Abstract
Rationale: There is an urgent need to understand the risk of viral transmission during nebulizer treatment of patients with coronavirus disease 2019 (COVID-19).
Objectives: To assess the risk of transmitting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), SARS, Middle East respiratory syndrome (MERS), and influenza with administration of drugs via nebulizer.
Methods: We searched multiple electronic databases, including PubMed®, China National Knowledge Infrastructure, Wanfang, preprint databases, and clinicaltrials.gov through December 1, 2020. Any study design in any language describing the risk of viral transmission with nebulizer treatment was eligible. Data were abstracted by one investigator and verified by a second.
Results: We identified 22 articles: 1 systematic review, 7 cohort/case-control studies, 7 case series, and 7 simulation-based studies. Eight individual studies involved patients with SARS, five involved MERS, and one involved SARS-CoV-2. The seven cohort/case-control studies (four high risk of bias [ROB], three unclear ROB) found mixed results (median odds ratio 3.91, range 0.08–20.67) based on very weak data among a small number of health care workers (HCWs) with variable use of personal protective equipment (PPE). Case series had multiple potential contributors to transmission. Simulation studies found evidence for droplet dispersion after saline nebulization and measureable influenza viral particles up to 1.7 m from the source after 10 minutes of nebulization with a patient simulator. Study heterogeneity prevented meta-analysis.
Conclusions: Case series raise concern of transmission risk, and simulation studies demonstrate droplet dispersion with virus recovery, but specific evidence that exposure to nebulizer treatment increases transmission of coronaviruses similar to COVID-19 is inconclusive. Tradeoffs balancing HCW safety and patient appropriateness can potentially minimize risk, including choice of delivery method for inhaled medications (e.g., nebulizer vs. metered dose inhaler) and PPE (e.g., N95 vs. surgical mask).
Keywords: aerosol-generating procedure, nebulizers, personal protective equipment, severe acute respiratory syndrome
Introduction
More than 7000 health care workers (HCWs) have died worldwide from coronavirus disease 2019 (COVID-19); more than 14% of these deaths have occurred in the United States alone.(1) Reduction in transmission risk to HCWs has been a priority during this pandemic. Our focus of transmission risk reduction has been around the delivery of therapeutic agents via nebulizers. Nebulizers are of particular concern due to the risk of aerosol generation and dispersion, leading to potential transmission of infection.(2) Importantly, there is a distinct difference between “bioaerosols” or therapeutic particles released during nebulizer treatment (which is expected) and airborne pathogens containing aerosols that develop as a byproduct of the nebulization process (e.g., viral aerosols). Often, the discussion of aerosolization or nebulization conflates the development of bioaerosols with viral aerosols. Although it is important to disambiguate between aerosol subtypes, our review focuses on the concern for the release of viral aerosols during the process of nebulization. Viral aerosol development poses a significant risk, because viral aerosols remain transmissible for up to 3 hours in the same enclosed environment without atmospheric turnover and travel distances farther than droplets due to their smaller size (<5 μm).(3–5) Because multiple professional organizations have identified nebulization as an aerosol-generating procedure (AGP),(6,7) its use in clinical care has been significantly restricted and, when used, requires high levels of personal protective equipment (PPE; i.e., fit-tested particulate respirators). These requirements for higher levels of PPE lead to increased equipment and personnel time costs.(8)
However, the administration of inhaled therapeutics remains a core component of the management of multiple acute respiratory conditions.(7) Nebulization offers particular benefits, such as delivery of medications directly to the site of action, reduced systemic availability, and has faster onset of action.(9) Nebulizers are often the preferred delivery mechanism when a patient is unable to perform required technique for metered dose inhalers (MDIs; e.g., poor coordination, agitation, or exacerbation severity).(10) Because of the potential infection risk, many providers are currently opting to use alternative, and presumed safer, modalities for patients with COVID-19, such as MDI or dry powder inhalers (DPI). MDIs and DPIs have less aerosolization risk but require training for proper patient administration.(9) However, the actual risk of infection transmission for COVID-19 with nebulization of therapeutic medications—and the necessity of current precautions—is unknown.
Understanding whether nebulizers pose sufficient infection transmission risk to require current stringent precautions is critical to delivering high-quality care while minimizing risk to HCWs and optimizing the use of potentially scarce resources. Thus, we sought to synthesize the evidence about the risk of transmitting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with nebulizer use. Because of the likely limited literature on risk associated with COVID-19, we also considered infections caused by related coronaviruses such as SARS and Middle East respiratory syndrome (MERS), as well as more a common and long-standing human viral infection, influenza. Our key question was: What is the risk of transmitting SARS-CoV-2, SARS, MERS, and influenza during administration of drugs via nebulizer?
Methods
This review was originally requested as a rapid review(11,12) by the national Department of Veterans Affairs (VA) operations leadership managing COVID-19 clinical care procedures and policies. We made the following modifications to streamline the systematic review processes: (1) Our a priori protocol was not published before review conduct, (2) a single review at title-and-abstract level, and (3) a single reviewer data abstraction, with second reviewer verification. Although there are no agreed-upon reporting guidelines for rapid reviews, we followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines for systematic reviews.(13) The method details are available in the extended technical report: https://covid19reviewsorg.files.wordpress.com/2020/04/risk-of-transmitting-covid-19-during-nebulizer-treatment-apr-10-2020.pdf. The technical report underwent an internal programmatic review but did not benefit from the peer-review process.
Data sources and searches
For our initial search in early April 2020, we conducted a broad search, including COVID-19 search terms and terms for additional respiratory viruses in anticipation of limited published data related to the emergent outbreak. In consultation with expert medical librarians, we initially conducted two searches in MEDLINE (via PubMed®): the first search for SARS-CoV-2, SARS, and MERS (last updated on December 1, 2020); and a second search for influenza (conducted on April 16, 2020) (Supplementary Table S1). Searches had no restrictions by study design or language. The developed search strategy was applied with adjustments to additional databases: China National Knowledge Infrastructure and Wanfang. We hand-searched pertinent clinical guidelines (e.g., Surviving Sepsis Campaign); systematic reviews(14,15); and all eligible studies and selected excluded studies to identify potentially relevant articles not identified otherwise.(4,8,14,16–28) Subsequently, the MEDLINE and China National Knowledge Infrastructure and Wanfang database searches have been limited to COVID-19 terms and are updated through September 1, 2020. We also reviewed multiple online databases for rapid reviews related to COVID-19.(11,29–43) To identify relevant work on COVID-19 in the Chinese-language resources, we hand-searched the Chinese Medical Journal Network. To identify emerging literature, we reviewed bioRxiv and medRxiv in April and conducted a search of the preprint server collections within the NIH iCite from inception through September 1, 2020. In addition, we reviewed clinicaltrials.gov for all studies involving SARS or SARS-CoV-2 to check for studies not identified by our other methods and that could provide additional data for this review once completed (August 31, 2020). Study selection was based on prespecified eligibility criteria listed in Table 1.
Table 1.
