Summary
Background
Surgical antibiotic prophylaxis (SAP) is one of the measures used for preventing surgical site infections. SAP has high impact but there is low compliance with antimicrobial guidelines in many developing countries like the Democratic Republic of the Congo. This study aimed to assess the compliance of antibiotics used for surgical site infection prophylaxis with international guidelines among patients undergoing surgery at the “Cliniques Universitaires du Graben” (CUG).
Methods
This was a retrospective study including all patients who underwent surgery and received SAP between January 2017 and December 2018 at CUG. Surgical and Gynaecology-Obstetric patients were included. A total of 265 patients were included in the analysis. A standardized questionnaire was used for collecting pre-, per-, and post-operative data. The compliance of SAP was assessed for all patients. Data were analysed using SPSS version 22.
Results
The compliance rate ofSAP among patients undergoing surgery at CUG was 18.1%. Emergency surgery increased the risk of SAP non-compliance by three fold (OR=3.5, 95% CI: 1.0–11.8, p = 0.033). The most frequent antibiotics used in SAP were ampicillin, cloxacillin, gentamicin and ceftriaxone, alone or in combination. Categories of non-compliance included; inappropriate initial dose of antibiotic (compliance rate of 23.8%) and incorrect duration of antibiotic use (compliance rate of 30.9%). Among the included patients, 22 (8.3%) presented with a surgical site infection, of those 20 (90.9%) had received non-compliant SAP.
Conclusion
The correct use of SAP among patients undergoing surgery at CUG is low. Implementing measures to optimize adherence to SAP guidelines should be encouraged. A high rate of surgical site infections is observed in cases where the SAP is prescribed or administered in a non-compliant manner.
Keywords: Surgical prophylaxis, Antibiotics, Surgical site infection, Compliance, CUG
Background
Surgical site infection (SSI) is a proliferation of pathogenic microbes on the incision site within a month after surgery or after one year in case of implant placement. SSI may be superficial (within the subcutaneous fat) or deep (muscular facial layer). It may involve an organ or a cavity when it occurs after implant placement [1,2]. SSI constitutes a major complication of surgical procedures and increases the morbidity, mortality and healthcare costs [3]. Despite infection prevention and control (IPC) measures such as the use of better instrument sterilization methods, improvements in operating theatre practices, and better surgical technique, SSI remains a significant cause of hospital-acquired infections (HAIs). Its rate is increasing even within high standard hospitals (those using standard protocols of preoperative preparation and surgical antibiotic prophylaxis (SAP)) [2]. There is a scarcity of information on the burden of HAIs in Africa, but the few available data show high morbidity and mortality due to SSI. Preoperative and postoperative asepsis is vital in the prevention of SSI [4]. The SSI compromises the surgical procedure done, prolongs the hospitalization stay and increases the healthcare cost [5]. Due to the complex origin of SSI, highly effective preventive measures are promoted but their implementation remains poor in several African countries [6].
SAP consists of an administration of antibiotics before the surgical procedure with the aim of preventing SSIs and does not include preoperative decolonisation or treatment of established infections [7]. Several studies have demonstrated the effectiveness of SAP for most surgical procedure types for the prevention of SSI [[8], [9], [10]]. The SAP aim in surgical procedures is not to sterilize tissues, but to lower the microbial burden introduced at the time of surgery [11].
Inadequate prescriptions, inappropriate times, incorrect dosage and duration of SAP remain significant problems in the practice [12,13]. Although SAP reduces the risk of SSI, it is an important source of prescription errors. The irrational use of antibiotics for surgical prophylaxis contributes to the development of antibiotic resistance and the increased incidence of SSI [14].
Before using an antibiotic in surgical prophylaxis, there should be evidence showing that it reduces postoperative infection risks. The used antibiotic should have shown its safety and efficiency on pathogens responsible for SSI and should be cost-effective. The serum and tissue concentrations of the antibiotic used should be optimal before the incision and the therapeutic concentrations should be maintained during the surgical procedure and few hours following the incision suture [13,15].
In developing countries, some practitioners prescribe long-term SAP (7–10 days) in an attempt to overcome the breakage of asepsis sometimes found in operating theatres [16]. This practice considerably increases the cost of surgery and the risk of developing resistance to antibiotics used in therapy [16].
In the Democratic Republic of the Congo (DRC), the use of SAP in the prevention of SSI is not documented. In addition, several hospitals (e.g. “Cliniques Universitaires du Graben” (CUG)) do not have locally written SAP guidelines but use international ones. Therefore, this study aims to assess the compliance of SAP used for surgical site infection prophylaxis with international guidelines (among patients undergoing surgery at the CUG).
