Abstract
The objective of the study was to compare psychiatric outcomes in adults with and without history of pediatric traumatic brain injury (TBI). Youth ages 6 to 14 years hospitalized for TBI from 1992 to 1994 were assessed at baseline and at 3, 6, 12, and 24 months post-injury. In the current study, psychiatric assessments were repeated at 24 years post-injury with the same cohort, now adults ages 29 to 39 years. A control group of healthy adults also was recruited for one-time cross-sectional assessments. Outcome measures included: 1) presence of a psychiatric disorder since the 24-month assessment not present before the TBI (“novel psychiatric disorder,” NPD), or in the control group, the presence of a psychiatric disorder that developed after the mean age of injury of the TBI group plus 2 years; and 2) Time-to-Event for onset of an NPD during the same time periods. In the TBI group, NPDs were significantly more common, and presence of a current NPD was significantly predicted by presence of a pre-injury lifetime psychiatric disorder and by abnormal day-of-injury computed tomography (CT) scan. Compared with controls, the TBI group also had significantly shorter Time-to-Event for onset of any NPD. These findings demonstrate that long-term psychiatric outcomes in adults previously hospitalized for pediatric TBI are significantly worse when compared with adult controls without history of pediatric TBI, both in terms of prevalence and earlier onset of NPD. Further, in the TBI group, long-term NPD outcome is predicted independently by presence of pre-injury psychiatric disorder and abnormal day-of-injury CT scan.
Keywords: adolescent TBI, child TBI, long-term psychiatric outcome, psychiatric disorder, traumatic brain injury
Introduction
Pediatric traumatic brain injury (TBI) is a major public health issue, with an annual incidence of about 280 per 100,000 globally.1 Among children and adolescents in the United States, TBI is among the leading causes of morbidity and mortality.2,3 For youth, post-TBI complications can include new-onset psychiatric disorders, neuropsychological deficits, poor school performance, and deficits in social competence and adaptive function.4–7 Large birth cohort and population long-term studies of pediatric TBI typically demonstrate adverse outcomes, including within the domains of psychiatric disorders, low educational attainment, disability, premature mortality, dementia, and Parkinson's disease.8,9 Studies examining psychiatric outcomes in youth with TBI have revealed that new-onset psychiatric disorders, which we have termed novel psychiatric disorders (NPDs), following injury are common, with incidence varying depending on severity of injury. Among children and adolescents who have suffered severe TBI, about 50 to 60% develop at least one NPD,10 compared with 10 to 36% of youth with mild-to-moderate TBI, and 5 to 9% of orthopedic injury controls in the 2 years after injury.4,11 In the first 2 years following pediatric TBI, onset of NPD also has been associated with several pre-injury variables, including lifetime psychiatric disorders, family function, adaptive function, family psychiatric history, and socioeconomic status (SES).12–15
Long-term psychiatric outcomes following pediatric TBI are more poorly understood, with the longest prospective studies of pediatric TBI based on follow-up assessments with psychiatric interviews being around 2 years.4,13 Prospective investigations of outcomes in adulthood of pediatric TBI have been completed up to approximately 20 years post-injury, but these studies have examined behavioral, not psychiatric, domains of function in unselected cohorts.16–22 Both behavioral and psychiatric studies are important, in part, because the former generally consist of analyses of self-reported traits as continuous measures, and the latter include clinician-rated clinical impairment associated with categorical diagnoses. Associations have been identified between severity of TBI, neuroimaging findings, and long-term psychosocial outcomes, and among domains of pre- and post-injury psychosocial variables including SES, family and adaptive functioning, social communication, internalizing and externalizing behaviors, and emotion perception.20-22 For example, in adults with history of pediatric TBI, neuroimaging evidence of decreased posterior corpus callosum volume and frontal lobe pathology, lower SES and less intimate family environment have been associated with poorer emotional perception,21 and pre-injury adaptive function was associated with long-term internalizing problems.20 These findings are consistent with patterns of anatomical damage from TBI to the “social brain network.”23-25 Thus far, long-term studies of outcomes following pediatric TBI have been limited by high rates of attrition (34-69%) or by referral bias.26–29
The current study aims to extend our prospective longitudinal study of psychiatric outcomes following pediatric TBI from 2 years to 24 years post-injury by examining the natural history, occurrence, and phenomenology of NPDs, along with biopsychosocial predictors of NPDs, in adults with and without exposure to TBI as youth. This investigation expands the analyses and findings of our first investigation that described the long-term psychiatric outcome only in the TBI cohort.30 The first investigation found lifetime pre-injury psychiatric disorder and increased severity of injury were the two of six domains tested that predicted NPD in the TBI cohort. The present study has used a different analytic approach and has included a control group. Here, based upon a review of the extant pediatric TBI psychiatric literature and findings that the social brain network is vulnerable to TBI, we hypothesized that the TBI group would have a significantly higher prevalence of ongoing NPD at 24 years (“Current NPD”, or NPD-C) compared with the control group. Second, we further hypothesized that the TBI group would have significantly greater occurrence of NPD at any point in the follow-up interval from 2 years to 24 years (“Any NPD,” or NPD-A). Related to the first two hypotheses was the expectation that the mean number of years of NPD exposure (i.e., the number of years each participant would manifest at least one NPD during the interval under investigation) would be significantly greater in the TBI group. Third, we hypothesized that NPD-C would be significantly related to some of the 14 injury and pre-injury predictor variables examined at the baseline assessment (see Fig. 1 for list of predictor variables). Finally, we hypothesized that the TBI group would have a greater hazard of onset of NPD-A (and therefore a shorter time to development of NPD-A) compared with the control group during the follow-up period.
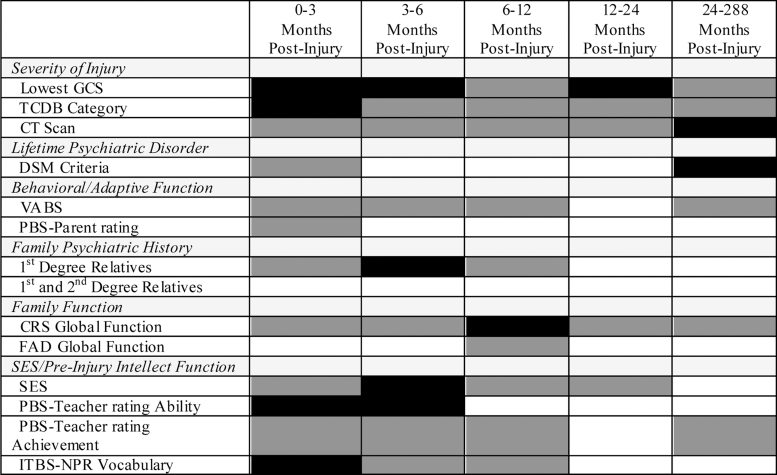
FIG. 1.
