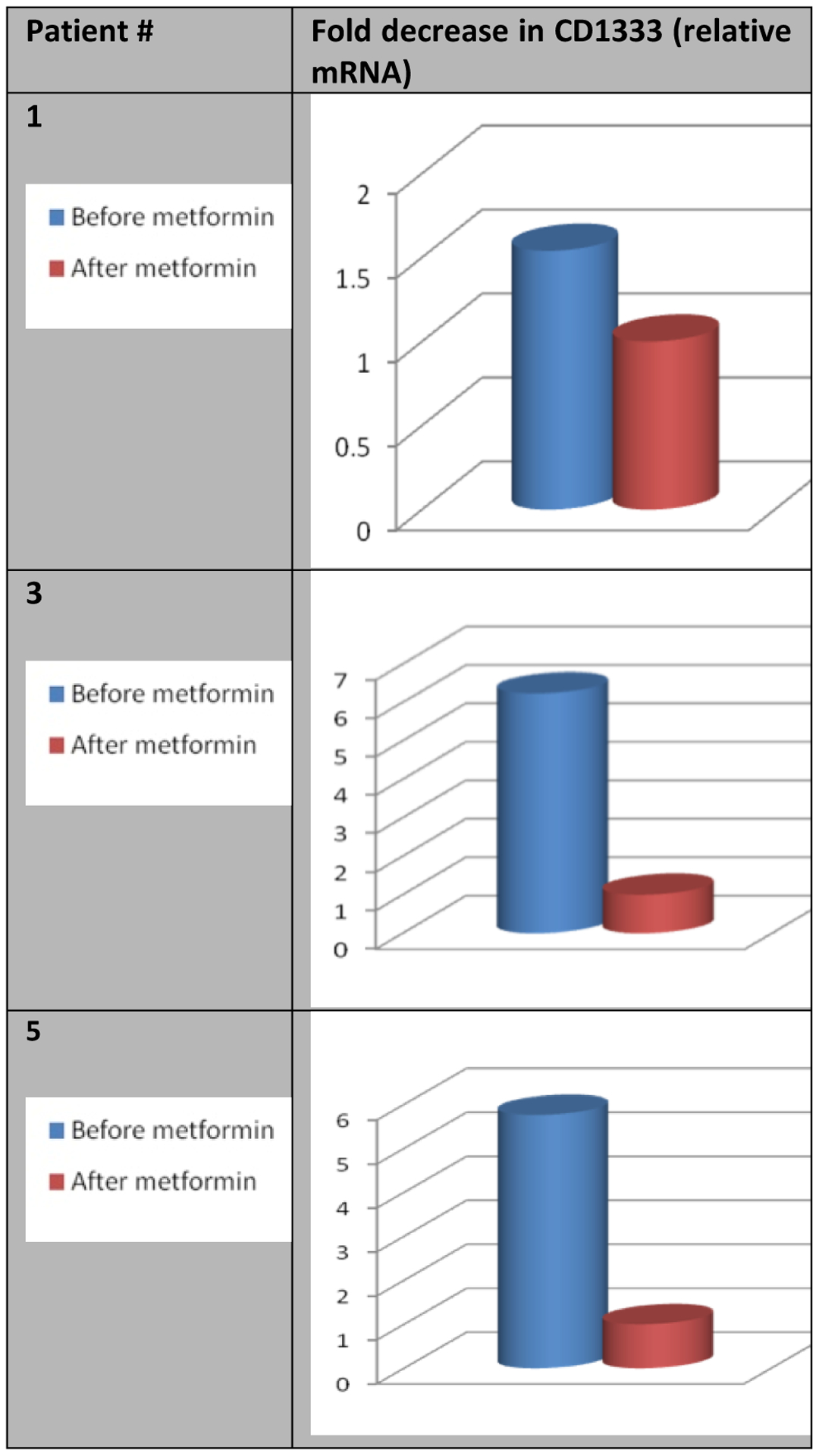
Effect of metformin on the relative mRNA levels of OCT4 (POU5F1).
