Abstract
Chimeric antigen receptor (CAR) T cell therapy has revolutionized the treatment of hematological malignancies, but solid tumors continue to pose significant challenges. Oncolytic viruses (OVs) have generated significant excitement in the field of cancer treatment recently. In particular, OVs can help CAR T cells overcome some of the immunosuppressive mechanisms within the tumor microenvironment through OV intrinsic effects or delivery of immunostimulatory agents. Numerous preclinical studies demonstrate that combining CAR T cells with OVs can increase CAR T cell trafficking, antitumor activity, and elimination of antigen-negative tumor cells. Despite promising preclinical results, only one clinical trial (NCT03740256) investigating CAR T and OV combination therapy is underway, highlighting the challenges of translating this approach to the clinic. Antiviral immunity and the route of OV administration, in addition to concerns about cost and safety, limit the clinical application of this approach. Strategies to reduce the production cost of both CAR T cells and OVs, as well as molecularly modifying OVs to enhance their bioavailability, will likely encourage further exploration of this combination therapy in clinical trials.
Keywords: oncolytic viruses, CAR T cells, immunotherapy
Introduction
Chimeric antigen receptor (CAR) T cells have demonstrated impressive successes in hematological malignancies.1 The current “gold standard” in the field is the CD19-specific CAR, and in pediatric B cell acute lymphoblastic leukemia, CD19-specific CAR T therapy resulted in between 70% and 90% complete response.1,2 While CD19 CAR T therapy has produced excellent response rates, between 30% and 50% of patients relapse one year after treatment.3
Two broad categories to disease relapse exist: antigen-positive and antigen-negative relapse. Antigen-positive relapse, where the target antigen remains on the tumor cell surface, is believed to occur as a result of limited CAR T persistence and CAR T cell dysfunction/exhaustion.3,4 Alternatively, antigen-negative relapse occurs when tumor cells either lose expression of the target antigen (e.g., CD19) or modify the antigen such that the CAR T cells no longer recognize it, and this can occur through a variety of mechanisms (see Shah and Fry3 and Xu et al.4 for more details).
Numerous avenues are being pursued to counter both antigen-positive and antigen-negative relapse, such as enhancing CAR T persistence in vivo, targeting multiple antigens, and optimizing costimulatory domains.5 Thus, improving CAR T therapy for hematological malignancies remains essential to achieve better clinical outcomes.
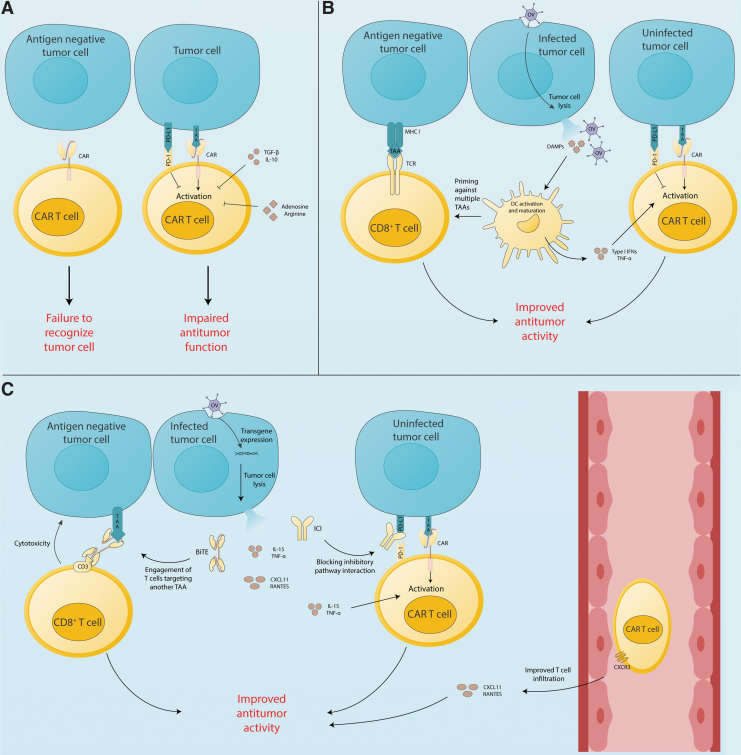
Despite the overall success in targeting hematological malignancies, CAR T therapy continues to struggle in the context of solid tumors. Various factors within the tumor microenvironment (TME) dampen T cell function (Fig. 1A).6 Tumor cells are known to upregulate programmed death ligand-1 (PD-L1), which negatively regulates T cell function, and tumor cells also induce tumor-associated macrophages to polarize toward an immunosuppressive phenotype, further impeding T cell function through a variety of surface receptors (e.g., cytotoxic T lymphocyte-associated antigen 4 [CTLA-4]) and cytokines (e.g., transforming growth factor-β [TGF-β]).
