Summary
Background
Modified measles is rarely reported and thought to be an attenuated, less transmissible form of measles. The occupational safety and management of previously immunized healthcare providers (HCP) facing the global reemergence of measles is controversial and unclear.
Aim: We report a measles outbreak with an unusual presentation among our vaccinated HCP at Saint George Hospital University Medical Center (SGHUMC) in Lebanon that occurred during a nationwide measles epidemic.
Methods
We recorded cases at SGHUMC, a 333-bed tertiary-care center, from April 2018 to June 2018. We established a measles clinic for investigating all febrile patients. HCP exposure was linked to influx of index cases through our Emergency Department. Modified measles was defined as any variation in the classic presentation with a pinpoint/vesicular rash, documented exposure and evidence of prior immunity. We performed serology testing to diagnose and/or document immunity and implemented outbreak controls measures including PPE, airborne isolation, and mass notification.
Findings
We diagnosed 8 inpatients with classic measles, and 9 affected HCP. We diagnosed 8 HCP with modified measles. One previously immunized HCP developed classic measles despite being immunized and having a positive IgG titer. Our contact tracing revealed a total of 96 exposed HCP with 27 HCP showing non-specific signs of viral illness. We required all the 9 affected HCP to undergo home isolation.
Conclusion
We believe it is a top priority to achieve adequate measles immunity, especially among HCP that are at the frontline of healthcare systems. This necessitates revisiting vaccination schedules and achieving seroprotective titers to reclaim proper herd immunity.
Keywords: Modified measles, Measles outbreak, Measles immunity, Vaccination, Infection control, Measles
Abbreviations: ED, Emergency Department; EMR, Eastern Mediterranean Region; HCP, Healthcare Providers; MOPH, Lebanese Ministry of Public Health; NSVI, Non-specific Signs of Viral Illness; PPE, Personal Protective Equipment; SGHUMC, Saint George Hospital University Medical Center
Introduction
Measles outbreaks have been observed across the globe for more than a decade now. The World Health Organization (WHO) reports a 300% rise in the global average of measles cases in 2019, with a 100% rise observed in the Eastern Mediterranean Region (EMR) alone [1].
Measles is a vaccine-preventable disease. Susceptibility to Measles infection can be prevented through three routes: vaccination, herd immunity, or infection with the wild type virus. The recommended two-dose vaccine (adult & paediatric) is long thought to provide lifelong protection [2]. However, vaccine failures have been identified and studied over the years [3].
In Lebanon, measles is a notifiable communicable disease actively surveyed by The Epidemiologic Surveillance Unit at the Lebanese Ministry of Public Health (MOPH). Routine vaccination was nationally introduced in 1987 based on international guidelines. On March 19 2018, MOPH announced a nationwide measles outbreak with cases reported across the country [4].
Healthcare providers (HCP) are the front-liners during public health emergencies. Transmission of measles in nosocomial settings is common and poses a threat to HCP [5]. They are at high risk of exposure while caring for patients in the early outbreak days, especially when no pathognomonic signs of measles have yet manifested. Thus HCP have an increased risk of developing serious complications including pneumonia and death, while also endangering colleagues and patients [6].
Considering this background of outbreaks and questionable life-long immunity, we report a hospital-based outbreak of measles disease with an unusual presentation among vaccinated HCP at Saint George Hospital University Medical Centre (SGHUMC) in Lebanon. We provide a descriptive analysis of our local outbreak detailing the multiple facets of outbreak recognition and response.
Materials and methods
Setting
We recorded cases at SGHUMC, a 333-bed tertiary care centre in Beirut, Lebanon from April 2018 till June 2018. The Infection Control and Infectious Diseases teams performed outbreak investigations and contact tracing starting in April 2018 until four weeks after the admission of the last case of measles (end of July 2018).
Case definitions
We defined cases according to a combination of clinical and serologic criteria from the available literature and the CDC [7,8].
