Abstract
Background
Infective endocarditis (IE) is more common in patients with cancer as compared with the general population. Due to an immunocompromised state, the need for invasive procedures, hypercoagulability and the presence of indwelling catheters, patients with cancer are particularly predisposed to the development of IE.
Objectives
Limited information exists about IE in patients with cancer. We aimed to evaluate the characteristics of patients with cancer and IE at our tertiary care centre, including a comparison of the microorganisms implicated and their association with mortality.
Methods
A retrospective chart review of patients with cancer who had echocardiography for suspicion of endocarditis was conducted. A total of 56 patients with a confirmed diagnosis of cancer and endocarditis, based on the modified Duke criteria, were included in the study. Baseline demographics, risk factors for developing IE, echocardiography findings, microbiology and mortality data were analysed.
Results
Following the findings of vegetations by echocardiography, the median survival time was 8.5 months. Staphylococcus aureus was the most common organism identified as causing endocarditis. The mitral and aortic valves were the most commonly involved sites of endocarditis. Patients with S. aureus endocarditis (SAE) had a significantly poorer survival when compared with patients without SAE (p=0.0217) over the 12-month period from diagnosis of endocarditis.
Conclusions
Overall survival of patients with cancer and endocarditis is poor, with a worse outcome in patients with SAE.
Keywords: endocarditis, infection, epidemiology
Key questions.
What is already known about this subject?
Infective endocarditis (IE) occurs in more commonly in patients with cancer as compared with the general population due to a myriad of factors including an immunocompromised state, the need for invasive procedures, hypercoagulability and the presence of indwelling catheters.
What does this study add?
There is limited information about the characteristics of IE in patients with cancer. Our study shows that overall survival of patients with cancer and endocarditis is poor (median survival of 8.5 months), IE occurs most commonly on the mitral and aortic valves, and that Staphylococcus aureus endocarditis has worse outcome.
How might this impact on clinical practice?
Our study illustrates that there must be a high index of suspicion and a thorough investigation in patients with cancer with suspected IE. Given the poor prognosis of IE in patients with cancer, it is of the utmost importance that these patients are efficiently diagnosed and treated.
Introduction
Endocarditis is a relatively uncommon but potentially fatal disease affecting 3–10 per 100 000 people, with a 1-year mortality rate of almost 30%.1–4 The demographics of patients infected with endocarditis have been changing. Until the 1980s, infective endocarditis (IE) was most prevalent in patients between 40 and 50 years of age and was mainly due to streptococcal organisms.1 5 6 IE is now most common in patients older than 70 years.7 Advances in therapy across many areas of medicine have led to the increasing use of indwelling devices such as prosthetic heart valves and long-term intravenous lines, which in turn have led to increased prevalence of staphylococcal bacteraemia, a precursor to endocarditis.8–10 This has also led to the emergence of Staphylococcus aureus as the most common organism in IE in some series, with >25% of cases being due to this organism.4
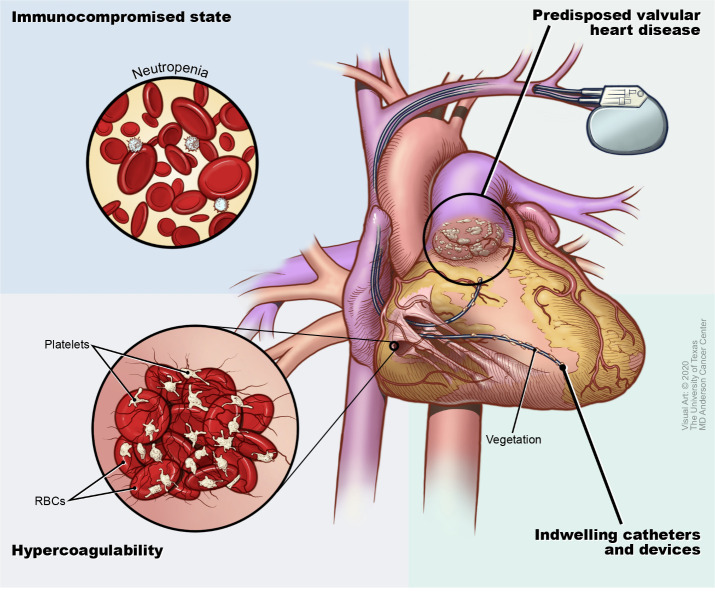
Due to an immunocompromised state, the need for invasive procedures, hypercoagulability, the presence of indwelling catheters and pre-existing valvular heart disease (VHD) (figure 1), patients with cancer are particularly predisposed to the development of IE.11–13 In addition, patients with cancer often develop recurrent local or systemic infections, which increases the possibility of IE. IE is common in patients with cancer, with a prevalence of about 18% in one study.14 In another prospective, multicentre study, 5.6% of patients with endocarditis had cancer.15 IE also has grave consequences in patients with cancer, as it is associated not only with increased mortality as compared with non-cancer patients but also cessation of chemotherapy and denial of more aggressive interventions.2 14 15 Limited information exists about the associated risk of IE with different types of cancer and the microorganisms commonly involved in IE in patients with cancer. A better understanding of predisposing factors may lead to improved identification and management of IE in this at-risk population of patients. Herein, we aimed to evaluate the characteristics of patients with cancer and IE at our tertiary care centre, including a comparison of the microorganisms implicated and their association with 12-month survival following the diagnosis of endocarditis.
Figure 1.
Conditions in patients with cancer that are conducive to infective endocarditis. RBC, red blood cells.
