Abstract
Background
Diagnostic ionizing radiation is a risk factor for breast cancer (BC). BC risk increases with increased dose to the chest and decreases with increased age at exposure, with possible effect modification related to familial or genetic predisposition. While chest X-rays increase the BC risk of BRCA1/2 mutation carriers compared to non-carriers, little is known for women with a hereditary predisposition to BC but who tested negative for a BRCA1 or BRCA2 (BRCA1/2) mutation.
Methods
We evaluated the effect of chest X-rays from diagnostic medical procedures in a dataset composed of 1552 BC cases identified through French family cancer clinics and 1363 unrelated controls. Participants reported their history of X-ray exposures in a detailed questionnaire and were tested for 113 DNA repair genes. Logistic regression and multinomial logistic regression models were used to assess the association with BC.
Results
Chest X-ray exposure doubled BC risk. A 3% increased BC risk per additional exposure was observed. Being 20 years old or younger at first exposure or being exposed before first full-term pregnancy did not seem to modify this risk. Birth after 1960 or carrying a rare likely deleterious coding variant in a DNA repair gene other than BRCA1/2 modified the effect of chest X-ray exposure.
Conclusion
Ever/never chest X-ray exposure increases BC risk 2-fold regardless of age at first exposure and, by up to 5-fold when carrying 3 or more rare variants in a DNA repair gene.
Further studies are needed to evaluate other DNA repair genes or variants to identify those which could modify radiation sensitivity. Identification of subpopulations that are more or less susceptible to ionizing radiation is important and potentially clinically relevant.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-021-01456-1.
Keywords: Breast cancer, X-ray exposure, Low dose, High-risk population, DNA repair genes
Introduction
Medical diagnostic ionizing radiation is a known risk factor for the development of primary breast cancer (BC). BC risk associated with exposure to such radiation increases with radiation dose and decreases with age of exposure [1, 2]. Periods of high breast cell proliferation, such as during puberty and pregnancy, are associated with increased levels of DNA synthesis and thus may make breast tissue particularly susceptible to the carcinogenic effects of radiation [1, 2]. This susceptibility to radiation may be exacerbated for women with a familial/genetic predisposition [3–7] and particularly for women carrying genetic variants altering DNA repair mechanisms that may lead to cellular radio-sensitivity [8]. Among studies conducted in the general population, few have evaluated the effect of medical radiation exposures according to family history of BC [6, 7, 9–12], and only two studies found a stronger dose response for patients with relatives affected than for patients with no family history [7, 9]. Among studies involving women carrying a BRCA1 or BRCA2 (BRCA1/2) mutation, some found an association between chest X-ray exposure and BC; almost all of these studies showed that early exposure may be a risk factor for BC [3–5, 13–18]. For women with a non-BRCA1/2 hereditary predisposition to BC, little is known about the effect of chest radiation exposures and knowledge of such an effect may have clinical relevance. Therefore, we evaluated the effect of low-dose radiation exposure from diagnostic medical procedures on BC risk in women attending family cancer clinics, but not carrying a BRCA1/2 mutation [19]. We also evaluated whether carrying a rare variant in a DNA repair gene other than BRCA1/2 modified the effect of chest X-ray exposure.
Methods
Study population
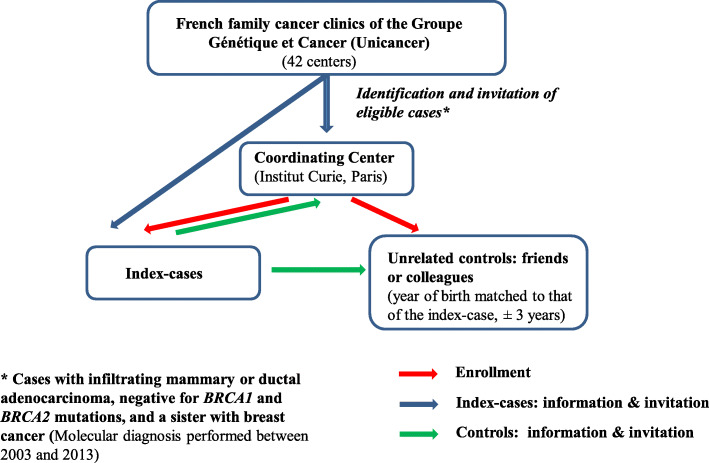
The GENESIS (for GENE SISter) study was initially set up to investigate the missing heritability of BC in a high-risk population with unrelated controls for conducting association studies [19]. GENESIS involved the recruitment of a study population enriched for susceptibility factors by case selection based on familial criteria (Supplementary Method Section), with consideration of environmental factors. Index cases were identified by the national network of family cancer clinics (Genetics and Cancer Group of UNICANCER) (i.e., 42 centers) when eligible, i.e., when diagnosed with infiltrating mammary or ductal adenocarcinoma, negative for BRCA1 and BRCA2 mutations, and had a sister with BC. The mutation screening strategy used was similar for all the clinics. Each family cancer clinic of the national network invited index cases to participate in the GENESIS study by letter or during consultations informing patients of their BRCA1/2 negative results and referred them to the coordinating center (Curie Institute, Paris, France) if index cases consented to participate. Index cases contacted unrelated unaffected friends or colleagues with years of birth matched to ±3 years and invited them to participate and referred those who accepted to participate to the coordinating center. The coordinating center organized the enrollment of index cases and their unrelated controls, collection of questionnaires, family, and clinical data of participants (Fig. 1).
Fig. 1.
Recruitment process for index cases and unrelated controls
All women completed a questionnaire on environmental, lifestyle and reproductive factors, and family history of cancer. Blood samples were collected at participation (see Supplementary Methods Section in Additional file 1.doc). We considered only women reporting European ancestry (i.e., over 95% of the study population) for this evaluation.
Exposure to low-dose radiation to the chest
Participants reported their history of chest X-ray exposure from diagnostic/screening medical procedures in a detailed questionnaire at the time of their recruitment. We considered procedures where the thoracic region was exposed such as conventional radiography, fluoroscopy, computed tomography, and scintigraphy (excluding mammograms). Age at exposure, number of exposures, type of procedure, and reason(s) for performing the examination were also documented.
To estimate lifetime exposure, pulmonary radiological examinations, preoperative radiological examinations, and radiological examinations of the heart and thoracic vessels were taken into account for all the reported procedures.
To exclude procedures that could have been performed because of BC diagnosis, we included exposures that occurred up to 1 year prior to BC diagnosis for cases and 1 year prior to the date of questionnaire completion for controls.
For each type of procedure, information on lifetime exposure (ever/never), number of exposures, and age at first exposure were collected. Variables considered in the analyses were ever versus never exposed, number of exposures, age at first exposure, and timing of first exposure relative to the first full-term pregnancy (FFTP).
We excluded 52 women who underwent radiotherapy for a benign disease 1 year prior to age at censoring (2.19% cases, 1.23% controls). Among cases, we also excluded 10 women (0.63%) who underwent radiotherapy for a cancer other than BC before their BC diagnosis.
DNA repair-related variants
We previously assessed the contribution of rare germline deleterious or likely deleterious variants (with minor allele frequency >0.5% in controls) in 113 DNA repair genes in familial BC by performing targeted sequencing of the entire coding sequence in 1207 cases and 1199 controls from GENESIS. Detailed information on the selection of genes, sequencing procedure and variants filtering and annotation is described in Girard et al. [20] (see Supplementary Methods Section in Additional file 1.doc). Published results of the association tests per gene are shown in Table 1. Sequencing data were available for 82.5% of the GENESIS subjects investigated in the present study. There was no difference in the distribution of the characteristics between the subsets of cases and controls with and without sequencing data (see Supplemental Table 1 in Additional file 2.doc).
Table 1.
