Abstract
Background
The impact of therapeutic drug management (TDM) on reducing toxicity and improving efficacy in colorectal cancer (CRC) patients receiving fluorouracil-based chemotherapy is still unclear.
Material/Methods
A total of 207 patients (Study Group n=54, Historical Group n=153) with metastatic colorectal cancer were enrolled. All of them received 6 administrations of the 5-FU based regimens. Initial 5-FU dosing of all patients was calculated using body surface area (BSA). In the Study Group, individual exposure during each cycle was measured using a nanoparticle immunoassay, and the 5-FU blood concentration was calculated using the area under the curve (AUC). We adjusted the 5-FU infusion dose of the next cycle based on the AUC data of the previous cycle to achieve the target of 20–30 mg×h/L.
Results
In the fourth cycle, patients in the target concentration range (AUC mean, 26.3 mg×h/L; Median, 28 mg×h/L; Range, 14–38 mg×h/L; CV, 22.4%) accounted for 46.8% of all patients, which were more than the ones in the first cycle (P<0.001). 5-FU TDM significantly reduced the toxicity of chemotherapy and improved its efficacy. The Study Group (30/289) showed a lower percentage of severe adverse events than that in the Historical Group (185/447) (P<0.001). The incidences of complete response and partial response in the Study Group were higher than those in the Historical Group (P=0.032).
Conclusions
TDM in colorectal cancer can reduce toxicity, improve efficacy and clinical outcome, and can be routinely used in 5-FU-based chemotherapy.
Keywords: Area Under Curve, Colorectal Neoplasms, Drug Monitoring, Fluorouracil
Background
5-Fluorouracil (5-FU) is widely applied in the treatment of various cancers, including colorectal cancer (CRC) [1,2]. Colorectal cancer is the fifth most prevalent cancer in China, after lung, gastric, esophageal, and liver cancers [2]. FOLFOX/FOLFIRI, consisting of fluorouracil, oxaliplatin, and irinotecan, combined with bevacizumab, is a first-line anti-tumor treatment for advanced colorectal cancer [3,4]. Due to the general condition of the patient and other factors, including adverse drug reactions and patient compliance, a safe and efficacious dose of chemotherapy drugs cannot always be calculated according to the patient’s body surface area (BSA), often resulting in empirical dosing reduction [5,6]. Many studies have found that BSA-based drugs administration can cause pharmacokinetic variations, resulting in increased suboptimal dosing of patients [7,8].
In other countries, monitoring of fluorouracil plasma concentration has resulted in improved individual fluorouracil dosing. Clinical studies show that therapeutic drug management (TDM) improves drug efficacy and reduces toxic adverse effects [9,10]. Gamelin et al [11–16] found that when the target area under the curve (AUC) of fluorouracil is in the range of 20–30 mg×h/L, the toxicity and adverse effects of 5-FU are relatively minor and the anti-tumor efficacy is highest. Preferably, when the AUC is lower than the target range, the subsequent dosage of 5-FU should be increased, and, vice versa, when it is higher, the dosage of 5-FU can be decreased.
Kline et al [10] showed that patients with stage II/III cancer who underwent adjuvant therapy had significantly improved disease-free survival and significantly fewer adverse events after TDM. In addition, for patients treated with FOLFOX6 (leucovorin, 5-FU, and oxaliplatin), 5-FU TDM not only reduced the incidence of serious toxic events (grade 3–4), but also increased the objective response rate by 52% and the median overall survival by 6 months [17].
Patients received fluorouracil blood concentration monitoring since this technology was implemented in our hospital. To evaluate the effect of TDM practice, we compared the fluorouracil plasma concentration monitoring results, rate of serious adverse reactions, and anti-tumor efficacy between patients who received TDM (the Study Group) and patients who did not receive TDM (the Historical Group). This retrospective provides evidence that may support the design of randomized controlled trials and the use of TDM in routine clinical practice.
Material and Methods
Chemotherapy and Patients
From March 2016 to August 2018, advanced CRC patients who received chemotherapy containing 5-FU (Jinyao Pharma, Tianjin, China) at Affiliated Hospital of Nantong University were enrolled in the study (Study Group, n=54). Baseline information, including sex, age, BSA, combined disease, and chemotherapy regimen and dosage, were collected. Inclusion criteria were as follows: treatment with mFOLFOX6, FOLFOX4, or FOLFIRI; at least 6 cycles of anti-tumor therapy; at least 5 fluorouracil blood concentrations obtained during hospitalization; and signed informed consent from either the patients or their legal representatives. The main exclusion criteria included incomplete data in medical records, treatment discontinuation other than for serious adverse reactions or complications, and a significant lack of compliance with the treatment regimen. The characteristics of the patients in the Study Group are shown in Table 1. The study was approved by the Ethics Committee of the Affiliated Hospital of Nantong University. All patients provided sign informed consents before the study began.
