Abstract
The biosynthesis of sialic acid (Neu5Ac) leads to the intracellular production of cytidine-5′-monophospho-N-acetylneuraminic acid (CMP-Neu5Ac), the active sialic acid donor to nascent glycans (glycoproteins and glycolipids) in the Golgi. UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase myopathy is a rare autosomal recessive muscular disease characterized by progressive muscle weakness and atrophy. To quantify the intracellular levels of CMP-Neu5Ac as well as N-acetylmannosamine (ManNAc) and Neu5Ac in human leukocytes, we developed and validated robust liquid chromatography–tandem mass spectrometry methods. A fit-for-purpose approach was implemented for method validation. Hydrophilic interaction chromatography was used to retain three hydrophilic analytes. The human leukocyte pellets were lysed and extracted in a methanol–water mixture and the leukocyte extract was used for LC–MS/MS analysis. The lower limits of quantitation for ManNAc, Neu5Ac and CMP-Neu5Ac were 25.0, 25.0 and 10.0 ng/ml, respectively. These validated methods were applied to a clinical study.
Keywords: cytidine-5′-monophospho-N-acetylneuraminic acid, human leukocytes, hydrophilic interaction chromatography, LC–MS/MS
1 |. INTRODUCTION
The biosynthesis of N-acetylneuraminic acid (sialic acid; Neu5Ac) involves an intracellular pathway comprising complex enzymatic reactions. N-Acetylmannosamine (ManNAc), the first committed pre-cursor, is biosynthesized from UDP-N-acetylglucosamine (UDP-GlcNAc) by the rate-limiting, bifunctional enzyme UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase, or GNE (Stasche et al., 1997). ManNAc, a pathway intermediate, undergoes several cytoplasmic enzymatic modifications, resulting in the formation of Neu5Ac. Neu5Ac is converted in the cell nucleus by CMP-Neu5Ac synthetase to the end-product of this intracellular pathway, CMP-Neu5Ac (Li & Chen, 2012). The activated CMP-Neu5Ac leaves the nucleus, and cytoplasmic CMP-Neu5Ac can be transported into the Golgi by the CMP-Neu5Ac transporter (SLC35A1) (Zhao, Chen, Vertel, & Colley, 2006). In the Golgi, CMP-Neu5Ac serves as substrate for ~20 human sialyltransferases to sialylate nascent glycans (glycoproteins and glycolipids) (Li & Chen, 2012). Sialylated glycans are either secreted or inserted into membranes, where they perform important biological functions (Varki, 2008). Cytoplasmic CMP-Neu5Ac can also feedback-inhibit UDP-GlcNAc-2-epimerase activity by binding to the allosteric site of GNE, thereby tightly controlling Neu5Ac biosynthesis (Seppala, Lehto, & Gahl, 1999).
Several rare diseases are associated with the sialic acid biosynthesis pathway. For example, mutations in SLC35A1 cause congenital disorder of glycosylation IIf, characterized by hyposialylation of certain glycans (Martinez-Duncker et al., 2005). Dominant mutations within the allosteric site of UDP-GlcNAc-2-epimerase lead to a binding defect of CMP-Neu5Ac and cause sialuria, a severe disorder of sialic acid storage manifested by multi-organ involvement and hypersialylated O-glycans (Wopereis et al., 2006). GNE myopathy is an autosomal recessive inborn disorder of sialic acid metabolism with an estimated prevalence of ~6/1,000,000 caused by bi-allelic mutations in GNE, which lead to decreased sialic acid (Neu5Ac) production and subsequent hyposialylation of muscle glycans, which is the putative cause of muscle deterioration in the disease (Noguchi et al. 2004). GNE myopathy is characterized by progressive skeletal muscle atrophy and weakness, resulting in significant disability. There are no approved therapies or biomarkers for this debilitating disorder.
Previous studies using mouse models of GNE myopathy demonstrated that administration of Neu5Ac, ManNAc or sialyllactose prevented development of the disease and offered prospects for treatment (Malicdan, Noguchi, Hayashi, Nonaka, & Nishino, 2009). In support of a Phase 1 clinical trial to evaluate the safety, pharmacokinetics and pharmacodynamics of ManNAc for subjects with GNE myopathy (Xu et al., 2017), two LC–MS/MS methods to quantify ManNAc and free Neu5Ac (the predominant mammalian form of sialic acid (Varki, 2008) were developed and validated in human plasma (Shi et al., 2015). Hydrophilic interaction chromatography (HILIC) was applied to retain ManNAc and Neu5Ac on the HPLC column since both analytes have low molecular weights and multiple hydroxyl groups.
Here we describe the development and validation of an LC–MS/MS method to quantify CMP-Neu5Ac in human leukocytes.Because CMP-Neu5Ac synthetase is found almost exclusively in the cell nucleus, nucleated cells would be necessary to measure intracellular CMP-Neu5Ac (Munster et al., 2002). Leukocytes were selected because they are easily accessible nucleated cells and have the same GNE isoform expression as skeletal muscle cells (Yardeni et al., 2011), the major affected cell-type in GNE myopathy. The previously developed methods in human plasma were adapted to quantify ManNAc and Neu5Ac in human leukocytes.
HILIC chromatography was utilized for all three analytes. Owing to the solid nature of the leukocyte pellets, strategies different from the previous methods (Shi, Xu et al. 2015) were applied. The three methods were validated with a “fit-for-purpose” approach (Cummings, Ward, & Dive, 2010; Houghton, Horro Pita, Ward, & Macarthur, 2009; Jian, Edom, & Weng, 2012; Lee et al., 2006; Wang et al., 2009) and successfully applied to support GNE myopathy clinical trials.
2 |. EXPERIMENTAL
2.1 |. Chemicals
ManNAc (purity 100%) and Neu5Ac (purity 99.1%) were supplied by New Zealand Pharmaceuticals, Ltd (Palmerston North, New Zealand). CMP-Neu5Ac reference standard (purity 98.4%) was acquired from EMD Millipore Corporation (San Diego, CA, USA). Stable isotope-labeled internal standards ManNAc-13C-d3, Neu5Ac-d3 and CMP-NeuAc-13C3 were supplied by Ricerca Biosciences, LLC (Concord, OH, USA), Medical Isotopes Inc. (Pelham, NH, USA) and Omicron Biochemicals, Inc. (South Bend, IN, USA), respectively. Figure 1 depicts the chemical structures of all six compounds. Acetonitrile, methanol and acetic acid were obtained from Fisher Scientific (Pittsburgh, PA). Trifluoracetic acid was obtained from EMD Chemicals Inc. (Gibbstown, NJ). Formic acid (purity 99.9%), ammonium acetate (NH4Ac) (purity >98%), ammonium hydroxide (NH4OH), ammonium chloride (NH4Cl), potassium bicarbonate, ethylenediaminetetraacetic acid (EDTA) and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Lysing buffer (10×) for leukocyte collection was prepared by dissolving 41.5 g ammonium chloride, 5.0 g potassium bicarbonate and 1 ml of 0.5 M EDTA in 500 ml deionized water.
