Abstract
Acquired haemophilia A (AHA) is an uncommon but severe acquired bleeding disorder caused by the development of antibodies against clotting factor VIII, impairing secondary haemostasis. It is more common in older individuals and characteristically presents with spontaneous soft tissue bleeding that can rapidly become life-threatening. Definitive treatment requires immunosuppression to eradicate anti-FVIII antibodies, while providing haemostatic support to manage bleeding. Transfusions of fresh frozen plasma or cryoprecipitate, typically used to treat severe bleeding, are ineffective in patients with AHA. Instead, highly specialised clotting factor concentrates are required. While the appearance and extent of the soft tissue bleeding and the markedly prolonged activated partial thromboplastin time are characteristic, lack of familiarity with this disease process can lead to significant treatment delays. We report the clinical course and management of a 65-year-old woman who presented with severe anaemia of unclear aetiology with unrecognised soft tissue bleeding who was subsequently diagnosed with AHA.
Keywords: malignant and benign haematology, haematology (incl blood transfusion), haematology (drugs and medicines)
Background
Acquired haemophilia A (AHA) is a rare haematological disorder affecting about one in a million people per year.1 It is a condition in which there is spontaneous development of autoantibodies against clotting factor VIII, thereby impairing secondary haemostasis. The age of presentation is bimodal, with a significant portion emerging in the postpartum setting and the majority arising in patients older than 50 years of age.2 Considering this disorder is autoimmune in nature, about 50% of patients present with other conditions such as systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis or inflammatory bowel disease. It also occurs in the context of malignancy, particularly haematologic, and during the postpartum period. Around half of patients have an idiopathic presentation.
Severe bleeding develops in 70% of cases and is fatal in about 5%–10% of cases.3 When considering other factors and comorbidities, the overall mortality rate is estimated to be up to 33%.4 Successful management requires specialised haematological care and access to high-cost clotting factor concentrates. Failure to quickly recognise and treat this condition due to unfamiliarity with the classical clinical and laboratory findings can lead to high morbidity and mortality, as well as very high costs of medical care.
This case report details the presentation and management of a patient that was admitted to the hospital with severe anaemia of unclear aetiology who was diagnosed with AHA, where extensive soft tissue bleeding was not recognised at initial presentation.
Case presentation
The patient is a 65-year-old woman with a medical history of obesity, stroke with no residual neurological deficits on low-dose aspirin, dyslipidaemia, chronic kidney disease and gastro-oesophageal reflux disease, who presented to the emergency department with extreme weakness, dizziness and recent unintentional weight loss following a foodborne illness. She had fallen at home and was unable to get up for over 72 hours. She reported that 4 months prior, she had spontaneously developed diffuse bruising on her arms, legs and torso. On admission, she was found to have a haemoglobin of 40 g/L with a normal mean corpuscular volume and she was admitted to the hospital for an anaemia workup. She did not report gastrointestinal bleeding, haematuria or other bleeding sources. The haematology service was consulted for an anaemia workup as well as an elevated activated partial thromboplastin time (aPTT) of 71.5 s (reference range: 25–35 s) that was obtained in the emergency department but had not been worked up further.