Baseline variables tested as predictors of NPD-C in 24 years following pediatric traumatic brain injury. Dark gray shading represents intervals during which each variable was selected for univariable binary logistic regression analysis (p < 0.20). Black shading represents variables assessed at baseline that independently significantly predict NPD-C at the indicated post-injury assessment (p < 0.05). Backward logistic regression was used for the 24-year follow-up data. Forward stepwise logistic regression analyses were used for the 3-, 6-, 12-, and 24-month assessments because of lack of separation with backward logistic regression analyses. CRS, Clinical Rating Scale of the McMaster Interview of Family Function; FAD, family assessment device; GCS, Glasgow Coma Scale; ITBS-NPR, Iowa Test of Basic Skills-National Percentile Rank; NPD-C, novel psychiatric disorder, current; PBS, Pediatric Behavior Scale; SES, socioeconomic status; TCBD, traumatic coma databank; VABS, Vineland Adaptive Behavior Scales.
Methods
Recruitment at baseline
The TBI group consisted of individuals recruited from 1992 to 1994 as children and adolescents ages 6 to 14 years consecutively hospitalized for mild to severe TBI at an academic medical center and three regional hospitals. Additional eligibility criteria included having completed a head computed tomography (CT) scan during initial hospitalization and English as a primary language. Exclusion criteria included penetrating TBI, loss of consciousness greater than 3 months, prior TBI requiring hospitalization, history of child abuse, history of intellectual disability, or history of another neurologic or serious medical illness. The study was approved by the University of Iowa and University of California, San Diego institutional review boards. Written informed consent was obtained from parents, and youth provided written assent when able to demonstrate decision-making capacity to do so.
During the course of the recruitment period, 87 patients met eligibility criteria, and 50 participants enrolled in the study. Participating and non-participating youth did not differ in terms of age, sex, race, SES, pre-injury psychiatric disorder or treatment, but did differ in terms of TBI severity, with participants being more likely to have severe TBI compared with non-participants. Over-representation of severe TBI in study participants was likely related to individuals and families affected by milder injuries feeling that study participation was unnecessary due to the youth having returned to baseline, and also to the main study site being a tertiary care hospital with a large catchment area.13
Assessments at baseline
Youth with pediatric TBI were initially evaluated between 1992 and 1994. Comprehensive neurologic, psychiatric, family and adaptive functioning assessments were conducted (mean = 14 days post-injury; standard deviation [SD] = 13 days) to assess severity of injury and several domains of pre-injury functioning. Fourteen variables, which are described below and also listed in Figure 1, were measured and used to predict NPD following pediatric TBI.
Three of the variables were related to severity of injury, including: 1) lowest post-resuscitation Glasgow Coma Scale (GCS) score; 2) Traumatic Coma Data Bank (TCDB) categorization; and 3) normal or abnormal day-of-injury CT scan. The GCS is a standard measure of acute brain injury severity, and scores range from 3 (unresponsive) to 15 (normal).31 Lowest post-resuscitation score for each participant was obtained from the medical record. A board-certified radiologist classified the initial day-of-injury CT scans as either showing an intracranial traumatic lesion or not. The radiologist additionally classified the CT scans according to the TCDB categorization, which incorporates the degree of brain edema and focal lesions into a single severity rating on a scale from 1 to 6.32 For descriptive purposes, severe injury was defined by a lowest post-resuscitation GCS score <8, moderate injury by a lowest post-resuscitation GCS score of 9 to 12, or a score of 13 to 15 with an intracranial lesion or depressed skull fracture on initial CT scan. Mild injury was defined by a lowest post-resuscitation GCS score of 13 to 15, regardless of associated linear skull fracture.
The “lifetime psychiatric disorder” variable refers to any psychiatric disorder present prior to TBI. Baseline psychiatric assessments were completed using a standardized, semi-structured psychiatric interview, the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Epidemiologic Version (K-SADS-E),33 supplemented by a post-traumatic stress disorder (PTSD) module. Psychiatric diagnoses were based on American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM-III-R) criteria.34 The Neuropsychiatric Rating Schedule (NPRS) was also administered at baseline. The NPRS is a measure designed specifically to identify symptoms and subtypes of personality change following TBI.35 All assessments were conducted by author JEM, a board-certified adult and child and adolescent psychiatrist.
Pre-injury adaptive and behavioral function were assessed using the Vineland Adaptive Behavior Scale (VABS) interview,36 and the parent-completed Pediatric Behavior Scale (PBS), a behavioral rating scale designed specifically for use with pediatric neurological and other medical disorders.37
Family psychiatric history was assessed using the Family History Research Diagnostic Criteria interview,38,39 with parents acting as the informants. We summarized family ratings for first-degree relatives only, and for a combined grouping of first- and second-degree relatives, on a 4-point scale with higher scores indicating worse family psychiatric history.40
Family functioning was assessed using the McMaster Structured Interview of Family Functioning.41 The interviewer used the Clinical Rating Scale (CRS) to rate each of six domains and global family functioning on a 7-point Likert scale, where higher scores indicated better functioning. Global family function was the predictive variable used for analyses. The Family Assessment Device (FAD) questionnaire also was completed by family members at least 12 years of age,41 and scores were used to calculate a mean global functioning dimension score for each family. Higher scores indicated greater dysfunction.
Socioeconomic class was assessed using the Four Factor Index.42 Measures used to assess intellectual function included teacher-report of pre-injury intellectual ability and academic achievement on the PBS, along with pre-injury national percentile rank (NPR) for vocabulary on the Iowa Tests of Basic Skills (ITBS), the latter of which is highly correlated with verbal intelligence quotient (IQ).43
Assessments at 3, 6, 12, and 24 months post-injury
TBI participants completed repeat psychiatric evaluations at 3, 6, 12, and 24 months post-injury. Instruments administered included the K-SADS-P,44 supplemented by the attention-deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), alcohol and substance abuse modules of the K-SADS-E,33 a PTSD module, and the NPRS.35 Psychiatric diagnoses were based on DSM-III-R criteria. At each follow-up assessment, NPD was the outcome variable of interest, and a designation of NPD was applied in one of two conditions. First, this could occur in a participant with no lifetime psychiatric disorders at the baseline assessment who later manifested a psychiatric disorder following injury. Second, this could occur in the case of a participant with a lifetime psychiatric disorder at baseline, but who manifested a new-onset psychiatric disorder that was not present before the TBI (e.g., a participant with a lifetime history of ODD who developed generalized anxiety disorder (GAD) post-injury). Results from assessments in the first 2 years following TBI have been published elsewhere.12–15
Recruitment at 24 years post-injury
Pediatric TBI participants who completed at least the baseline psychiatric assessment from 1992 to 1994 (n = 50) and/or a first-degree relative were invited from 2016 to 2018 to participate in a 24-year post-injury psychiatric assessment. At this long-term follow-up, we studied 86% of TBI participants in person (n = 43), as well as the sibling of an original participant who died approximately 3 years prior to this phase of the study, and the parent of another participant who did not respond to our invitation to participate (n = 45). Significant others (e.g., parent, partner, friend) also were recruited for participants with TBI because of the possibility that awareness of deficits may be compromised after TBI.45 A total of 31 significant others participated. The interval from injury to long-term assessment was 23.92 ± 2.17 years (mean ± SD).