A case of “classic measles” is an individual presenting with a fever (temperature >38.3°C), cough, conjunctivitis, or coryza and a generalized maculopapular rash lasting for three days or more.
A case of “modified measles” is defined as an individual who presents with a combination of: fever, cough, conjunctivitis, coryza, and an unusual rash that might be pinpoint or vesicular, in addition to a self-reported history of vaccination against measles and a documented exposure to a patient/colleague diagnosed with classic/modified measles.
Diagnostic testing was performed within 48 hours of presentation. We relied on a positive serologic test for measles immunoglobulin G antibody (IgG) to determine immunity against measles in affected individuals when available.
Non-specific signs of viral illness (NSVI) consist of fever, cough, sore throat, or fatigue.
Outbreak response measures
We launched a dedicated “measles clinic” to screen any incoming suspicious cases with a viral prodrome, as well as exposed HCP who were in contact with an index case or were experiencing symptoms suggestive of measles. We positioned the clinic at an area isolated from the rest of the hospital to minimize exposure.
The Infection Control team enforced the use “personal protective equipment” (PPE) in the Emergency Department (ED), measles clinic, and on medical wards during suspicious encounters. This included N95-type masks, adequate airborne isolation and proper hand hygiene/disinfection practices [9].
We declared the measles outbreak through a hospital-wide email notification to all hospital staff, advising all individuals to report to the measles clinic in case of febrile illness or rash.
We determined measles immunization status based on recall and IgG titres [9]. No official documentation of MMR vaccination was available among all those affected during the outbreak.
We placed confirmed cases under airborne isolation, in rooms with negative pressure ventilation and an adjacent anteroom, from diagnosis until four days after the appearance of their rash [9].
After examining and diagnosing affected HCP, we required them to follow strict home isolation with instructions to return to work four days after the appearance of their rash.
We attempted to trace the contacts of all clinical and laboratory-confirmed cases.
Laboratory testing
We performed an automated quantitative determination of measles-specific antibodies using the Anti-Measles Virus IgG and Anti-Measles Virus IgM Abs EIA assays (Orgentec Diagnostika GmbH, Germany), as per the manufacturer's instructions. The positive cut-off values of measles-specific IgG antibodies was 250 mIU/mL while that of IgM was 25 U/mL. We compared measles IgG titers at SGHUMC to the values reported by MOPH from January 2017 till December 31st 2018.
Ethical considerations
This study was part of a public health response in an outbreak setting and not considered clinical research. Ethical approval was not sought. Written patient consent was obtained for the use of clinical photographs. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Results
During this April–June 2018 measles outbreak, we diagnosed a total of 17 cases of measles among patients and HCP.
We diagnosed eight patients with classic measles. The index case, I1, was admitted to the hospital on April 18th 2018, through the ED, for management of fever without a focus with an unknown vaccination history. On the second day of admission, the patient developed a classic measles rash. IgM titres were positive (35.7 U/mL) while IgG titre was negative (76.2 mIU/mL). The second case, I2, presented to the ED and was misdiagnosed as a drug reaction and discharged. Two days later, I2 presented again with persistence of symptoms and was diagnosed with classic measles. The remaining six cases, I3–I8, had similar presentations with fever and maculopapular rash being the most frequent symptoms present on admission. Case I5 presented with a clinical picture consistent with classic measles.
Table I describes the characteristics of confirmed cases of classic measles who were admitted to the hospital during the outbreak.
Table I.