FIGURE 1.
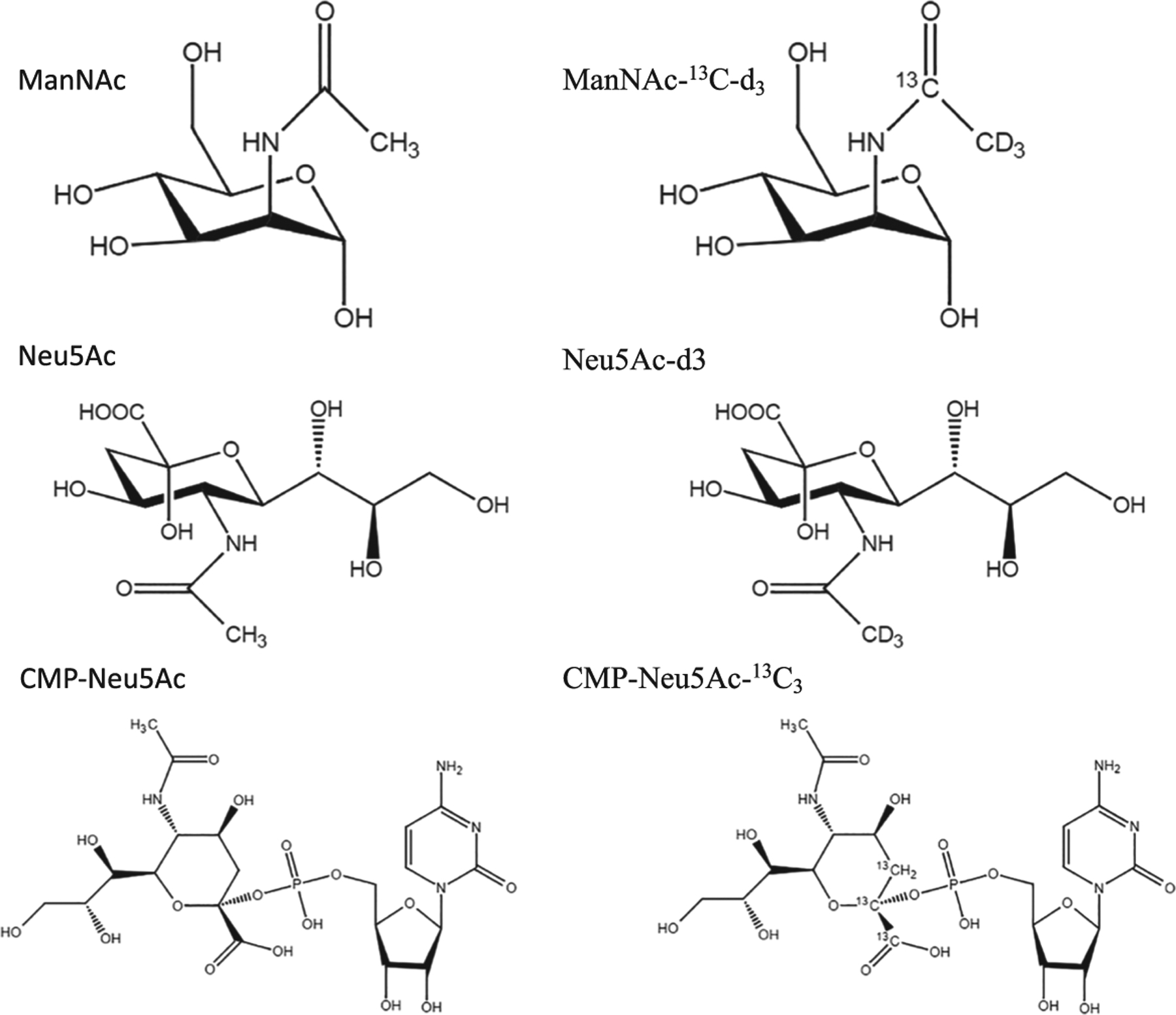
Chemical structures of N-acetylmannosamine (ManNAc), ManNAc-13C-d3, N-acetylneuraminic acid (Neu5Ac), Neu5Ac-d3, cytidine-5′-monophospho-sialic acid (CMP-Neu5Ac) and CMP-Neu5Ac-13C3
2.2 |. Leukocyte collections
Blood samples were collected into tubes with potassium K2EDTA as an anticoagulant. Samples were obtained from healthy donors at the Blood Bank of the National Institutes of Health (Bethesda, MD, USA) and from subjects with GNE myopathy enrolled under NIH protocol 15-HG-0068 “An Open-Label Phase 2 Study of ManNAc in Subjects with GNE Myopathy” (ClinicalTrials.gov NCT 02346461). The protocol was reviewed and approved by the National Human Genome Research Institute Institutional Review Board. All subjects were evaluated at the NIH Clinical Center and gave written, informed consent.
Within 30 min of blood collection, 10 ml whole blood was placed in a 35 ml lysing buffer for 30 min in a 50 ml conical tube. The tube was centrifuged for 5 min at 2000g, and the supernatant was discarded. A 600 μl aliquot of PBS was added to transfer the leukocyte pellet to a 2 ml Eppendorf tube, which was then centrifuged for 2 min at 1500g and PBS was discarded. The leukocyte pellet was washed twice more with 600 μl PBS. Leukocyte pellets were frozen at approximately −80°C and shipped on dry ice for analysis.
2.3 |. LC–MS/MS system
LC–MS/MS analyses were performed on an LC-20 AC HPLC system (Shimadzu, Kyoto, Japan) coupled with a Sciex API 4000 Q Trap mass spectrometer (AB Sciex, Foster City, CA, USA). HILIC chromatography was established on an XBridge Amide column (3.5 μm, 100 × 2.1 mm; Waters Co., Milford, MA, USA) for ManNAc (method 1), and on an Atlantis HILIC Silica column (5 μm, 50 × 3.0 mm; Waters Co., Milford, MA, USA) for Neu5Ac (method 2) and CMP-Neu5Ac (method 3). The HPLC columns were kept at ambient temperature.
2.4 |. LC–MS/MS conditions
For the LC separation of CMP-Neu5Ac from the matrix interference, the two mobile phases used were 0.05% NH4OH and 10 mM NH4Ac in water (mobile phase A) and 0.05% NH4OH with 10 mM NH4Ac in acetonitrile–water 99:1 (mobile phase B). The flow rate was 0.8 ml/min. Mobile phase B concentration was initially set at 90% and decreased to 75% in 0.3 min and kept for 2.3 min before being further decreased to 50% at 2.7 min and kept for 0.5 min. The gradient was ended at 4.5 min after holding at 90% B for 1.2 min.
