Abstract
Refractory intracranial hypertension (RIH) is a dramatic increase in intracranial pressure (ICP) that cannot be controlled by treatment. Recent reports suggest that the autonomic nervous system (ANS) activity may be altered during changes in ICP. Our study aimed to assess ANS activity during RIH and the causal relationship between rising in ICP and autonomic activity. We reviewed retrospectively 24 multicenter (Cambridge, Tromso, Berlin) patients in whom RIH developed as a pre-terminal event after acute brain injury (ABI). They were monitored with ICP, arterial blood pressure (ABP), and electrocardiography (ECG) using ICM+ software. Parameters reflecting autonomic activity were computed in time and frequency domain through the measurement of heart rate variability (HRV) and baroreflex sensitivity (BRS). Our results demonstrated that a rise in ICP was associated to a significant rise in HRV and BRS with a higher significance level in the high-frequency HRV (p < 0.001). This increase was followed by a significant decrease in HRV and BRS above the upper-breakpoint of ICP where ICP pulse-amplitude starts to decrease whereas the mean ICP continues to rise. Temporality measured with a Granger test suggests a causal relationship from ICP to ANS. The above results suggest that a rise in ICP interacts with ANS activity, mainly interfacing with the parasympathetic-system. The ANS seems to react to the rise in ICP with a response possibly focused on maintaining the cerebrovascular homeostasis. This happens until the critical threshold of ICP is reached above which the ANS variables collapse, probably because of low perfusion of the brain and the central autonomic network.
Keywords: autonomic nervous system, Granger causality, refractory intracranial hypertension, upper breakpoint of ICP
Introduction
According to Monro-Kellie doctrine, intracranial pressure (ICP) is the result of the craniospinal system ability to maintain a constant volume of craniospinal components.1 A rise in the ICP above the normal range (intracranial hypertension or ICH) can be attributed to an increase in the one or more of three volume components of craniospinal space: parenchyma (cytotoxic or vasogenic edema, brain tumor, contusion), blood (hemorrhage, vasodilatation, venous congestion) or cerebrospinal fluid (CSF, acute hydrocephalus).2,3 During the last few decades different phenomena of ICP elevation have been studied, and different pathophysiological pathways have been identified underlying the concept that not all the ICP elevations are the same.4–6
In the clinical context after ABI, ICH necessitates medical or surgical interventions after acute brain injury to avoid low cerebral perfusion and risk of herniation and death.7 Refractory intracranial hypertension (RIH) is a severe increase in ICP that happens after an ABI and is usually resistant to medical or surgical treatment. The RIH commonly leads to brain death or major brain damage.8,9 The ICP runs from normal or moderately increased values to a dramatic ICH. Detrimental effects of elevation in ICP per se can be attributed to the development of a transtentorial pressure gradient with damage on the brainstem; an increase in cerebral pressure causes the compression of bridging veins and a reduction in cerebral blood flow (CBF).10,11 The complete pathophysiological picture of this cascade, however, remains unclear at this time.
The autonomic nervous system (ANS) could be one of the potential factors involved in the refractory elevation of ICP. Variability in the beat-by-beat period of heart contraction is an intrinsic characteristic of a healthy neurocardiological system. The ANS activity has been demonstrated to correlate with outcome in acute brain-injured patients,12–15 with ANS impairment associated with higher mortality and long-term outcome. The causal relationship of autonomic changes on ABI sequelae remains hypothetical: ABI-related ANS dysfunction affects crucial organs of our body—specifically, the heart.16,17
According to guidelines,18 we can assess the autonomic system through the analysis of HRV and BRS which has been proposed as a marker of healthy ANS. A significant number of studies have been conducted since the first studies performed by Lowensohn and colleagues (1977) and Leipzig and Lowensohn (1986)19,20 exploring the relationship between ANS and ICP. More recent reports showed that ANS activity is altered during changes in ICP.12,21–23 The mechanisms involved, however, have not been clarified. Different methods have been used during the last decades producing results that have not been always consistent. It follows that more translational research is necessary, given that understanding the relationships between ANS and ICP and potential therapeutic targets will certainly improve patient outcomes.12,24–27
The primary aim of our study was to assess changes in autonomic activity during the development of RIH and to explore the causal relationship between ICP and autonomic activity in patients with ABI. We focused our analysis on physiological data occurring during RIH as a pre-terminal event. Clinical variables such as medical or surgical interventions, ABI etiology, different physiopathological brain injury features had not been taken into account.
Methods
Data collection
The study was conducted as a retrospective analysis of a prospectively maintained database cohort (2009–2018) in which physiological monitoring data had been archived in three different hospitals: Department of Neurosurgery, Charite Hospital, Berlin, Germany; Department of Intensive Care, University Hospital, Tromso, Norway, and Neurocritical Care, Addenbrooke's University Hospital, Cambridge. Monitoring was conducted using ICM+® software (Cambridge Enterprise, Ltd, Cambridge, UK, www.icmplus.neurosurg.cam.ac.uk).
Acute brain-injured patients with a clinical need for ICP monitoring and computerized signal were included. The monitoring was part of standard patient care and archived in an anonymized way. All demographic/clinical data were extracted from the hospital records and were anonymized fully, no data on patient identifiers were available, and therefore formal patient or proxy consent and institutional ethics approval were not obtainable. Institutional reviewing was not required because of the retrospective design of the study that consisted of the analysis of data acquired during routine care.
Patients were monitored with at least invasive intraparenchymal ICP, invasive ABP, and ECG. There were 61 patients with RIH selected initially, and 37 were excluded because of either the absence of the ECG signal, frequent artefacts, or absence of baseline ICP recording before RIH evolved, which was crucial for dynamic analysis of ANS behavior. Therefore, 24 patients with ABI were included in the final analysis. The patients in whom RIH developed had an initial baseline of ICP (mean ICP <20) followed by a rise to more than 40 mm Hg and then either fulfilled criteria of brain death or died after the withdrawal of treatment or cardiac arrest.