Excluded participants included one subject who remained in a persistent vegetative state, and was therefore not eligible to participate. Two of the 45 TBI participants were siblings and therefore one sibling was dropped only from the pre-injury predictive analyses because some variables could not be considered independent for the sibling pair. Four remaining originally enrolled participants (two male, two female) declined participation in the 24-year follow-up, and characteristics of these individuals are as follows. One participant had a severe TBI, no pre-injury psychiatric disorder, and developed secondary mania, which has been described previously.46 This individual initially agreed to participate and volunteered that he was divorced with children, and that he owned a small business; however, he did not attend his study assessment. Another non-participant at the long-term follow-up had a severe TBI, along with pre-injury agoraphobia, overanxious disorder, and major depressive disorder (MDD) with psychotic features, which evolved after the TBI into schizo-affective disorder with further evolution to a residual state. He also developed NPDs of personality change due to TBI, and ADHD during the 2-year follow-up. Family shared that he was employed but had ongoing unspecified problems, and would not wish to participate due to not wanting to be reminded of his injury.
The last two non-participants both had mild TBI, no pre-injury psychiatric disorders, and neither had participated beyond their baseline assessments. The third individual had not participated in the first 2 years post-injury due to avoiding memories of a sibling who was deceased at the time of her injury. At long-term follow-up recruitment, she volunteered that she was married, employed, and felt unaffected by the TBI; she declined to participate further due to being too busy. The fourth non-participant volunteered that she also felt unaffected by her TBI. She initially agreed to participate, but later declined.
Between 2017 and 2018, a control group of adults without history of neurological disorders—including no meningitis, encephalitis, brain tumors, or TBI—was recruited from the same geographic area. The Ohio State University TBI Identification Method was used to exclude potential controls with a history of TBI.47 For each TBI group participant, attempts were made to enroll a control participant of the same biologic sex and race, within 3 years of age, and within 1 point on the 5-point scale measure of SES.42 This method was used so that the TBI and control group characteristics would be similar, but we did not assume there would be an individually matched sample with the corresponding need for pair-wise statistical analyses. More specifically, we kept the original SES rating of the family of the child with TBI if the current SES rating of the adult proband with TBI was at the same level or worse. If the SES of the adult proband was better than their family of origin, we used the current rating for matching purposes. The rationale was to avoid missing real proband–control cognitive and possibly psychiatric differences in those with psychosocial drift,48 and to avoid finding spurious proband–control differences in those probands whose SES improved compared with their family of origin.
Assessments at 24 years post-injury
At the long-term follow-up assessment, all participants completed a questionnaire regarding demographic and medical information. We collected participants age, sex, marital status, education, socio-economic status, employment level, school attendance, living situation, primary source of income, and previous and intermittent medical history including a list of regular medications.
Psychiatric assessments were completed by author JEM using the Mini-International Neuropsychiatric Interview,49 and psychiatric diagnoses were made based on American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria.50 The NPRS was used to diagnose personality change due to TBI.35 In cases where individuals with TBI and a significant other participated, “best estimate” diagnoses were assigned based on integration of self-report and significant other report.51 Detailed inquiry was made in the interview assessments to document ages of onset and offset of every psychiatric diagnosis recorded. An inter-rater reliability study was conducted by a board-certified psychiatrist (EAT), which relied on ratings of videotaped interviews of TBI and control group participants. EAT was blind to group status and rated the videotaped interviews of every seventh TBI (n = 7) and control (n = 7) subject. Inter-rater reliability for diagnoses was excellent (ĸ = 0.962). There was perfect agreement on diagnoses in 12/14 (86%) cases, as well as agreement on 54/56 (96%) specific diagnoses.
We defined baseline as time of injury for the TBI group. For consistency with the TBI group, in the control group we regarded baseline as the mean age of injury of the TBI group (10.3 years). The determination of Any NPD (NPD-A) and Current NPD (NPD-C) at this most recent assessment wave of the study is defined according to psychiatric diagnoses assessed to have manifested during the interval “baseline plus 2 years” through the present assessment for participants in either group. With regard to assignment of the above NPD classifiers, consider the following examples. If an individual met criteria for only ADHD at the baseline and also at 24-year follow-up assessments, ADHD is coded as a lifetime psychiatric disorder because it was present prior to injury (TBI group) or prior to baseline (age 10.3 years) in the Control group. If at the 24-year follow-up evaluation, the same individual describes meeting criteria for MDD at some point since “baseline plus 2 years,” but which is currently in remission, along with GAD that is still interfering with functioning, then MDD is coded as an NPD-A, while GAD is coded as both an NPD-A and NPD-C.
Outcome measures of interest included: 1) NPD-C in each group (TBI and Controls); 2) NPD-A in each group; and 3) Post-Injury NPD exposure defined as the total number of years each participant manifested at least one NPD in the interval “baseline plus 2 years” through the present assessment for each group; and 4) Time-to-Event for onset of an NPD-A.
Statistical analysis
The prevalence of NPD during follow-up was compared between the TBI and control groups (hypotheses one and two) using Pearson's χ2 test. Additionally, the mean number of years of NPD exposure was compared between the groups using an independent samples t–test.
The association between the injury and pre-injury variables and NPD-C at 24-year follow-up was assessed using single- and multi-predictor logistic regression. The multi-predictor model was built using backward model selection with elimination threshold of p value <0.20.52 The starting multi-predictor model included all variables with single-predictor analysis p value <0.20. NPD-C was the primary outcome, rather than NPD-A, because during the 22-year follow-up interval other variables (e.g., life events) could transiently influence presentation of psychiatric disorders and mask findings related to more sustained problems. For completeness and relevance to the NPD-A outcome variable in our previous publications,12–15 similar analyses were performed with NPD-A at 3, 6, 12, 24, and 288 months post-baseline. In the NPD-A analyses, backward model selection failed due to separation in the starting model (due to cells generated by the combination of covariates in the model with 0% or 100% outcomes), and forward model selection was used instead.
The distribution of the time from “baseline plus 2 years” to development of NPD-A was estimated separately for the TBI and control groups using the Kaplan-Meier method, and compared between groups using the log-rank test. The hazard ratio of NPD-A between the TBI and control groups, unadjusted and adjusted for baseline factors, was computed (together with 95% confidence intervals) using the Cox proportional hazards model. The adjusted analyses used a similar backward model selection method as described above. In all analyses of the time to NPD-A, time was measured from “baseline plus 2 years.” We used SPSS (Version 25) for the statistical analyses.
Results
Pre-injury and novel psychiatric disorder
Demographic data for all participants are displayed in Table 1. The TBI and control groups did not significantly differ in age, sex, ethnicity, or social class. Table 2 shows the distribution of pre-injury psychiatric disorders (both lifetime and current at the time of injury for TBI participants, and respectively any time before age 10.3 years and at age 10.3 years for control participants), as well as NPD-C and NPD-A for all participants. The TBI group had significantly higher rate of pre-injury psychiatric disorder in their lifetime (23/45 [51%]) compared with the control group (13/45 [29%]; χ 2 = 4.63; df = 1; p = 0.031). These pre-injury psychiatric disorders persisted to the 24-year follow-up assessment significant less commonly in the TBI group versus the control group (5/23 [22%] vs. 8/13 [62%] respectively; Fisher's exact, p = 0.03). However, the groups did not differ in rate of pre-injury psychiatric disorders which remained current at the time of injury for the TBI group, and which were present at age 10.3 years in the control participants (16/45 [36%] vs. 13/45 [29%]; χ2 = 0.46; df = 1; p = 0.499).