Unlike ManNAc and Neu5Ac (Shi, Xu et al. 2015), CMP-Neu5Ac was monitored in negative-ion electrospray mode using selected reaction monitor. The optimized declustering potential and collision energy were −65 V and −26 eV, respectively. Turbo ion spray voltage was −4500 V, and the source temperature was 550°C. Curtain gas, nebulizing gas and auxiliary gas were 30 50, and 70 psi, respectively.The selected reaction monitoring for CMP-Neu5Ac was m/z 613.2 → 322 and for CMP-Neu5Ac-13C3 was 616.2 → 322.
For ManNAc and Neu5Ac, the previously developed methods for detecting these analytes in human plasma were adapted (Shi, Xu et al. 2015).
3 |. SAMPLE PREPARATION
3.1 |. Human leukocyte extract and surrogate blank matrix preparation
To prepare human leukocyte extracts, 100 μl of water and 1.00 ml of methanol were added to each tube that contained ~100 μl of packed human leukocyte pellet. The tubes were vortexed at 1650 rpm for 3 min and centrifuged at 3000 rpm for 5 min to lyse human leukocytes and pellet precipitated proteins. The extract (supernatant) was transferred to a clean tube. The extracts of normal human leukocytes were pooled and used as control matrix to prepare medium, high and dilution quality control (QC) samples.
A 2% BSA in water solution was used as the surrogate blank sample. To every 100 μl of 2% BSA solution, 100 μl of water and 1.00 ml of methanol were added. The solution was vortexed at 1650 rpm for 3 min and centrifuged at 3000 rpm for 5 min to precipitate proteins. The supernatant was transferred to a clean tube and used as surrogate blank matrix to prepare calibration standards and low QCs.
3.2 |. Stock solution, calibration standards and quality control
Stock solutions of CMP-Neu5Ac and CMP-Neu5Ac-13C3 were prepared and stored similar to ManNAc and Neu5Ac and their internal standards (Shi, Xu et al. 2015). The calibration ranges were 25.0–10,000 ng/ml for ManNAc and Neu5Ac, and 10.0–1000 ng/ml for CMP-Neu5Ac. Calibration standards, lower limit of quantitation QC (LLOQ-QC) and low QC (LQC), were prepared in surrogate blank matrix (methanol extract of 2% BSA solution). Medium QC (MQC), high QC (HQC) and dilution QC (DQC) were prepared in pooled leukocyte extract. The spiked-in concentrations of the five levels of QCs were 25.0, 75.0, 200, 8000 and 50,000 ng/ml for ManNAc and Neu5Ac, and 10.0, 30.0, 200, 500 and 2000 ng/ml for CMP-Neu5Ac, respectively. For the QC samples prepared in the pooled human leukocyte extract, the endogenous levels of ManNAc, Neu5Ac and CMP-Neu5Ac were pre-quantified and added to the spiked-in concentrations to calculate the nominal QC concentrations. All samples were stored at −80°C before analysis.
3.3 |. Sample preparation for LC–MS/MS analysis
To prepare samples for HILIC LC–MS/MS analysis, aliquots of 20 μl of pooled leukocyte extract, calibration standards, QCs and unknown sample extracts were transferred to a clean 96-well plate, and 20 μl of internal standard working solution in 50:50 acetonitrile–water (v/v) (2000 ng/ml ManNAc-13C-d3 for ManNAc samples, 2000 ng/ml Neu5Ac-d3 for Neu5Ac samples, or 2000 ng/ml CMP-Neu5Ac-13C3 for CMP-Neu5Ac samples) was added to each sample followed by 500 μl of reconstitution solution [50:50 acetonitrile–water (v/v) for ManNAc and Neu5Ac samples, or 0.05% NH4OH and 10 mM NH4Ac in 50:50 acetonitrile–water (v/v) for CMP-Neu5Ac samples]. The plates were sealed and vortexed at 1650 rpm for 3 min for ManNAc and Neu5Ac LC–MS/MS analysis. For CMP-Neu5Ac samples, 100 μl of the solution was transferred to a clean plate and further diluted with 200 μl of the reconstitution solution before LC–MS/MS analysis.
4 |. RESULTS AND DISCUSSION
4.1 |. Challenges and strategies for assay development
CMP-Neu5Ac, as well as ManNAc, and Neu5Ac, are endogenous cellular compounds in human leukocytes; thus it is difficult to determine the sensitivity and selectivity and evaluate the matrix effect and recovery during method development of these analytes. Unlike the validation of LC–MS/MS assays for small molecule pharmacokinetic studies, no specific regulatory guidance is yet available for endogenous compound method validation. Instead, a “fit-for-purpose” validation approach is usually adopted, in which the level of validation can be adjusted based on the actual application of the assay (Cummings et al., 2010; Houghton et al., 2009; Jian et al., 2012; Wang et al., 2009; Lee et al., 2006). Within this study, a surrogate matrix, which was known to be free of the endogenous compounds, was utilized to prepare the standards, LLOQ-QC, and LQC, while MQC, HQC and DQC were prepared in pooled leukocyte extract so that the assay performance in leukocyte extract was reflected. The endogenous levels of the three analytes were pre-quantified in the pooled leukocyte extract used for preparations of MQC, HQC and DQC and the final nominal concentrations of these QC samples calculated. Stable isotope labeled internal standards were implemented in all three methods to track ionization efficiency and minimize potential matrix effect.
Compared with assay development in matrices like plasma or serum, another significant challenge in human leukocyte assay development is that the solid state of the original human leukocyte pellet makes it impossible for the study sample to be conveniently aliquoted by volume or even by weight, since the homogeneity is uncertain for the analyte distribution in the pellet. In this study, a mixture of 1.00 ml of methanol and 100 μl of water was used to extract approximately 100 μl of human leukocyte pellet. With this strategy, the pool of methanol extract of human leukocyte was used for MQC, HQC and DQC preparation. Correspondingly, a similar extract of 2% BSA aqueous solution was used as the surrogate matrix for STDs, LLOQ-QC and LOQ preparation. One advantage of this strategy is that the methanol extract is mass spectrometry-friendly; it only requires further dilution before injection and thus simplifies the assay procedure. Using methanol extract as the matrix also facilitates the conversion of the final concentration unit. Since leukocytes make up ~1% of the total blood volume in an adult (Alberts et al., 2002), a volume of ~100 μl of leukocyte pellet is the predicted harvest from 10 ml of whole blood collected. Thus, using a fixed volume of 1.10 ml extraction solvent (1.00 ml of methanol and 100 μl of water) to extract each study sample generates a similar constitution. With the assumption that the final volume of methanol extract is 1.10 ml, and the exact blood volume (ml) and leukocyte counts (million cells/ml) for each subject, we converted the observed concentration (ng/ml) from the LC–MS/MS assay to the concentration in leukocytes (ng/million cells).
A HILIC silica column was used to achieve the HILIC separation in this study for CMP-Neu5Ac, and the mobile phases were prepared in basic condition to increase the mass spectrometer’s sensitivity in the negative ionization mode. The combination of the HILIC column and basic mobile phases helped to achieve good peak shape, sufficient retention time and acceptable background noise.