Methods
Study setting and design
This was a retrospective cross-sectional study, carried out from January 2017 to December 2018 at the CUG located in Butembo, North-Kivu province, DRC. CUG is a tertiary level, referral and teaching hospital affiliated to a private university, the “Université Catholique du Graben” (UCG). Overall, CUG has approximately 125 beds allocated to surgical departments. About 260 surgical procedures are performed annually in the department of Surgery at the CUG, and 327 in Gynaecology and Obstetrics.
Study population
All patients who underwent a surgical procedure (with infectious risk) and received SAP during the study period constituted the study population. The infectious risk was determined by the degree of bacterial contamination, general conditions of the patient and factors related to the surgical procedure.
Inclusion and exclusion criteria
All patients who underwent a surgical procedure and for whom antibiotics were used in prophylaxis were included in this study. Practically, files of patients who underwent surgery were checked manually if antibiotics were used for SAP. We excluded patients with incomplete files (i.e missing one or more variables studied), patients who did not receive antibiotics and those referred to CUG for follow up or postoperative complications (after surgery done in other hospitals). Patients with immunosuppression risk factors that may favour SSI (e.g. diabetes mellitus, prolonged corticosteroid therapy, progressive neoplasia, chronic inflammatory diseases and HIV) were excluded.
Sample size
Sample size estimation was done using Fischer's formula in a single population, assuming that the non-compliance of SAP is 20%, within a 95% confidence interval and 5% marginal error. Hence, the minimum sample size was of 246. By adding 10% of margin, the sample size was of 270. The stratified random sampling method (without replacement) was used. Each department was considered as a stratum. In each stratum, patients complying with inclusion criteria had an equal chance to be selected. Therefore, 265 patients were included and distributed as follow: 129 in surgery and 136 in gynaecology and obstetrics.
Data collection
Data were collected using a pre-tested data extraction questionnaire. Relevant data were retrieved from the patient's files (medical record, pre-anaesthesia file and surgical protocol). We collected socio-demographic data, clinical data, and data regarding the SAP.
The socio-demographic data collected included the sex and age of the patient. Clinical data included American Society of Anaesthesiologists (ASA) score and data regarding the surgical procedure. Surgical procedure data included: preoperative diagnosis, emergency or elective surgery, wound classification (according to Alteimeier classification), implant placement (prosthesis, drain, plate, nail), actual and predicted duration of the procedure (found on the National Nosocomial Infections Surveillance System (NNIS) (Supplemental file 1: Table S1)). For surgical procedures that were not on the NNIS list, we applied a predicted duration of 2 hours.
Data regarding the SAP included the prescribed antibiotic (before, during or after the surgical procedure), its dosage and duration, and the administration route. SAP compliance was measured against evidenced-based guidelines developed by the National Institute for Health and Care Excellence (NICE) [17], and Stanford Health Care (SHC) [18] (the guidelines were adapted for our setting). Post-operative SSIs were diagnosed by surgeons within one month post-operation or one year for implant placement procedures, using the Centres for Disease Control and Prevention (CDC) definitions of SSI [1].
Operational definitions
Six common variables of SAP practice were assessed. These were indication for use of prophylaxis, initial dosage and administration route, time of administration, duration of utilization and the starting time of SAP. Procedures were labelled "compliant" if all the six variables were individually compliant with the guidelines. A procedure in which one or more of the six variables were not practised according to the guidelines was labelled non-compliant [19,20]. Evidenced-based SAP guidelines developed by the NICE [17], and SHC [18] were used as a reference in our study but adapted in our setting. These guidelines recommend that SAP should be given to patients before clean surgery involving the placement of a prosthesis or implant, clean-contaminated surgery and contaminated surgery. SAP should not be used routinely for clean non-prosthetic uncomplicated surgery. Before giving SAP, the timing and pharmacokinetics (for example, the serum half-life) should be taken into account and necessary infusion time of the antibiotic. The antibiotic should be given within 30–60 minutes before the incision. It is recommended to give a repeat dose of antibiotic prophylaxis when the operation is longer than the half-life of the antibiotic given. Antibiotic treatment (in addition to prophylaxis) should be given to patients having surgery on a pre-existing dirty or infected wound [17,18].
The surgical wound was classified according to Altemeier classification as recommended by the CDC [21]. Four classes are considered: clean wound, clean-contaminated wound, contaminated wound and dirty wound.