REFERENCES
- 1.Siegel RL, Miller KD, Sauer GA, et al. (2020) Colorectal cancer statistics. CA: A Cancer Journal for Clinicians 70: 145164. [DOI] [PubMed] [Google Scholar]
- 2.Willett C, Tepper JE, Cohen A, et al. (1984) Local failure following curative resection of colonic adenocarcinoma. International Journal of Radiation Oncology, Biology, Physics 10(5): 645–651. [DOI] [PubMed] [Google Scholar]
- 3.Benson AB, Schrag D, Somerfield MR, et al. (2004) American society of clinical oncology recommendations on adjuvant chemotherapy for stage II colon cancer. Journal of Clinical Oncology 22(16): 3408–3419. [DOI] [PubMed] [Google Scholar]
- 4.André T, Boni C, Navarro M, et al. (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. Journal of Clinical Oncology 27(19): 3109–3116. [DOI] [PubMed] [Google Scholar]
- 5.Nordlinger B, Sorbye H, Glimelius B, et al. (2008) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 371(9617): 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin EH, Jiang Y, Deng Y, et al. (2008) Cancer stem cells, endothelial progenitors, and mesenchymal stem cells: “Seed and soil” theory revisited. Gastrointestinal Cancer Research 2(4): 169–174. www.tridhascholars.org | May-2021 [PMC free article] [PubMed] [Google Scholar]
- 7.Vermeulen L, Sprick MR, Kemper K, et al. (2008) Cancer stem cells - old concepts, new insights. Cell Death & Differentiation 15(6): 947–958. [DOI] [PubMed] [Google Scholar]
- 8.Shipitsin M, Polyak K (2008) The cancer stem cell hypothesis: In search of definitions, markers, and relevance. Laboratory Investigation 88: 459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch HA, Iliopoulos D, Tsichlis PN, et al. (2009) Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Research 69(19): 7507–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zakikhani M, Dowling RJ, Sonenberg N, et al. (2008) The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prevention Research (Phila Pa) 1(5): 369–375. [DOI] [PubMed] [Google Scholar]
- 11.Buzzai M, Jones RG, Amaravadi RK, et al. (2007) Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Research 67(14): 6745–6752. [DOI] [PubMed] [Google Scholar]
- 12.Tomimoto A, Endo H, Sugiyama M, et al. (2008) Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Science 99(11): 2136–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currie CJ, Poole CD, Gale EA (2009) The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52(9): 1766–1777. [DOI] [PubMed] [Google Scholar]
- 14.Evans JM, Donnelly LA, Smith EAM, et al. (2005) Metformin and reduced risk of cancer in diabetic patients. BMJ 330 (7503): 1304–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehenni H, Gehrig C, Nezu J, et al. (1998) Loss of LKB1 kinase activity in Peutz-Jeghers syndrome, and evidence for allelic and locus heterogeneity. American Journal of Human Genetics 63(6): 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw RJ, Kosmatka M, Bardeesy N, et al. (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proceedings of the National Academy of Sciences of the United States of America 101(10): 3329–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods A, Johnstone SR, Dickerson K, et al. (2003) LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Current Biology 13(22): 2004–2008. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien CA, Pollett A, Gallinger S, et al. (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445(7123): 106–110. [DOI] [PubMed] [Google Scholar]
- 19.Dalerba P, Dylla SJ, Park IK, et al. (2007) Phenotypic characterization of human colorectal cancer stem cells. Proceedings of the National Academy of Sciences of the United States of America 104(24): 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanzoni G, Roda G, Belluzzi A, et al. (2008) Inflammatory bowel disease: Moving toward a stem cell-based therapy. World Journal of Gastroenterology 14(29): 4616–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin AH, Miraglia S, Zanjani ED, et al. (1997) AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90(12): 5002–5012. [PubMed] [Google Scholar]
- 22.Lin EH, Hassan M, Li Y, et al. (2007) Elevated circulating endothelial progenitor marker CD133 messenger RNA levels predict colon cancer recurrence. Cancer 110(3): 534–542. [DOI] [PubMed] [Google Scholar]