Methods
Patient selection and data collection
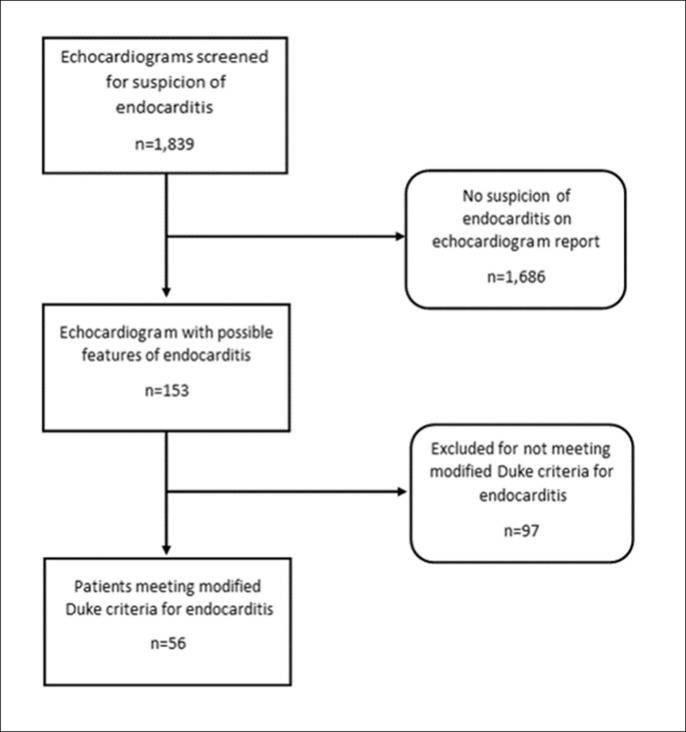
We conducted a retrospective chart review of 1839 patients with cancer who had echocardiography for suspicion of endocarditis from May 2001 to December 2006 and from July 2015 to December 2018 at The University of Texas MD Anderson Cancer Center (figure 2). Records in between these dates were not accessible due to electronic health record transition made by our institution during the study period. Among these patients, we identified 56 with a confirmed diagnosis of cancer as well as endocarditis, based on the modified Duke criteria.16 Data were extracted from the patient charts and analysed to confirm the diagnosis of endocarditis as per the modified Duke criteria. Data collected included baseline demographics (including age at diagnosis of cancer and IE, sex and race/ethnicity), findings of echocardiography (including transthoracic and transoesophageal studies), type of cancer, use of chemotherapeutics, use of steroids and mortality data. We also analysed the traditional risk factors for IE, including VHD, a pacemaker, a central line, dental surgery, recent invasive procedures, recent surgery, diabetes mellitus, HIV, heart failure and chronic kidney disease. Lastly, we evaluated the presence of modified Duke major and minor criteria for endocarditis, baseline serum chemistry data, septic emboli based on imaging studies, as well as blood culture results.
Figure 2.
Flow diagram for patient inclusion.
Patient and public involvement
Patients and the public were not involved in the design, conduct, or reporting of this study as it was done retrospectively.
Microbiology
The blood cultures were processed by the Microbiology Laboratory at MD Anderson Cancer Center at the time of IE diagnosis. After the samples were processed, standard techniques were employed to determine which pathogen was responsible, as well as antimicrobial sensitivities.17 18
Definitions
The modified Duke criteria were used to identify patients with endocarditis.16 19 Blood cultures that grew S. aureus and other typical organisms associated with endocarditis were considered clinically significant as the likely causative organism for IE. Patients with platelet counts less than 50 000/µL were considered thrombocytopaenic. Neutropaenia was defined to be absolute neutrophil count (ANC) of less than 500 cells/µL. Active cancer was defined as any cancer that was diagnosed or being managed by cancer therapeutics at the time of diagnosis of IE. Culture-negative IE was defined as endocarditis without microbiological aetiology following inoculation of samples in a standard blood-culture system with negative cultures after 7 days of incubation and subculturing. Predisposing valvular heart disease (VHD) was defined as patients at risk of VHD (stage A) or with progressive VHD (stage B) to severe asymptomatic (stage C) and symptomatic (stage D) VHD as per the American College of Cardiology/American Heart Association (AHA) societal guidelines, including patients with patients history of rheumatic heart disease, mitral valve stenosis, mitral regurgitation, bicuspid aortic valve, aortic stenosis, aortic regurgitation, prosthetic heart valve, history of IE, history of congenital heart disease or abnormal thickening of heart valves with greater than mild regurgitation.
Statistical analysis
Patient demographic and other characteristics were summarised by S. aureus status (presence or absence) using descriptive statistics. Survival time over 12 months (12-month survival) from diagnosis of endocarditis was calculated from first transthoracic or transoesophageal study to death or last follow-up occurred within 12 months. For those who survived longer than 12 months were censored at 12 months. Univariate and multivariate Cox proportional hazards regression models were used to identify factors associated with 12-month survival. Multivariate Cox models included factors with a significant p value based on univariate Cox models. The proportional hazards assumption was checked by the interaction between each covariate and the logarithm of survival time. When the proportional hazards assumption was violated, the time–covariate interaction was included in the multivariate model. A p<0.05 indicated statistical significance. SAS V.9.4 software (SAS Institute) was used for statistical analysis.
