Abstract
As we are over a year into the COVID-19 pandemic, we have made many forward strides in therapeutics. These treatments, such as monoclonal antibodies, have help mitigate the detrimental and often fatal consequences of COVID-19. The current indication for the use of monoclonal antibodies is mild to moderate COVID-19 infection within 10 days of symptom onset in those who are at high risk of progression to severe disease. However, their role in patients with prolonged symptoms is not clear. We present a unique case of monoclonal antibodies use after 54 days of symptom onset in an immunosuppressed patient with persistent COVID-19 infection despite standard treatment. This case illustrates the potential use of monoclonal antibodies outside of the current recommended therapeutic window in immunosuppressed patients, who may have difficulty with viral clearance.
Keywords: COVID-19, pneumonia (respiratory medicine), drugs: respiratory system
Background
COVID-19 is an emergent infectious disease caused by the SARS-CoV-2. With the diagnosis of COVID-19, there is concern for progression to acute respiratory failure, which can be fatal. Worldwide, there has been over 110 million cases with 2.5 million deaths thus far.1 As we pass the anniversary of the first reported case and encounter of the emerging variants, there have been many advances in the treatment of COVID-19, including the use of passive immunity with monoclonal antibodies which is most effective early in the disease process.2 On 21 November 2020, the Food and Drug Administration (FDA) granted emergency use authorisation (EUA) of monoclonal neutralising antibodies for the treatment of mild to moderate COVID-19 in patients who are at high risk of developing severe infection within 10 days of symptom onset.3–5
Though we now have guidelines for the use of monoclonal antibodies in early COVID-19, there are still limited data on how to treat those beyond 10 days of symptoms. Patients who are immunosuppressed present a particularly difficult challenge as they are unable to mount their own immune defence against COVID-19, and thus have a prolonged and indolent course.
Case presentation
A 35 year-old man presented via telehealth on 7 February 2021 with persistent fevers and malaise after a recent hospitalisation related to COVID-19 pneumonia. His history is significant for thymoma with metastasis to the pleura, which had previously been treated with chemotherapy and multiple surgical interventions including radical thymectomy complicated by bilateral phrenic nerve injury requiring diaphragmatic plication. For myasthenia gravis refractory to standard treatment, he receives rituximab two times per year (last given October 2020), which controls his symptoms.
He initially experienced upper respiratory symptoms on 25 December 2020 and was prescribed a course of levofloxacin by his primary care provider. Symptoms continued and progressed to fever thus prompting an urgent care visit on 31 December 2020, where he was prescribed antitussives. COVID-19 testing was not performed at that time due to limited availability. Because of persistent fevers, he returned to the urgent care centre and tested positive for COVID-19 by PCR on 3 January 2021. He was given a course of azithromycin for possible superimposed bacterial pneumonia with initial improvement. Fevers recurred, and the patient was referred to our outpatient teleservice, the Coronavirus-related Outpatient Work Navigators (CROWN) Programme, on 12 January 2021.6 At this visit, it had been 19 days since symptom onset, and therefore he was not a candidate for monoclonal antibodies. During his outpatient course, he was treated with intravenous fluids, prophylactic dose of rivaroxaban and empirical course of antibiotics. Despite these treatments, the patient continued to have cough and persistently high fevers to 39.2°C. He was noted to have an oxygen saturation of 88%, requiring supplemental oxygen. Remdesivir and dexamethasone were started on 24 January 2021 as an outpatient, but because of progressive dyspnoea, the patient was admitted to the hospital. During his hospitalisation, he completed a 5-day course of remdesivir and a 10-day course of dexamethasone. He also received amoxicillin/clavulanate to cover empirically for possible bacterial pneumonia, which was later discontinued given low procalcitonin and negative blood and sputum cultures. On discharge, he no longer required oxygen and appeared to be improving until he had a recurrence of fever up to 39.6°C on 6 February 2021.
Investigations
Notable laboratory investigations included white cell count 5.33×109/L, haemoglobin 144 g/L, platelet count 305×109/L and C reactive protein 17.65 mg/dL. Procalcitonin was 0.10 ng/mL during hospitalisation. D-dimer was initially 386 µg/L D-dimer units (DDU) on 13 January 2021 and peaked at 2288 µg/L DDU on 25 January 2021.