Characteristics of index cases of classic measles during the April 2018–June 2018 outbreak
| Case | Vaccination status∗ | Date of rash appearance | Date of IgM | IgM titre (U/mL) | IgG titter (mIU/mL) | Symptoms† |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fever | Cough | Coryza | Conjunctivitis | Maculopapular rash | ||||||
| I1 | ? | 18-Apr-18 | 19-Apr | 35.7 | 76.2 | + | + | − | + | + |
| I2 | + | 29-Apr-18 | 1-May | 169.6 | 443.5 | + | + | − | − | + |
| I3 | − | 3-May-18 | 4-May | 33.8 | 138.9 | + | − | − | − | + |
| I4 | + | 11-May-18 | 11-May | 3.9 | > 5000 | + | − | − | + | + |
| I5 | ? | 18-May-18 | – | – | – | + | + | + | + | + |
| I6 | + | 30-May-18 | 13-Jun | 92.5 | – | + | + | − | + | + |
| I7 | + | 2-Jun-18 | 18-Jun | Equivocal | – | + | + | + | + | + |
| I8 | ? | 6-Jun-18 | 8-Jun | 4.4 | <0.1 | + | + | + | − | + |
∗ +: Vaccinated; −: Not Vaccinated; ?: Unknown Status; † +: Present; -: Absent; Fever: >38.9°C.
In Table II, we demonstrate the characteristics of HCP who were exposed and affected during the outbreak. All the affected HCP were nurses, residents, and medical students born between 1985 and 1995. They all reported a history of MMR vaccination.
Table II.
Characteristics of documented classic/modified measles among healthcare professionals during the April 2018–June 2018 outbreak
| Case | Vaccination status∗ | Date of rash appearance | IgG titre (mIU/mL) | Symptoms† |
Diagnosis |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fever | Cough | Coryza | Conjunctivitis | Maculopapular rash | Pinpoint/Vesicular rash | |||||
| CA | + | 19-Jun-2018 | 364.6 | + | + | − | + | + | − | Classic measles |
| A | + | 30-Apr-2018 | >5000 | + | − | − | − | − | + | Modified Measles |
| B | + | 30-Apr-2018 | 2003 | + | + | − | − | − | + | Modified Measles |
| C | + | 3-May-2018 | >5000 | + | − | − | − | − | + | Modified Measles |
| D | + | 5-May-2018 | 24.4 | + | − | − | − | − | + | Modified Measles |
