Abstract
Objective
To evaluate the impact of drug diversity on treatment effectiveness in relapsing-remitting multiple sclerosis (RRMS) in Germany.
Design
This study employs real-world data captured in-time during clinical visits in 67 German neurology outpatient offices of the NeuroTransData (NTD) multiple sclerosis (MS) registry between 1 January 2010 and 30 June 2019, including 237 976 visits of 17 553 patients with RRMS. Adherence and clinical effectiveness parameters were analysed by descriptive statistics, time-to-event analysis overall and by disease-modifying therapies (DMTs) stratified by administration modes (injectable, oral and infusion). Three time periods were compared: 2010–2012, 2013–2015 and 2016–2018.
Results
Between 2010 and 2018, an increasing proportion of patients with RRMS were treated with DMTs and treatment was initiated sooner after diagnosis of MS. Introduction of oral DMT temporarily induced higher readiness to switch. Comparing the three index periods, there was a continuous decrease of annualised relapse rates, less frequent Expanded Disability Status Scale (EDSS) progression and increasing periods without relapse, EDSS worsening and with stability of no-evidence-of-disease-activity 2 and 3 criteria, lower conversion rates to secondary progressive MS on oral and on injectable DMTs.
Conclusion
Sparked by the availability of new mainly oral DMTs, RRMS treatment effectiveness improved clinically meaningful between 2010 and 2018. As similar effects were seen for injectable and oral DMTs more than for infusions, a better personalised treatment allocation in many patients is likely. These results indicate that there is an overall beneficial effect for the whole patient with MS population as a result of the greater selection of available DMTs, a benefit beyond the head-to-head comparative efficacy, resulting from an increased probability and readiness to individualise MS therapy.
Keywords: therapeutics, adult neurology, neurology, multiple sclerosis
Strengths and limitations of this study.
The descriptive real-world data study in patients with relapsing remitting multiple sclerosis (RRMS) evaluates overall effects of the increasing number of disease modifying therapies (DMTs) in multiple sclerosis (MS) on quality of clinical care between 2010 and 2018 in Germany.
Pseudonymised data of the German NeuroTransData registry are employed including the MS core data set as recommended by the European medical agency (EMA/548474/2017).
Sufficient patient numbers, high frequency of visits, standardised web-based data capturing by trained staff, consistency of formats and definitions of the data over the study period and multiple data quality assurance steps mitigate the risks of errors and biases.
Limitations to the study are the inclusion of only German RRMS outpatients, application of German DMT labels and regulatory specifications, varying follow-up times, immanent uncertainties of the exact time when RRMS switches into secondary progressive MS and the risk of residual confounding of the results by unknown confounders.
Introduction
The field of multiple sclerosis (MS) treatment has seen dynamic developments over the last three decades. (1) Since the introduction of the first interferon-β1a (IF-b1a) in 1994, treatment options for patients with relapsing remitting MS (RRMS) have expanded to 14 different disease modifying therapies (DMTs) registered in Europe. (2) Regulatory authorities have defined new MS subgroups such as high-disease activity (HDA) course of RRMS, relapsing MS, RRMS and relapsing forms of secondary progressive MS (SPMS), for the definition of drug labels. (3) Regulatory authorities have also developed legislative and administrative initiatives such as the ‘AMNOG (Arzneimittelmarktneuordnungsgesetz, ie, the price finding legislation for drug) procedure’ to control costs of drugs, in the face of drug costs in Germany raising from €30.2 billion to €43.9 billion from 2010 to 2018.1 (4) Patients, physicians and payers expect allocation of the most effective DMT for the individual patient while minimising adverse events. While reduction of relapse activity was the treatment goal in the 1990s, current treatment goals strive for ‘no evidence of disease activity’.
However, little is known about the impact of these developments on real-world treatment pattern and effectiveness on disease activity in RRMS. This analysis investigates treatment pattern and effectiveness over time by comparing three time periods between 2010 and 2018 (defined by availability of new DMTs entering the German market) of real-world data (RWD) from the physician’s network NeuroTransData (NTD) in Germany.
Methods
Database: the NTD MS registry
This project employed real-world clinical data captured by the NTD MS registry. NTD is a Germany-wide physicians’ network founded in 2008 and run by physicians in the fields of neurology and psychiatry (www.neurotransdata.com). Governance principles are defined. NTD generates revenue by its members’ participation in phase II–IV clinical trials, investigator initiated trials, and RWD analytic projects in cooperation with pharmaceutical industry, payers and other players in the German and international health systems.
Currently, 132 specialists work in 67 NTD practices throughout Germany, serving approximately 600 000 outpatients per year. Each practice is certified according to network-specific and International Organization for Standardization (ISO) 9001 criteria. An external certified organisation audits compliance annually. The NTD MS registry includes approximately 25 000 patients with MS, representing about 15% of all patients with MS in Germany. NTD captures demographic, clinical history, patient-related outcomes and clinical variables in real time during clinical visits. Standardised clinical assessments of functional system scores and Expanded Disability Status Scale (EDSS) calculation are performed by certified raters (http://www.neurostatus.net). Data are entered into the web-based registry either manually or directly from digital sources. Data quality is monitored by the NTD data management team, checking for inconsistencies and errors using an error analysis programme. Both automatic and manually executed queries are implemented to further ensure data quality, for example, checks for inconsistencies and requests for missing information. High data completeness is achieved by definition of minimum data sets, mandatory data entry fields, positive missing data confirmation. Advanced dynamic web-based data capturing, regular training of doctors and nurses, interactive chat forum for nurses and doctors, automated and manual feedback query system, daily-automated analysis of data plausibility and correctness, and annual on-site audit of procedures and source data by an external process quality certifier organisation contribute to high data consistency. The NTD data capturing platform is also used as patient management system in the daily care of patients in NTD offices, thus guaranteeing timeliness of data.