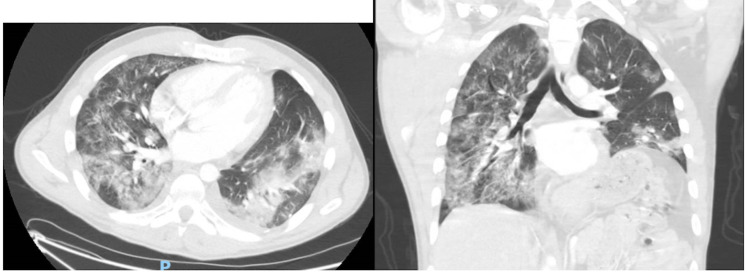
Ultrasound of lower extremities showed a right soleal deep vein thrombosis (DVT). CT angiography of the chest showed bilateral ground-glass opacities and consolidation consistent with COVID-19 pneumonia but no pulmonary embolism was identified (figure 1). Infectious workup included (1,3)-β-d-glucan <31 pg/mL, Aspergillus galactomannan antigen <0.500 index, as well as blood and sputum cultures which had no growth.
Figure 1.
CT of the chest. The patient’s CT of the chest shows post-surgical changes in the left hemithorax along with acute development of ground-glass opacities in the setting of an active COVID-19 infection.
The patient had initial positive COVID-19 PCR test on 3 January 2021 and had consistently positive results on 1 February 2021 and 15 February 2021. COVID-19 antibody test to the nucleocapsid protein was negative on 7 February and 15 February.
Differential diagnosis
Given the patient’s immunocompromised state, opportunistic bacterial and fungal infections were considered as a potential cause for his persistent fevers and hypoxemic respiratory failure. However, the patient was treated with multiple courses of antibiotics without significant improvement, cultures were negative, and a low procalcitonin and low (1,3)-β-d-glucan decreased the likelihood of these infections. Though the patient was found to have a DVT, thromboembolic disease was not felt to be the cause of his fever since the DVT was small and distal. Additionally, the D-dimer trended downwards, and pulmonary embolism was not noted on CT angiography. Another diagnostic consideration was progression of his underlying metastatic thymoma given the chronicity of symptoms and lack of response to standard treatment for COVID-19, but the CT of the chest did not show new nodules or any other features to suggest malignant disease.
With various therapies received, transient improvement of symptoms was noted but eventually fever and cough would re-emerge. Ultimately, persistent SARS-CoV-2 infection was suspected as he continued to test positive for COVID-19 by PCR and had negative COVID-19 antibodies after 53 days of symptom onset. The patient had received rituximab, depleting him of B cells, suggesting that the patient’s persistent symptoms and lack of response to therapy were due to an inability to mount a sustained immune response.
Treatment
Because of high suspicion of persistent SARS-CoV-2 infection, therapy with monoclonal antibody was considered despite being outside of the recommended therapeutic window. Compassionate use of casirivimab–imdevimab, two non-competing antibodies which target the receptor-binding domain of spike protein on the SARS-CoV-2, was authorised by Regeneron and EUA was granted by the FDA. The patient received the monoclonal antibody infusion on 16 February 2021, and he tolerated it well.
Outcome and follow-up
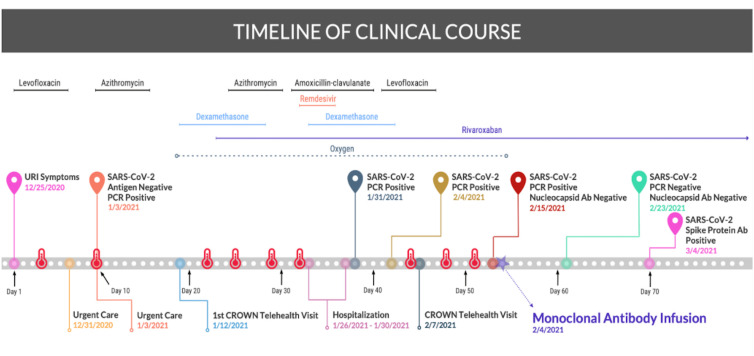
The patient reported complete resolution of fever, cough and malaise 24 hours after the monoclonal antibody infusion. One week following the infusion, the patient had a negative COVID-19 PCR. COVID-19 antibody testing to the nucleocapsid protein was negative, suggesting he was still unable to mount his own antibody response, but the antibody to the spike protein, the target of the monoclonal antibody, was positive. The status of the spike protein antibody was not checked earlier in the disease course as it was not available in our laboratory at the time. The patient has since been doing well and has remained symptom free for over 4 months post-infusion. A timeline of the patient’s clinical course is depicted below (figure 2).