Association of rare coding variants with breast cancer, for the 113 DNA repair genes sequenced in the GENESIS population [20]
| Gene | Any variant | ||||
|---|---|---|---|---|---|
| Control carriers | Case carriers | ORa (95% CI) | P value | Groupb | |
| BABAM1/MERIT40 | 4 | 2 | 0.5 (0.1, 2.8) | 0.43 | Low |
| BACH1 | 22 | 21 | 0.8 (0.4, 1.5) | 0.55 | Low |
| BRCC3/BRCC36 | 3 | 1 | 0.3 (0.0, 3.2) | 0.34 | Low |
| BRE | 11 | 7 | 0.6 (0.2, 1.6) | 0.35 | Low |
| CDH1 | 14 | 11 | 0.8 (0.4, 1.7) | 0.54 | Low |
| CDKN1A | 13 | 11 | 0.8 (0.4, 1.9) | 0.66 | Low |
| COBRA1 | 6 | 4 | 0.7 (0.2, 2.4) | 0.55 | Low |
| DLG1 | 25 | 15 | 0.6 (0.3, 1.2) | 0.12 | Low |
| ESR1 | 10 | 6 | 0.6 (0.2, 1.5) | 0.26 | Low |
| EXO1 | 45 | 37 | 0.8 (0.5, 1.3) | 0.41 | Low |
| FAM175A/ABRAXAS | 7 | 5 | 0.7 (0.2, 2.3) | 0.56 | Low |
| FANCA | 19 | 15 | 0.8 (0.4, 1.6) | 0.53 | Low |
| FANCD2 | 20 | 15 | 0.7 (0.4, 1.4) | 0.33 | Low |
| FANCF | 6 | 5 | 0.8 (0.3, 2.8) | 0.77 | Low |
| FANCG | 6 | 4 | 0.7 (0.2, 2.4) | 0.55 | Low |
| FANCI | 22 | 13 | 0.6 (0.3, 1.2) | 0.13 | Low |
| IRS2 | 13 | 9 | 0.7 (0.3, 1.5) | 0.33 | Low |
| KIAA1967 | 22 | 17 | 0.8 (0.4, 1.5) | 0.41 | Low |
| LIG4 | 15 | 13 | 0.7 (0.3, 1.6) | 0.46 | Low |
| MLH3 | 21 | 10 | 0.5 (0.2, 1.0) | 0.06 | Low |
| MUS81 | 10 | 7 | 0.6 (0.2, 1.6) | 0.30 | Low |
| MYC | 2 | 2 | 0.8 (0.1, 5.5) | 0.78 | Low |
| NAT1 | 3 | 1 | 0.4 (0.0, 3.4) | 0.37 | Low |
| PMS2 | 22 | 16 | 0.7 (0.3, 1.3) | 0.24 | Low |
| POLH | 13 | 5 | 0.4 (0.1, 1.1) | 0.07 | Low |
| POLQ | 43 | 36 | 0.8 (0.5, 1.2) | 0.43 | Low |
| PRKAA2 | 8 | 3 | 0.4 (0.1, 1.5) | 0.16 | Low |
| RAD51D/RAD51L3 | 9 | 4 | 0.4 (0.1, 1.4) | 0.17 | Low |
| RAD54L | 21 | 12 | 0.6 (0.3, 1.2) | 0.14 | Low |
| RTEL1 | 20 | 8 | 0.4 (0.2, 0.9) | 0.03 | Low |
| TIMELESS | 28 | 23 | 0.8 (0.5, 1.4) | 0.44 | Low |
| TP53BP1 | 27 | 21 | 0.8 (0.5, 1.5) | 0.51 | Low |
| TP63 | 6 | 3 | 0.5 (0.1, 2.0) | 0.33 | Low |
| TTI2 | 9 | 7 | 0.8 (0.3, 2.2) | 0.67 | Low |
| WDR48 | 15 | 10 | 0.7 (0.3, 1.5) | 0.36 | Low |
| XRCC1 | 31 | 21 | 0.7 (0.4, 1.2) | 0.14 | Low |
| APEX1 | 10 | 10 | 1.0 (0.4, 2.4) | 0.98 | No effect |
| AR | 32 | 31 | 1.0 (0.6, 1.6) | 0.94 | No effect |
| ATR | 30 | 29 | 1.0 (0.6, 1.6) | 0.92 | No effect |
| BAP1 | 4 | 4 | 1.0 (0.3, 4.0) | 1.00 | No effect |
| BLM | 35 | 31 | 0.9 (0.5, 1.4) | 0.59 | No effect |
| CDC27 | 12 | 12 | 1.0 (0.5, 2.3) | 0.98 | No effect |
| CDKN2A | 3 | 3 | 1.0 (0.2, 4.9) | 0.99 | No effect |
| EIF4G1 | 26 | 29 | 1.1 (0.7, 1.9) | 0.67 | No effect |
| EP300 | 21 | 18 | 0.9 (0.5, 1.6) | 0.66 | No effect |
| ERCC6 | 47 | 53 | 1.1 (0.8, 1.7) | 0.55 | No effect |
| FANCB | 9 | 9 | 0.9 (0.4, 2.4) | 0.87 | No effect |
| FANCC | 11 | 10 | 0.9 (0.4, 2.2) | 0.87 | No effect |
| FANCE | 11 | 10 | 0.9 (0.4, 2.2) | 0.86 | No effect |
| FANCL | 9 | 10 | 1.1 (0.4, 2.8) | 0.84 | No effect |
| FLNA | 25 | 24 | 1.0 (0.6, 1.7) | 0.94 | No effect |
| MAGI3 | 26 | 28 | 1.1 (0.6, 1.8) | 0.82 | No effect |
| MAST2 | 46 | 50 | 1.1 (0.7, 1.7) | 0.66 | No effect |
| MCM4 | 25 | 29 | 1.1 (0.7, 1.9) | 0.70 | No effect |
| MCPH1 | 27 | 28 | 1.0 (0.6, 1.8) | 0.92 | No effect |
| MDC1 | 24 | 22 | 0.9 (0.5, 1.7) | 0.81 | No effect |
| MSH2 | 18 | 17 | 0.9 (0.5, 1.8) | 0.76 | No effect |
| MSH6 | 16 | 16 | 0.9 (0.5, 1.9) | 0.86 | No effect |
| NBN | 26 | 27 | 1.0 (0.6, 1.8) | 0.87 | No effect |
| PHLPP2 | 32 | 34 | 1.0 (0.6, 1.7) | 0.97 | No effect |
| POLK | 22 | 22 | 1.0 (0.5, 1.8) | 0.99 | No effect |
| RAD51B/REC2/RAD51L1 | 6 | 5 | 0.9 (0.3, 2.8) | 0.80 | No effect |
| RECQL4 | 49 | 55 | 1.1 (0.8, 1.7) | 0.59 | No effect |
| RINT1 | 8 | 8 | 1.0 (0.4, 2.8) | 0.95 | No effect |
| SETX | 25 | 24 | 1.0 (0.6, 1.7) | 0.91 | No effect |
| TELO2 | 17 | 18 | 1.1 (0.5, 2.1) | 0.89 | No effect |
| XRCC2 | 7 | 6 | 0.9 (0.3, 2.7) | 0.84 | No effect |
| APLF | 7 | 11 | 1.5 (0.6, 3.9) | 0.40 | High |
| ATM | 40 | 77 | 1.9 (1.3, 2.9) | 0.001 | High |
| BARD1 | 7 | 9 | 1.3 (0.5, 3.6) | 0.59 | High |
| BRIP1/FANCJ | 16 | 25 | 1.5 (0.8, 2.8) | 0.25 | High |
| CHEK1 | 4 | 6 | 1.2 (0.3, 4.5) | 0.75 | High |
| CHEK2 | 22 | 62 | 3.0 (1.9, 5.0) | 0.00001 | High |
| CHGB | 9 | 11 | 1.2 (0.5, 3.0) | 0.65 | High |
| DCLRE1C | 9 | 14 | 1.6 (0.7, 3.7) | 0.28 | High |
| DGKZ | 33 | 38 | 1.2 (0.7, 1.9) | 0.52 | High |
| ERCC2 | 17 | 27 | 1.6 (0.9, 3.0) | 0.13 | High |
| EYA3 | 6 | 7 | 1.2 (0.4, 3.5) | 0.77 | High |
| FANCM | 23 | 38 | 1.7 (1.0, 2.8) | 0.06 | High |
| FEN1 | 6 | 7 | 1.2 (0.4, 3.6) | 0.74 | High |
| FOXO1 | 6 | 7 | 1.8 (0.5, 6.0) | 0.38 | High |
| FOXO3 | 0 | 8 | - | - | High |
| FOXO4 | 0 | 4 | - | - | High |
| MAST1 | 8 | 17 | 2.2 (0.9, 5.1) | 0.07 | High |
| MCM7 | 10 | 18 | 1.8 (0.8, 4.0) | 0.13 | High |
| MLH1 | 15 | 19 | 1.3 (0.6, 2.5) | 0.52 | High |
| MRE11A | 12 | 14 | 1.2 (0.6, 2.6) | 0.64 | High |
| MSH3 | 25 | 30 | 1.2 (0.7, 2.1) | 0.49 | High |
| NTHL1 | 18 | 22 | 1.2 (0.6, 2.2) | 0.65 | High |
| NUMA1 | 36 | 51 | 1.4 (0.9, 2.2) | 0.12 | High |
| PALB2 | 9 | 30 | 3.5 (1.7, 7.5) | 0.001 | High |
| PIK3R1 | 1 | 4 | 4.3 (0.5, 38.3) | 0.20 | High |
| PMS1 | 6 | 10 | 1.5 (0.6, 4.3) | 0.41 | High |
| PPM1D | 4 | 6 | 1.5 (0.4, 5.4) | 0.53 | High |
| PTEN | 0 | 4 | - | - | High |
| RAD50 | 30 | 37 | 1.2 (0.7, 2.0) | 0.44 | High |
| RAD51C | 7 | 10 | 1.5 (0.6, 4.0) | 0.41 | High |
| RAD9B | 4 | 6 | 1.5 (0.4, 5.2) | 0.55 | High |
| RECQL5 | 20 | 29 | 1.5 (0.9, 2.7) | 0.14 | High |
| REV3L | 31 | 39 | 1.3 (0.8, 2.1) | 0.30 | High |
| RNF168 | 13 | 16 | 1.2 (0.6, 2.6) | 0.59 | High |
| RPA1 | 9 | 14 | 1.5 (0.7, 3.6) | 0.32 | High |
| SLX4/FANCP | 36 | 44 | 1.2 (0.8, 1.9) | 0.38 | High |
| STK11 | 1 | 2 | 2.1 (0.2, 22.9) | 0.55 | High |
| TGFB1 | 5 | 9 | 1.6 (0.5, 4.9) | 0.38 | High |
| TOP3A | 22 | 31 | 1.4 (0.8, 2.5) | 0.23 | High |
| TP53 | 3 | 6 | 2.0 (0.5, 8.0) | 0.34 | High |
| TSC2 | 45 | 56 | 1.3 (0.9, 1.9) | 0.23 | High |
| TTI1 | 26 | 30 | 1.2 (0.7, 2.0) | 0.57 | High |
| UIMC1/RAP80 | 12 | 15 | 1.2 (0.6, 2.7) | 0.58 | High |
| USP8 | 9 | 16 | 1.7 (0.7, 3.8) | 0.23 | High |
| WRN | 47 | 59 | 1.3 (0.9, 1.9) | 0.23 | High |
| XRCC3 | 4 | 7 | 1.8 (0.5, 6.2) | 0.36 | High |
Abbreviations: OR (95%CI) odds ratio (95% confidence interval)
aReference group: non-carrier of a variant in the tested gene
bGroup “Low”: OR <0.9; Group “No Effect”: 0.9≤ OR ≤1.1; Group “High”: OR >1.1
Because each gene has very low deleterious or likely deleterious variant frequencies (frequency of the pool of variants for each gene ranged between 0% and 4.1% in controls) and thereby stratification by X-ray exposure and by gene led to very small numbers of subjects or even no subjects, we grouped the genes according to the value of their association with BC, i.e., the odds ratio (OR) point estimate obtained in the study by Girard et al. (Table 1) and classified them as follows: Group “Low” including genes with OR<0.9; Group “No Effect,” including genes with 0.9≤OR≤1.1 and Group “High,” including genes with OR>1.1. An individual could be assigned to more than one group if carrying variants in genes belonging to different groups.