Study Eligibility Criteria
| Study characteristic | Include criteria | Exclude criteria |
|---|---|---|
| Population | Adults, children, or simulated patients with probable or confirmed COVID-19 (SARs-CoV-2), SARS, MERS, or influenza. For experimental studies (simulation studies) examining effects of nebulization on droplet dispersal, these studies may include non-patients or patient simulators. | Animal studies |
| Intervention | Nebulized delivery of a medication (e.g., albuterol) or placebo solution (e.g., saline). Nebulizers include pneumatic jet compression, ultrasonic, vibrating mesh/horn and microprocessor-controlled breath actuated types. | NIV (e.g., BiPAP, CPAP); high-flow nasal oxygen; large-volume nebulizers |
| Comparator | None or other drug delivery (e.g., metered dose inhalers, dry powder, or slow-mist inhaler) | None |
| Outcomes | Confirmed and probable cases of the specific viral infection (i.e., measures of transmission risk). For experimental/simulation studies, virus recovery (e.g., by RNA sequencing) or droplet dispersion at different distances/time points. | None |
| Setting | Any inpatient setting, emergency department or outpatient setting. Experimental/simulation studies may take place in nonclinical settings. | Studies of fugitive emissions that did not involve patient simulation |
| Timing | Any | None |
| designs | Systematic reviews, trials (randomized and nonrandomized), cohort studies, case-control studies, case series, case reports, experimental/simulation studies of droplet/virus dispersion. | None |
BiPAP, bilevel positive airway pressure; COVID-19, coronavirus disease 2019; CPAP, continuous positive airway pressure; MERS, Middle East respiratory syndrome; NIV, noninvasive ventilation; RNA, ribonucleic acid; SARS, severe acute respiratory syndrome; SARs-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data extraction
We used Covidence (www.covidence.org), a web-based software to screen title/abstracts for identified citations, then full-text articles for inclusion. Citations/abstracts were screened by a single reviewer unless uncertain, in which case a second reviewer was consulted. At full-text review, articles were screened by two reviewers. At all steps of eligibility determination, reviewers maintained an open dialogue to clarify eligibility criteria (as needed) and to discuss articles of uncertain eligibility.
We collected the following study characteristics, such as author/year, location/setting, number of patients, and intervention characteristics (nebulizer type). We also abstracted outcomes, such as the rate of COVID-19 transmission or distance to aerosolized viral particles from simulation studies. Data elements were abstracted into a shared document by a single investigator and verified by a second investigator.
Data synthesis and analysis
For comparison of results across studies, we primarily used reported odds ratios (ORs) with 95% confidence interval (CI) or, when not provided, we calculated ORs and 95% CI from the provided primary data (e.g., case rates and at risk population numbers). RevMan 5.3 was used for calculations and forest plot generation. We did not calculate a pooled estimate due to limited data and heterogeneity in key factors, such as controlling for major confounders (e.g., use of PPE), virus studied, and setting; instead, we narratively synthesized the evidence.
Risk-of-bias assessment
We used the Newcastle-Ottawa Scale for Cohort Studies and case-control studies to assess study risk of bias (ROB). We did not complete a formal ROB assessment for case series or simulation studies but identified important strengths and limitations of these narratively.
Results
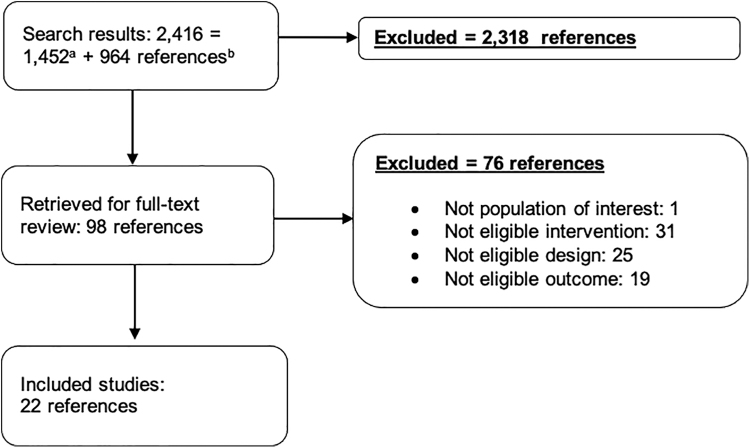
We identified 2416 publications through our systematic database searches of published literature and hand-searches of the literature. We also identified 542 studies through the literature searches. Of these, we retrieved 98 references for full-text review. Of the 22 included studies, 1 was a systematic review, 7 were cohort or case-control studies, 7 were case series, and 7 were simulation-based studies (Fig. 1). Eight individual studies involved patients with SARS, five involved MERS, and one involved SARS-CoV-2. We screened titles from the preprint servers bioRxiv and medRxiv, five of which were reviewed at full text. One was included.(44) In addition, we reviewed LitCOVID and found no additional studies. We reviewed the collection of 474 articles on COVID-19 within the Chinese Medical Journal Network, screened 4 articles at full text, and included none. We also did not identify any relevant completed COVID-19 evidence syntheses. There were no relevant systematic reviews underway. Similarly, our clinicaltrials.gov search found no relevant studies.
FIG. 1.
Literature flow chart. aSearch results from MEDLINE (1,041), iCite (388), and identified from relevant articles (23) were combined. bCNKI-China National Knowledge Infrastructure (382), Wanfang (108), and Chinese Medical Journal Network (474).
Next, we describe results by study type: cohort and case-control studies, case series studies, and experimental and simulation studies. Within each study type, we report findings by infectious illness.
Cohort and case-control studies (n = 7)
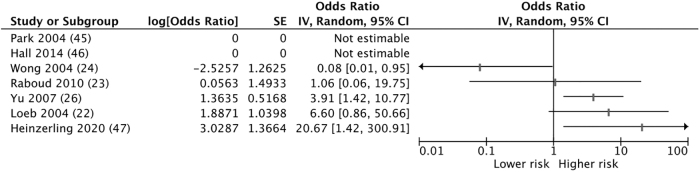
Of the seven studies, five were retrospective cohorts(22,23,25,45,46) and two were case-control studies(26,47) (Tables 2 and 3 and Fig. 2). Of the five cohort studies, three were high ROB(22,45,46) and two were unclear ROB.(23,25) One case-control study examined experiences with SARS-CoV-2(47) and was found to be high ROB. The other case-control study had uncertain ROB.(26) Common contributing causes of ROB included exposure details that were typically acquired via unblinded interviews, rare comprehensive matching or controlling for confounders, small sample sizes, inconsistent data about use of PPE and comorbidities of at-risk HCWs, and lack of information about potential community exposures. Supplementary Figures S1–S4 show details of the ROB assessment. Overall, these seven case-control and cohort studies had weak methods and reported inconsistent findings, with two studies showing an association between nebulizer use and viral transmission, three showing no association, and two showing no association with wide CIs.
Table 2.