4.2 |. Calibration curves, selectivity and sensitivity
The calibration curves with eight different concentrations ranged from 25.0 to 10,000 ng/ml for ManNAc and Neu5Ac, and from 10.0 to 1000 ng/ml for CMP-Neu5Ac. A 1/x2 weighted linear regression was used for all three methods. For all of the validation runs, the correlation coefficient was >0.997 for all three analytes.
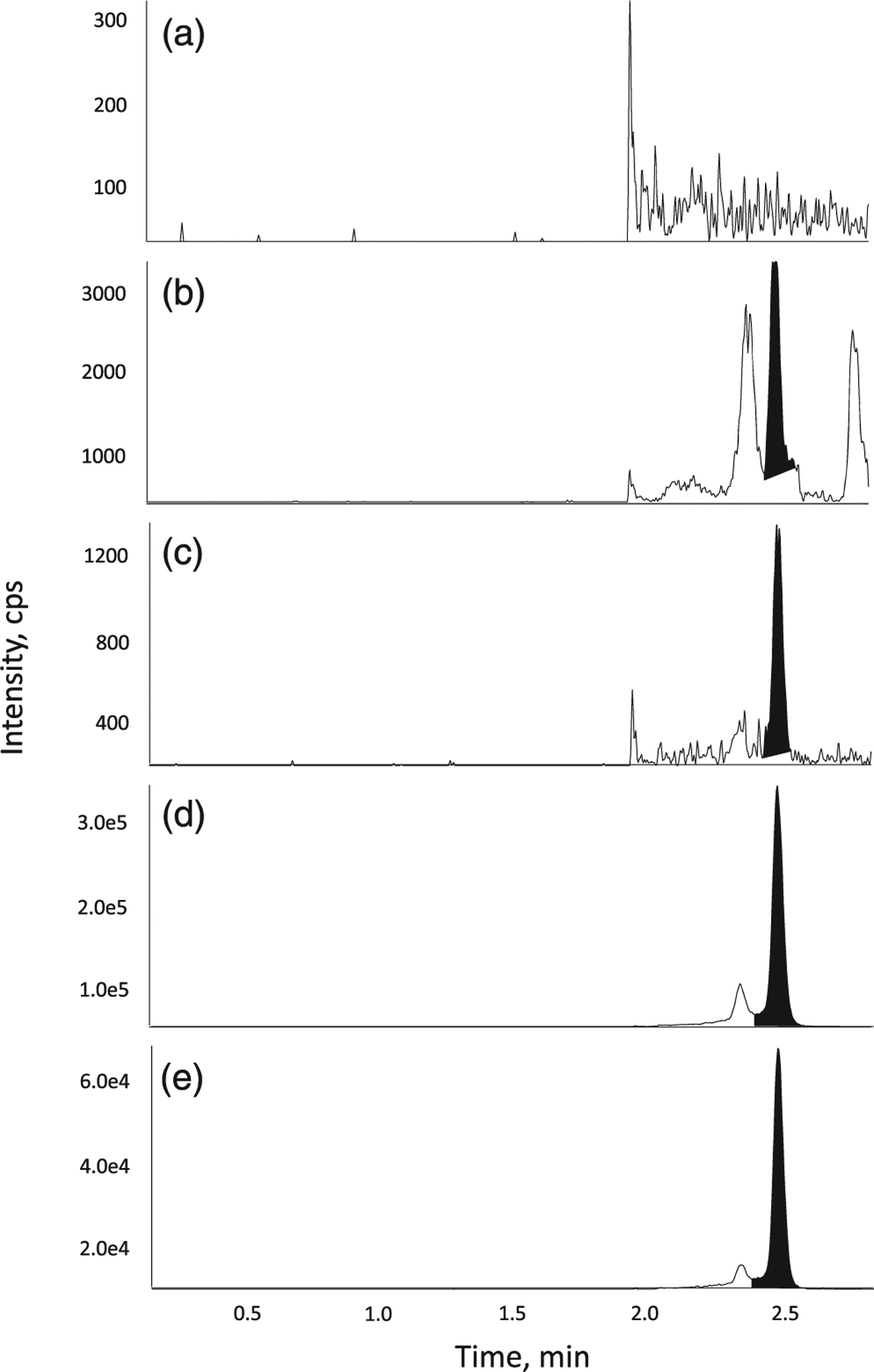
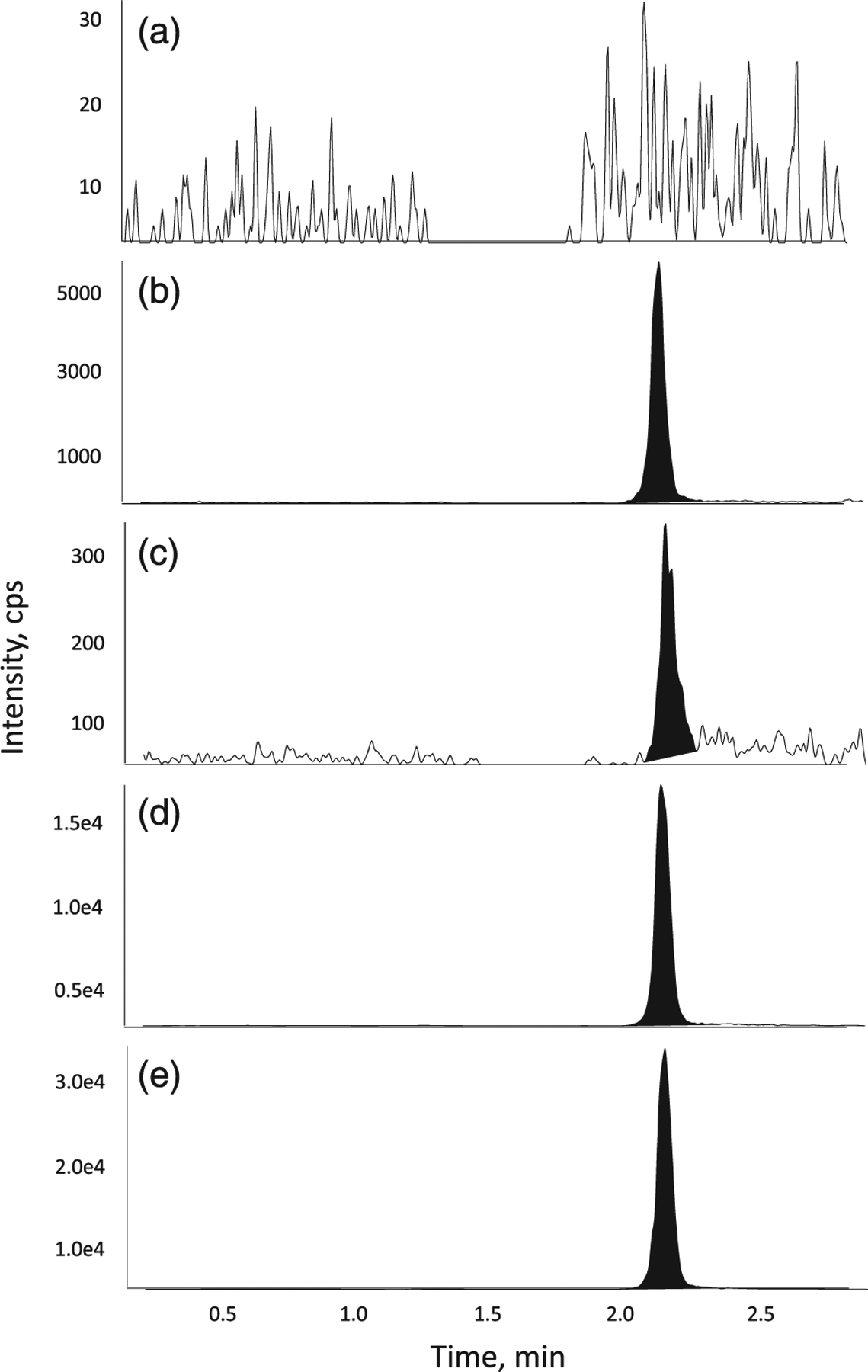
Representative chromatograms of a surrogate blank matrix (a), a pooled human leukocyte extract sample (b), an LLOQ sample (c), a ULOQ sample (d) and the internal standard in human leukocyte extract (e) are shown in Figures 2–4 for ManNAc, Neu5Ac and CMP-Neu5Ac, respectively. An acceptable assay selectivity was confirmed with no interference with ManNAc, Neu5Ac and CMP-Neu5Ac using multiple MRM for each analyte during method development. To evaluate the sensitivity at the LLOQ level, LLOQ-QC samples were assayed in six replicates in one batch. The signal-to-noise ratio was >5 for all three methods. The measured concentrations had acceptable accuracy and precision for all three analytes.