Statistical analyses
To assess the association between chest X-ray exposure and risk of BC, we used logistic regression models. To assess whether the association varied according to tumor estrogen receptors (ER) status, we used multinomial regression models. Analyses were adjusted for age at censoring, which was calculated as the age at diagnosis for cases, and the age at interview for controls. Other adjustment variables were education level (not graduated, basic level, intermediate/high level), birth cohort (≤1945, 1946–1959, ≥1960), body mass index at diagnosis for cases, and at interview for controls (<18.5, 18.5–24.99, 25–29.99, ≥30), number of full-term pregnancies (nulliparous, 1–2, >2), age at FFTP (<20, 20–24, 25–29, ≥30), mammography exposure at least 1 year before censoring (ever/never), and family history of BC. For this latter variable, the number of first- or second-degree relatives affected with BC was generated. Since cases had an affected sister by design, we excluded one affected sister from the family history count to assess cases’ BC family history distribution unbiased by the study design and classified BC family history as none affected, at least one 1st degree relative affected, or only 2nd degree relatives affected. We also adjusted for the number of chest X-ray exposures (≤5 vs. >5) when appropriate.
We assessed associations by birth cohort, age at censor, family history of BC, and DNA repair gene group; we used likelihood ratio tests to test for heterogeneity. Additionally, we adjusted for other gene groups when the analysis was stratified by the gene group.
We assessed heterogeneity between estrogen receptor (ER) tumor status using a multinomial logistic regression model and tested equality of coefficients between equations by difference between the log-likelihoods assuming a chi-square distribution with 1 degree of freedom (df) for never/ever exposed, 2 df for timing to first full-term pregnancy (FFTP) and age at first exposure, and 3 df for number of exposures.
To minimize potential survival bias, we also conducted an analysis restricted to cases diagnosed at most 5 years before enrollment in GENESIS.
Finally, as the DNA repair gene groups were defined using a priori bounds for ORs, we performed sensitivity analyses using different bounds (i.e., Group “Low”: OR<0.8 or <1.0; Group “No Effect”: 0.8≤OR≤1.2 or OR=1.0; Group “High”: OR>1.2 or >1.0).
To evaluate the effect of missing information on the observed results, we performed multiple imputations using the chained equations method (MICE) [21, 22] as implemented in STATA [23]. This method uses a Gibbs-like algorithm [24] to obtain 100 imputed datasets with complete observations for each outcome. ORs estimated on the imputed data sets were pooled together using Rubin’s rules to obtain valid statistical inferences [25].
All P values were two-sided and a 5% level of significance was used. All analyses were performed using Stata software version 14 [23].
Results
Characteristics of the study population are described in Table 2. Most of the cases were prevalent with a mean delay between diagnosis and interview of 8.3 years (SD:±7.1). The mean age at BC diagnosis was 50.2 years (SD:±9.3), and the mean age at interview for the controls was 55.8 years (SD:±9.9).
Table 2.
Characteristics of GENESIS participants
| Characteristics | Cases N = 1,552 |
Controls N = 1,363 |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Birth cohort | ||||
| ≤1945 | 488 | 31.4 | 294 | 21.6 |
| 1946–59 | 797 | 51.4 | 706 | 51.8 |
| ≥1960 | 267 | 17.2 | 363 | 26.6 |
| Age at censoring, years | ||||
| Mean (sd) | 50.2 (9.3) | 55.8 (9.9) | ||
| ≤45 | 513 | 33.1 | 201 | 14.8 |
| 46–50 | 336 | 21.7 | 197 | 14.5 |
| 51–60 | 473 | 30.5 | 485 | 35.6 |
| >60 | 230 | 14.8 | 480 | 35.2 |
| Education level | ||||
| Intermediate/high | 780 | 50.3 | 916 | 67.2 |
| Basic | 714 | 46.0 | 434 | 31.8 |
| Not graduated | 58 | 3.7 | 12 | 0.9 |
| Missing | 0 | 0.0 | 1 | 0.1 |
| Body mass index | ||||
| 18.5–24.9 | 1,019 | 65.6 | 869 | 63.8 |
| <18.5 | 69 | 4.5 | 32 | 2.3 |
| ≥25 and <30 | 341 | 22.0 | 345 | 25.3 |
| ≥30 | 120 | 7.7 | 117 | 8.6 |
| Missing | 3 | 0.2 | 0 | 0.0 |
| Smoking | ||||
| No | 832 | 53.6 | 680 | 49.9 |
| Current | 159 | 10.2 | 158 | 11.6 |
| Past | 550 | 35.4 | 514 | 37.7 |
| Missing | 11 | 0.7 | 11 | 0.8 |
| Number of full term pregnancies | ||||
| ≥2 | 445 | 28.7 | 411 | 30.2 |
| 1–2 | 921 | 59.3 | 763 | 56.0 |
| 0 | 185 | 11.9 | 187 | 13.7 |
| Missing | 1 | 0.1 | 2 | 0.1 |
| Age at first full-term pregnancy, years | ||||
| <20 | 179 | 11.5 | 102 | 7.5 |
| 20–24 | 628 | 40.5 | 531 | 39.0 |
| 25–29 | 393 | 25.4 | 392 | 28.8 |
| ≥30 | 165 | 10.6 | 149 | 10.9 |
| No full-term pregnancy | 185 | 11.9 | 187 | 13.7 |
| Missing | 2 | 0.1 | 2 | 0.1 |
| Family history of breast cancera | ||||
| None | 427 | 27.5 | 959 | 70.4 |
| 1st degree | 818 | 52.7 | 187 | 13.7 |
| Only 2nd degree | 307 | 19.8 | 216 | 15.9 |
| Missing | 0 | 0.0 | 1 | 0.1 |
| Tumor estrogen receptors (ER) | ||||
| ER+ | 818 | 52.7 | ||
| ER- | 168 | 10.8 | ||
| Missing | 566 | 36.5 | ||
| Gene groupb | ||||
| Group “Low” | ||||
| 0 | 723 | 46.6 | 721 | 52.9 |
| > 1 | 274 | 17.7 | 441 | 32.4 |
| Group “No Effect” | ||||
| 0 | 575 | 37.1 | 659 | 48.4 |
| >1 | 422 | 27.2 | 503 | 36.9 |
| Group “High” | ||||
| 0 | 422 | 27.2 | 667 | 48.9 |
| >1 | 575 | 37.1 | 495 | 36.3 |
| Missing | 555 | 35.8 | 201 | 14.8 |
aExcluding one affected sister per index case, “none” means no history of BC for controls or no additional BC case in the family for cases; “1st degree” means 1st degree family history for controls or additional 1st degree relative for cases and “2nd degree” means only 2nd degree family history for controls or only additional 2nd degree family history for cases
bIndividuals carrying at least one variant in one of the Gene Groups: Group “Low” (OR<0.9); Group “No Effect” (0.9≤OR≤1.1); Group “High” (OR>1.1)
Compared to controls, cases were more likely to have a basic education level, lower BMI, younger age at the FFTP, and as expected, stronger family history of BC. Regarding birth cohort, cases were more likely to be born before 1945 than controls. Among the subset of participants who were sequenced for the 113 DNA repair genes (74.1%), 20% of women did not carry any variant and 30.9% of women carried only one variant. Among those who carried at least two variants, 21.9% of them carried variants in the same group, and 14.7% carried variants in genes from all three groups (data not shown). Cases carried variants from Group “High” more often than controls (57.7% and 42.6%, respectively) (Table 2).
The distribution of chest X-ray exposures, by type of medical procedure, is shown in Table 3. When considering conventional radiography plus fluoroscopy, the mean age at first chest X-ray exposure was significantly lower for cases than for controls (20.4 and 22.0 years, respectively; P=0.003) with a higher percentage of cases exposed before age 20 years than controls (37.0% and 31.9%, respectively, P<10-3; data not shown).
Table 3.
Chest diagnostic/screening X-ray exposure characteristics by medical procedures
| Characteristicsa | Cases N = 1552 |
Controls N = 1363 |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Conventional radiography + fluoroscopy | ||||
| Never | 213 | 13.7 | 242 | 17.8 |
| Ever | 1,296 | 83.5 | 1,097 | 80.5 |
| Missing | 43 | 2.8 | 24 | 1.8 |
| Number of lifetime exposures | ||||
| 1–3 | 377 | 24.3 | 379 | 27.8 |
| 4–9 | 248 | 16.0 | 195 | 14.3 |
| ≥10 | 258 | 16.6 | 213 | 15.6 |
| Missing | 456 | 29.4 | 334 | 24.5 |
| Age at first exposure (years) | ||||
| Mean (sd) | 20.4 (10.9) | 22.0 (12.5) | ||
| Missing | 291 | 18.8 | 202 | 14.8 |
| Tomography | ||||
| Never | 1,389 | 89.5 | 1,276 | 93.6 |
| Ever | 48 | 3.1 | 40 | 2.9 |
| Missing | 115 | 7.4 | 47 | 3.5 |
| Number of lifetime exposures | ||||
| 1 | 37 | 2.4 | 30 | 2.2 |
| ≥2 | 9 | 0.6 | 9 | 0.7 |
| Missing | 117 | 7.5 | 48 | 3.5 |
| Age at first exposure (years) | ||||
| Mean (sd) | 40.0 (15.8) | 42.8 (15.4) | ||
| Missing | 115 | 7.4 | 47 | 3.5 |
| Scintigraphy | ||||
| Never | 1,416 | 91.2 | 1,294 | 94.9 |
| Ever | 21 | 1.4 | 22 | 1.6 |
| Missing | 115 | 7.4 | 47 | 3.5 |
| Number of lifetime exposures | ||||
| 1 | 18 | 1.2 | 18 | 1.3 |
| ≥2 | 3 | 0.2 | 4 | 0.3 |
| Missing | 115 | 7.4 | 47 | 3.5 |
| Age at first exposure (years) | ||||
| Mean (sd) | 50.8 (10.2) | 53.4 (12.0) | ||
| Missing | 115 | 7.4 | 47 | 3.5 |
| Mammography | ||||
| Never | 266 | 17.1 | 102 | 7.5 |
| Ever | 1271 | 81.9 | 1252 | 91.9 |
| Missing | 15 | 1.0 | 9 | 0.7 |
a Lifetime exposures up to one year prior to diagnosis for cases and up to one year prior to date of questionnaire completion for controls
We found that exposure to chest X-rays was associated with a 2-fold increased odds of BC (P<10-3) compared to non-exposed women (Table 4). Each additional procedure was associated with a 3% increased odds of BC (P<10-3).