Figure 1.
Opportunities for synergy between OVs and CAR T cells. (A) CAR T cells recognize antigen-expressing tumor cells, but immune checkpoints (e.g., PD-1), immunosuppressive cytokines (e.g., IL-10 and TGF-β), and inhibitory metabolites (e.g., adenosine and arginine) impair CAR T cell function. Furthermore, antigen-negative tumor cells are not killed by CAR T cells. (B) OV particles infect tumor cells leading to tumor cell lysis and the release of DAMPs, as well as OV virions that infect more tumor cells. DAMPs promote DC activation, maturation, and priming of a polyclonal host T cell response. DCs then release proinflammatory cytokines (e.g., TNF-α) that support CAR T cell function. (C) Armed OVs cause tumor cells to release immunostimulatory agents like BiTEs, ICIs, chemokines (e.g., CXCL11), and proinflammatory cytokines (e.g., IL-15). BiTEs redirect endogenous T cells against a different antigen than the CAR, countering antigen escape. ICIs and proinflammatory cytokines promote CAR T cell function. Chemokines and cytokines augment CAR T cell infiltration of the tumor. BiTE, bispecific T cell engager; CAR, chimeric antigen receptor; DAMP, damage-associated molecular pattern; DC, dendritic cell; ICI, immune checkpoint inhibitor; IL, interleukin; OV, oncolytic virus; PD-L1, programmed death ligand-1; TNF-α, tumor necrosis factor-α.
In addition, tumors recruit myeloid-derived suppressor cells to the TME where they block T cell infiltration and metabolically inhibit T cells. Chronic antigen exposure in the TME promotes regulatory T cell (Treg) differentiation, and these Tregs contribute to the immunosuppressive environment by secreting interleukin (IL)-10 and TGF-β, metabolically disrupting conventional T cells, and lysing effector T cells.7 The TME also induces an immunosuppressive phenotype, characterized by upregulation of PD-L1 and indoleamine 2,3 dioxygenase (IDO), which hampers the immune response.6
Furthermore, the TME excludes T cells by disrupting T cell extravasation and trapping T cells within a dense stromal network.8 Overcoming these physical and molecular mechanisms of immunosuppression would likely improve the efficacy of CAR T cell therapy in solid tumors.
For both hematological and solid malignancies, additional modifications to the T cells are being tested to enhance CAR T function. Further engineering of CAR T cells, such as expression of cytokines or costimulatory ligands, has been developed to engage other immune cells (e.g., IL-12 engaging macrophages) or improve persistence/function of the CAR T cells themselves (e.g., IL-15).9–11 Investigators have also genetically modified CAR T cells to produce single-chain variable fragments (scFvs) that block signaling through the PD-1/PD-L1 axis, and this strategy improved survival compared to CAR T therapy alone.12
However, incorporation of additional transgenes into the CAR construct increases the amount of genetic material to be transferred, which limits the transduction efficiency of both lentiviral13 and retroviral vectors,14 as well as electrotransfer efficiency.15 Therefore, providing help to CAR T cells using other biological agents is extremely appealing.
Oncolytic viruses (OVs) are viruses that selectively infect and replicate in cancerous tissue, while sparing normal tissue,16,17 and numerous clinical trials involving OVs are currently underway.18 Strategies to design a tumor-specific OV are reviewed in references 17, 19, and 20.17,19,20
Briefly, defective innate antiviral pathways within tumor cells, such as an inhibited interferon (IFN) response, allow attenuated viruses to replicate in tumor tissue. Alternatively, viral genes can be placed under tumor-specific promoters, like the human telomerase reverse transcriptase promoter, to prevent viral gene transcription in normal cells. A more targeted approach to specify viral tropism is adding tissue-specific microRNA to the viral genome that silences viral genes in tissues that experience OV-related toxicity, preventing off-tumor side effects.21
OVs exert their antitumor effects by directly lysing tumor cells and stimulating the immune response within the TME.22 OV-mediated tumor cell lysis results in the release of tumor-associated antigens (TAAs), pathogen-associated molecular patterns, and damage-associated molecular patterns (DAMPs) into the TME, which augments immune cell recruitment and activation.23,24 OVs also induce production of type-I IFNs, tumor necrosis factor-α (TNF-α), and other cytokines to promote DC maturation, which results in the activation and recruitment of CD8+ T cells.25,26 These proinflammatory molecules reinvigorate the local immune response and provide activation signals for both innate and adaptive immune cells, which aids in clearing tumor cells (Fig. 1B).22
Of note, OVs can also induce an immune response against viral epitopes, which limits OV intratumoral spread, although antiviral immunity has been observed to enhance the antitumor effect of OV treatment.27 It remains unclear to what extent anti-OV immunity aids the antitumor response and how this should be balanced with the reduction in OV spread.