Data processing
The signals were acquired digitally with a sampling frequency of at least 100 Hz. The time-averaged values of ICP and ABP were calculated on a 10-sec calculation window. The PRx was calculated as the moving Pearson correlation between ABP and ICP of a 5 min window, updated every minute.28 The amplitude (AMP) of the cardiac pulse in ICP and ABP were determined as the fundamental harmonic of the Fourier transform of the pulse of ICP. The right arterial pressure (RAP) was calculated as the moving correlation coefficient between slow changes in ICP pulse AMP and mean ICP (10 sec average data) over a period of 5 min, updating every minute.29
The artefacts were cleaned manually in the raw data: in the ABP and ICP signal, the non-pulsatile chunks were removed. From the ECG, long, visible arrhythmic events and flat lines were removed manually; single ectopic beats were automatically detected by the software.
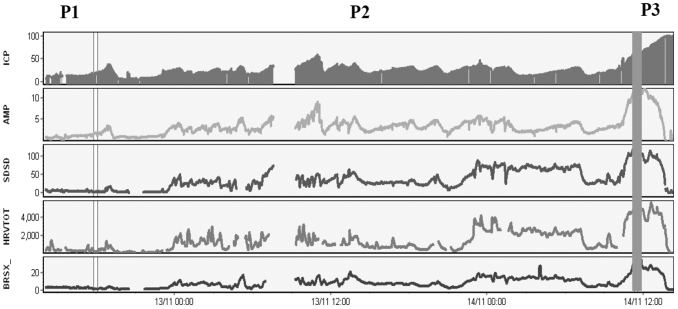
For each patient, the recording was divided into three different segments: the first was called “baseline” or period one (P1) in which the ICP mean was lower than 20 mm Hg; the second period (P2) was the period during which the ICP started to rise, more or less continuously, until the elevated value of ICP. The third period (P3) was defined starting from the ICP/AMP “upper breakpoint” onward.6,30 The upper breakpoint is a value of ICP and AMP of ICP above which pulse AMP started to decrease with an ongoing increase in the mean ICP. If the upper breakpoint was not found, the second period ended with the end of the recording when dramatic values of ICP were reached and which was followed by patient death.
Autonomic variable calculation
Secondary parameters reflecting autonomic activity were computed in time and frequency domain through the continuous measurements of HRV. According to the guidelines,18 we analyzed HRV both in the time and in the frequency domain.
The analysis of oscillatory components of the ECG signal enables the assessment of the autonomic system because it is the primary regulator of cardiac chronotropy.31,32 The interval between R waves in the ECG is the most commonly used to represent cardiac chronotropy and can be analyzed both in the time domain and/or in the frequency domain.
In time domain, we analyzed global indexes of HRV such as standard deviation (SD), the standard deviation of the difference between sequential beats (SDSD), and square root of the mean squared difference between sequential beats (RMSSD). In the frequency domain, we calculated the total power of the HRV spectrum; moreover, we calculated frequency-specific indexes. The high-frequency (HF) component (0.15–0.4 Hz) is thought to be modulated by the parasympathetic system, whereas the low-frequency (LF) component (0.04–0.15Hz) is modulated by both the sympathetic and the parasympathetic system. The ratio between the two (LF/HF ratio) seems to mirror the sympathetic activity.33,18
The HRV in the time domain was analyzed using a 300-sec time series of R-R intervals that were updated every 10 sec. In the frequency domain, the Lomb-Scargle periodogram was used to calculate the spectral power of the R-R interval time series.18 Baroreflex sensitivity, which can be described as the magnitude of response in the heart-beat interval to a change in blood pressure, was measured using the cross-correlation method, which had been shown to have the lowest intra- and inter-individual variability in the EUROBAVAR database.34
The x-BRS calculation algorithm was implemented into the ICM+ software using a 10-sec window moving along the time axis. To remove the influence of an unknown time delay of the baroreceptor response, a cross-correlation function was used to maximize the correlation coefficient, which meant that the actual total window length used in each calculation was 17 sec. Valid x-BRS was returned only if the correlation coefficient is significant at p < 0.01.
Statistical analysis
The R statistical language was used to perform the statistical analysis (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. www.R-project.org/version 3.3.3). Alpha was set at 0.05 for significance.
The non-normal distribution of the data was established by the Shapiro-Wilk test.35 The Wilcoxon test was used for comparisons after having extracted variables as mean value ± SD during the three different periods. The correlation between physiologic parameters was assessed using the Spearman method.
For establishing the direction of potential causal interactions in the time series, we used the model developed by Granger36 capable of causal inference. Understanding not only functional connectivity but also directional connectivity is becoming more and more important and popular, particularly in neuroscience.37 Granger causality is a statistical method for identifying the significance of directional information flow between a given set of time series. According to Granger, a time series X is called to Granger-cause another time series Y if the past value of X contains information that helps to predict future values of Y. The Granger test was applied to stationary time series between ICP and autonomic variables during P1 and P2. The calculation of the directionality index (DI) was then applied.
Results
We analyzed 24 patients with ABI who all died during RIH (four fulfilling brain death criteria, 11 after the withdrawal of intensive care treatment for catastrophic brain injury, while the cause of death is not reported about nine patients; however, their death occurred during RIH). The mean age was 37years (SD ±15 years). There were 21 patients who had a traumatic brain injury (TBI); three patients had a subarachnoid hemorrhage. Five patients underwent a decompressive craniectomy before the recorded time series, 17 did not undergo decompressive craniectomy, and we do not have information about surgical intervention for two patients. Five patients had cerebrospinal fluid (CSF) drainage with external ventricular drainage.
Mean ICP at baseline (P1) was 15 mm Hg (SD ±8 mm Hg) and increased by 25 mm Hg (SD ±14mmHg) during the transition period (P2). The third period (P3), after the upper breakpoint of ICP/AMP, was visible in 11 patients of 24. The upper breakpoint was reached at a different level of ICP. This means that the upper breakpoint identified in this population ranged from 21 mm Hg to 100 mm Hg. The overall mean value of ICP during the third period was 49 mm Hg but with a high SD of 22 mm Hg. With regard to the cerebral perfusion pressure (CPP), the lowest value was 10 mm Hg and the higher value of 74 mm Hg, with the mean value of 49 mm Hg during the third period (Table 1).