| E | + | 5-May-2018 | – | + | − | − | − | − | + | Modified Measles |
| F | + | 7-May-2018 | >5000 | + | − | + | − | − | + | Modified Measles |
| G | + | 19-Jun-2018 | >5000 | + | − | − | − | − | + | Modified Measles |
| H | + | – | – | + | − | − | − | − | − | Modified Measles |
∗ +: Vaccinated; -:Not Vaccinated; ?: Unknown Status; † +: Present; -: Absent; Fever: >38.9°C.
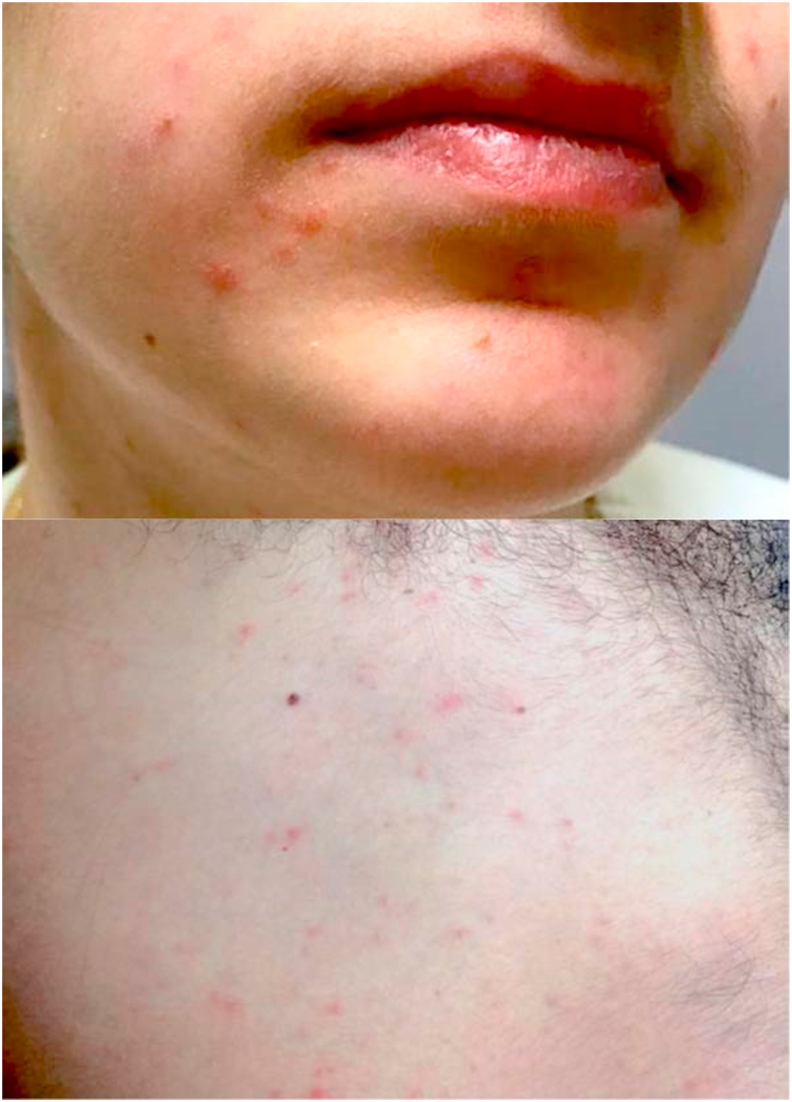
Case CA, a medical student, was in direct contact with case I7 and developed classic measles. CA had a positive IgG titre (364.6 mIU/mL). The maculopapular rash appeared seventeen days after the exposure. The remainder of the affected HCP were diagnosed with modified measles. Eight out of nine cases developed an unusual pinpoint/vesicular rash that started on their face and spread down over their body (Figure 1).
Figure 1.
Pinpoint/Vesicular rash of modified measles observed in healthcare providers during the outbreak.
Cases A and B were medical students rotating in the ED when the index patient I1 presented for evaluation. The rash appeared in both A and B 12 days after their exposure. Both had a positive IgG titre (>5000 mIU/mL; 2003 mIU/mL). Case C developed a rash after three days from exposure while case D after 5 days from exposure. Case E developed a rash, unlike case H. Cases F and G were nurses in contact with multiple index patients. Interestingly, Case G presented with fever and rash seventeen days after exposure to I7.
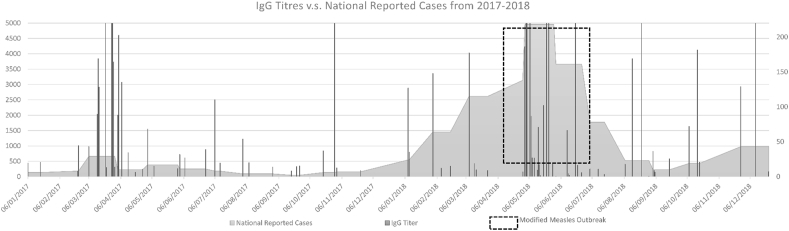
Figure 2 demonstrates the overall measles IgG titres measured at the SGHUMC laboratory overlaying the number of measles cases nationally reported to MOPH from January 2017 till December 2018. During the national outbreak in 2018, a cluster of elevated IgG titres >5000 mIU/mL was observed.
Figure 2.
Measles IgG Titres versus National Reported Cases of Measles to Lebanese Ministry of Public Health from January 2017 till December 2018.
