Summary
Background
The spread of carbapenemase-producing Enterobacterales (CPE) is a global health problem. Gastrointestinal tract carriage makes faeces or rectal swabs the recommended screening methods.
Aim
To assess the impact of three laboratory screening strategies for CPE on positivity rates and infection prevention and control in a hospital setting in North West England from 2015 to 2017.
Methods
In a retrospective study, time to CPE-positive and -negative results, number of new CPE-positive patients identified, and number of hospital bed-days lost/wards affected were measured for each of three CPE screening strategies; culture plus phenotypic tests, culture plus polymerase chain reaction (PCR), and PCR only (phases 1, 2 and 3, respectively).
Findings
The fastest time to CPE results was PCR only (median: 4.0 h), then culture plus PCR (median: 47.6 h), then culture plus phenotypic tests (median: 49.8 h) (P < 0.001). The mean numbers of hospital bed-days lost per month decreased between phases 2 and 3 (P = 0.01). The mean number of wards/units affected by CPE increased from phase 1 (2.57) to phase 2 (7.71), then decreased in phase 3 (3.86). The percentage positivity rate for phases 1, 2, and 3 were 2.01, 1.38, and 1.55 respectively. From May to October, the number of new CPE-positive patients was lower for phases 1 and 3 than for phase 2. During all three phases there was a peak in the number of newly identified CPE carriers in August.
Conclusion
This study provides evidence that using a rapid PCR to screen rectal or faeces swabs enables more timely infection prevention and control measures when compared with culture-based methods. A reduction in bed-days lost due to CPE was observed when rapid molecular screening was introduced.
Keywords: Carbapenemase-producing Enterobacterales, Laboratory, Hospital, Time to result, Infection prevention
Introduction
The spread of carbapenemase-producing Enterobacterales (CPE) is a global health problem of great concern. Gastrointestinal tract carriage makes faeces or rectal swabs the recommended screening methods [1]. Among risk factors for CPE colonization are length of hospital stay and hospitalization overseas [2]. The prevalence of CPE carriage in Europe has increased due to increased spread [3]. The percentage of carbapenem non-susceptible Klebsiella pneumonia isolates in England that were notified to Public Health England (PHE) increased from 3.8% to 5.8% between March 2016 and January 2018 (PHE Antimicrobial Surveillance workbook November 2018, unpublished results), with Merseyside in North West England reporting non-susceptibility of 8.5%. Similarly, non-susceptibility of Escherichia coli isolates to imipenem and meropenem in Merseyside at 0.6% is higher than the average for England (PHE Antimicrobial Surveillance workbook November 2018). Increasing prevalence of CPE colonization brings with it myriad clinical and infection prevention and control (IPC) challenges for healthcare facilities, notably preventing the spread of the organism. Clinical implications for a CPE carrier could arise if the patient develops a CPE infection with limited antimicrobial treatment options. These infections could increase hospital length of stay, increasing cost, and the risk of further resistant organism exposure [4].
In recognition of the global spread of CPE over the previous ten years, several guidelines have been published. The 2013 Acute Trust Toolkit for the Early Detection, Management and Control of Carbapenemase-Producing Enterobacteriaceae recommends that patients at high risk of CPE carriage, or contacts of CPE carriers, be nursed in isolation until three consecutive negative results several days apart are obtained [5]. More recently, the European Communicable Disease Centre (ECDC) recommended use of ‘sensitive and timely’ CPE screening methods but did not recommend a specific type of method owing to a lack of consensus [6]. The World Health Organization (WHO) recommend that surveillance is essential to limit spread by rapidly identifying CPE colonization [7].