The NNIS index of each patient had a value of 0–3. It was defined by 4 categories of risk (0–3) and was obtained by summing the mark obtained in each of the following parameters [17]:
-
•
Altemeier wound classification: 1 if ≥ III and 0 if < III
-
•
ASA score: 1 if ≥ 3 and 0 if < 3
-
•
Duration of the surgical procedure: 1 if > T and 0 is ≤ T
The duration “T” corresponds to the value of percentile 75 for the considered surgical procedure, according to data of the NNIS. The duration “T” of some surgical procedures is found in the supplemental file 1 (Table S1).
Data analysis
Descriptive analyses were performed using SPSS v22.0 (IBM Corp, Armonk, NY, US) to determine the patients' clinical characteristics and variables related to SAP. Odds ratios and p-values were calculated where appropriate. A p-value ≤0.05 was considered statistically significant. The surgical site infection rate was calculated by the number of infections that is equal to the cases identified in the study.
Ethical considerations
The protocol of this study was approved by the ethical board of the faculty of Medicine at the UCG and the research ethics committee of the Cliniques Universitaires du Graben. Confidentiality and anonymity of patients and patient information were maintained.
Results
One thousand five hundred patients' files were screened, of which 265 were included in the final analysis. The mean age of patients was 29.3 ± 17.3 years and 180 (67.9%) were female. One hundred and twenty-five (47.2%) patients were in the second class of ASA score and 116 (43.8%) in the first class, 186 (70.2%) had a clean-contaminated wound and 210 (79.2%) patients had an NNIS index of zero (as shown in Table 1). Among the patients, 22 (8.3%)presented a surgical site infection (SSI). From the 22 cases with SSIs, the non-compliance of SAP was observed in 20 (90.9%) cases (Table 2).
Table 1.
Socio-demographic and clinical characteristics of patients at surgical wards of CUG
| Variables | n (%), N=265 |
|---|---|
| Age in years, Mean ± SD | 29.3 ± 17.3 |
| Gender | |
| Female | 180 (67.9) |
| Male | 85 (32.1) |
| ASA Score | |
| ASA I | 116 (43.8) |
| ASA II | 125 (47.2) |
| ASA III | 21 (7.9) |
| ASA IV | 3 (1.1) |
| Altemeier wound class | |
| Clean | 60 (22.6) |
| Clean-contaminated | 186 (70.2) |
| Contaminated | 10 (3.8) |
| Dirty | 9 (3.4) |
| NNIS index | |
| 0 | 210 (79.2) |
| 1 | 43 (16.2) |
| 2 | 9 (3.4) |
| 3 | 3 (1.1) |
SD: Standard deviation, ASA: American Society of Anaesthesiologists, NNIS: National Nosocomial Infections Surveillance System.
Table 2.
Compliance of antibiotics used in SAP in different surgical departments at CUG and surgical site infection
| Variables | Total, N=265 | Antibiotics use in SAP, n (%) |
OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Noncompliance | Compliance | ||||
| Type of surgery | |||||
| Emergency | 44 (16.6) | 41 (93.2) | 3 (6.8) | 3.5 (1.0–11.8) | 0.033 |
| Elective | 221 (83.4) | 176 (79.6) | 45 (20.4) | Ref | |
| Departments | |||||
| Surgery | 129 (48.7) | 98 (76.0) | 31 (24.0) | 2.2 (1.2–4.24) | 0.017 |
| Gynaecology-Obstetrics | 136 (51.3) | 119 (87.5) | 17 (12.5) | Ref | |
| Surgical site infection | |||||
| Present | 22 (8.3) | 20 (90.9) | 2 (9.1) | 0.4 (0.09–1.9) | 0.386 |
| Absent | 243 (91.7) | 197 (81.1) | 46 (18.9) | Ref | |
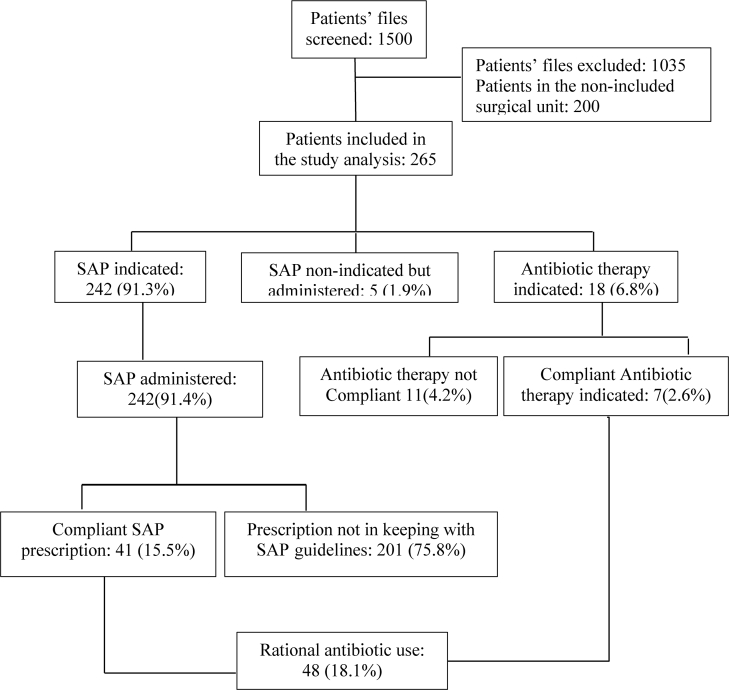
SAP was administered in compliance of standard principles of good practice in 48 (18.1%) cases. Two hundred and forty-two (91.3%) patients had a valid SAP indication, 5 (1.9%) patients had received SAP when it was not indicated, and 18 (6.8%) patients had an indication for a course of antibiotic therapy. Two hundred and seventeen (81.9%) patients received an incorrect choice of antibiotic therapy or antibiotic prophylaxis (Figure 1).