Table 4.
Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure, and the first full-term pregnancy
| Number of | Multiple Imputation | |||||
|---|---|---|---|---|---|---|
| Cases | Controls | ORa | 95% CI | ORa | 95% CI | |
| Chest X-ray exposureb | ||||||
| Never | 208 | 239 | 1 | 1 | ||
| Ever | 1304 | 1104 | 2.05 | 1.55–2.73 | 2.05 | 1.54–2.72 |
| Number of exposures | ||||||
| 0 | 208 | 239 | 1 | 1 | ||
| 1–3 | 392 | 390 | 1.70 | 1.23–2.34 | 1.62 | 1.18–2.23 |
| 4–9 | 251 | 200 | 2.52 | 1.76–3.61 | 2.29 | 1.65–3.16 |
| ≥10 | 263 | 215 | 2.37 | 1.64–3.43 | 2.70 | 1.89–3.87 |
| Continuous | 1.03 | 1.01–1.04 | 1.03 | 1.02–1.05 | ||
| Age at first exposure, yearsc | ||||||
| No exposure | 208 | 239 | 0.55 | 0.40–0.76 | 0.58 | 0.42–0.80 |
| ≥20 | 485 | 490 | 1 | 1 | ||
| 15–19 | 288 | 222 | 1.02 | 0.75–1.37 | 1.01 | 0.75–1.36 |
| <15 | 290 | 219 | 1.11 | 0.83–1.50 | 1.08 | 0.80–1.45 |
| Continuous | 1.00 | 0.99–1.01 | 1.01 | 0.99–1.02 | ||
| According to first full-term pregnancy (FFTP)c | ||||||
| Only after FFTPd | 268 | 232 | 1 | 1 | ||
| Before (incl. no FTP) | 825 | 725 | 0.81 | 0.61–1.08 | 0.85 | 0.64–1.12 |
| According to first FFTP and number of exposures | ||||||
| Only after and ≤5d | 186 | 178 | 1 | 1 | ||
| Only after and >5d | 56 | 43 | 1.77 | 0.98–3.18 | 1.68 | 0.99–2.85 |
| Before and ≤5 (incl. no FTP) | 442 | 412 | 0.95 | 0.67–1.36 | 0.89 | 0.63–1.25 |
| Before and >5 (incl. no FTP) | 272 | 215 | 1.12 | 0.77–1.61 | 1.33 | 0.96–1.85 |
Abbreviations: OR (95%CI) odds ratio (95% confidence interval)
aAdjusted for age at censoring, birth cohort (≤1945; 1946–1959; ≥1960), number of full-term pregnancies (>2; 1–2; 0), mammography use (never; ever), educational level (intermediate/high; basic; not graduated), BMI (18.5–24.9; <18.5; ≥25 and <30; ≥30), smoking (no; current; past), and breast cancer family history (0;1st degree; 2nd degree)
bChest X-ray exposure includes pulmonary radiological examinations in the field of preventive/occupational medicine or for lung disease, preoperative radiological examinations, and radiological examinations of heart and thoracic vessels for all the reported procedures
cAdjusted as in a plus number of exposures (≤5;>5)
dAfter = also includes chest X-ray exposure that occurred during the same year of first full-term pregnancy
When analyses were performed according to birth cohort, the association between chest X-ray exposure and BC risk was significant for later birth cohorts, i.e., women born after 1945 (ever vs. never, 1946–1959: OR=1.65, ≥1960: OR=2.54), with a significantly higher risk for women born after 1960 (Phet=0.024) (Table 5). We also found significant heterogeneity between birth cohorts for the effect of the number of exposures on BC risk (Phet=0.041) with each additional procedure associated with a 6% increased odds of BC (P<10-3) for women born after 1960. When analyses were performed according to age at censoring and family history of BC (see Supplemental Tables 2-3 in Additional file 2.doc), none of the heterogeneity tests were significant. However, the effect of chest X-ray exposure was significant only for women over the age of 60 with each additional procedure being associated with a 2% increased odds (P=0.036).
Table 5.
Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure, and the first full-term pregnancy by birth cohort
| Number of | Number of | Number of | Pb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Ctrls | ORa | 95% CI | Cases | Ctrls | ORa | 95% CI | Cases | Ctrls | ORa | 95% CI | ||
| Birth cohort | |||||||||||||
| ≤ 1945 | 1946–1959 | ≥ 1960 | |||||||||||
| Chest X-ray exposure | |||||||||||||
| No | 47 | 31 | 1 | 96 | 92 | 1 | 65 | 116 | 1 | ||||
| Yes | 431 | 259 | 1.57 | 0.72-3.43 | 678 | 605 | 1.65 | 1.06–2.56 | 195 | 240 | 2.54 | 1.63–3.98 | 0.024 |
| Number of exposures | |||||||||||||
| 0 | 47 | 31 | 1 | 96 | 92 | 1 | 65 | 116 | 1 | ||||
| 1–3 | 134 | 81 | 1.72 | 0.74–3.98 | 179 | 197 | 1.24 | 0.75–2.05 | 79 | 112 | 2.04 | 1.22–3.40 | |
| 4–9 | 81 | 46 | 2.04 | 0.82–5.07 | 122 | 111 | 1.70 | 0.98–2.94 | 48 | 43 | 3.77 | 2.03–7.01 | 0.041 |
| ≥10 | 105 | 64 | 1.44 | 0.60–3.45 | 142 | 130 | 2.26 | 1.31–3.90 | 16 | 21 | 2.77 | 1.19–6.47 | |
| Continuous | 1.01 | 0.99–1.03 | 1.04 | 1.02–1.06 | 1.06 | 1.01–1.12 | 0.013 | ||||||
| Age at first exposure, yearsc | |||||||||||||
| No exposure | 47 | 31 | 0.52 | 0.23–1.21 | 96 | 92 | 0.77 | 0.47–1.25 | 65 | 116 | 0.47 | 0.28–0.78 | |
| ≥20 | 163 | 119 | 1 | 237 | 261 | 1 | 85 | 110 | 1 | ||||
| 15–19 | 87 | 45 | 0.74 | 0.38–1.42 | 159 | 123 | 1.07 | 0.69–1.66 | 42 | 54 | 1.13 | 0.64–2.00 | 0.67 |
| <15 | 122 | 64 | 0.83 | 0.47–1.46 | 140 | 114 | 1.40 | 0.89–2.18 | 28 | 41 | 0.91 | 0.47–1.79 | |
| Continuous | 1.01 | 0.99–1.02 | 1.00 | 0.98–1.01 | 1.02 | 0.99–1.05 | 0.49 | ||||||
| According to first full-term pregnancy (FFTP)c | |||||||||||||
| Only after FFTPd | 109 | 59 | 1 | 124 | 139 | 1 | 35 | 34 | 1 | ||||
| Before (incl. no FTP) | 268 | 175 | 0.48 | 0.27–0.86 | 429 | 373 | 0.95 | 0.63–1.44 | 128 | 177 | 0.86 | 0.45–1.63 | 0.19 |
Abbreviations: OR (95% CI) odds ratio (95% confidence interval
aAdjusted for age at censoring (when by birth cohort analysis) and for birth cohort (continuous) (when by age at censure analysis), number of full-term pregnancies (>2; 1–2; 0), mammography use (never; ever), educational level (intermediate/high; basic; not graduated), BMI (18.5–24.99; <18.5; ≥25 and <30; ≥30), smoking (no; current; past), and family history of breast cancer (0;1st degree; 2nd degree)
bP value for heterogeneity test
cAdjusted as in a plus number of exposures (≤5; >5)
dAfter = also includes chest X-ray exposure that occurred during year of first full-term pregnancy
Interestingly, when stratifying on the gene group, we found that for women carrying at least one variant in Group “High” (i.e., OR>1.1) (see Supplemental Table 4 in Additional file 2.doc), the effect of chest X-ray exposures on BC risk was significantly higher than for those carrying at least one variant in the other groups (Phet=0.0038) (Table 6) (ever vs. never: Group “Low” (OR<0.9):OR=2.02; Group “No Effect”(0.9≤OR≤1.1): OR=1.62; Group “High” (OR>1.1): OR=3.31). Having had ten or more exposures also doubled the BC risk for women with a variant from Group “High” (OR>1.1) compared with women in the other two groups (Phet=0.022).
Table 6.
Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure, and the first full-term pregnancy by gene group
| Number of | Number of | Number of | Pb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Ctrls | ORa | 95% CI | Cases | Ctrls | ORa | 95% CI | Cases | Ctrls | ORa | 95% CI | ||
| Gene group | |||||||||||||
| Group “Low”f | Group “No Effect”f | Group “High”f | |||||||||||
| Chest X-ray exposure | |||||||||||||
| No | 34 | 67 | 1 | 61 | 81 | 1 | 65 | 91 | 1 | ||||
| Yes | 234 | 371 | 2.02 | 1.16–3.52 | 354 | 416 | 1.62 | 1.03–2.55 | 495 | 400 | 3.31 | 2.14–5.12 | 0.0038 |
| Number of exposures | |||||||||||||
| 0 | 34 | 67 | 1 | 61 | 81 | 1 | 65 | 91 | 1 | ||||
| <10 | 119 | 183 | 2.01 | 1.12–3.59 | 184 | 215 | 1.53 | 0.95–2.46 | 256 | 219 | 2.98 | 1.89–4.70 | |
| ≥10 | 47 | 75 | 2.19 | 1.09–4.40 | 67 | 85 | 2.04 | 1.14–3.66 | 94 | 78 | 3.88 | 2.23–6.75 | 0.022 |
| Continuous | 1.02 | 0.99–1.04 | 1.02 | 1.00–1.04 | 1.04 | 1.02–1.06 | 0.032 | ||||||
| Age at first exposure, yearsd | |||||||||||||
| No exposure | 34 | 67 | 0.55 | 0.30–1.01 | 61 | 81 | 0.83 | 0.51–1.37 | 65 | 91 | 0.38 | 0.23–0.61 | 0.43 |
| ≥20 | 90 | 163 | 1 | 124 | 188 | 1 | 179 | 180 | 1 | ||||
| <20 | 101 | 143 | 1.24 | 0.80–1.91 | 166 | 165 | 1.38 | 0.94–2.03 | 222 | 159 | 1.27 | 0.90–1.80 | |
| Continuous | 1.00 | 0.9–1.02 | 0.99 | 0.98–1.01 | 1.00 | 0.98–1.01 | 0.78 | ||||||
| According to first full-term pregnancy (FFTP)d | |||||||||||||
| Only after FFTPe | 52 | 75 | 1 | 68 | 91 | 1 | 95 | 92 | 1 | ||||
| Before (incl. no FTP) | 142 | 242 | 0.82 | 0.49–1.38 | 231 | 271 | 0.90 | 0.57–1.40 | 311 | 257 | 1.04 | 0.69–1.57 | 0.48 |
Abbreviations: OR (95% CI) odds ratio (95% confidence interval)
aAdjusted for age at censoring, birth cohort (≤1945; 1946–1959; ≥1960), number of full-term pregnancies (>2; 1–2; 0), mammography use (never; ever), educational level (intermediate/high; basic; not graduated), BMI (18.5–24.99; <18.5; ≥25 and <30; ≥30), smoking (no; current; past), plus two other DNA repair rare variants groups (when analysis by group of DNA repair rare variants), with missing included in reference categories for each variable
bBecause of the non-exclusivity of the 3 groups, P value for heterogeneity test was performed on the ranked Group variable : at least 1 variant in Group “High,” no variant in Group “High,” and at least 1 variant in Group “No Effect,” only at least 1 variant in Group “Low”
dAdjusted as in a plus number of exposures (≤5;>5)
eAfter = also includes chest X-ray exposure that occurred during the same year of first full-term pregnancy
fIndividuals carrying at least one variant in one of the Gene Groups: Group “Low” (OR<0.9) ; Group “No Effect” (0.9<=OR<=1.1); Group “High” (OR>1.1)
When considering chest X-ray exposure (ever vs. never) and number of variants simultaneously, BC risk increased with increasing number of variants from Group “High” (OR>1.1) with a significant 66% increased odds of BC for each additional variant. Inversely, BC risk decreased with increasing number of variants from Group “Low” (OR<0.9) with a significant 31% decreased odds of BC for each additional variant (Table 7).
Table 7.
Combined effect of chest X-ray exposure and genetic variants
| Chest X-ray exposure & number of DNA repair rare variants | Number of | Multiple imputation | ||||
|---|---|---|---|---|---|---|
| Cases | Ctrls | ORa | 95%CI | ORa | 95%CI | |
| Group ‘Low’b | ||||||
| Ever & 0 variant | 604 | 580 | 1 | 1 | ||
| Ever & 1 variant | 197 | 288 | 0.67 | 0.52-0.86 | 0.67 | 0.52-0.87 |
| Ever & 2 variants | 34 | 69 | 0.52 | 0.32-0.84 | 0.49 | 0.30-0.79 |
| Ever & ≥3 variants | 3 | 14 | 0.25 | 0.06-1.00 | 0.25 | 0.06-0.99 |
| Never | 135 | 195 | 0.50 | 0.37-0.68 | 0.31 | 0.18-0.52 |
| Continuouse | 0.69 | 0.57-0.84 | 0.69 | 0.58-0.83 | ||
| Never & 0 variant | 101 | 128 | 1 | 1 | ||
| Never & 1 variant | 27 | 50 | 0.56 | 0.30-1.06 | 0.56 | 0.30-1.05 |
| Never & 2 variants | 7 | 16 | 0.52 | 0.17-1.57 | 0.48 | 0.16-1.44 |
| Never & ≥3 variants | 0 | 1 | - | - | ||
| Ever & 0 variant | 604 | 580 | 1.67 | 1.19-2.34 | 1.66 | 1.18-2.34 |
| Ever & 1 variant | 197 | 288 | 1.12 | 0.77-1.62 | 1.12 | 0.77-1.64 |
| Ever & 2 variants | 34 | 69 | 0.86 | 0.50-1.51 | 0.82 | 0.46-1.43 |
| Ever &≥3 variants | 3 | 14 | 0.42 | 0.10-1.71 | 0.42 | 0.10-1.71 |
| Group ‘No Effect’c | ||||||
| Ever & 0 variant | 484 | 535 | 1 | 1 | ||
| Ever & 1 variant | 258 | 318 | 0.96 | 0.76-1.22 | 0.97 | 0.76-1.23 |
| Ever & 2 variants | 81 | 78 | 1.12 | 0.76-1.65 | 1.15 | 0.77-1.70 |
| Ever & ≥3 variants | 15 | 20 | 1.21 | 0.55-2.70 | 1.28 | 0.57-2.87 |
| Never | 135 | 195 | 0.58 | 0.43-0.78 | 0.61 | 0.40-0.94 |
| Continuouse | 1.02 | 0.86-1.21 | 1.05 | 0.90-1.21 | ||
| Never & 0 variant | 74 | 114 | 1 | 1 | ||
| Never & 1 variant | 45 | 67 | 1.08 | 0.62-1.90 | 1.08 | 0.61-1.89 |
| Never & 2 variants | 14 | 12 | 1.40 | 0.55-3.59 | 1.50 | 0.59-3.84 |
| Never & ≥3 variants | 2 | 2 | 1.90 | 0.23-15.8 | 1.93 | 0.23-16.1 |
| Ever & 0 variant | 484 | 535 | 1.85 | 1.27-2.70 | 1.85 | 1.27-2.71 |
| Ever & 1 variant | 258 | 318 | 1.77 | 1.19-2.65 | 1.78 | 1.19-2.66 |
| Ever & 2 variants | 81 | 78 | 2.07 | 1.25-3.42 | 2.13 | 1.29-3.54 |
| Ever & ≥3 variants | 15 | 20 | 2.24 | 0.95-5.32 | 2.39 | 1.01-5.64 |
| Group ‘High’d | ||||||
| Ever & 0 variant | 343 | 551 | 1 | 1 | ||
| Ever & 1 variant | 327 | 291 | 2.06 | 1.62-2.63 | 2.07 | 1.62-2.65 |
| Ever & 2 variants | 122 | 93 | 2.34 | 1.66-3.31 | 2.28 | 1.60-3.23 |
| Ever & ≥3 variants | 46 | 16 | 4.61 | 2.34-9.08 | 4.65 | 2.35-9.22 |
| Never | 135 | 195 | 0.85 | 0.63-1.16 | 0.74 | 0.49-1.13 |
| Continuouse | 1.67 | 1.42-1.96 | 1.66 | 1.44-1.90 | ||
| Never & 0 variant | 70 | 104 | 1 | 1 | ||
| Never & 1 variant | 46 | 62 | 0.90 | 0.51-1.58 | 0.88 | 0.50-1.54 |
| Never & 2 variants | 13 | 23 | 0.66 | 0.27-1.57 | 0.58 | 0.24-1.41 |
| Never & ≥3 variants | 6 | 6 | 2.01 | 0.54-7.48 | 2.01 | 0.54-7.45 |
| Ever & 0 variant | 343 | 551 | 1.12 | 0.75-1.65 | 1.10 | 0.75-1.63 |
| Ever & 1 variant | 327 | 291 | 2.30 | 1.54-3.44 | 2.28 | 1.53-3.40 |
| Ever & 2 variants | 122 | 93 | 2.61 | 1.63-4.19 | 2.51 | 1.56-4.03 |
| Ever & ≥3 variants | 46 | 16 | 5.15 | 2.43-10.9 | 5.10 | 2.41-10.8 |
Abbreviations: OR (95%CI) odds ratio (95% confidence interval)
aAdjusted for age at censoring, birth cohort (≤1945; 1946-1959; ≥1960), number of full-term pregnancies (>2; 1-2; 0), mammography use (never; ever), educational level (intermediate/high; basic; not graduated), BMI (18.5-24.99; <18.5; ≥25 and <30; ≥30), smoking (no; current; past), and two other DNA repair genes groups
bat least one variant in a gene from Group ‘Low’
cat least one variant in a gene from Group ‘No Effect’
dat least one variant in a gene from Group ‘High’
e“Never” excluded
When analyses were performed according to tumor ER status (Table 8), the heterogeneity tests were not significant when we compared ORs between ER− and ER+ tumors for any chest X-ray exposure variables, although there was a suggestive stronger association in the ever vs. never analysis for women with ER+ tumors when compared to women with ER− tumors.
Table 8.
Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure, and the first full-term pregnancy by estrogen receptor tumor status
| Estrogen receptor (ER) tumor status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ER negative | ER positive | Unknown | |||||||||
| Controls | Cases | ORa | 95% CI | Cases | ORa | 95% CI | Pb | Cases | ORa | 95% CI | |
| Chest X-ray exposure | |||||||||||
| No | 239 | 32 | 1 | 107 | 1 | 69 | 1 | ||||
| Yes | 1104 | 132 | 1.50 | 0.94–2.39 | 692 | 2.13 | 1.56–2.91 | 0.16 | 480 | 2.21 | 1.51–3.24 |
| Number of exposures | |||||||||||
| 0 | 239 | 32 | 1 | 107 | 1 | 69 | 1 | ||||
| 1–3 | 390 | 43 | 1.31 | 0.77–2.25 | 207 | 1.73 | 1.22–2.46 | 0.67 | 142 | 1.87 | 1.21–2.87 |
| 4–9 | 200 | 29 | 1.99 | 1.10–3.62 | 140 | 2.65 | 1.80–3.92 | 82 | 2.51 | 1.55–4.07 | |
| ≥10 | 215 | 21 | 1.51 | 0.79–2.90 | 124 | 2.23 | 1.49–3.33 | 118 | 3.27 | 2.03–5.28 | |
| Continuous | 1.01 | 0.99–1.04 | 1.02 | 1.00–1.03 | 0.58 | 1.03 | 1.01–1.05 | ||||
| Age at first exposure, yearsc | |||||||||||
| No exposure | 239 | 32 | 0.70 | 0.41–1.19 | 107 | 0.53 | 0.38–0.75 | 69 | 0.53 | 0.35–0.81 | |
| ≥20 | 490 | 52 | 1 | 263 | 1 | 170 | 1 | ||||
| 15–19 | 222 | 33 | 1.06 | 0.64–1.78 | 154 | 1.07 | 0.78–1.47 | 0.66 | 101 | 0.86 | 0.58–1.27 |
| <15 | 219 | 27 | 1.13 | 0.66–1.94 | 139 | 1.04 | 0.75–1.44 | 124 | 1.25 | 0.86–1.82 | |
| Continuous | 1.01 | 0.99–1.03 | 1.01 | 0.99–1.02 | 0.39 | 1.00 | 0.99–1.01 | ||||
| According to first full-term pregnancy (FFTP)c | |||||||||||
| Only after FFTPd | 232 | 35 | 1 | 141 | 1 | 92 | 1 | ||||
| Before (incl. no FTP) | 725 | 79 | 0.66 | 0.41–1.07 | 435 | 0.83 | 0.61–1.13 | 0.22 | 311 | 0.84 | 0.58–1.22 |
Abbreviations: OR (95% CI) odds ratio (95% confidence interval)
aAdjusted for age at censoring, birth cohort (≤1945; 1946–1959; ≥1960), number of full-term pregnancies (>2; 1–2; 0), mammography use (never; ever), educational level (intermediate/high; basic; not graduated), BMI (18.5–24.99; <18.5; ≥25 and <30; ≥30), smoking (no; current; past), and breast cancer family history (0;1st degree; 2nd degree)
bP value for χ2 heterogeneity test between ER-negative and ER-positive tumors
cAdjusted as in a plus number of exposures (≤5;>5)
dAfter = also includes chest X-ray exposure that occurred during the same year of first full-term pregnancy
In all analyses (Tables 4, 5, 6, and 8), there was no significant difference in the BC risk by age at first exposure, nor by timing according to the FFTP.
Because some chest X-ray variables had a high fraction of missing data, we reran the above analyses after imputing the missing data (Tables 4 and 7; Supplemental Tables 4-10 in Additional file 2.doc). The magnitude and direction of the effect estimates based on analyses using an extra class for missing data or a multiple imputation strategy were similar.
We also performed analyses restricted to cases diagnosed at most 5 years before enrollment in the study. Again, the magnitude and direction of the effect estimates were unchanged (see Supplemental Table 10 in Additional file 2.doc).
Discussion
We found that chest X-ray exposure doubles the risk of BC in women with a hereditary predisposition to BC unexplained by a BRCA1/2 mutation. This risk increases with the number of exposures with an increase of 3% for each additional exposure. Being born after 1960, over age 60 or a carrier of at least one variant in the DNA repair genes group associated with an increased risk of BC increased the effect of chest X-ray exposure on BC risk.
Our study confirms that low-dose ionizing radiation to the thoracic region increases the risk of BC among high-risk women, as pointed out by other studies [1, 7, 8, 18]. In contrast to Ma et al. [6] and John et al. [11], we did not find that younger age at first chest X-ray exposure was significantly associated with higher ORs compared to those initially exposed at an older age. However, we found a suggestive association between having been exposed at an early age in the subgroup of women born between 1946 and 1959 or those older than 50 years at censoring and in the subgroup of women without a family history of BC (i.e., only one sister affected for cases and none for controls) but due to the self-report exposures and potential recall bias, these results should be taken with cautious.
We also observed a difference by birth cohort on radiation-induced risk of BC, with significantly higher risks for women born after 1960, which was similar to BC risk in women carrying a BRCA1/2 pathogenic variant and born after 1950 [3]. However, this finding was not subsequently confirmed in the Pijpe et al. study [5]. Even if radiation exposure levels were higher in the past, the decrease over the generations in the number of exposed subjects by outcome status appeared different, especially in the younger birth cohort. This may be due to the reluctance of doctors to reduce radiological examinations in women at high risk of cancer, more often classified accordingly since the discovery of the first BC predisposing genes in the 1990s [26, 27].
When stratifying on tumor ER status, we found a suggestive stronger effect of chest X-ray exposure for women with an ER+ tumor, consistent with Sigurdson et al.’s findings that common variants in estrogen metabolizing genes may modify the association between ionizing radiation exposure and BC risk [28].
Several strengths and weaknesses should be considered in the interpretation of our results. First, we did not include mammography in the chest X-ray exposure because we were concerned about confounding by indication, i.e., self-selection for early mammography in women with a strong family history of BC. However, all analyses were adjusted for mammography to avoid confounding. Confounding by indication for other diagnostic procedures is expected to be highly unlikely.
Potential weaknesses also include the fact that most of the cases were prevalent cases which could lead to estimates biased toward the null if radiation exposure was associated with poorer survival. Unfortunately, very little is known about the influence of exposure to ionizing radiation at any doses on overall survival and BC specific survival in high-risk BC families. Nevertheless, we performed sensitivity analyses on a subgroup of cases diagnosed within 5 years before enrollment in the study and results remained unchanged. Another potential weakness is the selection of nonrandom friends or colleagues as controls. The advantage of such controls was the greater feasibility for finding a suitable control than through a random selection in the general population, and a higher comparability for unmeasured factors with, however, the risk of sharing some risk factors with the index cases. However, friends or colleagues’ selection should not have X-ray radiation exposures related to friend or colleague relationships, and if any, BC relative risks associated with X-ray exposures would be expected to be biased towards the null. Finally, information on lifetime X-ray exposures was self-reported with accompanying potential recall biases and exposure misclassification. We relied on self-reports rather than review of medical records because of the difficulties in accessing medical records for the various diagnostic procedures. Even if methodological studies showed that the extent of misclassification was small and mainly non-differential by disease status [29, 30], an indication of relatively poorer reporting among controls, particularly for certain types of X-ray examinations and for large numbers of such examinations, was shown by Berrington et al., although it did not translate into large differences in the estimated risks [31]. Therefore, we cannot totally rule out such a bias, and results on number of exposures and age at exposures should be interpreted with caution.
One important strength of our study is that it was conducted in a homogeneous sample of high-risk women and population controls with detailed information on diagnostic procedures at different age periods. We also dealt with missing values by performing multiple imputation, which showed results with similar magnitudes and direction of effects.
Another strength was the availability of sequencing data for 113 DNA repair genes for an important subset of the study population [20]. Indeed, our study is the first to investigate the joint effect of chest X-ray exposure and rare deleterious or likely deleterious variants in DNA repair genes in women at high risk of BC. Nevertheless, we cannot exclude potential biases due to the classification of the genes according to the ORs calculated in the same population.
Moreover, we fixed a large range of ORs around 1 for the group of DNA repair genes defined as conferring no effect on BC and this might have an impact on the findings. Therefore, sensitivity analyses were performed changing the boundaries and we found similar trends in the difference in the BC risk between groups (Supplemental Table 11). We also performed a sensitivity analysis that excluded from the “High” Group the genes that were significantly (or borderline) associated with BC in our population (i.e., ATM, CHEK2, PALB2, FANCM, MAST1) to test whether the differential effect was driven by those genes. Again, results were unchanged (Supplemental Table 12. This analysis also pointed out, for the first time, that carriers of a rare variant in the well-established BC susceptibility genes ATM, CHEK2, or PALB2 may be more radiosensitive than non-carriers.
Unlike previous reports, we did not find that the effect of chest X-ray exposure on BC risk was modified by family history [7, 9, 32]. This may be due to greater homogeneity in BC family history of the current sample compared with the previous studies.
Conclusions
Our results showed that chest X-ray exposure increases BC risk 2-fold and suggested that, independent of family history, carrying rare deleterious or likely deleterious variant(s) in some DNA repair genes may modify the effect of chest X-ray exposure. Further studies are needed to evaluate other DNA repair genes or variants to identify those which could increase radiation sensitivity. Identification of sub-populations that are more or less susceptible to ionizing radiation is important and clinically relevant.
Supplementary Information
Additional file 1. doc includes ‘Supplementary Method Section’ on the eligibility criteria for admission of BC patients to family cancer clinics and DNA repair-related variants identification.
Additional file 2: doc includes ‘Supplementary tables’. Supplemental Table 1. Comparison of the distribution of the characteristics between the subset of cases and controls with and without sequenced genes. Supplemental Table 2. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by age at censor. Supplemental Table 3. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by family history of breast cancer. Supplemental Table 4. Effect of variant carrier status on breast cancer in the GENESIS population. Supplemental Table 5. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by birth cohort, after imputation of missing data. Supplemental Table 6. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by age at censoring, after imputation of missing data. Supplemental Table 7. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by family history of breast cancer and by variant carrier status, after imputation of missing data. Supplemental Table 8. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy stratified by variant carrier status, after imputation of missing data. Supplemental Table 9. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by status of tumor estrogen receptors, after imputation of missing data. Supplemental Table 10. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy among cases diagnosed within 5 years before enrollment in GENESIS. Supplemental Table 11. Sensitivity analyses with varying bounds of OR for the definition of genetic variant group: effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy. Supplemental Table 12. Sensitivity analyses by variants group, excluding variants from the ‘High’ Group in genes individually statistically (or borderline) associated with an increased risk of breast cancer in GENESIS population.