Table 1.
Summary of Mean Values ± Standard Deviation Parameters and p Value of Wilcoxon Test between Mean Values of Period 1 (Baseline) Versus Period 2 (Increasing Intracranial Pressure) and Period 2 Versus Period 3 (above Upper Breakpoint of Amplitude-Mean Intracranial Pressure Relationship)
| Variable | Period 1 | Period2 | Period3 | p P1 vs P2 | p P2 vs P3 |
|---|---|---|---|---|---|
| CPP, mm Hg | 77 ± 14 | 70 ± 14 | 46 ± 28 | < 0.05 | < 0.01 |
| ICP, mm Hg | 15 ± 8 | 25 ± 14 | 49 ± 22 | < 0.01 | < 0.01 |
| ABP mm Hg | 93 ± 13 | 95 ± 12 | 95 ± 18 | 0.06 | 0.8 |
| PRx | 0.2 ± 0.4 | 0.4 ± 0.4 | 0.8 ± 0.2 | 0.1 | < 0.01 |
| Baroindex MS/mm Hg | 9 ± 8 | 12 ± 15 | 7 ± 11 | < 0.01 | < 0.01 |
| RAP | 0.4 ± 0.3 | 0.4 ± 0.3 | 0.3 ± 0.4 | 0.18 | < 0.05 |
| AMP | 2.2 ± 1.3 | 3 ± 2 | 4 ± 4.1 | < 0.01 | 0.1 |
| HR bpm | 71 ± 23 | 66 ± 19 | 93 ± 30 | 0.2 | < 0.01 |
| HRV HF POWER MS2 | 152 ± 295 | 473 ± 1092 | 99 ± 202 | < 0.01 | < 0.05 |
| HRV LF POWER MS2 | 55 ± 95 | 163 ± 415 | 149 ± 300 | < 0.01 | < 0.05 |
| HRV ratio POWER MS2 | 1.06 ± 1.2 | 1.05 ± 1.4 | 1.6 ± 1.5 | 0.7 | 0.6 |
| HRV TOT POWER MS2 | 388 ± 611 | 1094 ± 2290 | 529 ± 932 | < 0.01 | < 0.05 |
| HRV SD | 14 ± 11 | 24 ± 18 | 15 ± 14 | < 0.01 | < 0.05 |
| HRV SDSD | 15 ± 14 | 30 ± 26 | 12 ± 12 | < .01 | < 0.01 |
CPP, cerebral perfusion pressure; ICP, intracranial pressure; ABP, mean arterial blood pressure; PRx, autoregulation index; RAP, correlation between the AMP and ICP mean; AMP, amplitude of ICP waveform; HR bpm, heart rate beat for minute; HRV HF, heart rate variability high frequency range; HRV LF, heart rate variability low frequency range; HRV ratio, ratio between low frequency and high frequency of heart rate variability; HRV TOT, total power of heart rate variability; HRV SD, standard deviation of heart rate variability; HRV SDSD, standard deviation of the difference between sequential beats.
The mean values of variables in the three different periods are illustrated in Table1. Comparison of the mean values between baseline (P1) versus transition period (P2) of increasing ICP showed that the rise in ICP is associated with a significant rise in the global index of HRV both in time and frequency domain (p < 0.001) and BRS (p < 0.001). In terms of the frequency-specific index, a significant difference was found in the HF and LF of HRV, whereas no significant difference was found in the LF/HF ratio (Fig. 1, Table 1).
FIG. 1.
Neuromonitoring showing a time trend of intracranial pressure (ICP) rising to refractory values followed by the rise of the main autonomic variables: SDSD (standard deviation of the difference between sequential beats), HRV-TOT (total PSD, power spectral density, of heart rate variability), BRSX (baroindex). The figure also shows an upper breakpoint above which amplitude of ICP (AMP) starts to decrease together with the autonomic variables, while mean ICP continues to rise. The baseline period in which the ICP is around normal values is defined as P1, transitional period in which ICP starts to increase to high value is P2, the period after the upper breakpoint of ICP amplitude (AMP) is P3.
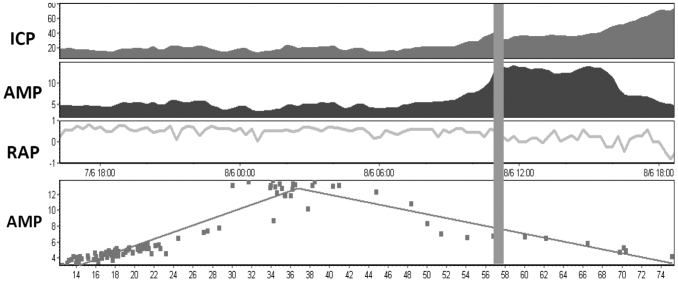
In 13 of 24 patients, an upper breakpoint of ICP AMP was identified above which ICP pulse AMP starts to decrease whereas ICP mean continues to rise (Fig. 2). The increase of autonomic variables during the rise in ICP was followed by a significant decrease of HRV and BRS after the upper breakpoint (P3) (p < 0.05 of the total power of HRV, HF, LF, SDSD) (Fig.1). The RAP index, which is the correlation between the AMP of ICP waveform and mean ICP, decreased toward zero or negative level after the upper breakpoint of ICP (Fig. 2). This seems to occur when the cerebral autoregulatory capacity is exhausted and is consistent with previous descriptions.29,38,39
FIG. 2.
Neuromonitoring showing a time trend of intracranial pressure (ICP), amplitude of ICP waveform (AMP), RAP (the correlation coefficient between amplitude and mean ICP) and scatter plot of AMP and ICP. The figure illustrates the upper breakpoint of AMP with a reduction of RAP, which goes near zero around the upper breakpoint and below zero after the upper breakpoint when AMP markedly decreases.