All data are pseudonymised and pooled. The Institute for Medical Information Processing, Biometry and Epidemiology (Institut für medizinische Informationsverarbeitung, Biometrie und Epidemiologie) at the Ludwig Maximilian University in Munich, Germany, manages codes and acts as an external trust centre. Pooled data are stored on NTD servers and other NTD-controlled storage technology. Written informed consent is obtained from each patient contributing data for the registry. This data acquisition and management protocol was approved by the ethical committee of the Bavarian Medical Board (Bayerische Landesärztekammer, 14 June 2012, ID 11144) and reapproved by the ethical committee of the Medical Board North-Rhine (Ärztekammer Nordrhein, 25 April 2017, ID 2017071). Compliance with European and German legislation (Bundesdatenschutzgesetz (BDSG), Europäische Datenschutz-Grundverordnung (EU-DSGVO)) is warranted including patient rights and informed consent requirements. Patient participation, informed consent procedures, data capturing, management and analytics fulfil the ‘Guidelines for Good Pharmacoepidemiology Practices (GPP) of the International Society for Pharmacoepidemiology’,2 the Strengthening the Reporting of Observational Studies in Epidemiology guidelines,3 the European Medicines Agency requirements for the ‘Use of patient disease registries for regulatory purposes—methodological and operational considerations4’ and the ethical principles laid down in the Declaration of Helsinki.5
Data for this project were captured between 1 January 2010 and 30 June 2019.
Data quality of the NTD MS registry
The main components for data quality of medical RWD registries proposed by European Medicine Agency4 are fulfilled by the NTD MS registry. The NTD also realises the quality criteria of the EunetHTA REQueST (Registry Evaluation and Quality Standards Tool)6 with 14 of 14 points in section ‘Methodological Information’, 23 of 24 points in section ‘Essential Standards’ and 5 of 6 points in section ‘Additional Requirements’.
Patient population
All patients with diagnosis of relapsing-remitting or SPMS documented in the NTD MS registry between 1 January 2010 and 30 December 2018 with at least one clinical visit were included. In patients with RRMS, the McDonald criteria as defined at the time of diagnosis of MS had to be fulfilled and documented in the registry. Seventeen thousand five hundred fifty-three patients with RRMS were included.
From this population 12 181 patients with RRMS were identified in whom a DMT was initiated between 2010 and 2018. This group was stratified in three populations according to their time of initiation of DMT (see next section).
As there is no accepted and validated diagnostic procedure to confirm SPMS, the generally applied diagnostic criteria for SPMS were applied by the treating neurologists to establish this diagnosis. Time of switch from RRMS to SPMS is defined as the first clinical visit, when in the treating neurologists’ judgement the criteria for manifest SPMS were fulfilled.
Data analysis
Analysis was performed in three time periods, reflecting different spectra of DMTs available during the respective period.
2010–2012 (index period 10–12)
Era of early treatment initiation at the stage of clinically isolated syndrome with IFs and glatiramer acetate and escalation with natalizumab approved since 2006 and fingolimod approved since 2011 for HDA patients. HDA is defined by the European Medicines Agency drug label as active disease despite treatment with at least one DMT or disease activity with two or more disabling relapses in 1 year without therapy, and with one or more Gadolinium enhancing lesions on brain MRI or a significant increase in T2 lesion load as compared with a previous recent MRI.
2013–2015 (index period 13–15)
Era of therapy diversification with introduction of alemtuzumab as an infusion for HDA patients, teriflunomide and dimethyl fumarate as oral drugs for all stages of RRMS.
2016–2018 (index period 16–18)
Era of consolidated DMT spectrum. Cladribine, an oral HDA activity drug, was newly approved in August 2017. Daclizumab, which became available in July 2016, was restricted in July 2017 and withdrawn in March 2018, was not considered as numbers of patients were very small and a temporary distortion of results in the injectable group had to be excluded.
Parameters characterising treatment acceptance and adherence were analysed for each index period.
Impact on treatment effectiveness was analysed between 2010 and 2018 and for each index period for the strata ‘all DMT’, ‘injectables’ including IFs-ß-1a, IFs-ß-1b, glatiramer acetate, ‘orals’ including fingolimod, teriflunomide, dimethyl fumarate, cladribine, ‘infusions’ including natalizumab, alemtuzumab, based on the European labels of these DMTs.
Treatment effectiveness was analysed for patients with RRMS on DMT by annualised relapse rate (ARR), time-to-first-relapse on DMT, percentage of patients with 6 months confirmed disability-progression (6mCDP, CDP defined as at least 1.0-point EDSS score increases for patients with baseline EDSS score 0‒5.5 EDSS and at least 0.5-point EDSS score increases for patients with baseline EDSS score greater than 5.5), time-to-6mCDP on DMT, time-from-first symptom to EDSS≥3–5 and >5 (in month), time-to-no-evidence-of-disease-activity (NEDA) 2 and 3 failure on DMT being started in the index periods. NEDA 2 is defined as no clinical evidence of relapse activity or disability progression. For NEDA 3 status no evidence of MRI activity, either new lesions or Gadolinium enhancing lesions, is required in addition to NEDA 2 criteria. Risk rates for discontinuation were calculated as ratio of number of patients with discontinuation of DMT divided by all patients.