FIGURE 2.
Representative chromatograms of ManNAc in (a) surrogate blank matrix, (b) blank matrix, (c) a LLOQ sample, (d) a ULOQ sample and (e) internal standard in matrix
FIGURE 4.
Representative chromatograms of CMP-Neu5Ac in (a) surrogate blank matrix, (b) blank matrix, (c) a LLOQ sample, (d) a ULOQ sample and (e) internal standard in matrix
4.3 |. Accuracy and precision of QC samples
For all three analytes, QC samples were used to assess intraday and interday assay accuracy and precision. Each QC sample was analyzed as six independent replicates on three different days. The assay accuracy was calculated by comparing the measured concentrations withtheir nominal values, and the intraday and interday precisions were depicted as percentage coefficient of variation (CV; Table 1). The accuracy was 100 ± 5.9, 100 ± 5.6 and 100 ± 7.0% for ManNAc, Neu5Ac and CMP-Neu5Ac, respectively. The intraday variation (CV) was <12.0, 5.2 and 5.8% for ManNAc, Neu5Ac and CMP-Neu5Ac, respectively. The interday variation (CV) was <4.6, 2.9 and 6.6% for ManNAc, Neu5Ac and CMP-Neu5Ac, respectively.
TABLE 1.
Assay accuracy and precision (CV) of LQC, MQC and HQC samples for N-acetylmannosamine (ManNAc), N-acetylneuraminic acid (Neu5Ac) and cytidine-5′-monophospho-sialic acid (CMP-Neu5Ac)
| ManNAc | LQC (75.0 ng/ml) | MQC (267 ng/ml) | HQC (8067 ng/ml) |
|---|---|---|---|
| Mean (ng/ml) | 79.4 | 278 | 8480 |
| Accuracy (%) | 105.9 | 104.1 | 105.1 |
| Intra-assay precision (CV, n = 6) | <10.1 | <12.0 | <5.7 |
| Inter-assay precision (CV, n = 18) | 4.6 | 2.6 | 1.0 |
| Neu5Ac | LQC | (75.0 ng/ml) | |
| MQC (1175 ng/ml) | HQC | (8975 ng/ml) | |
| Mean (ng/ml) | 79.2 | 1120 | 8830 |
| Accuracy (%) | 105.6 | 95.3 | 98.4 |
| Intra-assay precision (CV, n = 6) | <4.0 | <5.2 | <3.5 |
| Inter-assay precision (CV, n = 18) | 0.4 | 2.9 | 2.2 |
| CMP-Neu5Ac | LQC | (30.0 ng/ml) | |
| MQC (471 ng/ml) | HQC | (771 ng/ml) | |
| Mean (ng/ml) | 31.4 | 438 | 743 |
| Accuracy (%) | 104.7 | 93.0 | 96.4 |
| Intra-assay precision (CV, n = 6) | <5.8 | <5.3 | <4.7 |
| Inter-assay precision (CV, n = 18) | 6.6 | 2.5 | 4.1 |
4.4 |. Matrix effect and extraction recovery
The matrix effect and the recoveries in surrogate matrix and leukocyte extract were evaluated following the procedures described previously (Shi, Xu et al. 2015). Briefly, the matrix factor was calculated as the ratio of the mean peak area of the analyte spiked in blank matrix extract and the mean peak area of the neat solutions at the same concentration. For ManNAc and ManNAc-13C-d3 in surrogate matrix, the matrix factors were 100.0 and 98.6%, respectively. The matrix factors for Neu5Ac and Neu5Ac-d3 in surrogate matrix were 97.4 and 91.6%, respectively; and the matrix factors for CMP-Neu5Ac and CMP-Neu5Ac-13C3 in surrogate matrix were 102.0 and 102.0%, respectively. The normalized matrix factor (analyte to internal standard) was 101.4% for ManNAc, 106.3% for Neu5Ac and 100.0% for CMP-Neu5Ac, which demonstrated that the matrix effect was reduced when a stable isotope labeled internal standard was used in each assay. The recoveries of all three analytes and their respective internal standards in surrogate blank matrix and leukocyte extract were >90%. The recovery data for all three analytes in LQC and HQC are summarized in Table 2.
TABLE 2.
Extraction recovery for ManNAc, Neu5Ac and CMP-Neu5Ac and their respective internal standards at a low concentration in surrogate blank matrix and at a high concentration in blank matrix
| Recovery (%) | ||
|---|---|---|
| LQC | HQC | |
| ManNAc | 89.3 | 81.1 |
| ManNAc-13C-d3 | 106.7 | 85.5 |
| Neu5Ac | 114.3 | 87.6 |
| Neu5Ac-d3 | 108.7 | 78.4 |
| CMP-Neu5Ac | 103.9 | 111.0 |
| CMP-Neu5Ac-13C3 | 106.2 | 117.7 |
4.5 |. Stability
The stabilities of all three analytes in surrogate blank matrix (LQC) and pooled leukocyte extract (HQC) were evaluated with six replicates. As summarized in Table 3, all three analytes showed acceptable results in surrogate matrix and lysate for freeze–thaw stability, autosampler stability, extracted sample stability, short-term storage stability and long-term storage stability at −80°C.