Table 1.
Patients’ characteristics and treatment regimens in the Study Group.
| Variable | FOLFOX4 (n=14) | mFOLFOX6 (n=26) | FOLFIRI (n=14) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 7 (50.0%) | 11 (42.3%) | 8 (57.1%) |
| Female | 7 (50.0%) | 15 (57.7%) | 6 (42.9%) |
| Age (years) | |||
| Median | 65 | 70 | 67 |
| Range | 57–84 | 62–87 | 55–86 |
| Weight (kg) | |||
| Mean | 68.5 | 71.9 | 69.7 |
| SD | 15.3 | 14.5 | 16.6 |
| Height (cm) | |||
| Mean | 167.8 | 169.4 | 165.5 |
| SD | 8.4 | 8.2 | 8.9 |
| Baseline BSA (m2) | |||
| Mean | 1.83 | 1.86 | 1.82 |
| SD | 0.20 | 0.18 | 0.19 |
| Pretreatment ECOG status | |||
| 0 | 7 | 15 | 8 |
| 1 | 5 | 8 | 5 |
| 2 | 2 | 3 | 1 |
| Starting infusional 5-FU dose (mg/m2) | |||
| Average | 2350 | 2330 | 2520 |
| SD | 158 | 170 | 163 |
| Line of treatment, n (%) | |||
| First-line | 9 | 16 | 7 |
| Second-line | 3 | 6 | 5 |
| Third-line or more | 2 | 4 | 2 |
SD – standard deviation; FOLFOX – oxaliplatin, folinate, 5-FU; FOLFIRI – irinotecan, folinate, 5-FU.
Colorectal cancer patients who had received chemotherapy (Historical Group, n=153) without drug concentration monitoring were enrolled retrospectively from 2015 to 2018 at the Affiliated Hospital of Nantong University. Inclusion and exclusion criteria were similar to those of the Study Group, except that the fluorouracil blood drug concentration was not monitored in these patients. The characteristics of the patients in the Historical Group are shown in Table 2. A flow chart of excluded patients is showed as Supplementary Figure 1.
Table 2.
Comparison of patient between the Study Group and the Historical Group.
| Variable | Historical Group (n=153) | Study Group (n=54) | P value |
|---|---|---|---|
| Sex, n (%) | 0.307 | ||
| Male | 86 (56.2) | 26 (48.1) | |
| Female | 67 (43.8) | 28 (51.9) | |
| Age (years) | 0.423 | ||
| Median | 66 | 68 | |
| Range | 51–90 | 55–87 | |
| Weight (kg) | 0.204 | ||
| Mean | 68.5 | 70.4 | |
| SD | 17.2 | 15.6 | |
| Height (cm) | 0.363 | ||
| Mean | 166.7 | 168.0 | |
| SD | 9.7 | 8.5 | |
| Baseline BSA (m2) | 0.427 | ||
| Average | 1.82 | 1.84 | |
| SD | 0.21 | 0.19 | |
| Pretreatment ECOG status | 0.624 | ||
| 0 | 75 | 30 | |
| 1 | 45 | 18 | |
| 2 | 24 | 6 | |
| Starting infusional 5-FU dose (mg/m2) | 0.125 | ||
| Average | 2453 | 2384 | |
| SD | 182 | 164 | |
| Protocol of chemotherapy, n | 0.835 | ||
| FOLFOX4 | 39 (4) | 14 | |
| mFOLFOX6 | 68 () | 26 | |
| FOLFIRI | 46 () | 14 | |
| Treatment cycles | 0.999 | ||
| 1 | 87 | 54 | |
| 2 | 85 | 54 | |
| 3 | 78 | 50 | |
| 4 | 70 | 47 | |
| 5 | 65 | 44 | |
| 6 | 62 | 40 | |
| Total | 447 | 289 | |
| Adverse events | <0.001* | ||
| No toxicity or mild toxicity | 262 | 259 | |
| Severe toxicity | 185 | 30 | |
| Chemotherapy response | 0.032* | ||
| CR+PR | 82 | 38 | |
| SD+PD | 71 | 16 |
P<0.05 represents statistical difference.
SD – standard deviation; FOLFOX – oxaliplatin, folinate, 5-FU; FOLFIRI – irinotecan, folinate, 5-FU.