Moreover, in two patients, we observed an unexpected phenomenon: the upper breakpoint of ICP with a decrease in AMP of ICP and main autonomic variables was then followed by a “recovery” in AMP that started to increase again together with the ICP mean and sympathetic activity (Supplementary Fig. S1 and Supplementary Table S1). ICP and global indexes of HRV were significantly correlated during the final steep rise in ICP when it was present (20 patients) with a strong correlation between ICP and SDSD (R > 0.7) in 10 of 21 patients, moderate correlation (0.4< R < 0.7) in five of 21 patients, and weak correlation (R < 0.4) in five patients.
The Granger test was applied showing a directional connectivity from ICP and autonomic variable in the majority of patients. In 15 patients, the directionality index was directed from ICP to ANS, in five patients it was from ANS to ICP, in four the test was not significant. This directionality does not change significantly between P1 (baseline) and P2 (rise in ICP).
Discussion
Our results suggest that there is a relationship with the rise in ICP and the ANS; more precisely, this relationship seems to favor predictive causality from ICP to autonomic variables. To our knowledge, this is the first study that explores the causality in this area. Our results showed that the rise in ICP is associated with an increase in HRV (both in time and frequency domain) and in the BRS with the most significant rise involving the HF range of HRV, which represents the parasympathetic branch of ANS. This relationship has been previously confirmed by Sykora and associates,12 who have shown a positive correlation between ICP and the HF of HRV. Our results are also consistent with the study of Tymko and colleagues,23 which also demonstrated an increase in the global index of HRV and BRS during high ICP episodes of plateau waves.
On the other hand, an increase in the LF/HF ratio after the infusion of saline into the cerebrospinal system was found in the experimental studies of Guild and coworkers22 and Schmidt and colleagues.21 It was speculated by these authors that ICP might be a determinant of sympathetic output as a novel intracranial baroreflex. Other findings suggest a sympathetic control of CSF formation in experimental hydrocephalus.40
Based on this previous evidence, it is highly likely that there are sensitive intracranial receptors that can respond to reduced CBF and/or a rise in ICP activating ANS.41 In rat cerebral arteries, mitochondria-rich nerve varicosities were interpreted as sensory in nature. These nerve terminal varicosities have been postulated to represent nerve specializations for pressure or tension reception based on the structural analogy they share with sensory or baroreceptor nerve terminals.42–44 If these findings were to be reported also in humans, in terms of nature and functionality, we would have the anatomical explanation of the starting point of a “brain driven response” likely involving ANS.
In addition, our results reinforce this concept underlying that temporality between ICP and ANS might suggest a causal relationship from ICP to ANS. We cannot exclude, however, the presence of a third unmeasured or untested cause of change in both ICP and ANS; therefore, we did not measure true causality with the Granger test.
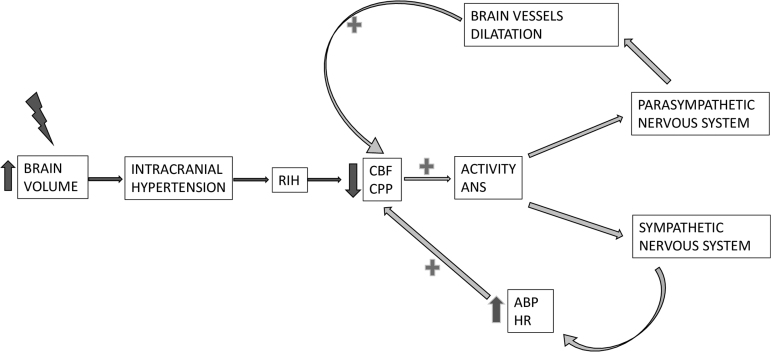
The HF of HRV was raised during the development of RIH; we speculate that the parasympathetic system might be triggered by the increase of ICP, or decrease in CPP, via stimulation of sensory nerves of the cerebrovascular system.42 It is well known that parasympathetic fibers innervate cerebral blood vessels exerting a vasodilatory action via nerves coming from the sphenopalatine and optic ganglia.43 The rise in parasympathetic activity might, therefore, attempt to produce vasodilatation as an attempt to preserve the CBF in the context of CBF deterioration. This vasodilatation produces an increase in arterial brain blood volume and consequently a further increase in ICP (Fig. 3).
FIG. 3.
Potential mechanisms involving the autonomic nervous system that might attempt to restore homeostasis in the context of cerebral blood flow (CBF) and cerebral perfusion pressure (CPP) derangement. A decrease in CPP and/or CBF might activate the two branches of the autonomic nervous system (ANS). The parasympathetic system causes brain vessel vasodilatation focused on maintaining brain CBF. At the same time, the sympathetic system increases arterial blood pressure (ABP) and therefore CPP. These assumptions could be considered in the context of not deranged ANS. HR, heart rate.