Results
Patient characteristics
We included 56 patients with a concurrent diagnosis of cancer and IE (table 1). The mean patient age was 54 years at diagnosis of cancer and 60 years at diagnosis of endocarditis. Most patients were white (45%) and male (55%). Thirty-six (64%) had active cancer. More than half of the patients had an underlying solid cancer (68%), while 18 patients (32%) had a haematological malignancy. The most common comorbidities among the cohort were diabetes (77%), chronic kidney disease (18%), and congestive heart failure (13%). Thirty-nine patients (70%) had culture-positive endocarditis and 17 (30%) had culture-negative endocarditis. S. aureus was the most common organism isolated in this cohort (20 patients), while 10 patients had Enterococcus species, 7 had Streptococcus viridans, 1 had a HACEK organism (Klebsiella) and 1 had Streptococcus gallolyticus. Among patients with S. aureus endocarditis (SAE), 11 patients were infected with methicillin-resistant S. aureus (MRSA). The most commonly involved site was the mitral valve, in 23 (41%) patients, followed by the aortic valve in 20 (36%) patients and the tricuspid valve in 9 (16%) patients. Two patients (4%) had pacemaker lead-associated endocarditis. Of the 23 patients with mitral valve endocarditis, seven (30%) had evidence of systemic embolisation, and four patients had vegetations ≥10 mm (17%). Three of four patients with vegetations ≥10 mm had systemic emboli. Of the 20 patients with aortic valve endocarditis, four (20%) had systemic emboli, one had large vegetations ≥10 mm and one had an abscess. Of the nine patients with tricuspid valve endocarditis, two (22%) had evidence of pulmonary infarcts, suggesting small pulmonary emboli. Twenty of the 56 patients (36%) had a central venous catheter at the time of IE diagnosis. Twenty-one patients had an indwelling foley catheter (38%), with five patients having a nephrostomy tube (9%) and one patient having a urostomy with an ileal conduit (2%).
Table 1.
Patient characteristics
| Patient characteristics (n=56) | Mean±SD or No (%) |
| Age at cancer diagnosis (years) | 53.74±16.18 |
| Age at endocarditis diagnosis (years) | 59.54±15.32 |
| Sex | |
| Male | 31 (55.4%) |
| Female | 25 (44.6%) |
| Race/ethnicity | |
| Non-black (white/other) | 36 (64.3%) |
| Black | 20 (35.7%) |
| Diabetes mellitus | 43 (76.8%) |
| Congestive heart failure | 7 (12.5%) |
| Chronic kidney disease | 10 (17.9%) |
| Type of cancer | |
| Haematologic cancer | 18 (32.1%) |
| Solid cancer | 38 (67.9%) |
| Cancer status | |
| Cancer in remission | 20 (35.7%) |
| Active cancer | 36 (64.3%) |
| Chemotherapy treatment | |
| Not active | 22 (39.3%) |
| Active | 34 (60.7%) |
| Pacemaker | 4 (7.1%) |
| Catheter/line presence | 20 (35.7%) |
| Endocarditis location | |
| Mitral valve | 23 (41.1%) |
| Aortic valve | 20 (35.7%) |
| Tricuspid valve | 9 (16.1%) |
| Pacemaker lead | 2 (3.6%) |
| Superior Vena Cava | 2 (3.6%) |
| Vegetation >10 mm | 5 (8.9%) |
| Systemic emboli | 11 (19.6%) |
| Blood microorganism | |
| Streptococcus viridans | 7 (12.5%) |
| Streptococcus gallolyticus | 1 (1.8%) |
| Klebsiella | 1 (1.8%) |
| Staphylococcus aureus | 20 (35.7%) |
| Enterococcus | 10 (17.9%) |
| Negative culture | 17 (30.4%) |
Table 2 compares the characteristics of patients with SAE and those without S. aureus. Patients with SAE had a significantly higher white cell count (WCC) (14.68 K/µL vs 11.29 K/µL in those without SAE; p=0.044) and had a significantly lower percentage of predisposing VHD (0% vs 22% in those without SAE; p=0.04). Otherwise, there were no significant differences between the two groups in age, sex, race/ethnicity, cancer status, comorbidities, indwelling catheter or location of IE involvement.
Table 2.
Comparison of patient characteristics with and without Staphylococcus aureus endocarditis
| Variable | No S. aureus (n=36) | S. aureus (n=20) | P value |
| Patient/diagnosis data | |||
| Age at cancer diagnosis (years) | 52.9±16.82 | 55.25±15.27 | 0.8844 |
| Age at endocarditis diagnosis (years) | 60.08±15.59 | 58.57±15.16 | 0.4069 |
| Time from cancer diagnosis to endocarditis diagnosis (months) | 86.6±146.12 | 40.31±51.54 | 0.5158 |
| Sex | 0.5478 | ||
| Male | 21 (58.3%) | 10 (50%) | |
| Female | 15 (41.7%) | 10 (50%) | |
| Race/ethnicity | 0.61 | ||
| Non-black (white/other) | 24 (66.7%) | 12 (60%) | |
| Black | 12 (33.3%) | 8 (40%) | |
| Comorbidities | |||