Patient and public involvement
There was no patient involvement.
Role of funding source
This study was conducted by NTD without additional funding or guidance by external sponsors.
Results
Data quality
Exemplary frequencies of data captured constantly over time for several data items (see table 1) underline the high data quality and consistency over time. The mean duration of follow-up was 5.07 years (SD 4.46). A total of 59 928 DMT treatment cycles were documented between 2010 and 2018.
Table 1.
Numbers of patients with relapsing remitting multiple sclerosis (RRMS), visits per year and therapy cycles with disease-modifying treatments (DMTs) captured in the NeuroTransData multiple sclerosis registry between 2010 and 2018
| Index year | Number patients with RRMS | Visits documented per year | DMT cycles per year | Relapses per year | MRI per year |
| 2010 | 5170 | 16 377 | 4168 | 1821 | 3096 |
| 2011 | 6648 | 24 296 | 5441 | 2638 | 4004 |
| 2012 | 7017 | 23 298 | 5893 | 2600 | 3107 |
| 2013 | 7532 | 25 840 | 6410 | 2433 | 3866 |
| 2014 | 7591 | 28 261 | 7536 | 2076 | 3989 |
| 2015 | 8074 | 28 313 | 7443 | 1972 | 3879 |
| 2016 | 8401 | 29 715 | 7566 | 1795 | 3781 |
| 2017 | 9021 | 31 199 | 7862 | 1707 | 3575 |
| 2018 | 8946 | 30 677 | 7609 | 1487 | 4102 |
| 2010–2018 | 237 976 | 59 928 | 18 529 | 33 399 | |
| Mean/patient/year | 3.48 | 0.88 | 0.27 | 0.49 |
Patient population
A total of 17 553 patients with RRMS were identified between 2010 and 2018 (73.6% women, 26.4% men). Mean age at diagnosis of RRMS was 34 years (SD 10.66), mean ARR between 2010 and 2018 was 0.27 (SD 0.6). From this group, in 12.181 patients a DMT was initiated in this period. Table 2 shows consistency and completeness of data of this patient group stratified into the three time periods between 2010 and 2018.
Table 2.
Means and percentages of relapsing remitting multiple sclerosis patient characteristics of the NeuroTransData multiple sclerosis (MS) registry in time periods between 2010 and 2018 at initiation of disease-modifying therapy (DMT) (=index event)
| Characteristic | 10–12 (N=3942) |
13–15 (N=5101) |
16–18 (N=3138) |
All patients (N=12 181) |
| Female, % | 73.17 | 73.74 | 71.86 | 73.07 |
| Age, years (SD) | 44.95 (10.21) | 43.93 (10.88) | 40.7 (11.03) | 43.59 (10.94) |
| EDSS (SD) | 2.12 (1.59) | 2.10 (1.62) | 1.89 (1.53) | 2.05 (1.59) |
| Relapses (SD) before index event | 1.93 (2.55) | 2.26 (2.63) | 2.21 (2.67) | 2.14 (2.62) |
| Months MS duration (SD) | 87.78 (85.87) | 101.78 (93.62) | 98.73 (94.99) | 96.46 (91.75) |
| DMTs before index event (SD) | 0.8 (1.01) | 1.08 (1.14) | 1.2 (1.26) | 1.02 (1.14) |
| MRI around index event, % | 39.93 | 43.5 | 37.38 | 40.77 |
| MRI with progression around index event, % | 20.83 | 20.78 | 18.36 | 20.17 |
EDSS, Expanded Disability Status Scale.
Treatment acceptance
Overall proportions of patients with RRMS actively treated with DMT increased steadily: 10–12, 70,7%; 13–15, 78.1%; 16–18, 80.1%. Proportions of DMT types by application changed during the three time periods 10–12/13–15/16–18 with percentages of patients on injectables 88/69/46, orals 13/44/54, infusions 12/10/10. Total percentages per period exceed 100% as some patients received more than one DMT per period (see section ‘Persistence on DMT’). Proportions of patients with RRMS receiving so-called HDA DMTs increased continuously: 10–12, 23%; 13–15, 27%; 16–18, 31%.
Initiation of DMT after diagnosis of RRMS
More patients started on a DMT within 6 months after diagnosis of RRMS (10–12, 62%; 13–15, 72%; 16–17, 66%), with shorter periods between first symptom and initiation of first DMT (10–12, 178±295 days; 13–15, 121±174 days; 16–18, 115±112 days). Orals were increasingly preferred as first DMT as they became available during the 3 periods of time 10–12/13–15/16–18 with percentages of patients on injectables 74/44/40, orals 19/52/55, infusions 7/4/5.