TABLE 3.
Stability results for ManNAc, Neu5Ac, and CMP-Neu5Ac in LQC (surrogate blank matrix) and in HQC (blank matrix)
| LQC (75.0 ng/ml) | HQC (8067 ng/ml) | |
|---|---|---|
| ManNAc | Deviation (%) | Deviation (%) |
| Room temperature for 18 h | 8.7 | 4.7 |
| Four freeze-thaw cycles | 3.7 | 1.8 |
| Autosampler at 4°C for 67 h | 12.4 | 9.2 |
| Processed sample at 4°C for 71 h | 6.5 | 8.5 |
| Long-term −80°C for 199 days | −1.9 | 1.4 |
| Neu5Ac | LQC (75.0 ng/ml) | HQC (8975 ng/ml) |
| Deviation (%) | Deviation (%) | |
| Room temperature for 18 h | 7.1 | −5.7 |
| Seven freeze-thaw cycles | −8.7 | −13.0 |
| Autosampler at 4°C for 114 h | 7.7 | 3.1 |
| Processed sample at 4°C for 100 h | 2.8 | −3.1 |
| Long-term −80°C for 198 days | 2.1 | 2.7 |
| CMP-Neu5Ac | LQC (30.0 ng/ml) | HQC (771 ng/ml) |
| Deviation (%) | Deviation (%) | |
| Room temperature for 21 h | 2.0 | −10.9 |
| Five freeze/thaw cycles | −4.3 | −3.5 |
| Autosampler at 4°C for 73 h | −2.7 | −10.6 |
| Processed sample at 4°C for 73 h | 3.7 | −9.7 |
| Long-term −80°C for 181 days | 0.0 | −3.6 |
4.6 |. Assay reproducibility and application
The three validated methods were implemented to quantify the levels of ManNAc, Neu5Ac and CMP-Neu5Ac in leukocytes to support Phase 2 clinical study in patients after oral administration of ManNAc (ClinicalTrials.gov Identifier NTC02346461). In this clinical trial, leukocytes were collected to assess pharmacodynamics. The levels of Man-NAc, Neu5Ac and CMP-Neu5Ac in leukocytes of healthy volunteers are listed in Table 4.
TABLE 4.
Measured concentrations (ng/ml) of ManNAc, Neu5Ac & CMP-Neu5Ac in healthy human leukocytes
| Sample ID | ManNAc (ng/ml) | Neu5Ac (ng/ml) | CMP-Neu5Ac (ng/ml) |
|---|---|---|---|
| Subject 369 | BLOQ | 162 | 203 |
| Subject 365 | BLOQ | 197 | 315 |
| Subject 374 | BLOQ | 105 | 217 |
| Subject 379 | BLOQ | 98 | 201 |
| Subject 381 | BLOQ | 85 | 144 |
| Subject 388 | 35.2 | 632 | 340 |
| Subject 390 | BLOQ | 168 | 178 |
| Subject 400 | BLOQ | 116 | 207 |
| Subject 401 | BLOQ | 252 | 225 |
| Subject 3 | 46.8 | 279 | 137 |
| Subject 4 | BLOQ | 283 | 108 |
| Subject 5 | BLOQ | 397 | 170 |
| Subject 6 | 39.2 | 456 | 150 |
| Subject 7 | 29.2 | 439 | 110 |
| Subject 8 | BLOQ | 336 | 129 |
| Subject 9 | BLOQ | 316 | 108 |
| Subject 10 | BLOQ | 264 | 116 |
| Subject 11 | 29.4 | 298 | 125 |
| Subject 12 | 50.7 | 471 | 145 |
| Subject 15 | BLOQ | 288 | 123 |
| Subject 16 | BLOQ | 1280 | 216 |
| Subject 18 | 48.7 | 300 | 127 |
| Subject 19 | 31.4 | 303 | 121 |
| Subject 20 | 50.6 | 279 | 124 |
BLOQ, Below limit of quantification (25.0 ng/ml for ManNAc).
For incurred sample reanalysis, 23 study samples were reanalyzed for the concentrations of Neu5Ac and CMP-Neu5Ac to assess assay reproducibility. Since the ManNAc concentration in all the samples was lower than 3× the LLOQ (<75 ng/ml), no incurred sample reanalysis test was required for ManNAc samples. As depicted in Figure 5, excellent reproducibility was confirmed with 100% (23 of 23) of the re-assayed samples for Neu5Ac and 96% (22 of 23) of the re-assayed samples for CMP-Neu5Ac meeting the acceptance criteria, where the initial and repeated values were 100 ± 20% of the mean values.
FIGURE 5.
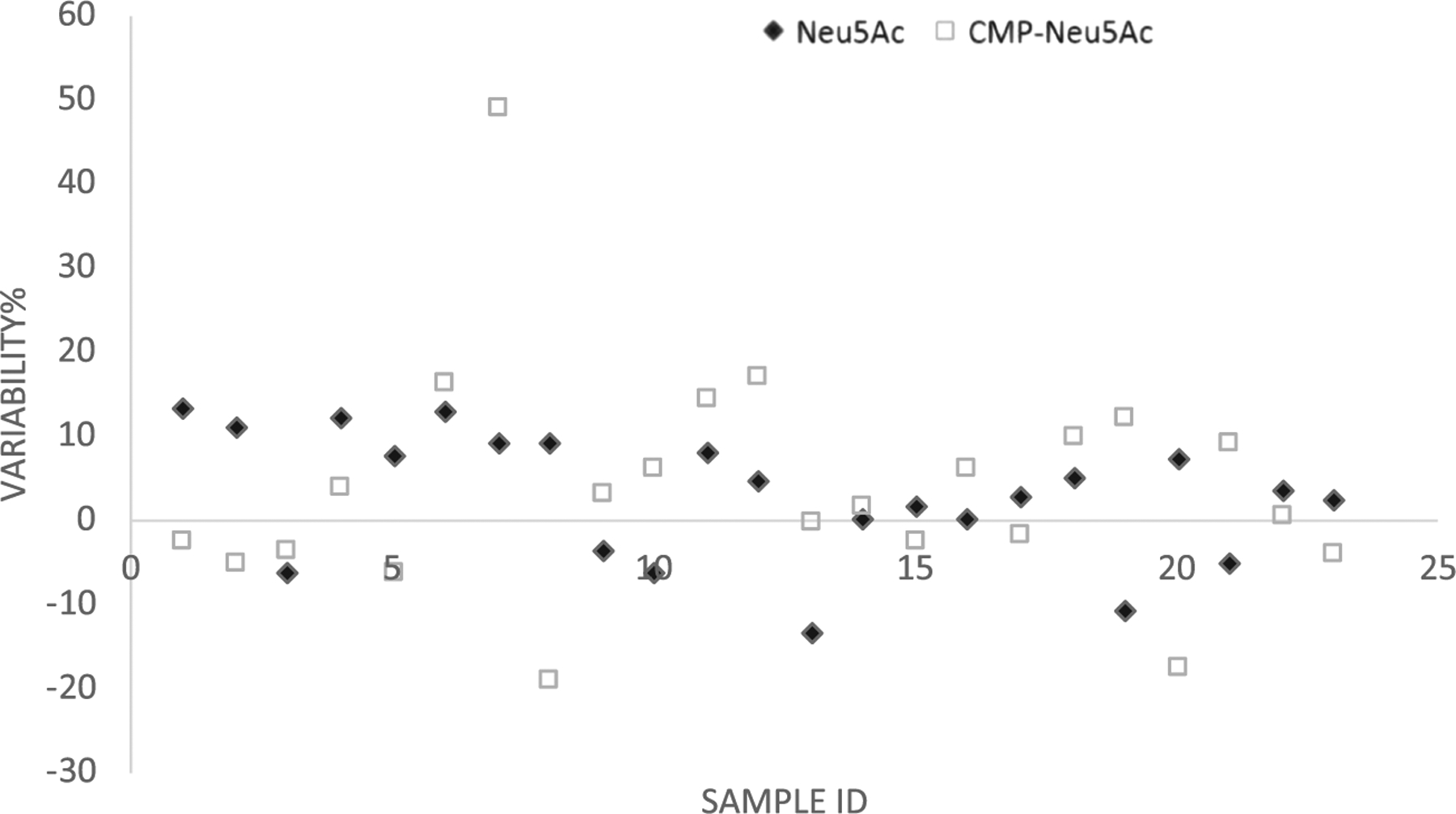
The incurred sample reanalysis results from 23 samples demonstrated assay reproducibility: 100% (23 of 23) for Neu5Ac and 96% (22 of 23) for CMP-Neu5Ac