Concentration Detection and AUC Calculation
All patients started receiving fluorouracil treatment uniformly at 15: 00–16: 00. We collected 5 mL of blood into a polyethylene tube pretreated with gimeracil, collected on the third day of continuous intravenous infusion at 8: 00–9: 00. Another blood sample was drawn 4 h before the end of the infusion. The plasma was obtained after centrifugation and stored frozen at −80°C for subsequent experiments.
The fluorouracil plasma concentrations were determined by a nanoparticle immunoassay (My 5-Fu; Saladax Biomedical, Bethlehem PA). Then, the AUC calculation of fluorouracil and the adjustment of fluorouracil doses for the next cycle were performed as reported previously [13–15].
Adverse Reactions and Efficacy Evaluation
The CTCAE 4.03 criteria were used to evaluate the overall serious adverse reactions to fluorouracil (including neutropenia, anemia, thrombocytopenia, diarrhea, oral mucositis, hand-foot syndrome).
The efficacy evaluation was performed according to the 1.1 version of Response Evaluation Criteria in Solid Tumors (RECIST), which was divided into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
Dose of Fluorouracil and Dose Adjustment for Fluorouracil
The initial dose of fluorouracil in the mFOLFOX6 and FOLFIRI regimens was: 400 mg/m2 for 4 days, intravenously (i.v.), and then 2400 mg/m2 for 1 day, for a continuous intravenous infusion for 44 to 48 h. FOLFOX4: on days 1–2, 400 mg/m2 per day, intravenous injection, and then another 600 mg/m2 per day, continuous intravenous infusion, for 22 h. Every 14 days is a treatment cycle.
All these dose determinations of fluorouracil in subsequent cycles were done independently by 2 experienced oncologists who received assistance from clinical pharmacists, referring to calculated indicators (DI, TI and RDI) combined with the fluorouracil concentration of blood concentration monitoring and adverse reactions after the previous treatment cycle. Briefly, according to the drug AUC of the previous cycle, the dose in the subsequent cycle was adjusted to the target range (20–30 mg×h/L, as reported by Kaldate and Denda et al) [13–15]. If neutropenia and/or grade 3–4 hematological and/or non-hematological toxicity occur (except inadequately treated nausea and vomiting or alopecia), drug therapy should be suspended until the toxic adverse effects return to NCCICTAE level 1. If the fluorouracil AUC was greater than 30 mg×h/L, the fluorouracil was appropriately reduced.
Statistical Analysis
The statistical analysis of the study was done using SPSS 19.0 software. Normally distributed data are expressed as mean±standard deviation. Non-normally distributed data are expressed in median and quartile. Curve regression coefficient analysis was performed using Spearman analysis. The relationship between fluorouracil concentration in blood and serious adverse reactions, as well as between fluorouracil concentration and chemotherapy efficacy, were compared using Pearson χ2 tests. P<0.05 was deemed as a statistically significant difference.
Results
Baseline Characteristics of the Patients in the Study Group
The Study Group included 54 patients (26 males and 28 females). The average age of the patients was 69±9.3 years. The number of patients treated with either FOLFOX4, mFOLFOX6, or FOLFIRI was 14, 26, and 14, respectively. Thirty-two of these were as a first-line treatment, 14 as a second-line treatment, and 8 as a third-line treatment (Table 1). In these 54 patients, a total of 289 blood samples were taken. The average values of the indicators, DI, TI, and RDI, for the amount of fluorouracil used in cycle 1, were 83.3±16.5%, 76.1±20.4%, and 63.1±21.1%, respectively.
The Impact of Dose Adjustment on 5-FU Exposure
The AUC obtained per cycle for the 5-FU peripheral blood concentration is shown in Figure 1 for all patients. The dose of 5-FU used in the first cycle was calculated based on BSA. As shown in Table 3, 32 patients (57.4%) had an AUC <20 mg×h/L, and 7 (13%) had an AUC ≥30 mg×h/L. Only 16 patients (29.6%) were within the target AUC range (20–30 mg×h/L). After 1 dose adjustment, the proportion of patients in the target AUC range increased to 23 (42.6%). However, the improvement in cycle 2 was not significant (Table 3, P=0.064). After 2 dose adjustments, in cycle 3 the number of patients within the target AUC was significantly improved, compared with cycle 1 (Table 3, P=0.006). There were 24 (48.0%) patients in the target AUC range in cycle 3, with a CV of 25.7%. By the fourth cycle, the average AUC was 26.3 mg×h/L (median, 28 mg×h/L; range, 14–38 mg×h/L; CV, 22.4%), with 46.8% of patients within the target 5-FU AUC range (Table 3). As shown in Figure 2, the incidence of grade III/IV toxicity decreased gradually with the dosing cycle (in cycles 1–6, Grade III toxicity was 35.2, 22.2, 18.0, 10.6, 9.1, and 12.5%, respectively, and Grade IV toxicity was 18.5, 7.4, 4.0, 2.1, 2.5, and 0%, respectively).