Figure 2.
Timeline. Graphic illustration of the patient’s COVID-19 course, including reported fevers indicated by the red thermometer, various treatments and COVID-19 testing. CROWN, Coronavirus-related Outpatient Work Navigators; URI, upper respiratory infection.
Discussion
COVID-19 is an emergent infectious disease caused by SARS-CoV-2 infection. Patients with pre-existing comorbidities and immunosuppression may be at greater risk of developing severe manifestations of SARS-CoV-2, including often fatal respiratory failure.7–10 High-risk population includes those with active malignancy, and receiving chemotherapy and disease-modifying therapies for rheumatological or inflammatory conditions. The course of the disease and clinical outcomes in this group of patients are not well described. Some studies have shown no significant difference in clinical outcomes compared with the general population.11 However, other reports suggest a more protracted and severe course, particularly in patients with haematological malignancies and in those receiving anti-CD20 therapy.12–16
Rituximab is a chimeric anti-CD20 monoclonal antibody that effectively treats many haematological malignancies such as chronic lymphocytic leukaemia and non-Hodgkin’s lymphoma as well as several inflammatory and rheumatological conditions such as granulomatosis with polyangitis and rheumatoid arthritis.17 18 It leads to the complete eradication of peripheral B lymphocytes, thereby suppressing B cell functions such as immunoglobulin production. There are a few case reports in the literature that have described patients on maintenance therapy with rituximab who presented with prolonged symptoms of COVID-19, as in our patient.12–16 One study found a high prevalence of severe disease and death due to COVID-19 in patients with rheumatological disorders treated with rituximab within the last 12 months. Of the 13 patients described, 61.5% developed severe disease requiring hospitalisation. Among those with severe disease, 62.5% fulfilled criteria for acute respiratory distress syndrome and three developed critical disease and died.16
In most cases of COVID-19, virus-specific IgM and IgG are detectable in the serum between 7 and 14 days after the onset of symptoms.19 Patients with immunodeficiency or those who are on active immunosuppressive therapy may fail to mount an antibody response. Similar to other cases reported in the literature, our patient did not develop COVID-19 antibodies even after 7 weeks from the onset of symptoms.20 21 Rituximab suppresses B cell lymphocyte function and therefore antibody production which may be necessary to recover from the infection. Our case supports the hypothesis described in other case reports that patients on rituximab may experience persistent and severe symptoms due to the inability to produce neutralising antibodies secondary to B cell depletion.
Extensive workup was performed to exclude alternative aetiologies that could explain the persistent symptoms in our patient, and were all negative including procalcitonin. However, the sensitivity of procalcitonin decreases when patients are immunosuppressed or leucopenic.22 Therefore, a low procalcitonin in an immunosuppressed patient does not completely exclude infection and the patient was empirically treated for possible bacterial pneumonia. Ideally, a bronchoscopy with bronchoalveolar lavage should have been performed to complete the infectious workup. It was considered at the time of his hospitalisation. However, given the temporary clinical improvement after starting remdesivir and steroids, it was not pursued. The restrictions within our healthcare system to perform elective outpatient procedures during the pandemic limited the ability to perform a bronchoscopy once fevers recurred after the hospital discharge.
The patient continued to test positive for COVID-19 by PCR and had negative COVID-19 antibodies after 53 days of symptom onset, suggesting that he had persistent COVID-19 infection without adequte immunological response. COVID-19 PCR tests can remain positive for days, weeks or even longer periods of time. This can represent prolonged shedding of viral fragments and may not necessarily represent ongoing infection or infectivity.23 However, in our case, the patient remained symptomatic without the development of COVID-19 antibodies favouring persistent infection instead.