The number of CPE screening methods available to microbiologists has increased over time and a recent survey of screening methods in English hospitals revealed wide variation in screening protocols and no consensus on best practice [8]. When CPE were first reported, common laboratory practice was solid medium culture for 18–24 h with confirmation using phenotypic tests. This approach takes two to three days to yield a result for hospital IPC teams. It is also labour intensive and may become increasingly costly if the number of confirmation methods to accurately identify CPE increases. A more recent alternative to culture is direct real-time PCR of patient samples targeting the most common CPE types, namely Klebsiella pneumoniae carbapenemase (KPC), New Delhi metallo-β-lactamase (NDM), Verona integron-encoded metallo-β lactamase (VIM), oxacillinase-48-type carbapenemases (OXA-48), and imipenemase (IMP) metallo-β-lactamase. One or more of these types accounted for >99% of isolates referred to PHE in 2017 [9]. PCR is a sensitive and specific method which can produce a result within 2 h of sample collection [10]. Despite studies showing the advantages of rapid molecular tests, there is currently insufficient evidence to recommend them for CPE screening [1], [10], [11].
The aims of this study were to assess three laboratory CPE detection and typing approaches used between March 2015 to December 2017 and to measure:
-
–
the time to CPE-positive and -negative results;
-
–
the number of samples tested, new CPE-positive patients identified, and CPE genotype detected;
-
–
CPE carrier identification and impact on IPC measures by the number of hospital bed-days lost and wards affected.
Methods
Hospital setting
The Royal Liverpool and Broadgreen University Hospital NHS Trust (RLBUHT) comprises the Royal Liverpool Hospital (RLH) in central Liverpool and Broadgreen Hospital (BH) in the city's suburbs. RLH was built in 1978, while BH consists of buildings of different ages, the oldest built in 1903. RLH is a tertiary hospital with 750 beds and more than 40 wards with specialist acute cardiology, respiratory care, general surgery, nephrology, renal transplant, orthopaedics, tropical and infectious diseases, haemato-oncology, gastrointestinal disease, intensive therapy and high dependency units. BH has 180 beds with specialist urology, orthopaedics and rehabilitation units. Patients are transferred between the two sites. All wards have four- or six-bed bays except some specialist units with only single rooms. The number of single rooms available for isolation of patients with transmissible organisms such as CPE, Clostridium difficile, and meticillin-resistant Staphylococcus aureus varies but is typically four-five per ward. Availability may be limited especially during winter months, as cases of influenza and norovirus increase. The testing laboratory is on the RLH site, samples from BG require transportation by courier so the transportation time could impact on the time to issue a result.
CPE screening strategies: laboratory testing
Three different laboratory CPE detection and typing approaches were used between March 2015 and December 2017. Laboratory testing phase 1 was from March 2015 to February 2016, when CPE screening samples were cultured on Brilliance CPE chromogenic agar (Oxoid, Basingstoke, UK) aerobically at 37°C with a 10 μg meropenem disc (Oxoid) for 18–24 h, repeated for 36–48 h if required. Any colonies suggesting meropenem resistance defined as a zone of inhibition of <32 mm around the meropenem disc after 24–48 h were tested using the algorithm in Figure 1 [12]. The Rapidec® Carba NP assay (bioMérieux, Marcy l’Etoile, France) and Rosco KPC/MBL, OXA-48 Confirm Kit (ROSCO, Taastrup, Denmark) were both used for phenotypic confirmation. For a small number of isolates where CPE status could not be determined using these tests, an in-house PCR targeting KPC, OXA-48, NDM, and VIM was used on an infrequent and ad-hoc basis [13], [14]. Results were released from the laboratory information management system (LIMS) manually by a biomedical scientist.
Figure 1.
Workflow for culture-positive samples in phase 1 with testing algorithm based on different combinations of results for CarbaNP/Rosco carbapenemase-producing Enterobacterales (CPE) tests. Polymerase chain reaction (PCR) was not a significant methodology in phase 1 and was not in used in real time but on an ad-hoc basis. ZOI, zone of inhibition; BSAC, British Society for Antimicrobial Chemotherapy.