Figure 1.
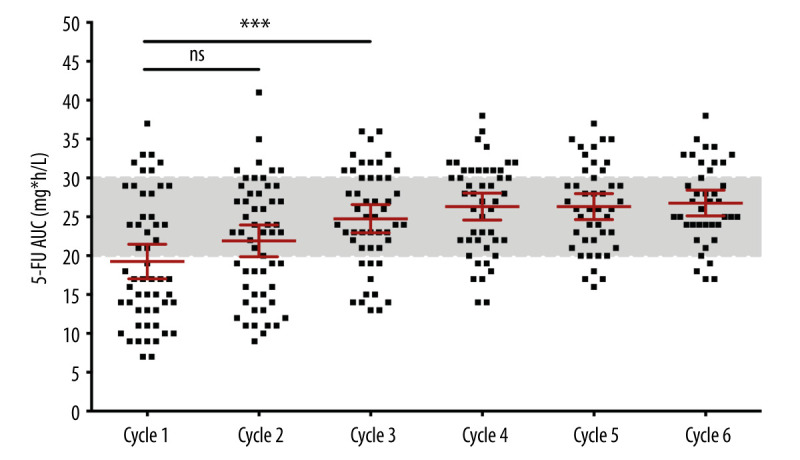
Distribution of measured 5-FU exposure (AUC) in every cycle of the Study Group. The AUC mean of the third cycle was significantly increased compared to the one at the first cycle (P=0.006).
Table 3.
Area under the curve of 5-Fluorouracil concentration in blood (actual exposure) in every cycle.
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Cycle 6 | |
|---|---|---|---|---|---|---|
| N | 54 | 54 | 50 | 47 | 44 | 40 |
| Mean | 19.3 | 21.9 | 24.8 | 26.3 | 26.3 | 26.8 |
| SD | 8.2 | 7.5 | 6.4 | 5.9 | 5.5 | 5.2 |
| Median | 17 | 23 | 24.5 | 28 | 26 | 26.5 |
| Range | 7–37 | 9–41 | 13–36 | 14–38 | 16–37 | 17–38 |
| CV | 42.4% | 34.2% | 25.7% | 22.4% | 21.0% | 19.4% |
| AUC ≤19 | 32 | 22 | 11 | 7 | 4 | 4 |
| 57.4% | 40.7% | 22.0% | 14.9% | 9.1% | 10.0% | |
| AUC 20–29 | 16 | 23 | 24 | 22 | 28 | 24 |
| 29.6% | 42.6% | 48.0% | 46.8% | 63.6% | 60.0% | |
| AUC ≥30 | 7 | 9 | 15 | 18 | 12 | 12 |
| 13.0% | 16.7% | 30.0% | 38.3% | 27.3% | 30.0% |
Figure 2.
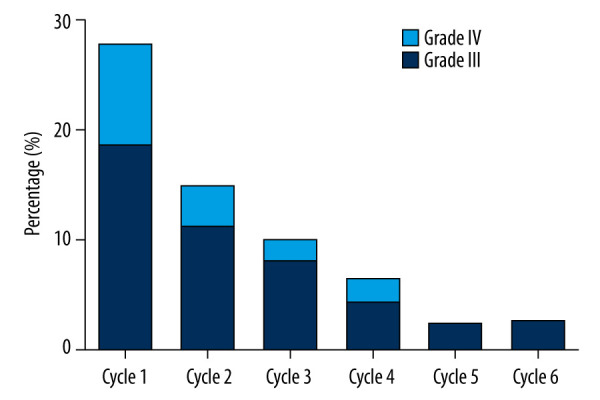
Percentage of the Study Group patients developing severe adverse events at all cycles.
