Summary
Background
Indiscriminate antimicrobial use is one of the greatest contributors to antimicrobial resistance. A low level of asepsis in hospitals and inadequate laboratory support have been adduced as reasons for indiscriminate use of antimicrobials among surgical patients. At present, there are no guidelines for presumptive antibiotic use in Nigeria and sub-Saharan Africa.
Aim
Surgical inpatients at the study hospital were surveyed to determine the level of antimicrobial use and degree of compliance with prescription quality indicators.
Methods
A cross-sectional survey was conducted among all surgical inpatients in May 2019 using a standardized tool developed by the University of Antwerp to assess the point prevalence of antimicrobials. Inpatients who were admitted from 08:00 h on the day of the survey were included. Data on patients' demographics, indication for antimicrobial use, reason for antimicrobial use, stop/review date, adherence to guidelines and laboratory use were collected. The prevalence of antimicrobial use in the surgical department was estimated.
Results
Eighty-two inpatients were included in the survey. Of these, 97.6% were receiving at least one antimicrobial agent. Only 5.4% of the prescriptions were targeted, and 37.6% of prescriptions were for empirical treatment of infections. Approximately half (50.7%) of the patients were receiving presumptive antibiotics, and 6% were receiving prophylactic antibiotics. In total, 58.7% of prescriptions were administered parenterally, and 98.2% of patients had documentation of a stop/review date. Metronidazole (P=32.3%, T=29.2%), ceftriaxone (P=28.4%, T=19.8%) and ciprofloxacin (P=14.2%, T=14.6%) were the most common antimicrobials used.
Conclusions
There is a high rate of antimicrobial use among surgical inpatients, and the rate of indiscriminate antimicrobial prescribing among these patients needs to be reduced. This can be achieved by developing antimicrobial guidelines for presumptive antimicrobial therapy.
Keywords: Antimicrobial guidelines, Antimicrobial resistance, Developing world, Presumptive antimicrobial therapy, Surgical inpatients
Introduction
Antimicrobials are widely used in surgical practice, with attendant risks of resistance [1]. Antimicrobials can be used for prophylaxis, empirical therapy and targeted therapy [2]. The presumptive antimicrobial role is relatively new and is controversial with respect to global concerns regarding resistance [3]. Presumptive antimicrobial use is defined as ‘administration of antimicrobials to prevent infection in trauma patients who already have tissue microbial inoculation, without adequate antimicrobial concentration prior to contamination’ [4,5].
Standard protocols for presumptive antimicrobial use in surgical patients are non-existent or generally not well defined in sub-Saharan Africa and low-income countries. Presumptive antimicrobial use in penetrating trauma injuries and open fractures is an accepted and widespread practice to reduce the incidence of infection. It has therefore become necessary to develop a standard protocol for presumptive antimicrobial use in resource-constrained settings. The primary indication for presumptive antimicrobials is to forestall frank infection from developing in open wounds [5,6]. This is necessitated by contamination of wounds which, if left, may progress to active infection. This form of antibiotic therapy is neither targeted nor prophylactic. This novel role of antimicrobials is classed as presumptive use [5]. Locally, the presumptive antibiotic choice is at the discretion of the surgeon as there are no guidelines in place. This may lead to antibiotic misuse.
The presumptive antibiotic role must be well defined to set boundaries and reduce the risk of antimicrobial resistance (AMR), which is a growing public health threat. The misuse of antimicrobials among surgeons is common, despite opportunities to get involved in standard antimicrobial stewardship [7], which would comply with the World Health Organization's global movement for reversal of the dangers of AMR [3]. Surveillance of AMR is not established in Africa, making it more difficult to assess the contribution of presumptive antibiotic use to AMR in the continent [8]. This is worsened by the paucity of studies in the subregion on antibiotic stewardship and resistance. Previous studies have focused on specific organisms and their resistance profiles in different countries [[9], [10], [11]]. However, in Nigeria, no studies have reported the full burden and associated socio-economic implications of AMR [12]. Presumptive antibiotic use without guidelines could contribute significantly to AMR due to antibiotic misuse [7,13].