The temporary clinical improvement during the patient’s hospitalisation may be explained by the viral suppressive effects of remdesivir. It is likely that this effect was not sustained because remdesivir can only suppress viral load, but antibodies are necessary to achieve viral clearance. One of the limitations of our case is the inability to demonstrate ongoing SARS-CoV-2 infection. The use of quantitative PCR testing and cycling threshold has been described as a marker for viral load and infectivity.24 25 As in many laboratories in the USA, the microbiology department in our institution does not report this value. This is because it remains unclear on how cycling threshold should be applied in clinical settings and no standardisation exists across different tests. In addition, cycling threshold has certain limitations, as it can vary depending on the method of specimen collection, specimen source, transport and the time from infection to collection to analysis.25
Currently, there are no clinical guidelines on how to manage this subset of patients who have persistent COVID-19 infection. Kos et al reported a case of a patient with lymphoma on therapy with rituximab who had prolonged symptoms of COVID-19 and improved after the administration of intravenous immunoglobulins.14 There are similar reports of prolonged COVID-19 symptoms in patients on rituximab who showed rapid clinical recovery after the use of convalescent plasma.26–28 Convalescent plasma is derived from individuals who had COVID-19 and mounted an immune response against it and can be administered to provide passive immunity for patients who develop acute SARS-CoV-2 infection. Another treatment using the neutralising effects of the immune system is monoclonal antibodies against COVID-19 which currently includes bamlanivimab, bamlanivimab–etesevimab and casirivimab–imdevimab. The use of monoclonal neutralising antibodies has been granted EUA by the FDA for the treatment of mild to moderate COVID-19 within 10 days of symptom onset in patients who are at high risk of progressing to severe disease or hospitalisation. This includes individuals with body mass index ≥35 kg/m2, chronic kidney disease, diabetes mellitus, immunosuppressive disease, current use of immunosuppressive treatment, aged ≥65 or ≥55 years with cardiovascular disease, hypertension, chronic obstructive pulmonary disease or other chronic respiratory disease.3 4 However, their role in patients with prolonged symptoms is not clear and is not currently part of the EUA by the FDA.
Despite treatment with dexamethasone and remdesivir earlier in the disease course, our patient remained symptomatic with persistent fever. We applied for compassionate use of the monoclonal neutralising antibodies (casirivimab–imdevimab) and was granted EUA by the FDA which he received on day 54 of his clinical course. Following the monoclonal antibody infusion, he reported clinical improvement after 24 hours and fevers did not recur. His subsequent COVID-19 PCR test was negative. The nucleocapsid antibodies remained negative but demonstrated positive antibodies to the SARS-CoV-2 spike protein after the infusion, which is the target of the monoclonal antibodies.29 Antibodies to the SARS-CoV-2 spike protein were initially unavailable in our laboratory but negative antibodies to the SARS-CoV-2 nucleocapsid protein suggest that the immunosuppressive effects of rituximab prevented the patient’s native antibody production. Active immunity would have produced antibodies to both the SARS-CoV-2 nucleocapsid and spike proteins, but the patient did not develop antibodies even on day 53 of symptoms. The patient’s recovery was likely due to passive immunity via the infusion of monoclonal antibodies, as only the SARS-CoV-2 spike protein was positive after the infusion. Theoretically, it is feasible that only time was needed to achieve clearance of SARS-CoV-2. However, the temporal relationship between the infusion, symptomatic improvement and clearance of SARS-CoV-2 by PCR is hard to ignore.
To the best of our knowledge, this is the first case in the literature to report clinical improvement after the use of monoclonal neutralising antibodies in patients with prolonged COVID-19 symptoms with an inability to mount an antibody response due to B cell suppression from rituximab use. Our case also highlights the potential benefit of the use of monoclonal antibodies outside of the current recommended therapeutic window in immunosuppressed patients who may have difficulty with viral clearance, but further studies are needed to demonstrate their role in this subset of patients.