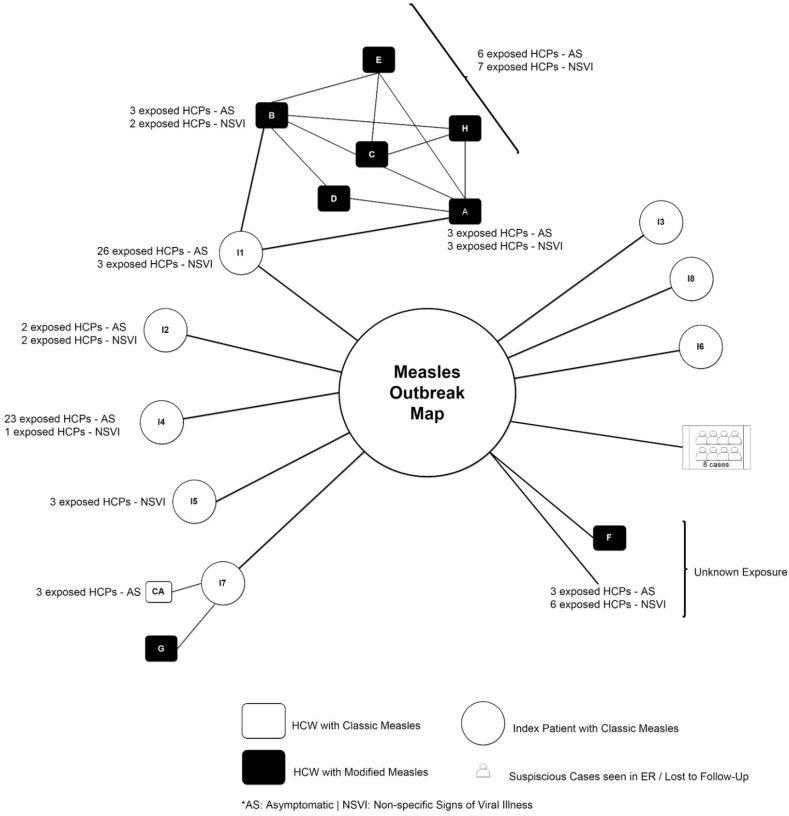
Figure 3 visually illustrates the outbreak with details of contact tracing of index cases and HCP with modified measles. We identified ninety-six HCP contacts. Sixty-nine remained asymptomatic while twenty-seven HCP reported non-specific signs of viral illness (NSVI).
Figure 3.
Measles outbreak map with contact tracing.
No complications or death were observed during this outbreak.
Discussion
In this study, we share our experience with a nosocomial HCP measles outbreak during the Spring 2018 measles epidemic in Lebanon. The transmissibility and characteristics of this measles variant was unusual on many levels. The evaluation and admission of eight cases of classic measles to SGHUMC triggered a rapid spread of modified measles among our vaccinated front-liners. How do we diagnose an outbreak of a highly transmissible febrile illness with random rash severity and distribution, in individuals with passive immunity, exposed to a nationwide classic measles outbreak?
In airborne diseases, early case recognition prevents unnecessary exposures and limits transmission. This is further highlighted when the disease has multiple presentations and variants. Classic measles is the most common form and is defined as an unvaccinated individual presenting with fever, cough, coryza, conjunctivitis, followed by the appearance of an erythematous maculopapular rash starting on the face and spreading down the body. Modified measles is defined as an infection in previously vaccinated individuals reporting features of classic measles with highly variable severity and characteristics [8]. Atypical Measles is defined as infection in individuals vaccinated with the killed-virus vaccine that was used in the 1960s, presenting with prolonged fever and a maculopapular rash appearing on the body before the face [10]. We find that this variety in presentations complicates diagnosing measles, especially in an early outbreak setting where the full clinical syndrome is not observed, as in case I2. The diagnosis is further complicated by a presumably adequate immunization history.
In this outbreak, we defined modified measles based on the basic features of classic measles coupled with the pinpoint/vesicular rash that we observed in eight out of nine affected HCP. The evidence is diverse on labelling a case as modified measles. Therefore, we built our definition for modified measles based on the observed variation in the classic presentation of measles coupled with confirmatory testing, documented exposure, and evidence of prior immunity. Individuals presenting with symptoms not meeting our case definition were identified as having NSVI.