In addition to OV intrinsic effects, numerous groups have engineered OVs with transgenes designed to help T cells overcome the immunosuppressive TME (Fig. 1C).20 The immune-stimulatory properties of OVs, and the potential to arm OVs with therapeutic transgenes, make them excellent partners to boost CAR T cells in vivo. Herein, we summarize current OVs in clinical trials, and discuss how OVs partner with CAR T cells in preclinical models to improve tumor control. In addition, we highlight promising combinations that remain unexplored and discuss translational challenges facing OV and CAR T combination therapy.
Clinical outcomes of OV monotherapy
The use of OVs arose from case studies in the early 20th century suggesting that influenza infection could provide beneficial effects to leukemia patients.28 Some OVs tested before the advent of genetic engineering exhibited antitumor effects, although they were often weak or transient.28 However, the vast majority of OVs currently in clinical trials are genetically altered to enhance their tumor-specific tropism. An oncolytic herpes simplex virus type 1 (oHSV-1) derivative was the first OV to obtain FDA approval for treatment of melanoma.29 The oHSV-1 was generated by deleting neurovirulence genes to prevent pathogenicity, and deletion of the viral-infected cell protein 47 (ICP47) provides tumor specificity, as ICP47 normally interferes with antigen loading and presentation.30
While numerous clinical trials demonstrated the safety of native oHSV-1, it did not meaningfully improve patient outcomes.31 However, “arming” the oHSV-1 with the granulocyte-macrophage colony-stimulating factor (GM-CSF) gene to create talimogene laherparepvec (TVEC) was key to achieving clinical efficacy against melanoma.30 GM-CSF enhances T cell activation and the maturation of dendritic cells,32 and tumor cells infected with TVEC release GM-CSF into the TME after viral lysis, significantly improving patient outcomes.33,34 Monotherapies with other OVs follow a similar story—as native viruses, they exhibit limited efficacy in vivo, but arming them with immunostimulatory transgenes enhances their antitumor effects.20,35
An extensive review of OV therapy can be found in Ref. 22. To briefly summarize, there are several important lessons from OV monotherapy. One major limitation of OV therapy is limited intratumoral spread due to antiviral immunity. The mode of OV delivery and modifications to the OV can delay clearance of the OV, but clinical studies suggest that antiviral immunity is intimately related to antitumor immunity, requiring a balance of these two immune responses to be achieved.18 Another key finding is that even though OVs directly lyse tumor cells, various studies have demonstrated that T cells play an essential role in the antitumor effect of OVs.36 Furthermore, OVs increase CD8+ T cell infiltration to the tumor, which can render tumors more susceptible to T cell-mediated immunotherapies.37
Thus, despite limited success as monotherapies, OVs have the potential to synergize with other immunotherapies, like adoptive cellular therapy and immune checkpoint inhibitors (ICIs).38,39
CAR T AND ARMED OV COMBINATION THERAPY
Enhancing activation and trafficking to solid tumors
Many solid tumors interfere with immune cell infiltration by impairing adhesion molecule expression on endothelium, reducing chemokine production, and secreting extracellular matrix (ECM) components to physically exclude T cells from reaching tumor cells.8
Our group was one of the first to describe an armed OV used in combination with CAR T therapy (Table 1).40 We loaded genes for RANTES, a chemokine that promotes effector and memory T cell migration, and IL-15, which has been shown to enhance CAR T cell activity, into an oncolytic adenovirus (Ad5Δ24). We showed that, in a neuroblastoma xenograft model, local treatment with armed OV combined with systemic infusion of GD2-specific CAR T cells led to improved tumor control due to production of both RANTES and IL-15, which enhanced CAR T cell trafficking to the tumor and persistence of these cells within the TME.
Table 1.