| Diabetes mellitus | 10 (27.8%) | 3 (15%) | 0.3392 |
| HIV | 0 (0%) | 1 (5) | 0.3455 |
| Congestive heart failure | 5 (13.9%) | 2 (10%) | 1 |
| Chronic kidney disease | 6 (16.7%) | 4 (20%) | 0.7234 |
| Oncologic data | |||
| Type of cancer | 0.798 | ||
| Haematological cancer | 12 (33.3%) | 6 (30%) | |
| Solid cancer | 24 (66.7%) | 14 (70%) | |
| Cancer status | 0.0963 | ||
| Cancer in remission | 10 (27.8%) | 10 (50%) | |
| Active cancer | 26 (72.2%) | 10 (50%) | |
| Active chemotherapy treatment | 22 (61.1%) | 12 (60%) | 0.935 |
| Steroid treatment | 3 (8.3%) | 3 (15%) | 0.4055 |
| Invasive procedures/endocarditis risk factors | |||
| Preexisting valvular heart disease | 8 (22.2%) | 0 (0%) | 0.0405 |
| Pacemaker presence | 3 (8.3%) | 1 (5%) | 1 |
| Bone marrow treatment/procedure | 8 (22.2%) | 2 (10%) | 0.3036 |
| Dental extraction | 0 (0%) | 2 (10%) | 0.1234 |
| Cystoscopy | 0 (0%) | 2 (10%) | 0.1234 |
| Upper GI endoscopy | 1 (2.8%) | 2 (10%) | 0.2879 |
| Lower GI endoscopy | 1 (2.8%) | 1 (5%) | 1 |
| Indwelling catheter/line | 10 (27.8%) | 10 (50%) | 0.0742 |
| Lab values | |||
| Haemoglobin (g/dL) | 9.96±2 | 10.24±2.04 | 0.6772 |
| White blood cell count (K/µL) | 11.29±13.68 | 14.68±11 | 0.0442 |
| Platelets (K/µL) | 163.12±120.13 | 205.70±141.10 | 0.2865 |
| Blood urea nitrogen (mg/dL) | 26.47±19.26 | 25.85±18.63 | 0.8019 |
| Creatinine (mg/dL) | 1.43±1.4 | 1.25±0.74 | 0.7506 |
| Alanine aminotransferase (U/L) | 59.72±111.25 | 47.3±45.82 | 0.5156 |
| Aspartate aminotransferase (U/L) | 60.63±85.59 | 44.05±31.28 | 0.8359 |
| Total bilirubin (mg/dL) | 0.97±1.15 | 1.48±3.09 | 0.374 |
| Site of involvement | |||
| Aortic valve | 13 (36.1%) | 7 (35%) | 0.9337 |
| Mitral valve | 17 (47.2%) | 6 (30%) | 0.2094 |
| Tricuspid valve | 6 (16.7%) | 3 (15%) | 1 |
| Pacemaker lead | 1 (2.8%) | 1 (5%) | 1 |
GI, gastrointestinal.
IE outcomes
After diagnosis of IE by echocardiography, the median survival time for our cohort was 8.5 months. In univariate analysis, black race was associated with significantly poorer 12-month survival when compared with all other races/ethnicities (HR 2.320; 95% CI 1.114 to 4.833; p=0.0246). Over the 12 months after diagnosis of IE, patients with active cancer had significantly poorer survival when compared with patients in remission (HR 2.497; 95% CI 1.062 to 5.868; p=0.0358).
Furthermore, increasing WCC, blood urea nitrogen, alanine aminotransferase (ALT), aspartate aminotransferase (AST), WCC <4000 cells/µL and >11 000 cells/µL, ALT ≥41 U/L, AST ≥40 U/L and bilirubin ≥1.2 mg/dL (above-normal institutional laboratory values) were significantly associated with increased risk of death within 12 months (table 3). A total of seven patients had cardiac valve surgery for endocarditis; six of them had SAE. Surgical treatment was not significantly associated with increased risk of death (HR 0.671; 95% CI 0.086 to 5.242; p=0.7036).
Table 3.
Univariate Cox regression analysis: variables that were significantly associated with survival within 12 months
| Variable | Level | HR (95% CI) | P value |
| WCC (K/µL) | In 1-unit increase | 1.052 (1.026 to 1.080) | <0.0001 |
| BUN (mg/dL) | In 1-unit increase | 1.020 (1.002 to 1.039) | 0.0284 |
| ALT (U/L) | In 1-unit increase | 1.004 (1.001 to 1.007) | 0.0035 |
| AST (U/L) | In 1-unit increase | 1.006 (1.002 to 1.010) | 0.0015 |
| Race/ethnicity | Black vs non-black | 2.320 (1.114 to 4.833) | 0.0246 |
| Cancer status | Active vs remission | 2.497 (1.062 to 5.868) | 0.0358 |
| Organism | S. aureus vs no S. aureus | 2.305 (1.107 to 4.802) | 0.0257 |
| Steroid usage | Yes vs No | 8.151 (3.059 to 21.719) | <0.0001 |
| WCC (K/µL) | <4 vs 4–11 | 3.845 (1.233 to 11.990) | 0.0203 |
| >11 vs 4–11 | 6.723 (2.783 to 16.241) | <0.0001 | |
| ALT (U/L) | ≥41 vs <41 | 4.032 (1.836 to 8.854) | 0.0005 |
| AST (U/L) | ≥40 vs <40 | 2.276 (1.071 to 4.836) | 0.0325 |
| Total bilirubin (mg/dL) | ≥1.2 vs <1.2 | 3.014 (1.285 to 7.070) | 0.0112 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; WCC, white cell count.
Although all patients were followed by an infectious diseases specialist and treated accordingly to AHA guidelines,20 patients with SAE had significantly worse 12-month survival when compared with those without SAE (HR 2.305; 95% CI 1.107 to 4.802; p=0.0257). The use of steroids was also associated with an increased risk of death within 12 months (HR 8.151; 95% CI 3.059 to 21.719; p<0.0001).