Table 1.
Characteristics of Participants at 24-Year Post-Injury Assessment
| TBI (n = 45) | Control (n = 45) | |
|---|---|---|
| Demographic variables | ||
| Age: mean (SD) | 34.29 (2.72) | 34.07 (3.01) |
| Sex: n male (%) | 29 (64.4) | 29 (64.4) |
| Ethnicity: n Caucasian (%) | 44 (97.8) | 44 (97.8) |
| Social class: mean (SD) | 2.18 (.98) | 2.18 (.98) |
| Injury severity | ||
| Mild TBI: n (%) | 24 (53) | n/a |
| Moderate TBI: n (%) | 9 (20) | n/a |
| Severe TBI: n (%) | 12 (27) | n/a |
TBI, traumatic brain injury; SD, standard deviation; n/a, not applicable.
Table 2.
Psychiatric Disorders in Participants at 24 Years Post-Injury Assessment
| TBI (n = 45) | Control (n = 45) | Statistical test, p value | |
|---|---|---|---|
| Pre-injury psychiatric disorder | |||
| Pre-injury lifetime: n (%) | 23 (51) | 13 (29) | χ2 = 4.63, p = 0.031 |
| Pre-injury current: n (%) | 16 (36) | 13 (29) | χ2 = 0.46, p = 0.499 |
| Novel psychiatric disorder (NPD) | |||
| NPD-A: n (%) | 37 (82) | 26 (58) | χ2 = 6.40, p = 0.011 |
| NPD-C: n (%) | 24 (53) | 6 (13) | χ2 = 16.2, p = 0.000 |
| Post-Injury NPD exposure | |||
| Mean years (SD) | 10.80 (9.28) | 3.96 (5.80) | Ind. t-test = 4.19, p = 0.000 |
| Time to development of NPD-A | |||
| Median years | 3.0 | 8.7 | Log rank test = 8.2, p = 0.004 |
Pre-injury psychiatric disorder in the Control group refers to disorders present before age 10.3 years (mean age of injury of the original TBI cohort). Pre-injury Current refers to psychiatric disorder that was present at the time of injury in the TBI group and refers to psychiatric disorder that was present at age 10.3 years in the Control group. The interval for assessing for novel psychiatric disorder is from 2 years post-injury to the 24-year assessment for TBI participants and from age 12.3 years to the current assessment for Control participants.
TBI, traumatic brain injury; Ind., independent sample; NPD-A, novel psychiatric disorder, any; NPD-C, novel psychiatric disorder, current; SD, standard deviation.
The TBI group had a significantly higher rate of NPD-C compared with the control group (24/45 [53%] vs. 6/45 [13%]; χ2 = 16.2; df = 1; p < 0.0005), along with significantly greater rate of NPD-A (37/45 [82%] vs. 26/45 [58%]; χ2 = 6.4; df = 1; p = 0.011). Number of years of NPD exposure during the preceding 22-year interval was also significantly greater for the TBI versus control group (10.8 [± 9.28] vs. 3.96 [± 5.80]; independent t-test = 4.19; df = 88; p < 0.0005).
Predictive variables for novel psychiatric disorders
For the TBI group, we conducted univariable analyses of the 14 injury and pre-injury predictive variables of NPD-C. Figure 1 shows which variables were associated with NPD-C (p < 0.2) based on the likelihood ratio test for all post-injury assessments (3, 6, 12, and 24 months, and 24 years post-injury). Variables associated with NPD-C (p < 0.2) at 24 years included lowest post-resuscitation GCS score (p = 0.079), TCBD (diffuse axonal injury rating) category (p = 0.154), abnormal day-of-injury CT scan (p = 0.067), pre-injury lifetime psychiatric disorder (p = 0.035), VABS (adaptive function) standard score (p = 0.115), CRS (family function interview) score (p = 0.071), and PBS (teacher report of pre-injury school achievement) score (p = 0.178). The backward stepwise logistic regression produced a significant final model (χ2 = 17.42; df = 2; p < 0.0005), which included pre-injury lifetime psychiatric disorder (Wald χ2 = 6.68; df = 1; p < 0.010) and abnormal day-of-injury CT scan (Wald χ2 = 6.69; df = 1; p < 0.010).
Since pre-injury lifetime psychiatric disorder was significantly more common in the TBI group and was significantly associated with NPD-C, we conducted a logistic regression analysis with NPD-C as the outcome variable examining pre-injury lifetime psychiatric disorder controlling for group affiliation (TBI vs. Control). This was to assess whether the significant difference in the TBI versus control rate of NPD-C was confounded by the pre-injury lifetime psychiatric disorder rate difference between the groups. The regression was significant (χ2 = 29.63; df = 2; p < 0.0005), and pre-injury lifetime psychiatric disorder (Wald χ2 = 11.50; df = 1; p = 0.001) and group (Wald χ2 = 10.99; df = 1; p = 0.001) significantly and independently accounted for NPD-C. Inspection of the data showed that NPD-C was found in 9/54 (16.7%) participants with no pre-injury lifetime psychiatric disorder versus 21/36 (53.3%) participants with a pre-injury psychiatric disorder. More specifically, individuals in the TBI group with no lifetime pre-injury psychiatric disorder developed NPD-C at a significantly higher rate than the control group counterparts with no lifetime pre-injury psychiatric disorder (8/22 [36%] vs. 1/32 [3%]; Fisher's exact, p = 0.002). However, the corresponding analysis for NPD-A was not significant (16/22 [73%] vs. 16/32 [50%]; χ2 = 2.8; df = 1; p = 0.095).
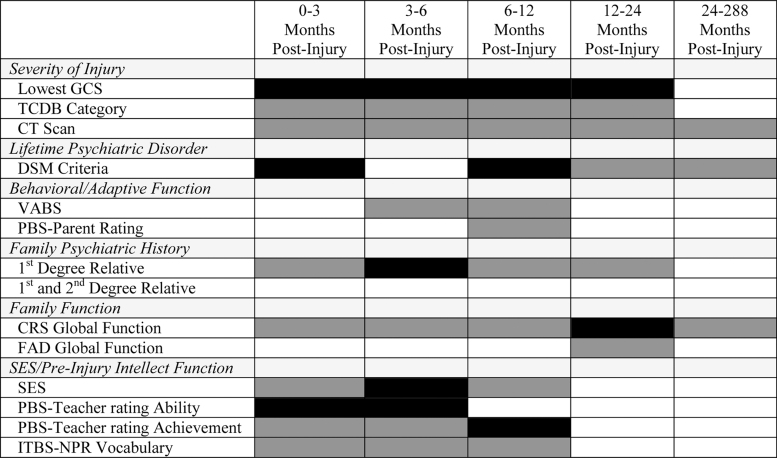
While NPD-C was the primary outcome variable of interest, we also repeated the univariable analyses with NPD-A as the outcome variable. Figure 2 shows which variables were associated with NPD-A (p < 0.2) based on the likelihood ratio test for each of the post-injury assessments. Variables associated with NPD-A at 24 years (p < 0.2) included abnormal day-of-injury CT scan (p = 0.200), pre-injury lifetime psychiatric disorder (p = 0.166), and CRS (family function interview) score (p = 0.171). Backward stepwise logistic regression produced a non-significant final model (χ2 = 3.53; df = 2; p = 0.172). For completeness, Figure 2 also shows the corresponding relationship of each variable with NPD-A at the 3, 6, 12, and 24-month assessments.