Results of Cohort and Case-Control Studies
| Study/country | Study design/setting | Virus | Population characteristics | Intervention/exposure and comparator | Outcomes | Strengths | Weaknesses |
|---|---|---|---|---|---|---|---|
| Heinzerling et al.(47)/United States | Case-control/hospital | SARS-CoV-2 | HCWs who had contact with index patient, N = 121 | Unspecified nebulizer treatment type | 2/3 HCWs with COVID-19 had nebulizer exposure versus 3/34 without (p = 0.04) | Standardized interviews of HCWs; lab confirmation of COVID-19; good effort to track down all HCWs tested | No PPE or transmission precautions during exposure; not all exposed were tested; six who were tested were not interviewed |
| Hall et al.(46)/Saudi Arabia | Retrospective cohort/multiple hospital settings | MERS | HCWs who had contact with index patient, N = 48 | Unspecified nebulizer treatment type | 0/14 HCWs present during nebulizer treatment; 0/34 HCWs not present during nebulizer treatment; outcome = EIA serum testing for MERS | Adequate follow-up time; representative sample with exposed and non-exposed from the same population; antibody testing in all included HCWs | Exposure not verified by medical records; PPE use was variable and not described in relation to exposure |
| Raboud et al.(23)/Canada | Retrospective cohort/multiple hospitals | SARS | HCWs, N = 624 (9 exposed) | Exposed to nebulizera versus not exposed (exposed = in room during treatment) | 0/26 cases (+ SARS); 9/598 controls (− SARS); outcome = positive convalescent antibodies or SARS case definition criteria (SARS-CoV) | Adequate follow-up; risk from multiple clinical activities considered; standardized case definition with confirmatory lab testing; triangulated identification of eligible HCWs via multiple sources | Variable use of PPE, training on use of PPE, and PPE removal behaviors; PPE use not reported in relation to nebulizer exposure; multiple infected HCWs cared for multiple patients with SARS; likely recall bias at an unreported duration after exposure event; generalizability limited to patients who were intubated |
| Loeb et al.(22)/Canada | Retrospective cohort/two ICUs | SARS | Critical care nurses, N = 43 (32 exposed) | Exposure to nebulizer treatmenta (type of nebulizer treatment not specified) | 3/5 Exposed (+ SARS); 5/27 not exposed (− SARS); relative risk = 3.24 (1.11–9.42); p = 0.09; outcome = Health Canada/WHO case definition | Exposure reports validated by medical records; standardized case definition; convalescent antibody titers; likely adequate follow-up | A few exposures; variable PPE use; no report of PPE specifically during exposure to nebulizer treatment |
| Yu et al.(26)/China; Hong Kong | Case-control/hospital wards with and without nosocomial super-spreader events of SARS | SARS | Inpatient wards, N = 124 wards (26 hospitals) | Environmental factor = nebulizera used at least once versus never; host factor = use of nebulizer (yes/no) | Environmental: OR 1.37 (0.66–2.85); p = 0.40; host: OR 3.91 (1.42–10.78); p = 0.006; risk factors from multivariate analyses: distance between beds, staff working with symptoms, oxygen therapy, BiPAP; nebulizer use not associated with super spreading event; outcome = super spreader eventb | Adequate follow-up time; cases appear representative and were drawn from the same community as the controls; case definition used was appropriate | Data collection conducted 1–2 years after event; unclear whether interviews were blinded; not reported how many distinct nebulizer events occurred |
| Wong et al.(25)/Hong Kong | Retrospective cohort/hospital ward | SARSc | Exposed medical students and assessors, N = 66 students who visited ward | Index patient received QID 30-minute jet nebulizer treatment from March 6, 2003 to March 12, 2003 | No association between risk of infection and presence on ward when nebulizer was in use; 6/10 students (and 5/5 assessors) who visited ward before start of nebulizer treatments contracted SARS versus 1/9 (and 3/5 assessors) who visited the day after nebulizer treatment started; note the 1 infected after visiting on 3/7 was present during the nebulizer administration time | Adequate follow-up time; representative sample with exposed and non-exposed from the same population; students without SARS at study outset, and exposure data were confirmed at least partially by school records | PPE use not described |
| Park et al.(45)/United States | Retrospective cohort/8 of 9 U. S. health care facilities that cared for known SARS patients in 2003 | SARS | HCW with exposure within 3′ of a patient with laboratory-confirmed SARS-CoV, N = 110 | Exposure either protected by full PPE or unprotected; full PPE defined as all recommended equipment, including full-length gown, gloves, N95 or higher respirator, eye protection | 4 HCW reported exposure to aerosolized medication delivery (1 without a respirator, and 1 without gown, gloves and eye protection); 0 of 103 patients tested positive for SARS (unknown whether the 4 exposed to nebulizers were included in the 103 tested) | 85% of estimated HCW with high-risk exposure participated; convalescent antibodies tested for 103 interviewed HCWs; data collected across multiple facilities; not restricted to symptomatic HCWs | One facility caring for the eight SARS-positive patients in the United States in 2003 did not participate; risk of recall bias as exposure data collected by interview without medical record corroboration; few nebulizer exposure events, 4 of 110 HCWs; self-reported adherence to PPE recommendations; no paired antibody samples |
Type of nebulizer not specified.
≥3 Cases within 2–10 days of admission of index patient +SARS or cluster 3+ cases in 8 days without known index.
Case definition: fever+pneumonia on imaging.
EIA, enzyme immunoassay; HCW, health care worker; ICU, intensive care unit; OR, odds ratio; PPE, personal protective equipment; QID, four times daily; WHO, World Health Organization.
Table 3.