Figure 1.
Flow chart for the compliance of antibiotics use at CUG (SAP: surgical antibiotic prophylaxis).
Table 2 summarizes the compliance of SAP in different surgical departments. Emergency surgery increased the risk of SAP non-compliance by three fold (OR=3.5, 95% CI: 1.0–11.8, p = 0.033) while elective surgery was associated with compliant use of antibiotics for SAP. The criteria for non-compliance of SAP at CUG were inappropriate initial dosage of the antibiotic (with a compliance rate of 23.8%) and duration of antibiotic use (with a compliance rate of 30.9%) (Table 3).
Table 3.
Measurement of SAP compliance at CUG
| Variables | Compliance with SAP guidelines | Total (N=265) | Surgery | Ob-gyn |
|---|---|---|---|---|
| Administration route | ||||
| Intravenous | Yes | 220 (83.0) | 105 (47.7) | 115 (52.3) |
| Intramuscular/Per os | No | 45 (17.0) | 24 (53.3) | 21 (46.7) |
| Dosage (initial dosage) | ||||
| Double | Yes | 63 (23.8) | 37 (58.7) | 26 (41.3) |
| Simple | No | 202 (76.2) | 92 (45.5) | 110 (54.5) |
| The starting time of SAP | ||||
| Preoperative | Yes | 210 (79.2) | 108 (51.4) | 102 (48.6) |
| Per-/Post-operative | No | 55 (20.8) | 21 (38.2) | 24 (61.8) |
| Peroperative readministrationa | ||||
| Yes | Yes | 7 (2.6) | 4 (57.1) | 3 (42.9) |
| No | Yes | 258 (97.4) | 125 (48.4) | 133 (51.6) |
| Duration of antibiotic use, Mean ± SD = 5.23±2.95 | ||||
| ≤ 2 days | Yes | 82 (30.9) | 16 (19.5) | 66 (80.5) |
| ≥ 3 days | No | 183 (69.1) | 113 (61.7) | 70 (38.3) |
A repeated dose of antibiotic prophylaxis when the operation is longer than the half-life of the antibiotic given.
All patients received one or more antibiotics. Antibiotic use patterns in SAP and therapy in the different surgical departments at CUG are summarized in Table 4. Overall, the most frequent antibiotics used were ampicillin (43.8%), cloxacillin (13.2%), gentamicin (9.4%) and ceftriaxone (9.1%), alone or in combination. The most frequently used combination therapy was ceftriaxone and tazobactam (4.2%).
Table 4.