Summary of preclinical studies combining oncolytic viruses with chimeric antigen receptor T cell treatment
| Viral Species | Transgene Products | Malignancy (Cell Line) | CAR Antigen | Refs. |
|---|---|---|---|---|
| Adenovirus | IL-15, RANTESa | Neuroblastoma (CHLA-255) | GD2 | 40 |
| Vaccinia | CXCL11 | Lung cancer (TC1-meso) | Mesothelin | 41 |
| Adenovirus | IL-2, TNF-α | PDA (AsPC-1) | PDA | 42 |
| Adenovirus | PD-L1 ICI | Prostate cancer (PC-3, SiHa) | HER2 | 43 |
| Adenovirus | EGFR-BiTE | Colon (HCT116), pancreatic (Panc-1) | Folate-receptor-α | 44 |
| Adenovirus | IL-12, PD-L1 ICI | HNSCC (FaDu, SCC-47) | HER2 | 48 |
| VSV, Vaccinia | None | Murine breast cancer (D2F2) | HER2 | 49 |
| Vaccinia | CD19 | Murine melanoma (B16) | CD19 | 51 |
| Vaccinia | CD19 | Breast cancer (MDA-MB-468), murine colon cancer (MC38) | CD19 | 52 |
| Adenovirus | CD44-BiTE, IL-12, PD-L1 ICI | HNSCC (FaDu) | HER2 | 58 |
| VSV | IFN-β | Murine melanoma (B16-EGFRvIII) | EGFRvIII | 59 |
Alternative name for CCL5.
BiTE, bispecific T cell engager; CAR, chimeric antigen receptor; CXCL11, C-X-C motif chemokine 11; EGFRvIII, epidermal growth factor receptor variant III; HER2, human epidermal growth factor receptor 2; HNSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibitor; IL, interleukin; PDA, pancreatic ductal adenocarcinoma; OV, oncolytic virus; PD-L1, programmed death ligand-1; TNF-α, tumor necrosis factor-α; VSV, vesicular stomatitis virus.
A similar approach used a vaccinia OV armed with another chemokine, CXCL11, combined with mesothelin-specific CAR T cells.41 CXCL11 is another chemokine that attracts effector T cells through interaction with CXCR3, which is known to be highly expressed by effector T cells.41 Thus, enrichment of chemokines matched with receptors expressed on effector T cells promotes CAR T cell recruitment to the tumor, resulting in improved tumor control.
An adenovirus armed with TNF-α and IL-2 combined with mesothelin-specific CAR T cells also demonstrated improved tumor control in a pancreatic ductal adenocarcinoma model.42 While both TNF-α and IL-2 do not directly attract T cells, they are known to induce production of chemokines, and in their model, the authors reported increased intratumoral CXCL10 and macrophage chemoattractant protein-1, both of which function as chemoattractants for effector T cells.42 Overall, these results reveal that, even in the context of immunosuppressive solid tumors, OVs armed with proinflammatory cytokines and chemokines can support CAR T cell infiltration and activation.
Countering immunosuppressive signals within the TME
Numerous molecular inhibitory pathways within the TME negatively regulate T cell function, including immune checkpoint receptor signaling (e.g., PD-1) from various cells within the TME.6
The remarkable clinical activity of ICIs in reinvigorating T cells within the TME prompted the development of OVs armed with ICIs, with the goal of delivering ICIs more selectively to the TME and thus avoiding toxicities associated with their systemic delivery. Many solid tumors express PD-L1,6 and IFN-γ produced by activated CAR T cells further promotes PD-L1 expression.43 Treatment with oncolytic adenovirus armed with a PD-L1 mini-antibody (HDPDL1) combined with human epidermal growth factor receptor-2 (HER2)-specific CAR T cells resulted in enhanced antitumor efficacy in a prostate cancer model, and delivery of the ICI through the OV vector was superior to systemic administration of the ICI.43 Thus, OVs armed with ICIs can enhance CAR T cell function by intratumorally blocking the PD-1/PD-L1 inhibitory pathway.
Preventing antigen-negative relapse
Treatment with CAR T cells, like other antigen-specific therapies, promotes tumor immunoediting, leading to the emergence of antigen-negative tumor cells.3,4 Furthermore, in solid tumors, antigen escape is prompted by the high heterogeneity of tumor cell composition. OVs directly lysing tumor cells and causing a proinflammatory environment increase priming against TAAs.