Availability of bioanalytical methods for quantitation of human (intracellular) Neu5Ac pathway intermediates may assist in better understanding biochemical alterations of various inborn errors of sialic acid metabolism. Genetic diseases including CDG-IIf, sialuria and GNE myopathy have alterations in ManNAc, Neu5Ac and its activated nucleoside sugar, CMP-Neu5Ac (Martinez-Duncker et al., 2005; Wopereis et al., 2006). It has also been shown that adding ManNAc in cell culture increases the intracellular concentration of CMP-Neu5Ac in normal cells and cells lacking GNE activity (Gu, Harmon, & Wang, 1997; Hinderlich, Berger, Keppler, Pawlita, & Reutter, 2001). In addition, altered sialic acid regulation has been identified in certain cancers (Harvey & Thomas, 1993). Therefore, intracellular CMP-Neu5Ac levels may be an informative early marker of restoration of the biosynthesis of biologically active sialic acid, particularly after ManNAc administration, and may also be utilized to determine intracellular regulation of glycan sialylation in various disorders.
5 |. CONCLUSIONS
We have validated the first LC–MS/MS methods to quantify concentrations of the monosaccharides ManNAc, Neu5Ac and CMP-Neu5Ac in leukocytes. These methods are sensitive, specific, rapid, reproducible and robust. All three analytes showed good stability in human leukocyte extracts. The methods were successfully applied in the analyses of leukocyte samples from a Phase 2 clinical study of Man-NAc in patients with GNE myopathy. The ability to assess intracellular concentrations of these key sialylation intermediates provides a more representative tool to evaluate disease mechanisms than previously established plasma bioanalytical methods for ManNAc and Neu5Ac. The methods could be readily adapted in other studies of sialic acid metabolism.
FIGURE 3.
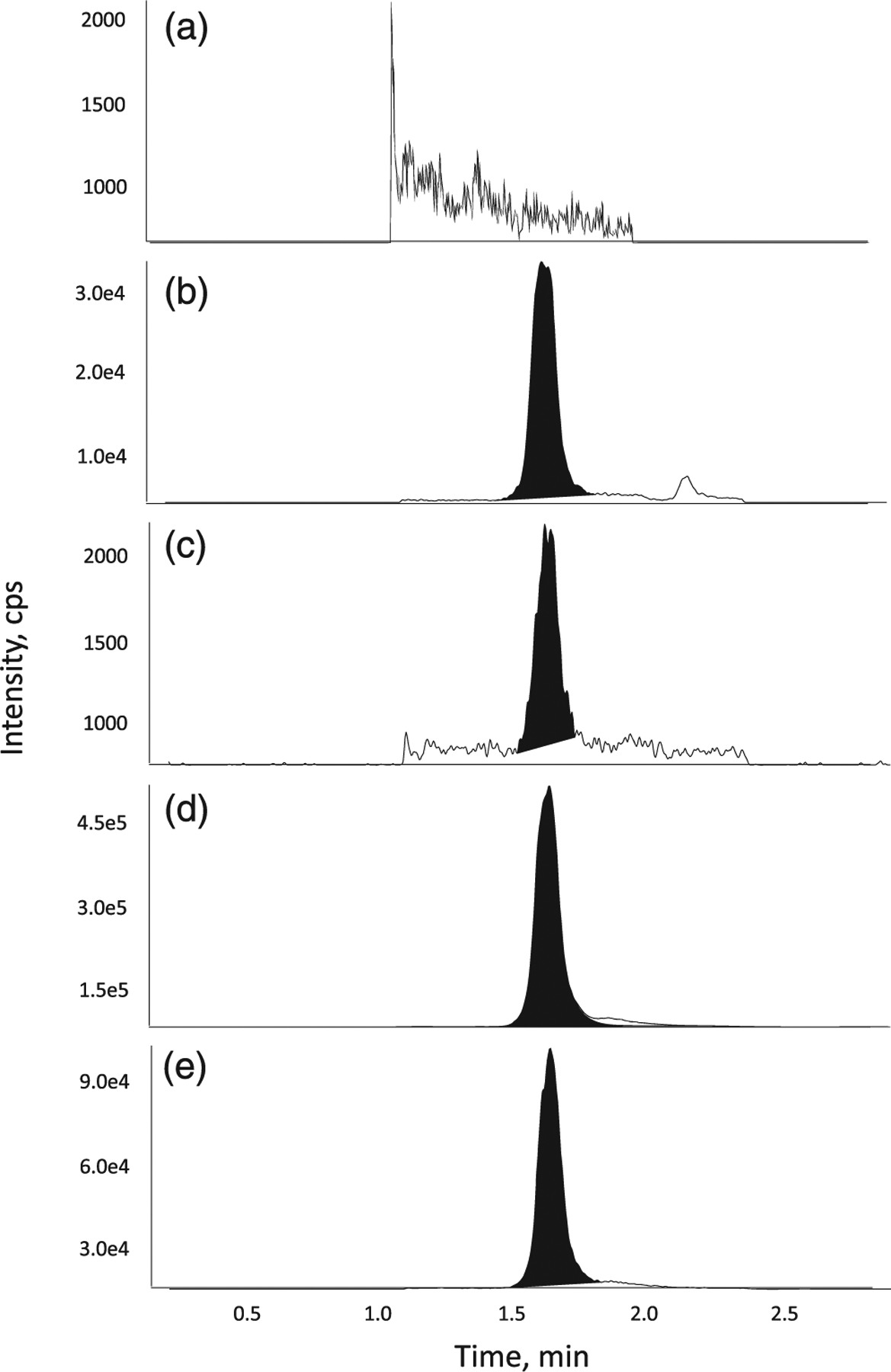
Representative chromatograms of Neu5Ac in (a) surrogate blank matrix, (b) blank matrix, (c) a LLOQ sample, (d) a ULOQ sample and (e) internal standard in matrix
ACKNOWLEDGEMENTS
This project was supported by the Intramural Research Programs of the National Center of Advancing Translational Sciences and the National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does the mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Funding information
National Center for Advancing Translational Sciences; National Human Genome Research Institute
REFERENCES
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, & Walter P (2002). Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN-10: 0-8153-4072-9. [Google Scholar]
- Cummings J, Ward TH, & Dive C (2010). Fit-for-purpose biomarker method validation in anticancer drug development. Drug Discovery Today, 15(19–20), 816–825. 10.1016/j.drudis.2010.07.006 [DOI] [PubMed] [Google Scholar]
- Gu X, Harmon BJ, & Wang DI (1997). Site- and branch-specific sialylation of recombinant human interferon-gamma in Chinese hamster ovary cell culture. Biotechnology and Bioengineering, 55(2), 390–398. [DOI] [PubMed] [Google Scholar]
- Harvey BE, & Thomas P (1993). Inhibition of CMP-sialic acid transport in human liver and colorectal cancer cell lines by a sialic acid nucleoside conjugate (KI-8110). Biochemical and Biophysical Research Communications, 190(2), 571–575. 10.1006/bbrc.1993.1086 [DOI] [PubMed] [Google Scholar]
- Hinderlich S, Berger M, Keppler OT, Pawlita M, & Reutter W (2001). Biosynthesis of N-acetylneuraminic acid in cells lacking UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. Biological Chemistry, 382(2), 291–297. 10.1515/BC.2001.036 [DOI] [PubMed] [Google Scholar]
- Houghton R, Horro Pita C, Ward I, & Macarthur R (2009). Generic approach to validation of small-molecule LC-MS/MS biomarker assays. Bioanalysis, 1(8), 1365–1374. [DOI] [PubMed] [Google Scholar]
- Jian W, Edom RW, & Weng N (2012). Important considerations for quantitation of small-molecule biomarkers using LC-MS. Bioanalysis, 4(20), 2431–2434. [DOI] [PubMed] [Google Scholar]
- Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, … Wagner JA (2006). Fit-for-purpose method development and validation for successful biomarker measurement. Pharmaceutical Research, 23(2), 312–328. 10.1007/s11095-005-9045-3 [DOI] [PubMed] [Google Scholar]