Antibiotic use patterns in SAP and therapy in different surgical departments at CUG
| Antibiotics | Total | Departments |
|
|---|---|---|---|
| Surgery | Ob-gyn | ||
| Ampicillin | 116 (43.8) | 40 (35.5) | 76 (65.5) |
| Cloxacillin | 35 (13.2) | 33 (94.3) | 2 (5.7) |
| Gentamicin | 25 (9.4) | 3 (12) | 22 (88) |
| Ceftriaxone | 24 (9.1) | 19 (79.2) | 5 (20.8) |
| Amoxicillin | 12 (4.5) | 9 (75) | 3 (25) |
| Amoxiclav | 10 (3.8) | 0 (0.0) | 10 (100) |
| Ciprofloxacin | 6 (2.3) | 4 (66.7) | 2 (33.3) |
| Metronidazole | 3 (1.1) | 1 (33.3) | 2 (66.7) |
| Azitromycin | 2 (0.7) | 2 (100) | 0 (0.0) |
| Levofloxacin | 2 (0.7) | 2 (100) | 0 (0.0) |
| Doxycycline | 1 (0.4) | 0 (0.0) | 1 (100) |
| Neomycin | 1 (0.4) | 1 (100) | 0 (0.0) |
| Ceftriaxone + Tazobactam | 11 (4.2) | 3 (27.3) | 8 (72.7) |
| Ceftriaxone + Gentamicin | 6 (2.3) | 3 (50) | 3 (50) |
| Ampiciline + Cloxacillin | 6 (2.3) | 4 (66.7) | 2 (33.3) |
| Ampicillin + Gentamicin | 1 (0.4) | 1 (100) | 0 (0.0) |
| Total | 265 (100) | 129 (48.7) | 136 (51.3) |
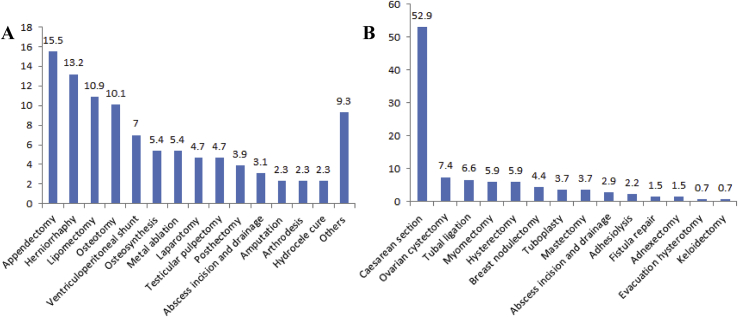
In surgery, the most frequent surgical procedures were appendectomy (15.5%), herniorrhaphy (hernia repair) (13.2%), lipomectomy (10.9%) and osteotomy (10.1%). In Gynaecology and Obstetrics, caesarean section (52.9%) and ovarian cystectomy (7.3%) were the most encountered surgical procedures (Figure 2).
Figure 2.
Type of surgical procedures done in different surgical departments at CUG (A: Surgery department including general surgery, orthopaedics and traumatology; B: Gynaecology and obstetrics department).
Discussion
This study reported the compliance of SAP compared to current guidelines to provide evidence for recommendations that may help to improve the healthcare of patients undergoing surgery at CUG. To our knowledge, this is the first report of SAP compliance in the DRC. These findings are the baseline for future studies. In this study, we used NICE and SHC guidelines for assessing SAP compliance [17,18].
This study showed that most patients received SAP in a non-compliant manner compared to guidelines and evidence-based practice for SSI prevention. Only one-fifth of patients received SAP in compliance with guidance. This study may have under/overestimated the problem of inadequate SAP because of the inclusion and exclusion criteria. However, the findings remain alarming and further actions should be implemented for increasing SAP compliance in practice.
These results are similar to those reported by Jallil et al. who found a low level of compliance with hospital-adapted SAP guidelines in Jordan [22]. Despite the availability of evidence for optimizing patient care and development of therapeutic guides on antimicrobial prophylaxis in surgery, several studies have demonstrated non-compliance and poor adherence to these guidelines [[23], [24], [25]]. In our setting, the non-compliance to SAP best practice may be explained by the fact that there are no locally written SAP guidelines. The NICE and SHC guidelines [17,18] are adapted in our setting and this on the experience of the surgeon and/or the person who will administer the SAP. The availability of recommended antibiotics may pose a challenge in the choice of SAP antibiotic. Regarding the measure of non-compliance, other studies reported inadequate prescriptions, inappropriate times, dosage and duration of SAP [12,13]. In this study, the non-compliance was more likely to be due to the inappropriate initial dosage and administration duration of antibiotic.
Non-compliance to SAP guidelines was statistically significant (OR= 2.2, 95% CI: 1.2–4.24, p = 0.017) in the surgical department. It has been reported that the facility type and surgical speciality are associated with suboptimal SAP practice [26]. The significant non-compliance to SAP guidelines in the surgery department may be explained by the lack of a surgical safety program in our setting. Recent studies have demonstrated that implementation of a surgical unit-based safety program (including policy and pharmacy interventions, infection prevention and control (IPC), and antimicrobial stewardship) may inspire global strategies for SSI prevention in resource-limited settings [26,27].
The rate of SSIs found in this study was 8.3% and was more observed among patients with non-compliant SAP. This rate was lower than the finding of a similar study conducted in Ethiopia [28] where the rate of infection reported was 23.5%. Moreover, it was also lower than the other studies carried out in countries such as India [29] and Tanzania [30] where the infection rates were 16% and 20% respectively. The low rate of SSIs observed in this study may be explained by the high proportion of patients in a low infectious risk category. This was confirmed by the fact that 210 (79.2%) patients reported in this study had an NNIS index of zero. Furthermore, our findings showed that more than two-thirds of patients had an ASA score ≤2. It has been reported that a low ASA score predicts a low risk of SSIs [31].