5-FU Dose Adjustment Reduces Toxicity
To observe the impact of TDM on toxicity reduction, we retrospectively enrolled colorectal cancer patients who had received chemotherapy (Historical Group, n=153) without TDM from 2015 to 2018. Monitoring and adjustment of the 5-FU concentration significantly reduced toxicity. The Study Group (30/289) showed a lower percentage of severe adverse events than that in the Historical Group (185/447) (Table 2, P<0.001). Across all cycles of the 3 regimens, the incidences of neutropenia, diarrhea, anemia, and thrombocytopenia were 3.5% (10 cases), 4% (12 cases), 2.8% (8 cases), and 0.1% (3 cases), respectively. There were no cases of either mucositis or hand-foot syndrome. In the Historical Group, the incidences of neutropenia, diarrhea, anemia, thrombocytopenia, mucositis, and hand-foot syndrome were 13.6%, 12.1%, 6.5%, 4.3%, 2.0%, and 2.9%, respectively (Figure 3). Therefore, 5-FU concentration monitoring led to a 74.3%, 66.9%, 56.9%, and 97.7% decrease in neutropenia, diarrhea, anemia, and thrombocytopenia incidence, respectively.
Figure 3.
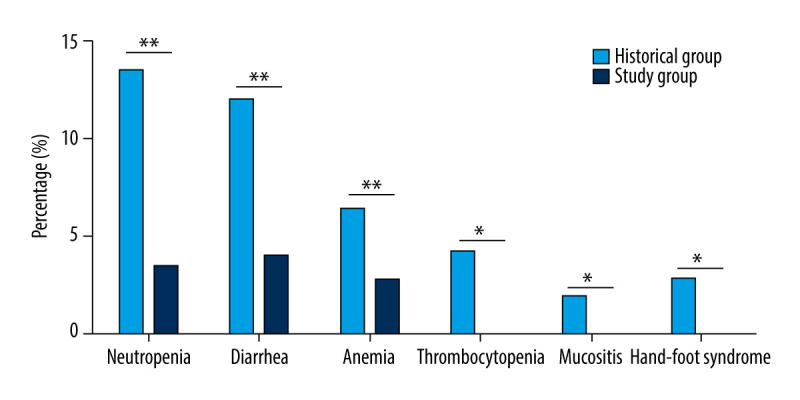
Incidence rate of severe adverse events (Grade 3 and 4) between the Study Group and the Historical Group. ** P<0.01 represents statistical difference
5-FU Dose Adjustment improves the Efficacy of the Therapy
Monitoring and adjustment of the 5-FU concentration significantly improved the efficacy of chemotherapy. The response rate (CR+PR: 38/54) in the Study Group was higher than that in the Historical Group (CR+PR: 82/153) (Table 2, P=0.032). Across the 3 dosing regimens in the Study Group, the incidences of CR, PR, SD, and PD were 5.6% (3), 64.8% (35), 24.1% (13), and 5.6% (3), respectively. Compared with the use of the 3 regimens in the Historical Group, in which there was a CR in 2.0% of patients, a PR in 54.2%, SD in 32.7%, and PD in 11.1% (Figure 4), 5-FU concentration monitoring reduced SD and PD by 26.3% and 49.5%, respectively.
Figure 4.
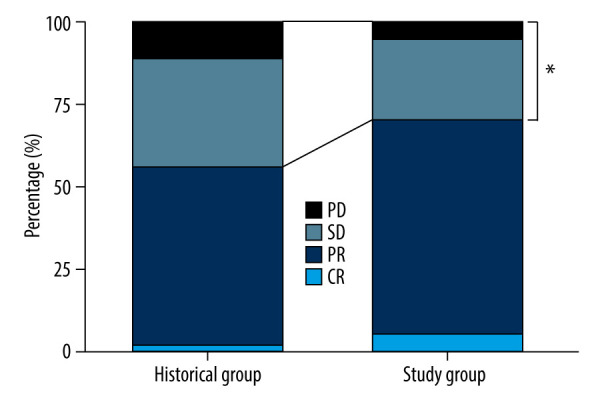
Percentage of all kinds of therapeutic outcomes between the Study Group and the Historical Group. CR – complete response; PR – partial response; SD – stable disease; PD – progressive disease. * P<0.05 represents statistical difference.