Results of Case Series Studies
| Study/country | Study design details | Virus | Setting | Population characteristics | Exposure | Outcomes | Comments |
|---|---|---|---|---|---|---|---|
| Assiri et al.(51)/Saudi Arabia | Case series | MERS | 4 Hospitals: 3 general and 1 regional referral | 23 Confirmed cases of MERS and 11 probable cases | 4 MERS patients in ICU of one hospital received unspecified type nebulizer treatments while also receiving CPAP | 2 Additional cases were diagnosed among patients in ICU at the same time during which no special isolation procedures were noted. | No further cases developed after implementation of infection control procedures; genome sequencing employed |
| Hunter et al.(52)/United Arab Emirates | Case series | MERS | 3 Hospitals with health care associated MERS clusters | 30 Cases of MERS transmitted in health care setting (n = 19 HCW) | Exposure to either inhaler or unspecified type of nebulizer treatment | 14 HCWs who developed MERS; 2 administered metered-dose inhaler or nebulizer treatment. | 13 of 14 HCWs were exposed before diagnosis of index patient; PPE use variable among 14 HCWs; genome sequencing employed |
| Nam et al.(49 )/South Korea | Case series | MERS | 2 Hospitals | 1 Index patient admitted to both hospitals and 25 secondary cases of MERS | Lidocaine inhalation using jet nebulizer before bronchoscopy on second day of Hospital B | 25 Total secondary cases (14 inpatients, 9 commercial/family caregivers, 2 hospital employees); 5 patients in same ward room in which nebulizer was used developed MERS Hospital B. | Hospital B had a higher case fatality rate versus Hospital A. Hospital B ward room had lower air ventilation and higher density of patients than Hospital A; no cases among bronchoscopy HCWs (all wore surgical masks, gloves, vinyl gowns) |
| Park et al.(50)/South Korea | Case series | MERS | 2 Hospitals | 1 Index patient admitted to both hospitals and 23 secondary cases and 3 tertiary cases | Lidocaine inhalation using jet nebulizer before bronchoscopy on second day of Hospital B | 13 Secondary cases at Hospital A; 10 secondary cases at Hospital B (5 patients and 3 caregivers in same room infected). | Similar attack rates between hospitals (15.8% hospital A; 14.3% hospital B, p = 0.51); incidence rate higher in hospital B than A (7.7/100 vs. 3.4/100 exposure days, IRR = 2.3, p < 0.001)a; no secondary cases among HCW |
| Lee et al.(21)/Hong Kong | Case seriesb | SARS | Medical ward with isolation facilities | Secondary/tertiary cases: male 66, female 72; mean age 39.3 (SD 16.8), N = 156 with SARS (138 secondary or tertiary cases) | Jet nebulizer 6 L/min; QID administered to index patient | 112 SARS patients with direct exposure to index patient (69 HCW, 16 medical students all with “unremarkable medical histories”). | Nebulizer speculated as important in transmission |
| Varia et al.(48)/Canada | Case series | SARS | Secondary-care community hospital in Toronto | N = 128, male N = 51, female N = 77; mean age 44.8; HCWs N = 47 household/social contacts N = 38 | Index patient received nebulizerc salbutamol while in the general observation area of the emergency department | 128 SARS patients that resulted from exposure to index patient, including 2 nearby patients from the Emergency Room (ER) (all cared for by the same nurse). | Highest transmission rate was observed in CCU nurses (60%) owing to prolonged exposure to severely ill patients. Unclear PPE use during the period of exposure |
| Wong and Hui(24)/Hong Kong | Case report | SARS | General medical ward in a tertiary care hospital; moved to negative pressure isolation room on day 8 of admission; N95+disposable gloves used day 8 forward | SARS patient (N = 1), limited information about patient | Jet nebulizer at 6 L/min for bronchodilation used QID until day 8 | 100 SARS patients linked to index patient. | Setting not clear regarding poor PPE standards and lack of isolation before diagnosis |
Overlapping cases with Nam et al.(49)
Case definition based on CDC, fever, lung consolidation on imaging, and exposure to index or secondary case.
Type of nebulizer not specified.
CCU, coronary care unit; IRR, incidence rate ratio; SD, standard deviation.
FIG. 2.
Odds ratios of viral transmission after nebulizer treatment exposure. IV, inverse variance; SE, standard error.
The 1 study of transmission of COVID-19 was a high ROB, case-control study of 121 hospital-based HCWs exposed to the first known U.S. case.(47) Authors report that 2 of the 3 COVID-positive HCWs were exposed to nebulizer treatment compared with three of 34 exposed HCWs who were COVID-negative (OR 20.7; 95% CI 1.4–300.9 by our calculation). None of the HCWs wore currently recommended PPE for COVID-19. The three HCWs with COVID-19 also had a higher median duration of contact than those without COVID-19 (120 minutes vs. 25 minutes; p = 0.06).
Five studies reported risk of transmission during the 2003 SARS outbreak. One uncertain ROB study compared nebulizer exposure during the 28 hours around patient intubation among HCWs who did, and did not, acquire SARS.(23) There were zero nebulizer exposures among 26 SARS cases and 9 exposures among HCWs who did not contract SARS (see Table 2 for additional study level details). The second uncertain ROB cohort study included 66 medical students who had visited the hospital ward of the index patient with SARS in China.(25) Medical school records were cross-referenced with nebulizer treatment timing to verify exposure. No association was found, though no students examined the index patient directly. Among a subset of 19 students with clear and limited exposures to SARS-infected patients, 6 of 10 who visited the ward before the patient started receiving nebulizer treatments contracted SARS compared with one of nine students who visited the ward the day after treatment initiation (OR 0.08; 95% CI 0.01–0.95 by our calculation). One high ROB cohort study examined the SARS attack rate of 43 critical care nurses in 2 Canadian intensive care units.(22) Only 5 of 32 nurses who entered the room of an infected patient also had medical record validated nebulizer exposure with a relative risk of 3.24 (95% CI 1.11–9.42; p = 0.09). The other high ROB cohort study of SARS included 110 HCWs from 8 of 9 facilities that cared for 6 U.S. patients with SARS.(45) Four of 110 HCWs with high-risk exposures to a SARS-positive patient also reported exposure while the patient received aerosolized medications, one without a respirator and one without gloves, gown, or eye protection. None of the 103 HCWs tested for convalescent antibodies were positive. The final study related to SARS was an unclear ROB case-control study that examined factors associated with nosocomial super-spreader events on adult inpatient wards in Hong Kong (n = 38) and China (n = 86).(26) In univariate analyses, the authors report an OR of 1.37 (95% CI 0.66–2.85; p = 0.40) for nebulizer exposure as an environmental factor and for as a host factor an OR 3.91 (95% CI 1.42–10.78; p = 0.006). Neither was significantly associated with a super-spreader event in the final multivariate analyses.
One high ROB cohort examined 48 HCWs with and 48 HCWs without contact with an index patient infected with MERS.(46) Fourteen (29%) of HCWs with index-patient contact also had exposure via nebulizer treatment. No HCWs with index-patient contact developed MERS (exposed or unexposed).
Case series studies (n = 7)
We identified three case series of patients with SARS(21,24,48) and four with MERS(49–52) (Table 3). Two of the three case series reported on the nosocomial transmission of SARS from the same index patient in China,(21,24) and a third described the nosocomial outbreak in Canada.(48) The MERS case series described nosocomial transmissions in Saudi Arabia,(51) the United Arab Emirates,(52) and South Korea.(49,50) All case series described hospitals with widespread nosocomial transmission and index cases who received nebulizer treatments, but in none of the studies were HCWs clearly using appropriate PPE. Overall, these studies raise the possibility that nebulizer treatment contributed to viral transmission and support the need for further evaluation via stronger study designs.
Experimental and simulation studies (n = 7)
There were two experimental studies on live human patients and five human simulation studies. Overall, these studies support the concept that nebulizers could increase transmission of viral infection as evidenced by the presence of aerosols in the vicinity of a patient (or patient simulator) receiving nebulizer treatments and one study found recovery of virus measured by polymerase chain reaction from viral transport medium (VTM).
One experimental study exposed 3 groups of live adult patients (11 healthy, 11 with coryzal symptoms, and 21 with acute exacerbations of chronic respiratory illness) to a series of respiratory procedures in variable order, including nebulization in a standard ward room without an external window or ventilation system.(53) Droplet dispersion was measured during each procedure with an optical particle sizer at 20 cm and 1 m from the patient (Table 4). The authors reported a significant increase (p < 0.0001) in mean pre/during intervention droplet counts (normalized difference) at sizes 0.3–0.5, 0.5–1, 1–3, and 2–5 μm across all patient groups at both distances. None of the included subjects had documented viral infections, nor was the presence of viral particles measured. A second experimental study, identified through a preprint server, sought to characterize the pattern of droplet dispersion with human participants of unknown clinical status receiving selected common airway management procedures during routine care, one of which was nebulization.(44) Droplet patterns were captured by a high-speed camera of illuminated droplets against a black background. They found no evidence of droplet dispersion during nebulization, though they noted that fine aerosols were detected but not quantified due to “abundance.”