- 23.Mehra N, Penning M, Maas J, et al. (2006) Progenitor marker CD133 mRNA is elevated in peripheral blood of cancer patients with bone metastases. Clinical Cancer Research 12(16): 4859–4866. [DOI] [PubMed] [Google Scholar]
- 24.Horst D, Kriegl L, Engel J, et al. (2008) CD133 expression is an independent prognostic marker for low survival in colorectal cancer. British Journal of Cancer 99: 1285–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445(7123): 111–115. [DOI] [PubMed] [Google Scholar]
- 26.Munro MJ, Wickremesekera SK, Peng L, et al. (2019) Cancer stem cell subpopulations in primary colon adenocarcinoma. PLoS ONE 14(9): e0221963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boman BM, Huang E (2008) Human colon cancer stem cells: A new paradigm in gastrointestinal oncology. Journal of Clinical Oncology 26(17): 2828–2838. [DOI] [PubMed] [Google Scholar]
- 28.Fujino S, Miyoshi N (2019) Oct4 gene expression in primary colorectal cancer promotes liver metastasis. Stem Cells International 2019: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Levasseur DN, Orkin SH (2008) Requirement of nanog dimerization for stem cell self-renewal and pluripotency. Proceedings of the National Academy of Sciences of the United States of America 105(17): 6326–6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5×11.pdf .
- 31.Higurashi T, Hosono K, Takahashi H, et al. (2016) Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicenter double-blind, placebo-controlled, randomised phase 3 trial. The Lancet Oncology 17(4): 475–483. [DOI] [PubMed] [Google Scholar]
- 32.Saif MW, Rajagopal S, Caplain J, et al. (2019) A phase I delayed-start, randomized and pharmacodynamic study of metformin and chemotherapy in patients with solid tumors. Cancer Chemotherapy and Pharmacology 84(6): 1323–1331. [DOI] [PubMed] [Google Scholar]
- 33.Lee AJ (1996) Metformin in noninsulin-dependent diabetes mellitus. Pharmacotherapy 16(3): 327–351. [PubMed] [Google Scholar]
- 34.Wang J, Levasseur DN, Orkin SH. (2008) Requirement of nanog dimerization for stem cell self-renewal and pluripotency. Proceedings of the National Academy of Sciences of the United States of America 105(17): 6326–6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bijwaard KE, Aguilera NS, Monczak Y, et al. (2001) Quantitative real-time reverse transcription-PCR assay for cyclin D1 expression: Utility in the diagnosis of mantle cell lymphoma. Clinical Chemistry 47(2): 195–201. [PubMed] [Google Scholar]
- 36.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445(7123): 111–115. [DOI] [PubMed] [Google Scholar]
- 37.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 414(6859): 105–111. [DOI] [PubMed] [Google Scholar]
- 38.Chen W, Dong J, Haiech J, et al. (2016) Cancer stem cell quiescence and plasticity as major challenges in cancer therapy. Stem Cells International 2016: 1740936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto T, Nakamura S, Jo Y, et al. (2003) Chromoscopy might improve diagnostic accuracy in cancer surveillance for ulcerative colitis. The American Journal of Gastroenterology 98(8): 1827–1833. [DOI] [PubMed] [Google Scholar]
- 40.Jiralerspong S, Palla SL, Giordano SH, et al. (2009) Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. Journal of Clinical Oncology 27(20): 3297–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Spratt, Zhang C, Zumsteg ZS, et al. (2013) Metformin and prostate cancer: Reduced development of castration-resistant disease and prostate cancer mortality. European Urology 63(4): 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storozhuk Y, Hopmans SN, Sanli T, et al. (2013) Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. British Journal of Cancer 108(10): 2021–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lei Y, Yi Y, Liu Y, et al. (2017) Metformin targets multiple signaling pathways in cancer. Chinese Journal of Cancer 36: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horst D, Kriegl L, Engel J, et al. (2008) CD133 expression is an independent prognostic marker for low survival in colorectal cancer. British Journal of Cancer 99(8): 1285–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]