Acknowledgements
We wish to thank the genetic epidemiology platform (the PIGE, Plateforme d’Investigation en Génétique et Epidémiologie: S. Eon-Marchais, M. Marcou, D. Le Gal, L. Toulemonde, J. Beauvallet, N. Mebirouk, E. Cavaciuti, A. Fescia), the biological resource center (C. Verny-Pierre, L. Barjhoux, V. Sornin, N. Mebirouk, F. Lesueur), and all the GENESIS collaborating cancer clinics (Clinique Sainte Catherine, Avignon: H. Dreyfus; Hôpital Saint Jacques, Besançon: M-A. Collonge-Rame; Institut Bergonié, Bordeaux: M.Longy, A. Floquet, E. Barouk-Simonet; CHU, Brest: S. Audebert; Centre François Baclesse, Caen: P. Berthet; Hôpital Dieu, Chambéry: S. Fert-Ferrer; Centre Jean Perrin, Clermont-Ferrand: Y-J. Bignon; Hôpital Pasteur, Colmar: J-M. Limacher; Hôpital d’Enfants CHU – Centre Georges François Leclerc, Dijon: L. Faivre-Olivier; CHU, Fort de France: O. Bera; CHU Albert Michallon, Grenoble: D. Leroux; Hôpital Flaubert, Le Havre: V. Layet; Centre Oscar Lambret, Lille: P. Vennin†, C. Adenis; Hôpital Jeanne de Flandre, Lille: S. Lejeune-Dumoulin, S. Manouvier-Hanu; CHRU Dupuytren, Limoges: L. Venat-Bouvet; Centre Léon Bérard, Lyon: C. Lasset, V. Bonadona; Hôpital Edouard Herriot, Lyon: S. Giraud; Institut Paoli-Calmettes, Marseille: F. Eisinger, L. Huiart; Centre Val d’Aurelle – Paul Lamarque, Montpellier: I. Coupier; CHU Arnaud de Villeneuve, Montpellier: I. Coupier, P. Pujol; Centre René Gauducheau, Nantes: C. Delnatte; Centre Catherine de Sienne, Nantes: A. Lortholary; Centre Antoine Lacassagne, Nice: M. Frénay, V. Mari; Hôpital Caremeau, Nîmes: J. Chiesa; Réseau Oncogénétique Poitou Charente, Niort: P. Gesta; Institut Curie, Paris: D. Stoppa-Lyonnet, M. Gauthier-Villars, B. Buecher, A. de Pauw, C. Abadie, M.Belotti; Hôpital Saint-Louis, Paris: O. Cohen-Haguenauer; Centre Viggo-Petersen, Paris: F. Cornélis; Hôpital Tenon, Paris: A. Fajac; GH Pitié Salpétrière et Hôpital Beaujon, Paris: C. Colas, F. Soubrier, P. Hammel, A. Fajac; Institut Jean Godinot, Reims: C. Penet, T. D. Nguyen; Polyclinique Courlancy, Reims: L. Demange†, C. Penet; Centre Eugène Marquis, Rennes: C. Dugast; Centre Henri Becquerel, Rouen: A. Chevrier, T. Frebourg††, J. Tinat, I. Tennevet, A. Rossi; Hôpital René Huguenin/Institut Curie, Saint Cloud: C. Noguès, L. Demange†, E. Mouret-Fourme; CHU, Saint-Etienne: F. Prieur; Centre Paul Strauss, Strasbourg: J-P. Fricker, H. Schuster; Hôpital Civil, Strasbourg: O. Caron, C. Maugard; Institut Claudius Regaud, Toulouse: L. Gladieff, V. Feillel; Hôpital Bretonneau, Tours: I. Mortemousque; Centre Alexis Vautrin, Vandoeuvre-les-Nancy: E. Luporsi; Hôpital de Bravois, Vandoeuvre-les-Nancy: P. Jonveaux; Gustave Roussy, Villejuif: A. Chompret†, O. Caron).
We wish to pay tribute to Olga M. Sinilnikova who was one of the initiators and principal investigators of GENESIS and who died prematurely on June 30, 2014.
Finally, we are very indebted to Dr. Alisa Goldstein (Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA) for agreeing to review and proofread this manuscript.
†: deceased prematurely
††: suddenly passed away on March 13, 2021.
Abbreviations
- BC
Breast cancer
- DNA
Deoxyribonucleic acid
- OR
Odds ratio
- CI
Confidence interval
- FFTP
First full-term pregnancy
- BRCA1/2
BRCA1 or BRCA2
Authors’ contributions
MRG and NA were responsible for the analyses, conducted the statistical analyses and wrote the report. DSL and NA led the GENESIS study and contributed to the protocol, design and search for funding. EC contributed to the protocol and design of the GENESIS study. NA and SEM were responsible for coordination of the study. DSL, BB, MGV, OC,CA, IC, PJ, CN, EMF, VB,CL, PG, AL, CD, JPF, CM, LF, EL, ML, PB, CD, JT, CC,YJB, VM, LVB,FE, DL, LG, SNH, IM, PP, TDN, JC, HD, SFF, LD, JLB, FS, VL, OCH, FP, FC, PJ, OB are geneticists at family cancer clinics who made major contributions to the invitation of index cases. SEM, JB, DL and NM were responsible for inclusion of participants, data collection and data entry. AT provided expertise for X-ray exposures. MGD was responsible for data checking and preparation. SM, LB, FD, NM and FL were responsible for the biological resource center and FL for the strategy to identify and classify the DNA repair gene variants. JC, FL provided critical readings of the manuscript and writing support. All authors reviewed the report critically and approved the final version of the manuscript.
Funding
The Brazilian National Council for the Improvement of Higher Education – CAPES, for the fellowship (process number: BEX 5852/15-3) to M.R. Guerra.
Financial support for GENESIS was provided by the Ligue Nationale contre le Cancer (3 grants: PRE05/DSL and PRE07/DSL to D. Stoppa-Lyonnet; PRE11/NA to N. Andrieu), the French National Institute of Cancer (INCa, Grant b2008-029/LL-LC), and the comprehensive cancer center SiRIC (Site de Recherche Intégrée sur le Cancer: Grant INCa-DGOS-4654) to N. Andrieu.
Availability of data and materials
The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the appropriate ethics committee (Comité de Protection des Personnes Ile-de-France III, 3 October 2006, agreement n°2373). Written informed consent was obtained for all women included in the study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thierry Frebourg is deceased.
References
- 1.Drooger JC, Hooning MJ, Seynaeve CM, Baaijens MH, Obdeijn IM, Sleijfer S, et al. Diagnostic and therapeutic ionizing radiation and the risk of a first and second primary breast cancer, with special attention for BRCA1 and BRCA2 mutation carriers: a critical review of the literature. Cancer Treat Rev. 2015;41(2):187–196. doi: 10.1016/j.ctrv.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Gray JM, Rasanayagam S, Engel C, Rizzo J. State of the evidence 2017: an update on the connection between breast cancer and the environment. Environ Health. 2017;16(1):94. doi: 10.1186/s12940-017-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrieu N, Easton DF, Chang-Claude J, Rookus MA, Brohet R, Cardis E, Antoniou AC, Wagner T, Simard J, Evans G, Peock S, Fricker JP, Nogues C, Veer LV', van Leeuwen FE, Goldgar DE. Effect of chest X-rays on the risk of breast cancer among BRCA1/2 mutation carriers in the international BRCA1/2 carrier cohort study: a report from the EMBRACE, GENEPSO, GEO-HEBON, and IBCCS Collaborators’ Group. J Clin Oncol. 2006;24(21):3361–3366. doi: 10.1200/JCO.2005.03.3126. [DOI] [PubMed] [Google Scholar]
- 4.Lecarpentier J, Nogues C, Mouret-Fourme E, Stoppa-Lyonnet D, Lasset C, Caron O, et al. Variation in breast cancer risk with mutation position, smoking, alcohol, and chest X-ray history, in the French National BRCA1/2 carrier cohort (GENEPSO) Breast Cancer Res Treat. 2011;130(3):927–938. doi: 10.1007/s10549-011-1655-3. [DOI] [PubMed] [Google Scholar]
- 5.Pijpe A, Andrieu N, Easton DF, Kesminiene A, Cardis E, Nogues C, et al. Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: retrospective cohort study (GENE-RAD-RISK) BMJ. 2012;345:e5660. doi: 10.1136/bmj.e5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma H, Hill CK, Bernstein L, Ursin G. Low-dose medical radiation exposure and breast cancer risk in women under age 50 years overall and by estrogen and progesterone receptor status: results from a case-control and a case-case comparison. Breast Cancer Res Treat. 2008;109(1):77–90. doi: 10.1007/s10549-007-9625-5. [DOI] [PubMed] [Google Scholar]
- 7.Ronckers CM, Doody MM, Lonstein JE, Stovall M, Land CE. Multiple diagnostic X-rays for spine deformities and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(3):605–613. doi: 10.1158/1055-9965.EPI-07-2628. [DOI] [PubMed] [Google Scholar]
- 8.Jansen-van der Weide MC, Greuter MJ, Jansen L, Oosterwijk JC, Pijnappel RM, de Bock GH. Exposure to low-dose radiation and the risk of breast cancer among women with a familial or genetic predisposition: a meta-analysis. Eur Radiol. 2010;20(11):2547–2556. doi: 10.1007/s00330-010-1839-y. [DOI] [PubMed] [Google Scholar]
- 9.Boice JD, Jr, Rosenstein M, Trout ED. Estimation of breast doses and breast cancer risk associated with repeated fluoroscopic chest examinations of women with tuberculosis. Radiat Res. 1978;73(2):373–390. doi: 10.2307/3574828. [DOI] [PubMed] [Google Scholar]
- 10.Hill DA, Gilbert E, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, Glimelius B, Andersson M, Wiklund T, Lynch CF, van't Veer M, Storm H, Pukkala E, Stovall M, Curtis RE, Allan JM, Boice JD, Travis LB. Breast cancer risk following radiotherapy for Hodgkin lymphoma: modification by other risk factors. Blood. 2005;106(10):3358–3365. doi: 10.1182/blood-2005-04-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John EM, Phipps AI, Knight JA, Milne RL, Dite GS, Hopper JL, Andrulis IL, Southey M, Giles GG, West DW, Whittemore AS. Medical radiation exposure and breast cancer risk: findings from the Breast Cancer Family Registry. Int J Cancer. 2007;121(2):386–394. doi: 10.1002/ijc.22668. [DOI] [PubMed] [Google Scholar]