Findings of this study demonstrated that SAP non-compliance was significantly associated with emergency surgeries compared to elective. Similar findings have been reported in other countries such as Jordania [22], Cameroon [16], Australia [24] and India [29]. In our setting, emergency surgery increased the risk of SAP non-compliance by three fold (OR= 3.5, 95% CI: 1.0–11.8, p= 0.033). This could be explained by the fact that there may be suboptimal preoperative preparation during emergency surgeries. In elective surgeries, the surgical team (surgeons and anaesthesiologists) have more time to discuss SAP antibiotic choice and refer to guidelines.
One hundred and eighty-six (70.2%) patients had clean-contaminated surgical sites. These results are similar to those reported in Cameroon [16], Ethiopia [2] and Australia [24]. Several SAP guidelines [19,20] recommend the administration of SAP for clean-contaminated surgery and contaminated surgery. Meanwhile, it has been reported that surgeons prefer to give SAP in clean surgery, although not recommended [32]. This study has shown that 60 (22.6%) patients had clean wounds but received unnecessary SAP. Other studies have reported the non-compliance with SAP guidelines in patients undergoing clean surgery [23].
This study showed that ampicillin, cloxacillin, gentamicin, and ceftriaxone were the most commonly used antibiotics. This is similar to other studies. Cephalosporins are prescribed when there is a risk of severe infection or in acute infection while waiting for the results of cultures [33,34]. A recent study suggested the use of ampicillin in SAP as its efficacy did not differ to the one of ceftriaxone in the prevention of caesarean section SSIs [35]. The use of ampicillin prevents the overuse of broad-spectrum antibiotics which is involved in the emergence of multidrug-resistant bacteria [36]. Ampicillin has also the advantage of being cheaper and accessible in developing countries. Several reports recommend the use of aminoglycosides in SAP when there is a risk of contamination with gram-negative bacteria [37].
Conclusion
In conclusion, the compliance of SAP (compared to international evidenced based guidance) at CUG is low. In this study, the non-compliance was caused by inappropriate initial dosage of the antibiotic used, incorrect duration and poor timing of antibiotic administration. This may have an impact on the effectiveness of SAP in preventing SSIs within the health institution. Implementing measures to optimize adherence to SAP guidelines should be encouraged.
Study limitations and perspectives
This study has certain limitations. It was performed in a single centre and therefore the results cannot be generalized to other hospitals. The retrospective nature of the study constituted another limitation.
However, the results create a starting point to improve current practice. A study on the knowledge and awareness of healthcare workers to SAP guidelines should be conducted. In addition, a study on the antimicrobial susceptibility pattern of isolates from SSIs should be done to help in developing effective local guidelines. Establishing and implementing an antimicrobial stewardship program at CUG will help to improve the adherence to SAP guidelines in this hospital. A multidisciplinary team (comprising pharmacists, infection prevention and control specialists, and clinical microbiologists) to provide education and strict and rigorous evaluation of antibiotic prescriptions will improve the use of antibiotics in surgical departments at CUG.
Funding
This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' contributions
GKB participated in the conception and design of the study, coordination of the study, data analysis and interpretation, and drafted the manuscript. MPM participated in the design of the study, acquired data, participated in data analysis and interpretation. CKM and SAU participated in the study coordination. All the authors read and approved the submitted manuscript.
Competing interests
The author GKB reports support from the Else-Kroner-Fresenius Stiftung through the BEBUC Excellence Scholarship. The grant reported is outside of the submitted work. The authors declare that they have no competing interests.