Laboratory testing phase 2 was from March 2016 to December 2016. As in phase 1, CPE screening samples were cultured on Brilliance chromogenic agar with a 10 μg meropenem disc. Any colonies indicating meropenem resistance (defined as a zone of inhibition after 18–24 h or 36–48 h incubation) were tested using the Cepheid Carba-R PCR on the GeneXpert System (Cepheid, Wooburn Green, UK) targeting the KPC, OXA-48, VIM, NDM, and IMP-1 genes [12]. Results were released from the LIMS by a biomedical scientist.
Laboratory testing phase 3 was from January 2017 to December 2017. Rectal or faeces swabs were tested using Cepheid Carba-R PCR on the GeneXpert System, which was interfaced to the LIMS to enable automatic release of laboratory results when the Carba-R test was complete. Culture on Brilliance agar with a 10 μg meropenem disc was performed on any sample that was positive for CPE by Carba-R PCR and suspected CPE isolates referred to PHE AMRHAI reference unit.
The total number of samples received each month for each phase and the percentage of those with a detected CPE were recorded as well as the number of new positive CPE patients by type and month.
Background to CPE detection 2011–2015
To place the three phases of CPE testing into context, it is important to understand prior local CPE epidemiology. The first CPE isolate at RLBUHT was an OXA-48 isolated from an orthopaedic patient's mid-stream urine sample in 2011. Between then and the end of 2013, five more CPE (two NDM, two KPC, one OXA-48) from four patients were isolated. During 2014, eight CPE were isolated from clinical (non-screening) samples (six KPC; two OXA-48). Screening of rectal swabs from patients suspected of being at increased risk of colonization with CPE as defined by PHE began in March 2015 [5]. Over the next six months, the number of screening swabs sent to the microbiology laboratory from patients at increased risk of colonization with CPE and contacts of carriers increased following implementation of the PHE recommendation of three screening samples within seven days of hospital admission.
IPC measures at RLBUHT
The IPC CPE screening policy/measures at RLBUHT were based on the recommendations of the Acute Trust Toolkit for the Early Detection, Management and Control of carbapenemase-producing Enterobacteriaceae [5].
Wherever possible, IPC measures were applied to minimize disruption to patient flow through the hospital. Patients were immediately placed in isolation if assessed to be high risk for CPE colonization. In phases 1 and 2, this was until the third negative screen laboratory culture result was available. In phase 3, this was until the PCR result was available. Criteria for high CPE risk include:
-
–
previous admission to a UK hospital with known CPE spread. In phases 2 and 3, this expanded to include all UK hospitals (including RLUH and BH);
-
–
hospitalization abroad in the previous 12 months;
-
–
previously identified CPE carriage or infection;
-
–
close contact with a CPE-colonized or CPE-infected person.
IPC measures observed included:
-
–
notification of CPE colonization to relevant staff;
-
–
isolation of the patient in a side room with en-suite facilities;
-
–
strict adherence to the CPE management plan including barrier nursing with gloves and gowns for patient contact;
-
–
immediate assessment to ascertain any epidemiological link to other cases.
This study calculated the number of ‘lost bed-days’ due to CPE. A lost bed-day was counted when a hospital bed remained unoccupied for 24 h because its use had been blocked by the IPC team due to the presence of a CPE-colonized patient in the bay who could not be isolated or because the room or bay was waiting to be cleaned and misted with hydrogen peroxide vapour. The numbers of lost bed-days for any ward/unit affected by CPE were recorded by the IPC team. Data collection started in July 2015 when the number of identified CPE carriers increased.