Moreover, the baroreflex was observed to rise together with the development of RIH and HRV HF, which suggests an intact baroreflex loop.45 The rise in both BRS and HRV HF is consistent because vagal activity has been shown to play a major role in BRS.13 It is also well known that the relative stability of CBF despite fluctuations in blood pressure is maintained by two regulatory mechanisms: the baroreflex and cerebral autoregulation (CA).13
Potential interactions between CA and ANS need further investigations. Baroreflex controls blood pressure in the short term by the extent of the stretch of receptors in the walls of carotid arteries and of the aorta; it discharges differently to the central nervous system. Changes in baroreflex discharge trigger modulation of the HR, cardiac contractility, and vascular tone and venous return through the modulation of the parasympathetic and sympathetic nervous system.46,47
Our results suggest the attempt of the autonomic system—specifically, the parasympathetic system and the baroreflex—to maintain a constant CBF in response to a CBF impairment. In terms of the potential “protective effect” of the parasympathetic nervous system, activation via vagal nerve stimulation has been proposed as a strategy to reduce the adverse effects of TBI-induced sympathetic hyperactivity.16 Lopez and associates48 speculated that stimulating the parasympathetic response may help alleviate the adverse effects on the blood–brain barrier that occur with hypersympathetic autonomic dysfunction by decreasing its disruption. Some other studies suggest that vagal nerve stimulation attenuates post-TBI intestinal permeability and intestinal dysfunction after ABI.49
The assumption that HF of HRV mirrors the parasympathetic branch whereas LF/HF ratio represents the sympathetic branch must be made with caution given the complex non-linear interactions between the sympathetic and parasympathetic systems. It is likely that brain injury alters the fine balance between the sympathetic and parasympathetic arms of the autonomic nervous system, resulting in an imbalance of the homeostatic mechanisms that maintain normal organ system function and their interactions with each other.16
Another important finding of our study was that the increase in autonomic variable and BRS during ICP rise was followed by a significant decrease after the upper breakpoint of ICP. An upper breakpoint above which ICP pulse AMP starts to decrease whereas ICP mean continues to rise has been observed both experimentally50 and clinically.29 It has been speculated that this phenomenon is related to the terminal closing of the cerebral arterial bed when the critical closing pressure approaches ABP.6
Another hypothesis is that it is strongly related to the state of the cerebrovascular system and point of autoregulation exhaustion at low perfusion pressure.51,52 If we assume these concepts are true, the sudden derangement of autonomic functionality after the upper breakpoint might be attributed to the cerebrovascular ischemic damage of the central autonomic network. This can occur at a different level of ICP/CPP depending on the hemodynamic response to acute ICH in different areas. The large variability of ICP breakpoint value in our subjects implies large compliance differences before herniation occurs in the traumatized brain.
Although a great extent of the literature53–56 has been focused on CPP only as the product rather than the driver of blood pressure dynamics, substantial experiments have been conducted demonstrating the paramount concept about the brain task to protect its own flow first and foremost. This is called “selfish brain theory.” According to Prof. Cushing and other more recent authors, the selfish brain theory supports the idea that ICP can influence ABP by influencing the autonomic system.57,22
Donnelly and colleagues6 described the cerebral hemodynamic in rabbits during artificial CSF infusion showing a response that, at a lower level of ICP, tries to maintain CBF reducing wall tension while at a higher level of ICP increases ABP. In the study of Schmidt and coworkers,21 conducted in both animals and humans, ICP is described by the authors as a reversible determinant of efferent sympathetic outflow even at relatively low ICP levels.21 Rosner and Becker58 demonstrate in laboratory observations in cats a gradual and sustained increased in ABP directed to restore CPP likely driven by sympathetic activity (Fig. 3).
It can be supposed that we could not see any significant increase in sympathetic activity in the majority of the patients assessed in our study given the small number of patients and a possible condition of sympathetic system derangement.58 Therefore, our observations, supported by the Granger results, might suggest the presence of sophisticated mechanisms that underpin the concept of a “brain driven” rescue mechanism involving both parasympathetic and sympathetic system playing a crucial role attempting to increase the CBF.
It still remains to be clarified whether what we call “the Cushing response” is an acute and terminal pathological reflex to brain ischemia or part of this fine mechanism for ABP regulation, capable of sensing and integrating information possibly involving not only the sympathetic branches.59 Transduction mechanisms are clearly not fully resolved but may include astrocytic–neuronal, as well as vascular–neuronal and vascular–astrocytic–neuronal signalling pathways, involving mediators such as ATP lactate, nitric oxide, shear stress, and stretch-activated cation channels.60 Recent findings suggest the astrocytes could be potentially classified as baroreceptors responding to change in ICP or CPP.61
Our study suggests that further investigation needs to be performed also to better assess the relationship and the directionality between ICP, autonomic systems, and therefore ABP and CBF; also to assess the behavior of autoregulation in this peculiar setting and interpret the autoregulation indexes that have been used during the last decades.
Limitations
Our data are retrospective, with a small patient number, recorded in three different hospitals, having obvious differences in management protocols. Even if all the patients were treated according to the Brain Trauma Foundation Guideline, 4th edition,62 the physiology described here does not represent RIH in its purest form, because each patient was subjected to various treatment that could have influenced ICP, CPP, and ECG data recorded, therefore precluding any reliable analysis on CA. Physiological data could be subject to clinical noise such as sedation, drugs, mechanical ventilation, temperature, and changes in body position. Moreover, the small number of patients did not enable us to provide reliable statistical analysis in terms of autonomic behaviors in different clinical subsets, such as treatments or physiopathological brain injury features or etiology. Despite these potential confounders, however, we were able to reconfirm findings coming from previous literature.
Concerning the two patients with the recovery of ICP AMP and rise in LF/HF ratio: It could be hypothesized theoretically that noradrenaline may have been administered to raise the ABP and the CPP after the rise in ICP, which can influence sympathetic nervous system activity and then LF/HF ratio. To explore this hypothesis, we analyzed the same method in a patient in whom ICP was stable, but noradrenaline was doubled in a couple of minutes for clinical reasons. It is shown that there is no difference in terms of LF/HF ratio change (Supplementary Fig. S2). These are, however, single observations. Dedicated studies are needed to confirm these descriptive results.
Conclusion
Rises in ICP are associated with changes in autonomic activity: increase in HRV and BRS. This association takes place mainly through the interaction between ICP and the parasympathetic system, which possibly attempts to restore deteriorating CBF. This happens until the upper breakpoint of the AMP-pressure relationship is reached, after which the autonomic system variables collapse possibly because of low brain perfusion of the central autonomic network. Further, temporality between ICP and ANS might suggest a causal relationship from ICP to ANS.
The presence of sophisticated mechanisms that underpin the concept of a brain driven rescue process involving the ANS needs further investigation in a large multi-centric prospective study.
Supplementary Material
Funding Information
F.A. Zeiler receives research support from the United States National Institutes of Health (NIH) through the National Institute of Neurological Disorders and Stroke (NINDS), the Canadian Institutes for Health Research (CIHR), the Canadian Foundation for Innovation (CFI), the University of Manitoba Centre on Aging, the University of Manitoba VPRI Research Investment Fund (RIF), the University of Manitoba Rudy Falk Clinician-Scientist Professorship, and the Health Sciences Centre Foundation Winnipeg. Soojin Park's research program is supported by NIH K01ES026833.