- Li Y, & Chen X (2012). Sialic acid metabolism and sialyltransferases: natural functions and applications. Applied Microbiology and Biotechnology, 94(4), 887–905. 10.1007/s00253-012-4040-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, & Nishino I (2009). Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nature Medicine, 15(6), 690–695. [DOI] [PubMed] [Google Scholar]
- Martinez-Duncker I, Dupre T, Piller V, Piller F, Candelier JJ, Trichet C, … Mollicone R (2005). Genetic complementation reveals a novel human congenital disorder of glycosylation of type II, due to inactivation of the Golgi CMP-sialic acid transporter. Blood, 105(7), 2671–2676. 10.1182/blood-2004-09-3509 [DOI] [PubMed] [Google Scholar]
- Munster AK, Weinhold B, Gotza B, Muhlenhoff M, Frosch M, & Gerardy-Schahn R (2002). Nuclear localization signal of murine CMP-Neu5Ac synthetase includes residues required for both nuclear targeting and enzymatic activity. The Journal of Biological Chemistry, 277(22), 19688–19696. 10.1074/jbc.M201093200 [DOI] [PubMed] [Google Scholar]
- Seppala R, Lehto VP, & Gahl WA (1999). Mutations in the human UDP-N-acetylglucosamine 2-epimerase gene define the disease sialuria and the allosteric site of the enzyme. American Journal of Human Genetics, 64(6), 1563–1569. 10.1086/302411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Xu X, Fang M, Zhang M, Li Y, Gillespie B, … Wang AQ (2015). Quantitative hydrophilic interaction chromatography–mass spectrometry analysis of N-acetylneuraminic acid and N-acetylmannosamine in human plasma. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 1000, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasche R, Hinderlich S, Weise C, Effertz K, Lucka L, Moormann P, & Reutter W (1997). A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. Molecular cloning and functional expression of UDP-N-acetyl-glucosamine 2-epimerase/N-acetylmannosamine kinase. The Journal of Biological Chemistry, 272(39), 24319–24324. [DOI] [PubMed] [Google Scholar]
- Varki A (2008). Sialic acids in human health and disease. Trends in Molecular Medicine, 14(8), 351–360. 10.1016/j.molmed.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lee J, Burns D, Doherty D, Brunner L, Peterson M, & DeSilva B (2009). “Fit-for-purpose” method validation and application of a biomarker (C-terminal telopeptides of type 1 collagen) in denosumab clinical studies. The AAPS Journal, 11(2), 385–394. 10.1208/s12248-009-9115-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wopereis S, Abd Hamid UM, Critchley A, Royle L, Dwek RA, Morava E, … Wevers RA (2006). Abnormal glycosylation with hypersialylated O-glycans in patients with Sialuria. Biochimica et Biophysica Acta, 1762(6), 598–607. 10.1016/j.bbadis.2006.03.009 [DOI] [PubMed] [Google Scholar]
- Xu X, Wang AQ, Latham LL, Celeste F, Ciccone C, Malicdan MC, … Carrillo N (2017). Safety, pharmacokinetics and sialic acid production after oral administration of N-acetylmannosamine (ManNAc) to subjects with GNE myopathy. Molecular Genetics and Metabolism, 122(1–2), 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardeni T, Choekyi T, Jacobs K, Ciccone C, Patzel K, Anikster Y, … Huizing M (2011). Identification, tissue distribution, and molecular modeling of novel human isoforms of the key enzyme in sialic acid synthesis, UDP-GlcNAc 2-epimerase/ManNAc kinase. Biochemistry, 50(41), 8914–8925. 10.1021/bi201050u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Chen TL, Vertel BM, & Colley KJ (2006). The CMP-sialic acid transporter is localized in the medial-trans Golgi and possesses two specific endoplasmic reticulum export motifs in its carboxyl-terminal cytoplasmic tail. The Journal of Biological Chemistry, 281(41), 31106–31118. 10.1074/jbc.M605564200 [DOI] [PubMed] [Google Scholar]