FIG. 2.
Baseline variables tested as predictors of NPD-A in 24 years following pediatric traumatic brain injury. Dark gray shading represents intervals during which each variable was selected for univariable binary logistic regression analysis (p < 0.20). Black shading represents variables assessed at baseline that independently significantly predict NPD-C at the indicated post-injury assessment (p < 0.05). Backward logistic regression was used for the 24-year follow-up data. Forward stepwise logistic regression analyses were used for the 3-, 6-, 12-, and 24-month assessments because of lack of separation with backward logistic regression analyses. CRS, Clinical Rating Scale of the McMaster Interview of Family Function; FAD, family assessment device; GCS, Glasgow Coma Scale; ITBS-NPR, Iowa Test of Basic Skills-National Percentile Rank; NPD-A, novel psychiatric disorder, any; PBS, Pediatric Behavior Scale; SES, socioeconomic status; TCBD, traumatic coma databank; VABS, Vineland Adaptive Behavior Scales.
Time-to-NPD analyses
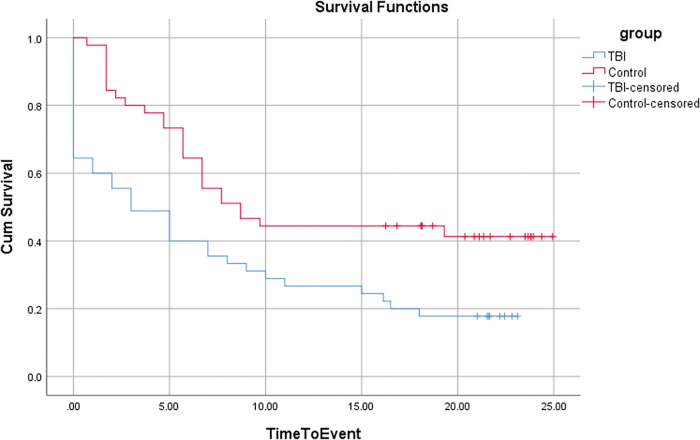
Time to NPD-A (survival) was significantly shorter and the hazard ratio for developing NPD-A significantly greater in the TBI group compared with the control group in analyses unadjusted for baseline factors (log rank test = 8.2; df = 1; p = 0.004; hazard ratio [HR] = 2.01; 95% CI, 1.214-3.328; Table 2 and Fig. 3) and adjusted for baseline factors of pre-injury lifetime psychiatric disorder, sex, SES, race, and age at injury (HR = 1.90; 95% CI, 1.125-3.221).
FIG. 3.
Survival function curves for traumatic brain injury (TBI) versus Control groups. Participants with TBI and Controls not developing novel psychiatric disorder (NPD) by their long-term follow-up, or by their one-time assessment, respectively, were censored at that time-point. Inspection of the survival function curves reveals the TBI group initially had a steeper change in events (onset of NPD-any) compared with the control group, but over the 10 to 15 years post-injury, the slopes are similar for both groups. Overall, the TBI group has a shorter survival with regard to onset of NPD-any. Color image is available online.
Discussion
There were three primary findings from this prospective longitudinal 24-year follow-up study of individuals who suffered a TBI requiring hospitalization at ages 6 to 14 years. First, we found that NPDs, both NPD-C and NPD-A, were significantly more common in the TBI group compared with controls, which supports our first and second hypotheses. Second, morbidity was significantly greater for the TBI group, as evidenced by significantly greater number of years of post-injury NPD exposure. Third, in the TBI group, NPD-C at 24 years post-injury was significantly and independently predicted by pre-injury lifetime psychiatric disorder assessed shortly after injury, and by an abnormal day-of-injury CT scan. These findings support our third hypothesis that some pre-injury and injury-related variables assessed at baseline can predict long-term psychiatric outcomes. Further, group differences in NPD-C remained after controlling for pre-injury lifetime psychiatric disorder. Finally, in support of our fourth hypothesis, survival analyses demonstrated a significantly shorter survival before development of an NPD, along with a greater hazard ratio for developing an NPD, in the TBI versus control group.
Short-term follow-up studies of pediatric TBI have previously demonstrated increased risk of NPDs following pediatric TBI.4,11,13 The current study has documented a 4-fold higher rate of NPD-C in adults with history of pediatric TBI compared with controls (53% vs. 13%), suggesting that exposure to TBI during development is a risk factor for mental illness at least into early adulthood. Further, close relationships between acquired brain injury and “adverse childhood events” have been found but require elucidation.53 In addition to increased frequency of NPDs, we also demonstrated that adults with history of pediatric TBI have a shorter duration to development of NPD-A and longer duration of exposure to NPD-A compared with control subjects, suggesting that the overall morbidity of psychiatric illness is significantly increased following pediatric TBI. These findings emerged in spite of the fact that the control group had high rates of psychiatric illness (29% met criteria for a “pre-injury” or lifetime psychiatric disorder, and 58% met criteria for a psychiatric disorder at some point over the interval “baseline plus 2 years” (age 12.3 years for controls) through the assessment at a mean (standard deviation) age of 34.07 (3.01) years.
The fact that significant differences emerged between the TBI and control groups despite not using a “super-normal” control group suggests that our findings are robust and unlikely to be spurious.54 This is because studies consistently show higher rates of new psychiatric disorders emerging by natural history in children and adolescents with versus those without psychiatric disorders.55 The rate of “pre-injury” lifetime psychiatric disorder in the controls (29%) is in the range of the 1-year prevalence (approximately 25%) in general population epidemiological samples of children and adolescents,56 whereas corresponding pre-injury lifetime psychiatric disorder rates in pediatric TBI samples are typically higher as in the present sample.10,57 We shall detail and compare specific pre-injury and NPD diagnoses in the TBI versus control groups in a follow-up article.
The finding that pre-injury lifetime psychiatric disorder and abnormal day-of-injury CT scan variables significantly and independently predict psychiatric outcomes 24 years following pediatric TBI underscores the importance of maintaining a comprehensive biopsychosocial model of psychopathology.58 This result is in line with previous studies which have demonstrated the importance of assessing psychosocial variables in pediatric TBI outcome studies.4,5,59
Pre-injury lifetime psychiatric disorder has previously been associated with NPD onset in the first years following pediatric TBI.4 The current analyses also extend our own findings at earlier time-points in this prospective longitudinal study. Pre-injury psychiatric history predicted NPD in the first 3 months, at 2 years, and now at 24 years post-injury, but not at 6 or 12 months post-TBI.12–15 Taken together, our findings suggest that pre-injury psychiatric history may influence pediatric TBI outcomes in both the short- and long-term. The finding that the specific pre-injury lifetime psychiatric disorder persisted to the 24-year follow-up assessment significantly less commonly in the TBI group versus the control group is likely to be a function of the prospective versus cross-sectional methodology in these groups respectively.