Investigations
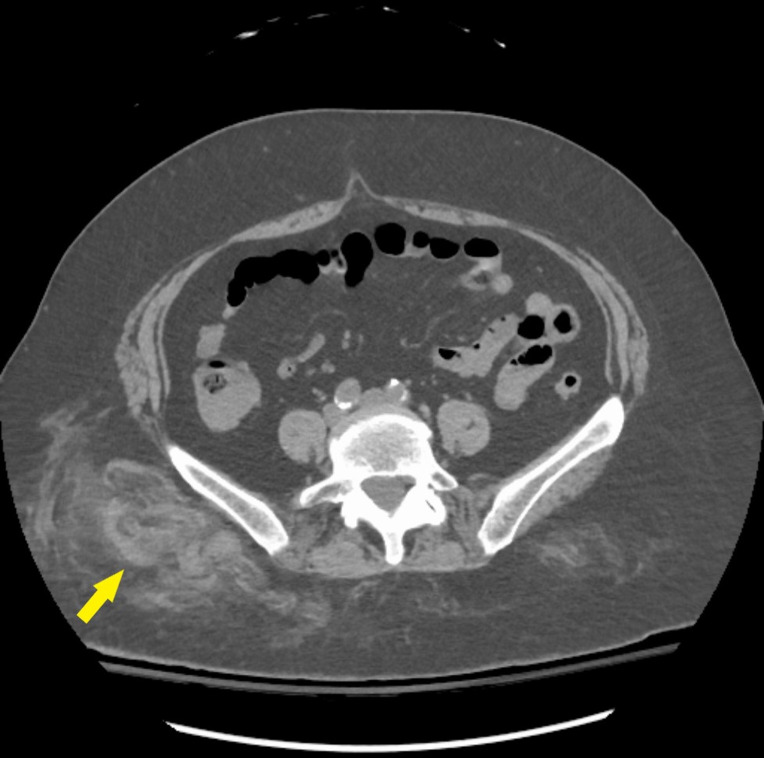
Without any clinical signs of apparent bleeding, normal white cell and platelet count, no laboratory evidence of haemolysis or chronic iron deficiency, and normal liver enzymes, the patient’s prolonged aPTT in the setting of a normal prothrombin time (PT) remained unexplained. An aPTT mixing study was performed and showed lack of correction after 1 hour of incubation. Additionally, a CT of the abdomen and pelvis revealed deep subcutaneous haematomas present in the right gluteus and flank without retroperitoneal haemorrhage (figure 1). These findings prompted the suspicion of AHA. Factor VIII activity was confirmed to be 1% with no lupus anticoagulant present and a FVIII inhibitor titre, which resulted a few days later, was 54 Bethesda units (BU). Forty-eight hours after initial presentation, extensive ecchymoses were apparent on the right flank and gluteus which had not been examined at the time of hospital admission.
Figure 1.
Ill-defined, mildly hyperdense fat stranding involving the right lower flank subcutaneous fat and extending down overlying the right gluteal region subcutaneous fat likely representing subcutaneous haemorrhage/haematoma seen on non-contrast CT for workup of anaemia of unclear aetiology.
Differential diagnosis
An isolated prolonged aPTT (with normal PT) should first be confirmed in duplicate as preanalytical factors are a common source of clotting time abnormalities. When confirmed in duplicate, the presence of a non-specific factor inhibitor such as lupus anticoagulant is the main differential diagnosis; however, platelets with lupus anticoagulants are usually asymptomatic or develop thrombosis, not bleeding. Severe congenital deficiency of factor FVIII, FIX or FXII can also lead to aPTT prolongation; however, these patients usually have a lifelong history of severe bleeding.
Treatment
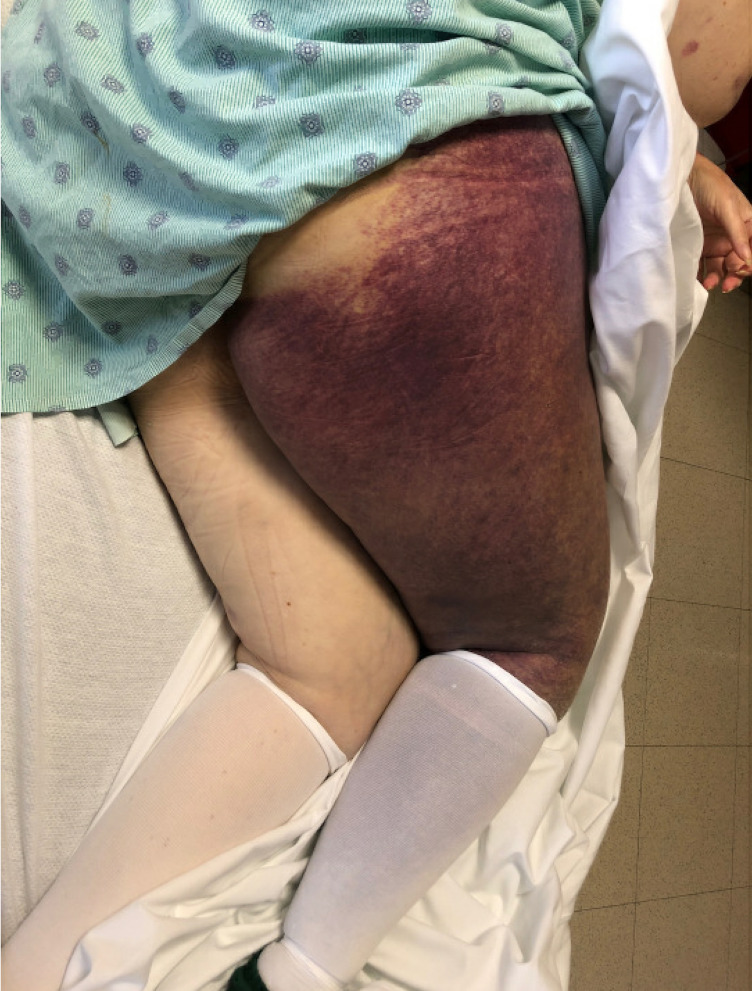
Considering the severity of the bleed and the availability of recombinant porcine FVIII (rpFVIII) at our institution, this was chosen as first-line haemostatic therapy. She responded well to an initial dose of 100 U/kg every 8 hours for 48 hours, with decreasing transfusion requirements and subsequent stabilisation of her haemoglobin. She received high-dose prednisone (1 mg/kg for 4 weeks with a slow taper) and started a rituximab induction course. After completion of 2 weeks of daily rpFVIII treatment at a dose of 80 U/kg every 12 hours, she was discharged home, only to be readmitted 2 days later with a large right thigh haematoma (figure 2). By that time, the inhibitor had started tittering down and she was able to be treated with recombinant FVIII with trough levels above 60%. She then completed 2 weeks of treatment after in order to achieve stabilisation of her haemoglobin. To allow her to discharge from the hospital and participate in physical rehabilitation after this acute illness and prolonged period of immobility, she received a loading dose of emicizumab as prophylaxis until the inhibitor was fully eradicated.