This study aimed to determine the prevalence of presumptive antimicrobial use among surgical inpatients, and evaluate the level of compliance with prescription quality indicators. A survey was conducted in the surgical department at Alex Ekwueme Federal University Teaching Hospital, Abakaliki. This is a 720-bedded tertiary care hospital that provides specialized care to patients in different surgical subspecialties, in addition to other medical specialties. Surgical subspecialties include burns and plastic surgery, general surgery, urology, orthopaedics, neurosurgery, cardiothoracic and paediatric surgery. There are 161 surgical inpatient beds, six operating suites, daily outpatient surgical clinics and emergency services at the study hospital.
Methods
A cross-sectional study was undertaken, including all surgical inpatients admitted to the hospital. Ethical approval was granted by the Research and Ethics Committee of Alex Ekwueme Federal University Teaching Hospital, Abakaliki (Reference No. FETHA/REC/Vol.2/2018/134).
All patients admitted to hospital from 08:00 h on the day of the survey were included in this study. Day-surgery patients were excluded.
The Global Point Prevalence ward and patient questionnaires (https://www.global-pps.com) were used in this study. Information was collected regarding the number of patients admitted to each ward, age, sex, clinical diagnosis, type of antimicrobial used, indication for antimicrobial use, route of administration, adherence to prescription order, documentation of stop/review date, type of antimicrobial use (empirical, targeted or prophylactic/presumptive), selected microbial resistance under surveillance and use of biomarkers. The erythrocyte sedimentation rate is the commonly used biomarker at the study hospital.
Data were analysed using Epi Info Version 7.2.3 after online data entry, and validated using the Global Point Prevalence interface developed at the University of Antwerp, Belgium. The antimicrobial prevalence rate was calculated, and the level of compliance with the antimicrobial prescription quality indicators was examined.
Results
The use of antimicrobials among surgical inpatients was generally high regardless of sex and age. The prevalence of antimicrobial use among surgical inpatients was 97.6% (Table I). The majority (94.6%) of prescriptions were made on an empirical basis [i.e. without a laboratory diagnosis or microbiological sensitivity testing (Table II)], and over half (58.7%) of prescriptions were administered parenterally. There was good documentation of a stop/review date among the prescriptions (98.2%).
Table I.
Prevalence of antimicrobial use by surgical ward
| Ward | Patients admitted (N) | Prevalence of antimicrobial use (%) |
|---|---|---|
| Male surgical | 41 | 97.6 |
| Female surgical | 19 | 100.0 |
| Paediatric surgical | 15 | 93.3 |
| Plastic surgery | 7 | 100.0 |
| Total | 82 | 97.6 |
Table II.