However, OVs can also be armed with bispecific T cell engagers (BiTEs) to selectively target TAAs, which may further reduce tumor immune escape through loss of antigen. BiTEs consist of a CD3 scFv and an scFv targeting a specific TAA, so they transiently localize T cells to the tumor cell and activate the T cell, leading to tumor cell lysis.44
Investigators demonstrated that folate receptor-α-specific CAR T cells combined with an adenovirus armed with an EGFR targeting BiTE enhanced tumor control in a colon cancer xenograft model by CAR T cells directly killing tumor cells and the recruitment of non-CAR T cells by the BiTE targeting the second antigen.44
Similar to BiTEs, membrane-integrated T cell engagers (MiTEs) localize T cells to the surface of tumor cells through a CD3 scFv, resulting in tumor cell lysis. However, MiTEs remain on the membrane of tumor cells, so only tumor cells infected with an OV armed with MiTEs would be targeted for lysis. While this limits T cell activation to cells infected by the OV, this approach could limit on-target, off-tumor effects compared to BiTEs since activation is dependent on viral tropism rather than expression of a specific surface marker.19 To summarize, these studies suggest that OVs armed with MiTEs or BiTEs could counter antigen-negative relapse by targeting multiple TAAs.
CURRENT LIMITATIONS AND FUTURE DIRECTIONS
Translational challenges
Despite numerous preclinical studies with promising results, there is only one approved clinical trial evaluating an OV in combination with CAR T therapy for HER2-positive cancers (NCT03740256). Translation of preclinical results to clinical trials is often difficult because oftentimes preclinical models use immunodeficient NOD scid gamma (NSG) mice, and this experimental system cannot model the interactions between OV and CAR T cells in the context of a fully competent immune system.
In addition, combination therapy with OVs and CAR T cells raises questions about the safety of combining two potent proinflammatory immunotherapies. While CD19-directed CAR T cells have gained FDA approval, they still pose a risk for life-threatening adverse events, like cytokine release syndrome and severe neurotoxicity.1 Although the only FDA-approved OV (TVEC) is relatively well tolerated,31 armed OVs designed to enhance T cell function could increase the rate and/or severity of CAR T cell side effects. Thus, it is critical to establish safe doses of these agents in combination with each other.
Another important consideration is cost. Current strategies to generate CAR T cells for therapy have a high cost per unit,45 and a full course of TVEC treatment is estimated to cost around $65,000.46 Combining these therapeutic agents also combines their cost, which could create a significant barrier to widespread access.
Delivery
One challenge to OV therapy is successfully delivering virus to tumor sites. While many clinical trials are investigating intratumoral administration of OVs, systemic administration is preferable to target metastatic cancer.24 However, in the blood, OVs encounter neutralizing factors in serum, become sequestered in the liver and spleen, and have impaired extravasation into tumors. Systemic administration can also cause cytokine storm responses, which can lead to severe systemic inflammation and even death.47 While strategies like carrier cells, polymer coatings, and targeting of tumor vessel endothelium are being employed to circumvent these challenges,24 OVs in combination with CAR T therapy may not need to infiltrate metastatic sites to promote their clearance.
As Rosewell et al. found in their study, an OV armed with a PD-L1 ICI did not infiltrate lymph node metastases, but HER2. CAR T cells from mice that received this OV still demonstrated improved control over metastases.48 Perhaps increasing activity of CAR T cells at primary tumor sites is sufficient to improve CAR T cell clearance of metastases, although this should be further studied using other CAR constructs and tumor models.
To counter barriers to OV delivery, one group used CAR T cells to deposit virus at tumor sites.49 They successfully load CAR T cells with OVs and deliver OVs to tumor cells without impairing CAR T function in vitro, and synergy between HER2.CAR T cells and an oncolytic vesicular stomatitis virus (VSV) is observed against a breast cancer cell line. Although this interesting system shows promise in vitro, its utility in vivo may be limited to immunologically hot tumors since in this scenario the OV cannot enhance CAR T infiltration without the cells first infiltrating the tumor to deposit the OV.
Expanding the combinations of armed OVs with CAR T cells
The primary mechanisms of resistance to CD19-specific CAR T cell therapy are believed to stem from CAR T cell dysfunction, lack of CAR T cell persistence, and antigen loss by tumor cells.3,4 Arming an OV with a cytokine that increases CAR T cell persistence, like IL-18,50 could also improve CD19-specific CAR T therapy for B cell malignancies. While OV therapy for hematological malignancies faces the same challenges as systemic delivery of OVs to target metastases (see Delivery section), OV engineering advances could improve the feasibility of using OV and CAR T cell combination therapy for hematological cancers in the future.