Patients
The patients selected for CPE testing were those considered at high risk of CPE colonization. Only high-risk patients were tested in the interest of feasibility and cost in an NHS Trust with more than 900 beds; however, as the epidemiology of carriage changed, additional patients were considered high risk. A contact patient had shared a multi-bed area and/or toilet facilities with a person colonized or infected with CPE or had been cared for by nursing staff simultaneously caring for CPE-colonized patient(s) without contact precautions. For phases 1 and 2, patients who had been in contact with a known CPE-colonized patient were screened as soon as the index colonization was reported. During phase 3 only those in contact with CPE-colonized patients for >24 h were screened. Patients who had been in contact with a CPE carrier but who were discharged from hospital before the carrier was identified were not screened. Patients in critical care and haemato-oncology units were excluded from this analysis as they followed a pre-existing surveillance protocol.
For the purposes of this study, only CPE-positive patients not previously identified as carriers were included in the analysis. The assumption was made that a patient known to be previously CPE-colonized would still carry the organism(s), so IPC measures would be implemented upon hospital admission without testing. Including previously positive patients would hinder accurate assessment of laboratory testing improvements (reflected in time to result) achieved during each phase. Patients with a first positive CPE result were identified using a combination of laboratory and IPC data. Time to result was calculated as time from receipt in the laboratory to result authorization. Data were analysed for each CPE type by month/year and hospital ward/unit.
Statistical analysis
Time-to-result data were not normally distributed, even when log-transformed, and therefore did not meet the assumptions for parametric statistical tests. Therefore, the non-parametric Kruskal–Wallis test was used to assess whether there was a statistically significant difference between median time to results for the three phases calculated overall and separately for positive and negative results. Wilcoxon signed-rank pairwise test was then performed with Bonferroni correction. Analysis was using R software (v3.5.2, R Core Team, Vienna, Austria). To assess bed-days lost due to CPE, the mean number of days lost per month for each phase was compared using one-way analysis of variance. A t-test (independent two-group) was calculated for bed-days lost in phase 1 with phase 2, phase 1 with phase 3, and phase 2 with phase 3, using GraphPad at https://www.graphpad.com/quickcalcs.
Results
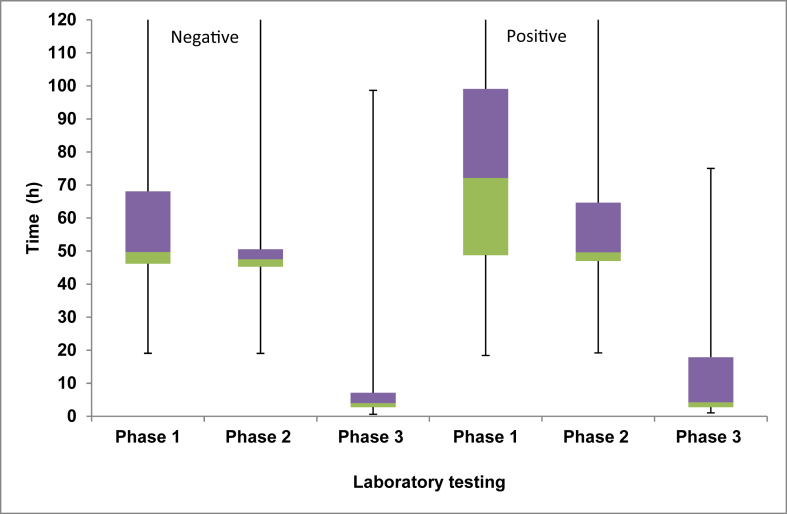
The total number of samples tested between March 2015 and December 2017 was 21,865. Using PCR (phase 3) to test for CPE resulted in the fastest time to results (median: 4.0 h) followed by culture plus PCR (phase 2) (median: 47.6 h). The slowest method was using culture followed by phenotypic test confirmation of a suspected CPE (phase 1) (median: 49.8 h) (Table I and Figure 2). This was also the case when looking at only the positive results or the negative results (Table I and Figure 2). The differences in median time to result were statistically significant between all the three phases (i.e. phase 1 vs 2, 1 vs 3, and 2 vs 3) (P < 0.001) and when comparing only the positive results (P < 0.001) or only the negative results (P < 0.001). The median time taken to issue a positive result decreased by 22.5 h from phase 1 to phase 2 and by a further 45.3 h from phase 2 to phase 3 (Table I and Figure 2). During phase 1, the time to confirm a positive CPE result was greater than that for a negative result (median time positive 72.1 h vs median time negative 49.7 h; Kruskal–Wallis, P < 0.001). This was due to the additional time needed to perform the phenotypic tests in order to determine CPE type (Table I).