Table 4.
Results of Experimental and Simulation Studies
| Study/country | Study design details | Virus | Setting | Population characteristics | Exposure | Outcomes | Comments |
|---|---|---|---|---|---|---|---|
| Simonds et al.(53)/England | Non-randomized trial | NA among control patients; not specified for coryzal patients or those with acute infective exacerbation of chronic respiratory disease | Single inpatient room on a respiratory ward | N = 11 normal controls; N = 11 healthy patients with infective symptoms; N = 21 chronic lung disease with infective symptoms | All patients received: oxygen therapy, NIV, modified NIV (add-on viral/bacterial filter to NIV), nebulized saline, physiotherapy for patients with chronic respiratory illness. A standard jet nebulizer with compressor was used to deliver 4 mL of normal saline. | Droplets were detected per intervention by using an optical particle sizer. Droplet sampling was carried out over 30 seconds at 5-minute intervals at baseline and during interventions. At 20 cm from patient's mask and at 1 m at 45° lateral plane. Nebulizer therapy: In all groups, there was a significant rise in droplets and aerosolization (ranges: 0.3–3.5 μm) at 20 cm and at 1 m. | Select patients did not undergo NIV due to claustrophobia |
| Hui et al.(19)/Hong Kong | Human patient simulator experiment | NA | Negative pressure hospital isolation room | Adult high-fidelity human patient simulator: normal, mild and severe lung injury | Jet nebulizer Air flow: 6 L/min | Exhale air particles measured by laser light: max distance ≤0.45 m (normal lung) to >0.8 m (severe lung injury). | More distant leakage through nebulizer side vents with severe lung injury, but less high concentration smoke particles |
| Hui et al.(18)/Hong Kong | Human patient simulator experiment | NA | Double-door negative pressure isolation room | Adult high-fidelity human patient simulator: normal, mild and severe lung injury | Jet nebulizer Air flow: 6 L/min | Exhale air particles measured by laser light: max distance ≤0.45 m (normal lung) to >0.8 m (severe lung injury). | Possible companion paper; same methods, authors, and results as Hui et al.19 report |
| Mueller et al.(44)/Germanya | Non-randomized trial | NA | Sterile clean room; patient in seated position | Patients undergoing airway management procedure as part of routine clinical care | Portable home jet nebulizer plus oxygen mask | Droplets measured by high-speed (1000 frames/s) camera with light against black background; no droplets visualized; fine aerosols detected but not quantified due “to abundance.” | N = 8 patients were included; unclear how many times each patient was evaluated (up to five times) |
| McGrath et al.(55)/Ireland | Human patient simulator experiment | NA | Room with no external doors/windows, mechanically ventilated | Breathing simulator via absolute filter set to adult respiratory rate, tidal volume and inspiratory:expiratory ratio | Vibrating Mesh nebulizer with aerosol chamber (air flow 6 L/min) and Jet nebulizer (8 L/min); combined with both facemask and mouthpiece (filtered and unfiltered); 2.5 mL albuterol sulfate nebulized | Aerosols measured by two aerodynamic particle sizers at 0.8 and 2.2 m from simulated patient over 25 minutes. Vibrating mesh nebulizer had fewer fugitive emissions than jet nebulizer. Unfiltered mouthpiece had fewer than facemask. No increase versus baseline in aerosol concentration with VMN/filtered mouthpiece. Size of fugitive emission aerosols ranged from 0.860 to 1.437 μm across combinations. | Focus on potential exposure of medical aerosols that potentially contain viral particles rather than direct measure of infectious aerosols |
| Blood et al.(56)/United States | Proof-of-concept simulator experiment | NA | Negative pressure clinic procedure rooms without high-efficiency particulate air (HEPA)-filters | Manikin (type unspecified) | 4 Minutes normal saline nebulizer via piston compression Nebulizer and Breath Actuated nebulizer; with and without addition of suction (120 mmHg) and oxygen (10 L/min) on | Control measurements at 3″ above height of isolation chamber bag (appears to be 31″) from bed without isolation chamber found peak particular measurements of 59,627 particulate/cm3 for the Power Nebulizer 2 at 1 minute and 214,020 particulate/cm3 for the breath actuated nebulizer at 5 minutes; measurements decreased to 4193 (SD 2260) and 4903 (SD 326) at 9 minutes and 927 (SD 2225) and 1030 (SD 131) at 13 minutes, respectively. | Manikins did not simulate human breathing. No information about size of manikins. |
| Tang et al.(54)/Finland | Human patient simulator | LAIV as surrogate virus tracer | Mock isolation room with mixed ventilation at 12 air exchanges per hour | Breath simulation produced by nebulizer with LAIV aerosols | Portable home jet nebulizer (air flow 6–8 L/min) nebulizing distilled water | Air-sampling for 10 minutes by three biosamplers into viral transport medium at 0.4 m near head, 1.10 m near abdomen, and 1.7 m near the feet. Over five repetitions, average viral loads in viral transport medium were 7.34 ± 0.28 × 104 copies/mL (head), 2.09 ± 0.41 × 104 copies/mL (abdomen), and 1.41 ± 0.23 × 104 copies/mL (feet). | Five repetitions over 2 days |
Preprint article.
LAIV, licensed live-attenuated influenza vaccine; NA, not applicable.
We found five publications describing simulations of patients undergoing nebulizer treatment(18,19,54–56) (Table 4). First, Tang et al. used live-attenuated influenza vaccine as a surrogate virus tracer in a simulation model with nebulized, distilled water from a portable home nebulizer.(54) They used biosamplers collecting into VTM over 10 minutes of nebulization within a mock isolation room at distances of 0.4, 1.1, and 1.7 m from the manikin's nose. They found decreasing average viral loads at samplings as distance increased: 7.34 ± 0.28 × 104 copies/mL VTM (0.4 m), 2.09 ± 0.41 × 104 copies/mL VTM (1.1 m), and 1.41 ± 0.23 × 104 copies/mL VTM (1.7 m). Second, McGrath et al. characterized aerosol emissions during administration of albuterol sulfate with combinations of open facemask, jet nebulizer versus a valved facemask, and vibrating-mesh nebulizer, while comparing each nebulizer type by using a mouthpiece with and without a filter.(55) Aerosol emissions were measured via ultraviolet spectrophotometry during three trials of all combinations. Open facemask, jet nebulizers had greater total aerosol emissions than closed facemask, vibrating-mesh nebulizers. Further, same-type nebulizers with an unfiltered mouthpiece displayed greater aerosol emission than their filtered mouthpiece counterpart. Taken together, those with an open facemask, jet nebulizer had the greatest level of aerosol concentration whereas those with a filtered mouthpiece, vibrating-mesh nebulizer had the lowest aerosol emission. The vibrating-mesh nebulizer combined with a filtered mouthpiece showed no increase in aerosol concentration at 0.8 m over baseline. Third, Blood et al. measured particles inside and outside a patient isolation system placed over a manikin's head in multiple treatment conditions, including nebulizer administration of normal saline.(56) Experiments were conducted at two hospitals, one with a piston-compression nebulizer via face mask and the other with a breath-actuated nebulizer via mouthpiece. Particulate counts were measured at baseline and every 2 minutes for 12 minutes, of which the first 4 minutes were during nebulizer treatment. In five trials without the isolation chamber, peak particulate measurements of 59,627 particulate/cm3 for the piston compression nebulizer 2 at 1 minute, and 214,020 particulate/cm3 for the breath-actuated nebulizer at 5 minutes; measurements decreased to 4193 (standard deviation [SD] 2260) and 4903 (SD 326) at 9 minutes and 927 (SD 2225) and 1030 (SD 131) at 13 minutes, respectively. Their isolation chamber system reduced >99% of nebulized particles in the surrounding environment. The fourth and fifth simulation publications by Hui et al. described experiments administering jet nebulization of sterile water using a human patient simulator that modeled variable lung damage (normal, mild, and severe injury) and used smoke particles to visualize exhaled air and aerosolized dispersion by laser light visualization.(18,19) In both publications with overlapping authors, they reported air particles measured at up to 0.45 m for normal lung function and >0.8 m with simulated severe lung injury. The severe lung injury simulation had fewer high-concentration particles than healthier lung function models.