Persistence on DMT
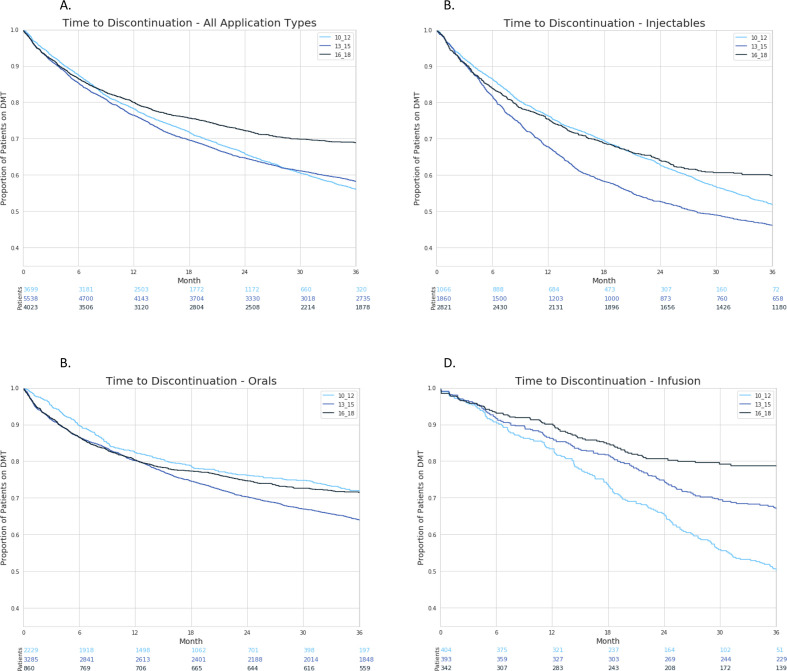
Availability of oral DMT increased the proportion of switches between DMTs from 16% of patients on treatment in 10–12% to 24% in 13–15, while in 16–18, 14% of patients on DMT switched. In parallel, time to discontinuation remained stable within these 3 years periods: in 10–12 mean time to discontinuation 8.49 months (SD 7.14); in 13–15, 8.10 months (SD 6.92); and in 16–18, 8.49 months (SD 7.71). There was a trend for patients staying longer on overall treatment for the most recent time period. This trend was driven by longer persistence of patients on infusion therapies in the most recent time period (figure 1).
Figure 1.
Time to discontinuation of disease-modifying treatments (DMTs) in patients with relapsing remitting multiple sclerosis for time periods 2010–2012, 2013–2015, 2016–2018; all DMT (A) and by injectables (B), orals (C), infusions (D).
Non-medical reasons for discontinuation, such as patients’ perceptions and wishes, decreased over time from 71% to 51%. Lack of effectiveness is increased as a motivation for switching DMTs, as well as adverse events or pregnancy/family planning (table 3).
Table 3.
Reasons overall and risk rates by application type of disease-modifying treatment for discontinuation for three time periods 2010–2012, 2013–2015 and 2016–2018
| Reasons for discontinuation, % | 10–12 | 13–15 | 16–18 |
| Antibodies/JCV-virus titre | 1.28 | 1.85 | 1.51 |
| family planning | 4.10 | 5.48 | 6.34 |
| Adverse events | 5.13 | 15.89 | 13.12 |
| Lack of effectiveness | 18.21 | 19.31 | 27.90 |
| Freedom of disease activity | NA | 0.34 | 0.36 |
| Non-medical reasons | 71.28 | 57.11 | 50.76 |
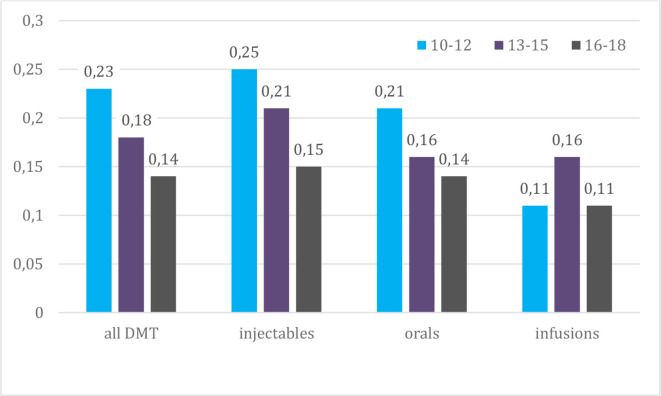
| Risk rates for discontinuation | |||
| Injectables | 0.59 | 0.54 | 0.33 |
| Orals | 0.39 | 0.36 | 0.21 |
| Infusions | 0.59 | 0.37 | 0.17 |
Non-medical reasons summarise patients’ perceptions and wishes.
JCV, John Cunningham Virus; NA, not applicable as criteria was not captured.
Risk rates for discontinuation decreased continuously for all types of DMT over the three time periods, reaching a decrease of 44% for injectables, 46% for orals and 71% for infusions between 2010–2012 and 2016–2018.