Table I.
Total number of tests performed and median time to results for the three testing phases
| Variable | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
| Duration of phase | 12 months | 10 months | 12 months |
| CPE tests performed | 5168 | 9810 | 6887 |
| Positive samples/patients | 104/62 (2.01%) | 135/96 (1.38%) | 107/91 (1.55%) |
| Negative samples | 5064 (97.99%) | 9675 (98.62%) | 6780 (98.45%) |
| Time to result (h) | |||
| Overall | |||
| Median | 49.8 | 47.6 | 4.0 |
| Range | 18.4–165.7 | 19.1–165.7 | 1.0–98.7 |
| IQR | 46.2–68.4 | 45.3–51.8 | 2.8–7.2 |
| Positive results | |||
| Median | 72.1 | 49.6 | 4.3 |
| Range | 18.4–153.8 | 19.2–140.2 | 1.1–75.0 |
| IQR | 48.8–99.1 | 47.0–64.7 | 2.8–17.9 |
| Negative results | |||
| Median | 49.7 | 47.5 | 4.0 |
| Range | 19.1–165.7 | 19.1–165.7 | 1.0–98.7 |
| IQR | 46.2–68.1 | 45.3–50.6 | 2.8–7.1 |
CPE, carbapenemase-producing Enterobacterales; IQR, interquartile range.
Figure 2.
Positive and negative results for each testing phase: box plot shows median value, interquartile range (IQR) between the upper quartile (purple box) and lower quartile (green box) and range (minimum and maximum values represented by whiskers). Laboratory testing phase 1 is culture followed by phenotypic testing, phase 2 is culture followed by genotypic testing, and phase 3 is polymerase chain reaction only.
Number of samples tested and percentage positive
The number of samples tested increased ten-fold during phase 1 (range: month 1, phase 1: 77; month 12, phase 1: 779) then stabilized during phase 2 (mean: 981 samples/month) with a decrease during phase 3 (mean: 574 samples/month) (Figure 3). The percentage positivity rate for phases 1, 2, and 3 was 2.01, 1.38, and 1.55, respectively (Table I). The number of samples tested was greater than the number of patients sampled in phases 1 and 2; this difference was not evident in phase 3 (Figure 3).
Figure 3.
Number of patients (red bars) and samples (blue bars) tested by month and year. Yellow line shows carbapenemase-producing Enterobacterales percentage positivity of the number of patients tested.
Number of new CPE carriers in each laboratory testing phase
From May to October, the number of new CPE-positive patients identified was higher in phase 2 than either phase 1 or 3 (Figure 4). During all three phases there was a peak in the number of newly identified CPE carriers in August (Figure 4). For each of the three phases, this increase was not associated with an outbreak of a single CPE type rather different types or mixtures of types (e.g. OXA-48 and KPC) across different wards.
Figure 4.
Number of new carbapenemase-producing Enterobacterales carriers in each laboratory testing phase. Blue: phase 1 (March 2015 to February 2016); red: phase 2 (March 2016 to December 2016); green: phase 3 (January 2017 to December 2017).
Number of new positives per month by genotype
In phase 1, KPC was the most prevalent genotype in newly identified CPE carriers (Figure 5). In phase 2, KPC predominated at the start but from June 2016 (phase 2) OXA-48 became the predominating genotype. This continued throughout phase 3 where a marked reduction in newly identified patients carrying the KPC genotype was seen (Figure 5). A small number of patients were identified carrying up to three different CPE genotypes (Figure 5).