The fact that abnormal day-of-injury CT scan predicted long-term psychiatric outcome is striking, given that this is a relatively crude imaging modality for assessing brain injury. Newer imaging modalities including magnetic resonance imaging (MRI) with sequences such as diffusion tensor imaging (DTI), and magnetoencephalography are considered to be much more robust in detecting subtle changes following brain injury.60,61 We have previously found that the DTI-derived fractional anisotropy measure is significantly related to NPD development at shorter-term follow-up intervals, while other structural MRI variables including cortical thickness, lesion volume, gray matter volume, and white matter volume were not related to NPD onset.11 We plan to report DTI findings from the current study in relation to NPD-C at 24 years post-injury as well.
The findings of the current study should be interpreted in light of its limitations. First, the sample was relatively small (n = 50), and findings therefore require replication in larger samples. Second, the control group was not studied prospectively. Third, most prospective longitudinal TBI studies have the limitation of requiring a retrospective assessment of pre-injury variables after the injury has occurred. However, in this study baseline assessments were completed within a mean of 14 days following the incident trauma. Fourth, the psychiatrist (JEM) who assessed participants was not blinded to TBI versus control group affiliation. However, another board-certified psychiatrist (EAT) was blinded to group status and rated the videotape of every seventh participant to establish inter-rater reliability. Fifth, the psychiatric diagnoses that were applied during the first 2 years of follow-up for TBI participants were according to DSM-III-R criteria, while long-term follow-up diagnoses were applied for all participants based on DSM-5 criteria. Finally, consistent with the distribution of race in Iowa, most participants were Caucasian, which potentially limits the generalizability of study findings to more diverse populations.
There were several notable strengths of the study. First, this was the only long-term prospective longitudinal psychiatric interview study of pediatric TBI, and attrition was exceptionally low (8%) at the 24-year follow-up. Other long-term studies have examined behavioral domains of function, but not psychiatric disorders, in unselected cohorts, and have suffered from much higher rates of attrition, up to 69%.16–22 In this study, the psychiatric assessment is a strong aspect of the study methodology for several reasons. First, all psychiatric interviews from baseline through 24-year follow-up were completed by the same board-certified general and child and adolescent psychiatrist. Second, diagnoses were made only in the face of true impairment, which requires clinical judgment. Third, significant others provided history for most TBI participants, which served to address potential under-reporting due to lack of awareness of impairment in this group.45 Finally, excellent inter-rater reliability was achieved for psychiatric diagnoses with a second, board-certified psychiatrist rating 16% of all interviews while blinded to group affiliation. Recruitment of a well-matched control group without history of TBI, was also a strength, in that it allowed for comparison of NPD frequency and morbidity between groups. The use of predictive variables for NPD in the TBI cohort was a strong point, as they were based on comprehensive and clinically-relevant biopsychosocial data derived from multiple sources including participants, parents, and teachers.
In conclusion, our long-term outcome findings from a cohort of consecutively hospitalized children for mild to severe TBI were that they showed significantly greater burden of long-term psychiatric sequelae compared with age-, sex-, race-, and SES-matched controls. Variables assessed at the time of injury which predicted psychiatric outcome in adulthood included pre-injury lifetime psychiatric disorder and abnormal day-of-injury CT scan. Baseline assessments may therefore help guide early and targeted interventions for those individuals most at risk of developing psychiatric complications following pediatric TBI. Further longitudinal follow-up of pediatric TBI cohorts would extend our understanding of the morbidity of psychiatric outcomes into early and late middle-age for these individuals.
Acknowledgments
The authors thank Dr. David Sheehan for granting us permission to use the Mini-International Neuropsychiatric Interview.
Funding Information
This study was funded by a gift from Big Blue Sky Foundation. Drs. Max, Vaida, Wilde, and Hesselink receive support from the National Institute of Child Health and Development (R-01 HD088438). Dr. Troyer receives support through the National Institute of Mental Health (T32MH018399). Dr. Tymofiyeva and Dr. Yang receive support through the National Center for Complementary and Integrative Health (R21AT009173, R61AT009864), and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1TR001872.
Author Disclosure Statement
Dr. Bigler receives book royalties from Oxford University Press. Dr. Bigler and Dr. Max conduct traumatic brain injury medico-legal consultations.
For the other authors, no competing financial interests exist.
References
- 1. Dewan, M.C., Mummareddy, N., Wellons, J.C. 3rd, and Bonfield, C.M. (2016). Epidemiology of global pediatric traumatic brain injury: qualitative review. World Neurosurg. 91, 497–509 e491. [DOI] [PubMed] [Google Scholar]
- 2. Langlois, J.A., Rutland-Brown, W., and Thomas, K.E. (2005). The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil. 20, 229–238 [DOI] [PubMed] [Google Scholar]
- 3. Shi, J., Xiang, H., Wheeler, K., Smith, G.A., Stallones, L., Groner, J., and Wang, Z. (2009). Costs, mortality likelihood and outcomes of hospitalized US children with traumatic brain injuries. Brain Inj. 23, 602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown, G., Chadwick, O., Shaffer, D., Rutter, M., and Traub, M. (1981). A prospective study of children with head injuries: III. Psychiatric sequelae. Psychol. Med. 11, 63–78 [DOI] [PubMed] [Google Scholar]
- 5. Fay, G.C., Jaffe, K.M., Polissar, N.L., Liao, S., Rivara, J.B., and Martin, K.M. (1994). Outcome of pediatric traumatic brain injury at three years: a cohort study. Arch. Phys. Med. Rehabil. 75, 733–741 [PubMed] [Google Scholar]
- 6. Fletcher, J.M., Ewing-Cobbs, L., Miner, M.E., and Levin, H.S. (1990). Behavioral changes after closed head injury in children. J. Consult. Clin. Psychol. 58, 93–98 [DOI] [PubMed] [Google Scholar]
- 7. Schwartz, L., Taylor, H.G., Drotar, D., Yeates, K.O., Wade, S.L., and Stancin, T. (2003). Long-term behavior problems following pediatric traumatic brain injury: prevalence, predictors, and correlates. J. Pediatr. Psychol. 28, 251–263 [DOI] [PubMed] [Google Scholar]