Discussion
Several studies have reported that 5-FU pharmacokinetic variability in different individuals has a great impact on the personal response to chemotherapy [7,13]. Patients with metastatic CRC who receive the common 5-FU regimens without drug monitoring often suffer from suboptimal exposure [7,10,13,17,18]. These studies all concluded that dosing of 5-FU based on the BSA is not the best strategy to achieve optimal efficacy of chemotherapy. Since a lack of monitoring of 5-FU exposure is highly related to toxicity [7,13], physicians are generally not willing to increase 5-FU doses without laboratory evidence of low exposure. A number of investigators have proposed regimens for adjusting the dose of fluorouracil according to the AUC (Table 4) [11–15]. Kaldate et al [7] suggested that an optimal concentration of 25 mg×h/L should be used for 5-FU in metastatic CRC, developing an adjustment algorithm for patients to reach an optimal exposure of 20–30 mg×h/L. Our study has not only shown the wide variability of 5-FU pharmacokinetics in the Chinese population, but has also demonstrated the efficacy of the Kaldate dose adjustment algorithm in this population. Our study shows that this dose adjustment strategy can achieve target 5-FU blood levels and reduce pharmacodynamic variability within 3 treatment cycles. The median 5-FU exposure in cycle 3 was 23 mg×h/L. The median concentration in the last 3 cycles finally stabilized (CV, 26%), which was 24–25 mg *h/L.
Table 4.
Dose adjustment strategy of fluorouracil based on the AUC.
| Gamelin, 2008 [11] | Wasif, 2009 [12] | Kaldate, 2012 [13] | Wilhelm, 201614 | Denda, 201615 | |||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | Adjustment range (%) | AUC | Adjustment range (%) | AUC | Adjustment range (%) | AUC | Adjustment range (%) | AUC | Adjustment range (%) |
| <4 | 70 | <5 | 150 | <8 | 30 | <8 | Repeat the original dose; if the AUC is <8 again, increase dose by 30% | <8 | 30 |
| 4–8 | 50 | 5–10 | 100 | 8–13 | 24 | 8–13 | 25 | 8–13 | 25 |
| 8–10 | 40 | 10–15 | 25 | 14–16 | 18 | 14–16 | 20 | 14–16 | 20 |
| 10–12 | 30 | 15–20 | 15 | 17–19 | 12 | 17–19 | 10 | 17–19 | 10 |
| 12–15 | 20 | 20–25 | 0 | 20–30 | 0 | 20–29 | 0 | 20–30 | 0 |
| 15–18 | 10 | 25–30 | −10 | 30–33 | −12 | 30–33 | −10 | 30–33 | −10 |
| 18–20 | 5 | 30–35 | −15 | 34–36 | −18 | 34–36 | −20 | 34–36 | −20 |
| 20–24 | 0 | 35–40 | −20 | 37–39 | −24 | 37–39 | −25 | 37–39 | −25 |
| 24–48 | −5 | – | – | ≥40 | −30 | ≥40 | −30 | ≥40 | −30 |
| 28–31 | −10 | – | – | – | – | – | – | – | – |
| >31 | −15 | – | – | – | – | – | – | – | – |
Although many studies have reported the exposure and reduced toxicity benefits of 5-FU concentration monitoring for patients with CRC, the association between monitoring and the efficacy of chemotherapy has rarely been addressed [19,20]. Wilhelm et al [14] studied only tumor marker concentrations (CEA and CA19-9) in response to chemotherapy. Although a significant increase or decrease in tumor marker levels was attributed to PD or a positive response (CR and PR), respectively, at the end of the study, this was not direct evidence. In our study, using simple grouping (treatment response group: CR and PR; treatment non-responder group: PD and SD) and the Pearson χ2 tests, it can be seen that 5-FU drug concentration monitoring significantly improves the efficacy of chemotherapy (Table 2, p=0.032). CR increased from 2.0% to 5.6%, and PR increased from 54.2% to 64.8% (Figure 4). SD and PD dropped by 26.3% and 49.5%, respectively (Figure 4).
Moreover, the present study also aimed to evaluate the feasibility of drug concentration monitoring and dose adjustment in routine practice, improving the benefits of 5-FU treatment for the patient. Fluorouracil blood concentration is characterized by day and night fluctuations, and also undulates during intravenous infusion [21]. In this study, the blood sample collection time was restricted to 8: 30-9: 30 (4.5–5.5 h before the end of intravenous pumping) to reduce the fluctuation interference, and to avoid early termination of the venous pump. Additionally, this restriction is convenient for blood sample collection during routine working hours. Because of the instability of fluorouracil in whole blood [22], the samples must be centrifuged as soon as possible, or stabilized using gimexin (an inhibitor of the fluorouracil metabolic enzyme, dihydrouracil hydrogenase) [23] in a polyethylene tube after collection. The overall process, including plasma transportation, result reporting, and decision making and dose adjustment by the physician, must be simple and repeatable. In our study, 48% of patients reached the target blood concentration in the fourth cycle and maintained this level for the next 3 cycles.