Author Disclosure Statement
Peter Smielewski and Marek Czosnyka receive part of the licensing fees for multi-modal brain monitoring software ICM+, licensed by Cambridge Enterprise Ltd, University of Cambridge, UK. For the remaining authors, no competing financial interests exist.
Supplementary Material
References
- 1. Andrews, P., and Citerio, G. (2004). Intracranial pressure. Part one: Hystorical overview and basic concept. Intensive Care Med. 9, 1730–1733 [DOI] [PubMed] [Google Scholar]
- 2. Kellie, G. (1824). An account of the appearances observed in the dissection of two of three individuals presumed to have perished in the storm of the 3rd and whose bodies were discovered in the vicinity of Leith on the morning of 4th November 1821; with some reflections on the pathology of the brain. Trans. Med. Chir. Soc. Edinb. 1, 84–122 [PMC free article] [PubMed] [Google Scholar]
- 3. Monro, A. (1783). Observation on the features and functions of the nervous system, illustrated with tables. Lond. Med. J. 4. 113–135 [Google Scholar]
- 4. Czosnyka, M., Smielewski, P., Piechnik, S., Schmidt, E.A., Al-Rawi, P.G., Kirkpatrick, P.J., and Pickard, J.D. (1999). Hemodynamic characterization of intracranial pressure plateau waves in head-injured patients. J. Neurosurg. 91, 11–19 [DOI] [PubMed] [Google Scholar]
- 5. Lemaire, J.J., Khalil, T., Cervenansky, F., Gindre, G., Boire, J.Y., Bazin, J.E., Irthum, B., and Chazal, J. (2002). Slow pressure waves in the cranial enclosure. Acta Neurochir. (Wien). 144, 243–254 [DOI] [PubMed] [Google Scholar]
- 6. Donnelly, J., Czosnyka, M., Harland, S., Varsos, G.V., Cardim, D., Robba, C., Liu, X., Ainslie, P.N., and Smielewski, P. (2017). Cerebral haemodynamics during experimental intracranial hypertension. J. Cereb. Blood Flow Metab. 37, 694–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stocchetti, N., and Maas, A.I. (2014). Traumatic intracranial hypertension. N. Engl. J. Med. 371, 972. [DOI] [PubMed] [Google Scholar]
- 8. Salih, F., Finger, T., Vajkoczy, P., and Wolf, S. (2017). Brain death after decompressive craniectomy: incidence and pathophysiological mechanisms. J. Crit. Care 39, 205–208 [DOI] [PubMed] [Google Scholar]
- 9. Salih, F., Holtkamp, M., Brandt, S.A., Hoffmann, O., Masuhr, F., Schreiber, S., Weissinger, F., Vajkoczy, P., and Wolf, S. (2016). Intracranial pressure and cerebral perfusion pressure in patients developing brain death. J. Crit. Care 34, 1–6 [DOI] [PubMed] [Google Scholar]
- 10. Nagao, S., Sunami, N., Tsutsui, T., Honma, Y., Momma, F., Nishiura, T., and Nishimoto, A. (1984). Acute intracranial hypertension and brain-stem blood flow. An experimental study. J. Neurosurg. 60, 566–571 [DOI] [PubMed] [Google Scholar]
- 11. Nakagawa, Y., Tsuru, M., and Yada, K. (1974). Site and mechanism for compression of the venous system during experimental intracranial hypertension. J. Neurosurg. 41, 427–434 [DOI] [PubMed] [Google Scholar]
- 12. Sykora, M., Czosnyka, M., Liu, X., Donnelly, J., Nasr, N., Diedler, J., Okoroafor, F., Hutchinson, P., Menon, D., and Smielewski, P. (2016). Autonomic impairment in severe traumatic brain injury. A multimodal neuromonitoring study. Crit. Care Med. 44, 1173–1181 [DOI] [PubMed] [Google Scholar]
- 13. Nasr, N., Gaio, R., Czosnyka, M., Budohoski, K., Liu, X., Donnelly, J., Sykora, M., Kirkpatrick, P., Pavy-Le Traon, A., Haubrich, C., Larrue, V., and Smielewski, P. (2018). Baroreflex impairment after subarachnoid hemorrhage is associated with unfavorable outcome. Stroke 49, 1632–1638 [DOI] [PubMed] [Google Scholar]
- 14. Szabo, J., Smielewski, P., Czosnyka, M., Jakubicek, S., Krebs, S., Siarnik, P., and Sykora, M. (2018). Heart rate variability is associated with outcome in spontaneous intracerebral hemorrhage. J. Crit. Care 48, 85–89 [DOI] [PubMed] [Google Scholar]
- 15. Colivicchi, F., Bassi, A., Santini, M., and Caltagirone, C. (2005). Prognostic implications of right-sided insular damage, cardiac autonomic derangement, and arrhythmias after acute ischemic stroke. Stroke 36, 1710–1715 [DOI] [PubMed] [Google Scholar]
- 16. Callaway, C.C.M., and Kosofsky, B.E. (2019). Autonomic dysfunction following traumatic brain injury: transational insight. Curr. Opin. Neurol. 6, 802–807 [DOI] [PubMed] [Google Scholar]
- 17. Megjhani, M., Kaffashi, F., Terilli, K., Alkhachroum, A., Esmaeili, B., Doyle, K.W., Murthy, S., Velazquez, A.G., Connolly, Jr, E.S., Roh, D.J., Agarwal, S., Loparo, K.A., Claassen, J., Boehme, A., and Park, S. (2020). Heart rate variability as a biomarker of neurocardiogenic injury after subarachnoid hemorrhage. Neurocrit. Care 32, 162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Camm, A.J., Bigger, J.T., Breithardt, G., Cerutti, S., Cohen, R.J., Coumel, P., Fallen, E.L., Kennedy, H.L., Kleiger, R.E., Lombardi, F., Malliani, A., Moss, A.J., Rottman, J.N., Schmidt, G., Schwartz, P.J., and Singer, D.H. (1996). Heart rate variability. Eur. Heart J. 17, 354–3818737210 [Google Scholar]
- 19. Leipzig, T.J., Lowensohn, R.I. (1986). Heart rate variability in neurosurgical patients. Neurosurgery 19, 356–362 [DOI] [PubMed] [Google Scholar]