DMT switching pattern
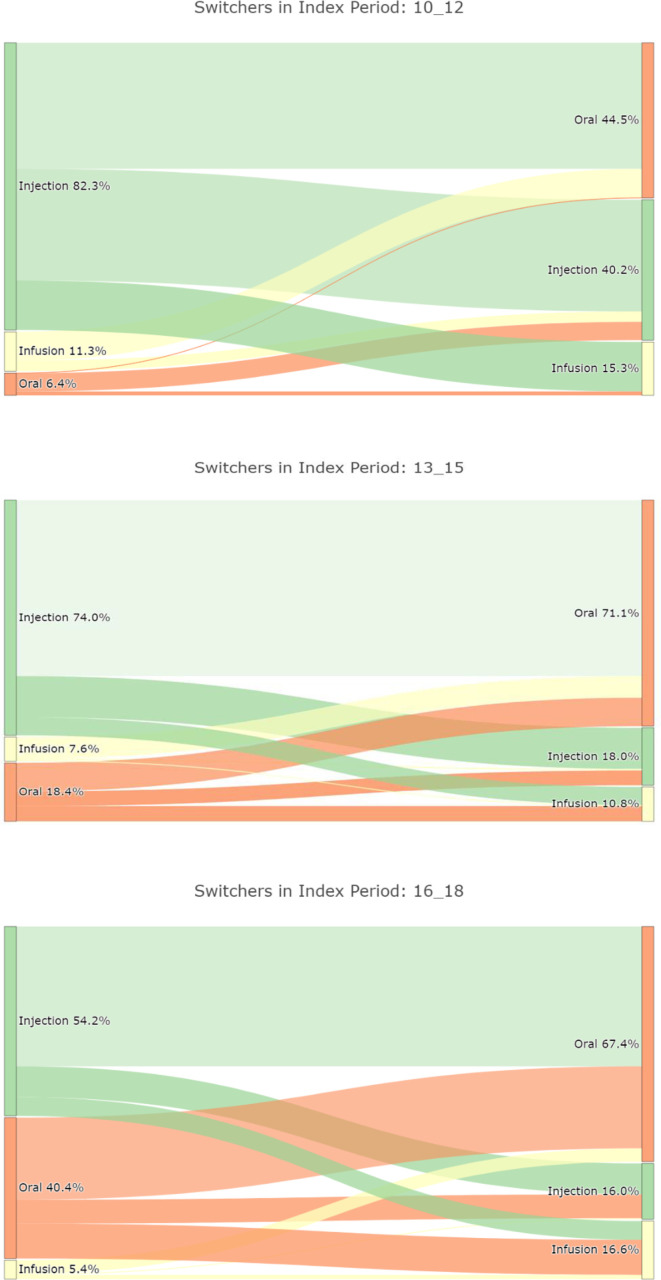
Patients increasingly switched from injectables to oral or infusion DMTs, while switches to injectables decreased. Follow-on DMTs after oral DMTs were predominantly oral DMT. If infusion therapy was discontinued, almost all patients continued with oral DMTs (see figure 2).
Figure 2.
Percentage of switches between injectable, oral and infusion disease-modifying therapies in relapsing remitting multiple sclerosis for time periods (A) 2010–2012, (B) 2013–2015, (C) 2016–2018.
Treatment effectiveness
Relapse activity
ARR decreased by a mean 39% overall, 40% for injectables and 30% for orals. Infusion therapies did not decrease (figure 3).
Figure 3.
Annualised relapse rate in three time periods 2010–2012, 2013–2015, 2016–2018 on disease-modifying therapy (DMT) overall and by application type.
Proportions of patients documented with 6mCDP, with progression of EDSS <3 to ≥3–5 as well as from EDSS <5 to ≥5 decreased by 39% and 23%, respectively, between the first and the last time period analysed. In parallel, times from first symptom of RRMS to reach the defined EDSS ranges increased by 22% and 15%, respectively (see table 4).
Table 4.
Six months confirmed disability progression (6 months confirmed disability-progression (6mCDP)
| EDSS <3 to ≥3–<5 | EDSS <5 to ≥5 | Months to 6mCDP EDSS ≥3–5 |
Months to 6mCDP EDSS ≥5 |
|||
| % Patients | % Patients | Mean | SD | Mean | SD | |
| 2010–2012 | 1.02 | 0.26 | 122.30 | 81.03 | 181.59 | 92.17 |
| 2013–2015 | 0.76 | 0.31 | 130.95 | 85.60 | 181.37 | 110.04 |
| 2016–2018 | 0.62 | 0.20 | 149.26 | 93.32 | 209.73 | 97.70 |
| Difference from 2010–2012 to 2016–2018, % | −39 | −23 | +22 | +15 | ||
EDSS increase of ≥1.0 for patients from previous EDSS): proportion of patients reaching EDSS≥3–5, reaching EDSS≥5 and months from first symptom of relapsing-remitting multiple sclerosis to 6mCDP in these strata.
EDSS, Expanded Disability Status Scale.