Another layer of complexity in defining cases lies in the choice of diagnostics. Most hospitals in Lebanon use a serological assay, especially since the prevalence of measles is historically low. Consequently, we had a very limited availability of testing kits during the outbreak throughout the country. The main challenge in using these serological assays is interpreting them in an outbreak setting since the antibody response observed among measles variants is variable. In classic measles, an IgM response in infected unvaccinated individuals is observed around 1–4 days after rash onset. However, in modified measles, there have been few reports of an absent IgM response, and a hyper-production of IgG antibodies early on during the illness. We suspect that this phenomenon occurred in some HCP who had an IgG titre beyond the range of detection of the assay (>5000 mIU/mL) [11].
At the time of the outbreak, we suspected that a direct transfer of modified measles occurred among HCP who were not directly exposed to classic measles (Cases C, D, E, and H). This contradicts multiple sources claiming that cases of modified measles have a lower risk of transmitting the disease to others [11]. It is known that virus particles are suspended in the air-space for an estimated two hours where an affected individual was present, explaining the high transmissibility of classic measles [12]. To this point, scarce literature describes the spread of modified measles from an index case of modified measles.
Various infection control protocols and interventions have aided in containing measles on the global front. Furthermore, the use of PPE in any clinical encounter with a febrile patient is an obligation [9]. Given the low prevalence of measles, many HCP usually fail to comply with basic PPE protocols when examining febrile patients. In our outbreak this was very well controlled by the Infection Control team. Referrals to the measles clinic enhanced control with the clinic successfully screening ninety-four HCP.
Measles is a vaccine-preventable illness after the recommended two-dose vaccination, but numerous reports of modified measles outbreaks questions this longstanding fact [3,11,13]. We had 8 presumably vaccinated HCP affected during our outbreak. A single-dose MMR vaccination is estimated to provide immunity against measles for twenty-five years, however the seroconversion and elevated IgG titres seemingly does not confer protection. [13] Case CA despite being vaccinated with a positive IgG titre of 364.6 mIU/mL was diagnosed with classic measles. Interestingly, a recent systematic review highlights the inaccuracy associated with the 120mIU/mL threshold that is commonly believed to be protective, especially in elimination zones. This sheds light on the imprecision of using history of vaccination or serologic titre alone to mark individuals as immune [3]. Furthermore, multiple studies link decreased protection in immunized individuals to primary or secondary vaccine failure, low vaccine efficacy, a disruption in cold chain management, and waning immunity [6,[13], [14], [15]]. Thus, waning immunity to the “life-long” protective vaccine calls for a major reconsideration of measles immunization schedule.
On another note, the ongoing socioeconomical and political conflict of the Eastern Mediterranean Region (EMR) weakens herd immunity and thus contributes to the spread of measles. In Lebanon, the MOPH created fixed and mobile vaccination stations to target affected populations. This has helped provide partial immunity against diseases like measles, however, many communities are still unprotected, thus providing a perfect milieu for measles transmission [16]. Thus HCP are at a great risk of being exposed to measles while providing medical care to these underserved populations [17].
Our outbreak response did not include revaccination or post-exposure prophylaxis because there is insufficient supporting evidence in the literature. Protecting HCP in their occupational setting should be a main aim since they are at an estimated 19-fold increased risk of acquiring measles as compared to the public [6]. Globally, occupational safety protocols include revaccination and proper documentation of immunity through proof of vaccination or determination of IgG titres in the pre-employment phase. Unfortunately, for HCP in Lebanon and the EMR, there are no protective national guidelines in place [18].