There have been several studies reporting the therapeutic benefit of 5FU TDM for CRC. The number of elderly patients who experienced severe toxic reactions (15%) dropped to 5% (the third cycle) after the use of TDM [24]. Compared with patients under 75 years old, TDM improved the tolerance of patients over 75 years old. The AUC of the initial dosing cycle reflects the patient’s individual drug metabolism characteristics. Patients with 5-FU AUC <8.4 h×mg/L at the first cycle exhibited significantly shorter disease-free survival [25]. However, cost-effectiveness must be considered in drug management. An Atlanta study reported that 5-FU TDM management is cost-effective in advanced CRC treatment [26]. In China, although there is no systematic analysis on the cost-benefit of 5-FU TDM, TDM has been added to individual medical insurance plans in some cities. There are obstacles to TDM implementation, but we believe that its cost-effectiveness will be clearly recognized. In countries such as France and the Netherlands, TDM is currently being incorporated into cancer clinical practice.
However, there are several limitations to our research. First, this study was carried out on a retrospective cohort. Our team is implementing a randomized controlled trial on 5-FU clinical drug monitoring, and we will publish the data when the trial completes. Second, due to the short study period, the survival data has not been obtained. We will expand the sample size and conduct long-term follow-up to optimize the research design.
Conclusions
TDM is routinely practiced when 5-FU is used in the clinic. This leads to the administration of higher doses of 5-FU, with lower toxicity and better therapeutic effects. Further randomized controlled trials are needed to verify its clinical value.
Supplementary Data
A flow chart of excluded patients.
Footnotes
Data Availability Statement
The datasets used in this study are available from the corresponding author on reasonable request.
Conflict of Interest
None.
Source of support: The research and manuscript preparation were funded by Jiangsu Provincial Government Scholarship Fund Project (Su Jiao Wai Han [2019] No. 43); Wu Jieping Medical Fund Project (No. 320.6750.2020-18-1); Nantong Science and Technology Planning Project (JCZ19092); Nantong Municipal Health Commission Project (MB2019054); Nantong Science and Technology Project (JC2020056)
References
- 1.Aguado C, Garcia-Paredes B, Sotelo MJ, et al. Should capecitabine replace 5-fluorouracil in the first-line treatment of metastatic colorectal cancer? World J Gastroenterol. 2014;20:6092–101. doi: 10.3748/wjg.v20.i20.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Elez E, Argiles G, Tabernero J. First-line treatment of metastatic colorectal cancer: Interpreting FIRE-3, PEAK, and CALGB/SWOG 80405. Curr Treat Options Oncol. 2015;16:52. doi: 10.1007/s11864-015-0369-x. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama G, Tanaka C, Uehara K, et al. The impact of dose/time modification in irinotecan- and oxaliplatin-based chemotherapies on outcomes in metastatic colorectal cancer. Cancer Chemother Pharmacol. 2014;73:847–55. doi: 10.1007/s00280-014-2416-x. [DOI] [PubMed] [Google Scholar]
- 5.Tosi D, Perez-Gracia E, Atis S, et al. Rational development of synergistic combinations of chemotherapy and molecular targeted agents for colorectal cancer treatment. Bmc Cancer. 2018;18:812. doi: 10.1186/s12885-018-4712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurk S, Peeters P, Stellato R, et al. Skeletal muscle mass loss and dose-limiting toxicities in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle. 2019;10(4):803–13. doi: 10.1002/jcsm.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saam J, Critchfield GC, Hamilton SA, et al. Body surface area-based dosing of 5-fluoruracil results in extensive interindividual variability in 5-fluorouracil exposure in colorectal cancer patients on FOLFOX regimens. Clin Colorectal Cancer. 2011;10:203–6. doi: 10.1016/j.clcc.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Kline CL, Sheikh HS, Scicchitano A, et al. Preliminary observations indicate variable patterns of plasma 5-fluorouracil (5-FU) levels during dose optimization of infusional 5-FU in colorectal cancer patients. Cancer Biol Ther. 2011;12:557–68. doi: 10.4161/cbt.12.7.18059. [DOI] [PubMed] [Google Scholar]