Antimicrobial quality indicators among surgical inpatients in a tertiary hospital
| Indicator | Antimicrobials (N) | Percentage |
|---|---|---|
| Stop/review date | ||
| Yes | 219 | 98.2 |
| No | 4 | 1.8 |
| Treatment | ||
| Empirical | 211 | 94.6 |
| Targeted | 12 | 5.4 |
| Reason given in notes | ||
| Yes | 214 | 96.0 |
| No | 9 | 4.0 |
| Route of administration | ||
| Oral | 92 | 41.3 |
| Parenteral | 131 | 58.7 |
| Indication | ||
| Prophylactic | 127 | 57.0 |
| Therapeutic | 96 | 43.0 |
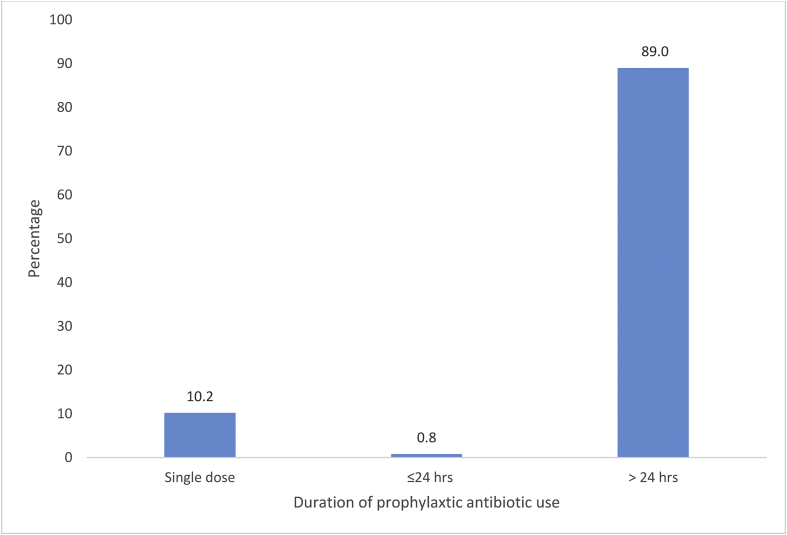
Table III shows the demographic characteristics of surveyed patients and indications for antibiotic use. There were more males than females, with the majority of patients being between 20 and 39 years of age. Only 17.9% were aged >50 years. Fifty-seven percent of prescriptions were administered for prophylactic indications. Of the prophylactic antimicrobial prescriptions, 89% were given for >24 h. This duration places 50.73% of all antimicrobial prescriptions in the presumptive antibiotic use category. Figure 1 shows the duration of prophylactic use. The remaining 37.6% of prescriptions were for therapeutic indications without a microbiological sensitivity result. At present, there are no local or national antibiotic guidelines for antibiotic use at the study hospital.
Table III.
Demographic characteristics of the participants and reasons for antibiotic use (N=223)a
| Variable | Frequency | Proportion |
|---|---|---|
| Age (years) | ||
| 0–4 | 13 | 5.8 |
| 5–9 | 8 | 3.6 |
| 10–14 | 16 | 7.2 |
| 15–19 | 11 | 4.9 |
| 20–24 | 30 | 13.5 |
| 25–29 | 26 | 11.7 |
| 30–34 | 20 | 9.0 |
| 35–39 | 37 | 16.6 |
| 40–44 | 7 | 3.1 |
| 45–49 | 15 | 6.7 |
| ≥50 | 40 | 17.9 |
| Sex | ||
| Male | 171 | 76.7 |
| Female | 52 | 23.3 |
| Indication for antibiotic use | ||
| Community-acquired infection | 76 | 34.1 |
| Hospital-associated infection 1 | 12 | 5.4 |
| Hospital-associated infection 2 | 5 | 2.2 |
| Hospital-associated infection 4 | 3 | 1.4 |
| Surgical prophylaxis 1 | 13 | 5.8 |
| Surgical prophylaxis 2 | 1 | 0.45 |
| Surgical prophylaxis 3 | 113 | 50.7 |
Surgical prophylaxis: 1, given as single dose; 2, given within 24 h; 3, given for >24 h.
The table is based on the 223 antimicrobial encounters experienced by the respondents; a patient may have had more than one encounter.
Figure 1.
Duration of prescription for antimicrobial prophylaxis.
Discussion
The use of antimicrobial agents among surgical inpatients was high in this study, regardless of sex or age. Similar findings have been documented previously in Ghana [14]. Although reasons for the high level of antimicrobial prescribing are not known, it is likely to be due to concerns about the low level of asepsis, poor infection prevention and control, and fear of wound contamination during a hospital stay resulting in healthcare-associated infections.