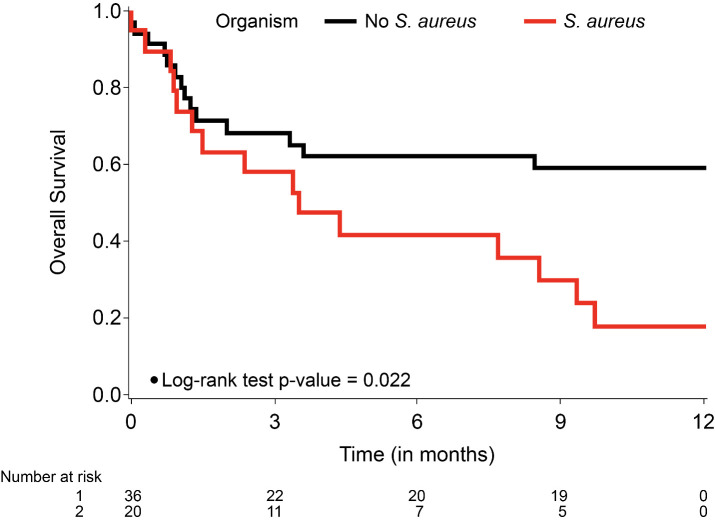
Multivariate Cox regression analyses revealed that the presence of SAE (HR 2.52; 95% CI 1.193 to 5.323; p=0.0015) and active cancer status (HR 2.967; 95% CI 1.253 to 7.025; p=0.013) and black race (HR 2.209; 95% CI 1.038 to 4.701, p=0.0396) remained significant risk factors for 12-month survival (table 4, model 1). The proportional hazards assumption was violated for SAE. Therefore, HR for SAE was interpreted as the weighted average over follow-up in the model without time–covariate interaction (table 4, model 1). In a second model (table 4, model 2), we considered time and SAE interaction (allowing two different HRs for two-time intervals, ≤4 months and >4 months of follow-up). Presence of SAE was not significantly associated with overall survival during the first 4 months but was significantly associated with overall survival after 4 months (HR 17.316; 95% CI 1.993 to 150.44; p=0.0097). Active cancer status (HR 2.892; 95% CI 1.217 to 6.875; p=0.0162) and black race (HR 2.140; 95% CI 1.008 to 4.453; p=0.0476) was significantly associated with 12-month survival. The Kaplan-Meier survival curve comparing patients with SAE versus non-SAE also reflects significantly poorer survival with SAE (p=0.0217) over the 12-month period from diagnosis of endocarditis (figure 3).
Table 4.
Multivariate Cox regression model
| Model 1, without time-covariate interaction | |||
| Model 1 | |||
| Covariate | Level | HR (95% CI) | P value |
| Organism | No Staphylococcus aureus | 1 | |
| S. aureus | 2.520 (1.193 to 5.323) | 0.0154 | |
| Cancer status | Remission | 1 | |
| Active | 2.967 (1.253 to 7.025) | 0.0134 | |
| Race/ethnicity | Non-black | 1 | |
| Black | 2.209 (1.038 to 4.701) | 0.0396 | |
| Model 2, with time-covariate interaction | ||||
| Model 2 | ||||
| Covariate | Time interval | Level | HR (95% CI) | P value |
| Cancer status | Remission | 1 | ||
| Active | 2.892 (1.217 to 6.875) | 0.0162 | ||
| Race/ethnicity | Non-black | 1 | ||
| Black | 2.140 (1.008 to 4.543) | 0.0476 | ||
| Organism | ≤4 months | No S. aureus | 1 | |
| S. aureus | 1.648 (0.713 to 3.810) | 0.2425 | ||
| >4 months | No S. aureus | 1 | ||
| S. aureus | 17.316 (1.993 to 150.440) | 0.0097 | ||
Figure 3.
Kaplan-Meier survival curves by presence of Staphylococcus aureus endocarditis. The curves were censored at 12 months after endocarditis diagnosis.
Discussion
Patients with cancer with IE have poor prognosis,15 and among such patients, those with SAE may in fact have the worst mortality after IE diagnosis. Our study showed a statistically significant association between mortality and SAE compared with non-SAE in patients with cancer. In fact, after 4 months patients with SAE were 20 times more likely to die compared with non-SAE patients.
Few studies have focused on IE in patients with cancer, and there is a relative paucity of data about the most prominent types of cancer associated with IE, which valves/sites are involved, and rates due to venous lines or cardiac devices. One study of 161 patients with cancer and IE showed that the majority of patients with solid tumours had intestinal cancers (42%) and the majority of those with haematological malignancies had lymphoma (42%).15 Studies have also showed that the vast majority of vegetations involve the mitral and aortic valves in patients with and without cancer.3 15 The source of the IE was documented to be associated with a central venous catheter in 23% of patients in a previous study of patients with cancer and IE, and up to 10% of the vegetations were associated with pacemaker leads.15 Our study showed that the mitral valve was most commonly involved, followed closely by the aortic valve, and 4% of patients had pacemaker-lead endocarditis.
The typical causative organism for non-cancer patients with IE has been found to be S. aureus.3 21 22 Some studies report Streptococcus spp most commonly, followed by S. aureus.15 23 One study of patients with cancer with IE showed equal prevalence of S. aureus and Streptococcus spp, with each comprising 23.6% of cases.15 Others have shown S. aureus to be the most common causative organism for IE in patients with cancer.24 Our study found that 20 (51%) of the 39 patients with culture-positive endocarditis had presence of S. aureus; this was 36% of all the patients (20 of 56).
SAE has been associated with a significant increase in in-hospital death in non-cancer patients and significantly increased 30-day mortality in surgical patients.3 22 23 Fernández-Cruz et al showed SAE to be a risk factor for 30-day mortality in patients with cancer with IE.15 Our study builds on this association of SAE and poor outcomes by showing significantly worse 12-month survival when compared with culture-negative IE and presence of other organisms. S. aureus is more problematic due to multiple factors, including its ability to adhere to and multiply on cardiac cells, form biofilms preventing eradication and rapidly develop resistance.22 25 26
Compared with the general population, patients with cancer are more likely to die from IE, but the mechanism is not completely known.27 Patients with cancer are more frequently neutropaenic, may be immunocompromised because of chemotherapy or the malignancy itself, and frequently have indwelling catheters that may serve as a source of infection.28 Specifically, cancer may predispose to IE by facilitating not only bacterial translocation into the bloodstream but also localisation to the endocardium through existence of non-bacterial thrombotic vegetations that may be preexisting due to the hypercoagulable state associated with cancer.27 29 Among patients with solid malignancies, patients with intestinal tumours have been found to be most likely to develop IE with Streptococcus gallolyticus (formerly known as S. bovis).15 This may be due to the tumour facilitating intestinal bacterial translocation to the bloodstream. In haematological malignancies, lymphoma has been found to be the most prevalent cancer associated with IE.15 Particularly, S. aureus IE has been associated with cutaneous portals of entry.30 This, combined with the frequency of indwelling catheters, may be the reason for patients with cancer with IE most frequently grow S. aureus.