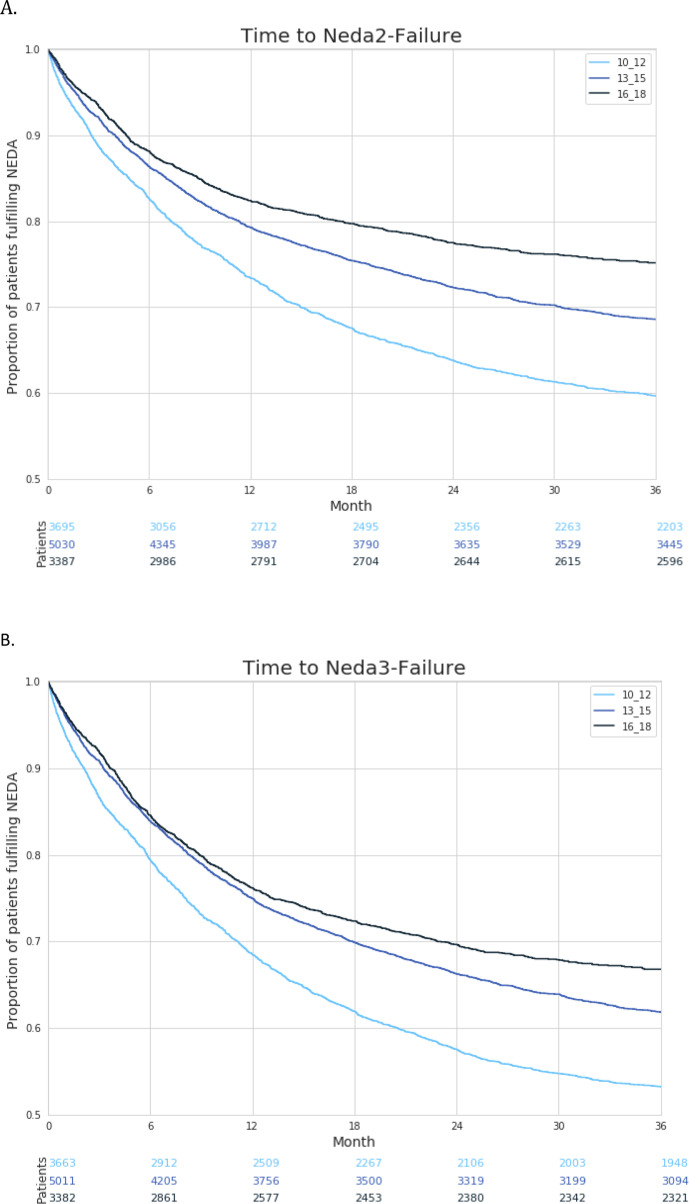
Maintenance of NEDA 2 and 3 criteria
There was a clear trend that patients who initated DMTs for a minimum of 3 months in these index periods remained more frequently and longer free of disease activity according to NEDA 2 (no relapse, no 6mCDP) and NEDA 3 (no relapse, no 6MCDP, no MRI progression) criteria over the three periods of time (figure 4).
Figure 4.
(A) Time to failure of no-evidence-of disease-activity (NEDA) 2 (no relapse, no confirmed disability progression) and (B) NEDA 3 (no relapse, no 6 months confirmed disability progression, no MRI worsening) criteria in patients with relapsing-remitting multiple sclerosis on disease-modifying treatments after a minimum treatment period of 3 months with treatment initiation within three time periods 2010–2012, 2013–2015, 2016–2018.
Mean times to NEDA 2 and 3 failure, censored for the three time periods, increased continuously. NEDA 2: 10–12, 6.92 months (SD 6.66); 13–15, 7.10 months (SD 6.55); 16–18, 7.43 months (SD 7.11). NEDA 3: 10–12, 6.70 months (SD 6.41); 13–15, 7.16 months (SD 6,42); 16–18, 7.49 months (SD 6.89).
Progression to SPMS
Between 2010 and 2018, overall 2.34% of 17 553 patients switched from RRMS to SPMS during a mean follow-up time of 5.31 years. The mean time from first symptom of MS to SPMS was 214 months (SD 113.77), almost 18 years. Time-to-SPMS progression analysis did not reveal time differences between the three index time periods (not shown here). There was a continuous trend towards lower numbers of patients switching to SPMS while on DMT for at least 12 months from 4.25% in 10–12, to 1.97% in 13–15, and to 1.46% in 16–18.
Discussion
The increasing number of new oral and intravenous DMTs was associated with continuously greater proportions of patients with RRMS being treated between 2010 und 2018 and with earlier initiation of first therapy after diagnosis of MS had been established. Orals were increasingly preferred as first and as switching therapies, reaching 55% and about 70% of treated patients, respectively. In the years 2013–2015 switching of DMTs increased by 50% including 24% of all treated patients compared with the previous period 2010–2012 as well as later on between 2016 to 2018 showing 14%. Lack of effectiveness was seen as an incremental driving motivation to switch, as well as adverse events and pregnancy or family planning. This raised readiness to adapt DMTs to the clinical course achieved a sustained drop of ARRs, frequency of EDSS progression and 6mCDP leading to more frequent and longer periods free of disease activity as defined by NEDA 2 and 3 criteria. Although the proportions of patients, who progressed to SPMS on therapy continuously declined as new DMTs become available, the time-to-SPMS progression of the affected patients remained unchanged at about 214 months.
Medical guidelines, regulatory processes and public discussion in Germany and other countries regarding clinical benefits, treatment strategies and drug pricing are often focused on results from the randomised controlled trials (RCTs) with an active comparator and vs placebo that led to registration of the drug.7 However, clinical usage in a broad natural spectrum of patients and the increasing complexity of treatment options are causing a knowledge gap that RCTs are unable to fill. Thus, qualified RWD are increasingly employed to evaluate optimisation of therapeutic strategies.8–11 The attempt to translate DMT efficacy studies into evidence-based clinical practice by meta-analysis of 123 unique RRMS studies provided very limited results. One main limitation was the paucity of efficacy data beyond 3 years of treatment.12 Other initiatives addressed methodological aspects of this efficacy–effectiveness gap between results of RCTs in selected patient groups and effectiveness in real-world usage.13 This is the first study to address population effects of a series of newly introduced DMTs in RRMS on adherence and clinical effectiveness.
Transparent data quality is the key stone of any scientific project. The physician-owned NTD MS registry can demonstrate constant data density, including a mean of 3.5 patient visits documented over the last 9 years, based on a defined minimal dataset and high data quality. This was achieved by utilisation of web-based in-time data capturing and continuous development of automated and manual quality assurance measures for capturing data from 8000 to 9000 RRMS outpatients per year in Germany.