Despite its shortcomings, since the CD19-specific CAR T cells exhibit the strongest preclinical and clinical efficacy, investigators generated an OV armed with a human CD19 transgene with the intent of forcing CD19 expression on solid tumor cells, making them targetable by CD19-specific CAR T cells. In a B16 melanoma model, an OV armed with murine CD19 allowed CD19-specific CAR T cells to slow tumor growth.51 Another group used a similar approach to deliver CD19 to MC38 tumors, and they observed improved antitumor efficacy when CD19-specific CAR T cells were infused in combination with their truncated CD19 armed OV.52
Even if this concept is conceptually appealing, its clinical potential remains questionable due to the unnecessary elimination of normal B lymphocytes and the limited biodistribution of the virus, which results in low infection rate of tumor lesions. Significant improvements in infectivity of OVs are likely necessary for this strategy to achieve clinical efficacy.
Beyond this creative approach, there are a few transgenes we believe would synergize well with CAR T cell therapy, which remain untested. In terms of improving trafficking to solid tumors, combination of CAR T cells with an OV targeting the physical barriers of the TME remains unexplored. OVs armed with hyaluronidase53 and matrix metalloproteinase-8,54 both of which degrade the dense ECM characteristic of solid tumors, exhibited increased intratumoral dissemination of the OV in preclinical models. Combining this OV with CAR T cells may also improve the infiltration of CAR T cells and thus their antitumor activity.
A monoclonal antibody (mAb) targeting CTLA-4 received FDA approval, along with a mAb targeting PD-1, for treatment of melanoma recently, which indicates that blocking CTLA-4 through an OV vector may also improve CAR T therapy.55 Engeland et al. describe an oncolytic measles virus (MV) armed with a full-length anti-CTLA-4 mAb, and they demonstrate that this OV improved tumor control compared to the native OV in an immunocompetent mouse model. However, the authors do report that systemic aCTLA-4 treatment combined with native MV resulted in better survival than MV-aCTLA-4.55 Nonetheless, using CAR T cells in combination with an OV encoding a blocking CTLA-4 agent should be explored.
Another potential approach is using OVs to modify the metabolism of the TME, making it more hospitable for CAR T cells. Studies in recent years have illustrated the importance of metabolism in T cell biology. For example, CD39 and CD73 hydrolyze extracellular ATP to immunosuppressive adenosine, which can accumulate in the TME and impair T cell function. A CD39 blocking antibody improved the efficacy of oxaliplatin chemotherapy treatment in an immunocompetent mouse model, prompting a clinical trial investigating the efficacy of this antibody in combination with a PD-1 blocking antibody (NCT04261075).56 Arming an OV with this antibody could represent a promising method to improve CAR T cell therapy by reducing adenosinergic signaling, while simultaneously stimulating T cells through OV intrinsic effects.
IDO is another enzyme that exerts its immunosuppressive effect through metabolism. IDO catalyzes the formation of immunosuppressive kynurenine and is commonly expressed on tumor cells. Disruption of IDO expression on tumor cells by micro-RNA targeting of its transcript improved CAR T cell therapy in a colon cancer xenograft model.57 Thus, there are multiple metabolic enzymes that could be targeted through arming an OV vector to synergize with CAR T cell therapy.
Furthermore, arming OV with multiple transgenes targeting nonredundant pathways could provide even greater tumor control. An OV armed with CD44-BiTE, IL-12, and PD-L1 ICI was used to boost HER2-specfic CAR T cell function.58 This OV combined with CAR T cells demonstrated improved tumor control in a metastatic head and neck squamous cell carcinoma model, highlighting the potential power of multiplexed immunotherapy. While this may represent a cost-effective method of delivering multiple immunotherapies, since the OV encodes for three different molecules, increasing the number of therapeutic molecules delivered by OVs also presents an increased risk for adverse reactions. Thus, safety concerns may limit the number of transgenes loaded into an OV until each is individually proven safe when combined with CAR T cell therapy.
Even though combining CAR T cells with OVs could overcome challenges within the TME, combination therapy can also present unique problems. Recent work describes a VSV encoding an IFN-β transgene that interferes with CAR T therapy by promoting apoptosis of CAR T cells; however, removal of the IFN-β transgene reduced CAR T cell attrition.59 Type-I IFNs, which include IFN-α and IFN-β, stimulate the immune system, but chronic type-I IFN signaling can lead to immune dysfunction.60
Proinflammatory and anti-inflammatory duality is a common feature of immunological molecules, which makes designing combination therapies tricky and requires a balance to be achieved. The heterogeneity of proteomes between malignancies, and potentially even patients with the same malignancy, could further complicate combination therapy. Careful consideration should be given to the potential immunosuppressive effects of transgenes used in armed OVs when combined with CAR T cell therapy.