Acknowledgements
Authors thank all the surgical team at CUG for their collaboration during the data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.infpip.2020.100075.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Horan T.C., Gaynes R.P., Martone W.J., Jarvis W.R., Emori T.G. CDC definitions of nosocomial surgical site infections, 1992; a modification of CDC definitions of surgical wound infections. Infection Control Hosp Epidemiol. 1992;(13):606–608. [PubMed] [Google Scholar]
- 2.Ayele Y., Taye H. Antibiotic utilization pattern for surgical site infection prophylaxis at Dil Chora Referral Hospital Surgical Ward, Dire Dawa, Eastern Ethiopia. BMC Res Notes. 2018;11:537. doi: 10.1186/s13104-018-3629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badia J.M., Casey A.L., Petrosillo N., Hudson P.M., Mitchell S.A., Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96(1):1–15. doi: 10.1016/j.jhin.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Organisation Mondiale de la Santé Pourquoi un défi mondial sur les infections nosocomiales. Infection liées aux soins de santé en Afrique : étude systématique. OMS. Oct. 2011;89(10):701–776. [Google Scholar]
- 5.Sanou J. Enquête de prévalence des infections nosocomiales dans les services de chirurgie au CHUYO. Rev Afr Anesth Med Urgence. 1999;4(1):8–13. [PubMed] [Google Scholar]
- 6.Allegranzi B., Aiken A.M., Zeynep Kubilay N., Nthumba P., Barasa J., Okumu G. A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: a multicentre, before-after, cohort study. Lancet Infect Dis. 2018 May;18(5):507–551. doi: 10.1016/S1473-3099(18)30107-5. [DOI] [PubMed] [Google Scholar]
- 7.Ierano C., Nankervis J., James R., Rajkhowa A., Peel T., Thursky K. Surgical antimicrobial prophylaxis. Aust Prescr. 2017;40(6):225–229. doi: 10.18773/austprescr.2017.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . WHO; 2016. Global guidelines for the prevention of surgical site infection. [PubMed] [Google Scholar]
- 9.Boonchan T., Wilasrusmee C., McEvoy M., Attia J., Thakkinstian A. Networkmeta-analysis of antibiotic prophylaxis for prevention of surgical-site infection after groin hernia surgery. Br J Surg. 2017;104(2):e106–e117. doi: 10.1002/bjs.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waddell T.K., Rotstein O.D. Antimicrobial prophylaxis in surgery. Committee on Antimicrobial Agents, Canadian Infectious Disease Society. Can Med Assoc J. 1994;151(7):925–931. [PMC free article] [PubMed] [Google Scholar]
- 11.Van Eyk N., Halifax N.E., Van Schalkwyk J., Vancouver C.B. Directive de la SOGC-Antibioprophylaxie dans le cadre d’interventions gynécologiques. J ObstetGynécol Can. 2012;275:S1–S12. [Google Scholar]
- 12.Thirion Daniel J.G., Frenette A.-J., Precourt A., Fillion A., Blais L. Évaluation de l’implantation d’un guide de pratique en antibioprophylaxie chirurgicale (projet Évidance). Le parrainage des antimicrobiens : vision 2010. Pharmactuel. 2009;42:41–52. Suppl 2(4) [Google Scholar]
- 13.Kallel H., Maaloul I., Bahloul M., Khemakhem A., Chelly H., Ksibi H. Evaluation de l'antibioprophylaxie péri-opératoire dans un hôpital universitaire. Antibiotiques. 2005;7(2):93–96. [Google Scholar]
- 14.Al-Abri S.S., Elsheikh M. Surgical Antimicrobial Prophylaxis Challenges in translating evidence to practice. SQU Med J. 2016;16(1):e1–2. doi: 10.18295/squmj.2016.16.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bratzler D.W., Dellinger E.P., Olsen K.M., Perl T.M., Auwaerter P.G., Bolon M.K. Clinical practise guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 16.Ngowa J.D., Ngassam A., Mbouopda R.M., Kasia J.M. Antibioprophylaxie dans les chirurgies gynécologiques et obstétricales propres et propres contaminées à l’Hôpital Général de Yaoundé, Cameroun. Pan Afr Med J. 2014 Sep 10;19:23. doi: 10.11604/pamj.2014.19.23.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NICE Surgical site infection. May;2019. https://www.nice.org.uk/guidance/qs49/resources/surgical-site-infection-pdf-2098675107781 2013 2013:40
- 18.Stanford Health Care . 2017. SHC surgical antimicrobial prophylaxis guidelines.http://med.stanford.edu/bugsanddrugs/guidebook/_jcr_content/main/panel_builder_584648957/panel_0/download/file.res/SHC_SurgProphylaxisGuidelines.pdf [May;2019] [Google Scholar]
- 19.Emori T.G., Culver D.H., Horan T.C. National nosocomial infection surveillance system (NNISS): description of surveillance methods. Am J Infect Control. 1991;19:19–35. doi: 10.1016/0196-6553(91)90157-8. [DOI] [PubMed] [Google Scholar]
- 20.Division of Healthcare Quality Promotion; National Center for Infectious Diseases; Centers for Disease Control and Prevention; Public Health Service US Department of Health and Human Services. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004, AJICspecial article. NNIS Report. 2004 Dec;32(8):470–484. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 21.Surgical Site Infection (SSI) Event: Center for Disease Control. 2010. http://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf?agree=yes&next=Accept Updated January 2015.