Antimicrobial use in this study was most commonly on an empirical basis, without laboratory results and antimicrobial sensitivity testing to ascertain the causative organisms and the agents to which such microbes are sensitive. These findings are similar to previous studies [14,15]. Empirical antimicrobial prescriptions should be made with caution in light of growing AMR. There are no local or national antimicrobial guidelines for antimicrobial use at the study hospital. There is a need to reduce empirical antimicrobial prescriptions, and an urgent need to develop local antimicrobial guidelines for empirical antimicrobial use in surgical practice. Having local guidelines (based upon local sensitivity data) in place would increase the quality of antimicrobial prescriptions and administration. Low levels of compliance with national and local antimicrobial guidelines have, however, been reported in Ghana and Ethiopia [14,16]. Higher rates of guideline compliance were reported in a multi-nation survey [17], but this may have been due to the inclusion of high-income countries.
This study found a high level of documentation of antimicrobial indication in patients' case notes, and this practice should be sustained. This was much higher than that reported in an earlier global multi-centre survey [17]. Similarly, this study found far higher documentation of the stop/review date compared with a global survey [17] (98.2% vs 38.3%, respectively). It is best practice to review antimicrobial use periodically and align prescriptions with the changing microbial profile to ensure effectiveness and patient safety.
The majority of antimicrobials were given parenterally. A multi-centre study conducted in northern Nigeria also found that the most common route of drug administration was parenteral [18]. These findings may reflect the fact that surgical prophylaxis requires immediate delivery of antimicrobial agents into the bloodstream, which may not be achieved easily with oral administration.
A high proportion of the antimicrobial prescriptions reviewed in this study were for surgical prophylaxis. The proportion of prophylactic antimicrobial use in this study was higher compared with previous studies [14,15,17]. In addition, this study found prolonged use of prophylactic antimicrobials, beyond the recommended 24 h. Prolonged surgical prophylaxis has been reported as common practice in previous studies [16,17,19,20]. A large proportion of cases of prolonged prophylactic antimicrobial use at the study hospital can be attributed to presumptive antibiotic use (Figure 1). This practice has been reported in previous studies [5,21,22].
The role of presumptive antibiotics is controversial among clinicians from various specialties [22]. Notwithstanding the low popularity of presumptive antimicrobials, they have been used to avoid potentially fatal infections that develop in traumatic injuries [4]. It is therefore necessary to understand this role and separate it from confusion with prophylactic antimicrobials. In this study, most antimicrobial prescriptions were for patients who did not have established infections.
The major challenge for presumptive antimicrobials in the developing world is the lack of guidelines for their prescription and administration. The development of guidelines will serve as the first step towards more inclusive antimicrobial stewardship programmes. A lack of guidelines creates confusion between the roles of presumptive and prophylactic antibiotic use [23,24]. This constitutes a major problem in low- and middle-income countries with considerable antimicrobial drug abuse, and the potential risk of development of AMR. The Eastern Association for the Surgery of Trauma, for instance, has developed guidelines for the use of presumptive antimicrobials in different types of traumatic injuries affecting various body parts [21,25].
At the study hospital, most patients were receiving prophylactic or presumptive antibiotics without any established guidelines. Prescribing was traditionally underpinned by the surgeons' clinical judgement, and the decision was based on normal microbial flora, hospital/community pattern of infection, and the cost and availability of antimicrobial drugs. This emphasizes the need for local and regional guidelines for presumptive antibiotic administration. While 24-h administration of antibiotic prophylaxis is generally accepted, such a clear description does not exist for presumptive antibiotics in the subregion.
Attempts have been made to develop guidelines to reduce infection in patients placed on presumptive antibiotics [21]. Both open and closed trauma injuries were found to be prone to infection due to physical anatomical breaches and disruptions, microfloral colonization and external contamination [26]. Infection as a primary outcome measure is very important. Postoperative infection has the tendency to cause significant morbidity and mortality, and to affect the cosmetic outcome [27]. Efforts should therefore be channelled towards the prevention of infection by developing and using presumptive antimicrobial guidelines. This is particularly important to forestalling AMR.
Limitations
This study had a small sample size and may not, in itself, account for the perceived need for standardization of presumptive antimicrobial guidelines. More studies or a meta-analysis may be needed to further establish the need for guidelines.