- 12.Kenney LB, Yasui Y, Inskip PD, Hammond S, Neglia JP, Mertens AC, Meadows AT, Friedman D, Robison LL, Diller L. Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141(8):590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 13.Gronwald J, Pijpe A, Byrski T, Huzarski T, Stawicka M, Cybulski C, van Leeuwen F, Lubiński J, Narod SA. Early radiation exposures and BRCA1-associated breast cancer in young women from Poland. Breast Cancer Res Treat. 2008;112(3):581–584. doi: 10.1007/s10549-008-9892-9. [DOI] [PubMed] [Google Scholar]
- 14.Narod SA, Lubinski J, Ghadirian P, Lynch HT, Moller P, Foulkes WD, Rosen B, Kim-Sing C, Isaacs C, Domchek S, Sun P, Hereditary Breast Cancer Clinical Study Group Screening mammography and risk of breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Lancet Oncol. 2006;7(5):402–406. doi: 10.1016/S1470-2045(06)70624-6. [DOI] [PubMed] [Google Scholar]
- 15.Goldfrank D, Chuai S, Bernstein JL, Ramon YC, Lee JB, Alonso MC, et al. Effect of mammography on breast cancer risk in women with mutations in BRCA1 or BRCA2. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2311–2313. doi: 10.1158/1055-9965.EPI-06-0176. [DOI] [PubMed] [Google Scholar]
- 16.Giannakeas V, Lubinski J, Gronwald J, Moller P, Armel S, Lynch HT, Foulkes WD, Kim-Sing C, Singer C, Neuhausen SL, Friedman E, Tung N, Senter L, Sun P, Narod SA. Mammography screening and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers: a prospective study. Breast Cancer Res Treat. 2014;147(1):113–118. doi: 10.1007/s10549-014-3063-y. [DOI] [PubMed] [Google Scholar]
- 17.John EM, McGuire V, Thomas D, Haile R, Ozcelik H, Milne RL, Felberg A, West DW, Miron A, Knight JA, Terry MB, Daly M, Buys SS, Andrulis IL, Hopper JL, Southey MC, Giles GG, Apicella C, Thorne H, for the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab) Whittemore AS. Diagnostic chest X-rays and breast cancer risk before age 50 years for BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1547–1556. doi: 10.1158/1055-9965.EPI-13-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colin C, Foray N, Di Leo G, Saranelli F. Radiation induced breast cancer risk in BRCA mutation carriers from low-dose radiological exposures: a systematic review. Radioprotection. 2017;52(4):231–240. doi: 10.1051/radiopro/2017034. [DOI] [Google Scholar]
- 19.Sinilnikova OM, Dondon MG, Eon-Marchais S, Damiola F, Barjhoux L, Marcou M, Verny-Pierre C, Sornin V, Toulemonde L, Beauvallet J, le Gal D, Mebirouk N, Belotti M, Caron O, Gauthier-Villars M, Coupier I, Buecher B, Lortholary A, Dugast C, Gesta P, Fricker JP, Noguès C, Faivre L, Luporsi E, Berthet P, Delnatte C, Bonadona V, Maugard CM, Pujol P, Lasset C, Longy M, Bignon YJ, Adenis C, Venat-Bouvet L, Demange L, Dreyfus H, Frenay M, Gladieff L, Mortemousque I, Audebert-Bellanger S, Soubrier F, Giraud S, Lejeune-Dumoulin S, Chevrier A, Limacher JM, Chiesa J, Fajac A, Floquet A, Eisinger F, Tinat J, Colas C, Fert-Ferrer S, Penet C, Frebourg T, Collonge-Rame MA, Barouk-Simonet E, Layet V, Leroux D, Cohen-Haguenauer O, Prieur F, Mouret-Fourme E, Cornélis F, Jonveaux P, Bera O, Cavaciuti E, Tardivon A, Lesueur F, Mazoyer S, Stoppa-Lyonnet D, Andrieu N. GENESIS: a French national resource to study the missing heritability of breast cancer. BMC Cancer. 2016;16(1):13. doi: 10.1186/s12885-015-2028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girard E, Eon-Marchais S, Olaso R, Renault AL, Damiola F, Dondon MG, Barjhoux L, Goidin D, Meyer V, le Gal D, Beauvallet J, Mebirouk N, Lonjou C, Coignard J, Marcou M, Cavaciuti E, Baulard C, Bihoreau MT, Cohen-Haguenauer O, Leroux D, Penet C, Fert-Ferrer S, Colas C, Frebourg T, Eisinger F, Adenis C, Fajac A, Gladieff L, Tinat J, Floquet A, Chiesa J, Giraud S, Mortemousque I, Soubrier F, Audebert-Bellanger S, Limacher JM, Lasset C, Lejeune-Dumoulin S, Dreyfus H, Bignon YJ, Longy M, Pujol P, Venat-Bouvet L, Bonadona V, Berthet P, Luporsi E, Maugard CM, Noguès C, Delnatte C, Fricker JP, Gesta P, Faivre L, Lortholary A, Buecher B, Caron O, Gauthier-Villars M, Coupier I, Servant N, Boland A, Mazoyer S, Deleuze JF, Stoppa-Lyonnet D, Andrieu N, Lesueur F. Familial breast cancer and DNA repair genes: insights into known and novel susceptibility genes from the GENESIS study, and implications for multigene panel testing. Int J Cancer. 2019;144(8):1962–1974. doi: 10.1002/ijc.31921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van BS, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681–694. doi: 10.1002/(SICI)1097-0258(19990330)18:6<681::AID-SIM71>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Raghunathan TE. What do we do with missing data? Some options for analysis of incomplete data. Annu Rev Public Health. 2004;25(1):99–117. doi: 10.1146/annurev.publhealth.25.102802.124410. [DOI] [PubMed] [Google Scholar]
- 23.StataCorp. 2015. Stata: Release 14. Statistical software. College Station, TX: StataCorp LP. 2015.
- 24.Roderick J. A. Little, Donald B. Rubin. Statistical analysis with missing data. Second Edition ed. John Wiley & Sons, Inc.; 2002.
- 25.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 26.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett L, Ding W, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 27.Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G, Barfoot R, Hamoudi R, Patel S, Rices C, Biggs P, Hashim Y, Smith A, Connor F, Arason A, Gudmundsson J, Ficenec D, Kelsell D, Ford D, Tonin P, Timothy Bishop D, Spurr NK, Ponder BAJ, Eeles R, Peto J, Devilee P, Cornelisse C, Lynch H, Narod S, Lenoir G, Egilsson V, Bjork Barkadottir R, Easton DF, Bentley DR, Futreal PA, Ashworth A, Stratton MR. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 28.Sigurdson AJ, Bhatti P, Chang SC, Rajaraman P, Doody MM, Bowen L, Simon SL, Weinstock RM, Linet MS, Rosenstein M, Stovall M, Alexander BH, Preston DL, Struewing JP. Polymorphisms in estrogen biosynthesis and metabolism-related genes, ionizing radiation exposure, and risk of breast cancer among US radiologic technologists. Breast Cancer Res Treat. 2009;118(1):177–184. doi: 10.1007/s10549-009-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman SA, Localio AR, Zhou L, Bernstein L, Coates RJ, Flagg EW, Marchbanks PA, Malone KE, Weiss LK, Lee NC, Nadel MR. Validation of self-reported screening mammography histories among women with and without breast cancer. Am J Epidemiol. 2003;158(3):264–271. doi: 10.1093/aje/kwg136. [DOI] [PubMed] [Google Scholar]
- 30.Pogoda JM, Preston-Martin S. Radiation exposure from diagnostic imaging: agreement between self-report and medical records. Health Phys. 2002;83(6):907–917. doi: 10.1097/00004032-200212000-00019. [DOI] [PubMed] [Google Scholar]
- 31.de GA B, Ekbom A, Glass AG, Galanti MR, Grimelius L, Allison MJ, et al. Comparison of documented and recalled histories of exposure to diagnostic X-rays in case-control studies of thyroid cancer. Am J Epidemiol. 2003;157(7):652–663. doi: 10.1093/aje/kwg026. [DOI] [PubMed] [Google Scholar]
- 32.IARC Working Group on the Evaluation of Carcinogenic Risks to Human. IARC Monogr Eval Carcinog Risks Hum 2012; 100(Pt D):7-303.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. doc includes ‘Supplementary Method Section’ on the eligibility criteria for admission of BC patients to family cancer clinics and DNA repair-related variants identification.
Additional file 2: doc includes ‘Supplementary tables’. Supplemental Table 1. Comparison of the distribution of the characteristics between the subset of cases and controls with and without sequenced genes. Supplemental Table 2. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by age at censor. Supplemental Table 3. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by family history of breast cancer. Supplemental Table 4. Effect of variant carrier status on breast cancer in the GENESIS population. Supplemental Table 5. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by birth cohort, after imputation of missing data. Supplemental Table 6. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by age at censoring, after imputation of missing data. Supplemental Table 7. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by family history of breast cancer and by variant carrier status, after imputation of missing data. Supplemental Table 8. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy stratified by variant carrier status, after imputation of missing data. Supplemental Table 9. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy by status of tumor estrogen receptors, after imputation of missing data. Supplemental Table 10. Effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy among cases diagnosed within 5 years before enrollment in GENESIS. Supplemental Table 11. Sensitivity analyses with varying bounds of OR for the definition of genetic variant group: effect of lifetime chest X-ray exposure (any exposure) on breast cancer risk according to the number of exposures, the age at first exposure and the first full-term pregnancy. Supplemental Table 12. Sensitivity analyses by variants group, excluding variants from the ‘High’ Group in genes individually statistically (or borderline) associated with an increased risk of breast cancer in GENESIS population.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.