Figure 5.
Number of positively tested carbapenemase-producing Enterobacterales genotypes for each month.
Number of hospital bed-days lost and wards/units affected by CPE by testing phase
The mean number of hospital bed-days lost per month was 10.0, 22.8, and 9.0 for phases 1, 2, and 3 respectively (Table II). There were significantly fewer bed-days lost in phase 3 compared to phase 2 (P = 0.01, independent t-test) but this was not seen when phases 1 and 2 (P = 0.08) and 1 and 3 (P = 0.8) were compared. The number of wards/units where a patient was identified as a CPE carrier increased as the number of CPE carriers increased, rising from a mean of 2.57 wards in phase 1 to 7.71 wards in phase 2. This decreased with the introduction of PCR as the primary screening method from January 2017 (phase 3) to a mean of 3.86. In phase 3, the number of bed-days lost due to CPE were 18, 20, and 25 in the first three months of the year. The numbers then fell to nine in April and remained below 12 per month for the remainder of phase 3.
Table II.
Number of CPE-positive patients and bed-days lost due to CPE
| Variable | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
| No. of months when data were collected | 6 | 10 | 12 |
| Total no. of positive patients | 41 | 156 | 128 |
| Mean no. positive patients/month | 6.8 | 15.6 | 10.6 |
| Total no. of bed closures | 60 | 228 | 108 |
| Mean bed-days lost per month (SD) | 10.0 (8.4) | 22.8 (13.6) | 9.0 (8.3) |
| Mean wards/units affected | 2.57 | 7.71 | 3.86 |
CPE, carbapenemase-producing Enterobacterales.
Discussion
This three-phased retrospective study during the period March 2015 to December 2017 was based on different CPE screening and identification laboratory methodologies. These were used in response to a real-life situation of increasing CPE identification owing to increased CPE spread and evolving laboratory testing methodologies. As more CPE carriers were identified, isolating them according to the hospital IPC policy increased pressure on isolation beds. IPC guidelines on CPE became outdated and there was no ‘reference standard’ testing approach.
This study has several strengths. First, it was conducted in a real-life setting where technological advances reduced laboratory turnaround times. Second, it adds to the available literature on the IPC impact of laboratory testing strategies. Berry et al. reported the diversity of UK CPE screening methods; evidence to support a preferred CPE testing algorithm is currently lacking but our study indicates that rapid molecular CPE screening of faeces or rectal swabs should be considered in hospitals with an increasing CPE burden where culture combined with phenotypic or genotypic testing is currently used [8]. Our finding of a median reduction in time to a positive result from 49.6 h for culture plus PCR to 4.3 h with PCR only would enable an earlier IPC response with more timely interventions. Although implementing PCR screening incurred high laboratory acquisition costs, there are likely to be savings made elsewhere as a result of expediting test results as well as improvements to the patient's experience. In phases 1 and 2 of this study the number of samples exceeded the numbers of patients screened as there was an aim to obtain three samples from each patient. This number was not always achieved as many patients were discharged before this number could be attained. In phase 3, PCR on a single sample was used to determine status of the patient. The use of a single sample is a method that has been reported to determine CPE colonization in other studies [1], [10]. This single result in phase 3 would suffice for infection management of patients so the numbers of samples and patients tested were more evenly balanced.
Despite a decrease in median time to results of only 2.2 h between phase 1 and phase 2, the number of bed-days lost per month increased by 12.8 days. This increase was likely due to testing nearly double the number of samples in phase 2 compared with phase 1 and the increased number of patients carrying CPE (Table I). There was a significant reduction in the mean number of bed-days lost between phases 2 and 3, from 22.8 to 9.0 (Table II). This may be due to the more rapid application of infection control measures, including rapid isolation of colonized patients, negating the need to close beds either for contact screening or for hydrogen peroxide vapour misting of bays.