In conclusion, this study found high prevalence of antimicrobial use, prolonged surgical prophylaxis, poor utilization of microbiological laboratory results and non-existence of antimicrobial guidelines among surgical inpatients at the study hospital. Urgent development of guidelines for therapeutic, prophylactic and presumptive antimicrobial use for surgical patients is recommended to ensure standardization and reduce the risks of antimicrobial abuse and AMR.
Acknowledgements
The Global Point Prevalence Survey used is coordinated at the University of Antwerp, Belgium.
Conflict of interest statement
None declared.
Funding sources
The authors and study hospital did not receive any direct funding for the survey. Data analysis and interpretation of the results were undertaken by the authors. However, bioMérieux provided unrestricted funding support for development of the Global Point Prevalence Survey Platform.
References
- 1.Gilmore O.J., Sanderson P.J. An antibiotic policy for surgical patients. Ann R Coll Surg Engl. 1975;57:204–211. [PMC free article] [PubMed] [Google Scholar]
- 2.Bohnen J. Surgical treatment: evidence-based and problem-oriented. In: Holzheimer R., Mannick J., editors. Zuchschwerdt; Munich: 2001. http://www.ncbi.nlm.nih.gov/pubmed/21028753 Available at: [last accessed November 2019] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO; Geneva: 2016. Global action plan on AMR. [Google Scholar]
- 4.Velmahos G.C., Toutouzas K.G., Sarkisyan G., Chan L.S., Jindal A., Karaiskakis M. Severe trauma is not an excuse for prolonged antibiotic prophylaxis. Arch Surg. 2002;137:537–542. doi: 10.1001/archsurg.137.5.537. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins T.L., Daley M.J., Rose D.T., Jaso T.C., Brown C.V.R. Presumptive antibiotic therapy for civilian trauma injuries. J. Trauma Acute Care Surg. 2016;81:765–774. doi: 10.1097/TA.0000000000001164. [DOI] [PubMed] [Google Scholar]
- 6.British Orthopaedic Association, British Association of Plastic, Reconstructive & Aesthetic Surgeons . BOA; London: 2017. Audit standards for trauma. [Google Scholar]
- 7.Leeds I.L., Fabrizio A., Cosgrove S.E., Wick E.C. Treating wisely: the surgeon’s role in antibiotic stewardship. Ann Surg. 2017;265:871–873. doi: 10.1097/SLA.0000000000002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . Global report on surveillance. WHO; Geneva: 2014. Antimicrobial resistance. [Google Scholar]
- 9.Workneh M.H., Bjune G.A., Yimer S.A. Prevalence and associated factors of tuberculosis and diabetes mellitus comorbidity: a systematic review. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gahamanyi N., Mboera L.E.G., Matee M.I., Mutangana D., Komba E.V.G. Prevalence, risk factors and antimicrobial resistance profiles of thermophilic Campylobacter species in humans and animals in sub-Saharan Africa: a systematic review. Int J Microbiol. 2020 doi: 10.1155/2020/2092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leopold S.J., Van Leth F., Tarekegn H., Schultsz C. Antimicrobial drug resistance among clinically relevant bacterial isolates in sub-Saharan Africa: a systematic review. J Antimicrob Chemother. 2014;69:2337–2353. doi: 10.1093/jac/dku176. [DOI] [PubMed] [Google Scholar]
- 12.Federal Ministries of Agriculture, Environment and Health . 2017. Antimicrobial use and resistance in Nigeria: situation analysis and recommendations.https://ncdc.gov.ng/themes/common/docs/protocols/56_1510840387.pdf accessed August 2020. [Google Scholar]