Moreover, hospital-based outbreaks are a highly resource-intensive, economic burden, especially with highly transmissible diseases like measles. In Australia, a single outbreak was estimated to cost an additional 10,300 $ to cover outbreak control measures relating to just 12 episodes [19]. Another financial loss is related to the medical leave of affected staff. All affected HCP in our outbreak were sent home until four days after onset of their rash. This led to a shortage in oncall-schedules and availability of HCP at the hospital. In Germany, a single outbreak was estimated to have led to a 700,000 € loss with 215,000 € attributed solely to medical leave of affected HCP [20]. In the US, a single outbreak consisting of seven infected HCP incurred a containment cost of around 800,000$ [21]. Thus, determining proper immune status of HCP can prevent an institution's financial and human resource losses, specifically in our region.
Our study had multiple limitations. Limited availability of diagnostic testing hindered the process of confirming the diagnosis. The most used diagnostic method globally is detecting measles virus RNA by real-time polymerase chain reaction (RT-PCR). However, this test is costly and not immediately available in a hospital setting. At our hospital, the available measles test was a serological assay. Limited staffing of the infection control team reduced our ability to perform an extensive contact tracing with complete follow-up on all contacts. Given the national outbreak setting, it was also challenging to clearly determine exposure and source of transmission. The number of affected cases was also not enough to draw reliable conclusions on the behaviour of modified measles.
Conclusion
In conclusion, measles poses a public heath dilemma and exerts significant pressure on the healthcare system. We believe it is a top priority to achieve adequate measles immunity, especially among HCP that are at the frontline of healthcare systems. Our study raises the question of adequacy of relying on serological testing and history of vaccination to determine an individual's immune status, especially when in resource-limited settings. Waning immunity might be best addressed by an additional booster dose of measles vaccine for HCP. Further studies are needed to redefine seroprotective titers, reclaim proper herd immunity, and understand the peculiar dynamics of hyperproduction of IgG measles antibody observed in modified measles.
Credit author statement
Omar Zmerli: Investigation, Writing – Original Draft, Formal Analysis.
Amanda Chamieh: Investigation, Writing – Original Draft, Validation.
Eliane Maasri: Investigation, Resources.
Eid Azar: Conceptualization, Methodology, Writing – Review & Editing, Supervision.
Claude Afif∗: Conceptualization, Visualization, Writing – Review & Editing, Methodology, Supervision.
Acknowledgments
We would like to acknowledge our hospital's Infection Control team and laboratory staff for their efforts.
Conflict of interest statement
All authors report no conflicts of interest relevant to this article. (Drs Zmerli, Chamieh, Azar, Afif, and Ms. Maasri have nothing to disclose.)
Financial support
None.
References
- 1.WHO . WHO; 2019. New measles surveillance data for 2019. [Google Scholar]
- 2.NHS . NHS; 2020. MMR (measles, mumps and rubella) vaccine.https://www.nhs.uk/conditions/vaccinations/mmr-vaccine/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolotin S., Hughes S.L., Gul N., Khan S., Rota P.A., Severini A. What is the evidence to support a correlate of protection for measles? a systematic review. J Infect Dis. 2020;221:1576–1583. doi: 10.1093/infdis/jiz380. [DOI] [PubMed] [Google Scholar]
- 4.MOPH Minister Office . 2018. MOPH warns against measles outbreak in Lebanon and assures it will secure vaccines free of charge.https://www.moph.gov.lb/en/DynamicPages/view_page/16415/21/moph-warns-against-measles-outbreak-in-lebanon- [Google Scholar]