- 20. Lowenshon, R.I., Weiss, M., and Hon, E.H. (1977). Heart rate variability in brain injured adults. Lancet 309, 626–628 [DOI] [PubMed] [Google Scholar]
- 21. Schmidt, E.A., Despas, J.M., Pavy-Le Traon, A., Czosnyka, Z., Pickard, J.D., Rahmouni, K., Pathak, A.F., and Senard, J.M. (2018). Intracranial pressure is a determinant of sympathetic activity. Front. Physiol. 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guild, S.J., Saxena, U.A., McBryde, F.D., Malpas, S.C., and Ramchandra, R. (2018). Intracranial pressure influences the level of sympathetic tone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R1049–R1053 [DOI] [PubMed] [Google Scholar]
- 23. Tymko, M.M., Donnelly, J., Smielewski, P., Zeiler, F.A., Sykora, M., Haubrich, C., Nasr, N., and Czosnyka, M. (2019). Changes in cardiac autonomic activity during intracranial pressure plateau waves in patients with traumatic brain injury. Clin. Auton. Res. 29, 123–126 [DOI] [PubMed] [Google Scholar]
- 24. Winchell, R.J., and Hoyt, D.B. (1997). Analysis of heart-rate variability: a noninvasive predictor of death and poor outcome in patients with severe head injury. J. Trauma 43, 927–933 [DOI] [PubMed] [Google Scholar]
- 25. Haji-Michael, P., Vincent, J., Degaute, J., and Van De Borne, P. (2000). Power spectral analysis of cardiovascular variability in critically ill neurosurgical patients. Crit. Care Med. 28, 2578–2583 [DOI] [PubMed] [Google Scholar]
- 26. Mowery, N.T., Norris, P.R., Riordan, W., Jenkins, J.M., Williams, A.E., and Morris, J.A. (2008). Cardiac uncoupling and heart rate variability are associated with intracranial hypertension and mortality: A study of 145 trauma patients with continuous monitoring. J. Trauma 65, 621–627 [DOI] [PubMed] [Google Scholar]
- 27. Biswas, A.K., Scott, W.A., Sommerauer, J.F., and Luckett, P.M. (2000). Heart rate variability after acute head injury in children. Crit. Care Med. 28, 3907–3912 [DOI] [PubMed] [Google Scholar]
- 28. Czosnyka, M., Smielewski, P., Kirkpatrick, P., Laing, R.J., Menon, D., and Pickard, J.D. (1997). Continuous assessment of the cerebral vasomotor reactivity index. Neurosurgery 41, 11–17 [DOI] [PubMed] [Google Scholar]
- 29. Czosnyka, M., Price, D.J., and Williamson, M. (1994). Monitoring of cerebrospinal dynamics using continuous analysis of intracranial pressure and cerebral perfusion pressure in head injury. Acta Neurochir. (Wien). 126, 113–119 [DOI] [PubMed] [Google Scholar]
- 30. Donnelly, J., Smielewski, P., Adams, H., Zeiler, F.A., Cardim, D., Liu, X., Fedriga, M., Hutchinson, P., Menon, D.K., and Czosnyka, M. (2020). Observations on the cerebral effects of refractory intracranial hypertension after severe traumatic brain injury. Neurocrit. Care 32, 437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malliani, A., Lombardi, F., and Pagani, M. (1994). Power spectrum analysis of heart rate variability : a tool to explore neural regulatory mechanisms (editorial). Br. Heart J. 71, 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karemaker, J.M. (2017). An introduction into autonomic nervous function. Physiol. Meas. 38, R89–R118 [DOI] [PubMed] [Google Scholar]
- 33. Draghici, A.E., and Taylor, J.A. (2016). The physiological basis and measurement of heart rate variability in humans. J. Physiol. Anthropol. 35, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Westerhof, B.E., Gisolf, J., Stok, W.J., Wesseling, K.H., and Karemaker, J.M. (2004). Time-domain cross-correlation baroreflex sensitivity: performance on the EUROBAVAR data. J. Hypertens. 22, 1371–1380 [DOI] [PubMed] [Google Scholar]
- 35. Shapiro, S., and Wilk, M.B. (1972). An analysis of variance test for normality (complete samples). Biometrika 52, 591–611 [Google Scholar]
- 36. Granger, C.W. (1969). title: Investigating causal relations by econometric models and cross-spectral methods. Econometrica 37, 424–438 [Google Scholar]
- 37. Cekic, S., Grandjean, D., and Renaud, O. (2018). Time, frequency, and time-varying Granger-causality measures in neuroscience. Stat. Med. 37, 1910–1931 [DOI] [PubMed] [Google Scholar]
- 38. Czosnyka, M., Guazzo, E., Whitehouse, M., Smielewski, P., Czosnyka, Z., Kirkpatrick, P., Piechnik, S., and Pickard, J.D. (1996). Significance of intracranial pressure waveform analysis after head injury. Acta Neurochir. (Wien). 138, 531–541 [DOI] [PubMed] [Google Scholar]
- 39. Balestreri, M., Czosnyka, M., Steiner, L.A., Schmidt, E., Smielewski, P., Matta, B., Pickard, J.D., Robertson, C.S., Dunn, L.T., and Chambers, I.R. (2004). Intracranial hypertension: What additional information can be derived from ICP waveform after head injury? Acta Neurochir. (Wien). 146, 131–141 [DOI] [PubMed] [Google Scholar]
- 40. Lindvall, M., and Owman, C. (1984). Sympathetic nervous control of cerebrospinal fluid production in experimental obstructive hydrocephalus. Exp. Neurol. 84, 606–615 [DOI] [PubMed] [Google Scholar]
- 41. Hoff, J.T., and Reis, D.J. (1970). Localization of regions mediating the Cushing response in CNS of cat. Arch. Neurol. 23, 228–240 [DOI] [PubMed] [Google Scholar]