Discussion
This systematic review included 14 publications examining patients with SARS or MERS, 1 publication with SARS-CoV-2, and 7 publications of studies examining dispersion of droplets with sham nebulizer treatments in human simulators or patients without known coronavirus infections. Evidence that nebulizer treatments increase risk of coronaviruses similar to COVID-19 is inconclusive, and there is minimal direct evidence about the risk for transmission of SARS-CoV-2. Specifically, across the seven cohort/case-control studies, we found that the risk of transmission due to nebulizer treatment exposure had a median OR of 2.4, and ranged from 0.08 (95% CI 0.01–0.95) to 20.7 (1.4–300.9) based on very weak data among a small number of HCWs with variable use of PPE. In addition, there were no studies that compared the transmission risk between MDIs and nebulizers, nor did any of the studies address transmission risk in the outpatient setting or whether this risk was modified when HCWs used PPE. Moreover, only one of the case-control or cohort studies considered asymptomatic spread and it was with SARS.
Our findings build on a 2012 systematic review addressed AGPs and the risk of transmitting acute respiratory infections to HCWs.(14) The authors identified three eligible retrospective cohort studies also included in this review that addressed risk of nebulizer treatments for HCWs, all judged to be very low quality.(22,23,25) A pooled estimate showed no increased risk (OR 0.9; 95% CI 0.1–13.6) with high statistical heterogeneity. A sensitivity analysis excluded the study by Wong et al.(25) on the basis that lack of training/description of infection control measures may have introduced bias, and showed an increased risk for transmission with nebulizer treatments (OR 3.7; 95% CI 0.7–19.5). The review by Tran et al. was protocol driven, used a comprehensive search, and employed standard systematic review methods. However, we note important weaknesses such as misapplication of Grading of Recommendations Assessment, Development, and Evaluation (GRADE) quality ratings(57) to individual studies; nonstandard ROB assessment; reporting a pooled estimate for highly heterogeneous studies (methodologically and statistically); and using a statistical approach shown to generate 95% CI that are often too narrow.(58)
We identified several gaps in the existing literature (see Table 5 for a modified gap framework.(59)) Of particular relevance is a lack of direct comparison between different types of nebulizer delivery systems and with other inhaled medication delivery mechanisms, as some may incur greater risk than others. For example, in the event of a faulty expiratory valve on the mouthpiece, there may be a greater theoretical risk for contamination of the liquid formulation within the jet nebulizer cup, compared with vibrating-mesh nebulizers that receive liquid formulations commonly through a syringe pump Luer-lock adaptor. In fact, the study by McGrath et al. found that aerosol emission was higher with jet nebulizers than with vibrating-mesh nebulizers and that the emission rate was attenuated by choice of patient applicators (i.e., filtered mouthpiece vs. facemask).(55) In the current COVID-19 pandemic, many providers and health care systems are recommending use of MDIs as they are believed to have lower risk for virus transmission,(16) a practice that has led to a shortage of MDIs.(60) In Wuhan, nebulizer treatments were used frequently in hospitalized patients with SARS-CoV-2,(61,62) but the relationship of these treatments to infection of hospital workers has not yet been reported. To strengthen this body of literature, observational studies could prospectively collect data on potential exposures while specifically characterizing the type of nebulizer equipment or inhaler used and PPE worn during potential exposures. In addition, observational studies could track and test asymptomatic exposed patients and explore more sophisticated and definitive methods to tie exposures to viral transmission such as molecular tracking.(51,52,61,62)
Table 5.
Evidence Gaps in Risk of Coronavirus Disease 2019 Transmission with Nebulizer Administration
| Study characteristic | Current evidence | Evidence gap |
|---|---|---|
| Population | Symptomatic patients with primarily non-COVID illnesses; HCWs during SARS/MERS epidemic and with variable PPE use | More data from patients with COVID-19; asymptomatic patients; variability in HCWs following current PPE standards |
| Intervention | Largely unspecified type of nebulizer | Comparison between types of nebulizer delivery (e.g., jet vs. vibrating mesh) |
| Comparator | No nebulizer exposure | Metered dose inhaler with/without spacer (vs. nebulizer) |
| Outcomes | New cases of disease with probable or documented exposure to known case receiving nebulizer treatment | Viable viral particles at different distances from patients with SARS-CoV-2 (or flu or other coronaviruses); molecular tracking via DNA sequencing to definitively identify transmission from person to person |
| Setting | Hospitalized patients | Outpatient setting, emergency department |
| Timing | Retrospective transmission tracking | Data collection ideally closer to time of exposure |
| Designs | Retrospective cohort and case-control studies | Prospective cohort studies |
Our review approach has limitations. Specifically, given the rapid production of literature specific to COVID-19, it is possible we missed relevant manuscripts despite searching various preprint servers. In view of the recent arrival of COVID-19, we also included data from other viral infections and simulation studies, which may not be directly applicable to patient care during this pandemic. We did not evaluate simulation studies of fugitive emissions that did not include patient simulation, which could provide additional information about the transmission risk with nebulizer use. Last, it is unknown as to what amount of viral load is required to transmit COVID-19 during human exposure, which significantly limits the interpretation of this review.
Conclusion
The possibility that nebulizers increase viral transmission cannot be ruled out. Specifically, there is a paucity of direct evidence regarding the risk for transmission of SARS-CoV-2 in infected patients requiring nebulizer therapy. Thus, prolonged exposures of patients with COVID-19 during nebulizer treatments should be minimized. In the absence of definitive evidence, clinical treatment decisions that utilize nebulizers should be driven by the most appropriate type of nebulizer for a given patient and the safest mode of medication delivery for exposed HCWs and patient caregivers. Additional investigations quantifying viral aerosolization from a variety of nebulizer delivery devices will be needed to better characterize the role that nebulizers play, if any, in the transmission of COVID-19.