Patient’s perspective.
As someone who had two serious medical conditions prior to contracting COVID-19, I had experience with the overall decrease in the baseline of my daily activity and general health. However, it has been my personal experience that, since my gradual decline, my body adapted…subconsciously acknowledging the change in my inability to start my day the way I would ‘normally.’ My routine altered to adjust to my new situation. It now would take 2 to 3 hours before I felt well enough to fully start my day in the outside world. Before my illnesses, like most average, healthy individuals, I would wake and feeling well was my ‘normal’, and only when I felt a minor illness like a head cold would my routine be out of the ordinary. With a severe illness, this becomes inverted and your ‘new normal’ is waking with debilitating pain or chronic exhaustion but it is barely acknowledged because that’s just how it is. It is only when you have a good day and get out of bed and do not feel the exhaustion or whatever other constant ailment that is always present upon waking up, that you are shocked to realize the pain is absent and you are then reminded…this is what my normal used to be. At that point it becomes very clear that the pain is what you expect and feeling good is a surprise…and a reminder of what you have lost.
Specifically, regarding my experience with COVID-19, almost immediately after opening my eyes, it was a struggle to breathe. For six weeks, there was a general decrease in my breathing. It started with going up my stairs and having to sit on the bed to regain my breath. At 35, even after having three bouts of stage 4 cancer, 3 months of chemotherapy and three subsequent surgeries, along with an auto-immune disorder that wreaked havoc on my neurological system…this was a terrifying, sudden phenomenon. Over the course of the illness I ended up on oxygen and adapted to the point where I didn’t think about needing the oxygen to breathe through the night or to move around because this became yet another ordinary aspect of my day. Then, I received this infusion. One day (not even 48 hours after the infusion) I woke up, took off the oxygen and made coffee. Just as suddenly as my breathing struggles began, they now faded. It took time for my mind to realize my body’s improvement and that I, again, had a new normal.
My situation continues to improve, and aside from the miracle of the pharmaceutical drugs I benefitted from having administered, there is no doubt in my mind that an important aspect of my treatment was confidence in my physicians. As someone who has met with over 12 different doctors in a given year, you begin to realize that some doctors you just ‘click with’. There is no replacing the calming effect of knowing your only responsibility is to stay alive because some person who you had never met weeks before is one hundred percent committed to your health. I very much doubt I would be writing this had I not had the tremendous luck of being treated by the doctors at LIJ. I am eternally grateful.
Learning points.
Persistent SARS-CoV-2 infection should be considered in immunocompromised individuals with recurrent fever.
Monoclonal antibody may have a role as a therapeutic option in patients unable to mount an antibody response to SARS-CoV-2.
Monoclonal antibody infusion may be effective outside of the usual recommended 10-day window from symptom onset in immunocompromised patients with persistent COVID-19, but further studies are needed to demonstrate their role in this subset of patients.
Footnotes
Contributors: CXR wrote and edited the manuscript. BXL wrote and edited the manuscript, and designed the images. SSH wrote and edited the manuscript, and designed the images. BN-M reviewed and edited the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021;384:229–37. 10.1056/NEJMoa2029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An EUA for Bamlanivimab-A monoclonal antibody for COVID-19. JAMA 2021;325:880–1. 10.1001/jama.2020.24415 [DOI] [PubMed] [Google Scholar]
- 4.L J. New name and packaging for Regeneron COVID-19 monoclonal antibodies (casirivimab with imdevimab) to be administer together: REGEN-COV™. In: Administration USFaD. Tarrytown, NY: Regeneron, 2021. https://www.fda.gov/media/145611/download [Google Scholar]
- 5.C-TG P, Disease C. COVID-19) treatment guidelines: National Institutes of health 2019. [PubMed]