Thirty per cent of the patients in our study had blood culture–negative endocarditis. Our group previously reported a 42% frequency of culture-negative endocarditis in a retrospective series of patients with cancer.6 This culture-negative rate may be due to a variety of factors including early administration of antibiotics,3 non-infectious aetiologies of the vegetations, and lack of use of PCR techniques to identify organisms.31 32 This yield may be increased by using reverse transcription-PCR in addition to culture, valvular biopsies, serological analysis and evaluation for non-infectious causes, including autoimmune and thrombotic.31 32 Some of these patients may have had non-bacterial thrombotic endocarditis, which can be found in patients ith cancer; previous studies have shown that this form of endocarditis can be associated with an embolic phenomenon in solid-tumour cancers such as lung, pancreatic and gastric cancer.33 34 Although culture negative patients in our study were classified as definitive IE by the modified Duke’s Criteria, its diagnostic specificity is estimated to only be 74% based on the results of a recent cohort study.35 This is further complicated by the fact that no single laboratory, imaging, nor clinical criteria can definitively diagnose marantic endocarditis. Therefore, distinguishing true culture negative IE from marantic endocarditis is challenging and often requires a high degree of clinical suspicion, with definitive distinction often made during autopsy.36 The use of fluoro-18-fluorodeoxyglucose positron emission tomography/CT and radiolabeled WCC scintigraphy has been explored to identify underlying infectious valvular foci, although the diagnostic utility of these imaging techniques appears to be greatest with suspected prosthetic valve and cardiac implantable electronic device (CIED) endocarditis.37
Endocarditis frequently is believed to originate from cutaneous portals of entry into the bloodstream.30 The most common healthcare-associated causes of cutaneous portals are vascular access and implantable cardiac devices and prosthetic valves.3 30 For example, within the first year of valve replacement, infections with coagulase negative staphylococci, S. aureus, HACEK organisms and fungi are much more common, whereas Streptococcus spp and Enterococcus infections occur less frequently.38 This microbiology reflects the nosocomial nature of prosthetic valve endocarditis acquired perioperatively. Of note, however, prosthetic valve endocarditis after the first year of valve replacement tends to be community acquired, as reflected by increased incidence of Streptococcus spp and Enterococcus infections.38 Antibiotic prophylaxis for staphylococci prior to implantation of a CIED is a class 1 recommendation by the AHA and was shown to be beneficial in a randomised clinical trial.39 40 However, antibiotic prophylaxis in minor invasive procedures has been challenged and remains under debate.26 It has been suggested that antibiotic prophylaxis be used in invasive procedures for those with congenital heart defects because of increased risk of infection.23 Perhaps prophylaxis with antibiotics should be considered before procedures in patients with cancer due to the myriad factors discussed above. However, both the Society of Interventional Radiology and American Society of Clinical Oncology (ASCO) recommend against routine antibiotic prophylaxis in the general cancer population in the setting of placement of a totally implantable vascular access device (ie, a chemotherapy port).41 42 Instead, they recommend ensuring a clean procedure is done with the use of central venous catheter clinical care bundles. These guidelines were based on prior surgical literature and retrospective studies demonstrating lack of clinical benefit with routine antibiotic prophylaxis and low baseline rate of device-associated infections. Further supporting evidence came from two randomised controlled studies that demonstrated no difference in the rate of device-associated infections between antibiotic-prophylaxis and placebo groups. However, these studies were limited by their small sample sizes, low incidence of infectious complications, exclusion of liquid tumours in one study, and exclusion of patients with neutropaenia in both.43 44 A meta-analysis of these studies combined with two prior retrospective studies recapitulated the lack of benefit of antibiotic prophylaxis.45–47 Interestingly, a recent clinical trial found that universal MRSA decolonisation in intensive care unit patients resulted in a greater reduction in all-cause bloodstream infections and MRSA clinical isolates compared with targeted decolonisation (MRSA screening followed by decolonisation) and isolation measures.48 However, although targeted MRSA decolonisation has been studied extensively in surgical populations, concerns for cost-effectiveness and the development of multidrug-resistant MRSA remain with widespread adoption of this approach.49
There is conflicting evidence regarding the risk of device-related infectious complications and neutropaenia at the time of device placement, but a 2019 retrospective single-centre cohort study addressed this question and found a significantly greater rate of infection-related port removal within 30 days of device placement in neutropaenic patients.50 However, the study was limited by its retrospective nature, a higher rate of liquid tumours in the neutropaenic cohort and lack of documentation of pre-procedure antibiotic prophylaxis. There are currently no specific recommendations by the Infectious Diseases Society of America or ASCO on the use of antibiotic prophylaxis in the neutropaenic and liquid tumour patient populations. Future studies are needed to help further assess which patients, if any, may benefit from antibiotic prophylaxis prior to invasive procedures.
Conclusions
Among patients with cancer treated at our tertiary care centre, the development of endocarditis portended poor survival, and the presence of SAE or active cancer further increased mortality. This study further contributes to the limited knowledge about endocarditis, particularly SAE, in the cancer population. Given these findings, we emphasise the need for further research in prevention and treatment of this life-altering complication.