Definition criteria of the three time periods chosen for this study are thought to reflect periods characterised by different sets of DMTs being available for the treatment of patients with RRMS. Between 2010 and 2018 the broader spectrum, in particular of oral DMTs motivated more patients to initiate DMT treatment and also to start earlier after diagnosis of RRMS. Availability of oral DMTs temporarily increased switches between DMTs in the years after their introduction from 16% in 2010–2012 to 24% of patients on DMT in 2013–2015, with a decline back to 14% of patients switching between 2016 and 2018. This is also reflected in the time-to-discontinuation analysis, showing more frequent and quicker discontinuation of injectables in 2013–2015. Lack of effectiveness and adverse events seem to have gained in importance over time as reasons for discontinuation of DMT, mirroring increasing expectations of doctors and patients regarding benefit/risk of DMTs. Persistence on classes of DMTs after 3 years improved most noticeable for infusions moving from 50% in 2010–2012 to almost 80% in 2015–2018, injectables increasing from less than 10%–60% and orals achieving stable persistence of about 72%. Risk rates for discontinuation decreased overall and for each application type. This suggests that over time the individual selection of efficient and well tolerated DMTs succeeds more often in all application modes of DMTs if a broader selection and better acceptance of substances is available.
Earlier initiation of treatment and more readiness to search for individual optimal therapy by switching between a greater diversity of drugs seems to have impacted treatment effectiveness. ARR decreased overall and for patients on injectables and orals approximately 30%–40% between 2010–2012 and 2016–2018. However, there was no change in ARR over time for infusions. Furthermore, worsening of disability could be controlled better in parallel. The proportion of patients with EDSS reaching total sum scores >3 and >5 decreased by 39% and 23%, respectively, and times from diagnosis to the 6mCDPs increased by 22% and 15%, respectively, when comparing time periods 2010–2012 and 2016–2018.
Comparing treatment cycles initiated in these three time periods, these positive developments are also reflected by continuously increasing proportions of patients maintaining NEDA 2 and NEDA 3 criteria. In addition, proportions of patients on DMT switching from RRMS to SPMS decreased each time period, but mean times to SPMS from diagnosis of RRMS remained unchanged at 17.8 years, corresponding with previous published data with a conversion time to SPMS on active treatment of 16.8 years.14 The potential risk reduction for SPMS conversion on a broad spectrum of DMTs will have to be reevaluated in more detail as longer observation times on the new therapies become available.
The parallel improvements of reduction in ARR and disability progression, longer maintenance of NEDA 2 and 3 status in all types of DMTs, independent of their application modes, indicate that the broader selection of DMTs enable a better individual disease control in RRMS. It can be reasonably assumed that the regulatory introduced definition of HDA labels further supported a more stringent application of the therapeutic options available. As expected, better treatment is associated with longer persistence. The observation that more efficient therapies achieved lower relapse activity in parallel with slower disability progression and longer persistence on DMTs is in line with a previous MSBase registry-based report in smaller groups of patients with RRMS with advanced EDSS scores between 3 and 6,15 as well as more recent data in earlier disease stages.16 Beside the individual patient’s fate, this is of great socioeconomic relevance, as costs and utility in MS are highly correlated with disease severity17 and progression inducing disease activity.18 In contrast, continuing IF-ß and glatiramer acetate therapy 10 years or longer without optimisation of therapy in response to disease activity results in an inevitable, almost linear increase in mean EDSS.19
This study demonstrates a clinically meaningful, population-based benefit resulting from the availability of a broader selection of DMTs over time. The introduction of oral DMTs sparked a dynamic development between 2013 and 2015 with temporarily higher proportions of DMT switches but also more readiness to initiate DMTs earlier after diagnosis of MS. The similar extent of improvement of effectiveness parameter for oral and injectable DMTs demonstrates that this population effect is based on a more effective personalised allocation in individual patients.
This study is descriptive by definition. Limitations to the study are the inclusion of only German RRMS outpatients, application of German DMT labels and regulatory specifications. The role of attrition bias due to varying follow-up times can not be ruled out, but constant mean times to discontinuation seem to reduce the risk. By including all patients with RRMS giving informed consent and as distributions of clinical characteristics are balanced, indication or selection bias appear to be mitigated. Although the established data sets to characterise patients and clinical course in MS were employed the risk of residual confounding of results by unknown confounders remains. As there is no validated, generally accepted definition of SPMS, the diagnosis of SPMS is made by clinical judegment of the treating physician base don best clinical knowledge, but remains per definition retrospective.
In conclusion, these descriptive results seem to indicate that there is an overall beneficial effect for the whole patient population with MS as a result of the greater selection of available DMTs, a benefit beyond the head-to-head comparative efficacy, seemingly driven by an increased probability and readiness to individualise MS therapy by doctors and patients. Nevertheless, the challenge in daily practice is the timely identification of the individually most effective DMT at a given time during the course of MS, particularly in patients with persistent disease activity on their current DMT, especially regarding the immanent risk of developing progressive disability or SPMS. Promising techniques emerge based on biomarker like neurofilament light chain20 or B-cell activity response21 or RWD-based statistical predictive algorithms.22 As treatment decisions are driven currently by European label definitions, national cost control regulations and perceptions of physicians and patients, personalised-data-based decision support is required to further improve individual care.