- 6.Lisker G, Narasimhan M, Greenberg H, et al. “Ambulatory Management of Moderate to High Risk COVID-19 Patients: The Coronavirus Related Outpatient Work Navigators (CROWN) Protocol”. Home Health Care Manag Pract 2021;33:49–53. 10.1177/1084822320964196 [DOI] [Google Scholar]
- 7.Shah V, Ko Ko T, Zuckerman M, et al. Poor outcome and prolonged persistence of SARS‐CoV‐2 RNA in COVID‐19 patients with haematological malignancies; King’s College Hospital experience. Br J Haematol 2020;190:268–888. 10.1111/bjh.16935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aries JA, Davies JK, Auer RL, et al. Clinical outcome of coronavirus disease 2019 in haemato‐oncology patients. Br J Haematol 2020;190:57–94. 10.1111/bjh.16852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia 2020;34:1637–45. 10.1038/s41375-020-0836-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haberman R, Axelrad J, Chen A, et al. Covid-19 in Immune-Mediated Inflammatory Diseases - Case Series from New York. N Engl J Med 2020;383:85–8. 10.1056/NEJMc2009567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meca-Lallana V, Aguirre C, Beatrizdel Río, et al. COVID-19 in 7 multiple sclerosis patients in treatment with anti-CD20 therapies. Mult Scler Relat Disord 2020;44:102306. 10.1016/j.msard.2020.102306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tepasse P-R, Hafezi W, Lutz M, et al. Persisting SARS-CoV-2 viraemia after rituximab therapy: two cases with fatal outcome and a review of the literature. Br J Haematol 2020;190:185–8. 10.1111/bjh.16896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kos I, Balensiefer B, Roth S, et al. Prolonged course of COVID-19-Associated pneumonia in a B-cell depleted patient after rituximab. Front Oncol 2020;10:1578. 10.3389/fonc.2020.01578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulze-Koops H, Krueger K, Vallbracht I. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-218075 [DOI] [PubMed] [Google Scholar]
- 16.Loarce-Martos J, García-Fernández A, López-Gutiérrez F, et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int 2020;40:2015–21. 10.1007/s00296-020-04699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cang S, Mukhi N, Wang K, et al. Novel CD20 monoclonal antibodies for lymphoma therapy. J Hematol Oncol 2012;5:64. 10.1186/1756-8722-5-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du FH, Mills EA, Mao-Draayer Y. Next-Generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Auto Immun Highlights 2017;8:12. 10.1007/s13317-017-0100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity 2020;52:910–41. 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasuda H, Tsukune Y, Watanabe N, et al. Persistent COVID-19 pneumonia and failure to develop Anti-SARS-CoV-2 antibodies during rituximab maintenance therapy for follicular lymphoma. Clin Lymphoma Myeloma Leuk 2020;20:774–6. 10.1016/j.clml.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benucci M, Quartuccio L, Li Gobbi F. Ann Rheum Dis 2020. [DOI] [PubMed] [Google Scholar]
- 22.Buhaescu I, Yood RA, Izzedine H. Serum procalcitonin in systemic autoimmune Diseases—Where are we now? Semin Arthritis Rheum 2010;40:176–83. 10.1016/j.semarthrit.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 23.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA 2020;323:2249–51. 10.1001/jama.2020.8259 [DOI] [PubMed] [Google Scholar]
- 24.Rhoads D, Peaper DR, She RC, et al. College of American pathologists (CAP) microbiology Committee perspective: caution must be used in interpreting the cycle threshold (CT) value. Clin Infect Dis 2021;72:e685–6. 10.1093/cid/ciaa1199 [DOI] [PubMed] [Google Scholar]
- 25.Binnicker MJ. Challenges and controversies to testing for COVID-19. J Clin Microbiol 2020;58. 10.1128/JCM.01695-20. [Epub ahead of print: 21 10 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betrains A, Godinas L, Woei-A-Jin FJSH, et al. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol 2021;192:1100–5. 10.1111/bjh.17266 [DOI] [PubMed] [Google Scholar]
- 27.Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood 2020;136:2290–5. 10.1182/blood.2020008423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark E, Guilpain P, Filip IL, et al. Convalescent plasma for persisting COVID-19 following therapeutic lymphocyte depletion: a report of rapid recovery. Br J Haematol 2020;190:e154–6. 10.1111/bjh.16981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian X, Li C, Huang A, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect 2020;9:382–5. 10.1080/22221751.2020.1729069 [DOI] [PMC free article] [PubMed] [Google Scholar]