- 42. Uddman, R. (1993). Vascular Innervation and Receptor Mechanisms: New Perspectives. Academic Press, Cambridge, MA [Google Scholar]
- 43. Gulbenkian, S., Uddman, R., and Edvinsson, L. (2001). Neuronal messengers in the human cerebral circulation. Peptides 22, 995–1007 [DOI] [PubMed] [Google Scholar]
- 44. Edvinsson, L., MacKenzie, E.T., and McCulloch, J. (1993). Cerebral Blood Flow and Metabolism. Raven Press: New York. J [Google Scholar]
- 45. Papaioannou, V., Giannakou, M., Maglaveras, N., Sofianos, E., and Giala, M. (2008). Investigation of heart rate and blood pressure variability, baroreflex sensitivity, and approximate entropy in acute brain injury patients. J. Crit. Care 23, 380–386 [DOI] [PubMed] [Google Scholar]
- 46. Benarroch, E.E. (2008). The arterial baroreflex: functional organization and involvement in neurologic disease. Neurology 71, 1733–1738 [DOI] [PubMed] [Google Scholar]
- 47. Lanfranchi, P.A., and Somers, V.K. (2002). Arterial baroreflex function and cardiovascular variability: interaction and implications. Am. J. Physiol. Regul. Comp. Physiol. 283, R815–R826 [DOI] [PubMed] [Google Scholar]
- 48. Lopez, N.E., Krzyzaniak, M.J., Costantini, T.W., Putnam, J., Hageny, A.M., Eliceiri, B., Coimbra, R., and Bansal, V. (2012). Vagal nerve stimulation decreases blood–brain barrier disruption after traumatic brain injury. J. Trauma Acute Care Surg. 72, 1562–1566 [DOI] [PubMed] [Google Scholar]
- 49. Bansal, V., Costantini, T., Ryu, S.Y., Peterson, C., Loomis, W., Putnam, J., Elicieri, B., Baird, A., and Coimbra, R. (2010). Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J. Trauma 68, 1059–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Avezaat, C.J., van Eijndhoven, J.H., and Wyper, D.J. (1979). Cerebrospinal fluid pulse pressure and intracranial volume-pressure relationships. J. Neurol. Neurosurg. Psychiatry 42, 687–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Czosnyka, M., Guazzo, E., Kirkpatrick, P., Smielewski, P., Whitehouse, H., and Pickard, J.D. (1994). Testing of cerebral autoregulation in head injury by waveform analysis of blood flow velocity and cerebral perfusion pressure. Acta Neurochir. Suppl. (Wien) 60, 468–471 [DOI] [PubMed] [Google Scholar]
- 52. Gray, W.J., and Rosner, M.J. (1987). Pressure-volume index as a function of cerebral perfusion pressure. J. Neurosurg. 67, 377–380 [DOI] [PubMed] [Google Scholar]
- 53. Czosnyka, M., Smielewski, P., Kirkpatrick, P., Menon, D.K., and Pickard, J.D. (1996). Monitoring of cerebral autoregulation in head-injured patients. Stroke 27, 1829–1834 [DOI] [PubMed] [Google Scholar]
- 54. Tzeng, Y.C., Ainslie, P.N., Cooke, W.H., Peebles, K.C., Willie, C.K., MacRae, B.A., Smirl, J.D., Horsman, H.M., and Rickards, C.A. (2012). Assessment of cerebral autoregulation: the quandary of quantification. Am. J. Physiol. Heart Circ. Physiol. 303, H658–H671 [DOI] [PubMed] [Google Scholar]
- 55. Czosnyka, M., Smielewski, P., Kirkpatrick, P., Laing, R.J., Menon, D., and Pickard, J.D. (1997). Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41, 11–17 [DOI] [PubMed] [Google Scholar]
- 56. Donnelly, J., Aries, M.J., and Czosnyka, M. (2015). Further understanding of cerebral autoregulation at the bedside: possible implications for future therapy. Expert Rev. Neurother. 15, 169–185 [DOI] [PubMed] [Google Scholar]
- 57. Saleem, S., Teal, P.D., Howe, C.A., Tymko, X.M.M., Ainslie, P.N., and Tzeng, Y. (2018). Is the Cushing mechanism a dynamic blood pressure-stabilizing system? Insights from Granger causality analysis of spontaneous blood pressure and cerebral blood flow. Am. J. Physiol. Regul Integr. Comp. Physiol. 315, R484–R495 [DOI] [PubMed] [Google Scholar]
- 58. Rosner, M.J., and Becker, D.P. (1984). Origin and evolution of plateau waves. Experimental observations and a Theoretical model. J. Neurosurg. 60, 312–324 [DOI] [PubMed] [Google Scholar]
- 59. Paton, J.F., Dickinson, C.J., and Mitchell, G. (2009). Harvey Cushing and the regulation of blood pressure in giraffe, rat and man : introducing ‘Cushing's mechanism.’ Exp. Physiol. 94, 11–17 [DOI] [PubMed] [Google Scholar]
- 60. McBryde, F.D., Malpas, S.C., and Paton, J.F. (2017). Intracranial mechanisms for preserving brain blood flow in health and disease. Acta Physiol. 219, 274–287 [DOI] [PubMed] [Google Scholar]
- 61. Marina, N., Christie, I.N., Korsak, A., Doronin, M., Brazhe, A., Hosford, P.S., Wells, J.A., Sheikhbahaei, S., Humoud, I., Paton, J.F., Lythgoe, M.F., Semyanov, A., Kasparov, S., and Gourine, A.V. (2020). Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat. Commun. 11, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carney, N., Totten, A.M., O'Reilly, C., Ullman, J.S., Hawryluk, G.W., Bell, M.J., Bratton, S.L., Chesnut, R., Harris, O.A., Kissoon, N., Rubiano, A.M., Shutter, L., Tasker, R.C., Vavilala, M.S., Wilberger, J., Wright, D.W., and Ghajar, J. (2017). Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80, 6–15 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.