- 5.Hahné S.J.M., Lochlainn L.M.N., Van Burgel N.D., Kerkhof J., Sane J., Yap K.B. Measles outbreak among previously immunized healthcare workers, the Netherlands, 2014. J. Infect. Dis. 2016;214:1980–1986. doi: 10.1093/infdis/jiw480. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- 6.Steingart K.R., Thomas A.R., Dykewicz C.A., Redd S.C. Transmission of Measles Virus in Healthcare Settings During a Communitywide Outbreak. Infect Control Hosp Epidemiol. 1999;20:115–119. doi: 10.1086/501595. [DOI] [PubMed] [Google Scholar]
- 7.Council of State and Territorial Epidemiologists (CSTE) 2012. Public health reporting and national notification for measles.https://cdn.ymaws.com/www.cste.org/resource/resmgr/PS/12-ID-07FINAL.pdf [Google Scholar]
- 8.Babbott F.L., Gordon J.E. Modern measles. Am J Med Sci. 1954;228:334–361. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention . US Dep Heal Hum Serv; 2019. Interim infection prevention and control recommendations for measles in healthcare settings.https://www.cdc.gov/infectioncontrol/guidelines/measles/index.html [Google Scholar]
- 10.Fulginiti V.A., Eller J.J., Downie A.W., Kempe C.H. Altered reactivity to measles virus: atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA J Am Med Assoc. 1967;202:1075–1080. doi: 10.1001/jama.1967.03130250057008. [DOI] [PubMed] [Google Scholar]
- 11.Rosen J.B., Rota J.S., Hickman C.J., Sowers S.B., Mercader S., Rota P.A. Outbreak of measles among persons with prior evidence of immunity, New York City, 2011. Clin Infect Dis. 2014;58:1205–1210. doi: 10.1093/cid/ciu105. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention . US Dep Heal Hum Serv; 2018. Measles (rubeola): for healthcare professionals.https://www.cdc.gov/measles/hcp/index.html [Google Scholar]
- 13.Mossong J., Nokes D.J., Edmunds W.J., Cox M.J., Ratnam S., Muller C.P. Modeling the impact of subclinical measles transmission in vaccinated populations with waning immunity. Am J Epidemiol. 1999;150:1238–1249. doi: 10.1093/oxfordjournals.aje.a009951. [DOI] [PubMed] [Google Scholar]
- 14.Pillsbury A., Quinn H. An assessment of measles vaccine effectiveness, Australia, 2006-2012. West Pacific Surveill Response J WPSAR. 2015;6:43–50. doi: 10.5365/WPSAR.2015.6.2.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidkin I., Jokinen S., Broman M., Leinikki P., Peltola H. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: A 20-year follow-up. J Infect Dis. 2008;197:950–956. doi: 10.1086/528993. [DOI] [PubMed] [Google Scholar]
- 16.MOPH Minister Office . 2017. Hasbani inspected a vaccination campaign at al masnaa border crossing.https://www.moph.gov.lb/en/Pages/17/13537/hasbani-inspected-a-vaccination-campaign-at-al-masnaa-border-crossing- [Google Scholar]
- 17.Bank T.W. vols. 1–189. 2013. (Lebanon - economic and social impact assessment of the Syrian conflict). [Google Scholar]
- 18.Chamat S., Salameh P., Haddad N., Berry A., Chedid P., Bouharoun-Tayoun H. Protection of medical and paramedical university students in Lebanon against measles, mumps, rubella and varicella: active measures are needed. J Infect Public Health. 2011;4:125–134. doi: 10.1016/j.jiph.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Stuart R.L., Bradford J., Leszkiewicz P., Wilson J., Gillespie E.E. The costs of containing measles within a health care service. Healthc Infect. 2010;15:43–46. doi: 10.1071/HI10008. [DOI] [Google Scholar]
- 20.Hiller U., Mankertz A., Köneke N., Wicker S. Hospital outbreak of measles – evaluation and costs of 10 occupational cases among healthcare worker in Germany, February to March 2017. Vaccine. 2019 doi: 10.1016/j.vaccine.2019.02.068. [DOI] [PubMed] [Google Scholar]
- 21.Chen S.Y., Anderson S., Kutty P.K., Lugo F., McDonald M., Rota P.A. Health care-associated measles outbreak in the United States after an importation: challenges and economic impact. J Infect Dis. 2011;203:1517–1525. doi: 10.1093/infdis/jir115. [DOI] [PubMed] [Google Scholar]