- 13.Tadasse B.T., Ashley E.A., Ongarello S., Havumaki J., Wijegoonewardena M., Gonzalez I.J. Antimicrobial resistance in Africa: a systematic review. BMC Infect Dis. 2017;17:616. doi: 10.1186/s12879-017-2713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bediako-Bowan A.A.A., Owusu E., Labi A.-K., Obeng-Nkrumah N., Sunkwa-Mills G., Bjerrum S. Antibiotic use in surgical units of selected hospitals in Ghana: a multi-centre point prevalence survey. BMC Publ Health. 2019;19:797. doi: 10.1186/s12889-019-7162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bin Y.B., Rozina A., Junaid M., Saima K. A study of unnecessary use of antibiotics at a tertiary care hospital: urgent need to implement antimicrobial stewardship programs. J Young Pharm. 2015;7:311–319. [Google Scholar]
- 16.Alemkere G. Antibiotic usage in surgical prophylaxis: a prospective observational study in the surgical ward of Nekemte referral hospital. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Versporten A., Zarb P., Caniaux I., Gros M.F., Drapier N., Miller M. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Heal. 2018;6:e619–e629. doi: 10.1016/S2214-109X(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 18.Abubakar U. Antibiotic use among hospitalized patients in northern Nigeria: a multicentre point-prevalence survey. BMC Infect Dis. 2020;20:86. doi: 10.1186/s12879-020-4815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talaam R.C., Abungana M.M., Ooko P.B. An antibiotic audit of the surgical department at a rural hospital in Western Kenya. Pan Afr Med J. 2018;29:219. doi: 10.11604/pamj.2018.29.219.14510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umeokonkwo C.D., Madubueze U.C., Onah C.K., Okedo-Alex I.N., Adeke A.S., Versporten A. Point prevalence survey of antimicrobial prescription in a tertiary hospital in South East Nigeria: a call for improved antibiotic stewardship. J Glob Antimicrob Resist. 2019;17:291–295. doi: 10.1016/j.jgar.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Moore F.O., Duane T.M., Hu C.K.C., Fox A.D., McQuay N., Lieber M.L. Presumptive antibiotic use in tube thoracostomy for traumatic hemopneumothorax: an Eastern Association for the Surgery of Trauma Practice Management Guideline. J Trauma Acute Care Surg. 2012;73(Suppl. 4):S341–S344. doi: 10.1097/TA.0b013e31827018c7. [DOI] [PubMed] [Google Scholar]
- 22.Cook A., Hu C., Ward J., Schultz S., Moore F.O.D., Funk G. Presumptive antibiotics in tube thoracostomy for traumatic hemopneumothorax: a prospective, multicenter American Association for the Surgery of Trauma study. Trauma Surg Acute Care Open. 2019;4 doi: 10.1136/tsaco-2019-000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opanga S.A., Mwang’ombe N.J., Okalebo F.A., Godman B., Oluka M., Kuria K.A.M. Determinants of the effectiveness of antimicrobial prophylaxis among neurotrauma patients at a referral hospital in Kenya: findings and implications. J Infect Dis Prev Med. 2017;5:169. [Google Scholar]
- 24.Kim Y., Morris M.C., Lee T.C., Earnest R.E. Surgical management of compound skull fracture with exposed brain matter in a third-world country. J Surg Case Rep. 2019;2019:rjz147. doi: 10.1093/jscr/rjz147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg S.R., Anand R.J., Como J.J., Dechert T., Dente C., Luchette F.A. Prophylactic antibiotic use in penetrating abdominal trauma: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(Suppl. 4):S321–S325. doi: 10.1097/TA.0b013e3182701902. [DOI] [PubMed] [Google Scholar]
- 26.Lane J.C., Mabvuure N.T., Hindocha S., Khan W. Current concepts of prophylactic antibiotics in trauma: a review. Open Orthop J. 2012;6:511–517. doi: 10.2174/1874325001206010511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altergott C., Garcia F.J., Nager A.L. Pediatric fingertip injuries: do prophylactic antibiotics alter infection rates? Pediatr Emerg Care. 2008;24:148–152. doi: 10.1097/PEC.0b013e3181666f5d. [DOI] [PubMed] [Google Scholar]