- 9.Patel JN, O’Neil BH, Deal AM, et al. A community-based multicenter trial of pharmacokinetically-guided 5-fluorouracil dosing for personalized colorectal cancer therapy. Oncologist. 2014;19:959–65. doi: 10.1634/theoncologist.2014-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kline CLB, Schiccitano A, Zhu JJ, et al. Personalized dosing via pharmacokinetic monitoring of 5-fluorouracil might reduce toxicity in early- or late-stage colorectal cancer patients treated with infusional 5-fluorouracil-based chemotherapy regimens. Clin Colorectal Canc. 2014;13:119–26. doi: 10.1016/j.clcc.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Gamelin E, Delva R, Jacob J, et al. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: Results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2099–105. doi: 10.1200/JCO.2007.13.3934. [DOI] [PubMed] [Google Scholar]
- 12.Saif MW, Choma A, Salamone SJ, Chu E. Pharmacokinetically-guided dose adjustment of 5-fluorouracil: A rational approach to improving therapeutic outcomes. J Natl Cancer I. 2009;101:1543–52. doi: 10.1093/jnci/djp328. [DOI] [PubMed] [Google Scholar]
- 13.Kaldate RR, Haregewoin A, Grier CE, et al. Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6. Oncologist. 2012;17:296–302. doi: 10.1634/theoncologist.2011-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilhelm M, Mueller L, Miller MC, et al. Prospective, multicenter study of 5-fluorouracil therapeutic drug monitoring in metastatic colorectal cancer treated in routine clinical practice. Clin Colorectal Canc. 2016;15:381–88. doi: 10.1016/j.clcc.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Denda T, Kanda M, Morita Y, et al. Pharmacokinetic dose adjustment of 5-FU in modified FOLFOX7 plus bevacizumab for metastatic colorectal cancer in Japanese patients: A-JUST phase II clinical trial. Cancer Chemoth Pharm. 2016;78:1253–61. doi: 10.1007/s00280-016-3184-6. [DOI] [PubMed] [Google Scholar]
- 16.Beumer JH, Chu E, Allegra C, et al. Therapeutic drug monitoring in oncology: international association of therapeutic drug monitoring and clinical toxicology recommendations for 5-fluorouracil therapy. Clin Pharmacol Ther. 2019;105:598–613. doi: 10.1002/cpt.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capitain O, Asevoaia A, Boisdron-Celle M, et al. Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: A phase II, proof-of-concept study. Clin Colorectal Canc. 2012;11:263–67. doi: 10.1016/j.clcc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Patel JN, O’Neil BH, Deal AM, et al. A community-based multicenter trial of pharmacokinetically-guided 5-fluorouracil dosing for personalized colorectal cancer therapy. Oncologist. 2014;19:959–65. doi: 10.1634/theoncologist.2014-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moloney M, Faulkner D, Link E, et al. Feasibility of 5-fluorouracil pharmacokinetic monitoring using the My-5FU PCM system in a quaternary oncology centre. Cancer Chemother Pharmacol. 2018;82:865–76. doi: 10.1007/s00280-018-3679-4. [DOI] [PubMed] [Google Scholar]
- 20.Macaire P, Morawska K, Vincent J, et al. Therapeutic drug monitoring as a tool to optimize 5-FU-based chemotherapy in gastrointestinal cancer patients older than 75 years. Eur J Cancer. 2019;111:116–25. doi: 10.1016/j.ejca.2019.01.102. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto H, Okumura H, Murakami H, et al. Fluctuation in plasma 5-fluorouracil concentration during continuous 5-fluorouracil infusion for colorectal cancer. Anticancer Res. 2015;35:6193–99. [PubMed] [Google Scholar]
- 22.Lee JJ, Beumer JH, Chu E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother Pharmacol. 2016;78:447–64. doi: 10.1007/s00280-016-3054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakata K, Someya M, Matsumoto Y, et al. Gimeracil, an inhibitor of dihydropyrimidine dehydrogenase, inhibits the early step in homologous recombination. Cancer Sci. 2011;102:1712–16. doi: 10.1111/j.1349-7006.2011.02004.x. [DOI] [PubMed] [Google Scholar]
- 24.Macaire P, Morawska K, Vincent J, et al. Therapeutic drug monitoring as a tool to optimize 5-FU-based chemotherapy in gastrointestinal cancer patients older than 75 years. Eur J Cancer. 2019;111:116–25. doi: 10.1016/j.ejca.2019.01.102. [DOI] [PubMed] [Google Scholar]
- 25.Di Paolo A, Lencioni M, Amatori F, et al. 5-fluorouracil pharmacokinetics predicts disease-free survival in patients administered adjuvant chemotherapy for colorectal cancer. Clin Cancer Res. 2008;14:2749–55. doi: 10.1158/1078-0432.CCR-07-1529. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein DA, Chen Q, Ayer T, et al. Cost effectiveness analysis of pharmacokinetically-guided 5-fluorouracil in FOLFOX chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer. 2014;13:219–25. doi: 10.1016/j.clcc.2014.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A flow chart of excluded patients.