CONCLUSIONS
The TME in solid tumors is characterized by multiple immunosuppressive mechanisms, which may require the simultaneous inhibition of different pathways to achieve tumor eradication. The significant cargo capacity of OVs can be leveraged to express multiple transgenes including chemokines, cytokine, ICIs, and BiTEs, as well as other molecules to shape the TME and facilitate the antitumor effects of CAR T cells.48 Furthermore, the high flexibility of the engineering process of OVs and CAR T cells allows tailoring the most appropriate combinations of genes and CAR T cell costimulation to the specific characteristics of the targeted tumor and TME. While the vast majority of OV studies target solid malignancies, using armed OVs with CAR T therapy against hematological malignancies could reduce disease relapse.
AUTHORS' CONTRIBUTIONS
K.M. performed the literature search and wrote the article. G.D. edited and approved the article.
AUTHOR DISCLOSURE
No competing financial interests exist.
FUNDING INFORMATION
This work was supported, in part, by 1R01CA243543-01 (G.D.), 5-R01-CA193140-03 (G.D.), and 1-R21-CA229938-01A1 (G.D.), and by AACR Stand Up to Cancer (G.D.).
References
- 1. June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med 2018;379:6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood 2016;127:3312–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol 2019;16:372–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu X, Sun Q, Liang X, et al. Mechanisms of relapse after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and its prevention and treatment strategies. Front Immunol 2019;10:2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol 2020;17:147–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol 2016;39:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008;8:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson KG, Stromnes IM, Greenberg PD. Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell 2017;31:311–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeku OO, Brentjens RJ. Armored CAR T-cells: utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochem Soc Trans 2016;44:412–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yeku OO, Purdon TJ, Koneru M, et al. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep 2017;7:10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pegram HJ, Park JH, Brentjens RJ. CD28z CARs and armored CARs. Cancer J 2014;20:127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rafiq S, Yeku OO, Jackson HJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 2018;36:847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar M, Keller B, Makalou N, et al. Systematic determination of the packaging limit of lentiviral vectors. Hum Gene Ther 2001;12:1893–1905 [DOI] [PubMed] [Google Scholar]
- 14. Coffin JM, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1997 [PubMed] [Google Scholar]
- 15. Lesueur LL, Mir LM, André FM. Overcoming the specific toxicity of large plasmids electrotransfer in primary cells in vitro. Mol Ther Nucleic Acids 2016;5:e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Russell L, Peng K-W. The emerging role of oncolytic virus therapy against cancer. Chin Clin Oncol 2018;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maroun J, Muñoz-Alía M, Ammayappan A, et al. Designing and building oncolytic viruses. Future Virol 2017;12:193–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martinez-Quintanilla J, Seah I, Chua M, et al. Oncolytic viruses: overcoming translational challenges. J Clin Invest 2019;129:1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Twumasi-Boateng K, Pettigrew JL, Kwok YYE, et al. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat Rev Cancer 2018;18:419–432 [DOI] [PubMed] [Google Scholar]
- 20. Zheng M, Huang J, Tong A, et al. Oncolytic viruses for cancer therapy: barriers and recent advances. Mol Ther Oncolytics 2019;15:234–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruiz AJ, Russell SJ. MicroRNAs and oncolytic viruses. Curr Opin Virol 2015;13:40–48 [DOI] [PubMed] [Google Scholar]
- 22. Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 2015;14:642–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raja J, Ludwig JM, Gettinger SN, et al. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer 2018;6:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Russell SJ, Peng K-W, Bell JC. Oncolytic virotherapy. Nat Biotechnol 2012;30:658–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zamarin D, Holmgaard RB, Subudhi SK, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med 2014;6:226ra232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rivadeneira DB, DePeaux K, Wang Y, et al. Oncolytic viruses engineered to enforce leptin expression reprogram tumor-infiltrating T cell metabolism and promote tumor clearance. Immunity 2019;51:548–560.e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ricca JM, Oseledchyk A, Walther T, et al. Pre-existing immunity to oncolytic virus potentiates its immunotherapeutic efficacy. Mol Ther 2018;26:1008–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther 2007;15:651–659 [DOI] [PubMed] [Google Scholar]
- 29. Eissa IR, Bustos-Villalobos I, Ichinose T, et al. The current status and future prospects of oncolytic viruses in clinical trials against melanoma, glioma, pancreatic, and breast cancers. Cancers (Basel) 2018;10:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Conry RM, Westbrook B, McKee S, et al. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum Vaccin Immunother 2018;14:839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu JCC, Coffin RS, Davis CJ, et al. A Phase I Study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 2006;12:6737. [DOI] [PubMed] [Google Scholar]