Figure 2.
Extensive spontaneous right lower extremity haematoma in this patient with acquired haemophilia A and undetectable FVIII activity.
Outcome and follow-up
During the first month after her hospital dismissal, she continued to have ecchymosis at the sites of venipuncture but did not develop major bleeding. The rituximab induction course (four weekly doses of 325 mg/m2) was completed and prednisone was slowly tapered. Three months after initial presentation, her factor FVIII activity had completely normalised. Additional testing to rule out an underlying haematologic or solid malignancy or an autoimmune disorder was performed and the result is negative. She remains under clinical observation with FVIII activity obtained every 3 months for the first year with the intention to detect an early relapse.
Discussion
The hallmark of AHA is spontaneous and extensive bleeding, usually in an older individual, involving the soft tissue. Patients can, however, also present with melena or haematuria.3
An isolated prolonged aPTT with a normal PT is typical. Prolonged aPTT can be seen in congenital haemophilias A and B, vitamin K deficiency, severe von Willebrand’s disease, liver disease, antiphospholipid syndrome, systemic lupus erythematosus and disseminated intravascular coagulation (DIC).5 A normal PT makes vitamin K deficiency, liver disease and DIC less likely. Mixing study, where the patient’s serum is combined with normal serum at a 1:1 ratio, is a relatively simple and widely available test. A PTT that fully corrects (returns to normal range) with mixing suggests a coagulation factor deficiency (without the presence of an inhibitor). A failure to correct suggests a coagulation inhibitor which can be non-specific (such as lupus anticoagulant) or specific (such as a coagulation factor-specific antibody). This initial set of laboratory studies should prompt further testing for confirmation of the acquired FVIII deficiency and include a FVIII activity followed by FVIII inhibitor (if low), as well as a lupus anticoagulant.
While the precise pathophysiology of this specific disorder remains unexplained, failure of the immune-tolerance mechanism has been postulated as the culprit. In the development of tolerance, there is recognition of self (FVIII) and if there is a strongly autoreactive lymphocyte, regulatory T cells will bind and eliminate the autoreactive cell. While it has been observed that healthy individuals can still possess low levels of antibodies to factor VIII, these are kept under the threshold for clinical significance by the activity of regulatory T cells.6 The immunogenicity of FVIII has been postulated to be due to its large size and other structural elements. Additionally, it has been suggested that its association with its carrier protein, von Willebrand factor, may also contribute.7
There are two parallel treatment goals in patient with AHA: provide haemostatic support and eliminate the inhibitor (antibody). For acute events with minor bleeding and a low factor VIII titre level (usually under 5BU), desmopressin may be administered.8 In cases with severe bleeding but low titre levels, recombinant factor VIII can be used. These high doses can ‘overwhelm’ the inhibitor and allow for resumption of physiological conditions for coagulation. In patients with severe bleeding and high inhibitor titres (a large proportion of patients at presentation), activated prothrombin complex concentrate, recombinant human factor VIIa or recombinant porcine factor VIII can be used.9 Recombinant human factor VIIa is more broadly available but carries risk of thromboembolic complications. Recombinant porcine factor VIII restores normal coagulation but can lack efficacy in patients who have cross-reactivity of the inhibitor with porcine factor. Recently, emicizumab, a bispecific humanised antibody which functions in a similar manner to endogenous factor VIII, has also been used (although off-label) in patients with AHA.10 To eradicate the inhibitor, immunosuppressive agents such as steroids, rituximab or cyclophosphamide are used.11 Inhibitor eradication takes weeks to months and patients remain at high risk of bleeding complications until their endogenous levels of FVIII begin to rise.