Supplementary Material
Footnotes
Contributors: SB planned the study, analysis and wrote the manuscript. FR assisted the data analysis, interpretation of results and drafting of the manuscript. HD performed the statistical analysis and aided data interpretation. AB aided in interpreting the results and worked on the manuscript. NTD study group collected the data, performed data cleansing and data extraction from the NTD MS registry. All authors discussed the results and commented on the manuscript.
Funding: This project was funded by NeuroTransData.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Statista , 2020. Available: https://de.statista.com/statistik/daten/studie/158096/umfrage/pharma-gesamtmarkt-umsatzentwicklung-seit-2006/
- 2. Public policy committee, international society of pharmacoepidemiology . Guidelines for good pharmacoepidemiology practice (Gpp). Pharmacoepidemiol Drug Saf 2016;25:2–10. 10.1002/pds.3891 [DOI] [PubMed] [Google Scholar]
- 3. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 4. The cross-committee task force on patient registries. discussion paper: use of patient disease registries for regulatory purposes – methodological and operational considerations, 2018. EMA/763513/. Available: https://www.ema.europa.eu/documents/other/discussion-paper-use-patient-disease-registries-regulatory-purposes-methodological-operational_en.docx
- 5. World Medical Association . WMA declaration of Helsinki – ethical principles for medical research involving human subjects. Available: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ [PubMed]
- 6. European network for health technology assessment . REQueST® tool and its vision paper. public consultation. Available: https://eunethta.eu/request-tool-and-its-vision-paper-are-now-available-for-public-consultation/
- 7. Wieseler B, McGauran N, Kaiser T. New drugs: where did we go wrong and what can we do better? BMJ 2019;366:l4340. 10.1136/bmj.l4340 [DOI] [PubMed] [Google Scholar]
- 8. Paolicelli D, Lucisano G, Manni A, et al. Retrospectively acquired cohort study to evaluate the long-term impact of two different treatment strategies on disability outcomes in patients with relapsing multiple sclerosis (RE.LO.DI.MS): data from the Italian MS register. J Neurol 2019;266:3098–107. 10.1007/s00415-019-09531-6 [DOI] [PubMed] [Google Scholar]
- 9. Boremalm M, Juto A, Axelsson M, et al. Natalizumab, rituximab and fingolimod as escalation therapy in multiple sclerosis. Eur J Neurol 2019;26:1060–7. 10.1111/ene.13936 [DOI] [PubMed] [Google Scholar]
- 10. Braune S, Grimm S, van Hövell P, et al. Comparative effectiveness of delayed-release dimethyl fumarate versus interferon, glatiramer acetate, teriflunomide, or fingolimod: results from the German NeuroTransData registry. J Neurol 2018;265:2980–92. 10.1007/s00415-018-9083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalincik T, Jokubaitis V, Spelman T, et al. Cladribine versus fingolimod, natalizumab and interferon β for multiple sclerosis. Mult Scler 2018;24:1617–26. 10.1177/1352458517728812 [DOI] [PubMed] [Google Scholar]
- 12. Claflin SB, Broadley S, Taylor BV. The effect of disease modifying therapies on disability progression in multiple sclerosis: a systematic overview of meta-analyses. Front Neurol 2018;9:1150. 10.3389/fneur.2018.01150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egger M, Moons KGM, Fletcher C, et al. GetReal: from efficacy in clinical trials to relative effectiveness in the real world. Res Synth Methods 2016;7:278–81. 10.1002/jrsm.1207 [DOI] [PubMed] [Google Scholar]
- 14. University of California, San Francisco MS-EPIC Team, Cree BAC, Gourraud P-A, et al. Long-Term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 2016;80:499–510. 10.1002/ana.24747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lizak N, Lugaresi A, Alroughani R, et al. Highly active immunomodulatory therapy ameliorates accumulation of disability in moderately advanced and advanced multiple sclerosis. J Neurol Neurosurg Psychiatry 2017;88:196–203. 10.1136/jnnp-2016-313976 [DOI] [PubMed] [Google Scholar]
- 16. Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 2003;61:1528–32. 10.1212/01.WNL.0000096175.39831.21 [DOI] [PubMed] [Google Scholar]
- 17. Kobelt G, Thompson A, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler 2017;23:1123–36. 10.1177/1352458517694432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ness N-H, Schriefer D, Haase R, et al. Differentiating societal costs of disability worsening in multiple sclerosis. J Neurol 2020;267:1035–42. 10.1007/s00415-019-09676-4 [DOI] [PubMed] [Google Scholar]
- 19. Palace J, Duddy M, Lawton M, et al. Assessing the long-term effectiveness of interferon-beta and glatiramer acetate in multiple sclerosis: final 10-year results from the UK multiple sclerosis risk-sharing scheme. J Neurol Neurosurg Psychiatry 2019;90:251–60. 10.1136/jnnp-2018-318360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019;92:e1007–15. 10.1212/WNL.0000000000007032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tacke S, Braune S, Rovituso DM. In vivo B-cell activity predicts response to treatment with glatiramer acetate and interferons in patients with relapsing-remitting multiple sclerosis (RRMS). Neurol Neuroimmunol Neuroinflamm 2021;8/3. 10.1212/NXI.0000000000000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stühler E, Braune S, Lionetto F, et al. Framework for personalized prediction of treatment response in relapsing remitting multiple sclerosis. BMC Med Res Methodol 2020;20:24. 10.1186/s12874-020-0906-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.