Acknowledgments
We thank Sunita Patterson, Research Medical Library, for editing this article.
Footnotes
Correction notice: Since the publication of this article, initials have been added to the following author names: Saamir A Hassan and Syed W Yusuf.
Contributors: CG and SAH contributed to the planning, conduct, writing and reporting of the report. SWY, JS, GMV, OU, JB, CTJ and SAH contributed to the planning, conduct and reporting of the work. SAH will serve as the guarantor of the work.
Funding: Supported in part by the National Institutes of Health/National Cancer Institute through award number P30CA016672 (used the Clinical Trials Office and Biostatistics Resource Group).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the institutional review board, and the requirement for patient consent was waived.
References
- 1.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016;387:882–93. 10.1016/S0140-6736(15)00067-7 [DOI] [PubMed] [Google Scholar]
- 2.García-Albéniz X, Hsu J, Lipsitch M, et al. Infective endocarditis and cancer in the elderly. Eur J Epidemiol 2016;31:41–9. 10.1007/s10654-015-0111-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International collaboration on Endocarditis-Prospective cohort study. Arch Intern Med 2009;169:463–73. 10.1001/archinternmed.2008.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selton-Suty C, Célard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 2012;54:1230–9. 10.1093/cid/cis199 [DOI] [PubMed] [Google Scholar]
- 5.Finland M, Barnes MW. Changing etiology of bacterial endocarditis in the antibacterial era. experiences at Boston City Hospital 1933-1965. Ann Intern Med 1970;72:341–8. 10.7326/0003-4819-72-3-341 [DOI] [PubMed] [Google Scholar]
- 6.Yusuf SW, Ali SS, Swafford J, et al. Culture-Positive and culture-negative endocarditis in patients with cancer: a retrospective observational study, 1994-2004. Medicine 2006;85:86–94. 10.1097/01.md.0000208503.06288.7b [DOI] [PubMed] [Google Scholar]
- 7.Correa de Sa DD, Tleyjeh IM, Anavekar NS, et al. Epidemiological trends of infective endocarditis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc 2010;85:422–6. 10.4065/mcp.2009.0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler VG, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005;293:3012-21. 10.1001/jama.293.24.3012 [DOI] [PubMed] [Google Scholar]
- 9.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro heart survey on valvular heart disease. Eur Heart J 2003;24:1231–43. 10.1016/S0195-668X(03)00201-X [DOI] [PubMed] [Google Scholar]
- 10.Pittet D, Wenzel RP. Nosocomial bloodstream infections. secular trends in rates, mortality, and contribution to total hospital deaths. Arch Intern Med 1995;155:1177–84. 10.1001/archinte.155.11.1177 [DOI] [PubMed] [Google Scholar]
- 11.Fowler VG, Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005;40:695–703. 10.1086/427806 [DOI] [PubMed] [Google Scholar]
- 12.Janszky I, Gémes K, Ahnve S, et al. Invasive procedures associated with the development of infective endocarditis. J Am Coll Cardiol 2018;71:2744–52. 10.1016/j.jacc.2018.03.532 [DOI] [PubMed] [Google Scholar]
- 13.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39:309–17. 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 14.Kim K, Kim D, Lee S-E, et al. Infective Endocarditis in Cancer Patients - Causative Organisms, Predisposing Procedures, and Prognosis Differ From Infective Endocarditis in Non-Cancer Patients. Circ J 2019;83:452–60. 10.1253/circj.CJ-18-0609 [DOI] [PubMed] [Google Scholar]
- 15.Fernández-Cruz A, Muñoz P, Sandoval C, et al. Infective endocarditis in patients with cancer: a consequence of invasive procedures or a harbinger of neoplasm?: a prospective, multicenter cohort. Medicine 2017;96:e7913. 10.1097/MD.0000000000007913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 17.Safdar A, Chaturvedi V, Koll BS, et al. Prospective, multicenter surveillance study of Candida glabrata: fluconazole and itraconazole susceptibility profiles in bloodstream, invasive, and colonizing strains and differences between isolates from three urban teaching hospitals in New York City (Candida susceptibility trends study, 1998 to 1999). Antimicrob Agents Chemother 2002;46:3268–72. 10.1128/AAC.46.10.3268-3272.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarrand JJ, Guillot C, Wenglar M, et al. Clinical comparison of the resin-containing BACTEC 26 plus and the isolator 10 blood culturing systems. J Clin Microbiol 1991;29:2245–9. 10.1128/jcm.29.10.2245-2249.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke endocarditis service. Am J Med 1994;96:200–9. 10.1016/0002-9343(94)90143-0 [DOI] [PubMed] [Google Scholar]
- 20.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American heart association. Circulation 2015;132:1435-86. 10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 21.Hoerr V, Franz M, Pletz MW, et al. S. aureus endocarditis: clinical aspects and experimental approaches. Int J Med Microbiol 2018;308:640–52. 10.1016/j.ijmm.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 22.Williams JB, Shah AA, Zhang S, et al. Impact of microbiological organism type on surgically managed endocarditis. Ann Thorac Surg 2019;108:1325–9. 10.1016/j.athoracsur.2019.04.025 [DOI] [PubMed] [Google Scholar]