In the context of a known or undiagnosed connective tissue disorder, such as rheumatoid arthritis, systemic lupus erythematosus or Sjogren’s,2 the immunosuppressive treatment should be focused on achieving the best control possible of the associated disorder. Complications may continue to arise throughout treatment. Recurrence is common, occurring in about 20% of cases.3 Furthermore, pneumonia and sepsis are responsible for nearly 50% of the mortality in AHA.12 Sepsis can occur secondary to treatment with chronic immunosuppressive therapies, therefore early detection of infectious disease processes is also of paramount importance.
Ultimately, while AHA is an uncommon disease rarely encountered by the general practitioner, the recognition of its prominent clinical and laboratory characteristics can allow for prompt referral to a specialist and initiation of treatment that maximises the chance of long-term survival.
Patient’s perspective.
Not all patients are stupid and being told you are wrong is hurtful. I felt belittled by a staff member after contesting taking my aspirin because I was worried this was contraindicated with my bleeding disorder. I was frustrated when someone stated that my bruising was due to a fall, despite me pointing out that I had never fallen. The best way to get the information is by listening and not making assumptions… Be mindful of your documentation and patients, be knowledgeable about your medical history to make sure your side of story is understood and shared accurately.
Learning points.
New extensive and spontaneous soft tissue bleeding (including ecchymoses) in an older individual who is not on anticoagulation warrants additional evaluation.
A prolonged activated partial thromboplastin time should be repeated and if confirmed to be prolonged, the aetiology of prolongation investigated.
Management of bleeding in patients with acquired haemophilia A requires specialised clotting factor concentrates and is best achieved in a tertiary centre that has haematologic expertise.
Footnotes
Contributors: HC and JPB both wrote the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Yousphi AS, Bakhtiar A, Cheema MA, et al. Acquired hemophilia A: a rare but potentially fatal bleeding disorder. Cureus 2019;11:e5442. 10.7759/cureus.5442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mo L, Bao GC. Acquired factor VIII deficiency: two case reports and a review of literature. Exp Hematol Oncol 2017;6:8. 10.1186/s40164-017-0068-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitting RL, Bent S, Li Y, et al. The prognosis and treatment of acquired hemophilia: a systematic review and meta-analysis. Blood Coagul Fibrinolysis 2009;20:517–23. 10.1097/MBC.0b013e32832ca388 [DOI] [PubMed] [Google Scholar]
- 4.Sakurai Y, Takeda T. Acquired hemophilia A: a frequently overlooked autoimmune hemorrhagic disorder. J Immunol Res 2014;2014:1–10. 10.1155/2014/320674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rountree KM, Yaker Z, Lopez PP. Partial Thromboplastin Time. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 6.Mahendra A, Padiolleau-Lefevre S, Kaveri SV, et al. Do proteolytic antibodies complete the panoply of the autoimmune response in acquired haemophilia a? Br J Haematol 2012;156:3–12. 10.1111/j.1365-2141.2011.08890.x [DOI] [PubMed] [Google Scholar]
- 7.Meeks SL, Cox CL, Healey JF, et al. A major determinant of the immunogenicity of factor VIII in a murine model is independent of its procoagulant function. Blood 2012;120:2512–20. 10.1182/blood-2012-02-412361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baudo F, Collins P, Huth-Kühne A, et al. Management of bleeding in acquired hemophilia A: results from the European acquired haemophilia (EACH2) registry. Blood 2012;120:39–46. 10.1182/blood-2012-02-408930 [DOI] [PubMed] [Google Scholar]
- 9.Hay CR, Negrier C, Ludlam CA. The treatment of bleeding in acquired haemophilia with recombinant factor VIIa: a multicentre study. Thromb Haemost 1997;78:1463–7. [PubMed] [Google Scholar]
- 10.Franchini M, Marano G, Pati I, et al. Emicizumab for the treatment of haemophilia A: a narrative review. Blood Transfus 2019;17:223–8. 10.2450/2019.0026-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchini M, Lippi G. Acquired factor VIII inhibitors. Blood 2008;112:250–5. 10.1182/blood-2008-03-143586 [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Chen J, Zhou X, et al. Acquired hemophilia a presenting as progressive intra-abdominal hemorrhage, muscle hemorrhage and hemothorax postpartum: a case report and literature review. Exp Ther Med 2019;17:633–8. 10.3892/etm.2018.7031 [DOI] [PMC free article] [PubMed] [Google Scholar]