- 8. Morissette, M.P., Prior, H.J., Tate, R.B., Wade, J., and Leiter, J.R.S. (2020). Associations between concussion and risk of diagnosis of psychological and neurological disorders: a retrospective population-based cohort study. Fam. Med. Community Health 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sariaslan, A., Sharp, D.J., D'Onofrio, B.M., Larsson, H., and Fazel, S. (2016). Long-term outcomes associated with traumatic brain injury in childhood and adolescence: a nationwide Swedish cohort study of a wide range of medical and social outcomes. PLoS Med. 13, e1002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Max, J.E. (2014). Neuropsychiatry of pediatric traumatic brain injury. Psychiatr. Clin. North Am. 37, 125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Max, J.E., Wilde, E.A., Bigler, E.D., Thompson, W.K., MacLeod, M., Vasquez, A.C., Merkley, T.L., Hunter, J.V., Chu, Z.D., Yallampalli, R., Hotz, G., Chapman, S.B., Yang, T.T., and Levin, H.S. (2012). Neuroimaging correlates of novel psychiatric disorders after pediatric traumatic brain injury. J. Am. Acad. Child Adolesc. Psychiatry 51, 1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Max, J.E., Lindgren, S.D., Robin, D.A., Smith, W.L.Jr., Sato, Y., Mattheis, P.J., Castillo, C.S., and Stierwalt, J.A. (1997). Traumatic brain injury in children and adolescents: psychiatric disorders in the second three months. J. Nerv. Ment. Dis. 185, 394–401 [DOI] [PubMed] [Google Scholar]
- 13. Max, J.E., Robin, D.A., Lindgren, S.D., Smith, W.L.Jr., Sato, Y., Mattheis, P.J., Stierwalt, J.A., and Castillo, C.S. (1997). Traumatic brain injury in children and adolescents: psychiatric disorders at two years. J. Am. Acad. Child Adolesc. Psychiatry 36, 1278–1285 [DOI] [PubMed] [Google Scholar]
- 14. Max, J.E., Robin, D.A., Lindgren, S.D., Smith, W.L.Jr., Sato, Y., Mattheis, P.J., Stierwalt, J.A., and Castillo, C.S. (1998). Traumatic brain injury in children and adolescents: psychiatric disorders at one year. J. Neuropsychiatry Clin. Neurosci. 10, 290–297 [DOI] [PubMed] [Google Scholar]
- 15. Max, J.E., Smith, W.L.Jr., Sato, Y., Mattheis, P.J., Castillo, C.S., Lindgren, S.D., Robin, D.A., and Stierwalt, J.A. (1997). Traumatic brain injury in children and adolescents: psychiatric disorders in the first three months. J. Am. Acad. Child Adolesc. Psychiatry 36, 94–102 [DOI] [PubMed] [Google Scholar]
- 16. Hessen, E., Anderson, V., and Nestvold, K. (2008). MMPI-2 profiles 23 years after paediatric mild traumatic brain injury. Brain Inj. 22, 39–50 [DOI] [PubMed] [Google Scholar]
- 17. Hessen, E., Nestvold, K., and Anderson, V. (2007). Neuropsychological function 23 years after mild traumatic brain injury: a comparison of outcome after paediatric and adult head injuries. Brain Inj. 21, 963–979 [DOI] [PubMed] [Google Scholar]
- 18. Klonoff, H., Clark, C., and Klonoff, P.S. (1993). Long-term outcome of head injuries: a 23 year follow up study of children with head injuries. J. Neurol. Neurosurg. Psychiatry 56, 410–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klonoff, H., Low, M.D., and Clark, C. (1977). Head injuries in children: a prospective five year follow-up. J. Neurol. Neurosurg. Psychiatry 40, 1211–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosema, S., Muscara, F., Anderson, V., Godfrey, C., Eren, S., and Catroppa, C. (2014). Agreement on and predictors of long-term psychosocial development 16 years post-childhood traumatic brain injury. J. Neurotrauma 31, 899–905 [DOI] [PubMed] [Google Scholar]
- 21. Ryan, N.P., Anderson, V., Godfrey, C., Beauchamp, M.H., Coleman, L., Eren, S., Rosema, S., Taylor, K., and Catroppa, C. (2014). Predictors of very-long-term sociocognitive function after pediatric traumatic brain injury: evidence for the vulnerability of the immature “social brain.” J. Neurotrauma 31, 649–657 [DOI] [PubMed] [Google Scholar]
- 22. Ryan, N.P., Anderson, V., Godfrey, C., Eren, S., Rosema, S., Taylor, K. and Catroppa, C. (2013). Social communication mediates the relationship between emotion perception and externalizing behaviors in young adult survivors of pediatric traumatic brain injury (TBI). Int. J. Dev. Neurosci. 31, 811–819 [DOI] [PubMed] [Google Scholar]
- 23. Ryan, N.P., Reyes, J., Crossley, L., Beauchamp, M.H., Catroppa, C., and Anderson, V.A. (2019). Unraveling the association between pediatric traumatic brain injury and social dysfunction: the mediating role of self-regulation. J. Neurotrauma 36, 2895–2903 [DOI] [PubMed] [Google Scholar]
- 24. Tuerk, C., Degeilh, F., Catroppa, C., Dooley, J.J., Kean, M., Anderson, V., and Beauchamp, M.H. (2020). Altered resting-state functional connectivity within the developing social brain after pediatric traumatic brain injury. Hum. Brain Mapp. 41, 561–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zamani, A., Mychasiuk, R., and Semple, B.D. (2019). Determinants of social behavior deficits and recovery after pediatric traumatic brain injury. Exp. Neurol. 314, 34–45 [DOI] [PubMed] [Google Scholar]
- 26. Cattelani, R., Lombardi, F., Brianti, R., and Mazzucchi, A. (1998). Traumatic brain injury in childhood: intellectual, behavioural and social outcome into adulthood. Brain Inj. 12, 283–296 [DOI] [PubMed] [Google Scholar]
- 27. Koskiniemi, M., Kyykka, T., Nybo, T., and Jarho, L. (1995). Long-term outcome after severe brain injury in preschoolers is worse than expected. Arch. Pediatr. Adolesc. Med. 149, 249–254 [DOI] [PubMed] [Google Scholar]
- 28. Nybo, T. and Koskiniemi, M. (1999). Cognitive indicators of vocational outcome after severe traumatic brain injury (TBI) in childhood. Brain Inj. 13, 759–766 [DOI] [PubMed] [Google Scholar]
- 29. Jonsson, C.A., Horneman, G., and Emanuelson, I. (2004). Neuropsychological progress during 14 years after severe traumatic brain injury in childhood and adolescence. Brain Inj. 18, 921–934 [DOI] [PubMed] [Google Scholar]
- 30. Max, J.E., Troyer, E.A., Arif, H., Vaida, F., Wilde, E.A., Bigler, E.D., Hesselink, J.R., Yang, T.T., Tymofiyeva, O., Wade, O., and Paulsen, J.S. (2021). Traumatic brain injury in children and adolescents: Psychiatric disorders 24 years later. J. Neuropsychiatry Clin. Neurosci. [Accepted.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teasdale, G. and Jennett, B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 32. Marshall, L.F., Marshall, S.B., Klauber, M.R., Van Berkum Clark, M., Eisenberg, H., Jane, J.A., Luerssen, T.G., Marmarou, A., and Foulkes, M.A. (1992). The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 9 Suppl 1, S287–S292 [PubMed] [Google Scholar]
- 33. Orvaschel, H., Puig-Antich, J., Chambers, W., Tabrizi, M.A., and Johnson, R. (1982). Retrospective assessment of prepubertal major depression with the Kiddie-SADS-e. J. Am. Acad. Child Psychiatry 21, 392–397 [DOI] [PubMed] [Google Scholar]