Supplementary Material
Disclaimer
The views expressed are those of the authors and do not reflect the official views or policy of the U.S. Department of Defense or its components.
Reproducible Research Statement
Data set is available from Ms. Gordon (e-mail: adelaide.gordon@va.gov).
Authors' Contributions
K.M.G., J.W.W., and J.M.G. designed the study. K.M.G., K.G., and J.W.W. drafted the article. K.M.G., K.G., J.W.W., H.M., Y.K., and T.M. screened citations. K.M.G., K.G., J.W.W., and H.M. abstracted data and analyzed data. S.C., M.V.I., Y.K., and T.M. designed and executed the literature search. A.G. and B.E. designed and executed data organization and data representation. All authors reviewed and edited the article.
Acknowledgments
The authors gratefully acknowledge Isaretta Riley, MD, MPH, Steve Taylor, MD, MPH, Victoria Mobley, MD, MPH, and Christopher Hostler, MD, for providing expertise; Liz Wing, MA, for editorial assistance; and Robin Paynter, MLIS, for search assistance.
Author Disclosure Statement
The authors declare they have no competing financial interests.
Funding Information
This project was funded by the Department of VA, Veterans Health Administration, Office of Research and Development, Quality Enhancement Research Initiative (ESP 09-010). This work was also supported by the Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT) at the Durham VA Health Care System (CIN 13-410). Dr. Goldstein's effort is supported by VA HSR&D CDA award number 13-263. Dr. Ghadimi's effort is supported by NIH T32 GM008600, by Duke Health for CT.gov: NCT03081052, and receives recurrent remuneration from Wolters Kluwer Health for work with UpToDate, Inc. The U.S. Department of VA, which funded this review, was not involved in the design, conduct, or analysis of the study.
Supplementary Material
Reviewed by:
Stephan Ehrmann
Ariel Berlinski
References
- 1. Amnesty International: Global: amnesty analysis reveals over 7,000 health workers have died from COVID-19. September 3, 2020. Available at: https://www.amnesty.org/en/latest/news/2020/09/amnesty-analysis-7000-health-workers-have-died-from-covid19 (accessed October16, 2020)
- 2. Reychler G, Vecellio L, and Dubus JC: Nebulization: a potential source of SARS-CoV-2 transmission. Respir Med Res. 2020;78:100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization: Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. Available at: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed October16, 2020)
- 4. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, and Munster VJ: Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Services Scotland: Aerosol generating procedures (AGPs). Available at: https://hpspubsrepo.blob.core.windows.net/hps-website/nss/2893/documents/1_tbp-lr-agp-v1.pdf (accessed October16, 2020)
- 6. Centers for Disease Control and Prevention (CDC): Potential exposure at work. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html (accessed October16, 2020)
- 7. Alhazzani W, Møller MH, Arabi YM, Loeb M, Ng Gong M, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, and Rhodes A: Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med. 2020;48:e440–e469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ari A: Promoting safe and effective use of aerosol devices in COVID-19: risks and suggestions for viral transmission. Expert Opin Drug Deliv. 2020;17:1509–1513 [DOI] [PubMed] [Google Scholar]
- 9. UpToDate: Delivery of inhaled medication in adults. Available at: https://www.uptodate.com/contents/delivery-of-inhaled-medication-in-adults (accessed October16, 2020)
- 10. Camargo CA, Rachelefsky G, and Schatz M: Managing asthma exacerbations in the emergency department. Proc Am Thorac Soc. 2009;6:357–366 [DOI] [PubMed] [Google Scholar]
- 11. Cochrane: Cochrane's work on rapid reviews in response to COVID-19. Available at: https://www.cochrane.org/cochranes-work-rapid-reviews-response-covid-19 (accessed October16, 2020)
- 12. Haby MM, Chapman E, Clark R, Barreto J, Reveiz L, and Lavis JN: What are the best methodologies for rapid reviews of the research evidence for evidence-informed decision making in health policy and practice: a rapid review. Health Res Policy Syst. 2016;14:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, and Altman DG; PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tran K, Cimon K, Severn M, Pessoa-Silva CL, and Conly J: Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PloS One. 2012;7:e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swedish Agency for Health Technology Assessment and Assessment of Social Services: Risk for transmission of viral infection during treatment with nebuliser or high-flow nasal cannula. Available at: https://www.sbu.se/en/publications/responses-from-the-sbu-enquiry-service/risk-for-transmission-of-viral-infection-during-treatment-with-nebuliser-or-high-flow-nasal-cannula2 (accessed October27, 2020)
- 16. Daugherty EL, Branson RD, Deveraux A, and Rubinson L: Infection control in mass respiratory failure: preparing to respond to H1N1. Crit Care Med. 2010;38(4 Suppl):e103–e109 [DOI] [PubMed] [Google Scholar]
- 17. Gamage B, Moore D, Copes R, Yassi A, and Bryce E; BC Interdisciplinary Respiratory Protection Study Group: Protecting health care workers from SARS and other respiratory pathogens: a review of the infection control literature. Am J Infect Control. 2005;33:114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hui D, Chan M, and Chow B: Aerosol dispersion during various respiratory therapies: a risk assessment model of nosocomial infection to health care workers. Hong Kong Med J. 2014;20:9–13 [PubMed] [Google Scholar]
- 19. Hui DS, Chow BK, Chu LCY, Ng SS, Hall SD, Gin T, and Chan MTV: Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest. 2009;135:648–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khoo SM, Tan LK, Said N, and Lim TK: Metered-dose inhaler with spacer instead of nebulizer during the outbreak of severe acute respiratory syndrome in Singapore. Respir Care. 2009;54:855–860 [DOI] [PubMed] [Google Scholar]
- 21. Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, and Sung JJY: A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994 [DOI] [PubMed] [Google Scholar]
- 22. Loeb M, McGeer A, Henry B, Ofner M, Rose D, Hlywka T, Levie J, McQueen J, Smith S, Moss L, Smith A, Green K, and Walter SD: SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raboud J, Shigayeva A, McGeer A, Bontovics E, Chapman M, Gravel D, Henry B, Lapinsky S, Loeb M, McDonald LC, Ofner M, Paton S, Reynolds D, Scales D, Shen S, Simor A, Stewart T, Vearncombe M, Zoutman D, and Green K: Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PloS One. 2010;5:e10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong RS, and Hui DS: Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10:339–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong TW, Lee CK, Tam W, Tak-fai Lau J, Yu T-S, Lui S-F, Chan PKS, Li Y, Bresee JS, Sung JJY, and Parashar UD; Outbreak Study Group: Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis. 2004;10:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu IT, Xie ZH, Tsoi KK, Chiu YL, Lok SW, Tang XP, Hui DS, Lee N, Li YM, Huang ZT, Liu T, Wong TW, Zhong NS, and Sung JJ: Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44:1017–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rahman SA: Transmission of Middle East respiratory syndrome coronavirus infections among healthcare personnel in the Middle East: a systematic review. Trop J Pharm Res 2018;17:731–739 [Google Scholar]