- 32. Kaufman HL, Ruby CE, Hughes T, et al. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer 2014;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaufman HL, Kim DW, DeRaffele G, et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol 2010;17:718–730 [DOI] [PubMed] [Google Scholar]
- 34. Andtbacka RHI, Collichio F, Harrington KJ, et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III–IV melanoma. J Immunother Cancer 2019;7:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mondal M, Guo J, He P, et al. Recent advances of oncolytic virus in cancer therapy. Hum Vaccin Immunother 2020:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kanerva A, Nokisalmi P, Diaconu I, et al. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF–coding oncolytic adenovirus. Clin Cancer Res 2013;19:2734. [DOI] [PubMed] [Google Scholar]
- 37. Bourgeois-Daigneault M-C, Roy DG, Aitken AS, et al. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci Transl Med 2018;10:eaao1641. [DOI] [PubMed] [Google Scholar]
- 38. Martin NT, Bell JC. Oncolytic virus combination therapy: killing one bird with two stones. Mol Ther 2018;26:1414–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harrington K, Freeman DJ, Kelly B, et al. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov 2019;18:689–706 [DOI] [PubMed] [Google Scholar]
- 40. Nishio N, Diaconu I, Liu H, et al. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res 2014;74:5195–5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moon EK, Wang L-CS, Bekdache K, et al. Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology 2018;7:e1395997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Watanabe K, Luo Y, Da T, et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight 2018;3:e99573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanoue K, Rosewell Shaw A, Watanabe N, et al. Armed oncolytic adenovirus-expressing PD-L1 mini-body enhances antitumor effects of chimeric antigen receptor T cells in solid tumors. Cancer Res 2017;77:2040–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wing A, Fajardo CA, Posey AD Jr, et al. Improving CART-cell therapy of solid tumors with oncolytic virus-driven production of a bispecific T-cell engager. Cancer Immunol Res 2018;6:605–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harrison RP, Zylberberg E, Ellison S, et al. Chimeric antigen receptor–T cell therapy manufacturing: modelling the effect of offshore production on aggregate cost of goods. Cytotherapy 2019;21:224–233 [DOI] [PubMed] [Google Scholar]
- 46. Orloff M. Spotlight on talimogene laherparepvec for the treatment of melanoma lesions in the skin and lymph nodes. Oncolytic Virother 2016;5:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Domingo-Musibay E, Yamamoto M. Gene and virotherapy for hematological malignancies. Int J Hematol 2016;104:29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosewell Shaw A, Porter CE, Watanabe N, et al. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T cells against metastatic head and neck cancer. Mol Ther 2017;25:2440–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. VanSeggelen H, Tantalo DGM, Afsahi A, et al. Chimeric antigen receptor–engineered T cells as oncolytic virus carriers. Mol Ther Oncolytics 2015;2:15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hu B, Ren J, Luo Y, et al. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep 2017;20:3025–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aalipour A, Le Boeuf F, Tang M, et al. Viral delivery of CAR targets to solid tumors enables effective cell therapy. Mol Ther Oncolytics 2020;17:232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Park AK, Fong Y, Kim S-I, et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Scie Transl Med 2020;12:eaaz1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guedan S, Rojas JJ, Gros A, et al. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol Ther 2010;18:1275–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cheng J, Sauthoff H, Huang Y, et al. Human matrix metalloproteinase-8 gene delivery increases the oncolytic activity of a replicating adenovirus. Mol Ther 2007;15:1982–1990 [DOI] [PubMed] [Google Scholar]
- 55. Engeland CE, Grossardt C, Veinalde R, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther 2014;22:1949–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perrot I, Michaud H-A, Giraudon-Paoli M, et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep 2019;27:2411–2425.e2419. [DOI] [PubMed] [Google Scholar]
- 57. Liu M, Wang X, Wang L, et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol 2018;11:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Porter CE, Rosewell Shaw A, Jung Y, et al. Oncolytic adenovirus armed with BiTE, cytokine, and checkpoint inhibitor enables CAR T cells to control the growth of heterogeneous tumors. Mol Ther 2020;28:1251–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Evgin L, Huff AL, Wongthida P, et al. Oncolytic virus-derived type I interferon restricts CAR T cell therapy. Nat Commun 2020;11:3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Snell LM, McGaha TL, Brooks DG. Type I interferon in chronic virus infection and cancer. Trends Immunol 2017;38:542–557 [DOI] [PMC free article] [PubMed] [Google Scholar]