- 23.Cahill TJ, Jewell PD, Denne L, et al. Contemporary epidemiology of infective endocarditis in patients with congenital heart disease: a UK prospective study. Am Heart J 2019;215:70–7. 10.1016/j.ahj.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 24.Mesa Del Castillo-Payá C, Rodríguez-Esteban M, Quijada-Fumero A, et al. Infective endocarditis in patients with oncological diseases. Enferm Infecc Microbiol Clin 2018;36:72–7. 10.1016/j.eimce.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya M, Wozniak DJ, Stoodley P, et al. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti Infect Ther 2015;13:1499–516. 10.1586/14787210.2015.1100533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saeed K, Bal AM, Gould IM, et al. An update on Staphylococcus aureus infective endocarditis from the International Society of antimicrobial chemotherapy (ISAC). Int J Antimicrob Agents 2019;53:9–15. 10.1016/j.ijantimicag.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 27.Sun L-M, Wu J-N, Lin C-L, et al. Infective endocarditis and cancer risk: a population-based cohort study. Medicine 2016;95:e3198. 10.1097/MD.0000000000003198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nørgaard M, Larsson H, Pedersen G, et al. Risk of bacteraemia and mortality in patients with haematological malignancies. Clin Microbiol Infect 2006;12:217–23. 10.1111/j.1469-0691.2005.01298.x [DOI] [PubMed] [Google Scholar]
- 29.Decousus H, Moulin N, Quenet S, et al. Thrombophilia and risk of venous thrombosis in patients with cancer. Thromb Res 2007;120 Suppl 2:S51–61. 10.1016/S0049-3848(07)70130-5 [DOI] [PubMed] [Google Scholar]
- 30.Delahaye F, M'Hammedi A, Guerpillon B, et al. Systematic search for present and potential portals of entry for infective endocarditis. J Am Coll Cardiol 2016;67:151–8. 10.1016/j.jacc.2015.10.065 [DOI] [PubMed] [Google Scholar]
- 31.Fournier P-E, Gouriet F, Casalta J-P, et al. Blood culture-negative endocarditis: improving the diagnostic yield using new diagnostic tools. Medicine 2017;96:e8392. 10.1097/MD.0000000000008392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fournier P-E, Thuny F, Richet H, et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis 2010;51:131–40. 10.1086/653675 [DOI] [PubMed] [Google Scholar]
- 33.Asopa S, Patel A, Khan OA, et al. Non-Bacterial thrombotic endocarditis. Eur J Cardiothorac Surg 2007;32:696–701. 10.1016/j.ejcts.2007.07.029 [DOI] [PubMed] [Google Scholar]
- 34.el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 2007;12:518–23. 10.1634/theoncologist.12-5-518 [DOI] [PubMed] [Google Scholar]
- 35.Shrestha N, Shakya S, Hussain S, et al. Sensitivity and specificity of Duke criteria for diagnosis of definite infective endocarditis: a cohort study. Open Forum Infect Dis 2017;4:S550–1. 10.1093/ofid/ofx163.1431 [DOI] [Google Scholar]
- 36.Bussani R, DE-Giorgio F, Pesel G, et al. Overview and comparison of infectious endocarditis and non-infectious endocarditis: a review of 814 autoptic cases. In Vivo 2019;33:1565–72. 10.21873/invivo.11638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galea N, Bandera F, Lauri C, et al. Multimodality imaging in the diagnostic work-up of endocarditis and cardiac implantable electronic device (CIED) infection. J Clin Med 2020;9:2237. 10.3390/jcm9072237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piper C, Körfer R, Horstkotte D. Prosthetic valve endocarditis. Heart 2001;85:590–3. 10.1136/heart.85.5.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American heart association. Circulation 2010;121:458–77. 10.1161/CIRCULATIONAHA.109.192665 [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira JC, Martinelli M, Nishioka Silvana Angelina D'Orio, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009;2:29–34. 10.1161/CIRCEP.108.795906 [DOI] [PubMed] [Google Scholar]
- 41.O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 2011;39:S1–34. 10.1016/j.ajic.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 42.Schiffer CA, Mangu PB, Wade JC, et al. Central venous catheter care for the patient with cancer: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2013;31:1357–70. 10.1200/JCO.2012.45.5733 [DOI] [PubMed] [Google Scholar]
- 43.Di Carlo I, Toro A, Pulvirenti E, et al. Could antibiotic prophylaxis be not necessary to implant totally implantable venous access devices? randomized prospective study. Surg Oncol 2011;20:20–5. 10.1016/j.suronc.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 44.Karanlik H, Kurul S, Saip P, et al. The role of antibiotic prophylaxis in totally implantable venous access device placement: results of a single-center prospective randomized trial. Am J Surg 2011;202:10–15. 10.1016/j.amjsurg.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 45.Covey AM, Toro-Pape FW, Thornton RH, et al. Totally implantable venous access device placement by interventional radiologists: are prophylactic antibiotics necessary? J Vasc Interv Radiol 2012;23:358–62. 10.1016/j.jvir.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scaife CL, Gross ME, Mone MC, et al. Antibiotic prophylaxis in the placement of totally implanted central venous access ports. Am J Surg 2010;200:719–23. 10.1016/j.amjsurg.2010.07.023 [DOI] [PubMed] [Google Scholar]
- 47.Johnson E, Babb J, Sridhar D. Routine antibiotic prophylaxis for totally implantable venous access device placement: meta-analysis of 2,154 patients. J Vasc Interv Radiol 2016;27:339–43. 10.1016/j.jvir.2015.11.051 [DOI] [PubMed] [Google Scholar]
- 48.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65. 10.1056/NEJMoa1207290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Septimus EJ, Schweizer ML. Decolonization in prevention of health care-associated infections. Clin Microbiol Rev 2016;29:201–22. 10.1128/CMR.00049-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez AW, Watchmaker JM, Brown DB, et al. Association between periprocedural neutropenia and early infection-related chest Port removal. Radiology 2019;291:513–8. 10.1148/radiol.2019182175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information