- 34. American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders. 4th, Text Revised Edition. American Psychiatric Press: Washington, DC [Google Scholar]
- 35. Max, J.E., Castillo, C.S., Lindgren, S.D., and Arndt, S. (1998). The Neuropsychiatric Rating Schedule: reliability and validity. J. Am. Acad. Child and Adolesc. Psychiatry 37, 297–304 [DOI] [PubMed] [Google Scholar]
- 36. Sparrow, S.S. and Cicchetti, D.V. (1985). Diagnostic uses of the Vineland Adaptive Behavior Scales. J. Pediatr. Psychol. 10, 215–225 [DOI] [PubMed] [Google Scholar]
- 37. Lindgren, S.D. and Koeppl, G.K. (1987). Assessing child behavior problems in a medical setting: Development of the pediatric behavior scale. Adv. Behav. Assess. Children Fam. 3, 57–90 [Google Scholar]
- 38. Andreasen, N.C., Rice, J., Endicott, J., Reich, T., and Coryell, W. (1994). The family history approach to diagnosis, in: Psychatric Epidemiology Assessment Concepts and Methods. Mezzich J.E., Jorge M.R., and Salloum I.M., (eds). John Hopkins University Press: Baltimore. MD and London U.K., pps. 349–367 [Google Scholar]
- 39. Andreasen, N.C., Endicott, J., Spitzer, R.L., and Winokur, G. (1977). The family history method using diagnostic criteria. Reliability and validity. Arch. Gen. Psychiatry 34, 1229–1235 [DOI] [PubMed] [Google Scholar]
- 40. Max, J.E., Arndt, S., Castillo, C.S., Bokura, H., Robin, D.A., Lindgren, S.D., Smith, W.L.Jr., Sato, Y., and Mattheis, P.J. (1998). Attention-deficit hyperactivity symptomatology after traumatic brain injury: a prospective study. J. Am. Acad. Child Adolesc. Psychiatry 37, 841–847 [DOI] [PubMed] [Google Scholar]
- 41. Miller, I.W., Kabacoff, R.I., Epstein, N.B., and Bishop, D.S. (1994). The development of a clinical rating scale for the McMaster Model of Family Functioning. Fam. Process 33, 53–69 [DOI] [PubMed] [Google Scholar]
- 42. Hollingshead, A. (1975). Four factor index of social status: unpublished working paper. www.academia.edu/927771/Four_Factor_Index_of_Social_Status (Last accessed April6, 2021)
- 43. Hieronymus, A.N. and Hoover, H.D. (1986). Manual for School Administrators Iowa Tests of Basic Skills, Forms G/H. Riverside Publishing Company: Chicago, IL [Google Scholar]
- 44. Chambers, W.J., Puig-Antich, J., Hirsch, M., Paez, P., Ambrosini, P.J., Tabrizi, M.A., and Davies, M. (1985). The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch. Gen. Psychiatry 42, 696–702 [DOI] [PubMed] [Google Scholar]
- 45. Prigatano, G. (1987). Psychiatric aspects of head injury: problem areas and suggested guidelines for research, in: Neurobehavioral Recovery from Head Injury. Grafman J., Levin H.S., and Eisenberg H.M. (eds). Oxford University Press: New York, NY, pps. 215–231 [Google Scholar]
- 46. Max, J.E., Smith, W.L., Sato, Y., Mattheis, P.J., Robin, D.A., Stierwalt, J.A.G., Lindgren, S.D., and Castillo, C. (1997). Mania and hypomania following traumatic brain injury in children and adolescents. Neurocase 119–126 [Google Scholar]
- 47. Corrigan, J.D. and Bogner, J. (2007). Initial reliability and validity of the Ohio State University TBI Identification Method. J. Head Trauma Rehabil. 22, 318–329 [DOI] [PubMed] [Google Scholar]
- 48. Kendler, K.S., Ohlsson, H., Karriker-Jaffe, K.J., Sundquist, J., and Sundquist, K. (2017). Social and economic consequences of alcohol use disorder: a longitudinal cohort and co-relative analysis. Psychol. Med. 47, 925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sheehan, D.V., Lecrubier, Y., Sheehan, K.H., Amorim, P., Janavs, J., Weiller, E., Hergueta, T., Baker, R., and Dunbar, G.C. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl 20, 22–33 [PubMed] [Google Scholar]
- 50. American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Press: Washington, DC [Google Scholar]
- 51. Leckman, J.F., Sholomskas, D., Thompson, W.D., Belanger, A., and Weissman, M.M. (1982). Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch. Gen. Psychiatry 39, 879–883 [DOI] [PubMed] [Google Scholar]
- 52. Maldonado, G. and Greenland, S. (1993). Simulation study of confounder-selection strategies. Am. J. Epidemiol. 138, 923–936 [DOI] [PubMed] [Google Scholar]
- 53. Guinn, A.S., Ports, K.A., Ford, D.C., Breiding, M., and Merrick, M.T. (2019). Associations between adverse childhood experiences and acquired brain injury, including traumatic brain injuries, among adults: 2014 BRFSS North Carolina. Inj. Prev. 25, 514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kessler, R.C., Coulouvrat, C., Hajak, G., Lakoma, M.D., Roth, T., Sampson, N., Shahly, V., Shillington, A., Stephenson, J.J., Walsh, J.K., and Zammit, G.K. (2010). Reliability and validity of the brief insomnia questionnaire in the America insomnia survey. Sleep 33, 1539–1549 [PMC free article] [PubMed] [Google Scholar]
- 55. Last, C.G., Perrin, S., Hersen, M., and Kazdin, A.E. (1996). A prospective study of childhood anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry 35, 1502–1510 [DOI] [PubMed] [Google Scholar]
- 56. Merikangas, K.R., Nakamura, E.F., and Kessler, R.C. (2009). Epidemiology of mental disorders in children and adolescents. Dialogues Clin. Neurosci. 11, 7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Max, J.E., Wilde, E.A., Bigler, E.D., MacLeod, M., Vasquez, A.C., Schmidt, A.T., Chapman, S.B., Hotz, G., Yang, T.T., and Levin, H.S. (2012). Psychiatric disorders after pediatric traumatic brain injury: a prospective, longitudinal, controlled study. J. neuropsychiatry Clin. Neurosci. 24, 427–436 [DOI] [PubMed] [Google Scholar]
- 58. Engel, G.L. (1979). The biopsychosocial model and the education of health professionals. Gen. Hosp. Psychiatry 1, 156–165 [DOI] [PubMed] [Google Scholar]
- 59. Yeates, K.O., Taylor, H.G., Rusin, J., Bangert, B., Dietrich, A., Nuss, K., and Wright, M. (2012). Premorbid child and family functioning as predictors of post-concussive symptoms in children with mild traumatic brain injuries. Int. J. Dev. Neurosci. 30, 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang, M.X., Robb Swan, A., Angeles Quinto, A., Huang, J.W., De-la-Garza, B.G., Huang, C.W., Hesselink, J.R., Bigler, E.D., Wilde, E.A., and Max, J.E. (2020). Resting-state magnetoencephalography source imaging pilot study in children with mild traumatic brain Injury. J. Neurotrauma 37, 994–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wilde, E.A., Hunter, J.V., Newsome, M.R., Scheibel, R.S., Bigler, E.D., Johnson, J.L., Fearing, M.A., Cleavinger, H.B., Li, X., Swank, P.R., Pedroza, C., Roberson, G.S., Bachevalier, J., and Levin, H.S. (2005). Frontal and temporal morphometric rindings on MRI in children after moderate to severe traumatic brain injury. J. Neurotrauma 22, 333–344 [DOI] [PubMed] [Google Scholar]