- 28. Wilson NM, Norton A, Young FP, and Collins DW: Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia. 2020;75:1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evidence Aid. Coronavirus (COVID-19): evidence collection. Available at: https://www.evidenceaid.org/coronavirus-covid-19-evidence-collection (accessed October16, 2020)
- 30. Evidence for Policy and Practice Information and Co-ordinating Centre: COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx (accessed October16, 2020)
- 31. Evidence Synthesis Program (ESP): COVID-19 evidence reviews. Available at: https://covid19reviews.org (accessed October16, 2020)
- 32. Centre for Evidence-Based Medicine: Oxford COVID-19 evidence service. Available at: https://www.cebm.net/covid-19 (accessed October16, 2020)
- 33. National Institute for Health and Care Excellence: Coronavirus (COVID-19). Available at: https://www.nice.org.uk/covid-19 (accessed October16, 2020)
- 34. EBSCO: COVID-10 updates and information. Available at: https://covid-19.ebscomedical.com (accessed October16, 2020)
- 35. ECRI: COVID-19 resource center. Available at: https://www.ecri.org/coronavirus-covid-19-outbreak-preparedness-center (Accessed October16, 2020)
- 36. National Center for Biotechnology Information, U.S. National Library of Medicine: LitCOVID: Available at: https://www.ncbi.nlm.nih.gov/research/coronavirus (accessed October16, 2020)
- 37. World Health Organization: Global research on coronavirus disease. Available at: https://search.bvsalud.org/global-research-on-novel-coronavirus-2019-ncov (accessed October16, 2020)
- 38. Trip: Medical database. Available at: https://www.tripdatabase.com (accessed October16, 2020)
- 39. COVID-NMA: Living mapping and living network meta-analysis of Covid-19 studies. Available at: https://covid-nma.com (accessed October16, 2020)
- 40. National Institute for Health Research: NIHR's response to COVID-19. Available at: https://www.nihr.ac.uk/covid-19 (accessed October16, 2020)
- 41. Health Technology Wales: Coronavirus (COVID-19). Available at: https://www.healthtechnology.wales/covid-19 (accessed October16, 2020)
- 42. Ministry of Health Singapore: Homepage. Available at: https://www.moh.gov.sg (accessed October16, 2020)
- 43. Norwegian Institute of Public Health: Homepage. https:/www.fhi.no/en/ (accessed October16, 2020)
- 44. Mueller SK, Veltrup R, Jakubass B, Kempfle JS, Kniesburges S, Huebner MJ, Iro H, and Döllinger M: Clinical characterization of respiratory droplet production during common airway procedures using high-speed imaging. medRxiv. 2020:2020.07.01.20144386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park AJ, Newbern EC, Feikin DR, Issakbaeva ET, Park BJ, Fehr J, LaMonte AC, Le TP, Burger TL, Rhodes LV, 3rd, Weltman A, Erdman D, Ksiazek TG, and Lingappa JR; SARS Pennsylvania Case Investigation Team: Lack of SARS transmission and U.S. SARS case-patient. Emerg Infect Dis. 2004;10:217–224 [DOI] [PubMed] [Google Scholar]
- 46. Hall AJ, Tokars JI, Badreddine SA, Saad ZB, Furukawa E, Al Masri M, Haynes LM, Gerber SI, Kuhar DT, Miao C, Trivedi SU, Pallansch MA, Hajjeh R, and Memish ZA: Health care worker contact with MERS patient, Saudi Arabia. Emerg Infect Dis. 2014;20:2148–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heinzerling A, Stuckey MJ, Scheuer T, Xu K, Perkins KM, Resseger H, Magill S, Verani JR, Jain S, Acosta M, and Epson E: Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient—Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Varia M, Wilson S, Sarwal S, McGeer A, Gournis E, Galanis E, and Henry B; Hospital Outbreak Investigation Team: Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ. 2003;169:285–292 [PMC free article] [PubMed] [Google Scholar]
- 49. Nam HS, Park JW, Ki M, Yeon M-Y, Kim J, and Kim SW: High fatality rates and associated factors in two hospital outbreaks of MERS in Daejeon, the Republic of Korea. Int J Infect Dis. 2017;58:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park SH, Kim YS, Jung Y, Choi SY, Cho N-H, Jeong HW, Heo JY, Yoon JH, Lee J, Cheon S, and Sohn KM: Outbreaks of Middle East respiratory syndrome in two hospitals initiated by a single patient in Daejeon, South Korea. Infect Chemother. 2016;48:99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DAT, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H, Madani H, Alhakeem R, Al-Tawfiq JA, Cotten M, Watson SJ, Kellam P, Zumla AI, and Memish ZA; KSA MERS-CoV Investigation Team: Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hunter JC, Nguyen D, Aden B, Al Bandar Z, Al Dhaheri W, Elkheir KA, Khudair A, Al Mulla M, El Saleh F, Imambaccus H, Al Kaabi N, Sheikh FA, Sasse J, Turner A, Wareth LA, Weber S, Al Ameri A, Amer WA, Alami NN, Bunga S, Haynes LM, Hall AJ, Kallen AJ, Kuhar D, Pham H, Pringle K, Tong S, Whitaker BL, Gerber SI, and Al Hosani FI: Transmission of Middle East Respiratory syndrome coronavirus infections in healthcare settings, Abu Dhabi. Emerg Infect Dis. 2016;22:647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simonds AK, Hanak A, Chatwin M, Morrell M, Hall A, Parker KH, Siggers JH, and Dickinson RJ: Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess. 2010;14:131–172 [DOI] [PubMed] [Google Scholar]
- 54. Tang JW, Kalliomaki P, Varila TM, Waris M, and Koskela H: Nebulisers as a potential source of airborne virus. J Infect. 2020;81:647–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McGrath JA, O'Sullivan A, Bennett G, O'Toole C, Joyce M, Byrne MA, and MacLoughlin R: Investigation of the quantity of exhaled aerosols released into the environment during nebulisation. Pharmaceutics. 2019;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Blood TC Jr., Perkins JN, Wistermayer PR, Krivda JS, Fisher NT, Riley CA, Ruhl DS, and Hong SS: COVID-19 airway management isolation chamber. Otolaryngol Head Neck Surg. 2021;164:74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, and Guyatt G: The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, and Goodman SN: Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160:267–270 [DOI] [PubMed] [Google Scholar]
- 59. Robinson KA, Saldanha IJ, and McKoy NA: Development of a framework to identify research gaps from systematic reviews. J Clin Epidemiol. 2011;64:1325–1330 [DOI] [PubMed] [Google Scholar]
- 60. Mahase E: Covid-19: increased demand for steroid inhalers causes “distressing” shortages. BMJ. 2020;369:m1393. [DOI] [PubMed] [Google Scholar]
- 61. Gao T, Xu Y, He X, Ma Y, Wang L, Jiang Y, Wu C, Zhang M, and Chen J: Epidemiological and clinical characteristics of 40 patients with coronavirus disease 2019 outside Hubei. Chin J Respir Crit Care Med. 2020;19:148–153 [Google Scholar]
- 62. Chen J, Ling Y, Xi X, Liu P, Li F, Li T, Shang Z, Wang M, Shen Y, and Lu H: Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Chin J Infect Dis. 2020;38:86–89 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.