The rise in bed-days lost and the number of wards affected immediately after the introduction of a rapid PCR (phase 3) as a first-line screening method could be due to identification of CPE carriers whose acquisition occurred prior to the change in testing methodology. A downward trend in bed-days lost and number of wards affected then started from three months after the introduction of the rapid PCR and continued for the remaining eight months of phase 3. These data therefore suggest that CPE transmission was somewhat controlled by the introduction of a faster sensitive test. Further studies are required to confirm the impact of faster result times on bed-days lost in a variety of settings; however, as CPE spreads more widely in the UK, such rapid screening methods should be considered to reduce transmission risk.
In our patient cohort we identified a small number of patients with more than one CPE type in the same sample. Patients with a combination of KPC and OXA-48 were detected during testing phases 2 and 3 (Figure 5). This combination has been reported by PHE to be increasing in the UK and was associated with an outbreak of K. pneumoniae in a London hospital [9]. The number and mixture of CPE types in our NHS Trust appeared to relate to sporadic cases, indicating that the spread was appropriately contained. We also noted an increase in the number of new positive patients detected in August of each phase (Figure 4). This may be coincidental or there may have been a genuine increase in the number of CPE acquisitions. Further studies to confirm this finding, to identify possible associations (such as travel to endemic areas), and to assess possible seasonality of CPE need to be undertaken.
This study has some limitations. First, it was a retrospective study based in a single hospital organization. However, hospitals face the same problems globally as CPE continues to spread. The phased approach to CPE detection was a response to escalating CPE positivity and the number of hospital bed-days lost due to IPC measures rather than planned. The move from culture with phenotypic confirmation of a CPE (phase 1) to culture followed by genotypic confirmation (phase 2) resulted in a decrease in the positivity rate in the subsequent nine months (Table I) but the number of bed-days lost, wards or units affected (Table II) and patients detected with CPE increased (Figure 5). Despite an improvement in result turnaround time from phase 1 to phase 2, this and the clinical need to further improve turnaround time facilitated the change to a rapid and sensitive test for CPE detection.
Second, only screening samples (faeces and rectal swabs) from patients who were at increased risk of CPE acquisition were included. Other patients could carry CPE but not be identified. It was not possible to screen all patients admitted due to financial constraints, nor is universal screening advocated in Acute Trust Toolkit for the Early Detection, Management and Control of Carbapenemase-Producing Enterobacteriaceae, so some transmission events could have been missed [5]. We also did not include patients in the analysis where a CPE was identified from a different anatomical site, as these were identified using laboratory culture and then reflex tested for CPE confirmation during the three phases.
Third, as this was a real-life response to an evolving situation it is likely that there were subtle changes in practice, as both IPC and ward-based staff became familiar with the management of increasing numbers of patients colonized with CPE. These unmeasured changes may have contributed to the success of IPC measures, as indicated by a reduction in lost bed-days during phase 3.
In conclusion, the findings in this retrospective study provide evidence that using a molecular test such as PCR to screen patient samples provides a rapid time to result. This benefits hospitals and patients by facilitating timely IPC measures, thereby reducing the risk of exposure and onward transmission to non-CPE patients and reduced bed-days lost.
Acknowledgements
We would like to thank Dr S. Patel (Cepheid, UK) for data analysis and Dr S. Huntington (Aquarius Population Health) for statistical analysis and constructive comments. We would also like to thank J. Sinnott (IPC team, RLBUHT) for providing IPC bed-day data.
Footnotes
Results presented in part at British Society for Antimicrobial Chemotherapy Infection Prevention 2018 meeting, Glasgow, 30.09-02.10.18 [conference, location, date].
Conflict of interest statement
C.E.C. and A.M.H. have no interests to declare. T.J.N. has received consultancy fees from Cepheid.
Funding sources
None.