- 22.Jallil M.H., Abu Ammour K., Alsous M., Hadadden R., Awad W., Bakri F. Noncompliance with surgical antimicrobial prophylaxis guidelines: a Jordanian experience in cesarean deliveries. Am J Infect Control. 2018;46(1):14–19. doi: 10.1016/j.ajic.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 23.Tourmousoglou C.E., Yiannakopoulou E.C., Kalapothaki V., Bramis J., Papadopoulos J. Adherence to guidelines for antibiotic prophylaxis in general surgery: A critical appraisal. J Antimicrob Chemother. 2008;61(1):214–218. doi: 10.1093/jac/dkm406. [DOI] [PubMed] [Google Scholar]
- 24.Friedman N.D., Styles K., Gray A.M., Low J., Athan E. Compliance with surgical antibiotic prophylaxis at an Australian teaching hospital. Am J Infect Control. 2013;41(1):71–74. doi: 10.1016/j.ajic.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Durando P., Bassetti M., Orengo G., Crimi P., Battistini A., Bellina D. Adherence to international and national recommendations for the prevention of surgical site infections in Italy: results from an observational prospective study in elective surgery. Am J Infect Control. 2012;40(10):969–972. doi: 10.1016/j.ajic.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Branch-Elliman W., Pizer S.D., Dasinger E.A., Gold H.S., Abdulkerim H., Rosen A.K. Facility type and surgical speciality are associated with suboptimal surgical antimicrobial prophylaxis practice patterns: a multi-centre, retrospective cohort study. Antimicrob Resist Infect Control. 2019;8:49. doi: 10.1186/s13756-019-0503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clack L., Willi U., Berenholtz S., Aiken A.M., Allegranzi B., Sax H. Implementation of a surgical unit-based safety programme in African hospitals: a multicenter qualitative study. Antimicrob Resist Infect Control. 2019;8:91. doi: 10.1186/s13756-019-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misganaw D., Linger B., Abesha A. Surgical antibiotic prophylaxis use and surgical site infection pattern in Dessier Referral Hospital , Dessie, Northeast of Ethiopia. Biomed Research International. 2020:7. doi: 10.1155/2020/1695683. Article ID 1695683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh M.P., Brahmchari S., Banerjee M. Surgical site infection among postoperative patients of tertiary care centre in Central India-a prospective study. Asian Journal of Biomedical and Pharmaceutical Sciences. 2013;3(17):41. [Google Scholar]
- 30.Mawalla B., Mshana S.E., Chalya P.L., Imirzalioglu C., Mahalu W. Predictors of surgical site infections among patients undergoing major surgery at Bugando Medical Centre in Northwestern Tanzania. BMC Surgery. 2011;11:21. doi: 10.1186/1471-2482-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodfeld J.C., Beshay N.M.Y., Pettigrew R.A., Plank L.D., van Rij A.M. American Society of anesthesiologists classification of physical status as a predictor of wound infection. ANZ J Surg. 2007;77:738–741. doi: 10.1111/j.1445-2197.2007.04220.x. [DOI] [PubMed] [Google Scholar]
- 32.Mangram A.J., Horan T.C., Pearson M.L., Silver L.C., Jarvis W.R. Guideline for prevention of surgical site infection, Hospital infection control practices advisory committee. Infect Control Hosp Epidemiol. 1999;20:250–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 33.Bratzler D.W., Houck P.M. Antimicrobial prophylaxis for surgery: an advisory statement from the national surgical infection prevention project. Clin Infect Dis. 2004;38(12):1706–1715. doi: 10.1086/421095. [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Aziz A., El-Menyar A., Al-Thani H., Zarour A., Parchani A., Asim M. Adherence of surgeons to antimicrobial prophylaxis guidelines in a tertiary general hospital in a rapidly developing country. Advances Pharmacol Sci. 2013 doi: 10.1155/2013/842539. Article ID 842593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assawapalanggool S., Kasatpibal N., Sirichotiyakul S., Arora R., Suntornlimsiri W., Apisarnthanarak A. The efficacy of ampicillin compared with ceftriaxone on preventing cesarean surgical site infections: an observational prospective cohort study. Antimicrob Resist Infect Control. 2018;7:13. doi: 10.1186/s13756-018-0304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolar M., Urbanek K., Latal T. Antibiotic selective pressure and development of bacterial resistance. Int J Antimicrob Agents. 2001;17(5):357–363. doi: 10.1016/s0924-8579(01)00317-x. [DOI] [PubMed] [Google Scholar]
- 37.Bratzler D.W., Houck P.M. Surgical infection prevention guidelines writers workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;38:1706–1717. doi: 10.1086/421095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.