References
- 1.Lowman W., Marais M., Ahmed K., Marcus L. Routine active surveillance for carbapenemase-producing Enterobacteriaceae from rectal swabs: diagnostic implications of multiplex polymerase chain reaction. J Hosp Infect. 2014;88:66–71. doi: 10.1016/j.jhin.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Nicolas-Chanoine M.H., Vigan M., Laouénan C., Robert J., on behalf of the E-carb Study Group Risk factors for carbapenem-resistant Enterobacteriaceae infections: a French case–control–control study. Eur J Clin Microb Infect Dis. 2019;38:383–393. doi: 10.1007/s10096-018-3438-9. [DOI] [PubMed] [Google Scholar]
- 3.Albiger B., Glasner C., Struelens M.J., Grundmann H., Monnet D.L. European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) working group in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill. 2015;20(45) doi: 10.2807/1560-7917.ES.2015.20.45.30062. [DOI] [PubMed] [Google Scholar]
- 4.Mauldin P.D., Salgado C.D., Hansen I.S., Durup D.T., Bosso J.A. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant Gram-negative bacteria. Antimicrob Agents Chemother. 2010;54:109–115. doi: 10.1128/AAC.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acute trust toolkit for the early detection, management and control of carbapenemase-producing Enterobacteriaceae. 2013. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/329227/Acute_trust_toolkit_for_the_early_detection.pdf on 13/11/18 Available at: [last accessed July 2019] [Google Scholar]
- 6.Magiorakos A.P., Burns K., Rodríguez Baño J., Borg M., Daikos G., Dumpis U. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: guidance from the European Centre for Disease Prevention and Control. Antimicrob Resist Infect Control. 2017;6:113. doi: 10.1186/s13756-017-0259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . WHO; Geneva: 2017. Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities. Licence: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 8.Berry C., Davies K., Woodford N., Wilcox M., Chilton C. Survey of screening methods, rates and policies for the detection of carbapenemase-producing Enterobacteriaceae in English hospitals. J Hosp Infect. 2019;101:158–162. doi: 10.1016/j.jhin.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 9.English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) 2018. ESPAUR 2018 report.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/749747/ESPAUR_2018_report.pdf Available at: [last accessed November 2018] [Google Scholar]
- 10.Tato M., Ruiz-Garbajosa P., Traczewski M., Dodgson A., McEwan A., Humphries R. Multisite evaluation of Cepheid Xpert Carba-R Assay for detection of carbapenemase-producing organisms in rectal swabs. J Clin Microbiol. 2016;54:1814–1819. doi: 10.1128/JCM.00341-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoyos-Mallecot Y., Ouzani S., Dortet L., Fortineau N., Naas T. Performance of the Xpert® Carba-R v2 in the daily workflow of a hygiene unit in a country with a low prevalence of carbapenemase-producing Enterobacteriaceae. Int J Antimicrob Agents. 2017;49:774–777. doi: 10.1016/j.ijantimicag.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 12.British Society for Antimicrobial Chemotherapy (BSAC) Methods for Antimicrobial Susceptibility Testing; version 14 January 2015. Available at: http://www.bsac.org.uk/wp-content/uploads/2012/02/BSAC-Susceptibility-testing-version-143.pdf [last accessed May 2019].
- 13.Swayne R.L., Ludlam H.A., Shet V.G., Woodford N., Curran M.D. Real-time TaqMan PCR for rapid detection of genes encoding five types of non-metallo-(class A and D) carbapenemases in Enterobacteriaceae. Int J Antimicrob Agents. 2011;38:35–38. doi: 10.1016/j.ijantimicag.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Diene S.M., Bruder N., Raoult D., Rolain J.-M. Real-time PCR assay allows detection of the New Delhi metallo-β-lactamase (NDM-1)-encoding gene in France. Int J Antimicrob Agents. 2011;37:544–546. doi: 10.1016/j.ijantimicag.2011.02.006. [DOI] [PubMed] [Google Scholar]